Abstract
BACKGROUND
The pyramided genetically modified maize (Zea mays [L.]) event MON 95379, expressing the Cry1B.868 and Cry1Da_7 proteins, was designed to protect against larval feeding damage by the fall armyworm, Spodoptera frugiperda (FAW). Here, we conducted laboratory, greenhouse, and field studies to assess the dose and field efficacy of MON 95379 against FAW and inform the development of insect resistance management plans.
RESULTS
The Cry1B.868 and Cry1Da_7 proteins were active against susceptible FAW neonates in diet‐incorporation bioassays: median lethal concentration [LC50] (95% CI) = 62.8 (42.6–87.6) μg/ml diet for Cry1B.868 and 9.4 (5.3–18.6) μg/ml diet for Cry1Da_7. In laboratory leaf disc bioassays, MON 95379 maize and experimental maize lines expressing the individual components were effective in controlling susceptible FAW. In whole‐plant assays, MON 95379 controlled FAW resistant to the Cry1A.105 and Cry2Ab2 proteins. Likewise, under field conditions, MON 95379 maize expressing Cry1B.868 and Cry1Da_7 was highly effective at protecting plants against the larval feeding of FAW.
CONCLUSIONS
The expression of Cry1B.868 and Cry1Da_7 in MON 95379 consistently protected maize plants against larval feeding by FAW and represents an alternative to manage trait resistance issues in South America. © 2021 Bayer Crop Science‐US. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: FAW, Spodoptera frugiperda, Bt pyramids, MON 95379
The pyramided genetically modified maize (Zea mays [L.]) event MON 95379, expressing the Cry1B.868 and Cry1Da_7 proteins was designed to protect against larval feeding damage of the fall armyworm, Spodoptera frugiperda (FAW). The expression of Cry1B.868 and Cry1Da_7 in MON 95379 consistently protected maize plants against larval feeding of FAW and represents an alternative to manage trait resistance issues in South America.

1. INTRODUCTION
The fall armyworm, Spodoptera frugiperda (FAW), is an insect indigenous to the western hemisphere1 that has long been considered a pest of maize and other economically important crops. FAW is a migratory and highly polyphagous pest species2, 3 that does not have the ability to enter diapause, an inactive state that allows insects to survive prolonged periods of unfavorable conditions such as extreme cold or drought.1, 4 In warm‐winter areas such as Central and South America, FAW is considered the primary maize pest5, 6 whereas in the United States, due to the absence of a diapause trait, this pest species must migrate northward annually to re‐infest temperate cropping areas.7, 8 Overwintering regions in the southern portions of Florida and Texas, extending into Mexico, are assumed to be the source of practically all FAW infestations in the United States and Canada.7 In recent years, this species has achieved the status of a global pest after the documentation of the presence and damage of FAW in maize plants across the eastern hemisphere (e.g., Africa and Asia).9, 10, 11, 12, 13 The high reproductive capacity, multivoltinism (multiple generations per year) and polyphagy2, 3, 14 of FAW all contribute to its status as a major global pest of maize.6, 7, 11, 13, 15 FAW causes extensive injury in the maize whorl as well as eventual direct damage to the ear, resulting in yield reductions ranging from 21% to 73%.16, 17 In addition, late FAW instars can behave as cutworms leading to severe reduction in seedling stand and counts.18
Synthetic insecticides have been the traditional primary control tactic used to manage FAW in maize and other crops in South America. However, the movement of FAW larvae into the maize whorl typically limits the efficacy of synthetic insecticides by making it difficult for the sprayed active ingredients to reach the insects.19 Moreover, the efficacy of systemic insecticides (e.g., seed treatments) is limited against FAW, and therefore may not be sufficient to delay or reduce foliar sprays to prevent damage by FAW.20 Furthermore, resistance to several distinct classes of insecticides has been documented in Brazil.21, 22, 23, 24
For these reasons, genetically modified (GM) plants expressing insecticidal proteins from the bacteria Bacillus thuringiensis (Bt) have become well accepted by growers for FAW control in South America.25, 26, 27, 28 A recurrent challenge threatening the long‐term durability of Bt crops is their high adoption rate coupled with high selection pressure on target insects and low compliance with non‐Bt structured refuge recommendations.29, 30 The assumption is that rare homozygous resistant insects that survive on Bt plants will mate with the relatively abundant homozygous susceptible insects in refuges. Providing that inheritance of resistance is recessive, the resulting heterozygous offspring will not survive on high‐dose Bt crops, substantially delaying the evolution of resistance. This dynamic is often called the “high dose‐refuge” strategy.29 The use of Bt maize plants with less‐than‐ideal IRM fit (e.g., less‐than‐high‐dose technologies, and components of Bt pyramids with cross‐resistance to other Bt proteins in the landscape) combined with low compliance with structured refuge recommendations seems to be a common theme across the resistance cases documented in FAW in Latin America.31, 32 FAW has developed field resistance to Cry1F in Puerto Rico,33 Brazil,31, 34 the United States35, 36 and Argentina,37 and to Cry1Ab maize in Brazil.38 Moderate levels of cross‐resistance between Cry1F and Cry1A.105 and less‐than‐high‐dose activity of Cry2Ab2 against FAW likely were key causes of resistance to a Bt maize technology expressing Cry1A.105 and Cry2Ab2.32, 39 Vip3Aa20 is currently the most effective mode of action against FAW in the field in Brazil and Argentina. However, researchers were able to isolate a Vip3Aa20 resistance allele from a natural field population of the pest, indicating the potential for relatively rapid evolution of resistance in FAW under scenarios of low refuge compliance.40, 41 These circumstances indicate the need to identify new insecticidal modes of action and deploy them rapidly as pyramids with suitable refuge strategies to sustain the benefits of Bt maize to growers in South America.42
There are several proteins from the Cry1B, Cry1C and Cry1D subclasses of Bt protein that are toxic to FAW.43 Thus, it may be possible to use different Cry1 protein subclasses in transgenic crops to overcome existing field resistance to certain Cry1 subclasses. Insect‐protected maize MON 95379 was developed to produce the insecticidal proteins Cry1B.868 and Cry1Da_7, which protect against feeding damage caused by targeted pest Lepidoptera. Cry1B.868 is a chimeric protein comprised of domains I and II from Cry1Be (Bt), domain III from Cry1Ca (Bt subsp. aizawai) and the C‐terminal protoxin domain from Cry1Ab (Bt subsp. kurstaki). Cry1Da_7 is a modified Cry1Da protein derived from Bacillus thuringiensis (Bt subsp. aizawai). The activity of Cry1B.868 and Cry1Da_7 against FAW was documented by Wang et al.44 Results from resistant insect bioassays, disabled insecticidal protein bioassays,45 and cell‐based assays using insect cells expressing individual receptors demonstrated that the receptor utilization of the newly modified Cry1Da_7 and Cry1B.868 proteins in FAW were distinct from each other and from commercially available Bt proteins such as Cry1F, Cry1A.105, Cry2Ab, and Vip3A. According to their results, the Cry1Da_7 and Cry1B.868 proteins when pyramided together should provide practical durability to MON 95379 in the field against this economically important pest.44 Here, we present the first in planta evaluations of MON 95379, a new GM maize pyramid of Cry1B.868 and Cry1Da_7 designed to protect against FAW larval feeding damage.
2. MATERIALS AND METHODS
2.1. Susceptibility of FAW to Cry1B.868 and Cry1Da_7 in diet‐incorporation bioassays
Bt‐produced Cry1B.868 protein at 2.5 mg/ml and Cry1Da_7 protein at 3.3 mg/ml were received frozen from the Protein Technology Team (Regulatory Science, Bayer Crop Science US). The Cry1B.868 protein was dissolved in a buffer solution containing 20 mm CAPS (pH 11.5), 10 mm dithiothreitol, 240 mm NaCl and 1 mm benzamidine. The Cry1Da_7 protein was dissolved in a buffer containing 25 mm sodium carbonate, pH 10.5. When not in use, the test substances were stored in a freezer at −80°C or on dry ice. FAW eggs were obtained from a Bayer Crop Science insect rearing facility. This susceptible S. frugiperda strain was established in 2007 and has been maintained on artificial diet without any exposure to Bt proteins. By the time of the experiments presented here, this strain had been in the laboratory for approximately 114–116 generations without infusion of wild genes. FAW eggs were incubated within environmental chambers with at target temperature of 10°C and 27°C, 60% relative humidity (RH), and a 14:10 h light/dark photoperiod to obtain the desired hatch time. The functional activity of the Cry1B.868 and Cry1Da_7 proteins was measured in 7‐day diet‐incorporation bioassays. Treatments were prepared by mixing 4 ml of dosing solution that contained Cry1B.868 or Cry1Da_7 protein at appropriate concentrations with 16 ml of lepidopteran diet (Southland) to achieve a final volume of 20 ml. The concentrations of Cry1B.868 used in the bioassay ranged from 0.01 to 5 μg/ml of artificial diet for the estimation of the effective concentration (EC) values, and from 10 to 160 μg/ml of artificial diet for the estimation of median lethal concentration (LC) values. The concentrations of Cry1Da_7 used in the bioassay ranged from 0.01 to 80 μg/ml of artificial diet for the estimation of the EC values, and from 0.32 to 80 μg/ml of artificial diet for the estimation of LC values. The prepared artificial diet was kept in a water bath to allow it to cool. The temperature of the artificial diet was measured and the Cry1B.868 or Cry1Da_7 dosing solutions were added when it reached below 46°C. Each dosing solution was thoroughly mixed in a tube containing artificial diet by vortexing. The bioassays consisted of a geometric series of protein dilutions, an untreated control and a buffer control. The treated diet mixture was dispensed in 1‐ml aliquots into 16 wells per treatment level in a 128‐well tray. Each well was infested with a single FAW larva, totaling 16 FAW neonates per concentration of Cry1B.868 or Cry1Da_7. Bioassay trays were incubated at a target temperature of 27°C, 60% RH, and a 14:10 h light/dark photoperiod for 7 days after infestation. The number of insects infested, the number of surviving insects, and the combined mass of the surviving insects were recorded after the 7‐day incubation period (assay termination). The concentration–response relationships for mortality and growth inhibition of surviving FAW larvae were analyzed using Probit analysis (PROC PROBIT) and a three‐parameter logistic regression (PROC NLMIXED), respectively, in SAS.46 Results from the concentration–response bioassays were used to estimate EC and LC values and their associated 95% confidence intervals (95% CI).
2.2. Efficacy of MON 95379, Cry1B.868 and Cry1Da_7 against FAW in leaf disc bioassays
Leaf discs of MON 95379 (containing the Cry1B.868 and Cry1Da_7 proteins), two experimental lines containing the single Bt proteins, namely Cry1B.868 (Cry1B.868_single) and Cry1Da_7 (Cry1Da_7_single), and a near‐isogenic negative check were taken from plants grown in a Bayer Crop Science greenhouse at Chesterfield, MO, USA. Both experimental lines (Cry1B.868_single and Cry1Da_7_single) express levels of their respective Bt protein comparable with those in the MON 95379 event (see Results). The presence of the Bt genes of interest, cry1B.868 or cry1Da_7, in these Bt maize lines was verified by polymerase chain reaction (PCR) assays. Leaf tissue was harvested when all plants reached the V8 growth stage and then stored in a freezer at –80°C. The content of Cry1B.868 and Cry1Da_7 in the leaf tissue of these maize line was analyzed by enzyme‐linked immunosorbent assay (ELISA) at Bayer Crop Science laboratories (Chesterfield, MO, USA). A pool of leaves from each maize line was frozen, milled, lyophilized and stored at −80°C prior to analysis. On the ELISA plate, each biological replicate was analyzed as a technical quadruplicate (i.e., 384‐well plates).
The same maize lines tested for expression of Cry1B.868 and Cry1Da_7 were used in leaf disc bioassays at a Bayer Crop Science Brazil facility in Santa Cruz das Palmeiras, São Paulo, Brazil. All maize lines tested were in the same genetic background, minimizing possible confounding effects in the bioassays. When maize plants reached the V4–V5 stage, the newest completely expanded leaves were removed from the greenhouse‐grown plants. Leaf discs measuring 2.2 cm in diameter were cut using a metallic cutter and placed on a non‐gelled mixture of water and agar at 2.0% (1 ml/well) in 12‐well acrylic plates (Costar®, Maizeing). Leaf discs were separated from the water–agar layer using a filter paper disc. One neonate larva (less than 24 h old) was placed in each well of the plate using a fine brush. Plates were sealed with plastic film and incubated in an environmental chamber (25 ± 1°C, 60% ± 10% RH, 14:10 h light/dark photoperiod). The experimental design was completely randomized with ten replicates per treatment, for a total of 120 neonates of the S. frugiperda susceptible strain tested on each maize event. This susceptible S. frugiperda strain was established by sampling larvae from non‐Bt maize plants in Conchal, São Paulo, Brazil in 2014. This susceptible strain has been maintained on artificial diet without any exposure to Bt proteins. By the time of the experiments presented here, this strain had been in the laboratory for approximately 50 generations without infusion of wild genes. Larval mortality, instar, and growth inhibition relative to the control were recorded at 5 days after leaf disc infestation (assay termination). Data were subjected to analysis of variance (ANOVA), and treatment averages were compared by the Tukey test (P ≤ 0.05), using JMP software (JMP®, v. 12; SAS Institute Inc.).47
2.3. Efficacy of MON 95379 against a MON 89034‐resistant FAW strain
A FAW strain (MON 89034‐R) resistant to MON 89034 maize (containing the Cry1A.105 and Cry2Ab2 proteins) was laboratory‐selected from a field population sampled in Casa Branca, São Paulo, Brazil. Insects were sampled on MON 89034 maize plants in 2016 and the F2 screen technique48 was used to identify FAW larvae carrying alleles conferring resistance to MON 89034 maize. Since the original selection on MON 89034, every generation of this strain has been reared on MON 89034 leaf tissue to maintain the selection pressure for Cry1A.105 and Cry2Ab2 resistance. In leaf disc tests, the survivorship of insects from this RR strain on MON 89034 and plants expressing Cry1A.105 and Cry2Ab2 individually was: 75.8 ± 5.6% on MON 89034; 76.6 ± 5.5% on Cry1A.105; 74.2 ± 6.5% on Cry2Ab2; and 80.8 ± 4.3% on non‐Bt maize in 5‐day leaf disk assays. F2 neonates were screened on leaf tissues of MON 89034 maize excised from greenhouse‐grown plants at V4–V8.32, 49
Greenhouse (whole‐plant) trials were performed to evaluate survival of the resistant FAW strain on MON 95379, MON 89034, and non‐Bt maize (near‐isoline). Plants in the greenhouse were cultivated in 4‐L plastic pots following a completely randomized experimental design. At the V5 growth stage, plants were artificially infested with 3 second‐instar MON 89034‐R FAW (less than 24 h old; four plants/entry) using a paintbrush. To minimize the influence of larval movement between plants, there was approximately 1‐m between the 4‐L plastic pots. At 9 days after infestation, the incidence of FAW larvae was determined by dissecting the maize plants, counting the number of live FAW larvae, and visually sorting them into the following size categories: medium (between 2 mm and 1.5 cm) and large (more than 1.5 cm). Leaf damage was scored using a scale of 0–9 (Davis scale).50 Means were compared by LSMeans contrasts (α = 0.05) for survival, number of larvae in each size class, and leaf damage using JMP software (JMP®, v. 12).47 To test the hypothesis that MON 95379 is protected against FAW, the first contrast compared survival of second‐instar MON 89034‐R FAW on MON 95379 maize with its survival on non‐Bt maize. The second contrast compared survival of second‐instar MON 89034‐R FAW on MON 89034 maize with its survival on non‐Bt maize. The third contrast compared survival of second‐instar MON 89034‐R FAW on MON 89034 maize with its survival on MON 95379 maize. Contrasts 4, 5 and 6 compared the number of large larvae on non‐Bt and MON 89034, non‐Bt and MON 95379, and MON 89034 and MON 95379, respectively. Contrasts 7, 8 and 9 compared leaf feeding damage on MON 95379 and non‐Bt maize, MON 89034 and non‐Bt maize, and MON 95379 and MON 89034 maize, respectively.
2.4. Efficacy of MON 95379 maize against FAW in naturally infested field trials
The efficacy of MON 95379 was evaluated under natural FAW infestations in Brazil. The field trials were conducted at the following Bayer Crop Science research sites in Brazil: Não‐Me‐Toque, RS; Rolândia, PR; Santa Cruz das Palmeiras, SP; Cachoeira Dourada, MG; Sorriso, MT and Luis Eduardo Magalhães, BA. The field trials were planted from September to November in the 2019 season (spanning 2018/2019) and the 2020 season (spanning 2019/2020). There were a total of six and four entries were in the original experimental designs in the 2019 and 2020 seasons, respectively; however, here we present only data comparing MON 95379, MON 89034 × MON 88017 and non‐Bt maize (near‐isoline). MON 89034 produces the Bt‐derived proteins Cry1A.105 and Cry2Ab2, which are active against lepidopteran pests. MON 88017 maize has been genetically modified to express a modified cry3Bb1 gene from Bt subsp. kumamotoensis. This gene encodes the protein Cry3Bb1 that protects maize plants against feeding damage by corn rootworm larvae (Diabrotica spp.). The experimental design had randomized blocks with four replicates per treatment. The plot size of each treatment during the 2019 season trials was 32 m2 (8 m length × 4 m width) with eight rows and a row spacing of 0.5 m. The useful area of each treatment during the 2020 season trials was 15 m2 (5 m length × 3 m width) with six rows and a row spacing of 0.5 m. Larval incidence and leaf damage (Davis scale) were monitored approximately every 7 days. Three evaluations were performed: between V3 and V4, between V5 and V6, and between V8 and V9. The incidence of FAW larvae was determined by dissecting the maize plants, counting the number of live FAW larvae and visually sorting them into the following size categories: small (2 mm or less) versus medium and large (more than 2 mm). During each evaluation (V3 and V4, V5 and V6, and V8 and V9) of larval incidence and damage, 15 plants in sequence per row, at each time point, were randomly evaluated in a single row per plot. Leaf damage was scored using the Davis 0–9 scale.50 Plants evaluated were in the center of each row, and their location was prescribed in the protocol. Means were compared by LSMeans contrasts (α = 0.05) for survival, size of larvae, and damage using JMP software (JMP®, version 12).47
3. RESULTS
3.1. Susceptibility of FAW to Cry1B.868 and Cry1Da_7 in diet‐incorporation bioassays
Both the Cry1B.868 and Cry1Da_7 proteins demonstrated a concentration‐dependent relationship with FAW growth inhibition (Figure 1A) and mortality (Figure 1B), but the slopes of the dose–response curve for growth inhibition and mortality were significantly different between Cry1B.868 and Cry1Da_7. The EC50 (95% CI) value was 0.15 (0.12–0.19) μg/ml diet for Cry1B.868 and 0.096 (0.087–0.10) μg/ml diet for Cry1Da_7 (Table 1). The EC90 (95% CI) value was 0.58 (0.33–0.82) μg/ml diet for Cry1B.868 and 0.26 (0.21–0.31) μg/ml diet for Cry1Da_7 (Table 1). The EC50 and EC90 values indicate that the proteins had similar growth inhibition activity against FAW. However, the Cry1Da_7 protein showed higher activity based on mortality than the Cry1B.868 protein (Figure 1B). The LC50 (95% CI) value was 9.4 (5.3–18.6) μg/ml diet for Cry1Da_7, compared with 62.8 (46.2–87.6) μg/ml diet for Cry1B.868. The LC90 (95% CI) value was 225.8 (84.1–1241) μg/ml diet for Cry1Da_7, compared with 179.3 (119.9–390.6) μg/ml diet for Cry1B.868 (Table 2).
FIGURE 1.
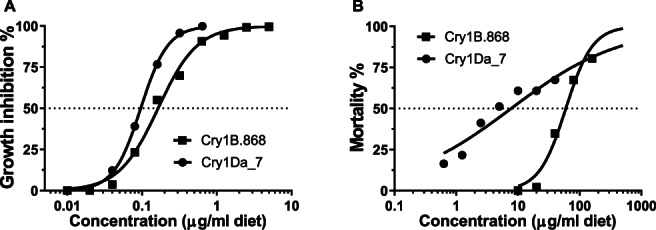
Concentration–response curves for growth inhibition (A) and mortality (B) with Cry1B.868 (black squares) and Cry1Da_7 (black circles) against Spodoptera frugiperda in 7‐day diet incorporation bioassays.
TABLE 1.
Concentration–growth inhibition response (EC μg/ml diet)a of Spodoptera frugiperda neonates exposed to the Cry1B.868 and Cry1Da_7 proteins incorporated into artificial diet
| Protein | n b | EC50 and 95% CI (μg/ml diet) | EC90 and 95% CI (μg/ml diet) | Slope ± SE |
|---|---|---|---|---|
| Cry1B.868 | 13 (204) | 0.15 (0.12–0.19) | 0.58 (0.33–0.82) | 1.7 ± 0.2 |
| Cry1Da_7 | 10 (158) | 0.096 (0.086–0.10) | 0.26 (0.21–0.31) | 2.2 ± 0.2 |
EC50 = effective concentration of Cry1B.868 and Cry1Da_7 (μg/ml diet) required to cause 50% growth inhibition in the observation period of 7 days. Similarly, EC90 is the effective concentration of Cry1B.868 and Cry1Da_7 required for 90% growth inhibition.
Number of groups of insects weighed, with total number of insects in parentheses. Additional concentrations were used to estimate the EC resulting in a larger number of insects tested relative to the determination of the LC values.
TABLE 2.
Concentration–mortality response (LC μg/ml diet)a of Spodoptera frugiperda neonates exposed to the Cry1B.868 and Cry1Da_7 proteins incorporated into artificial diet
| Protein | n b | LC50 and 95% CI (μg/ml diet) | LC90 and 95% CI (μg/ml diet) | Slope ± SE | dfc | ∑χ2 |
|---|---|---|---|---|---|---|
| Cry1B.868 | 128 | 62.8 (46.2–87.6) | 179 (119.9–390.6) | 2.8 ± 0.5 | 13 | 16 |
| Cry1Da_7 | 189 | 8.3 (4.5–16.7) | 225.8 (84.1–1241) | 0.9 ± 0.1 | 12 | 12.8 |
LC50 = concentration of Cry1B.868 and Cry1Da_7 (μg/ml diet) required to cause 50% mortality in the observation period of 7 days. Similarly, LC90 is the concentration of Cry1B.868 and Cry1Da_7 required for 90% mortality.
Number of insects tested.
Degrees of freedom.
3.2. Efficacy of MON 95379, Cry1B.868 and Cry1Da_7 against FAW in leaf disc bioassays
Expression of Cry1B.868 protein in greenhouse‐grown MON 95379 and in the experimental line Cry1B.868_single was 86 and 81 μg/g fw, respectively, whereas expression of Cry1Da_7 in MON 95379 and in the Cry1Da_7_single was 17 and 4.7 μg/g fw, respectively (Table 3). Seeds from the assayed plants were used to produce the plant material utilized in the leaf disc bioassays. In the leaf disc bioassays, survivorship of FAW neonates on all Bt maize materials was significantly lower than on non‐Bt maize (F = 539.03, df = 3,36; P < 0.0001) (Table 4). No FAW larvae survived on MON 95379 or on Cry1B.868_single leaf discs 5 days after infestation; however, one larva of 120 tested (0.8%) survived on Cry1Da_7_single (Table 4). The surviving larva exhibited significant growth inhibition indicated by weight reduction and instar relative to larvae developing on non‐Bt [approximately 67% weight reduction and at an earlier instar (L2) than most larvae on non‐Bt maize (87.2% at L3)] (Table 4). The higher LC90 of Cry1Da_7 compared with Cry1B.868 coupled with low expression of Cry1Da_7 in the Cry1Da_7_single may explain this surviving larva.
TABLE 3.
Cry1B.868 and Cry1Da_7 protein levels in leaf tissue of MON 95379, Cry1B.868_single, Cry1Da_7_single, and non‐Bt maize
| Protein | Entry | Mean concentration (μg/g fw)a , b |
|---|---|---|
| Cry1B.868 | MON 95379 | 86 |
| Cry1B.868_single | 81 | |
| Cry1Da_7_single | <LOQ | |
| Non‐Bt | <LOQ | |
| Cry1Da_7 | MON 95379 | 17 |
| Cry1B.868_single | <LOQ | |
| Cry1Da_7_single | 4.7 | |
| Non‐Bt | <LOQ |
LOQ, limit of quantitation.
Protein levels are expressed as the mean (μg) per gram (g) of tissue on a fresh weight (fw) basis.
Plants were grown in a controlled environment. Leaf tissue was harvested when all plants reached the V8 growth stage and then stored at –80°C in a freezer.
TABLE 4.
Survival of susceptible Spodoptera frugiperda neonates on leaf discs of MON 95379, Cry1B.868_single, Cry1Da_7_single and non‐Bt maize after 5 days
| Entry | n | Survival (%) | Larval stage of survivorsa | Weight (mg)b | ||
|---|---|---|---|---|---|---|
| L1 | L2 | L3 | ||||
| MON 95379 | 120 | 0.0 ± 0.0 b | n.a. | n.a. | n.a. | n.a. |
| Cry1B.868_single | 120 | 0.0 ± 0.0 b | n.a. | n.a. | n.a. | n.a. |
| Cry1Da_7_single | 120 | 0.8 ± 0.8 b | 0.0 | 100.0 | 0.0 | 1.7c |
| Non‐Bt | 120 | 89.1 ± 3.7 a | 0.0 ± 0.0 | 12.3 ± 3.1 | 87.2 ± 3.1 | 5.2 ± 0.5 |
n.a., not applicable.
Values represent means ± SE. A separate ANOVA (Tukey test, P ≤ 0.05) was conducted for treatments within survival column (means followed by the same letter in that column are not significantly different).
Mean weight of survivors.
Data from one survivor.
3.3. Efficacy of MON 97379 against a MON 89034‐R FAW strain
MON 95379 showed high efficacy against second‐instar larvae of the MON 89034‐R FAW strain (Contrast 1, t ratio = 4.92, df = 4,39; P < 0.0001), and there was no survival at 9 days after infestation (Table 5). There was no difference between MON 89034 and non‐Bt for survivorship (Contrast 2, t ratio = 0.82, df = 4,39; P = 0.42). There was a significant difference between survivorship on MON 89034 and MON 95379 (Contrast 3, t ratio = 4.1, df = 4,39; P < 0.0002). There was no difference among treatments in the number of medium‐size larvae and for Contrast 4 between the number of large larvae on non‐Bt and MON 89034 (Contrast 4, t ratio = 0.91, df = 4,39; P = 0.37), but there were significant differences between non‐Bt and MON 95379 (Contrast 5, t ratio = 4.55, df = 4,39; P < 0.0001) and between MON 89034 and MON 95379 for number of large larvae (Contrast 6, t ratio = 3.64, df = 4,39; P < 0.0008). MON 95379 demonstrated high efficacy against second‐instar resistant FAW, significantly reducing the leaf feeding damage compared with non‐Bt maize (Contrast 7, t ratio = 12.44, df = 4,39; P < 0.0001). There were also significant differences among treatments for leaf damage on MON 89034 and non‐Bt maize (Contrast 8, t ratio = 3.61, df = 4,39; P < 0.0009) and on MON 95379 and MON 89034 (Contrast 9, t ratio = 8.83, df = 4,39; P < 0.0001) at 9 days after infestation (Table 5).
TABLE 5.
Survival, size of surviving larvae and damage caused by MON 89034‐resistant Spodoptera frugiperda on MON 95379, MON 89034 and non‐Bt maize plants after 9 days
| Entry | Survival (%) | Size of surviving larvaea | Foliar damageb | |
|---|---|---|---|---|
| Mediumc | Larged | |||
| Non‐Bt | 50.00 ± 9.62 | 0.25 ± 0.25 | 1.25 ± 0.25 | 8.75 ± 0.25 |
| MON 89034 | 41.67 ± 8.33 | 0.25 ± 0.25 | 1.00 ± 0.41 | 6.50 ± 0.65 |
| MON 95379 | 00.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.00 |
Values represent mean number of larvae per plant ± SE.
Larvae >2 mm and <1.5 cm.
Larvae >1.5 cm.
Values represent mean damage per plant ± SE. Davis scale of 0 (no visible damage) to 9 (whorl and furl leaves almost destroyed) was used to assess maize leaf injury.
3.4. Efficacy of MON 95379 maize against FAW in naturally infested field trials
In the assessment of the efficacy of MON 95379 maize under natural FAW infestations, a reduction in the foliar damage caused by FAW relative to non‐Bt maize plants was evident across locations and years (Figures 2 and 3) despite the variable FAW pressure among locations and maize development stages. FAW pressure was categorized based on non‐Bt leaf feeding injury and indicated as either negligible (mean of 1 or less on the Davis scale) or high (mean above 3 on the Davis scale) (Figures 2 and 3). The threshold for insecticide applications to manage FAW in Brazil is a mean Davis rating on non‐Bt plants of 3 or more.19, 50 In both the 2019 and 2020 seasons, significantly less FAW damage was observed on MON 95379 plants relative to non‐Bt maize and MON 89034 × MON 88017, particularly under high FAW pressure (Figures 2 and 3). The mean damage due to FAW observed on MON 95379 plants was consistently negligible (1 or less on the Davis scale) even under high insect pressure (Figures 2 and 3). The mean FAW damage observed on MON 89034 × MON 88017 plants was significantly different from the damage observed on non‐Bt maize plants at most locations (Figures 2 and 3). However, in some locations with high FAW pressure (above 3 on the Davis scale) at some development stages of the maize plants, there was no difference between the MON 89034 × MON 88017 and non‐Bt treatments (Figures 2B,C and 3B,E). Likewise, the number of FAW larvae larger than 2 mm was significantly lower on MON 95379 plants than on non‐Bt maize (Figures 4 and 5) and MON 89034 × MON 88017 (Figures 4B,C and 5A,B,E). For details on the results of statistical analysis see Tables S1 and S2.
FIGURE 2.
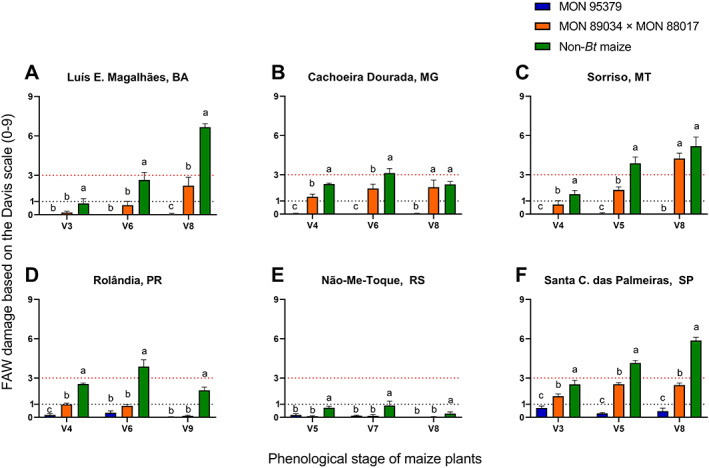
Mean Spodoptera frugiperda leaf damage (Davis scale) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2019 natural pressure field trials. *Statistically significant differences (LSMeans contrast, P ≤ 0.05) between MON 95379, MON 89034 × MON 88017 and non‐Bt maize at the indicated maize phenological stage in the 2019 season. Error bars represent standard error. Dashed lines represent negligible FAW pressure, and high pressure/insecticide action threshold.
FIGURE 3.
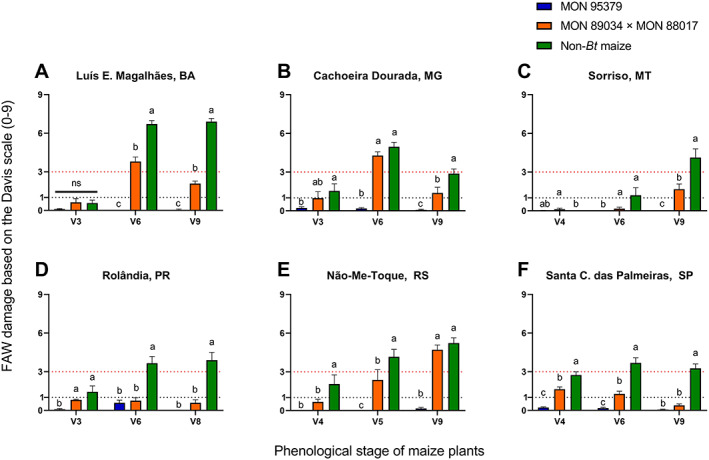
Mean Spodoptera frugiperda leaf damage (Davis scale) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2020 natural pressure field trials. *Statistically significant differences (LSMeans contrast, P ≤ 0.05) between MON 95379, MON 89034 × MON 88017 and non‐Bt maize at the indicated maize phenological stage in the 2020 season. Error bars represent standard error. Dashed lines represent negligible FAW pressure, and high pressure/insecticide action threshold.
FIGURE 4.
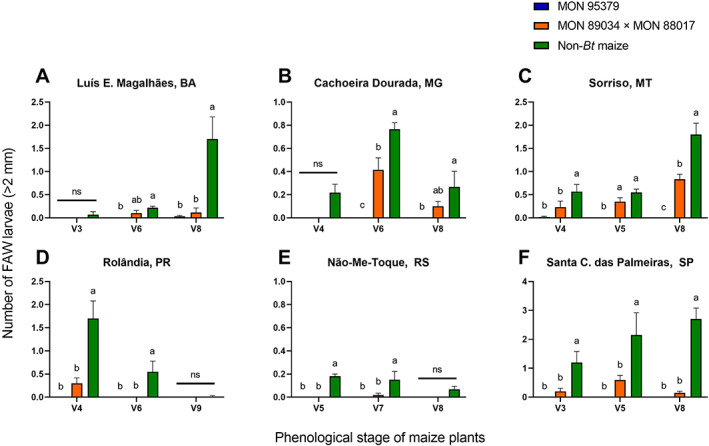
Mean number of Spodoptera frugiperda larvae (greater than 2 mm) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2019 natural pressure field trials. *Statistically significant differences (LSMeans contrast, P ≤ 0.05) between MON 95379, MON 89034 × MON 88017 and non‐Bt maize at the indicated maize phenological stage. Error bars represent standard error.
FIGURE 5.
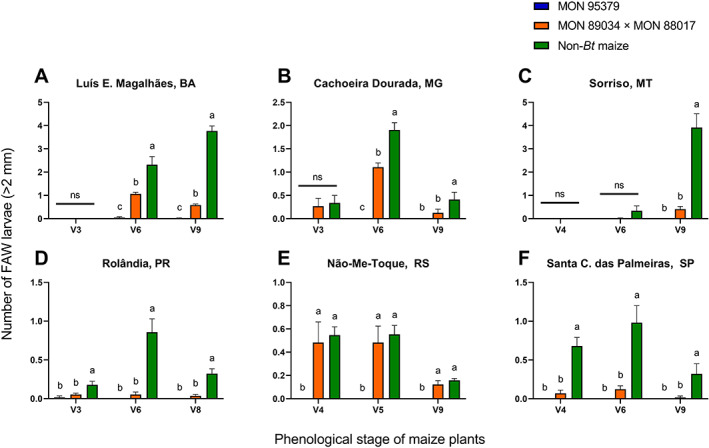
Mean number of Spodoptera frugiperda larvae (greater than 2 mm) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2020 natural pressure field trials. *Statistically significant differences (LSMeans contrast, P ≤ 0.05) between MON 95379, MON 89034 × MON 88017 and non‐Bt maize at the indicated maize phenological stage. Error bars represent standard error.
4. DISCUSSION
The results with the Cry1B.868 and Cry1Da_7 proteins incorporated into artificial diet demonstrated their high biological activity against FAW neonates. Overall, the diet‐incorporation bioassay results indicated FAW to be more sensitive to Cry1Da_7 protein than to Cry1B.868, except for the LC90 value which was greater for Cry1Da_7 than for Cry1B.868. In general, LC90 is an important metric in the context of the expected field performance of insecticidal products like Bt proteins, but Bt proteins also cause sublethal effects such as growth inhibition.51 Therefore, using only LC values as predictors of expected dose (i.e., killing power) of Bt plants will tend to underestimate dose because LC values do not account for larval growth inhibition.52, 53 EC values, which are estimates based on measuring mass reduction of surviving larvae relative to those in an untreated control, are a very sensitive measure of sublethal effects and can also be used as a predictor of total generational mortality.51 A response criterion that integrates growth inhibition as evidenced by molting inhibition (e.g., first‐instar larvae are considered dead) seems to more accurately characterize the expected response of pest species to Bt plants than do measurements based on mortality alone.51, 54, 55 In our study, the EC50;90 and LC50;90 values estimated for Cry1B.868 and Cry1Da_7 provided data on the potency of these Bt proteins against FAW neonates.
We utilized leaf disc bioassays and a susceptible laboratory strain of FAW to evaluate the dose (killing power) of Cry1B.868 and Cry1Da_7 when independently expressed and when combined in MON 95379 maize plants. Our results indicated complete mortality of FAW neonates on MON 95379 and Cry1B.868_single leaf discs, and approximately 99% mortality of the pest on Cry1Da_7_single leaf discs. These results are in line with mathematical modeling indicating that the concentration of each insecticidal protein in a Bt pyramid must be sufficiently high to kill at least 95% of susceptible individuals to maximize the delaying of insect resistance.56 In whole‐plant bioassays, MON 95379 completely controlled second‐instar larvae of MON 89034‐R, a resistant FAW strain capable of surviving and damaging MON 89034 plants expressing Cry1A.105 and Cry2Ab2. This result validated the conclusion of Wang et al.44 that pyramiding the Cry1B.868 and Cry1Da_7 proteins will offer an alternative to control FAW resistant to Cry1F, Cry1A.105 and or Cry2Ab2 in South America.
Resistance to the Cry1F protein in FAW is well characterized,31, 34, 57, 58 and documented to be both frequent and widespread across Brazil.59, 60 Because of partial cross‐resistance between Cry1F and Cry1A.10532, 39 and the continued use of Bt maize technologies such as MON 89034 with limited compliance with structure refuge recommendations in Brazil, the ability of Bt maize pyramids based on these proteins to protect plants against FAW damage was reduced.32, 49, 61, 62 A similar outcome is expected in other regions in South America where FAW is the major maize lepidopteran pest and where resistance to commercially available Bt maize technologies has already been observed in FAW.37, 63, 64 This chain of events considerably reduces the choices that growers have to effectively manage FAW. Based on the limited efficacy of currently available Bt technologies expressing Cry1F, Cry1A.105, and/or Cry2Ab2 proteins to manage FAW, Vip3Aa20 is currently the most effective mode of action against FAW in the field in Brazil and Argentina.40, 41 The expression of Cry1B.868 and Cry1Da_7 in MON 95379 consistently protected maize plants against larval feeding of FAW over 2 years of field trials in locations in different geographic regions and environments across Brazil. These field trials were conducted in 2019 (season 2018/2019) and 2020 (season 2019/2020), and the sites represented most of the locations with widespread resistance to Cry1F maize.34, 61, 65 Furthermore, MON 95379 was directly compared with MON 89034 × MON 88017 in our field trials, and the results indicate the higher level of efficacy of this technology against natural FAW populations likely carrying resistance alleles to Cry1F, Cry1A.105, and/or Cry2Ab2. Overall, the results presented herein indicate that MON 95379 maize, expressing a new generation of Bt traits, will be a highly effective additional tool to manage FAW in Brazil. Based on the recent history of resistance to Bt traits in FAW, it is extremely important that appropriate resistance management practices are implemented in South America if the benefits of MON 95379 and other effective FAW management technologies are to be sustained.
ACKNOWLEDGMENTS
We thank Marcos Barancelli, Fabiana Bacalhau, Adolfo Vieira, Fernando Prins, Luiz Bellini, Rafael Kotsubo, Sibila Grigolo, Daniel Sordi, Matheus Palhano and Daniel Soares for collecting the field efficacy data. We also thank Chen Meng for carrying out the statistical analysis of the diet‐incorporation bioassays.
Supporting information
TABLE S1 Mean Spodoptera frugiperda leaf damage (Davis scale) on MON 95379 and non‐Bt maize in 2019 and 2020 natural pressure field trials.
TABLE S2 Mean number of Spodoptera frugiperda larvae (greater than 2 mm) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2019 and 2020 natural pressure field trials.
REFERENCES
- 1.Sparks AN, A review of the biology of the fall armyworm. Fla Entomol 62:82–86 (1979). [Google Scholar]
- 2.Pogue GM, A World Revision of the Genus Spodoptera Guenée (Lepidoptera: Noctuidae), Vol. 43. American Entomological Society, Philadelphia, pp. 1–202 (2002). [Google Scholar]
- 3.Hardke JT, Temple JH, Leonard BR and Jackson RE, Laboratory toxicity and field efficacy of selected insecticides against fall armyworm (Lepidoptera: Noctuidae). Fla Entomol 94:272–278 (2011). [Google Scholar]
- 4.Luginbill P, The fall armyworm. USDA Tech Bull 34:91 (1928). [Google Scholar]
- 5.Cruz I, Figueiredo MLC, Silva RB, Silva IF, Paula CS and Foster JE, Using sex pheromone traps in the decision‐making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith] [Lepidoptera: Noctuidae]) larvae in maize. Int J Pest Manage 58:83–90 (2012). [Google Scholar]
- 6.Nagoshi RN, Rosas‐Garcia NM, Meagher RL, Fleischer SJ, Westbrook JK, Sappington TWet al., Haplotype profile comparisons between Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Rico, South America, and the United States and their implications to migratory behavior. J Econ Entomol 108:135–144 (2015). 10.1093/jee/tou044. [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi NN, Meagher RL and Hay‐Roe M, Assessing the resolution of haplotype distributions to delineate fall armyworm (Lepidoptera: Noctuidae) migratory behaviors. J Econ Entomol 107:1462–1470 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Westbrook JK, Nagoshi RN, Meagher RL, Fleischer SJ and Jairam S, Modeling seasonal migration of fall armyworm moths. Int J Biometeorol 60:255–267 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Goergen G, Kumar PL, Sankung SB, Togola A and Tamò M, First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11:e0165632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagoshi RN, Georgen G, Tounou KA, Agboka K, Koffi D and Meagher RL, Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern sub Saharan Africa. Sci Rep 8:1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganiger PC, Yeshwanth HM, Muralimohan K, Vinay N, Kumar ARV and Chandrashekara K, Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr Sci 115:621–623 (2018). [Google Scholar]
- 12.Nagoshi RN, Goergen G, Plessis HD, van den Berg J and Meagher R Jr, Genetic comparisons of fall armyworm populations from 11 countries spanning sub‐Saharan Africa provide insights into strain composition and migratory behaviors. Sci Rep 9:8311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagoshi RN, Htain NN, Boughton D, Zhang L, Xiao Y, Nagoshi BYet al., Southeastern Asia fall armyworms are closely related to populations in Africa and India, consistent with common origin and recent migration. Sci Rep 10:1421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montezano DG, Spech A, Sosa‐Gómez DR, Roque‐Spech VF, Sousa‐Silva JC, Paula‐Moraes SVet al., Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr Entomol 26:286–300 (2018). [Google Scholar]
- 15.Barros EM, Torres JB, Ruberson JR and Oliveira MD, Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol Exp Appl 137:237–245 (2010). [Google Scholar]
- 16.Williams WP and Davis FM, Response of maize to artificial infestation with fall armyworm and southwestern maize borer larvae. Southwest Entomol 15:163–166 (1990). [Google Scholar]
- 17.Cruz I, Figueiredo MLC, Oliveira AC and Vasconcelos CA, Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminium saturation. Int J Pest Manage 45:293–296 (1999). 10.1080/096708799227707. [DOI] [Google Scholar]
- 18.Ávila CJ, Degrande PE and Gomez AS, Insetos pragas: reconhecimento, comportamento, danos e controle. Informações Ténicas 5, Milho: (1997). [Google Scholar]
- 19.Burtet LM, Bernardi O, Melo AA, Pes MP, Strahl TT and Guedes JVC, Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag Sci 73:2569–2577 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Muraro DS, Stacke RF, Cossa GE, Godoy DN, Garlet CG, Valmorbida Iet al., Performance of seed treatments applied on Bt and non‐Bt maize against fall armyworm (Lepidoptera: Noctuidae). Environ Entomol 49:1137–1144 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Diez‐Rodríguez GI and Omoto C, Inheritance of lambda‐cyhalothrin resistance in Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop Entomol 30:311–316 (2001). [Google Scholar]
- 22.Carvalho RA, Omoto C, Field LM, Williamson MS and Bass C, Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda . PLoS One 8:e62268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nascimento ARB, Farias JR, Bernardi D, Horikoshi RJ and Omoto C, Genetic basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to the chitin synthesis inhibitor lufenuron. Pest Manage Sci 72:810–815 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Okuma DM, Bernardi D, Horikoshi RJ, Bernardi O, Silva AP and Omoto C, Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manage Sci 74:1441–1448 (2018). 10.1002/ps.4829. [DOI] [PubMed] [Google Scholar]
- 25.Murúa MG, Degano MFG, Pereira dÁ, Pero E, Willink E and Gastaminza G, Eficacia en campo del maíz Herculex® I para el control de Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) en el Noroeste Argentino. Rev Ind Agríc Tucumán 90:37–43 (2013). [Google Scholar]
- 26.Waquil JM, Dourado PM, Carvalho RA, Oliveira WS, Berger GU, Head GPet al., Manejo de lepidópteros‐praga na cultura do milho com o evento Bt piramidado Cry1A.105 e Cry2Ab2. Pesqui Agropecu Bras 48:1529–1537 (2013). [Google Scholar]
- 27.Fatoretto JC, Michel AP, Silva Filho MC and Silva N, Adaptive potential of fall armyworm (Lepidoptera: Noctuidae) limits Bt trait durability in Brazil. J Integr Pest Manage 8:17 (2017). [Google Scholar]
- 28.Marques LH, Santos AC, Castro BA, Moscardini VF, Rosseto J, Silva OABNet al., Assessing the efficacy of Bacillus thuringiensis (Bt) pyramided proteins Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 expressed in Bt maize against lepidopteran pests in Brazil. J Econ Entomol 112:803–811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabashnik BE and Carrière Y, Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol 35:926–935 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Carrière Y, Brown ZS, Downes SJ, Gujar G, Epstein G, Omoto Cet al., Governing evolution: a socioecological comparison of resistance management for insecticidal transgenic Bt crops among four countries. Ambio 49:1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farias JR, Andow DA, Horikoshi RJ, Sorgato RJ, Fresia P, Santos ACet al., Field‐evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot 64:150–158 (2014). [Google Scholar]
- 32.Bernardi D, Salmeron E, Horikoshi RJ, Bernardi O, Dourado PM, Carvalho RAet al., Cross‐resistance between Cry1 proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLoS One 10:e0140130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JWet al., Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 103:1031–1038 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Farias JR, Horikoshi JR, Santos AC and Omoto C, Geographical and temporal variability in susceptibility to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. J Econ Entomol 107:2182–2189 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Huang F, Qureshi JA, Meagher RL Jr, Reisig DD, Head GP, Andow DAet al., Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLoS One 9:e112958 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Reisig DD, Miao J, Gould F, Huang F and Feng H, Frequency of Cry1F on recessive resistance alleles in North Carolina field populations of Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS One 11:e0154492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasena DI, Signorini AM, Abratti G, Storer NP, Olaciregui ML, Alves APet al., Characterization of field‐evolved resistance to Bacillus thuringiensis‐derived Cry1F δ‐endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manage Sci 74:746–754 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM, Crivellari Aet al., Field‐evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manage Sci 72:1727–1736 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Hernández‐Rodríguez CS, Hernández‐Martínez P, Van Rie J, Escriche B and Ferré J, Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important maize pests, Ostrinia nubilalis and Spodoptera frugiperda . PLoS One 8:e68164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardi O, Bernardi D, Horikoshi RJ, Okuma DM, Miraldo LL, Fatoretto Jet al., Selection and characterization of resistance to the Vip3Aa20 protein from Bacillus thuringiensis in Spodoptera frugiperda . Pest Manage Sci 72:1794–1802 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Amaral FS, Guidolin AS, Salmeron E, Kanno RH, Padovez FE, Fatoretto JCet al., Geographical distribution of Vip3Aa20 resistance allele frequencies in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Pest Manage Sci 76:169–178 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Martinelli S, Carvalho RA, Dourado PM and Head GP, Resistance of Spodoptera frugiperda to Bacillus thuringiensis proteins in the Western hemisphere, in Bacillus thuringiensis and Lysinibacillus sphaericus: Characterization and Use in the Field of Biocontrol, ed. by Fiuza LM, Polanczyk RA and Crickmore N. Springer, Cham, Switzerland: (2017). [Google Scholar]
- 43.van Frankenhuyzen K, Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol 101:1–16 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Wang J, Fu X, Nageotte JR, Silverman J, Bretsnyder ECet al., Bacillus thuringiensis Cry1Da_7 and Cry1B.868 protein interactions with novel receptors allow control of resistant fall armyworms, Spodoptera frugiperda (J.E. Smith). Appl Environ Microbiol 85:e00579–e00519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jerga A, Evdokimov AG, Moshiri F, Haas JA, Chen M, Clinton Wet al., Disabled insecticidal proteins: a novel tool to understand differences in insect receptor utilization. Insect Biochem Mol Biol 105:79–88 (2019). [DOI] [PubMed] [Google Scholar]
- 46.SAS , Statistical Analysis System: Getting Started with the SAS Learning. SAS Institute, Cary, NC: (2000). [Google Scholar]
- 47.SAS , JMP Introductory Guide, Version 12.0. SAS Institute, Cary, NC: (2015). [Google Scholar]
- 48.Andow DA and Alstad DN, F2 screen for rare resistance alleles. J Econ Entomol 91:572–578 (1998). [Google Scholar]
- 49.Bernardi D, Bernardi O, Horikoshi RJ, Salmeron E, Okuma DM, Farias JRet al., Selection and characterization of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to MON 89034 × TC1507 × NK603 maize technology. Crop Prot 94:64–68 (2017). [Google Scholar]
- 50.Davis FM, Ng SS and Williams WP, Visual rating scales for screening whorl‐stage corn for resistance to fall armyworm. Miss Agric For Exp Stn Tech Bull 186:1–9 (1992). [Google Scholar]
- 51.Ali MI and Luttrell RG, Response estimates for assessing Heliothine susceptibility to Bt toxins. J Econ Entomol 102:1935–1947 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Moar WR, Roush R, Shelton A, Ferré J, MacIntosh S, Leonard BRet al., Field‐evolved resistance to Bt toxins. Nat Biotechnol 26:1072–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Sumerford DV, Head GP, Shelton A, Greenplate J and Moar W, Field‐evolved resistance: assessing the problem and moving forward. J Econ Entomol 106:1525–1534 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GUet al., Assessment of the high‐dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manage Sci 68:1083–1091 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Sims SR, Greenplate JT, Stone B, Caprio MA and Gould F, Monitoring strategies for early detection of Lepidoptera resistance to Bacillus thuringiensis insecticidal proteins, in Molecular Genetics and Evolution of Pesticide Resistance, ed. by Brown TM. American Chemical Society, Washington, DC, pp. 229–242 (1996). [Google Scholar]
- 56.Roush RT, Two‐toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos Trans R Soc Lond B Biol Sci 353:1777–1786 (1998). [Google Scholar]
- 57.Santos‐Amaya OF, Tavares CS, Monteiro HM, Teixeira TPM, Guedes RNC, Alves APet al., Genetic basis of Cry1F resistance in two Brazilian populations of fall armyworm, Spodoptera frugiperda . Crop Prot 81:154–162 (2016). [Google Scholar]
- 58.Santos‐Amaya OF, Tavares CS, Rodrigues JVC, Campos SO, Guedes RNC, Alves APet al., Fitness costs and stability of Cry1Fa resistance in Brazilian populations of Spodoptera frugiperda . Pest Manage Sci 73:35–43 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Farias JR, Andow DA, Horikoshi RJ, Bernardi D, Ribeiro RS, Nascimento ABet al., Frequency of Cry1F resistance alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Pest Manage Sci 72:2295–2302 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Santos‐Amaya OF, Tavares CS, Rodrigues JVC, Souza TC, Rodrigues‐Silva N, Guedes RNCet al., Magnitude and allele frequency of Cry1F resistance in field populations of the fall armyworm (Lepidoptera: Noctuidae) in Brazil. J Econ Entomol 110:1770–1778 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Santos‐Amaya OF, Rodrigues JVC, Souza TC, Tavares CS, Campos SO, Guedes RNCet al., Resistance to dual‐gene Bt maize in Spodoptera frugiperda: selection, inheritance and cross‐resistance to other transgenic events. Sci Rep 5:18243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horikoshi RJ, Bernardi D, Bernardi O, Malaquias JB, Okuma DM, Miraldo LLet al., Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management. Sci Rep 6:64864 (2016). 10.1038/srep34864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vassallo CN, Bunge FF, Signorini AM, Valverde‐Garcia P, Rule D and Babcock J, Monitoring the evolution of resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) to the Cry1F protein in Argentina. J Econ Entomol 112:1838–1844 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Jaramillo‐Barrios CI, Quijano EB and Andrade BM, Populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) cause significant damage to genetically modified maize crops. Rev Fac Nac Agron Medellín 72:8953–8962 (2019). [Google Scholar]
- 65.Huang F, Resistance of the fall armyworm, Spodoptera frugiperda, to transgenic Bacillus thuringiensis Cry1F corn in the America: lessons and implications for Bt corn IRM in China. Insect Sci 0:1–16 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Mean Spodoptera frugiperda leaf damage (Davis scale) on MON 95379 and non‐Bt maize in 2019 and 2020 natural pressure field trials.
TABLE S2 Mean number of Spodoptera frugiperda larvae (greater than 2 mm) on MON 95379, MON 89034 × MON 88017 and non‐Bt maize in 2019 and 2020 natural pressure field trials.


