Abstract
The tumor microenvironment (TME) has been identified as one of the driving factors of tumor progression and invasion. Within this microenvironment, cancer‐associated fibroblasts (CAF) have multiple tumor‐promoting functions and play key roles in drug resistance, through multiple mechanisms, including extracellular matrix (ECM) remodeling, production of growth factors, cytokines, and chemokines, and modulation of metabolism and angiogenesis. More recently, a growing body of evidence has shown that CAF also modulate immune cell activity and suppress anti‐tumor immune response. In this review, we describe the current knowledge on CAF heterogeneity in terms of identity and functions. Moreover, we analyze how distinct CAF subpopulations differentially interact with immune cells, with a particular focus on T lymphocytes. We address how specific CAF subsets contribute to cancer progression through induction of an immunosuppressive microenvironment. Finally, we highlight potential therapeutic strategies for targeting CAF subpopulations in cancer.
Keywords: cancer, cancer‐associated fibroblasts, heterogeneity, immunosuppression, immunotherapy, T lymphocytes
1. INTRODUCTION
Over the last few decades, multiple findings have improved our understanding of cellular and molecular hallmarks in cancer, with a better characterization of the tumor microenvironment (TME). Four major components of the TME have been identified, including the following: (1) an immune component, composed of a large variety of immune cells such as tumor‐associated macrophages, T and B cells, natural killer, and dendritic cells; (2) a vascular component formed by blood and lymphatic endothelial cells; (3) an extracellular matrix (ECM) made by complex collagen fibers and other glycoproteins; and (4) a stromal component that includes cancer‐associated fibroblasts (CAF) and mesenchymal stem cells (MSCs).1, 2, 3, 4, 5, 6, 7 CAF were originally considered as a homogeneous population uniformly driving tumorigenesis. In contrast, multiple recent studies revealed that CAF constitute a heterogeneous group of stromal cells, which differ in their origin, phenotype, functions, and quantity in different cancer types.5, 7, 8 Many theories have defined diverse origins of CAF, including tissue‐resident fibroblasts or bone marrow–derived MSC via transforming growth factor‐β (TGFβ), epithelial or endothelial cells through epithelial‐ or endothelial‐to‐mesenchymal transition.5, 6, 9, 10, 11, 12, 13, 14 CAF might also derive from trans‐differentiation of adipocytes or pericytes, which results in the upregulation of mesenchymal lineage‐committed genes, such as peroxisome proliferator–activated receptor‐gamma (PPARγ) and Runt‐related transcription factor‐2 (RUNX2).13, 15, 16, 17 Moreover, vitamin deficiency in certain cancer stromal cells, such as stellate cells, leads to the upregulation of smooth muscle actin (α‐SMA), which induces their differentiation into CAF.18, 19 CAF also originate from a variety of precursor cells recruited by tumor cells at primary and metastatic sites. Among these, MSCs represent an important source of CAF and can provide up to 20% of the CAF population in tumors.12, 20
Numerous studies recently highlighted that CAF are composed of several functionally different subpopulations, which either promote or restrain cancer growth (Figure 1).7, 14, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Indeed, even if a large number of studies currently support the tumor‐promoting effects of CAF, some evidence suggests that CAF can also decrease tumor growth.29, 31 For example, depletion of fibroblasts has been shown to accelerate pancreatic ductal adenocarcinoma (PDAC) growth, suggesting that some stromal cells such as normal fibroblasts can protect against cancer growth.22 Similarly, deletion of sonic hedgehog, a soluble ligand overexpressed by neoplastic cells in PDAC, increases tumor aggressiveness.23 Still, a number of evidence supporting multiple pro‐tumorigenic roles for CAF suggests that targeting CAF in human cancer could be a valuable strategy. Indeed, CAF secrete numerous growth factors, such as fibroblast growth factor‐2 (FGF‐2) and stromal cell–derived factor 1 (SDF‐1/CXCL12), resulting in cancer cell proliferation and metastatic spread. Moreover, CAF regulate angiogenesis, as well as immune cell recruitment and polarization in a pro‐tumorigenic manner by secreting interleukins and chemokines, such as CXCL12.27, 32, 33, 34, 35, 36 The abundance of α‐SMA+ CAF in TME is associated with poor prognosis in multiple cancers.30, 32, 37, 38 Moreover, tumors with high stromal signatures are linked to therapy resistance and disease relapse.39, 40, 41, 42 Finally, CAF have been recently identified as key components regulating immune infiltration in cancer.5, 43, 44 Indeed, specific CAF subsets are implicated in mediating an immunosuppressive microenvironment, characterized by immune cell evasion. Several studies including gene signature or mass spectrometry analysis have shown that CAF exhibit a particular immunomodulatory secretome, including chemokine C‐X‐C motif ligands (CXCL1, CXCL2, CXCL5, CXCL12), chemokine C‐C motif ligand CCL5, interleukin 1β (IL‐1 β), interleukin 6 (IL‐6), interleukin 10 (IL‐10), vascular endothelial growth factor (VEGF), and TGFβ.26, 27, 43, 44, 45, 46, 47, 48 This secretion profile plays a major role in modulating the TME, by regulating immune cell recruitment and functions within tumors. Moreover, beside this direct effect on immune cells, CAF construct ECM protein networks that serve as a physical barrier preventing immune cell infiltration in tumors43, 46, 47, 49, 50, 51, 52, 53 (Figure 1). In this review, we focus on the recent progress on CAF immunosuppressive activities, in particular on the cross talk with T lymphocytes, and we examine future therapeutic strategies.
FIGURE 1.
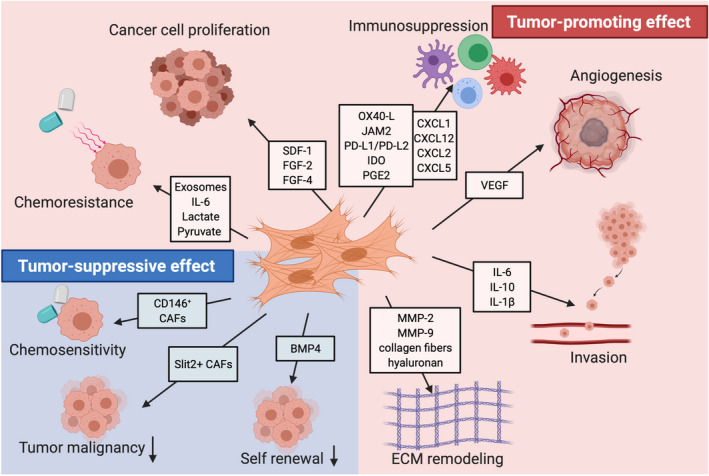
Functional heterogeneity of cancer‐associated fibroblasts. CAF can either promote or restrain cancer growth. CAF contribute to cancer progression through multiple mechanisms, including proliferation, invasion, metastasis, and vascularization (red section). CAF secrete numerous cell growth factors such as FGF and SDF‐1/CXCL12, promoting cancer cell growth and spread. CAF also regulate angiogenesis, as well as immune cell recruitment and polarization by secreting various cytokines and chemokines, such as CXCL12, IL‐6, and IL‐8. In addition, CAF exosomes or metabolic components, such as lactate, contribute to chemoresistance. CAF‐mediated ECM production and remodeling is additionally considered as a key tumor‐promoting activity. In contrast, most often, CAF do exert tumor‐suppressive effects (blue section). For instance, Slit2+ and CD146+ CAF suppress tumorigenesis and increase chemosensitivity, while molecules such as BMP4 reduce the self‐renewal of stem‐like cancer cells. FGF, fibroblast growth factor; SDF‐1, stromal cell–derived growth; MMP, Matrix metalloproteinase; IL‐6, Interleukin‐6; IL‐8, interleukin‐8; IL‐1β, Interleukin‐1β; ECM, extracellular matrix
2. CAF HETEROGENEITY: IDENTIFICATION OF DISTINCT CAF SUBSETS IN CANCER
CAF are actually defined as a set of heterogeneous mesenchymal cells, which are negative for epithelial, endothelial, and immune cell markers. Given their potential distinct cellular origins, various markers have been tested individually in tumors. This rapidly leads to the demonstration that CAF are heterogenous in cancer. Expression of several markers, such as α‐SMA, FAP (fibroblast activation protein), integrin β1/CD29, S100‐A4/FSP1 (fibroblast‐specific protein 1), PDGFRβ (platelet‐derived growth factor receptor‐β), and CAV1 (caveolin 1), was first analyzed individually. Studies using α‐SMA to stain CAF in human tumors showed that they accumulate in cancer of poor prognosis, in particular in breast cancer (BC).32, 41, 54 In addition to α‐SMA, high expression of PDGFRβ was associated with high risk and poor prognosis of in situ ductal carcinoma.55, 56 FAP is another well‐known marker, which is abundant in the stroma of aggressive BC.26, 57, 58, 59 Prognostic value of CAV1 or FSP1 expression in CAF has been demonstrated in BC, although with some conflicting information on patient survival.49, 60, 61 These different markers first analyzed alone were next combined to distinguish CAF subsets with key functional differences, highlighting the notion of CAF heterogeneity. A first study combining α‐SMA, PDGFRβ, and S100A4/FSP1 together showed that these markers exhibit a differential expression in CAF in PDAC and BC mouse models.62 In addition, based on the expression patterns of six specific markers (FAP, α‐SMA, FSP1, PDGFRβ, CD29, and CAV1), four different CAF subsets (referred to as CAF‐S1 to CAF‐S4) have been identified in breast and ovarian cancers.26, 27 Interestingly, these CAF subsets accumulate differentially according to the distinct BC subtypes.26, 59 Indeed, CAF‐S1 (FAPHigh CD29Med α‐SMAMed‐High FSP1Med PDGFRβMed‐High CAV1Low) and CAF‐S4 (FAPNeg‐Low CD29High α‐SMAHigh FSP1Low‐Med PDGFRβLow‐Med CAV1Low) subsets are detected at high level in aggressive BC (HER2 and triple negative (TN)) and in metastatic lymph nodes, while the CAF‐S2 subset (FAPNeg CD29Low α‐SMANeg FSP1Neg‐Low PDGFRβNeg CAV1Neg) is enriched in luminal BC subtype and CAF‐S3 fibroblasts (FAPNeg CD29Med α‐SMANeg FSP1Med‐High PDGFRβMed CAV1Low) accumulate in healthy tissues.26 The gene signature analysis of these CAF subsets revealed that CAF‐S1 are defined by extracellular matrix and inflammation signatures, while CAF‐S4 are characterized by a perivascular signature.26, 27 This explains their accumulation in aggressive BC, such as HER2 and triple‐negative BC, and their cooperative actions in metastatic spread.8, 58, 59, 63 The existence of these two major subpopulations CAF‐S1 and CAF‐S4 was validated in distinct cancer types, including PDAC, head and neck, and lung cancer.48, 64, 65, 66, 67, 68, 69, 70 Moreover, among the FAPHigh CAF (CAF‐S1) subpopulation, two spatially separated subtypes of CAF have been identified, based on the expression of α‐SMA. Inflammatory CAF (iCAF), characterized by low α‐SMA levels, secrete high levels of inflammatory factors and are located distantly from neoplastic cells. In contrast, myofibroblastic CAF (myCAF) are characterized by high α‐SMA expression and myofibroblast features and are located immediately adjacent to neoplastic cells.8, 26, 48, 59, 65, 67, 71 Similarly, two discrete populations of FAP+ mesenchymal cells can also be distinguished on the basis of podoplanin (PDPN) expression. Although both FAP+ PDPN+ and FAP+ PDPN‐ CAF subsets express high levels of ECM components, the PDPN+ CAF transcriptome is enriched in genes associated with TGFβ signaling. In addition, this CAF subset is enriched at the outer edge of the tumor, in close contact with T cells, whereas PDPN‐ CAF are localized around vessels.57 Similarly, the content in CAF characterized by PDPN+/FSP1+ ratio is associated with disease outcome and BRCA gene mutations in the 4T1 triple‐negative BC mouse model.72 In this study and in the most recent ones, authors used the single cell RNA sequencing (scRNAseq) method to unravel comprehensive mapping of CAF in BC. By performing scRNAseq from 768 CAF isolated from the genetically engineered MMTV‐PyMT BC mouse model, four transcriptionally distinct CAF subpopulations were identified and named vascular CAF (vCAF), matrix CAF (mCAF), cycling CAF (cCAF), and developmental CAF (dCAF).65 Notably, each CAF subset is discriminated by the expression of gene programs representing different functionality and unique spatial location within the tumor parenchyme: The vCAF subtype might originate from a pool of perivascular cells, which invade tumor stroma over tumor progression; cCAF represent the proliferative fraction of vCAF, in which genes involved in cell cycle regulation were upregulated; dCAF might originate from tumor cells that have undergone an epithelial‐to‐mesenchymal transition; and mCAF might derive from resident fibroblasts co‐opted by tumor cells.65 Similarly, 6 distinct clusters, including myCAF, iCAF, and antigen‐presenting CAF (apCAF) first identified in PDAC, 67, 69, 71 were detected in the 4T1 BC mouse model.73 As observed in these mouse models, scRNA‐seq from more than 18 000 FAP+ CAF (CAF‐S1) isolated from human BC revealed the existence of eight different cellular clusters within this population. Among them, three CAF‐S1 clusters belong to the iCAF subgroup and five to the myCAF subgroup. These clusters are characterized by high expression of genes involved in detoxification (detox‐iCAF), interleukin‐ (IL) (IL‐iCAF) or IFNγ‐signaling (IFNγ‐iCAF), ECM remodeling (ECM‐myCAF), TGF‐β‐dependent pathway (TGF‐β‐myCAF), wound healing (wound‐myCAF), IFNαβ‐signaling (IFNαβ‐myCAF), and acto‐myosin pathway (acto‐myCAF), respectively. The existence and the relative proportions of the five most abundant CAF‐S1 clusters (representing up to 91% of sequenced cells) have been confirmed in head and neck squamous cell carcinoma and in non‐small cell lung cancer (NSCLC), demonstrating the relevance of these CAF‐S1 clusters across different cancer subtypes.48 Similarly, the existence of FAP+ CAF characterized by high ECM content was confirmed in PDAC using scRNAseq.70 As observed for ECM‐myCAF in BC, these PDAC CAF express high levels of LRRC15 (leucine‐rich repeat containing 15), surround tumor islets, and are absent from normal pancreatic tissue.70 Finally, the IFNγ‐iCAF gene signature includes CD74, encoding the major histocompatibility class (MHC) II invariant chain, which also characterizes the antigen‐presenting CAF (apCAF) identified in PDAC.69 To summarize, CAF are a collection of diverse cell subpopulations, which respond to different stimuli, display distinct secretory phenotypes, and execute specific biological functions. By combining the study of several markers concomitantly and by performing scRNAseq, similar CAF subsets and clusters have been identified in distinct cancer types, thereby showing their relevance and potential clinical interest.
3. IMPACT OF CAF SUBSETS ON T CELL PHENOTYPES AND FUNCTIONS
As mentioned before, it is now well established that CAF exert a strong immunomodulatory regulation, modulating both immune cell infiltration and anti‐tumor functions within the TME. CAF‐mediated effects can be direct by increasing the content in suppressive T lymphocytes and counteracting effector T cell functions,47, 54, 74 or indirect by producing ECM components that form a physical barrier preventing immune cell infiltration 43, 46, 47, 49, 50, 51, 52, 53, 75, 76 (Figure 2). It is still important to note that these functions are not exclusive of CAF, as they can be exerted by other cells, including cancer and immune cells themselves,77 thereby highlighting a reciprocal cross talk between the different components of the TME.
FIGURE 2.
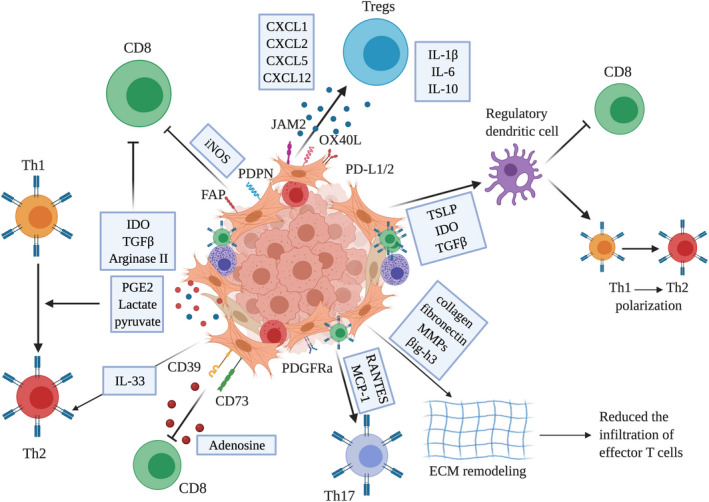
Immunosuppressive function of CAF subsets. CAF orchestrate an immunosuppressive tumor microenvironment through the secretion of numerous chemokines and cytokines, such as TGFβ, IL‐10, or CXCL‐12, thereby inhibiting cytotoxic CD8+ T cell function or polarizing T cell subset toward Th2. Some CAF subpopulations express PD‐L1/2, targets for immune checkpoint inhibitors. Metabolites or metabolic enzymes, such as IDO or arginase and nucleosides, such as adenosine, favor the recruitment and differentiation of Tregs. Finally, CAF can produce and remodel ECM components such as collagen, fibronectin, and MMPs, which in turn reduces the infiltration of effector T cells. TGFβ, Transforming growth factor‐β; IL‐10, Interleukin 10; PD‐L1/2, programmed cell death ligand 1/2; IDO, Indoleamine‐2,3 dioxygenase; Tregs, Regulatory T cells; ECM, extracellular matrix; MMPs, matrix metalloproteinases
3.1. Direct impact of CAF on T lymphocytes
CAF abundancy is often correlated with poor clinical outcome and reduced anti‐tumor immune response, as assessed by an increased FOXP3+ regulatory T cells to CD8+ T lymphocytes ratio.26, 70, 78 In accordance with CAF immunosuppressive function, there is a growing evidence that CAF promote the recruitment of pro‐tumoral immune cell populations, as shown by an increased Th2 response, at the expense of Th1. For example, interleukin 33 (IL‐33), a type 2 immune modulator, is upregulated in CAF in mouse models of spontaneous BC metastasis, as well as in BC patients with lung metastasis.79 Thus, IL‐33 promotes type 2 inflammation through a downregulation of T‐bet (T‐box expressed in T cells) expression, indicating a bias against Th1‐mediated immune responses.79 In addition, both CAF and cancer cells produce RANTES (regulated upon activation, normally T‐expressed, and presumably secreted) and MCP‐1 (monocyte chemotactic protein‐1), which preferentially mediate the recruitment and expansion of CD4+ Th17 T cells.80 Th17 cells, via IL‐17 secretion, promote tumor growth, through the induction of IL‐6 production, which in turn activates oncogenic signal transducer and STAT3 (signal transducer and activator of transcription 3)‐dependent transcription.81, 82 Despite these findings, the link between CAF subset heterogeneity and immune infiltration has only recently been analyzed in detail. FAP+ CAF have been first demonstrated to exert an immunosuppressive activity in several cancer types and their depletion allows immunological control of growth.26, 27, 28, 33, 46, 57, 83, 84, 85, 86, 87, 88 Among FAP+ stromal cells, PDPN+ CAF suppress proliferation of effector T lymphocytes in a nitric oxide–dependent manner, while FAP+ PDPN‐ CAF are not immunosuppressive.57 In addition, FAP+ CAF‐S1 potentiate the recruitment, survival, and differentiation of regulatory T cells, thereby contributing to a tumor‐promoting microenvironment in BC and high‐grade serous ovarian cancer.26, 27 By secreting CXCL‐12, FAP+ CAF attract and increase the survival of CD4+ CD25+ T lymphocytes.26, 27, 84 CXCL12‐dependent attraction of CD4+ CD25+ T lymphocytes confirms previous observations showing that CXCR4 inhibitors induce T cell accumulation and synergize with anti‐PD‐L1 treatment in mouse models.33, 71 Recent scRNAseq data from BC patients demonstrated that CXCL12 is highly secreted by some specific FAP+CAF‐S1 cellular clusters, referred to as inflammatory CAF.48 In addition to their capacity to attract T lymphocytes, FAP+ CAF also directly interact with T cells.26 Using functional assays in vitro, two types of interactions have been detected between FAP+ CAF and CD4+ CD25+ T lymphocytes, including short‐ and long‐term contacts.26 Persistent interactions between FAP+ CAF and T cells can exceed 14 hours and account for 20% of total contacts. Long‐term T cell retention at the surface of FAP+ CAF is mediated by strong expression of OX40L, programmed cell death ligand‐2 (PD‐L2), and junctional adhesion molecule 2 (JAM2) in FAP+ CAF.26
In addition to their impact on T cell attraction and retention, FAP+ CAF induce CD4+ CD25+ T cell differentiation into CD4+ CD25+ FOXP3+ regulatory lymphocytes and enhance their capacity to inhibit CD4+ effector T cell proliferation.26, 27 In line with the identification of different cellular clusters among FAP+ CAF, recent findings demonstrated that only specific FAP+ CAF cellular clusters, characterized by ECM accumulation, wound healing, and TGFβ signaling, are associated with T cell infiltration, in particular high content of CD4+ CD25+ FOXP3+ PD‐1+ or CTLA‐4+ T lymphocytes in BC patients.48 Interestingly, these 3 specific FAP+ CAF cellular clusters are associated with primary resistance to immunotherapy in melanoma and NSCLC patients.48 CAF promote expression of programmed cell death (PD‐1), cytotoxic T lymphocyte associated protein‐4 (CTLA‐4), and T cell immunoglobulin and mucin domain‐containing protein 3 (TIM3) in proliferating T cells in PDAC.89 Consistent with these data, ECM‐myCAF cluster from BC increases the expression of FOXP3, PD‐1, and CTLA‐4 at the surface of CD4+ CD25+ T cells.48 In addition, both FAP+ myCAF and iCAF clusters increase the content in TIGIT+ (T cell immunoreceptor with Ig and ITIM domain) cells among CD4+ CD25+ FOXP3+ T lymphocytes.48 FAP+ CAF from melanoma also induce TIGIT expression at the surface of cytotoxic T lymphocytes.90 These findings are totally consistent with previous observations in PDAC and several other cancer types showing that FAP+ CAF promote immune escape and that ECM and TGFβ signaling pathways are key determinants in resistance to immunotherapy.43, 45, 75, 76, 91, 92, 93, 94 Interestingly, another FAP+ CAF cluster characterized by IFNγ‐dependent signaling pathway activates CD4+ T cells in an antigen‐dependent manner.48, 69 In addition, CAF can modulate the recruitment of peripheral CD8+ T cells within tumors through secretion of numerous cytokines and chemokines. Indeed, FAP+ CAF secrete high amounts of CXCL12 that guide CD8+ T migration and sequestration in the stromal compartment surrounding the tumor, thereby reducing CD8+ T cell infiltration within tumor islets.33, 50, 95 Consistent with these results, preclinical studies in a PDAC murine model revealed that both pharmacological inhibition of CXCR4 (CXCL12 receptor) and genetic ablation of CXCL12 lead to CD8+ T cell accumulation within tumor and reduced tumor growth.33 Taken as a whole, these data highlight that considering the different CAF subsets identified in several cancer types, as well as the heterogeneity within the FAP+ CAF subset, is essential to understand T cell infiltration and better appreciate immunotherapy resistance.
3.2. Role of CAF on immune checkpoint molecules
As mentioned above, FAP+ CAF, in particular ECM‐myCAF and TGFβ ‐myCAF clusters, enhance expression of PD‐1 and CTLA‐4 at the surface of CD4+ CD25+ FOXP3+ Tregs. In addition, CAF influence T cell immunity through high expression of immune checkpoint ligands, such as PD‐L1 and PD‐L2. Both PD‐L1 and PD‐L2 bind to PD‐1 receptor expressed by T cells and drive their dysfunction resulting in enhanced tumor growth.26, 89 Among the genes highly expressed by FAP+ CAF, PD‐L2 and TNFSF4/OX40L ligands were identified as key players in long‐term interactions between CAF and CD4+ CD25+ T lymphocytes.26 Although OX40/OX40L signaling increases memory CD4+ T cells and acts on Treg homeostasis, OX40L can also enhance CD4+ CD25+ T cell retention at the surface of FAP+ CAF, suggesting the potential detrimental effect of OX40 agonist use in tumors enriched in FAP+ CAF. In addition to OX40L, expression of the immune checkpoint PD‐L2 is strongly increased in FAP+ CAF.26 PD‐L2 is also involved in CD4+ CD25+ T cell retention at the surface of FAP+ CAF. While most studies focus on PD‐L1/PD‐1, both PD‐L2 and PD‐L1 ligands can bind to PD‐1, leaving open the role of PD‐L2 in immunotherapy resistance.96 As immunotherapies based on PD‐L1 blockade may not prevent interaction of PD‐L2 with PD‐1, high PD‐L2 expression in FAP+ CAF could be a new mechanism of resistance to immunotherapies.26 PD‐L1 expression in CAF can be upregulated by IFNγ secreted by activated lymphocytes, confirming a reciprocal cross talk between these cell types.97 Moreover, consistent with FAP+ CAF immunosuppressive activity, several studies showed that CAF promote PD‐L1 expression in tumor cells through CXCL5 or CXCL2 secretion.36, 98 In addition, CAF‐produced exosomes increase PD‐L1 expression in BC cells, together with miR‐92 expression, which impairs T cell proliferation.99 The underlined mechanism involves large tumor suppressor kinase 2 (LATS2), important component of the Hippo pathway, which is directly targeted by miR‐92. Interestingly, LATS2 directly interacts with Yes‐associated protein 1 (YAP1) and prevents its nuclear translocation, thereby reducing PD‐L1 transcription.99
3.3. Role of metabolism in CAF‐mediated immunosuppression
Metabolites are emerging as key players in immune escape. In that sense, metabolic reprogramming is another mechanism by which CAF can trigger T cell immunosuppression. Indeed, glucose consumption by glycolytic CAF depletes glucose in the TME, thereby impairing effector T cell activity without affecting cancer cell survival, as cancer cells take advantage of release of lactate and pyruvate by CAF.100, 101 In prostate cancer, release of lactate by glycolytic CAF acts on CD4+ T cells and shapes T cell polarization, including reduction of Th1 and increase in Treg content.102 Tregs can survive under low glucose conditions and use lactate to fuel their metabolism.103 Moreover, CAF impair T cell function through increased activity of amino acid degrading enzymes, such as indoleamine 2,3‐dioxygenase (IDO) and arginase II (ARG2), which emerged as key players in the regulation of immune tolerance in tumors.104 Expression of IDO is upregulated by IFNγ and catalyzes the conversion of tryptophan into kynurenine, which inhibits T cell proliferation and function.105 IDO‐mediated tryptophan degradation is also able to promote Treg differentiation and activation, which in turn lead to cytotoxic T lymphocyte anergy and apoptosis.105 CAF also inhibit anti‐tumor effector T cell responses through ARG2, which hydrolyzes arginine to ornithine and urea.106 This depletes arginine content in TME, which is required for T cell functions.106 Accordingly, FAP+ CAF from melanoma interfere with cytotoxic T lymphocyte activity by impeding CD69 and granzyme B production, via increased arginase activity.90 Furthermore, activation of the COX2/PGE2 axis in CAF increases FOXP3 expression and Treg activity, suppressing effector T cell function.63, 107, 108, 109, 110 Moreover, prostaglandin 2 (PGE2), produced by CAF, drives an immunosuppressive TME by modulating cytokine secretion profiles in human T cells from anti‐tumoral Th1 toward pro‐tumoral Th2 phenotype.111 In addition, CAF express high levels of CD39 and CD73 ectonucleotidases at the cell membrane. Interestingly, this feature is associated with the ability to strongly suppress the proliferation, activation, and effector functions of cytotoxic T cells through the generation of large amounts of adenosine from adenosine triphosphate (ATP) hydrolysis.112 Finally, Galectin‐1, recently identified as overexpressed in CAF, contributes to tumor immune escape by promoting apoptosis of activated T cells.113, 114, 115, 116
3.4. Role of TGFβ signaling pathway in CAF‐mediated immunosuppression
As described above, a specific cellular cluster of FAP+ CAF expressing high levels of TGFβ (referred to as TGFβ‐myCAF) has recently been identified in BC.48 TGFβ ligands secreted by these FAP+ CAF act on both T cell immune response and resistance to immunotherapies. The content in TGFβ‐myCAF is indeed correlated with the proportion of CTLA‐4+ Tregs in BC and these CAF accumulate preferentially in melanoma and NSCLC patients who do not respond to immunotherapy.48 TGFβ1 attenuates effector functions of antigen‐specific and fully activates memory CD8+ T lymphocytes.117 Moreover, TGFβ1 reduces responsiveness of memory T cells by blocking CD28‐TCR signaling. This effect was reversed by an anti‐TGFβ1 neutralizing antibody or by TGFβ1 removal using a low PH buffer.118 In addition, TGFβ reduces T lymphocyte cytotoxicity by specifically inhibiting expression of cytolytic gene products, such as perforin, granzyme A and B, Fas ligand, and IFNγ.119 Indeed, repression of granzyme B and IFNγ involves binding of TGFβ‐activated SMAD and ATF1 transcription factors to their promoter regions, indicating a direct and selective regulation by the TGFβ/SMAD pathway. Interestingly, neutralization of systemic TGFβ in mice enables tumor clearance with restoration of antigen‐specific cytotoxic T cell activity.119 Moreover, in head and neck cancer, TGFβ secreted by CAF induces T cell apoptosis and enhances Tregs differentiation.74 On the other hand, TGFβ induces a switch of B cells from IgM toward IgA expressing cells.120 Interestingly, in hepatocarcinoma, these IgA+ cells express PD‐L1 and IL‐10 and directly suppress liver cytotoxic CD8+ T lymphocytes.121
3.5. Indirect effect of CAF on T cell activity
Another mechanism by which CAF inhibit T cell anti‐tumor immune response is by affecting the function of dendritic cells (DC), the most important antigen‐presenting cell involved in T lymphocyte activation. TGFβ secreted by CAF downregulates expression of MHC class II molecules and the costimulatory molecules CD40, CD80, and CD86 at cell surface of DCs. These immature cells promote formation of Tregs, which inhibit effector T cell function.122 Moreover, CAF derived from hepatocellular carcinoma (HCC) tumors facilitate the generation of regulatory DCs, which are characterized by low expression of costimulatory molecules, high suppressive cytokines production, and enhanced regulation of immune responses, including T‐cell proliferation impairment and promotion of Treg expansion via IDO upregulation.123, 124 CAF also secrete thymic stromal lymphopoietin (TSLP), which favors tumor‐promoting Th2 cell polarization, through myeloid dendritic cell conditioning.125
CAF also contribute to the formation of an immunosuppressed microenvironment through production and remodeling of ECM components, which serve as a physical barrier restricting access of immune cells to cancer cells.126, 127 Stromal ECM proteins influence anti‐tumor immunity by controlling T cells positioning and migration. In PDAC, dense collagen networks represent a physical barrier, rearranging T cell distribution in favor of tumor stroma. These mechanisms are mainly responsible for T cell trapping in stroma that might hinder efficacy of T cell–based immunotherapies.128 Similarly, in human lung cancer, peritumoral ECM fibers play an important role in limiting T cell access to tumor cells. Consistently, matrix reduction with collagenase increased the ability of T cells to contact cancer cells.50 Furthermore, different extracellular proteins highly produced by CAF acted directly on tumor specific CD8+ T cells by reducing the number of infiltrated cells within tumors.129, 130, 131 For example, CAF‐derived βig‐h3 restrains the anti‐tumor immune response by inhibiting CD8+ T cell immunity. βig‐h3 binds to and inhibits signals via integrin β3 (CD61), which is highly expressed on infiltrating CD8+ T cells and leads to increased Hic‐5 protein binding to Y505 phosphorylated Lck blunting the signal transduction.130 Interestingly, depletion of βig‐h3 protein in vivo using an antibody strategy is accompanied by an increase in granzyme B response.
Taken together, these studies demonstrate that CAF impede T cell activation, clonal proliferation, tumor localization, and cytotoxicity. Consequently, there is a growing interest in developing novel therapeutic strategies that target tumor stroma.
4. STRATEGIES TARGETING CAF
The pro‐tumoral functions that CAF exert during cancer development make them promising therapeutic targets in cancer treatment.5, 6, 132, 133 However, CAF targeting has faced numerous obstacles and challenges, in particular due to the lack of specific cell surface markers inducing their depletion without damaging normal tissue. However, with our increasing understanding of CAF phenotypic diversity and functional heterogeneity, a number of preclinical studies have been recently reported (Figure 3).
FIGURE 3.
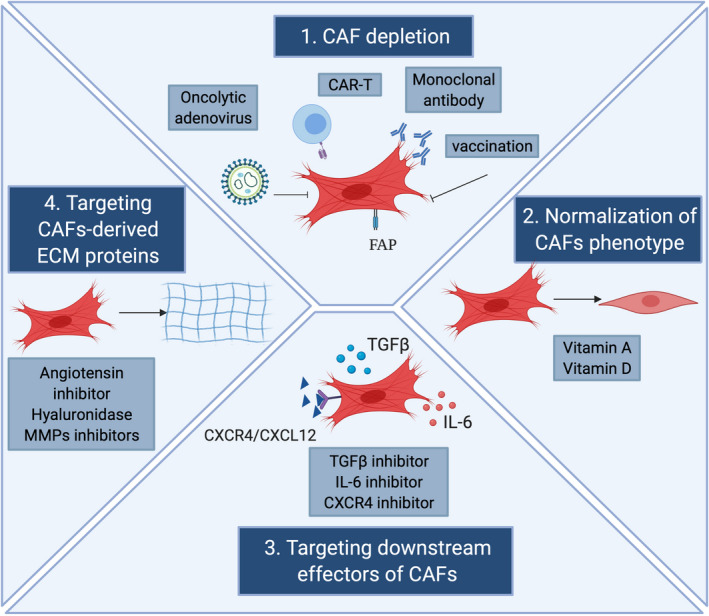
Principal strategies for CAF‐directed anti‐cancer therapies. Main anti‐cancer therapies targeting the stromal compartment in tumors are shown. CAF can be directly depleted by either transgenic technologies or immunotherapies. CAF can also be normalized and adopt an inactive phenotype through the use of molecules, such as vitamin A or vitamin D. Furthermore, targeting crucial signals and effectors of CAF such as chemokines and growth factor pathways can be used to inhibit CAF activation or functions. Finally, CAF‐derived extracellular matrix proteins and associated signaling can be targeted to induce stromal depletion and increase immune T cell infiltration. FAP, fibroblast activation protein; CAR, chimeric antigen receptor; IL‐6, Interleukin 6; MMP, matrix metalloproteinase; ECM, extracellular matrix
4.1. Depletion of CAF using specific surface markers
Anti‐CAF therapies have been primarily focused on CAF depletion by targeting specific surface markers. Indeed, genetic depletion of FAP causes rapid hypoxic necrosis of both cancer and stromal cells by a process involving IFNγ and TNFα.83 Interestingly, this process is capable of enhancing anti‐tumor T cell infiltrate and function in both PDAC and NSCLC.134 Moreover, elimination of FAP+ CAF using oral DNA vaccine targeting FAP increases CD8+ T cell infiltration within the TME and improves intra‐tumoral uptake of chemotherapeutic drugs in multi‐drug‐resistant model of colon and breast carcinoma.88, 135 Further FAP‐targeting strategies, such as FAP‐CAR‐T cell therapy or FAP‐targeted oncolytic adenovirus, promote a specific immune attack against FAP+ CAF, with concomitant anti‐tumor efficacy.136, 137, 138 Using these approaches, elimination of FAP+ CAF reverses CAF‐mediated immunosuppression, upregulates pro‐inflammatory cytokines and increases antigen presentation, T cell function, and trafficking. Additionally, a monoclonal antibody (FAP5‐DM1) targeting FAP+ CAF induces long‐lasting inhibition of tumor growth and complete regression in xenograft models of lung, pancreas, and head and neck cancer, with no sign of toxicity.139 Moreover, depletion of FAP+ stromal cells by FAP‐targeting immunotoxin αFAP‐PE38 in the mouse 4T1 BC model modifies recruitment of tumor‐infiltrating immune cells. In addition, combination of αFAP‐PE38 and paclitaxel potently inhibits tumor growth in vivo.86 As FAP is not exclusively expressed by CAF, this can substantially hinder the precision of the above‐mentioned strategies. In order to counteract this challenge, targeting a CAF subset correlated with chemoresistance and poor survival in breast and lung cancer by using a neutralizing monoclonal antibody against GPR77 showed promising results.28 Indeed, depleting CD10+ GPR77+ CAF reduces tumor formation and improves chemotherapy efficacy in mouse models.28
In order to reactivate the anti‐tumor immune response following CAF‐targeting strategy, recent studies investigated the use of bispecific antibodies, which target both CAF and immune cells.140, 141 RO6874281 consists of an interleukin‐2 variant (IL‐2v) domain that binds the IL‐2 receptor on immune cells and a FAP‐specific domain, which brings the antibody drug conjugate inside the tumor. This antibody stimulates a local immune response by activating effector CD8+ T lymphocytes and natural killer (NK) cells and reducing Treg activity.93, 142, 143 Given its promising results, RO6874281 is presently under clinical trials in combination with atezolumab (anti PD‐L1 antibody) or pembrolizumab (anti‐PD‐1) in several solid tumor indications (Clinical trial information: NCT03875079, NCT03063762, and NCT03193190). In addition, other clinical trials are ongoing to test RO6874281 in combination with chemo‐ or targeted therapies (NCT02627274 and NCT03063762). Moreover, a novel immune cell stroma bispecific antibody was designed, composed of a trimeric split 4‐1BB ligand, targeting 4‐1BBL (CD137), and a monovalent fragment that binds specifically to FAP. This component enhances T cell stimulation in vivo, through the hyper‐crosslinking of 4‐1BB expressed by T cells and FAP expressed by tumor stroma.144 Interestingly, combination of FAP‐4‐1BBL with tumor antigen‐targeted T cell bispecific molecules results in tumor remission in mouse models, accompanied by intra‐tumoral accumulation of activated effector CD8+ T lymphocytes.94, 144
4.2. Normalization of CAF activated phenotype
In addition to the direct depletion of CAF, a new CAF‐targeting strategy was developed in order to revert the activated state of the pro‐tumorigenic CAF into a quiescent state or a tumor‐suppressor phenotype. A first demonstration of the efficacy of this strategy was highlighted in PDAC.18, 19 Indeed, vitamin A deficiency in PDAC patients results in stromal cell activation; and incubation with all‐trans retinoic acid reverts CAF phenotype into a quiescent state and increases apoptosis of surrounding pancreatic cancer cells.18, 19 Similarly, treatment with vitamin D normalizes the activated phenotype of stromal cells and improves the uptake of chemotherapeutic drugs in PDAC mouse models, resulting in 57% increase in mice survival compared to chemotherapy alone, thereby reversing chemotherapeutic resistance.145 As such, reprogramming CAF, via normalization of their activated phenotype, may be a preferable therapeutic option rather than targeted ablation of CAF.
4.3. Targeting downstream effectors of CAF
Both CAF depletion and reversion of their functional states remain challenging therapeutic strategies. New approaches have been proposed in order to target downstream effectors of CAF, mainly CAF‐derived cytokines and chemokines. Novel agents that target IL‐6 and TGFβ have been developed in order to improve the anti‐tumor immune response. Interestingly, therapeutic co‐administration of TGFβ inhibitors with anti‐PD‐L1 immunotherapy reduces TGFβ signaling in stromal cells, facilitates T cell penetration within tumors, and enhances anti‐tumor immunity.91, 92, 146, 147, 148 Interestingly, multiple clinical trials using TGFβ‐based therapies are ongoing, highlighting the clinical importance of CAF‐targeted strategies in cancer treatment. In addition to TGFβ, high levels of IL‐6 are secreted by activated CAF in particular by FAP+ CAF.26, 71, 78 IL‐6 induces production of pro‐inflammatory cytokines and pro‐angiogenic factors, which increase cancer cell proliferation and metastasis and negatively regulate NK and T cell cytotoxic activity.149, 150 Thus, agents targeting IL‐6, IL‐6R, or JAK/STAT3 pathway downstream of IL‐6 have been approved by the US Food and Drug administration for the treatment of myeloproliferative diseases and autoimmune disorders.149 Similarly, mTOR‐4E‐BP1 pathway is responsible for protein synthesis in SMA+ CAF. Inhibition of this pathway using the multi‐receptor somatostatin analogue pasireotide (SOM230) in mouse models downregulates CAF‐secreted molecules, such as IL‐6, thereby abrogating CAF‐directed cancer cell resistance to chemotherapy and showing some efficacy and tolerability in first clinical trials.151, 152 In addition, targeting CXCL12‐CXCR4 axis with AMD3100 compound reverses FAP+ CAF‐mediated immunosuppression and synergizes with anti‐PD‐L1 immunotherapy in pancreatic cancer.33, 153, 154, 155 Collectively, drugs that target the stromal CAF signals and effectors have emerged as an important complement to anti‐tumor therapies.
4.4. Targeting CAF‐derived ECM proteins
Other anti‐stromal therapies consist in targeting ECM proteins that serve as a physical barrier preventing anti‐tumor immune cell access and therapeutic drug delivery. For example, the angiotensin inhibitor losartan reduces stromal collagen content and hyaluronan production.156, 157, 158 Consequently, losartan increases vascular perfusion and enhances drug and oxygen delivery to tumors, thereby potentiating chemotherapy in breast and pancreatic cancer models.159 Similarly, enzymatic ablation of hyaluronan by PEGPH20, a PEGylated recombinant hyaluronidase, leads to re‐expansion of the tumor micro‐vasculature and improves intra‐tumoral penetration of systemic chemotherapy.160, 161 Moreover, CAF‐controlled ECM remodeling cannot be achieved without the production of metalloproteases (MMPs). Thus, novel MMPs inhibitors are emerging in order to improve ECM stiffness to favor drug delivery.162, 163 On the whole, the above‐mentioned anti‐stromal therapies are designed based on the premise that CAF promote cancer development. Nevertheless, CAF heterogeneity reveals the existence of tumor‐suppressive CAF subtypes, which require further studies.
5. CONCLUSIONS AND PERSPECTIVES
It is becoming clear that research on CAF has recently reached an exciting and critical stage. Although challenging, rapid advances in the knowledge of CAF biology, in particular CAF heterogeneity, will help in developing novel therapeutic strategies targeting CAF. CAF are now considered as targets that could be manipulated for therapeutic benefit in cancer patients. There are currently many clinical trials involving CAF‐targeting agents in combination with existing therapies. Targeting CAF is expected first to improve access to either conventional or targeted therapies and second to enhance infiltration of active cytotoxic T/NK cells within tumor. Despite recent progress, we are still facing numerous challenges in developing adequate tools to modify stromal components in tumors. Multiple studies recently highlighted specific functions of distinct CAF subpopulations, bringing key insights on CAF cellular heterogeneity and revealing interesting new specific markers. As stromal cells are essential components for physiological processes, any therapy targeting stromal pro‐tumoral functions should be specific enough to spare stromal cells in healthy tissues. Using specific markers for targeting these distinct CAF subpopulations will pave the way to new promising therapeutic combinations in cancer.
CONFLICT OF INTEREST
F.M‐G. received research support from Innate‐Pharma, Roche, and Bristol‐Myers‐Squibb (BMS). RM declares no potential conflict of interest.
ACKNOWLEDGEMENTS
RM is supported by the Institut National du Cancer (INCa) and the Simone and Cino del Duca Foundation. The Stress and Cancer laboratory is supported by grants from the Ligue Nationale Contre le Cancer (Labelisation), Inserm (PC201317), Institut Curie (Incentive and Cooperative Program Tumor Micro‐environment PIC TME/T‐MEGA, PIC3i CAFi), ICGex (ANR‐10‐EQPX‐03), SIRIC (INCa‐DGOS‐4654), INCa (STROMAE INCa‐DGOS‐9963, CaLYS INCa‐11692, INCa‐DGOS‐Inserm‐12554), SIGN'IT 2019 from the Foundation ARC and the Foundation “Chercher et Trouver”. F.M‐G acknowledges both the Association « Le Ruban Rose », as well as the Simone and Cino del Duca Foundation for attribution of their “Grand Prix”. F.M‐G is very grateful to all her funders for providing support throughout the years.
Mhaidly R, Mechta‐Grigoriou F. Role of cancer‐associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer. Immunol Rev. 2021;302:259–272. 10.1111/imr.12978
This article is part of a series of reviews covering Immunological functions of fibroblasts in human health and disease appearing in Volume 302 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423‐1437. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309‐322. 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 3.Costa A, Scholer‐Dahirel A, Mechta‐Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23‐32. 10.1016/j.semcancer.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582‐598. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 5.Mhaidly R, Mechta‐Grigoriou F. Fibroblast heterogeneity in tumor micro‐environment: Role in immunosuppression and new therapies. Semin Immunol. 2020;48:101417. 10.1016/j.smim.2020.101417 [DOI] [PubMed] [Google Scholar]
- 6.Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer‐associated fibroblasts. Nat Rev Cancer. 2020;20:174‐186. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentric G, Mechta‐Grigoriou F. Tumor cells and cancer‐associated fibroblasts: An updated metabolic perspective. Cancers. 2021;13(3):399. 10.3390/cancers13030399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickman RE, Faget DV, Beachy P, et al. Deconstructing tumor heterogeneity: the stromal perspective. Oncotarget. 2020;11:3621‐3632. 10.18632/oncotarget.27736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano A, Pérez‐Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial‐mesenchymal transitions by repressing E‐cadherin expression. Nat Cell Biol. 2000;2:76‐83. 10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- 10.Quante M, Tu SP, Tomita H, et al. Bone marrow‐derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257‐272. 10.1016/j.ccr.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosaka K, Yang Y, Seki T, et al. Pericyte‐fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci U S A. 2016;113:E5618‐5627. 10.1073/pnas.1608384113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel AP, Ginestier C, Pommier RM, et al. A stemness‐related ZEB1‐MSRB3 axis governs cellular pliancy and breast cancer genome stability. Nat Med. 2017;23:568‐578. 10.1038/nm.4323 [DOI] [PubMed] [Google Scholar]
- 13.Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54‐63. 10.1172/jci93558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz Y, Cohen N, Shani O, et al. Bone marrow‐derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075‐3093. 10.1084/jem.20180818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd S, Spaeth E, Watson K, et al. Origins of the tumor microenvironment: quantitative assessment of adipose‐derived and bone marrow‐derived stroma. PLoS One. 2012;7:e30563. 10.1371/journal.pone.0030563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte‐derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657‐5668. 10.1158/0008-5472.can-13-0530 [DOI] [PubMed] [Google Scholar]
- 17.Strong AL, Pei DT, Hurst CG, Gimble JM, Burow ME, Bunnell BA. Obesity enhances the conversion of adipose‐derived stromal/stem cells into carcinoma‐associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int. 2017;2017:9216502. 10.1155/2017/9216502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Froeling FE, Feig C, Chelala C, et al. Retinoic acid‐induced pancreatic stellate cell quiescence reduces paracrine Wnt‐β‐catenin signaling to slow tumor progression. Gastroenterology. 2011;141(4):1486‐1497. 10.1053/j.gastro.2011.06.047 [DOI] [PubMed] [Google Scholar]
- 19.Carapuça EF, Gemenetzidis E, Feig C, et al. Anti‐stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol. 2016;239:286‐296. 10.1002/path.4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol. 2014;25:3‐9. 10.1016/j.semcancer.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392‐401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 22.Özdemir BC, Pentcheva‐Hoang T, Carstens JL, et al. Depletion of carcinoma‐associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719‐734. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735‐747. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer‐associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186‐196. 10.1016/j.addr.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 25.Richards KE, Zeleniak AE, Fishel Ml, et al. Cancer‐associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770‐1778. 10.1038/onc.2016.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa A, Kieffer Y, Scholer‐Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463‐479. 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 27.Givel AM, Kieffer Y, Scholer‐Dahirel A, et al. miR200‐regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun. 2018;9:1056. 10.1038/s41467-018-03348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su S, Chen J, Yao H, et al. CD10(+)GPR77(+) Cancer‐Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841‐856. 10.1016/j.cell.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Gieniec KA, Butler LM, Worthley DL, Woods SL. Cancer‐associated fibroblasts‐heroes or villains? Br J Cancer. 2019;121:293‐302. 10.1038/s41416-019-0509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizutani Y, Kobayashi H, Iida T, et al. Meflin‐positive cancer‐associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 2019;79:5367‐5381. 10.1158/0008-5472.can-19-0454 [DOI] [PubMed] [Google Scholar]
- 31.Miyai Y, Esaki N, Takahashi M, Enomoto A. Cancer‐associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci. 2020;111:1047‐1057. 10.1111/cas.14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toullec A, Gerald D, Despouy G, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211‐230. 10.1002/emmm.201000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP‐expressing carcinoma‐associated fibroblasts synergizes with anti‐PD‐L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212‐20217. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugihara H, Ishimoto T, Yasuda T, et al. Cancer‐associated fibroblast‐derived CXCL12 causes tumor progression in adenocarcinoma of the esophagogastric junction. Med Oncol. 2015;32:618. 10.1007/s12032-015-0618-7 [DOI] [PubMed] [Google Scholar]
- 35.Teng F, Tian W‐Y, Wang Y‐M, et al. Cancer‐associated fibroblasts promote the progression of endometrial cancer via the SDF‐1/CXCR4 axis. J Hematol Oncol. 2016;9:8. 10.1186/s13045-015-0231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Zhou J, Zhang J, et al. Cancer‐associated fibroblasts promote PD‐L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019;145:1946‐1957. 10.1002/ijc.32278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becht E, de Reyniès A, Giraldo NA, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057‐4066. 10.1158/1078-0432.ccr-15-2879 [DOI] [PubMed] [Google Scholar]
- 38.Qin X, Yan M, Wang X, et al. Cancer‐associated Fibroblast‐derived IL‐6 Promotes Head and Neck Cancer Progression via the Osteopontin‐NF‐kappa B Signaling Pathway. Theranostics. 2018;8:921‐940. 10.7150/thno.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518‐527. 10.1038/nm1764 [DOI] [PubMed] [Google Scholar]
- 40.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39‐49. 10.1016/j.drup.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, et al. Stromal Myofibroblasts are associated with poor prognosis in solid cancers: a meta‐analysis of published studies. PLoS One. 2016;11:e0159947. 10.1371/journal.pone.0159947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiori ME, Di Franco S, Villanova L, et al. Cancer‐associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70. 10.1186/s12943-019-0994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrett R, Puré E. Cancer‐associated fibroblasts: key determinants of tumor immunity and immunotherapy. Curr Opin Immunol. 2020;64:80‐87. 10.1016/j.coi.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker AT, Abuwarwar MH, Poly L, Wilkins S, Fletcher AL. Cancer‐associated fibroblasts and T cells: From mechanisms to outcomes. J Immunol. 2021;206:310‐320. 10.4049/jimmunol.2001203 [DOI] [PubMed] [Google Scholar]
- 45.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF‐β‐associated extracellular matrix genes link cancer‐associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9:4692. 10.1038/s41467-018-06654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer‐associated fibroblasts. Front Immunol. 2018;9:414. 10.3389/fimmu.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteran L, Erez N. The dark side of fibroblasts: cancer‐associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019;10:1835. 10.3389/fimmu.2019.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kieffer Y, Hocine HR, Gentric G, et al. Single‐cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020;10:1330‐1351. 10.1158/2159-8290.cd-19-1384 [DOI] [PubMed] [Google Scholar]
- 49.Goetz JG, Minguet S, Navarro‐Lérida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin‐1 favors tumor invasion and metastasis. Cell. 2011;146:148‐163. 10.1016/j.cell.2011.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899‐910. 10.1172/jci45817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvo F, Ege N, Grande‐Garcia A, et al. Mechanotransduction and YAP‐dependent matrix remodelling is required for the generation and maintenance of cancer‐associated fibroblasts. Nat Cell Biol. 2013;15:637‐646. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Attieh Y, Clark AG, Grass C, et al. Cancer‐associated fibroblasts lead tumor invasion through integrin‐β3‐dependent fibronectin assembly. J Cell Biol. 2017;216:3509‐3520. 10.1083/jcb.201702033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glentis A, Oertle P, Mariani P, et al. Cancer‐associated fibroblasts induce metalloprotease‐independent cancer cell invasion of the basement membrane. Nat Commun. 2017;8:924. 10.1038/s41467-017-00985-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazareth MR, Broderick L, Simpson‐Abelson MR, et al. Characterization of human lung tumor‐associated fibroblasts and their ability to modulate the activation of tumor‐associated T cells. J Immunol. 2007;178:5552‐5562. 10.4049/jimmunol.178.9.5552 [DOI] [PubMed] [Google Scholar]
- 55.Primac I, Maquoi E, Blacher S, et al. Stromal integrin α11 regulates PDGFR‐β signaling and promotes breast cancer progression. J Clin Invest. 2019;129:4609‐4628. 10.1172/jci125890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strell C, Paulsson J, Jin S‐B, et al. Impact of epithelial‐stromal interactions on peritumoral fibroblasts in ductal carcinoma in situ. J Natl Cancer Inst. 2019;111:983‐995. 10.1093/jnci/djy234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cremasco V, Astarita JL, Grauel AL, et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune‐excluded breast tumors. Cancer Immunol Res. 2018;6:1472‐1485. 10.1158/2326-6066.cir-18-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonneau C, Eliès A, Kieffer Y, et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020;22:76. 10.1186/s13058-020-01311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelon F, Bourachot B, Kieffer Y, et al. Cancer‐associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun. 2020;11:404. 10.1038/s41467-019-14134-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudland PS. Prognostic significance of the metastasis‐inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595‐1603. [PubMed] [Google Scholar]
- 61.Simpkins SA, Hanby AM, Holliday DL, Speirs V. Clinical and functional significance of loss of caveolin‐1 expression in breast cancer‐associated fibroblasts. J Pathol. 2012;227:490‐498. 10.1002/path.4034 [DOI] [PubMed] [Google Scholar]
- 62.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640‐1646. 10.4161/cbt.5.12.3354 [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, Shi C, Zeng L, et al. High COX‐2 expression in cancer‐associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol Carcinog. 2020;59:265‐280. 10.1002/mc.23150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Courtois ET, Sengupta D, et al. Reference component analysis of single‐cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708‐718. 10.1038/ng.3818 [DOI] [PubMed] [Google Scholar]
- 65.Bartoschek M, Oskolkov N, Bocci M, et al. Spatially and functionally distinct subclasses of breast cancer‐associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:5150. 10.1038/s41467-018-07582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24:1277‐1289. 10.1038/s41591-018-0096-5 [DOI] [PubMed] [Google Scholar]
- 67.Biffi G, Oni TE, Spielman B, et al. IL1‐Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282‐301. 10.1158/2159-8290.cd-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neuzillet C, Tijeras‐Raballand A, Ragulan C, et al. Inter‐ and intra‐tumoural heterogeneity in cancer‐associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248:51‐65. 10.1002/path.5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elyada E, Bolisetty M, Laise P, et al. Cross‐species single‐cell analysis of pancreatic ductal adenocarcinoma reveals antigen‐presenting cancer‐associated fibroblasts. Cancer Discov. 2019;9:1102‐1123. 10.1158/2159-8290.cd-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dominguez CX. Single‐cell RNA sequencing reveals stromal evolution into LRRC15(+) Myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232‐253. 10.1158/2159-8290.cd-19-0644 [DOI] [PubMed] [Google Scholar]
- 71.Öhlund D, Handly‐Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579‐596. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedman G, Levi‐Galibov O, David E, et al. Cancer‐associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nature Cancer. 2020;1:692‐708. 10.1038/s43018-020-0082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sebastian A, Hum NR, Martin KA, et al. Single‐cell transcriptomic analysis of tumor‐derived fibroblasts and normal tissue‐resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers. 2020;12:1307. 10.3390/cancers12051307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahashi H, Sakakura K, Kawabata‐Iwakawa R, et al. Immunosuppressive activity of cancer‐associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2015;64:1407‐1417. 10.1007/s00262-015-1742-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Jaeghere EA, Denys HG, De Wever O. Fibroblasts fuel immune escape in the tumor microenvironment. Trends Cancer. 2019;5:704‐723. 10.1016/j.trecan.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 76.Liu T, Han C, Wang S, et al. Cancer‐associated fibroblasts: an emerging target of anti‐cancer immunotherapy. J Hematol Oncol. 2019;12:86. 10.1186/s13045-019-0770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559‐572. 10.1038/nri.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kato T. Cancer‐associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T Cells Via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24:4820‐4833. 10.1158/1078-0432.ccr-18-0205 [DOI] [PubMed] [Google Scholar]
- 79.Shani O, Vorobyov T, Monteran L, et al. Fibroblast‐derived IL33 facilitates breast cancer metastasis by modifying the immune microenvironment and driving type 2 immunity. Cancer Res. 2020;80:5317‐5329. 10.1158/0008-5472.can-20-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su X, Ye J, Hsueh EC, et al. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630‐1641. 10.4049/jimmunol.0902813 [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Yi T, Kortylewski M, et al. IL‐17 can promote tumor growth through an IL‐6‐Stat3 signaling pathway. J Exp Med. 2009;206:1457‐1464. 10.1084/jem.20090207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Q, Han Y, Fei G, et al. IL‐17 promoted metastasis of non‐small‐cell lung cancer cells. Immunol Lett. 2012;148:144‐150. 10.1016/j.imlet.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 83.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein‐alpha. Science. 2010;330:827‐830. 10.1126/science.1195300 [DOI] [PubMed] [Google Scholar]
- 84.Fearon DT. The carcinoma‐associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2:187‐193. 10.1158/2326-6066.CIR-14-0002 [DOI] [PubMed] [Google Scholar]
- 85.Wang LC, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154‐166. 10.1158/2326-6066.cir-13-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang J, Xiao L, Joo K, et al. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int J Cancer. 2016;138:1013‐1023. 10.1002/ijc.29831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X, Lin Y, Shi Y, et al. FAP promotes immunosuppression by cancer‐associated fibroblasts in the tumor microenvironment via STAT3‐CCL2 signaling. Cancer Res. 2016;76:4124‐4135. 10.1158/0008-5472.can-15-2973 [DOI] [PubMed] [Google Scholar]
- 88.Duperret EK, Trautz A, Ammons D, et al. Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice. Clin Cancer Res. 2018;24:1190‐1201. 10.1158/1078-0432.ccr-17-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorchs L, Fernández Moro C, Bankhead P, et al. Human pancreatic carcinoma‐associated fibroblasts promote expression of co‐inhibitory markers on CD4(+) and CD8(+) T‐cells. Front Immunol. 2019;10:847. 10.3389/fimmu.2019.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Érsek B, Silló P, Cakir U, et al. Melanoma‐associated fibroblasts impair CD8+ T cell function and modify expression of immune checkpoint regulators via increased arginase activity. Cell Mol Life Sci. 2021;78:661‐673. 10.1007/s00018-020-03517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544‐548. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batlle E, Massagué J. Transforming growth factor‐β signaling in immunity and cancer. Immunity. 2019;50:924‐940. 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang HC, Chan LP, Cho SF. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front Oncol. 2019;9:1084. 10.3389/fonc.2019.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trüb M, Uhlenbrock F, Claus C, et al. Fibroblast activation protein‐targeted‐4‐1BB ligand agonist amplifies effector functions of intratumoral T cells in human cancer. J Immunother Cancer. 2020;8:e000238. 10.1136/jitc-2019-000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ene‐Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121‐1132. 10.1053/j.gastro.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275‐287. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teramoto K, Igarashi T, Kataoka Y, et al. Clinical significance of PD‐L1‐positive cancer‐associated fibroblasts in pN0M0 non‐small cell lung cancer. Lung Cancer. 2019;137:56‐63. 10.1016/j.lungcan.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 98.Inoue C, Miki Y, Saito R, et al. PD‐L1 induction by cancer‐associated fibroblast‐derived factors in lung adenocarcinoma cells. Cancers. 2019;11:1257. 10.3390/cancers11091257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dou D, Ren X, Han M, et al. Cancer‐associated fibroblasts‐derived exosomes suppress immune cell function in breast cancer via the miR‐92/PD‐L1 Pathway. Front Immunol. 2020;11:2026. 10.3389/fimmu.2020.02026 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Sakamoto A, Kunou S, Shimada K, et al. Pyruvate secreted from patient‐derived cancer‐associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. 2019;110:269‐278. 10.1111/cas.13873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Becker LM, O’Connell JT, Vo AP, et al. Epigenetic reprogramming of cancer‐associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 2020;31:107701. 10.1016/j.celrep.2020.107701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Comito G, Iscaro A, Bacci M, et al. Lactate modulates CD4(+) T‐cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38:3681‐3695. 10.1038/s41388-019-0688-7 [DOI] [PubMed] [Google Scholar]
- 103.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015;36:257‐264. 10.1016/j.it.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Timosenko E, Hadjinicolaou AV, Cerundolo V. Modulation of cancer‐specific immune responses by amino acid degrading enzymes. Immunotherapy. 2017;9:83‐97. 10.2217/imt-2016-0118 [DOI] [PubMed] [Google Scholar]
- 105.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2,3‐dioxygenase‐mediated tryptophan degradation. Blood. 2004;103:4619‐4621. 10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- 106.Ino Y, Yamazaki‐Itoh R, Oguro S, et al. Arginase II expressed in cancer‐associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146. 10.1371/journal.pone.0055146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S. Tumor cyclooxygenase‐2/prostaglandin E2‐dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211‐5220. 10.1158/0008-5472.can-05-0141 [DOI] [PubMed] [Google Scholar]
- 108.Karavitis J, Hix LM, Shi YH, et al. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2‐induction of regulatory T cell migration. PLoS One. 2012;7:e46342. 10.1371/journal.pone.0046342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miao J, Lu X, Hu Y, et al. Prostaglandin E(2) and PD‐1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget. 2017;8:89802‐89810. 10.18632/oncotarget.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li A, Chen P, Leng Y, Kang J. Histone deacetylase 6 regulates the immunosuppressive properties of cancer‐associated fibroblasts in breast cancer through the STAT3‐COX2‐dependent pathway. Oncogene. 2018;37:5952‐5966. 10.1038/s41388-018-0379-9 [DOI] [PubMed] [Google Scholar]
- 111.Snijdewint FG, Kaliński P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321‐5329. [PubMed] [Google Scholar]
- 112.de Lourdes Mora‐García M, García‐Rocha R, Morales‐Ramírez O, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14:302. 10.1186/s12967-016-1057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kovács‐Sólyom F, Blaskó A, Fajka‐Boja R, et al. Mechanism of tumor cell‐induced T‐cell apoptosis mediated by galectin‐1. Immunol Lett. 2010;127:108‐118. 10.1016/j.imlet.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 114.He XJ, Tao H‐Q, Hu Z‐M, et al. Expression of galectin‐1 in carcinoma‐associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1. Cancer Sci. 2014;105:1402‐1410. 10.1111/cas.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Büchel G, Schulte JH, Harrison L, et al. Immune response modulation by Galectin‐1 in a transgenic model of neuroblastoma. Oncoimmunology. 2016;5:e1131378. 10.1080/2162402x.2015.1131378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang D, Gao J, Wang S, et al. Cancer‐associated fibroblasts promote angiogenesis in gastric cancer through galectin‐1 expression. Tumour Biol. 2016;37:1889‐1899. 10.1007/s13277-015-3942-9 [DOI] [PubMed] [Google Scholar]
- 117.Ahmadzadeh M, Rosenberg SA. TGF‐beta 1 attenuates the acquisition and expression of effector function by tumor antigen‐specific human memory CD8 T cells. J Immunol. 2005;174:5215‐5223. 10.4049/jimmunol.174.9.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Broderick L, Bankert RB. Membrane‐associated TGF‐beta1 inhibits human memory T cell signaling in malignant and nonmalignant inflammatory microenvironments. J Immunol. 2006;177:3082‐3088. 10.4049/jimmunol.177.5.3082 [DOI] [PubMed] [Google Scholar]
- 119.Thomas DA, Massagué J. TGF‐beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369‐380. 10.1016/j.ccr.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 120.Stavnezer J, Kang J. The surprising discovery that TGF beta specifically induces the IgA class switch. J Immunol. 2009;182:5‐7. 10.4049/jimmunol.182.1.5 [DOI] [PubMed] [Google Scholar]
- 121.Shalapour S, Lin X‐J, Bastian IN. Inflammation‐induced IgA+ cells dismantle anti‐liver cancer immunity. Nature. 2017;551:340‐345. 10.1038/nature24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flavell RA, Sanjabi S, Wrzesinski SH, Licona‐Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554‐567. 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Popov A, Abdullah Z, Wickenhauser C, et al. Indoleamine 2,3‐dioxygenase‐expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116:3160‐3170. 10.1172/jci28996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng JT, Deng Y‐N, Yi H‐M, et al. Hepatic carcinoma‐associated fibroblasts induce IDO‐producing regulatory dendritic cells through IL‐6‐mediated STAT3 activation. Oncogenesis. 2016;5:e198. 10.1038/oncsis.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer‐associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469‐478. 10.1084/jem.20101876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395‐406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243‐1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hartmann N, et al. Prevailing role of contact guidance in intrastromal T‐cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20:3422‐3433. 10.1158/1078-0432.ccr-13-2972 [DOI] [PubMed] [Google Scholar]
- 129.Ecker BL, Kaur A, Douglass SM, et al. Age‐related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 2019;9:82‐95. 10.1158/2159-8290.cd-18-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goehrig D, Nigri J, Samain R, et al. Stromal protein βig‐h3 reprogrammes tumour microenvironment in pancreatic cancer. Gut. 2019;68:693‐707. 10.1136/gutjnl-2018-317570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaur A, Ecker BL, Douglass SM, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9:64‐81. 10.1158/2159-8290.cd-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanley CJ, Mellone M, Ford K, et al. Targeting the myofibroblastic cancer‐associated fibroblast phenotype through inhibition of NOX4. J Natl Cancer Inst. 2018;110:109‐120. 10.1093/jnci/djx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen X, Song E. Turning foes to friends: targeting cancer‐associated fibroblasts. Nat Rev Drug Discov. 2019;18:99‐115. 10.1038/s41573-018-0004-1 [DOI] [PubMed] [Google Scholar]
- 134.Gunderson AJ, Yamazaki T, McCarty K, et al. Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS One. 2019;14:e0211117. 10.1371/journal.pone.0211117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor‐associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955‐1962. 10.1172/jci26532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lo A, Wang L‐CS, Scholler J, et al. Tumor‐Promoting Desmoplasia Is Disrupted by Depleting FAP‐Expressing Stromal Cells. Cancer Res. 2015;75:2800‐2810. 10.1158/0008-5472.can-14-3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Freedman JD, Duffy MR, Lei‐Rossmann J, et al. An oncolytic virus expressing a T‐cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852‐6865. 10.1158/0008-5472.can-18-1750 [DOI] [PubMed] [Google Scholar]
- 138.de Sostoa J, Fajardo CA, Moreno R, et al. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein‐targeted bispecific T‐cell engager. J Immunother Cancer. 2019;7:19. 10.1186/s40425-019-0505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ostermann E, Garin‐Chesa P, Heider KH, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14:4584‐4592. 10.1158/1078-0432.ccr-07-5211 [DOI] [PubMed] [Google Scholar]
- 140.Melero I, Castanon Alvarez E, Mau‐Sorensen M, et al. Clinical activity, safety, and PK/PD from a phase I study of RO6874281, a fibroblast activation protein (FAP) targeted interleukin‐2 variant (IL‐2v). Ann Oncol. 2018;29:viii134‐viii135. 10.1093/annonc/mdy279.400 [DOI] [Google Scholar]
- 141.Soerensen MM, Ros W, Rodriguez‐Ruiz ME, et al. Safety, PK/PD, and anti‐tumor activity of RO6874281, an engineered variant of interleukin‐2 (IL‐2v) targeted to tumor‐associated fibroblasts via binding to fibroblast activation protein (FAP). J Clin Oncol. 2018;36:e15155. 10.1200/JCO.2018.36.15_suppl.e15155 [DOI] [Google Scholar]
- 142.Dasari S, Fang Y, Mitra AK. Cancer associated fibroblasts: naughty neighbors that drive ovarian cancer progression. Cancers. 2018;10:406. 10.3390/cancers10110406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horvath L, Thienpont B, Zhao L, Wolf D, Pircher A. Overcoming immunotherapy resistance in non‐small cell lung cancer (NSCLC) ‐ novel approaches and future outlook. Mol Cancer. 2020;19:141. 10.1186/s12943-020-01260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Claus C, Ferrara C, Xu W, et al. Tumor‐targeted 4–1BB agonists for combination with T cell bispecific antibodies as off‐the‐shelf therapy. Sci Transl Med. 2019;11(496):eaav5989. 10.1126/scitranslmed.aav5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor‐mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80‐93. 10.1016/j.cell.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ohshio Y, Teramoto K, Hanaoka J, et al. Cancer‐associated fibroblast‐targeted strategy enhances antitumor immune responses in dendritic cell‐based vaccine. Cancer Sci. 2015;106:134‐142. 10.1111/cas.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Colak S, Ten Dijke P. Targeting TGF‐beta signaling in cancer. Trends Cancer. 2017;3:56‐71. 10.1016/j.trecan.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 148.Wei Y, Kim TJ, Peng DH, et al. Fibroblast‐specific inhibition of TGF‐β1 signaling attenuates lung and tumor fibrosis. J Clin Invest. 2017;127:3675‐3688. 10.1172/jci94624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL‐6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234‐248. 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jones SA, Jenkins BJ. Recent insights into targeting the IL‐6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773‐789. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 151.Moatassim‐Billah S, Duluc C, Samain R, et al. Anti‐metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancer‐associated fibroblasts. Oncotarget. 2016;7:41584‐41598. 10.18632/oncotarget.9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feun LG. Phase II trial of SOM230 (pasireotide LAR) in patients with unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:9‐15. 10.2147/JHC.S153672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng Y, Li B, Liang Y, et al. Dual blockade of CXCL12‐CXCR4 and PD‐1‐PD‐L1 pathways prolongs survival of ovarian tumor‐bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. 2019;33:6596‐6608. 10.1096/fj.201802067RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Biasci D, Smoragiewicz M, Connell CM, et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci U S A. 2020;117:28960‐28970. 10.1073/pnas.2013644117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Z, Wang Y, Shen Y, et al. Targeting pulmonary tumor microenvironment with CXCR4‐inhibiting nanocomplex to enhance anti‐PD‐L1 immunotherapy. Sci Adv. 2020;6:eaaz9240. 10.1126/sciadv.aaz9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457‐1461. 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418‐429. 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim DJ, Kim J, Spaunhurst K, et al. Open‐label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745‐751. 10.1200/jco.2013.49.9525 [DOI] [PubMed] [Google Scholar]
- 159.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. 10.1038/ncomms3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112‐120. 10.1136/gutjnl-2012-302529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hingorani SR, Harris WP, Beck JT, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848‐2854. 10.1158/1078-0432.ccr-15-2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chiappori AA, Eckhardt SG, Bukowski R, et al. A phase I pharmacokinetic and pharmacodynamic study of s‐3304, a novel matrix metalloproteinase inhibitor, in patients with advanced and refractory solid tumors. Clin Cancer Res. 2007;13:2091‐2099. 10.1158/1078-0432.ccr-06-1586 [DOI] [PubMed] [Google Scholar]
- 163.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904‐927. 10.1038/nrd4390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


