Abstract
Objective
To determine whether children and youths with Type 1 diabetes (T1D) have early alterations of the corneal subbasal nerve plexus detectable with in vivo confocal microscopy (IVCM) and to investigate the role of longitudinally measured major risk factors for diabetes complications associated with these alterations.
Methods
One hundred and fifty children and youths with T1D and 51 age‐matched controls were enrolled and underwent IVCM. Corneal nerve fiber length (CNFL), corneal nerve fiber density (CNFD), corneal nerve branch density (CNBD), corneal fiber total branch density (CTBD), and corneal fiber fractal dimension (CNFrD) were measured. Risk factors for diabetes complications (blood pressure, BMI, HbA1c, lipoproteins, urinary albumin‐creatinine ratio) were recorded at IVCM and longitudinally since T1D onset. Unpaired t‐test was used to compare variables between the groups. Multiple regression models were calculated using IVCM parameters as dependent variables and risk factors as independent variables.
Results
All IVCM parameters, except CTBD, were significantly lower in the T1D patients. Glycometabolic control (HbA1c, visit‐to‐visit HbA1c variability, and mean HbA1c), and blood pressure were inversely correlated with IVCM parameters. Multiple regression showed that part of the variability in CNFL, CNFD, CTBD, and CNFraD was explained by HbA1c, blood pressure percentiles and age at IVCM examination, independent of diabetes duration, BMI percentile and LDL cholesterol. Comparable results were obtained using the mean value of risk factors measured longitudinally since T1D onset.
Conclusions
Early signs of corneal nerve degeneration were found in children and youths with T1D. Glycometabolic control and blood pressure were the major risk factors for these alterations.
Keywords: children and youths, corneal nerve plexus, in vivo confocal microscopy, neurodegeneration, Type 1 diabetes
Abbreviations
- ACR
albumin‐creatinine ratio
- BMI
body‐mass index
- CNBD
corneal nerve branch density
- CNFD
corneal nerve fiber density
- CNFL
corneal nerve fiber length
- CNFrD
corneal nerve fiber fractal dimension
- CTBD
corneal nerve fiber total branch density
- DBP
diastolic blood pressure
- HDL
high density lipoprotein
- ISPAD
International Society for Pediatric and Adolescent Diabetes
- IVCM
in vivo confocal microscopy
- LDL
low density lipoprotein
- SBP
systolic blood pressure
- T1D
Type 1 diabetes
- TG
triglycerides
1. INTRODUCTION
Diabetic retinopathy and neuropathy are two major microvascular complications of Type 1 diabetes (T1D). They have an insidious onset, with overt symptoms manifesting only when damage to the retina and peripheral nerves is at an advanced stage.1, 2 There is mounting evidences that impairment of microvascular components is preceded by very early neurodegenerative alterations primarily involving small nerve fibers.3, 4 In vivo confocal microscopy (IVCM) of the corneal subbasal nerve plexus has substantially corroborated these findings in adult patients with diabetes.5, 6 IVCM is a safe and non‐invasive imaging technique for the direct visualization of this corneal layer, which is predominantly composed of small and unmyelinated C‐fibers.7 Corneal nerve length and density parameters are altered in T1D patients with neuropathy compared to those without neuropathy, and there is a significant correlation between these parameters and the results of conventional tests8, 9 and intra‐epidermal nerve fiber density measured with skin biopsy.10 Early corneal neurodegenerative changes in T1D adults has been associated with worse glycemic control and higher blood pressure, two recognized major risk factors for diabetes complications.11 Moreover, longitudinal studies have demonstrated that improvement of these risk factors, as well as combined kidney and pancreas transplantation, can regenerate corneal nerve fibers12, 13 .
The acceptability, reliability, and reproducibility of IVCM in healthy and T1D children and adolescents have been demonstrated.14, 15 However, the few studies that have been conducted in young T1D patients have had modest sample size and inconsistent results for the possible predisposing role of the main risk factors for diabetes complications in causing corneal nerve fiber damage.16, 17, 18
The early detection of corneal nerve fiber damage in young patients with T1D is particularly valuable, given the limitations of conventional diagnostic tools for the diagnosis of early signs of diabetic neuropathy in childhood and adolescence and the importance of prompt intervention to prevent or delay its progression in childhood.2, 19 The primary aim of this study was to determine whether children and youths with T1D have early alterations of the corneal subbasal plexus compared to age‐matched healthy controls. The secondary aim was to investigate the role of longitudinally measured risk factors for diabetes complications in causing these alterations in a large cohort of children and youths with T1D.
2. METHODS
2.1. Study population
Children, adolescents, and young adults with T1D and a history of diabetes of at least 2 years were recruited from among patients attending the Regional Center for Pediatric Diabetes of the University Hospital, Verona, between June 2018 and August 2019. Exclusion criteria were: diagnosis of diabetic retinopathy based on mydriatic fundoscopy examination and/or diagnosis of diabetic neuropathy after evaluation of neurologic symptoms based on the diabetic neuropathy symptom score and standard clinical tests (assessment of ankle tendon reflexes and temperature, pinprick and vibration perceptions) with calculation of the neuropathy disability score,20, 21 as recommended by current International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines2; diagnosis of other forms of neuropathies, celiac disease with low compliance to gluten‐free diet, not‐euthyroid Hashimoto's thyroiditis, and other systemic chronic diseases other than T1D. Ophthalmological exclusion criteria were: history of corneal abnormality, ocular trauma or surgery, dry eye disease, and/or contact lens wear.
A healthy, age‐ and sex‐matched control group was enrolled from among outpatient children and youths attending the Eye Clinic, Department of Neurosciences, Biomedicine and Movement Sciences, Verona University, for minor ocular symptoms or general screening ophthalmic examination but with no history of relevant ocular and systemic disease (including diabetes).
All procedures were performed in accordance with the tenets of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Verona; participants and their parents/legal guardians provided written, informed consent after explanation of the nature of the study.
2.2. Clinical and biochemical data collection
At study enrolment, all participants underwent physical examination for the collection of anthropometric (height, weight, waist circumference, body‐mass index [BMI] and BMI percentile) and blood pressure measurement (systolic blood pressure [SBP], diastolic blood pressure [DBP], and percentile by sex, age, and height).22 In patients with T1D, urinary and venous blood samples were collected for analysis of the urinary albumin‐creatinine ratio (ACR), HbA1c, and lipid profile (triglycerides [TG], total cholesterol, high density lipoprotein [HDL] cholesterol, low density lipoprotein [LDL] cholesterol). Age at onset, duration of T1D, type of insulin therapy, and daily insulin dosage were recorded. In addition, these clinical and biochemical data annually collected starting from T1D onset were retrospectively retrieved and the mean of all the values recorded was calculated for each parameter. HbA1c values were also used for calculation of visit‐to‐visit HbA1c variability (HbA1c SD) as an index of long‐term glycometabolic variability.
2.3. Ophthalmologic examination and in vivo confocal microscopy
All participants underwent complete bilateral ophthalmologic examination, including slit lamp examination of the anterior segment and mydriatic fundoscopy. The corneal subbasal nerve plexus was assessed by IVCM (Heidelberg Retinal Tomography III with Rostock Cornea Module, Heidelberg Engineering, Heidelberg, Germany) by the same expert operator (TC) according to published standard protocols for examination, image acquisition, and analysis.23, 24 The subject's eyes were anesthetized with a drop of topical lidocaine hydrocloride 40 mg/ml (A23lfa Intes Pharmaceuticals, Casoria, Italy) and a drop of ophthalmic tear gel (Tear Gel carbomer 0.3%, Thea Pharmaceuticals, Clermont‐Ferrand, France) was used as coupling medium between the microscope objective lens and the corneal surface. Scans of the central and paracentral cornea were acquired using a sequence module for recording one image per second. To achieve the best possible quality of nerve images, the examiner manually focused the subbasal nerves while scanning and adjusting the axial depth dial (image resolution 384 × 384 pixels with a 400 μm2 field of view lens). The total duration of the IVCM examination was about 2 min per eye.
After the examination, the examiner chose six images per eye for each participant, selecting from among high‐contrast images without artefacts. The images were then processed using ACCMetrics Image Analysis Software v 2.0,25 to quantify five corneal nerve parameters for each eye: (1) corneal nerve fiber length (CNFL)‐the total length of all nerve fibers and branches (mm/mm2) within the scanned area; (2) corneal nerve fiber density (CNFD)‐the total number of major nerves per square millimeter of corneal tissue (n/mm2); (3) corneal nerve branch density (CNBD)‐the number of branches emanating from major nerve trunks per square millimeter of corneal tissue (n/mm2); (4) corneal nerve fiber total branch density (CTBD)‐the total number of branch points/mm2; and (5) corneal nerve fiber fractal dimension (CNFrD)‐a dimensionless measure of corneal nerve complexity. There were no significant differences between measurements of the left and the right eye; the average of each corneal nerve parameter from both eyes was entered in the data analysis, as previously described.15, 23
2.4. Statistical analysis
The normal distribution of all variables was assessed by the Kolmogorov–Smirnov test. Unless otherwise specified, the data are presented as the mean and SD in brackets. The data between the T1D patients and the healthy controls were compared using unpaired Student's t‐test for continuous variables and chi‐square test for categorical variables. The relationships between the variables related to the main clinical and biochemical risk factors for diabetes complications and IVCM parameters were assessed using Pearson's correlation coefficients. On the basis of the results of correlation analysis, outlining how variables are inter‐related, and of the known biological plausibility regarding the possible causal association between biological factors and early neurodegenerative changes, multiple regression analysis was performed using IVCM parameters as dependent variables and clinical and biochemical risk factors for diabetes complications as independent variables. All tests were two‐sided; a probability value (p) < 0.05 was accepted as statistically significant. Statistical analysis was performed using SPSS (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, version 20. Armonk, NY, USA: IBM Corp.).
3. RESULTS
A total of 150 children, adolescents, and young adults with T1D (age range, 10–22 years) were recruited. Table 1 presents the clinical and demographic characteristics of the T1D patients and the 51 age‐ and sex‐matched controls. There was no difference in age, sex, pubertal status distribution, anthropometric and blood pressure measurements between patients and controls. The scores for symptoms and/or signs of diabetic neuropathy and neuropathy disability were 0 in the T1D patients. Ophthalmologic findings were within the normal range for all participants. Mydiatric fundoscopy detected no signs of diabetic retinopathy in the T1D patients.
TABLE 1.
Clinical and demographic characteristics of T1D patients and healthy controls
| Characteristic | T1D patients (n = 150) | Controls (n = 51) | p‐value |
|---|---|---|---|
| Gender (m/f) | 77/73 | 25/26 | 0.77 |
| Puberty (pubertal/post‐pubertal) | 34/116 | 10/41 | 0.64 |
| Age (years) | 16.6 (4.0) | 16.3 (2.9) | 0.64 |
| BMI [kg/m2] | 22.0 (3.8) | 21.7 (2.7) | 0.62 |
| BMI [kg x (m2)] percentile | 61.2 (27.4) | 64.8 (28.2) | 0.43 |
| SBP (percentile) | 38.1 (25.2) | 39.3 (15.8) | 0.83 |
| DBP (percentile) | 57.0 (22.4) | 56.2 (20.5) | 0.78 |
| Age at onset (years) | 7.5 (3.6) | NA | — |
| Diabetes duration (years) | 8.7 (4.2) | NA | — |
| HbA1c (%; mmol/mol) | 8.07 (0.71); 65 (5.7) | NA | — |
| LDL cholesterol (mmol/L; mg/dl) | 1.98 (0.63); 76.6 (24.5) | NA | — |
| HDL cholesterol (mmol/L; mg/dl) | 1.53 (0.34); 59.6 (13.2) | NA | — |
| Triglycerides (mmol/L; mg/dl) | 2.03 (0.76); 67.5 (30.3) | NA | — |
| ACR (mg/mmol) | 1.85 (1.62) | NA | — |
Abbreviations: ACR, albumin/creatinine ratio; BMI, body‐mass index; DBP, systolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure.
Figure 1 presents the IVCM parameters. There was no significant difference in CTBD between the T1D patients and the controls (40.63 [16.59] vs. 43.67 [15.24] n/mm2; p = 0.254). All other parameters were significantly lower in the T1D patients: CNFL (14.51 [2.87] vs. 15.70 [2.26] mm/mm2; p = 0.005); CNFD (23.77 [5.43] vs. 27.93 [4.79] n/mm2; p < 0.001); CNBD (26.75 [12.34] vs. 29.83 [10.61] n/mm2; p = 0.013); CNFraD (1.481 [0.026] vs. 1.492 [0.019]; p = 0.002).
FIGURE 1.
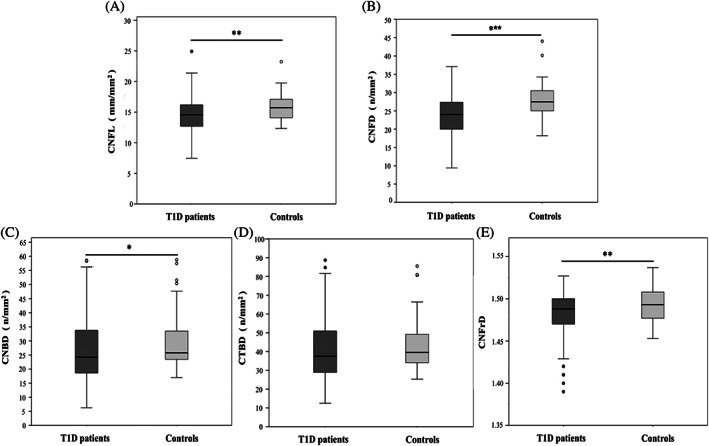
Box and whiskers plot of corneal nerve fiber length (CNFL) (A), corneal nerve fiber density (CNFD) (B), corneal nerve branch density (CNBD) (C), corneal nerve fiber total branch density (CTBD) (D), and corneal nerve fiber fractal dimension (CNFraD) (E) values in Type 1 diabetes (T1D) patients and healthy controls
Correlation analysis did not show significant correlations between IVCM parameters and age, diabetes duration, sex, pubertal status, BMI, lipid profile, and ACR measured at study enrolment and since the onset of T1D. A significant correlation was found between age at onset of diabetes and CNBD, with no comparable results for the other IVCM parameters. The SBP percentile measured at IVCM examination was inversely correlated with all IVCM parameters. In addition, the mean SBP percentile was significantly correlated with CNFraD. The DBP was not significantly associated with IVCM parameters. A significant inverse correlation was found between HbA1c measured at IVCM examination and all IVCM parameters. Comparable results were obtained for correlation analysis of visit‐to‐visit HbA1c variability (HbA1c SD) and mean HbA1c. No significant correlations were found between IVCM parameters and insulin requirements.
Table 2 and Supplementary Table 3 present the results of multiple regression analysis of clinical and biochemical risk factors for diabetes complications measured at study enrolment as independent variables. The inter‐individual variability of CNFL was explained by HbA1c, BP percentile, and age (R2 = 0.144; p = 0.03), independent of diabetes duration, BMI percentile, and lipid profile (LDL cholesterol). In detail, CNFL increased by 0.263 for each year of age and decreased by 0.744 for each 1% of HbA1c and by 0.019 for each percentile unit of systolic blood pressure. Comparable results were obtained using the same independent variables for CNFD (R2 = 0.133; p = 0.002), CTBD (R2 = 0.164; p < 0.001), and CNFraD (R2 = 0.126; p = 0.001), whereas for CNBD, diabetes duration, in addition to HbA1c, BP percentile, and age at IVCM examination, contributed to explain the inter‐individual variability of this IVCM parameter (R2 = 0.184; p < 0.001). Multiple regression analysis of clinical and biochemical risk factors for diabetes complications expressed as the mean of the values recorded since T1D onset showed comparable results with visit‐to‐visit HbA1c variability (HbA1c SD), mean BP percentiles, and age at IVCM examination significantly contributed to explain the inter‐individual variability of CNFL and CNFrAD parameters (CNFL R2 = 0.064, p = 0.046; CNFraD R2 = 0.071, p = 0.025) (Supplemental Table 4).
TABLE 2.
Multiple regression analysis of clinical and biochemical parameters for the risk factors for diabetes complications measured at IVCM evaluation
| Dependent variable | Variables in the model | B | 95% CI | p value |
|---|---|---|---|---|
| CNFL (R2 = 0.144, p = 0.03) | Age | 0.263 | 0.28 – 0.465 | 0.011 |
| Diabetes duration | −0.111 | −0.250 – 0.028 | 0.116 | |
| HbA1c | −0.744 | −1.430 to −0.057 | 0.034 | |
| BMI percentilea | −0.015 | −0.034 – 0.005 | 0.144 | |
| SBP percentilea | −0.019 | −0.038 – 0.001 | 0.043 | |
| LDL cholesterola | 0.010 | −0.011 – 0.030 | 0.371 | |
| CNFD (R2 = 0.133, p = 0.002) | Age | 0.566 | 0.160 – 0.972 | 0.007 |
| Diabetes duration | −0.314 | −0.592 to −0.037 | 0.077 | |
| HbA1c | −1.521 | −2.90 to −0.142 | 0.031 | |
| BMI percentilea | −0.039 | −0.075 – 0.003 | 0.077 | |
| SBP percentilea | −0.33 | −0.78 to −0.001 | 0.045 | |
| LDL cholesterola | 0.002 | −0.044 – 0.044 | 0.926 | |
| CNBD (R2 = 0.184, p < 0.001) | Age | 1.132 | 0.336 – 1.928 | 0.006 |
| Diabetes duration | −0.703 | −1.244 to −0.160 | 0.010 | |
| HbA1c | −3.814 | −6.477 to −1.150 | 0.005 | |
| BMI percentilea | −0.055 | −0.131 – 0.020 | 0.150 | |
| SBP percentilea | −0.099 | −0.173 to −0.024 | 0.018 | |
| LDL cholesterola | 0.029 | −0.054 – 0.111 | 0.492 |
Note: Data about CNFL, CNFD and CNBD are presented.
Abbreviations: BMI, body‐mass index; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; LDL, low density lipoprotein; SBP, systolic blood pressure.
Variables measured at the time of IVCM examination.
4. DISCUSSION
The main finding of this study is the evidence for significant alterations in corneal nerve fiber morphology measured on corneal subbasal nerve plexus images in the T1D patients compared to the healthy controls. Also, we noted a significant association between these parameters and glycometabolic control and blood pressure, two major risk factors for the development of diabetes complications.
Previous studies in T1D adult patients have demonstrated the diagnostic validity of IVCM for the detection of early alterations in corneal nerve fibers and the diagnosis of diabetic neuropathy at its earliest stages.6 To date, this ophthalmologic approach has seldom been used in children and youths with T1D, although some studies have demonstrated that it is acceptable, feasible, reliable, and reproducible in this patient subpopulation.14, 15 The few studies that have been conducted in young T1D patients have produced inconsistent results: Sellers et al. found no significant difference between healthy controls and children with T1D,14 whereas Gotze et al. reported a reduction only in CNFL with no significant alterations in other parameters.18 A study by Szalai et al. reported significantly lower values of corneal nerve fiber length, fiber density, and branch density.17 These studies had a modest sample size, with a low statistical power for detecting differences between T1D patients and healthy controls. Differently, our study presents results obtained in a large cohort of T1D children and adolescents and demonstrates a consistent reduction in fiber length and density parameters, with substantial evidence that early alterations of small corneal nerve fibers are detectable starting in children and youths with T1D. The depletion in the subbasal nerve plexus revealed in our study is documented also by the reduction in CNFrD, which is a recently defined parameter that measures corneal nerve structure complexity.26
These results are in line with evidence that the pathogenesis of diabetic neuropathy is characterized by early involvement of small and unmyelinated nerve fibers, followed by progressive and length‐dependent neurodegeneration.3 Since the damage is not detectable on conventional clinical and neurophysiological examinations, the identification and validation of new markers of small fiber neuropathy is particularly important for early diagnosis and prompt therapeutic intervention.5 In this scenario, IVCM is a valuable non‐invasive technique in clinical practice and for the study of pathogenic mechanisms underlying neurodegeneration in diabetes.
Analysis of the association between IVCM parameters and risk factors for T1D complications, longitudinally assessed starting at T1D onset, showed that glycometabolic control and blood pressure are major factors for early alteration of corneal nerves. In detail, HbA1c, measured at IVCM examination and since diabetes onset, was consistently and inversely associated with all IVCM parameters. This observation is shared by previous studies on the importance of long‐term glycometabolic control for the prevention of diabetic neuropathy27 and, more specifically, alterations of the corneal subbasal plexus.28, 29, 30
Moreover, in our cohort of young T1D patients, also visit‐to‐visit HbA1c variability (HbA1c SD), an index of long‐term glycometabolic variability, was significantly associated with IVCM parameters and significantly contributed to predict alterations, as demonstrated by multiple regression analysis. This novel finding further supports evidence for the independent predictive role of HbA1c variability in the development of microvascular complications in adolescents with T1D.31 We found a negative association between IVCM parameters and blood pressure, systolic values in particular, as reported by a study on the role of long‐term exposure to this risk factor.30
In this cohort of patients with T1D, age and diabetes duration, two other relevant but unmodifiable risk factors, emerged as significant predictors of IVCM parameters: age significantly contributed to an increase in IVCM parameters, whereas diabetes duration was negatively associated. These results are not comparable with those from previous studies of cohorts with a similar age range; longitudinal studies on the role of age and diabetes duration in T1D and healthy controls are desirable. Based on our data, we may speculate that diabetes with an early onset during childhood affects corneal innervation.
This study has some limitations: (1) despite the relatively small sample size of healthy controls, post‐hoc power analysis showed that the number of T1D patients and healthy controls allowed to detect possible differences in IVCM parameters with a statistical power of at least 80% (α error probability 0.05, estimated effect size for CNFL 0.460 with a statistical power of 88%); (2) the sample had European ancestry, so the results cannot necessarily be extended to children and adolescents with other ethnic backgrounds.
Numbering among the strengths of the study are: (1) application of IVCM with imaging collection and selection performed by the same expert operator according to standard protocols and simultaneous analysis of several IVCM parameters; (2) the relatively large sample of young patients with T1D; (3) the completeness of clinical and biochemical risk factors for diabetes complications assessed at IVCM examination and longitudinally, starting from T1D onset and including indices of long‐term glycometabolic control and variability.
In conclusion, IVCM proved useful in detecting early markers of neurodegeneration of the corneal subbasal nerve plexus in children and youths with T1D. The major risk factors are glycometabolic control and blood pressure, underlining the importance of maintaining good metabolic control starting in childhood. Longitudinal analysis of this cohort will document the evolution of nerve parameters in T1D patients and confirm the role of the risk factors associated with diabetes microvascular complications.
CONFLICT OF INTEREST
None of the authors declared conflicts of interest.
AUTHOR CONTRIBUTIONS
Tiziano Cozzini and Claudia Piona researched and analyzed the data and wrote the manuscript. Tommaso Merz, Tommaso Brighenti, Jacopo Bonetto, Marco Marigliano, Francesca Olivieri researched the data. Giorgio Marchini discussed and edited the manuscript. Emilio Pedrotti and Claudio Maffeis designed the study, co‐wrote and edited the manuscript. Emilio Pedrotti is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13219.
ETHICS STATEMENT
All procedures were performed in accordance with the tenets of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Verona; participants and their parents/legal guardians provided written, informed consent after explanation of the nature of the study.
Supporting information
Supplementary Table 3 Multiple regression analysis of clinical and biochemical parameters for the risk factors for diabetes complications measured at IVCM evaluation. Data on CTBD and CNFrD are presented.
Supplementary Table 4: Multiple regression analysis of clinical and biochemical parameters for the risk factors for diabetes complications measured as the mean of values recorded since T1D onset.
ACKNOWLEDGEMENT
We kindly thank the patients and their families who participated in the study.
Cozzini T, Piona C, Marchini G, et al. In vivo confocal microscopy study of corneal nerve alterations in children and youths with Type 1 diabetes. Pediatr Diabetes. 2021;22(5):780–786. 10.1111/pedi.13219
Claudia Piona, Tiziano Cozzini, Emilio Pedrotti, and Claudio Maffe contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Association AD . 11. Microvascular Complications and Foot Care. Diabetes Care. 2020;43(Suppl 1):S135‐S151. 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 2.Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 10 2018;19suppl 27:262–274. 10.1111/pedi.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227‐1239. 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 4.Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 01 2017;40(1):136–154. 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Clerck EE, Schouten JS, Berendschot TT, et al. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(8):653‐663. 10.1016/S2213-8587(15)00136-9. [DOI] [PubMed] [Google Scholar]
- 6.Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856‐1861. 10.1007/s00125-018-4653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521‐542. 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard N, Edwards K, Dehghani C, et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104(2):248‐256. 10.1016/j.diabres.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35(4):821‐828. 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra‐epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. 10.1371/journal.pone.0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Morphological changes of the peripheral nerves evaluated by high‐resolution ultrasonography are associated with the severity of diabetic neuropathy, but not corneal nerve fiber pathology in patients with type 2 diabetes. J Diabetes Investig. 2015;6(3):334‐342. 10.1111/jdi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabetes Med. 2011;28(10):1261‐1267. 10.1111/j.1464-5491.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30(10):2608‐2612. 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 14.Sellers EA, Clark I, Tavakoli M, Dean HJ, McGavock J, Malik RA. The acceptability and feasibility of corneal confocal microscopy to detect early diabetic neuropathy in children: a pilot study. Diabetes Med. 2013;30(5):630‐631. 10.1111/dme.12125. [DOI] [PubMed] [Google Scholar]
- 15.Pacaud D, Romanchuk KG, Tavakoli M, et al. The reliability and reproducibility of corneal confocal microscopy in children. Invest Ophthalmol Vis Sci. 2015;56(9):5636‐5640. 10.1167/iovs.15-16995. [DOI] [PubMed] [Google Scholar]
- 16.Deák EA, Szalai E, Tóth N, Malik RA, Berta A, Csutak A. Longitudinal changes in corneal cell and nerve fiber morphology in young patients with type 1 diabetes with and without diabetic retinopathy: a 2‐year follow‐up study. Invest Ophthalmol Vis Sci. 2019;60(2):830‐837. 10.1167/iovs.18-24516. [DOI] [PubMed] [Google Scholar]
- 17.Szalai E, Deák E, Módis L, et al. Early corneal cellular and nerve fiber pathology in young patients with type 1 diabetes mellitus identified using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2016;57(3):853‐858. 10.1167/iovs.15-18735. [DOI] [PubMed] [Google Scholar]
- 18.Götze A, von Keyserlingk S, Peschel S, et al. The corneal subbasal nerve plexus and thickness of the retinal layers in pediatric type 1 diabetes and matched controls. Sci Rep. 2018;8(1):14. 10.1038/s41598-017-18284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornstad P, Maahs DM. Diabetes complications in childhood diabetes‐new biomarkers and technologies. Curr Pediatr Rep. 2015;3(2):177‐186. 10.1007/s40124-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer JW, Bosma E, Lefrandt JD, et al. Clinical diagnosis of diabetic polyneuropathy with the diabetic neuropathy symptom and diabetic neuropathy examination scores. Diabetes Care. 2003;26(3):697‐701. 10.2337/diacare.26.3.697. [DOI] [PubMed] [Google Scholar]
- 21.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150‐154. 10.1007/bf00400697. [DOI] [PubMed] [Google Scholar]
- 22.Flynn JT, Kaelber DC, Baker‐Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 23.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non‐invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011;(47):2194. 10.3791/2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarr D, Lovblom LE, Ostrovski I, et al. Agreement between automated and manual quantification of corneal nerve fiber length: implications for diabetic neuropathy research. J Diabetes Complications. 2017;31(6):1066‐1073. 10.1016/j.jdiacomp.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi‐scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738‐747. 10.1016/j.media.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Graham J, Petropoulos IN, et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Invest Ophthalmol Vis Sci. 2018;59(2):1113‐1118. 10.1167/iovs.17-23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyllienmark L, Alstrand N, Jonsson B, Ludvigsson J, Cooray G, Wahlberg‐Topp J. Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care. 2013;36(10):3187‐3194. 10.2337/dc12-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Abnormal anterior corneal morphology in diabetes observed using in vivo laser‐scanning confocal microscopy. Ocul Surf. 2016;14(4):507‐514. 10.1016/j.jtos.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk factors associated with corneal nerve alteration in type 1 diabetes in the absence of neuropathy: a longitudinal in vivo corneal confocal microscopy study. Cornea. 2016;35(6):847‐852. 10.1097/ICO.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi F, Okino M, Ishibashi M, et al. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J Diabetes Investig. 2012;3(2):191‐198. 10.1111/j.2040-1124.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virk SA, Donaghue KC, Cho YH, et al. Association between hba1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3257‐3263. 10.1210/jc.2015-3604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 3 Multiple regression analysis of clinical and biochemical parameters for the risk factors for diabetes complications measured at IVCM evaluation. Data on CTBD and CNFrD are presented.
Supplementary Table 4: Multiple regression analysis of clinical and biochemical parameters for the risk factors for diabetes complications measured as the mean of values recorded since T1D onset.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


