Summary
We investigated the current role of interferon‐alpha (IFNα) in hairy cell leukaemia (HCL) in a retrospective analysis of patients with HCL. A cohort of 74 patients with HCL was divided in to three groups: (A) patients aged >65 years with first‐line treatment; (B) patients with comorbidities with first‐line treatment; (C) patients who were purine analogues resistant. In total, 94% achieved a response, with a complete response rate of 24%. After a median (range) follow‐up of 60 (7–236) months, 55 patients (78%) are still responding. The 5‐year progression‐free survival was 95%, 68%, and 96% in groups A, B and C respectively. A proportion of patients were monitored through their B‐Raf proto‐oncogene, serine/threonine kinase (BRAF)‐V600E status. IFNα remains a possible option in select patients with HCL, where minimal residual disease negativity is achievable.
Keywords: Hairy cell leukaemia, interferon, lymphoproliferative disease, MRD, BRAF
Introduction
Hairy cell leukaemia (HCL) is a rare clonal B‐cell disorder, presenting with cytopenia, splenomegaly and bone marrow (BM) involvement.1
According to the 2016 World Health Organization (WHO) classification there are two entities: classic HCL and HCL variant (HCLv). The V600E mutation of B‐Raf proto‐oncogene, serine/threonine kinase (BRAF) is the hallmark of HCL, found in exon 15 and, rarely, in exon 11, leading to activation of the RAF/mitogen‐activated protein kinase (MEK)‐extracellular signal‐regulated kinase (ERK) pathway.2
Current treatments include purine analogues (PA), rituximab and new agents.3, 4
Interferon alpha (IFNα), extensively used in the past, today is reserved for neutropenic patients and relapsed/refractory or frail patients not suitable for chemotherapy.5, 6 Recently, a few studies have investigated IFNα in HCL.7, 8
In the present study, we report on 74 patients with HCL/HCLv treated with IFNα administered at low dose until relapse or progression, evaluating safety and efficacy.
Methods
This retrospective analysis included patients with HCL or HCLv treated with IFNα monotherapy from 1990 to 2020. Diagnostic evaluation was performed according to criteria used at the time of presentation.5 The study was approved by Institutional Review Board and respects the ethical principles of 2008 Helsinki Declaration.
The IFNα was administered continuously until progression; only 10 patients were treated for a defined time (12 months). The initial dosage was 3 × 106 u subcutaneously three‐times a week; in responding patients the dose was progressively tapered to reach a very low maintenance dosage until reaching 0·1 × 106 u MUI three‐times a week.
Progression‐free (PFS) and overall survival (OS) were calculated from the first IFNα dose. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences (SPSS®), version 25.0 (IBM Corp., Armonk, NY, USA). Analysis of single groups was made following the D'Agostino–Pearson normality test, chi‐square test, if appropriate. The risk of event was assessed as survival functions (PFS, OS) using the Kaplan–Meier method. Multivariate analysis was conducted using Cox regression model.
Response evaluation was performed at 12 months according to the European Society of Medical Oncology (ESMO) guidelines. Treatment was started when at least one severe cytopenia occurred. A complete response (CR) was defined as normalisation of the full blood count (FBC) (neutrophils >1.5 × 109/l, platelets >100 × 109/l, haemoglobin 110 g/l); the absence of hairy cells in BM (<5%); resolution of organomegaly. A partial response (PR) was defined as normalisation of the FBC and a ≥50% reduction of organomegaly and BM HC infiltration.9 During follow‐up, disease assessment was planned according to clinical judgment; at least one BM biopsy was taken within 12 months. In a subgroup of responding patients, in order to evaluate minimal residual disease (MRD), BRAF mutation was assessed by allele‐specific polymerase chain reaction (PCR) in peripheral blood samples, with a detection threshold of 0·05% BRAF‐V600E mutant alleles.10
Three patient groups were identified:
Patients aged >65 years, at time of treatment, who received front‐line IFNα.
Patients aged <65 years with comorbidities or women desiring pregnancy who received front‐line IFNα.
PA‐resistant or relapsed patients, who received IFNα as second or further line of treatment.
Comorbidities were classified according to the Charlson Comorbidity Index (CCI). Patients with a CCI score of ≥3 were considered suitable for Group B.
Results
From 1990 to 2020, 74 patients were treated for a median (range) of 82 (3–230) months. The patients' clinical and haematological characteristics are reported in Table I.
Table I.
The patients' clinical and haematological characteristics.
| Characteristic |
Group A (n = 21, 28%) |
Group B (n = 25, 34%) |
Group C (n = 28, 38%) |
Total (n = 74, 100%) |
|---|---|---|---|---|
| Age at diagnosis, years, median (range) | 69 (61–96) | 53 (33–63) | 51·5 (29–82) | 58·5 (29–96) |
| HCL/HCLv, n | 18/3 | 22/3 | 24/4 | 64/10 |
| Male/female, n | 17/4 | 22/3 | 18/10 | 58/16 |
| Age at INFα start, years, median (range) | 73 (65–96) | 53 (33–63) | 60·5 (31–90) | 61(31–96) |
| Hb at IFNα start, g/l, median (range) | 125 (84–154) | 113 (75–145) | 119 (100–158) | 115 (84–158) |
| WBC at IFNα start, × 109/l, median (range) | 3.8 (1.6–32.6) | 3.1 (1.68–11.0) | 3.15 (1.47–12.6) | 3.16 (1.47–32.6) |
| Neutrophils at IFNα start, × 109/l, median (range) | 1.5 (0.25–4.0) | 1.0 (0.59–2.79) | 1.4 (0.28–4.7) | 1.6 (0.25–4.7) |
| Platelets at IFNα start, × 109/l, median (range) | 94 (23–178) | 76 (36–234) | 95 (25–217) | 95 (23–217) |
| Bone marrow infiltration at IFN start, %, median (range) | 60 (30–90) | 65 (35–90) | 35 (5x–70) | 55 (5x–90) |
| Spleen size, cm, median (range) | 14·5 (10–25) | 15 (10–20) | 12 (10–16) | 14 (10–25) |
| Charlson Comorbidity Index score at IFNα start | 4·7 | 3 | 3·5 | 3·5 |
| IFNα schedule: continuous/12 months, n | 19/2 | 19/6 | 26/2 | 64/10 |
| Duration of therapy, months, median (range) | 61 (5–214) | 75 (3–228) | 99 (4–230) | 82 (3–230) |
| BRAF tested after treatment, n | 6 | 7 | 9 | 22 |
| Treatment results, n (%) | ||||
| Response at 12 months (ORR) | 19 (90) | 24 (96) | 27 (96) | 70 (94) |
| CR rate at 12 months | 6 (28) | 4 (16) | 8 (28) | 18 (24) |
| PR rate at 12 months | 13 (62) | 20 (80) | 19 (68) | 52 (70) |
| NR rate at 12 months | 2 (9) | 1(3) | 1 (3) | 4 (5) |
BRAF, B‐Raf proto‐oncogene, serine/threonine kinase; CR, complete response; Hb, haemoglobin; HCL(v); hairy cell leukaemia (variant); IFNα, interferon‐alpha; NR, no response; ORR, overall response rate, PR, partial response; WBC, white blood cells.
Patients in Group A received IFNα after a median watch‐and‐wait period of 4 years following diagnosis.
Patients in Group B were women desiring pregnancy (three) or patients with comorbidities (22). This group of patients received IFNα front‐line usually immediately after diagnosis.
Patients in Group C had received a median of two prior lines of therapy, including PAs. In total, 25 patients were relapsed and three were refractory to PAs. The IFNα treatment was started at a median of 9 years after diagnosis.
At the start of treatment, at least one severe cytopenia and/or splenomegaly plus BM infiltration were present.
In total, 70 patients (94%) achieved a response; PR in 52 (70%) and CR in 18 (24%). Four non‐responders were salvaged with PAs.
The response rates, which were similar in the three groups, are shown in Table I.
Regarding the 10 patients receiving IFNα for a fixed period of 12 months, eight obtained a response (one CR and seven PR). At relapse these patients were re‐challenged after a median (range) of 7 (1–22) years with IFNα with a 100% overall response rate (ORR; one CR and seven PR). Regarding responding patients receiving IFNα continuously, after a median (range) follow‐up of 92 (7–236) months, 55 of them (89%) still maintained normal blood counts. Nine patients relapsed, one in Group A, seven in Group B and one in Group C.
For the whole cohort (n = 74), at a median follow‐up of 5 years, the median OS and PFS have not been reached, and survival duration is estimated at 95% for OS and 78% for PFS.
Patients in CR at 12 months of treatment (n = 18) showed a significantly superior PFS (89%) compared to those in PR (n = 52; PFS 71%; P = 0·001) (Figure S1). A significantly shorter PFS was recorded in the 10 patients receiving IFNα for a defined 12‐month period (40%; P < 0·001).
The PFS duration was lower for Group B compared to groups A and C (P = 0·005; Fig 1), the 5‐year PFS was 68%, 95% and 96% respectively. This difference parallels the numerically lower CR rate observed in Group B versus groups A and C. In the Group C patients previously treated with PAs, the median (range) PFS was 99 (4–230) months. Additional findings are shown in the supplementary data.
Fig 1.
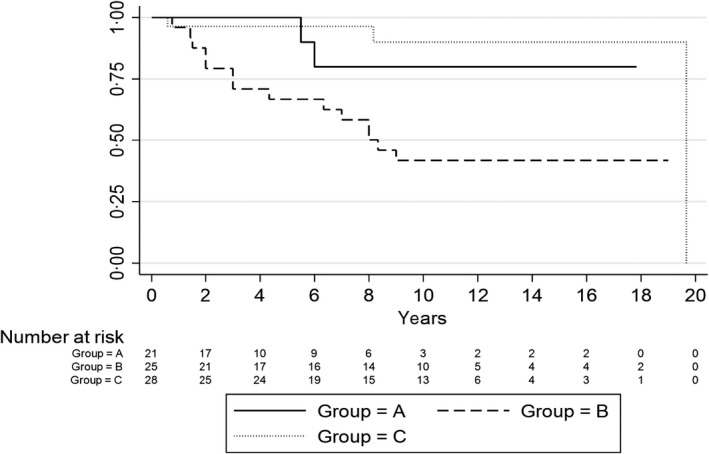
Progression‐free survival in patients in groups A, B and C.
The 10 patients with HCLv were equally distributed in the three groups. The median age at the start of IFNα treatment was 60 years and the median duration of therapy was 81 months. The ORR, CR and PFS at follow‐up were lower in HCLv with respect to HCL, being, respectively, 70%, 10% and 75%.
A total of 22 of the 55 responding patients during IFNα maintenance were tested for MRD according to their BRAF status in a peripheral blood samples taken after a median (range) of 120 (44–236) months from the first dose. Six patients were tested in Group A, seven in Group B and nine in Group C. In all, 32% (seven of 22) became negative during IFNα treatment. All patients with a negative MRD were in CR at 12 months after starting IFNα versus only six CRs in the remaining 15 patients (40%). No patient stopped IFNα administration because of MRD negativity. There were no relapses in MRD‐negative patients.
There were no Grade 3–4 toxicities during treatment. Extra‐haematological toxicities consisted of a flu‐like syndrome (25, 34%) in the first months of treatment and alopecia (one case). In total, 13 patients [17%; median (range) age: 76 (59–87)years] discontinued IFNα for Grade 1–2 toxicity after a median of 82 months of treatment. Four deaths occurred, two due to second neoplasms and two due to comorbidities in old patients.
Discussion
Few studies have reported the long‐term outcomes of IFNα in HCL. Benz et al.9 described 35 patients with HCL, concluding that IFNα induces a control of the disease rather than eradication. Damasio et al.8 reported on 64 patients, reporting a CR rate of only 13% with front‐line treatment. There were no relapses in patients who achieved a CR.
Our present results compare favourably with these and historical data,3, 6, 11 the good outcome observed could be explained by the use of prolonged IFNα administration in the setting of a relatively limited burden of disease as soon as patients are found to relapse during periodic follow‐up. A possible selection bias could relate to a heterogeneous clinical behaviour over a 30‐year period. The low incidence of side‐effects and the impact on quality of life was probably due to the low dosage, with few patients requiring discontinuation of treatment.
In our present study, obtaining a CR was confirmed to improve PFS, as reported with PAs.12 Although we reported limited numbers, IFNα in HCLv had an inferior outcome. We need to mention that soon IFNα will be probably available only as pegylated INFα‐2a.
The data of MRD evaluation by PCR detection of BRAF‐mutated cells, suggest that IFNα can induce a molecular remission, although in a limited proportion of patients. In other experiences, cladribine showed a 50% MRD negativity in the peripheral blood10 confirming a higher therapeutic impact of PAs.
We could speculate that a subset of patients with HCL with BRAF‐V600E negativity could discontinue IFNα by close MRD monitoring.
Given the retrospective nature of the present study, we need to be cautious of the evaluation of the response rate, but we can conclude that in young patients with comorbidities front‐line IFNα remains a possible option, although not as effective as PAs. In elderly patients, the continuous administration at a low dose allows remarkable results with acceptable toxicity. Also, in patients resistant to PAs, INFα is capable of inducing durable responses in a considerable proportion of patients. The data on MRD demonstrate the possibility of achieving a molecular response in a proportion of patients. Although the role of IFNα is further limited by the advent of new agents,13, 14 when administered continuously it still represents a valid option in specific subsets of patients with HCL. Furthermore, its potential could be re‐evaluated in the pandemic landscape, considering its inferior impact on the immune system compared to chemotherapy and its possible role in reducing coronavirus disease 2019 (COVID‐19)‐related inflammation as recently demonstrated.15
Conflict of interest
Alessandro Pulsoni: Advisory boards: Roche, Merck, Pfizer, Sandoz; Speakers bureau: Roche, Gilead, Bristol Meyer Squibb. The other authors have no conflict to declare.
Author contributions
Alessandro Pulsoni: treated the patients, conceived the study, analysed the results and wrote the paper. Giovanni M. Assanto: treated the patients, collected data, performed the statistical analysis, analysed the results and wrote the paper. Costantino Riemma: collected data, treated the patients, analysed results and co‐wrote the paper. Francesco Malaspina, Salvatore Perrone and Maria L. De Luca: collected data and treated the patients. Alessandra Pucciarini: performed the MRD evaluation. Giorgia Annechini and Gianna M. D'Elia: treated the patients. Maurizio Martelli and Robin Foà: contributed to manuscript's elaboration and critical review of the paper. Enrico Tiacci: supervisor of the MRD evaluation, contributed to manuscript's elaboration and critical review of the paper.
Supporting information
Table SI. Multivariate analysis of patient characteristics and PFS, multivariate analysis (Cox regression model stepwise: forward) of patients' characteristics at IFNα start confirms the impact of white blood cell count (WBC) >3.5 × 109/l. In the analysis are highlighted as well complete remission at 12 months as favourable and IFNα administration limited to 12 months as unfavourable.
Figure S1. OS and PFS of the whole population of patients treated with long‐term IFNα.
Figure S2. PFS according to response achieved after 12 months of therapy. Patients in CR at 12 months of treatment showed a significantly superior PFS (P = 0·001). The 10‐year PFS was 94% for patients in CR versus 73% for patients who obtained a PR during IFNα therapy.
Figure S3. PFS long‐term IFNα related to WBC count at the start of therapy; A significant difference in the whole population was observed in patients with a WBC of >3.5 × 109/l at the start of treatment, with an estimated median PFS of 236 versus 108 months (P = 0·004).
References
- 1.Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- 2.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy‐cell leukemia. N Engl J Med. 2011;364:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grever M, Kopecky K, Foucar MK, Head D, Bennett JM, Hutchison RE, et al. Randomized comparison of pentostatin versus interferon alfa‐2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol. 1995;13:974–82. [DOI] [PubMed] [Google Scholar]
- 4.Grever MR. How I treat hairy cell leukemia. Blood. 2010;115:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robak T, Matutes E, Catovsky D, Zinzani PL, Buske C. Hairy cell leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;1:v100–7. [DOI] [PubMed] [Google Scholar]
- 6.Quesada JR, Hersh EM, Manning J, Reuben J, Keating M, Schnipper E, et al. Treatment of hairy cell leukemia with recombinant α‐interferon. Blood. 1986;68:493–7. [PubMed] [Google Scholar]
- 7.Falini B, Tiacci E. New treatment options in hairy cell leukemia with focus on BRAF inhibitors. Hematol Oncol. 2019;37(Suppl. 1):30–7. [DOI] [PubMed] [Google Scholar]
- 8.Damasio EE, Clavio M, Masoudi B, Isaza A, Spriano M, Rossi E, et al. Alpha‐interferon as induction and maintenance therapy in hairy cell leukemia: a long‐term follow‐up analysis. Eur J Haematol. 2000;64:47–52. [DOI] [PubMed] [Google Scholar]
- 9.Grever MR, Abdel‐Wahab O, Andritsos LA, Banerji V, Barrientos J, Blachly JS, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chihara D, Arons E, Stetler‐Stevenson M, Yuan CM, Wang HW, Zhou H, et al. Randomized phase II study of first‐line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38:1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benz R, Siciliano RD, Stussi G, Fehr J. Long‐term follow‐up of interferon‐alpha induction and low‐dose maintenance therapy in hairy cell leukemia. Eur J Haematol. 2009;82:194–200. [DOI] [PubMed] [Google Scholar]
- 12.Saven A, Burian C, Koziol JA, Piro LD. Long‐term follow‐up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–26. [PubMed] [Google Scholar]
- 13.Kreitman RJ, Dearden C, Zinzani PL, Delgado J, Karlin L, Robak T, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiacci E, Park JH, De Carolis L, Chung SS, Broccoli A, Scott S, et al. Targeting mutant BRAF in relapsed or refractory hairy‐cell leukemia. N Engl J Med. 2015;373:1733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feld JJ, Kandel C, Biondi JM, Kozak RA, Zahoor MA, Lemieux C, et al. Peginterferon lambda for the treatment of outpatients with COVID‐19: a phase 2, placebo‐controlled randomised trial. www.thelancet.com/respiratory, 5 February 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Multivariate analysis of patient characteristics and PFS, multivariate analysis (Cox regression model stepwise: forward) of patients' characteristics at IFNα start confirms the impact of white blood cell count (WBC) >3.5 × 109/l. In the analysis are highlighted as well complete remission at 12 months as favourable and IFNα administration limited to 12 months as unfavourable.
Figure S1. OS and PFS of the whole population of patients treated with long‐term IFNα.
Figure S2. PFS according to response achieved after 12 months of therapy. Patients in CR at 12 months of treatment showed a significantly superior PFS (P = 0·001). The 10‐year PFS was 94% for patients in CR versus 73% for patients who obtained a PR during IFNα therapy.
Figure S3. PFS long‐term IFNα related to WBC count at the start of therapy; A significant difference in the whole population was observed in patients with a WBC of >3.5 × 109/l at the start of treatment, with an estimated median PFS of 236 versus 108 months (P = 0·004).


