Abstract
Since the completion of the Management of Myelomeningocoele Study, maternal‐fetal surgery for spina bifida has become a valid option for expecting parents. More recently, multiple groups are exploring a minimally invasive approach and recent outcomes have addressed many of the initial concerns with this approach. Based on a previously published framework, we attempt to delineate the developmental stage of the surgical techniques. Furthermore, we discuss the barriers of performing randomized controlled trials comparing two surgical interventions and suggest that data collection through registries is an alternative method to gather high‐grade evidence.
Key Points
What's already known about this topic?
The use of fetoscopy for spina bifida repair is adopted by an increasing number of centers worldwide. On the other hand, its efficacy has not been established within a randomized controlled trial and thus it is still considered experimental by others.
What does this study add?
Herein we describe a framework which could be used to determine the developmental stage of novel interventions in the field of maternal‐fetal surgery. Furthermore, we discuss the difficulties of gathering high level evidence for a fetoscopic closure of the spinal defect in fetuses with a spina bifida.
1. INTRODUCTION
After its publication in 2011, the Management of Myelomeningocele study (MOMS) has led to a widespread expansion of centers offering prenatal surgery for fetuses with a spina bifida.1 , 2 The benefits of prenatal surgery are a reduction in the need for ventriculo‐peritoneal (VP) shunt placement, as well as improved mental development, motor and urological outcomes.1 , 3 The downside of open maternal‐fetal surgery is the high maternal morbidity and the risk of uterine rupture due to the large hysterotomy in current and subsequent pregnancies.4 In addition, any fetal intervention results in an increased risk of preterm birth and complications of prematurity.
Because of these risks many groups have explored the possibility of a fetoscopic approach already since the 1990s.5 One initial attempt included a maternal laparotomy but due to high maternal and fetal risks it was nearly immediately abandoned in favor of open maternal‐fetal surgery.6 Yet, several researchers continued to explore a percutaneous fetoscopic approach.7, 8, 9, 10 With ongoing experience more steady techniques were established, though the benefits of a minimally invasive approach were initially offset by longer surgery times, increased risk of preterm premature rupture of membranes (PPROM), preterm delivery and higher fetal and neonatal mortality.7 , 8 , 10, 11, 12 These discouraging results temporized many centers in adopting an endoscopic approach.
In 2017, the first clinical series of an alternative fetoscopic technique was reported, combining maternal laparotomy with a two‐port fetoscopic closure (open fetoscopy).13 The technique is still evolving but recent results are promising, as they provided first, early evidence for a lower risk of PPROM and longer interval to delivery.13, 14, 15 In addition, albeit not evaluated in a clinical trial, the rate of VP shunting and motor outcome appeared to be in the range of what was observed in the MOMS trial.
Improving performance as well as ongoing criticisms from others triggered the idea to set up a consortium studying the outcomes of fetoscopic spina bifida closure.16 This raises the question on how exactly top‐level evidence will be gathered and, for instance, whether it is justifiable not to do so in a randomized controlled trial (RCT) with head to head comparison of the investigational (fetoscopic) technique versus what is the standard of care (the open approach). Conversely, one may consider introducing the fetoscopic approach based on the available literature. In this paper, we discuss the challenges of evidence‐based medicine in maternal‐fetal surgery and alternatives to performing an RCT. We also propose a framework that could be used as guidance to define the developmental stage of any novel treatment, provided a wide support, a spirit of collaboration, and honest registration can be established and techniques become more consistent.
2. CONCERNS ABOUT THE FETOSCOPIC APPROACH
The use of fetoscopy for spina bifida closure has been controversial from the moment it was introduced. The main concerns were about the safety of using CO2 for intra‐amniotic insufflation, the high intraoperative complications and fetal mortality rates, the high PPROM and preterm birth rates, the use of patches to cover the defect instead of anatomical closure and the fragmented and incomplete reporting of neurological outcomes. In the last years, many of these concerns are being addressed and more recent outcomes are encouraging (Table 1). The safety of CO2 insufflation was questioned because initial animal experiments observed the development of a normoxic fetal acidosis, particularly at higher pressures.20, 21, 22, 23
TABLE 1.
Overview of procedural, pregnancy and neonatal outcomes
| Postnatal repair | Open fetal surgery | Percutaneous fetoscopy | Open fetoscopy | ||
|---|---|---|---|---|---|
| Cases (N) | 921 , 17, 18, 19 | 911 , 17, 18, 19 | Germany: 718 (59)7 , 9 a | Brasil: 6016 (80)b | 58 (40/18)15 c |
| GA at surgery (weeks) | NA | 19–25.9d | 21.0–29.1d | 24–28.9d | 24.9 ± 0.7/25.0 ± 0.5 |
| Surgery times (min) | Not reported | 105 ± 23.2 | 140–315 | 83–450 | 261 ± 58/237 ± 47 |
| PPROM | 7.6% | 44% | 84.3% | 67% | 28%/29% |
| Fetal demise (N) | 0 | 1 | 0/71 | 1 (+1 TOP)/60 | 0/32//1/18 |
| GA at delivery (weeks) | 37.3 ± 1.1 | 34.0 ± 3.0 | 32+2 | 32.5 (26.9–40.7) | 36.5 ± 3.5//37.6 ± 3.1 |
| Vaginal birth | Not reported | 0% | 0% | 22% | 50%//47% |
| NND (N) | 2/92 | 1/91 | 2/71 | 0/60 | 0/32//0/17 |
| Dehiscence at repair site or CSF leakage | NA | 13/77 (13%) | 28% | 10% | 25%//0% |
| Treated for hydrocephalus | 84% | 44% | 45% | 14/30 (47%) | 47%//33%e |
Note: Data are presented as mean ± standard deviation; median (interquartile range) or absolute numbers (N), depending on what is published in the respective papers.
Abbreviations: CSF, cerebrospinal fluid; GA, gestational age; NND, neonatal death; PPROM, preterm premature rupture of membranes; TOP, termination of pregnancy; VP, ventriculo‐peritoneal.
Data are from two papers with two overlapping study populations, data depicted are for total cohort (N = 71) unless not available.
Data from learning curve (N = 20/80) not included.
Fourty cases between 2014 and 2017, 8 cases where surgery was not successful and outcomes for 32 cases are reported; 18 cases from 2017 onwards with triple layer repair.15
range.
only 12/17 cases were older than 12 months.
However, recent evidence has shown that this could be mitigated by reducing insufflation pressures and adjusting the humidity and temperature of the insufflated gas.23 In addition, the effect of CO2 in humans maybe less prominent as was recently demonstrated in a clinical case series.24 Nonetheless, the latter study analyzed venous samples which may not entirely reflect the fetal pH/pCO2 and there was a considerable delay between cessation of insufflation and sampling.24 As CO2 is highly soluble any increased values could have resolved at the time of sampling. Reassuringly, there is currently no evidence of immediate adverse effects on cerebral development based on postnatal MRI.25
The initial high intraoperative complications and perinatal mortality observed with the percutaneous approach have improved in the latest experience published by the two most experienced groups in the world.7, 8, 9 , 16 , 26 In the most recent series, excluding cases from learning curves, the procedure was technically feasible in almost all cases.7 , 16 Likewise, fetal and neonatal deaths (4/131, 3%; two fetal and two neonatal deaths) were similar to what is reported for open spina bifida repair (2/91, 2%; one fetal and one neonatal death).7 , 9 , 16 , 17 In the largest series of the exteriorised‐uterus fetoscopic approach, even including the learning curve, the procedure was technically feasible in 90% of cases with one fetal demise (Table 1).13 On the other hand, technical success is determined by the surgeon at the end of surgery, but the true benefit can only be defined by comparing outcomes.
The next big game changer is that PPROM rates are going down, in experienced hands even below the level observed following hysterotomy, and as a consequence a more advanced median gestational age at delivery. This particularly seems consistent for the fetoscopic approach with exteriorized uterus.13 , 16 The reason for this is not entirely clear. The use of heated and humidified CO2 could partially explain this as a recent animal experiment indeed reported reduced inflammatory cell reaction in the fetal membranes compared to cold (room temperature) and dry CO2.23 The exteriorized uterus also enables the transuterine fixation of the membranes prior to port placement and closure at the end of the operation. A third explanation could be the difference in shear stress applied to the membranes when positioning the trocar through only one layer instead of multiple layers with a percutaneous technique.27 , 28
These changes add to the generic advantage of all fetoscopic approaches to not mandate a caesarean section and indeed vaginal births have been reported in up to 70% of cases.16
Nevertheless, despite these promising observations there are still a number of concerns remaining. One of the main criticisms is the lack of a unified technique for the neurosurgical part of the intervention, which seems rather “eminence based.” This is however not only a privilege of fetoscopy. The neurosurgical techniques are rapidly evolving making it difficult to compare outcomes not only between centers, but also within the same center. A fetoscopic repair is technically more challenging and requires specific surgical skills necessitating extensive training and a longer learning curve, recently calculated to be at least 56 cases for the percutaneous approach compared to 35 for open surgery.29 This has consequences in terms of volume load of centers considering fetoscopic repair. This also translates into longer surgery times at a time that the fetal brain is very sensitive to external factors: during mid gestation there is extensive synaptogenesis described as brain growth spurt.30 , 31 It is unclear whether prolonged exposure to maternal anesthesia as well as CO2 has consequences on developmental outcomes.31
The lack of standardization does not pertain for the surgical technique but also to the reporting of postnatal outcomes. The MOMS trial included an extensive evaluation of postnatal neurodevelopmental outcomes in a standardized blinded manner and to a contemporary cohort of infants that were operated postnatally. The majority of these infants were included in a long‐term follow‐up study up to 10 years of age (MOMS 2).32 There were no differences in overall adaptive behavior; however the benefit on functional mobility seems to persist to school age. as the number of infants that could independently walk community distances was twice as high (51.3% vs. 23.1%).33 There were fewer surgeries for shunt replacement, yet the incidence of spinal cord tethering release surgery was higher in the prenatal group.32 Conversely, based on the current available evidence scattered in several publications and sometimes in absence of a control population it is difficult to determine whether the fetoscopic repair is not inferior to the open approach.8 , 34 , 35 The percutaneous approach is associated with a higher need of postnatal corrections for cerebrospinal fluid leakage, which maybe related to the closure technique. Its effect on the incidence of clinically relevant spinal cord tethering is yet to be determined by long‐term follow‐up studies. On the other hand, the reported VP shunt rates in recent series of both fetoscopic approaches are similar to that in the prenatal surgery arm of the MOMS.16 In general, there are also promising motor outcomes, but given the diversity in the used methodology it is hard to compare actual outcomes. Like in the MOMS‐trial a blinded assessment of outcomes should be performed. An option is an independent panel of experts reviewing videos, charts or direct examinations of the child at a determined age.
As a result of the above encouraging observations, fetoscopic surgery for spina bifida repair has progressed from a controversial and highly experimental treatment to an acceptable alternative that several centers consider and/or implement. This is mainly driven by the need for attenuating the obstetric consequences (uterine incision) and maternal morbidity (percutaneous approach). The current questions therefore remain on what exact technique provides optimal neuroprotection, best obstetric outcomes and reduced maternal morbidity. This prompts the next question about how the highest‐quality scientific data can be obtained and when one or more experimental surgical techniques will be sufficiently documented to be considered equivalent to the current gold standard of open fetal surgery?
3. BENEFITS OF AN RCT
Evidence‐based medicine has become the cornerstone of modern healthcare. The effectiveness of any intervention is ideally determined using the highest‐quality scientific data and in this respect RCTs are considered top level. In an RCT, patient subjects are randomly assigned to one of the compared treatments, mostly one “standard” treatment and one “experimental” treatment. The process of randomization eliminates the influence of selection bias and reduces confounding factors.36 This is not the case for prospective cohort series or retrospective case series providing a lower level of evidence, which is the main concern for the fetoscopic approach. In maternal‐fetal therapy there have been several landmark RCTs, including the Eurofoetus trial for twin‐to‐twin transfusion syndrome, the Solomon laser trial, the MOMS trial and the recently completed Tracheal Occlusion To Accelerate Lung growth (TOTAL) trial.1 , 37, 38, 39 All of these have contributed to the acceptance of maternal‐fetal therapy and formed the basis of ongoing collaborative consortia that strive to move this field of medicine forward. Conversely, they have taken long and often led to controversy. Also, some failed to recruit sufficient patients.40
4. LIMITATIONS OF AN RCT
Despite RCTs being often rated as the gold standard, this may not be applicable to every type of research.41 , 42 First, there are some concerns when it comes to RCTs evaluating surgical interventions in general. For example, all surgical RCTs are complicated by the fact that for every case there is a countless number of confounders (such as pathological findings, surgeon's expertise and surgical approach as well as shift in the management for the condition over time) which may have an effect on the standardization the investigated treatment and these are not controlled for by randomization.43 A second concern is the discrepancy between the time to gather evidence from an RCT and the pace of developing novel techniques. For all the RCTs in maternal‐fetal surgery mentioned above, slow recruitment made it challenging to complete enrollment of sufficient patients. The MOMS trial took nearly 10 years to complete and likewise the TOTAL trial evaluating the effect of fetoscopic tracheal occlusion for infants with a congenital diaphragmatic hernia has only recently finished recruitment after more than a decade. This could be explained partially by the rarity of these conditions but at least as much by the hesitancy of parents to undergo investigational procedures in pregnancy and physician's bias. On the other hand, most procedures will go through an experimental preclinical and clinical phase prior to the initiation of clinical trials, and this often inspires other centers to start clinical programs, who later on find it difficult to question that intervention. This so‐called “back‐door” phenomenon, that is, offering interventions outside of clinical trials, is common and weighs on recruitment rates. The powerful “technological imperative”—the idea that if something can be done it should be done—is an important aspect for both physician and patient and increases the urge to offer a not always thoroughly studied treatment.44 During the MOMS trial, a moratorium was imposed by the US maternal‐fetal surgical centers except for the three trial centers, but repeating a study like this to objectively assess the benefit of a fetoscopic repair seems difficult. Fetal therapy in general often generates (social) media attention and not uncommonly this could be a driving factor.
Moreover, when for instance designing an RCT to study the benefits of a fetoscopic approach it may become difficult to identify the most relevant primary outcome and a single RCT may not answer all research questions. The main justification for a fetoscopic intervention is to improve or have at least comparable obstetric outcomes, however it should not be non‐inferior concerning neurological outcomes. Therefore, two or more endpoints seem imperative.
Another reason why an RCT in maternal‐fetal surgery is challenging is that it is difficult to determine equipoise.45 Although there might theoretically be equipoise, clinical and patient equipoise might be difficult to achieve. The principle of clinical equipoise has been described by Freedman, arguing that in order to be able to enroll patients in RCTs, within the medical community there should be an overall state of uncertainty between the trial arms.46 Yet on the other hand, every individual investigator is also influenced by personal biases, opinions and “gut‐feelings” and technical skills making true clinical equipoise difficult to achieve.45, 46, 47 Moreover, often the results during the experimental stage of a novel intervention are promising and hyped, as such doctors (as well as patients) will unconsciously develop a positive bias towards one of the treatment arms. We expect that this well‐known issue of “dissemination first, evidence later” is one of the most challenging factors when designing a clinical trial for a fetoscopic closure. This inadvertently influences counselling and hinders an unbiased decision to participate in an RCT.
In addition, patient equipoise relies on the same degree of uncertainty about the two different treatments. A source of conflict lies in the fact that the only patient is the mother, yet it is the future child who will possibly benefit from the intervention. Though the mother benefit from a less invasive procedure, the fetus (future child) may potentially benefit more from an open procedure, as this technique has proven benefit. The potential psychological benefit for the mother related to doing everything possible for her unborn child out of altruism is a very difficult item to include in a risk benefit analysis as it is almost impossible to score. As the novel procedure aims to solve a limitation of the current standard treatment this translates in a positive bias towards the investigated treatment. It is important to note that her risks and benefits not only include the benefit for the fetus, but also the implications for other family members such as future siblings.
As was underlined by Rodrigues et al., when patient equipoise cannot be established independently of the existence of theoretical and clinical equipoise, consent to randomization might not be possible and therefore RCTs become difficult to organize. The concept of total (theoretical, clinical and patient) equipoise is therefore described as “an overwhelmingly fragile concept” particularly for unblinded interventional studies.46 Nevertheless, this should not be considered the only reason not to pursue randomization in maternal fetal surgery.
Another limitation is that most centers have invested in gaining expertise in one technique and thus it is challenging, maybe impossible, to find centers that can offer different treatments in an unbiased manner. This does not only apply to studies comparing open versus fetoscopic methods, but also studies that will compare the different fetoscopic techniques (percutaneous vs. open).
Taking all together, it seems that an RCT for the comparison of the different surgical methods for prenatal spina bifida closure will be very challenging, maybe impossible for several reasons. Hence, alternative options should also be explored on how to determine when an “experimental” therapy becomes the gold standard.
5. THE CONTINUUM OF RESEARCH
Most regulatory bodies depart from a dichotomous approach labelling treatments as either experimental or established. However, there is no clear consensus of the extent of this research phase and thus it remains unclear when available evidence is convincing enough to decide that a new technology or treatment is no longer to be labelled as “experimental”.48 To evaluate the implementation of new technologies in the field of reproductive medicine, a conceptual framework and a scoring tool were developed.49 We propose that with some minor adaptations, this framework can be used as a general tool for evaluating new treatments in other medical specialties, such as maternal‐fetal surgical interventions (Table 2). This framework describes that the development of novel techniques ideally follows a continuum that distinguishes three categories: experimental, innovative and established treatments.49 Experimental therapies should only be offered in a research setting, aiming at showing efficacy and safety in animals and case reports in humans (“proof of concept”), with approval of a medical research ethical committee. Innovative treatments have progressed from this initial phase and there is albeit limited evidence of efficacy and short‐term safety in humans. The label “innovative treatment” entails an intermediate phase of research in a novel treatment to provide generalizable knowledge, comparable to a phase III drug research study. Obviously, this should be done with continuous oversight of regulatory bodies. The scoring tool provides a means to determine the status of a novel treatment within this continuum rating the therapy in four criteria: efficacy, safety, procedural reliability and effectiveness.49
TABLE 2.
Scoring tool for distinguishing between treatments (modified for maternal‐fetal therapy, based on Provoost et al.49 )
| Criterion | Definition | Scoring |
|---|---|---|
| Efficacy | Proof of principle |
|
| Safety |
|
|
| Procedure | Procedural reliability and transparency: The similarity or variability of the procedure in different centers and the potential for implementation by other centers |
|
| Effectiveness | The likelihood of producing the desired outcome compared with outcome of conventional (postnatal), established therapies |
Threshold to move from experimental to innovative treatment.
Threshold to move from innovative treatment to an established treatment.
Within this framework, open fetal spina bifida closure is categorized as an established therapy (Figure 1). Both fetoscopic approaches would rank as innovative treatments, however based on the most recent series effectiveness could be scored as acceptable (Figure 1).15 , 16 , 50
FIGURE 1.
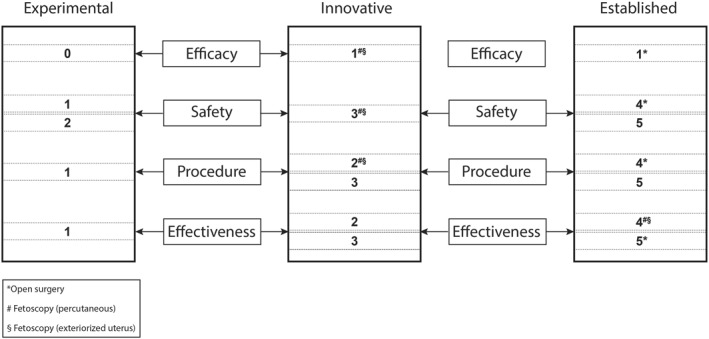
Adapted from Provoost et al.49 Assessment tool for the transition from an experimental treatment to an innovative treatment and to an established treatment
The following recommendations were suggested for interventions that fall in the innovative treatment category: (1) treatment should only be offered by expert centers; (2) there should be a commitment to closely monitor their practice conducting thorough follow‐up studies with the purpose of publishing the (positive and negative) results in peer‐reviewed journals; (3) patients should be adequately informed about all relevant aspects of the procedure (including surgical expertise) and about the status of the treatment (lack of long‐term outcomes should be emphasized) and (4) centers should always be prepared to stop treatment when there are signs of serious concerns based on their own studies or on published reports.49 This also implies that all patients should also be asked for their consent for any innovative treatment and also to be contacted in the future for follow‐up studies. Therefore, requiring formal approval of a medical research ethics committee is essential.
The difficulty of maternal‐fetal therapy is that the status of the treatment should be balanced between the benefits and risks for the fetus and the maternal risks, and this is evidently not incorporated in the framework of Provoost et al.49
6. INTERNATIONAL REGISTRIES
A pragmatic alternative for evaluating the effect of surgical interventions is to generate large, prospective, cohort registries with standardized data collection for key outcomes. The data acquired within the MOMS trial were collected with a scientific rigor that is currently not present for fetoscopic interventions. There are important inconsistencies in selection criteria, operative methodology and data points differ, there is overlap between series and the postnatal outcomes are not reported uniformly.
Regardless, every center developing an innovative therapy has a moral obligation to monitor its own outcomes. Given the relative scarcity of these maternal‐fetal interventions such registries need to be organized within (inter)national collaborations. Establishing collaborative networks stimulates transparency between competing centers and enabling generation of larger series assessing short‐ and long‐term follow‐up. It also offers the opportunity to unify the technique based on the collective experience of the participants, thereby accelerating the innovation phase. With this goal, a consortium of 25 North American Fetal Therapy Network centers agreed to collect data of all fetalmyelomeningocoele (MMC) interventions prospectively from 2012 onwards.18 In parallel, the International Fetoscopic Myelomeningocoele Repair Consortium was founded in 2018 to advance knowledge on the fetoscopic technique by providing data into a common registry.16 Such international collaborations are essential and all centers performing these interventions are strongly encouraged to participate. Moreover, every effort should be made to collate registries and in order to facilitate this a minimum data set should be agreed upon covering all aspects of the MMC repair including maternal views and experience. In addition, a continuous comparison to contemporary cohorts of infants with postnatal closure and open prenatal surgery remains essential. But more importantly it is our responsibility as scientific community to determine the most optimal outcome measure to compare the fetoscopic versus the open approach. This consensus outcome could then be used to calculate the number of cases needed to generate the evidence to favor one of the treatments with sufficient power without performing an RCT.
7. CONCLUSION
Maternal‐fetal surgery has become a widespread treatment option for fetuses with a spina bifida. In pursuit of minimizing maternal risks, fetoscopic techniques are being explored. We have discussed that these inventions have progressed from an experimental to an innovative stage, albeit that the optimal fetoscopic approach has not been determined. Nevertheless, the promising results created an expansion of centers offering such interventions, resulting in fragmentation of surgical experience and the publication of outcomes. Although an RCT is generally considered the highest level of evidence to evaluate the effects of any treatment, we foresee major difficulties organizing such a study for this indication. We propose that gathering data from high‐quality international registries with standardized outcomes and long‐term follow‐up could be a valuable alternative. Yet, until we are able to establish superiority of a fetoscopic approach, we cannot entirely discard the necessity of an RCT. In the absence of high‐quality evidence, we emphasize the importance of careful and balanced in‐depth counselling taking in consideration individual risks and benefits for every patient.
CONFLICT OF INTEREST
No conflicts of interests to disclose.
ACKNOWLEDGMENTS
We would like to thank Dr Wybo Dondorp for his comments on the adjustments of the framework previously published.48
Verweij EJ, de Vries MC, Oldekamp EJ, et al. Fetoscopic myelomeningocoele closure: Is the scientific evidence enough to challenge the gold standard for prenatal surgery? Prenatal Diagnosis. 2021;41(8):949–956. 10.1002/pd.5940
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.
REFERENCES
- 1.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacco A, Ahmed S, Deprest J, David AL. A study to assess knowledge and acceptability of foetal surgery for spina bifida amongst healthcare professionals in the UK. J Obstet Gynaecol. 2020;40(4):448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock JW, Thomas JC, Baskin LS, et al. Effect of prenatal repair of myelomeningocele on urological outcomes at school age. J Urol. 2019;202(4):812‐818. [DOI] [PubMed] [Google Scholar]
- 4.Goodnight WH, Bahtiyar O, Bennett KA, et al. Subsequent pregnancy outcomes after open maternal‐fetal surgery for myelomeningocele. Am J Obstet Gynecol. 2019;220(5):494.e1‐494.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruner JP, Richards WO, Tulipan NB, Arney TL. Endoscopic coverage of fetal myelomeningocele in utero. Am J Obstet Gynecol. 1999;180(1 I):153‐158. [DOI] [PubMed] [Google Scholar]
- 6.Bruner JP, Tulipan NB, Richards WO, Walsh WF, Boehm FH, Vrabcak EK. In utero repair of myelomeningocele: a comparison of endoscopy and hysterotomy. Fetal Diagn Ther. 2000;15(2):83‐88. [DOI] [PubMed] [Google Scholar]
- 7.Kohl T. Percutaneous minimally‐invasive fetoscopic surgery for spina bifida aperta—Part I Surgical technique and perioperative outcome. Ultrasound Obstet Gynecol. 2014;44:515‐524. [DOI] [PubMed] [Google Scholar]
- 8.Graf K, Kohl T, Neubauer BA, et al. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part III: neurosurgical intervention in the first postnatal year. Ultrasound Obstet Gynecol. 2016;47(2):158‐161. [DOI] [PubMed] [Google Scholar]
- 9.Degenhardt J, Schürg R, Winarno a, et al. Percutaneous minimal‐access fetoscopic surgery for spina bifida aperta. Part II: maternal management and outcome. Ultrasound Obstet Gynecol. 2014;44(5):525‐531. [DOI] [PubMed] [Google Scholar]
- 10.Pedreira DAL, Zanon N, Nishikuni K, et al. Endoscopic surgery for the antenatal treatment of myelomeningocele: the CECAM trial. Am J Obstet Gynecol. 2016;214(1):111.e1‐111.e11. [DOI] [PubMed] [Google Scholar]
- 11.Joyeux L, Engels AC, Russo FM, et al. Fetoscopic versus open repair for spina bifida aperta: a systematic review of outcomes. Fetal Diagn Ther. 2016;39(3):161–171. [DOI] [PubMed] [Google Scholar]
- 12.Kabagambe SK, Jensen GW, Julia Y, et al. Fetal surgery for myelomeningocele : A systematic review and meta‐analysis of outcomes in fetoscopic versus Open Repair. Fetal Diagn Ther. 2018;43:161‐174. [DOI] [PubMed] [Google Scholar]
- 13.Belfort MA, Whitehead WE, Shamshirsaz AA, et al. Fetoscopic open neural tube defect repair. Obstet Gynecol. 2017;129(4):734‐743. [DOI] [PubMed] [Google Scholar]
- 14.Kohn JR, Rao V, Sellner AA, et al. Management of labor and delivery after fetoscopic repair of an open neural tube defect. Obstet Gynecol. 2018;131(6):1062‐1068. [DOI] [PubMed] [Google Scholar]
- 15.Belfort MA, Whitehead WE, Shamshirsaz AA, et al. Comparison of two fetoscopic open neural tube defect (ONTD) repair techniques: single‐layer vs three‐layer closure. Ultrasound Obstet Gynecol. 2020;56(4):532‐540. [DOI] [PubMed] [Google Scholar]
- 16.Cortes MS, Lapa DA, Acacio GL, et al. Proceedings of the first Annual Meeting of the International fetoscopic myelomeningocele repair Consortium. Ultrasound Obstet Gynecol. 2019;53(6):855‐863. [DOI] [PubMed] [Google Scholar]
- 17.Farmer DL, Thom EA, Brock JW, et al. The Management of Myelomeningocele Study: full cohort 30‐month pediatric outcomes. Am J Obstet Gynecol. 2018;218(2):256.e1‐256.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldenhauer JS, Flake AW. Open fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol. 2019;58:121‐132. [DOI] [PubMed] [Google Scholar]
- 19.Tulipan N, Wellons JC, Thom EA, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. Pediatrics. 2015;16(6):613‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratacós E, Wu J, Devlieger R, Van de Velde M, Deprest JA. Effects of amniodistention with carbon dioxide on fetal acid‐base status during fetoscopic surgery in a sheep model. Surg Endosc. 2001;15(4):368‐372. [DOI] [PubMed] [Google Scholar]
- 21.Luks FI, Deprest J, Marcus M, et al. Carbon dioxide pneumoamnios causes acidosis in fetal lamb. Fetal Diagn Ther. 1994;9(2):105‐109. [DOI] [PubMed] [Google Scholar]
- 22.Skinner S, DeKoninck P, Crossley K, et al. Partial amniotic carbon dioxide insufflation for fetal surgery. Prenat Diagn. 2018;38(13):983‐993. [DOI] [PubMed] [Google Scholar]
- 23.Amberg BJ, Hodges RJ, Kashyap AJ, et al. Physiological effects of partial amniotic carbon dioxide insufflation with cold, dry vs heated, humidified gas in a sheep model. Ultrasound Obstet Gynecol. 2019;53(3):340‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baschat AA, Ahn ES, Murphy J, Miller JL. Fetal blood‐gas values during fetoscopic myelomeningocele repair performed under carbon dioxide insufflation. Ultrasound Obstet Gynecol. 2018;52(3):400‐402. [DOI] [PubMed] [Google Scholar]
- 25.Sanz Cortes M, Torres P, Yepez M, et al. Comparison of brain microstructure after prenatal spina bifida repair by either laparotomy‐assisted fetoscopic or open approach. Ultrasound Obstet Gynecol. 2020;55(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 26.Lapa Pedreira DA, Acacio GL, Gonçalves RT, et al. Percutaneous fetoscopic closure of large open spina bifida using a bilaminar skin substitute. Ultrasound Obstet Gynecol. 2018;52(4):458‐466. [DOI] [PubMed] [Google Scholar]
- 27.Luks F, Deprest J, Gilchrist B, et al. Access techniques in endoscopic fetal surgery. Eur J Pediatr Surg. 1997;7(3):131‐134. [DOI] [PubMed] [Google Scholar]
- 28.Bircher K, Merluzzi R, Wahlsten A, et al. Influence of osmolarity and hydration on the tear resistance of the human amniotic membrane. J Biomech. 2020;98:109419. [DOI] [PubMed] [Google Scholar]
- 29.Joyeux L, De Bie F, Danzer E, et al. Learning curves of open and endoscopic fetal spina bifida closure: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2019;55(6):730‐739. [DOI] [PubMed] [Google Scholar]
- 30.Rizzi S, Ori C, Jevtovic‐Todorovic V. Timing versus duration. Ann N Y Acad Sci. 2010;1199:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Veeken L, Van der Merwe J, Devroe S, et al. Maternal surgery during pregnancy has a transient adverse effect on the developing fetal rabbit brain. Am J Obstet Gynecol. 2019;221(4):355.e1‐355.e19. [DOI] [PubMed] [Google Scholar]
- 32.Houtrow AJ, Thom EA, Fletcher JM, Burrows PK, et al. Prenatal repair of myelomeningocele and school‐age functional outcomes. Pediatrics. 2020;145(2):e20191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houtrow AJ, MacPherson C, Jackson‐Coty J, et al. Prenatal repair and physical functioning among Children with myelomeningocele. JAMA Pediatr. 2021;15224:e205674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbeek RJ, Heep A, Maurits NM, et al. Fetal endoscopic myelomeningocele closure preserves segmental neurological function. Dev Med Child Neurol. 2012;54(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 35.Lapa DA. Endoscopic fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol. 2019;58:133‐141. [DOI] [PubMed] [Google Scholar]
- 36.JM K. Designing a research project: randomised controlled trials and their principles. Emerg Med J. 2003;20:164‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senat M‐V, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin‐to‐twin transfusion syndrome. N Engl J Med. 2004;351(2):136‐144. [DOI] [PubMed] [Google Scholar]
- 38.Slaghekke F, Lopriore E, Lewi L, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin‐to‐twin transfusion syndrome: an open‐label randomised controlled trial. Lancet. 2014;383(9935):2144‐2151. [DOI] [PubMed] [Google Scholar]
- 39.DeKoninck P, Gratacos E, van Mieghem T, et al. Results of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia and the set up of the randomized controlled TOTAL trial. Early Hum Dev. 2011;87(9):619‐624. [DOI] [PubMed] [Google Scholar]
- 40.Morris RK, Malin GL, Quinlan‐Jones E, et al. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet. 2013;382(9903):1496‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465‐475. [DOI] [PubMed] [Google Scholar]
- 42.Grossman J, Mackenzie FJ. The randomized controlled trial: gold standard, or merely standard? Perspect Biol Med. 2005;48(4):516‐534. [DOI] [PubMed] [Google Scholar]
- 43.Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the gold standard—lessons from the history of RCTs. N Engl J Med. 2016;374(22):2175‐2181. [DOI] [PubMed] [Google Scholar]
- 44.Chervenak FA, Mccullough LB, Birnbach DJ. Ethical issues in fetal surgery research. Best Pract Res Clin Anaesthesiol. 2004;18(2):221‐230. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues HCML, Deprest J, Berg PP. When referring physicians and researchers disagree on equipoise : the TOTAL trial experience. Prenat Diagn. 2011;31:589‐594. [DOI] [PubMed] [Google Scholar]
- 46.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141‐145. [DOI] [PubMed] [Google Scholar]
- 47.Lyerly AD, Mahowald MB. Maternal‐fetal surgery: the fallacy of abstraction and the problem of equipoise. Health Care Anal. 2001;9(2):151‐165. [DOI] [PubMed] [Google Scholar]
- 48.Luks FI, Johnson A, Polzin WJ. Innovation in maternal‐fetal therapy. Obstet Gynecol. 2015;125(3):649‐652. [DOI] [PubMed] [Google Scholar]
- 49.Provoost V, Tilleman K, D'Angelo A, et al. Beyond the dichotomy: a tool for distinguishing between experimental, innovative and established treatment. Hum Reprod. 2014;29(3):413‐417. [DOI] [PubMed] [Google Scholar]
- 50.Diehl D, Belke F, Axt‐Fliedner R, et al. Intrauterine total percutaneous fetoscopic repair of myelomeningocele: 30 months follow up data. Ultrasound Obstet Gynecol. 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable—no new data generated.


