Abstract
Rivaroxaban is a factor Xa inhibitor oral anticoagulant first approved for use in the United States in 2011. Under the drug class commonly termed direct oral anticoagulants, rivaroxaban is approved for the most indications within its class, 7 indications, which are: (1) reduction of risk of stroke and systemic embolism (SE) in nonvalvular atrial fibrillation, (2) treatment of deep vein thrombosis (DVT), (3) treatment of pulmonary embolism (PE), (4) reduction in the risk of recurrence of DVT and/or PE, (5) prophylaxis of DVT following hip or knee replacement surgery, (6) prophylaxis of venous thromboembolism in acutely ill medical patients at risk for thromboembolic complications not at high risk of bleeding, and (7) reduction of risk of major cardiovascular events in patients with chronic coronary artery disease or peripheral artery disease. Considering the relationship between cardiovascular disease, renal impairment, and the use of oral anticoagulants, the following targeted review was created. This review reports the results of the primary pharmacology, pharmacokinetic modeling, clinical safety and efficacy, and real‐world postmarketing effectiveness and safety of rivaroxaban in patients with various degrees of renal impairment. Based on these data, rivaroxaban is a viable option for when anticoagulation is needed in patients who have both cardiovascular disease and renal impairment. However, as with any therapy, the benefits and risks of intervention should be carefully assessed and balanced. Patients treated with rivaroxaban for several of its approved indications should have their kidney function assessed prior to and during continued therapy to ensure consistency with the drug label.
Keywords: efficacy, pharmacology, renal, rivaroxaban, safety
There is a well‐established link between the prevalence of cardiovascular disease (CVD) and chronic kidney disease (CKD). A recent 2018 analysis of the United States Renal Data System, a clinical data registry funded by the National Institutes of Health, reported that 64.5% of patients ≥ 66 years in the United States were diagnosed with both CVD‐related conditions and CKD.1 The CVD conditions commonly diagnosed with CKD include but are not limited to atrial fibrillation (AF), peripheral artery disease (PAD), coronary artery disease (CAD), venous thromboembolism (VTE), and pulmonary embolism (PE). This is significant, as the presence of CKD is associated with worse short‐ and long‐term prognosis for all these cardiovascular conditions.1
This cardiac‐renal relationship appears to be based on pathophysiologic mechanisms by which CKD may predispose patients to cardiovascular events. For example, CKD generally leads to alterations in sodium and fluid balance, leading to volume overload, vascular calcification, oxidative stress, uremic toxin retention, and inflammatory changes.2 These alterations are considered CKD‐specific nonclassical risk factors for CVD, and they can lead to the conditions previously mentioned in addition to atherosclerotic plaque destabilization, leading to potential life‐threating downstream events.1, 2
Based on the inherent risk of CVD conditions and subsequent events, anticoagulation is generally warranted in renally impaired patients with comorbid CVD and for whom therapy is indicated (eg, AF,3 VTE,4 hip/knee arthroplasty,5 CAD,6 PAD7). However, anticoagulant treatment in this patient population can be further complicated, as many of the currently approved medications are eliminated from the body through the kidneys. If normal drug elimination is compromised, systemic concentrations may increase, potentially leading to an adverse event. As such, additional care should be taken to routinely measure kidney function and adjust the dose of the anticoagulant, if appropriate.
The most recent class of anticoagulants, those categorized as direct oral anticoagulants, have shown a tremendous increase in use over the last 5 years. The use of these agents is driven by their comparable (if not better) safety and efficacy profiles, simplified treatment regimens, limited drug interactions, and lack of strict dietary restrictions, compared with vitamin K antagonists, the past standard of care. This category of drugs consists of rivaroxaban (Xarelto), apixaban (Eliquis), edoxaban (Savaysa), and dabigatran etexilate (Pradaxa). Each of these anticoagulants has some degree of renal clearance. Dabigatran leads this category with a renal clearance of 80%,8 followed by edoxaban at 50%,9 rivaroxaban at 36%,10 and apixaban at 27%.11 Dabigatran, edoxaban, and rivaroxaban allow for dose adjustment for renally impaired patients, dependent on both the indication and degree of impairment.8, 9, 10 Apixaban also allows for dose modification based on indication; however, this is not based on renal function alone, but with a paradigm in which at least 2 of the following 3 characteristics are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL.11
It is generally understood that although each compound provides direction on its use in this patient population, the degree of evidence that supports their respective labels is different and in general limited. Most labeling is based on data obtained from clinical pharmacology trials in otherwise healthy participants with various degrees of renal impairment down to end‐stage renal disease and/or dialysis. The additional use of pharmacokinetic (PK) and pharmacodynamic (PD) modeling and simulation along with data obtained from the large pivotal randomized, controlled trials (RCTs) further supports their use. Considering these limited data, it is important to expand our knowledge in this area via the use of real‐world evidence studies based on medical claims databases, electronic health care records, and prospective registries. The combination of all 3 data sources — (1) clinical pharmacology, (2) subpopulation analyses from RCTs, and (3) real‐world evidence studies — should provide greater understanding of these compounds in the treatment of CVD in renally impaired patients.
Rivaroxaban was chosen for this review due to its breadth of indications, which include: (1) to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular AF; (2) for the treatment of deep vein thrombosis (DVT); (3) for the treatment of PE; (4) for the reduction in the risk of recurrence of DVT and/or PE in patients at continued risk for recurrent DVT and/or PE after completion of initial treatment lasting ≥ 6 months; (5) for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery; (6) for the prophylaxis of VTE in acutely ill medical patients at risk for thromboembolic complications and not at high risk of bleeding; and (7) in combination with aspirin to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in patients with chronic CAD or PAD.10
Methods
The objective of this publication is to review the pharmacological profile, RCT efficacy and safety, and real‐world evidence postmarketing effectiveness and safety of rivaroxaban in patients with various degrees of renal impairment. The publications referenced in this review are those that support the approved indications listed in the current drug label for (1) the prophylaxis of DVT after hip or knee arthroplasty, (2) treatment of VTE (DVT and PE), (3) reduction in the risk of stroke and systemic embolism in AF, and (4) the reduction of risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in chronic CAD or PAD. As such, the publications describing the results from the phase 1 clinical pharmacology studies along with phases 2 and 3 efficacy and safety trials and the population PK analyses from each, were included. Real‐world evidence studies typically come after regulatory approval. Thus, the inclusion of these studies in this review play an important role in supporting the findings of the pivotal phase 3 studies.
CKD is classified by the severity of impairment. There are several ways to measure renal function and a few recognized guidelines that help to provide direction on how to properly classify the level of dysfunction. As this review will assess the data obtained from the various clinical trials conducted during drug development in support of the label, the extent of renal impairment was based on the recommendations from the guidelines for Industry when conducting new drug clinical trials. As such, an estimated creatinine clearance (CrCl) via the Cockcroft‐Gault equation that was used in all phase 1 through phase 3 clinical trials is reflected here, unless otherwise noted.12 There are known limitations when solely using the Cockcroft‐Gault equation to measure renal function, which will not be addressed in this review. However, it should be noted that the use of this equation was recommended from various regulatory agencies during drug development and was used in each clinical trial to ensure consistency unless otherwise stated.
Results
Effects of Renal Dysfunction on the Pharmacology of Rivaroxaban
During phase 1 clinical development, several studies were conducted to assess the PK and PD profile of rivaroxaban in otherwise healthy adult participants with various degrees of renal impairment. These original trials were small with a limited number of participants and designed to characterize (1) the impact of different stages of renal impairment, (2) the impact of regular dialysis, and (3) the impact of administering a combined P‐glycoprotein (P‐gp) and moderate cytochrome P 450 (CYP) 3A inhibitor with rivaroxaban.
The initial study was conducted by Kubitza et al and published in 2010.13 A total of 32 otherwise healthy adult participants with different degrees of renal function were enrolled and stratified based on measured CrCl: healthy control, ≥80 mL/min; mild renal impairment, 50‐79 mL/min; moderate renal impairment, 30‐49 mL/min; and severe renal impairment, <30 mL/min. Each participant received a single 10‐mg dose of rivaroxaban, followed by the serial collection of PK and PD plasma samples. The renal clearance of rivaroxaban was shown to decrease with decreasing renal function, leading to an increase in rivaroxaban plasma concentration area under the curve (AUC), a measure reflective of systemic exposure of the drug. When compared with the healthy control participants, these values increased by approximately 44%, 52%, and 64%, for subjects with mild, moderate, and severe renal impairment, respectively. The PD parameters prothrombin time and factor Xa inhibition also increased with decreasing renal function, however, with prothrombin time showing greater prolongation in those with moderate and severe renal impairment. Participants with a CrCl < 30 mL/min could not be on dialysis 4 weeks prior to and during the study.13
As the effects of dialysis were not characterized in this initial study, a separate clinical pharmacology study was conducted by Dias et al, in which a single 15‐mg dose of rivaroxaban was administered to participants prior to and following dialysis in otherwise healthy adult participants with end‐stage renal disease (ESRD).14 A total of 16 participants were enrolled and grouped based on renal function, as measured by CrCl. Eight participants had ESRD requiring maintenance hemodialysis 3 times a week, and 8 participants with a CrCl ≥ 80 mL/min acted as the control group. Participants with ESRD were administered a 15‐mg dose of rivaroxaban 2 ± 0.5 hours before a hemodialysis session and repeated 7‐14 days later 3 hours following a 4‐hour hemodialysis session. Control subjects received a single 15‐mg dose of rivaroxaban. In all participants, serial PK and PD plasma samples were collected.14
When compared with the control group, the mean rivaroxaban plasma concentration AUC of the ESRD participants increased by ≈56% following administration after dialysis. This increase reflects an approximate 35% decrease in overall drug clearance. Predialysis dosing resulted in only an approximate 5% lowering of AUC versus postdialysis dosing, thereby confirming that dialysis has minimal impact on the PK profile of rivaroxaban. Similar changes in the PD parameters prothrombin time, factor Xa inhibition, and anti‐Xa activity were also observed.14
Although the studies by both Kubitza et al and Dias et al helped to inform the current labeling for rivaroxaban, the data obtained were limited to a single‐dose PK and PD profile and did not address the potential for accumulation after multiple doses. This question was ultimately addressed by an independent investigator‐led study that assessed the steady‐state PK and PD of rivaroxaban in participants with ESRD on dialysis. The study, conducted by De Vriese et al in 2015, enrolled 18 adult participants who required dialysis 3 times a week.15 To assess rivaroxaban plasma concentrations after multiple dosing, participants were administered a 10‐mg dose once daily for 7 days. Serial plasma samples for PK were collected on days 1 and 7. After assessing the PK parameters following a single dose (day‐1) and after multiple dosing (day‐7), the author concluded that there was no significant accumulation of rivaroxaban.15
Considering the importance of both renal elimination and hepatic metabolism of rivaroxaban to ensure proper clearance of the drug, a study was conducted by Moore et al that investigated the PK and PD profile when both pathways were altered.16 The purpose of this drug‐drug‐disease interaction study was to assess the potential for a synergistic increase in exposure, as both renal impairment and the administration of erythromycin (a combined P‐gp and moderate CYP3A inhibitor), when assessed separately, displayed an increase in rivaroxaban exposure. Hence, this study investigated the pharmacological profile of rivaroxaban when both conditions were met. As such, rivaroxaban was coadministered with steady‐state erythromycin in subjects with either mild or moderate renal impairment.16 A total of 29 adult participants were enrolled and placed into groups (normal renal function, mild renal impairment, and moderate renal impairment) based on their respective renal function as measured by baseline CrCl. When assessing the coadministration of a single 10‐mg dose of rivaroxaban with steady state erythromycin in participants with mild or moderate renal impairment, rivaroxaban AUC∞ values increased by about 76% and 99%, respectively. Rivaroxaban maximum concentration (Cmax) increased by about 56% and 64%, respectively. All PD parameters displayed a similar trend.16 Although these increases did not appear synergistic, they were slightly more than additive and ultimately supported the current direction given in the drug label.
It is commonly criticized that the clinical impact of phase 1 pharmacology studies is limited, as the study participants are usually healthy volunteers. However, these data are critical in providing a firm foundation for the different pharmacological attributes a compound displays across different subpopulations. To further understand these attributes, the influence of renal function was assessed in diverse patient populations using population‐PK modeling. Studies in which the model was applied included (1) phase 2b clinical trials for the prevention of VTE after hip or knee replacement surgery,17, 18 (2) phase 2 clinical trials for acute DVT,19 (3) a phase 3 clinical trial in nonvalvular AF,20 and (4) a phase 2 trial in acute coronary syndrome.21
Using nonlinear mixed‐effects modeling, the population PK model was first described by Mueck et al in 2007 and was assessed with serial PK and PD data obtained from the phase 1 multiple‐ascending‐dose trial conducted in healthy participants.22 At this time, the population PK of rivaroxaban was well described by an oral 2‐compartment model with first‐order absorption and elimination from the central compartment. PD responses, which included FXa activity and changes in prothrombin time correlated with rivaroxaban plasma concentrations following both an inhibitory Emax and linear model, respectively.22 However, when assessed in larger phase 2 studies conducted in patients undergoing total hip or knee replacement surgeries, in which sparse sampling was used, the model was simplified to an oral 1‐compartment model with first‐order absorption and elimination, which in turn described the pharmacokinetics well in this patient population.17 With this model, both age and renal function were found to influence drug clearance.17, 18 The 1‐compartment model was subsequently assessed in other phase 2 and/or phase 3 trials that followed and continued to describe the drug kinetics and dynamics well.20, 21
This type of modeling allowed for broader understanding of the potential effects of different intrinsic patient characteristics on the pharmacology of rivaroxaban, beyond what was assessed in the initial phase 1 studies. When assessing the totality of these data, which included a far greater number of actual patients than previously assessed, those with decreased renal function (determined via serum creatinine and/or CrCl values), showed a moderate effect on drug clearance, which led to a corresponding increase in exposure.17, 18, 19, 20, 21 This was consistent with the trend observed in the phase 1 studies. Further population PK modeling led to the decision to reduce the dose of rivaroxaban assessed in the phase 3 ROCKET‐AF trial based on the patient's renal function.19
As drug modeling techniques continued to advance and additional data became available, a physiologically based pharmacokinetic model (PBPK) was developed. Unlike population PK modeling, which is essentially built to fit the data (top‐down approach), PBPK modeling uses what is already known about the anatomical and physiological structure of human organs and blood flow with the physiochemical properties of the drug to help to predict its distribution through the body (bottom‐up approach). Because of the detailed physiologic‐based nature of this modeling, it has become extremely helpful in predicting exposures across a broad range of different patient populations and conditions. A recent work by Willmann et al described this model for rivaroxaban and the predictive effects of both renal and hepatic impairment on its distribution.23 Although the PBPK model predicted slightly smaller changes in systemic exposure because of renal impairment, it remained generally consistent with the previously reported clinical data.23
Last, considering that oral phosphate binders, such as calcium salts or sevelamer (an anion exchange resin), are used in the majority of patients with kidney failure to prevent hyperphosphatemia, an in vitro dissolution study was performed with sevelamer carbonate and calcium acetate to assess the potential for a drug‐drug interaction with rivaroxaban. These results indicated that the 2 phosphate binders evaluated do not bind with rivaroxaban in vitro and therefore should not cause any change in efficacy for patients with ESRD taking phosphate binders with rivaroxaban.24
Efficacy and Safety of Rivaroxaban in Patients With Renal Impairment
Although large pivotal efficacy and safety studies that made up the phase 3 drug development program were not conducted solely in patients with various degrees of renal dysfunction, each of the trials conducted with rivaroxaban that led to approved treatment indication allowed for a rich analysis of results based on participants’ renal function.
Reduction in the Risk of Stroke and Systemic Embolism in AF
The ROCKET‐AF trial was a phase 3 randomized, noninferiority study designed to evaluate the efficacy and safety of rivaroxaban versus warfarin for the prevention of stroke and systemic embolism (SE) in patients with nonvalvular‐AF at moderate to high risk for stroke (CHADS2 score ≥ 2).25 Approximately 14 000 patients were enrolled and received either rivaroxaban 20 mg once daily (15 mg once daily in moderate renal impairment) or warfarin dosed to an international normalized ratio (INR) range of 2.0 to 3.0. Patients with a baseline CrCL< 30 mL/min were excluded.
Based on the primary efficacy analysis, rivaroxaban was determined to be noninferior to warfarin for the prevention of stroke or SE. Within the per‐protocol population, the primary efficacy end point (composite of stroke [ischemic or hemorrhagic] and SE) occurred in 188 patients in the rivaroxaban group (1.7% per year) and in 241 patients in the warfarin group (2.2% per year) with a hazard ratio (HR) of 0.79 and a 95% confidence interval (95%CI) of 0.66 to 0.96; P < .001 for noninferiority. As noninferiority was achieved, an analysis for superiority in the as‐treated safety population followed, in which the primary events occurred in 189 patients in the rivaroxaban group (1.7% per year) and in 243 patients in the warfarin group (2.2% per year) — HR, 0.79; 95%CI, 0.65‐0.95; P = .01 for superiority. The primary safety end point (bleeding outcomes) occurred in 1475 patients in the rivaroxaban group and in 1449 patients in the warfarin group (HR, 1.03; 95%CI, 0.96‐1.11; P = .44), with significant reductions in intracranial hemorrhage (0.5% vs 0.7%) and fatal bleeding (0.2% vs 0.5%) in the rivaroxaban group. Major bleeding from a gastrointestinal site was significantly greater in the rivaroxaban group compared with the warfarin group (3.2% vs 2.2%; P < .001).25
A study of the effect of rivaroxaban, compared with warfarin, for both the primary efficacy and safety outcomes was conducted in patients with varied renal function as part of a prespecified subgroup analysis. Patients were grouped by 3 CrCl categories: <50, 50‐80; and >80 mL/min. In each category, the primary efficacy and safety outcomes were consistent with those findings from the overall trial population (Figures 1 and 2).25
Figure 1.
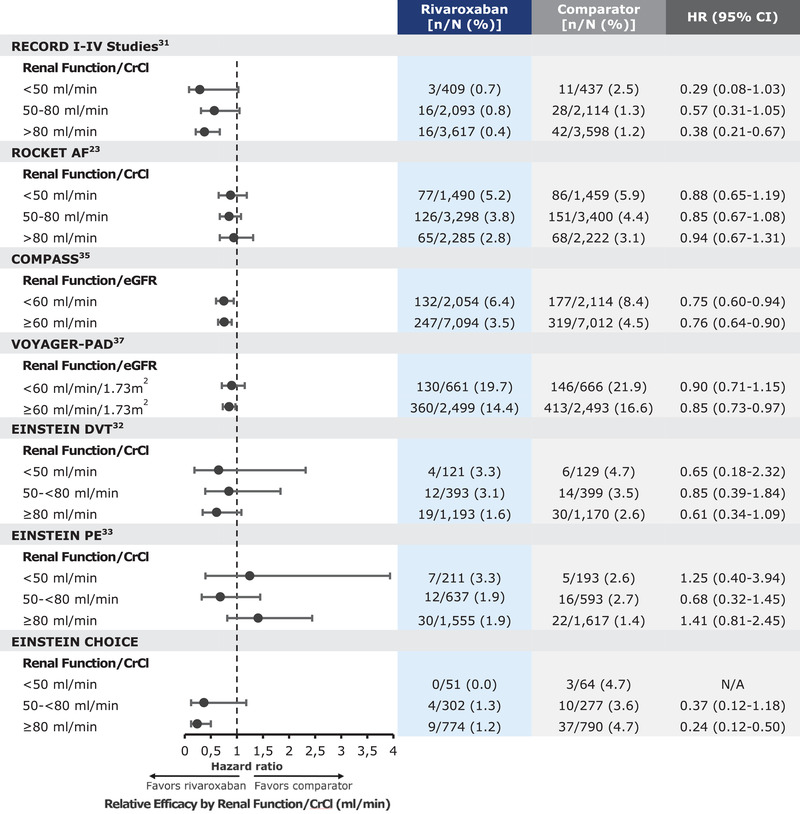
Rivaroxaban relative efficacy based on renal function.
Figure 2.
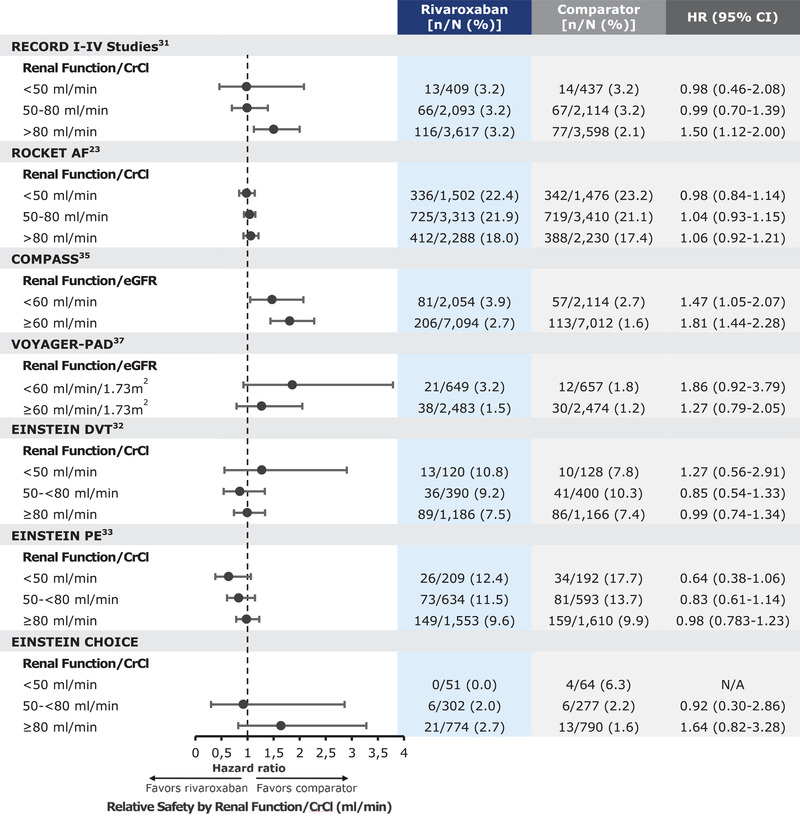
Rivaroxaban relative safety based on renal function.
Considering the adjusted rivaroxaban dose (15 mg once daily) for patients with moderate renal impairment compared with those with mild renal impairment or normal renal function (20 mg once daily), an additional secondary analysis conducted by Fox et al tested the efficacy and safety by treatment arm and renal function (moderate renal impairment, CrCl 30‐49 mL/min versus mild renal impairment and normal renal function, ≥50 mL/min).26 Approximately 21% of randomized patients had moderate renal impairment. When comparing the primary outcomes by treatment within the moderate renal impairment group, there were 2.32 events per 100 patient‐years with rivaroxaban compared with 2.77 per 100 patient‐years with warfarin, consistent with those observed in patients with mild renal impairment or normal renal function and also with the overall trial population.26 There was no significant increase in major and nonmajor clinically relevant bleeding or in individual bleeding outcomes in patients treated with rivaroxaban 15 mg compared with warfarin. In addition, those with moderate renal impairment, gastrointestinal bleeding was more frequent with rivaroxaban versus warfarin (4.1% vs 2.6%), and critical organ bleeding and fatal bleeding were less frequent with rivaroxaban versus warfarin.26
Following the analysis by Fox et al, an ad hoc analysis of the ROCKET‐AF trial was conducted by Fordyce et al, which evaluated the difference in the primary clinical outcomes between rivaroxaban and warfarin in patients with either stable renal function or worsening renal function.27 Patients with at least 1 follow‐up creatinine measurement were included in the analyses. Worsening renal function patients (26.3%) were defined as those with a decrease of ≥20% in CrCl from the screening visit, whereas stable renal function patients (73.7%) included all other patients.
Both the primary efficacy and safety end‐point measures remained the same as the overall trial. The rate of worsening renal function was similar in those randomized to rivaroxaban (26%) or warfarin (27%). However, there was a small, albeit statistically significant, decline in mean ± standard deviation (SD) CrCl values among patients receiving warfarin (−4.3 ± 14.6 mL/min) compared with patients receiving rivaroxaban (−3.5 ± 15.1 mL/min); P < .001. When assessing the primary efficacy and safety outcomes in renal function groups, there was no statistically significant difference between patients with worsening renal function and those with stable renal function. However, worsening renal function patients did have a higher incidence of other outcomes like vascular death; the composite end point of stroke, SE, vascular death, and myocardial infarction; and all‐cause mortality.27
When assessing the primary outcomes between treatment arms within worsening renal function and stable renal function groups, patients with worsening renal function and taking rivaroxaban had a reduction in stroke or systemic embolism compared with patients with worsening renal function taking warfarin (1.54 vs 3.25 events per 100 patient‐years). However, the same outcome was not observed in patients with stable renal function. There was no significant difference in patients with either worsening or stable renal function taking rivaroxaban or warfarin in the rates of major or nonmajor clinically relevant bleeding or intracerebral hemorrhage. However, worsening renal function patients receiving rivaroxaban had a greater incidence of gastrointestinal bleeding that was not seen in stable renal function patients (3.21 vs 1.28 events per 100 patient‐years for patients taking rivaroxaban vs warfarin). These data suggest that rivaroxaban may have a better renal risk‐benefit profile than warfarin in the setting of patients with worsening renal function.
A final ad hoc analysis of the different baseline renal populations in the ROCKET‐AF trial was conducted by Lindner et al.28 This analysis examined the primary efficacy and safety end points within patients now grouped by 4 different renal function/CrCl categories: moderate renal impairment, CrCl 30 to <50 mL/min; mild renal impairment, CrCl 50 to <80 mL/min; normal renal function, ≥80 mL/min; and high CrCl, CrCl > 95 mL/min).28 As expected, the rates of the primary efficacy end point increased with declining CrCl, regardless of treatment. Similarly, the rates of the primary safety end point also increased with declining CrCl, again regardless of treatment. No differences were observed between treatment arms, except a significantly lower risk of major bleeding was observed in the CrCl ≥ 80 mL/min normal renal function group treated with warfarin versus rivaroxaban (2.34 vs 3.10 events per 100 patient‐years).28
In addition, in patients with CrCl > 95 mL/min, rivaroxaban appeared to have a slightly, but not significantly, higher rate of stroke or systemic embolism than warfarin (1.28 and 0.86 events per 100 patient‐years, respectively; HR, 1.47; 95%CI, 0.81‐2.68). Consistent with the overall ROCKET‐AF trial results, rivaroxaban remains noninferior to warfarin for the principle efficacy end point across the full range of baseline CrCl included in the trial. Last, similar rates of the principle safety end point were observed across all renal function subgroups.28
DVT Prophylaxis Following Hip or Knee Replacement Surgery
The RECORD series is a group of 4 phase 3 global trials comparing the safety and efficacy of rivaroxaban with enoxaparin for VTE prevention following total hip arthroplasty or total knee arthroplasty.29, 30, 31, 32 The primary efficacy end points in these studies was a composite of any symptomatic VTE (DVT and PE) and all‐cause mortality. The primary safety end point was the incidence of major bleeding. Turpie et al33 completed a pooled analysis of these 4 trials, which included an assessment of the various intrinsic factors, including renal function, on the safety and efficacy of rivaroxaban. The 12 729 patients enrolled into these 4 studies were randomized to receive either rivaroxaban 10 mg once daily or enoxaparin 40 mg once daily (or 30 mg twice daily) following surgery. For this pooled analysis, 12 383 of these patients were included. Because of slight differences in each of the 4 trials, the authors provided 3 different grouped analyses of the data.33
When assessing the effects of renal function on the primary outcomes, the total treatment duration pool was used. The following data are based on that analysis. The safety population, which excluded patients who were randomized but did not receive any study medication, included 12 383 patients (6183 in the rivaroxaban group and 6200 in the enoxaparin group). Renal function was classified by baseline CrCl, creating 3 groups: <50, 50‐80, and >80 mL/min. In this pooled subgroup analysis across renal function, rivaroxaban demonstrated consistent reductions in the composite of symptomatic VTE and all‐cause mortality compared with enoxaparin, with the lowest incident rate observed in those with a CrCl > 80 mL/min (Figure 1).33 The incidence of major and nonmajor clinically relevant bleeding was comparable with rivaroxaban and enoxaparin across renal function groups, except for those with a CrCl > 80 mL/min, in which rivaroxaban displayed a higher rate (Figure 2).33
Treatment of VTE (DVT and PE)
The EINSTEIN trials were 2 global phase 3 noninferiority studies conducted to evaluate the efficacy and safety of rivaroxaban for the treatment of acute symptomatic DVT (EINSTEIN‐DVT)34 or PE (EINSTEIN‐PE).35 The primary efficacy outcome for both studies was recurrent VTE. The principal safety outcome was major bleeding or nonmajor clinically relevant bleeding. Both trials compared the use of rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily) with subcutaneous enoxaparin (1.0 mg/kg twice daily) followed by dose‐adjusted oral vitamin K antagonist (VKA; warfarin or acenocoumarol) for 3, 6, or 12 months. An extension study (EINSTEIN‐Extension) assessed the continued use of rivaroxaban (20 mg once daily) with use of the placebo over an additional 6 or 12 months of treatment for those who had already completed 6 to 12 months of initial VTE treatment.34
In both trials, rivaroxaban was found to be noninferior to enoxaparin/VKA therapy for the recurrence of VTE; EINSTEIN‐DVT (HR, 0.68; 95%CI, 0.44‐1.04; P < .001) and EINSTEIN‐PE (HR, 1.12; 95%CI, 0.75‐1.68; P = .003).34, 35 In the EINSTEIN‐Extension study, rivaroxaban had superior efficacy (8 events [1.3%] vs 42 with placebo [7.1%]; HR, 0.18; 95%CI, 0.09‐0.39; P < .001).34 In the EINSTEIN‐DVT study, rivaroxaban had the same principal safety outcome results (8.1% of patients) as enoxaparin/VKA therapy. Within the EINSTEIN‐PE study, rivaroxaban displayed a comparable principal safety outcome to enoxaparin/VKA therapy (10.3% vs 11.4%, respectively; HR, 0.90; 95%CI, 0.76‐1.07; P = .23).34, 35 In the EINSTEIN‐Extension study, when rivaroxaban was compared with placebo, 4 patients in the rivaroxaban group had nonfatal major bleeding (0.7%), versus none in the placebo group.34
Similar to the other rivaroxaban clinical trials, a prespecified subgroup analysis was conducted by renal function, defined by CrCl groups (<50, 50 to <80, ≥80 mL/min). In each renal function group, the results for the primary efficacy and safety outcomes were consistent with the main findings of the trials (Figures 1 and 2).34, 35
A prespecified subgroup analysis of the EINSTEIN‐DVT and EINSTEIN‐PE pooled data was conducted by Bauersach et al.36 This analysis specifically assessed the efficacy and safety of rivaroxaban, compared with enoxaparin/VKA, in patients with VTE with and without renal impairment. Primary outcomes were recurrent VTE (efficacy) and major or nonmajor clinically relevant bleeding (safety) in patients with normal renal function (67.3%) or mild (24.6%), moderate (7.7%), or severe (0.3%) renal impairment. Renal function was categorized via CrCl (normal, CrCl ≥ 80 mL/min; mild renal impairment, CrCl 50‐79 mL/min; moderate renal impairment, CrCl 30–49 mL/min; or severe renal impairment, CrCl < 30 mL/min).36
The risk of recurrent VTE and bleeding increased with declining renal function. The primary outcome, rate of recurrent VTE for both treatments combined, was 1.8%, 2.8%, 3.3%, and 4.8% in patients with normal renal function and mild, moderate, and severe renal impairment, respectively. When assessing the rates of recurrent VTE between rivaroxaban and enoxaparin/VKA, HRs were similar for those with normal renal function (HR, 0.95; 95%CI, 0.65‐1.41), mild renal impairment (HR, 0.77; 95%CI, 0.45‐1.30), and moderate renal impairment (HR, 1.05; 95%CI, 0.44‐2.47). For those in the severe renal impairment group, no event occurred in the rivaroxaban group, and 1 event occurred in the VKA group.36
In patients receiving rivaroxaban, major and nonmajor clinically relevant bleeding occurred 8.7%, 10.7%, 11.6%, and 22.2% of patients with normal renal function, mild renal impairment, moderate renal impairment, and severe renal impairment, respectively. In the enoxaparin/VKA group, the incidence was 8.8%, 12.3%, 13.9%, and 9.1%, respectively. When assessing the hazard ratios between the treatments, HR was 0.98 (95%CI, 0.82‐1.18) for patients with normal renal function, 0.85 (95%CI, 0.65‐1.09) for those with mild renal impairment, and 0.77 (95%CI, 0.49‐1.19) for those with moderate renal impairment.36
In patients receiving rivaroxaban, major bleeding occurred in 0.8%, 1.4%, 0.9%, and 0% of patient with normal renal function, mild renal impairment, moderate renal impairment, and severe renal impairment, respectively. Major bleeding rates in enoxaparin/VKA recipients were 1.0%, 3.0%, 3.9%, and 9.1%, respectively. There was a significant reduction in major bleeding observed with rivaroxaban compared with enoxaparin/VKA, particularly in patients with mild or moderate renal impairment.36
EINSTEIN‐CHOICE was the fourth study in the VTE series and was conducted to ascertain whether it is better to use full‐ or lower‐intensity anticoagulation therapy or aspirin for patients with VTE requiring extended treatment.37 This randomized, double‐blind phase 3 study was conducted in 3396 patients with a VTE. Patients were randomized to receive either once‐daily rivaroxaban (at doses of 20 or 10 mg) or 100 mg of aspirin. Patients were required to first complete 6 to 12 months of anticoagulation therapy and be in equipoise regarding the need for continued anticoagulation, at which time therapy was administered for up to an additional 12 months. The primary efficacy outcome was symptomatic recurrent fatal or nonfatal VTE, and the principal safety outcome was major bleeding.37
A total of 3365 patients were included in the final analyses. The primary efficacy outcome occurred in 1.5% of the patients receiving 20 mg of rivaroxaban and 1.2% receiving 10 mg of rivaroxaban, compared with 4.4% receiving aspirin. Rates of major bleeding were 0.5% in the rivaroxaban 20‐mg group, 0.4% in the rivaroxaban 10‐mg group, and 0.3% in the aspirin group. Ultimately, the lower 10‐mg dose of rivaroxaban was approved for use. Subgroup analyses by renal function (<50, 50 to <80, ≥80 mL/min) showed a consistent result for both primary efficacy and safety end points, with the overall study population (Figures 1 and 2).37
Reduction of Risk of Major Adverse Cardiovascular Events (CV Death, Myocardial Infarction, and Stroke) in Chronic CAD or PAD
The COMPASS trial was a global phase 3 study that evaluated the use of rivaroxaban, with or without concomitant aspirin, for the prevention of major adverse cardiovascular events (myocardial infarction, stroke, or CV death) and major bleeding events (using modified International Society on Thrombosis and Haemostasis [ISTH] criteria) in patients with a history of stable atherosclerotic vascular disease (CAD and/or PAD).38 A total of 27 395 patients were enrolled and randomized in a 1:1:1 ratio receiving either rivaroxaban 2.5 mg twice daily plus aspirin 100 mg once daily, rivaroxaban 5 mg twice daily alone, or aspirin 100 mg once daily. The study was stopped early by an independent data safety and monitoring board for evidence of superiority for the rivaroxaban + aspirin regimen after a mean follow‐up of 23 months.38
Patients randomized to the rivaroxaban + aspirin group had fewer primary outcome events (n = 379 patients [4.1%]) than the aspirin‐alone group (n = 496 patients [5.4%]) and the rivaroxaban‐alone group (n = 448 patients [4.9%]). When comparing the rivaroxaban + aspirin and aspirin‐alone groups, rivaroxaban displayed a significant reduction in events (HR, 0.76; 95%CI, 0.66‐0.86; P < .001).38 Major bleeding events occurred more frequently in the rivaroxaban + aspirin group (n = 288 patients [3.1%]) compared with the aspirin‐alone group (n = 170 [1.9%]; HR, 1.70; 95%CI, 1.40‐2.05; P < .001). Importantly, there was no significant difference in intracranial or fatal bleeding between these 2 groups. Last, there was a total of 313 deaths (3.4%) in the rivaroxaban + aspirin group compared with 378 (4.1%) in the aspirin‐alone group (HR, 0.82; 95%CI, 0.71‐0.96; P = .01).38
As part of the subgroup analyses for the trial, patients were grouped by their estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration formula. Two cohorts were established, <60 and ≥60 mL/min/1.73 m2. Subgroup analyses for the primary efficacy and safety outcomes only compared rivaroxaban + aspirin with aspirin alone. When assessing the primary efficacy end point in the eGFR < 60 mL/min/1.73 m2 cohort, a total of 132 events (6.4%) occurred in the rivaroxaban + aspirin group, compared with 177 events (8.4%) that occurred in the aspirin‐alone group (Figure 1). A similar trend was observed in the eGFR ≥ 60 mL/min/1.73 m2 cohort, in which a total of 247 event s (3.5%) occurred in the rivaroxaban + aspirin compared with 319 events (4.5%) that occurred in the aspirin‐alone group (Figure 1).38
When assessing the primary safety end point in the eGFR < 60 mL/min/1.73 m2 cohort, a total of 81 events (3.9%) occurred in the rivaroxaban + aspirin group, compared with 57 events (2.7%) that occurred in the aspirin‐alone group (Figure 2). A similar trend was observed in the eGFR ≥ 60 mL/min/1.73 m2 cohort, in which a total of 206 events (2.9%) occurred in the rivaroxaban + aspirin compared with 113 events (1.6%) that occurred in the aspirin‐alone group (Figure 2).38 In totality, the rivaroxaban + aspirin reatment, with its dual pathway (factor Xa inhibition and antiplatelet) benefits are preserved in patients with moderate renal dysfunction (eGFR < 60 mL/min/1.73 m2) without evidence of an excess hazard of bleeding.39
Further expanding the knowledge of rivaroxaban use in PAD, another phase 3 study, which assessed the use of rivaroxaban in patients who have undergone lower‐extremity revascularization, was conducted.40 VOYAGER‐PAD was a randomized, double‐blind clinical trial in which 6564 patients received either rivaroxaban 2.5 mg twice daily plus aspirin (n = 3286) or placebo plus aspirin (n = 3278). The primary efficacy outcome was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction (MI), ischemic stroke, and death from CV causes. The principal safety outcome was major bleeding, defined according to the Thrombolysis in Myocardial Infarction (TIMI) classification. In addition, major bleeding as defined by the ISTH criteria was assessed as a secondary safety outcome.40
Patients randomized to rivaroxaban + aspirin had fewer primary composite outcome events than those randomized to placebo + aspirin (rivaroxaban, 508; versus placebo, 584). The Kaplan‐Meier estimates of the incidence at 3 years were 17.3% for rivaroxaban and 19.9% for placebo (HR, 0.85; 95%CI, 0.76‐0.96; P = .009). The primary safety end point (TIMI major bleeding) occurred in 62 patients (2.65%) versus 44 patients (1.87%) randomized to the rivaroxaban and placebo groups, respectively (HR, 1.43; 95%CI, 0.97‐2.10; P = .07). The secondary safety outcome (ISTH major bleeding) occurred in 140 patients (5.94%) in the rivaroxaban group, compared with 100 patients (1.42%) in the placebo group (HR, 1.42; 95%CI, 1.10‐1.84; P = .007).40
Similar to the COMPASS trial, a subgroup analysis was conducted comparing the efficacy and safety end points between renal function groups. Patients were grouped by eGFR score < 60 or ≥ 60 mL/min/1.73 m2. When assessing the primary efficacy end point in the eGFR < 60 mL/min/1.73 m2 cohort, a total of 130 events (19.67%) occurred in the rivaroxaban + aspirin group, compared with 146 events (21.92%) that occurred in the aspirin‐alone group (Figure 1). When assessing the eGFR ≥ 60 mL/min/1.73 m2 cohort, a total of 360 events (14.41%) occurred in the rivaroxaban + aspirin group compared with 413 events (16.57%) that occurred in the aspirin‐alone group (Figure 1).40
When assessing the primary safety end point in the eGFR < 60 mL/min/1.73 m2 cohort, a total of 21 events (3.24%) occurred in the rivaroxaban + aspirin group, compared with 12 events (1.83%) that occurred in the aspirin‐alone group (Figure 2). A similar trend was observed in the eGFR ≥60 mL/min/1.73 m2 cohort, in which a total of 38 events (1.53%) occurred in the rivaroxaban + aspirin group compared with 30 events (1.21%) that occurred in the aspirin‐alone group (Figure 2).40 It is important to note that the data from the VOYAGER‐PAD study is currently being reviewed by the Food and Drug Administration and does not yet support a labeling claim.
Real‐World Evidence Studies Among Patients With Renal Dysfunction
The effectiveness and safety of rivaroxaban among patients with renal dysfunction have also been studied extensively in real‐world studies. One of the first real‐world studies conducted was a population‐based nested case‐control study among elderly patients in Ontario, Canada, with a history of CKD who received an oral anticoagulant between April 2006 and March 2013.41 Patients on hemodialysis were excluded from the study. Researchers assessed the odds of hospitalization (emergency room visit or hospital admission) for major bleeding among patients prescribed rivaroxaban, dabigatran, or warfarin 60 days prior to their bleeding‐related hospitalization. Study controls were selected from individuals without a hospitalization or emergency room visit for a major bleeding event, and up to 4 controls were matched to each case based on age and sex. Among the 237 409 elderly patients with CKD identified, ∼2% had a major bleeding event (n = 4470) and were matched to 14 460 controls. After matching, the majority had been exposed to warfarin (97%), whereas a total of 151 patients (0.8%) were exposed to rivaroxaban, and the remaining 2.2% were on dabigatran. No statistically significant difference in major bleeding was found with the use of rivaroxaban compared with warfarin (adjusted odds ratio [aOR], 1.22; 95%CI, 0.83‐1.79). Sensitivity analyses by dose and older age (>80 years) found no significant differences for rivaroxaban compared with warfarin (low‐dose aOR, 1.16; 95%CI, 0.74‐1.79; high‐dose aOR, 1.45; 95%CI, 0.66‐3.19; age < 80 years aOR, 0.94; 95%CI, 0.53‐1.65; age ≥ 80 years aOR, 1.57; 95%CI, 0.91‐2.69). A subset analysis of patients with AF also found a similar risk of major bleeding for rivaroxaban (aOR, 0.97; 95%CI, 0.44‐2.11).41
Several US‐based retrospective claims and electronic health care record (EHR) analyses have also assessed rivaroxaban's effectiveness and safety among patients with nonvalvular AF and renal dysfunction.42, 43, 44 Weir and colleagues assessed the impact of renal function on ischemic stroke and major bleeding rates in 2 integrated claims‐EHR databases: (1) Optum Integrated Claims Clinical Deidentified database from May 2011 to August 201442 and (2) IMS Health Real‐World Data Adjudicated Claims linked with Ambulatory EHR data from May 2011 to June 2015.43 In the Optum integrated analysis, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes were used to determine incidence rates of ischemic stroke and major bleeding hospitalizations. Patients with nonvalvular AF treated with warfarin (n = 2468) or rivaroxaban (n = 1290) were selected and stratified by baseline estimated creatinine clearance (eCrCl) levels. Confounding adjustments were made using inverse probability of treatment weighing. Among the overall population, patients treated with rivaroxaban had an ischemic stroke incidence rate of 1.9 per 100 person‐years, and patients treated with warfarin had a rate of 4.2 per 100 person‐years (HR, 0.41; 95%CI, 0.21‐0.80; P = .009). Rivaroxaban patients with an eCrCl ≤ 50 mL/min had an ischemic stroke rate of 0.8 per 100 person‐years, whereas the warfarin cohort rate was 6.0 per 100 person‐years (Table 1). Among the other renal function groups (ie, eCrCl, 51‐79 and ≥80 mL/min), ischemic stroke rates did not statistically differ between treatment groups (Table 1). Overall, major bleeding rates were 7.3 per 100 person‐years for rivaroxaban and 7.4 per 100 person‐years for warfarin (HR, 1.04; 95%CI, 0.72‐1.51; P = .84). Major bleeding events did not differ significantly between treatment cohorts when stratified by renal function (Table 2).42
Table 1.
Rivaroxaban Effectiveness Outcomes Among Patients With Renal Dysfunction in RWE Studies
| Rivaroxaban Event Rate (per 100 PY) | Warfarin Event Rate (per 100 PY) | HR (95%CI) | |
|---|---|---|---|
| Weir, 201739 | |||
| Ischemic stroke | |||
| eCrCl ≤ 5 mL/min | 0.8 | 6.0 | 0.09 (0.01‐0.72) |
| eCrCl 51‐79 mL/min | 2.7 | 4.3 | 0.46 (0.20‐1.10) |
| eCrCl ≥ 80 mL/min | 1.7 | 2.6 | 0.82 (0.31‐2.20) |
| Weir, 201840 | |||
| Ischemic stroke | |||
| Renal impairment (defined by ICD‐9‐CM diagnosis codes) | 2.6 | 5.4 | 0.55 (0.40‐0.77) |
| eCrCl < 60 mL/min (based on Cockcroft‐Gault formula) | 4.2 | 5.6 | 3.22 (0.50‐20.77) |
| Vaitsiakhovich, 201941 | |||
| Ischemic stroke | |||
| Cohort 1: CKD stages 3‐4 only | 0.77 | 1.06 | 0.77 (0.33‐1.82) |
| Cohort 2: extended patient identification with additional kidney diseases | 0.82 | 1.01 | 0.94 (0.50‐1.77) |
| Nielsen, 201742 | |||
| Ischemic stroke/systemic embolism (renal impairment subgroup) | NR | NR | 0.59 (0.25‐1.39) |
| Chan, 201944 | |||
| Subgroup of patients with CKD | |||
| Ischemic stroke/SE | NR | NR | 0.68 (0.42‐1.08) |
| Ischemic stroke only | NR | NR | 0.73 (0.44‐1.22) |
| Fatal ischemic stroke/SE | NR | NR | 0.74 (0.19‐2.86) |
| Coleman, 201946 | |||
| Stroke/SE | 1.10 | 2.16 | 0.55 (0.27‐1.10) |
| Ischemic stroke alone | 0.85 | 1.44 | 0.67 (0.30‐1.50) |
| Weir, 202047 | |||
| Ischemic stroke/SE | NR | NR | 0.93 (0.46‐1.90) |
| Rivaroxaban Event Rate (per 100 PY) | Warfarin Event Rate (per 100 PY) | Unadjusted RR (95%CI) | |
|---|---|---|---|
| Chan, 201545 | |||
| Total embolic events (ie, embolic stroke, arterial embolism) | 11.2 | 6.2 | 1.80 (0.89‐3.64) |
CI, confidence interval; CKD, chronic kidney disease; eCrCl, estimated creatinine clearance; HR, hazard ratio; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NR, not reported; PY, person‐years; RR, rate ratio; SE, systemic embolism.
Table 2.
Rivaroxaban Safety Outcomes Among Patients With Renal Dysfunction in RWE Studies
| Rivaroxaban Event Rate (per 100 PY) | Warfarin Event Rate (per 100 PY) | HR (95%CI) | |
|---|---|---|---|
| Weir 201739 | |||
| eCrCl ≤50 mL/min | 13.1 | 9.4 | 1.20 (0.66‐2.20) |
| eCrCl 51‐79 mL/min | 10.1 | 7.5 | 1.26 (0.75‐2.12) |
| eCrCl ≥80 mL/min | 3.1 | 5.7 | 0.73 (0.33‐1.63) |
| Weir 201840 | |||
| Major bleeding | |||
| Renal impairment (defined by ICD‐9‐CM diagnosis codes) | 10.8 | 13.2 | 0.98 (0.82‐1.16) |
| eCrCl <60 mL/min (based on Cockcroft‐Gault formula) | 14.9 | 11.3 | 0.86 (0.36‐2.05) |
| Vaitsiakhovich 201941 | |||
| Bleeding related hospitalization | |||
| Cohort 1:CKD stages 3‐4 only | 5.90 | 5.09 | 1.14 (0.83‐1.58) |
| Cohort 2: Extended patient identification with additional kidney diseases | 5.60 | 4.39 | 1.22 (0.95‐1.56) |
| Intracranial hemorrhage | |||
| Cohort 1:CKD stages 3‐4 only | 0.33 | 0.79 | 0.42 (0.12‐1.44) |
| Cohort 2: Extended patient identification with additional kidney diseases | 0.29 | 0.65 | 0.34 (0.12‐0.95) |
| Nielsen 201742 | |||
| Any bleeding (renal impairment subgroup) | NR | NR | 0.63 (0.38‐1.05) |
| Chan 201944 | |||
| Subgroup of patients with CKD | |||
| Intracranial hemorrhage | NR | NR | 0.54 (0.22‐1.36) |
| Major GI bleeding | NR | NR | 0.82 (0.39‐1.72) |
| Fatal bleeding | NR | NR | 0.88 (0.14‐5.45) |
| All major bleeding | NR | NR | 0.64 (0.37‐1.08) |
| Coleman 201946 | |||
| Major bleeding | 3.73 | 6.16 | 0.68 (0.47‐0.99) |
| Intracranial bleeding | 0.08 | 0.28 | 0.19 (0.02‐1.56) |
| GI bleeding | 3.39 | 4.52 | 0.87 (0.58‐1.30) |
| Weir 202047 | |||
| Major bleeding | NR | NR | 0.91 (0.65‐1.28) |
| GI bleeding | NR | NR | 1.14 (0.77‐1.69) |
| Intracranial bleeding | NR | NR | 0.60 (0.22‐1.68) |
| Other major bleeding | NR | NR | 0.64 (0.30‐1.36) |
| Rivaroxaban Event Rate (per 100 PY) | Warfarin Event Rate (per 100 PY) | Adjusted RR | |
|---|---|---|---|
| Chan 201545 | |||
| Major bleeding | 68.4 | 47.1 | 1.38 (1.03‐1.83) |
| Hemorrhagic death | NR | NR | 1.71 (0.94‐3.12) |
CI, confidence interval; CKD, chronic kidney disease; eCrCl, estimated creatinine clearance; GI, gastrointestinal; HR, hazard ratio; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NR, not reported; PY, person‐years; RR, rate ratio.
In the IMS Health Real‐World Data Adjudicated Claims linked with Ambulatory EHR analysis, 39 872 rivaroxaban‐ and 48 637 warfarin‐treated nonvalvular AF patients were identified and stratified based on renal function using 2 approaches: (1) among all patients with a relevant ICD‐9‐CM diagnosis code for renal dysfunction (rivaroxaban, n = 3572; warfarin, n = 8230), and (2) in patients with EHR data available with at least 1 creatinine value available to estimate CrCl using the Cockcroft‐Gault formula (eCrCl < 60 mL/min: rivaroxaban, n = 66; warfarin, n = 208). Inverse probability of treatment weighing was used to adjust for potential confounding. When renal function was categorized by diagnostic codes, the rate of ischemic stroke was lower in patients with renal impairment that were treated with rivaroxaban (Table 1). Similar bleeding rates were seen in patients with renal impairment (Table 2). When renal function was categorized by eCrCl values, ischemic stroke and major bleeding rates did not significantly differ between the rivaroxaban and warfarin groups among patients with eCrCl < 60 mL/min (Tables 1 and 2).43
Vaitsiakhovich et al evaluated the risk of ischemic stroke, intracranial hemorrhage, and bleeding‐related hospitalization among nonvalvular AF patients with renal dysfunction treated with 15 mg rivaroxaban versus warfarin from the IBM MarketScan database from January 2012 to September 30, 2017. Renal dysfunction patients were identified based on 2 criteria:cohort 1–CKD stages 3 and 4 only, and cohort 2–extended patient identification by including additional specific kidney diseases (ie, cystic kidney disease, unspecified kidney failure, chronic or unspecified nephritic syndrome, nephrotic syndrome, recurrent and persistent hematuria, nephropathy, chronic tubulointerstitial nephritis, and diabetes mellitus with kidney complications). Inverse probability of treatment weighing was used to adjust for potential confounding. Among 7372 patients (warfarin, n = 5906; rivaroxaban, n = 1466) identified in xcohort 1, the risk of all 3 outcomes was similar in the treatment groups (Tables 1 and 2). For the cohort 2 analysis a total of 15 985 patients (warfarin, n = 13 275; rivaroxaban, n = 2710) were identified and risk of all 3 outcomes were also similar in the treatment groups (Tables 1 and 2).44
Additional retrospective real‐world studies from Europe and Asia also assessed rivaroxaban's effectiveness and safety profile in renal dysfunction patients.45, 46, 47 A nationwide cohort study using linked data from 3 registries in Denmark identified patients with nonvalvular AF newly initiated on reduced dose direct oral anticoagulants compared with warfarin from August 2011 to February 2016. Inverse probability of treatment weighing was used to adjust for potential confounding. Nonvalvular AF patients with renal impairment that were on reduced‐dose rivaroxaban had a similar risk of ischemic stroke/systemic embolism (Table 1) and any bleeding (Table 2).45
Investigators in Italy conducted an observational longitudinal study of 347 consecutive nonvalvular AF patients with CKD stage 3b‐4 from 8 cardiac outpatient clinics from March 2015 to October 2017 to assess the clinical effectiveness (ie, ischemic stroke, VTE, or transient ischemic attack) and safety (intracranial hemorrhage, gastrointestinal or other bleeding) of rivaroxaban compared with warfarin. Overall, 247 patients were treated with rivaroxaban 15 mg and 100 with warfarin (median time in therapeutic range was 67.6%) for at least 12 months. Patients with ESRD, age > 75 years, cancer and chronic inflammatory diseases were excluded. Outcomes of interest were identified via primary diagnoses during hospitalization and emergency room visit (for TIA episode). Overall, 25 strokes (15 hemorrhagic and 10 ischemic) occurred among 24 warfarin‐treated patients compared with no strokes in the rivaroxaban group over a mean follow‐up of 16 ± 0.3 months (P ≤ .002). The warfarin group also had 5 DVT events compared with no events in the rivaroxaban group. Gastrointestinal hemorrhages were seen in 8 warfarin patients and 2 rivaroxaban patients (P = .001), whereas minor bleeding episodes (epistaxis) were found to be similar in treatment groups (4 warfarin and 6 rivaroxaban, P = .48).46
A nationwide cohort study using Taiwan's National Health Insurance Research Database (June 2012‐December 2017) assessed clinical outcomes among nonvalvular AF patients treated with direct oral anticoagulants compared with warfarin. Overall, 33 022 patients were treated with rivaroxaban (30.1% had CKD), and 19 761 were treated with warfarin (31.7% had CKD). Patients with ESRD, DVT, PE, joint replacement therapy, or valvular AF 6 months prior to index date were excluded. Subgroup analyses by presence of CKD found no significant differences for rivaroxaban in thromboembolism and major bleeding risk compared with warfarin (Tables 1 and 2).47
Real‐World Evidence Studies Among Patients With Advanced/ESRD
One key patient subgroup in which there has been a paucity of evidence pertaining to anticoagulation therapy is in patients with advanced CKD (ie, stage IV‐V CKD or on hemodialysis), given that this group of patients was excluded from RCTs. In recent years, real‐world evidence studies focused on advanced/ESRD patients have been published and may help better inform the prescribing community.48, 49, 50
An early real‐world analysis focused on oral anticoagulant (rivaroxaban, dabigatran, warfarin) prescribing patterns and rates of stroke, bleeding, and arterial embolism in chronic hemodialysis patients with AF used data from the Fresenius Medical Care North America ESRD database between October 2010 and October 2014. A total of 29 977 chronic hemodialysis patients with AF were identified. Among the 244 patients that were started on rivaroxaban, 67.8% received the reduced 15‐mg once‐daily dose. In covariate‐adjusted Poisson regression, rivaroxaban had a higher risk of major bleeding (Table 2) compared with warfarin. Dialysis patients who were prescribed the full dose of rivaroxaban 20 mg (32.2%) had a higher risk of major bleeding compared with patients who were on the 15‐mg rivaroxaban dose, which is the recommended dose for patients with renal impairment. Hemorrhagic death risk was numerically higher but not significant among rivaroxaban patients compared with warfarin (Table 2), and there were too few stroke and arterial embolism events in the study to detect a difference (Table 1).48
A retrospective claims analysis of US Truven Marketscan Commercial Claims and Medicare Supplemental databases from January 2012 to December 2017 identified nonvalvular AF patients with advanced CKD (stage IV, stage V, or undergoing hemodialysis) who were newly initiated on either rivaroxaban or warfarin. Differences in baseline covariates between treatment cohorts were adjusted using inverse probability of treatment weighing. A total of 1896 rivaroxaban (38.7% received a dose < 20 mg/d), and 4848 warfarin treated patients were included with the majority of patients (88%) having stage 5 CKD or undergoing hemodialysis. Over a median follow‐up time of 1.4 years (0.6, 2.7 years), similar reductions were seen with rivaroxaban compared with warfarin for stroke/systemic embolism risk and ischemic stroke alone (Table 1). Major bleeding risk was significantly reduced among rivaroxaban‐treated patients versus warfarin (Table 2). Intracranial bleeding and gastrointestinal bleeding did not significantly differ with warfarin treatment (Table 2).49
A recent analysis utilizing Optum Deidentified EHR data from November 2011 to June 2018 also assessed the effectiveness and safety of newly initiated rivaroxaban compared with warfarin in nonvalvular AF patients with advanced CKD. Propensity score matching was used to adjust for potential difference in baseline characteristics. A total of 781 rivaroxaban‐treated patients were matched to 1536 warfarin‐treated patients, with most patients having stage IV CKD (81.3%). Among the rivaroxaban cohort, 60.1% were on the recommended 15‐mg dose for patients with renal impairment, whereas 21.1% were on a dose <15 mg and 14.7% were on the 20‐mg dose. Mean follow‐up period was 389 days for rivaroxaban patients and 370 days for warfarin patients. No statistically significant differences were found in the risk of ischemic stroke/systemic embolism (or major bleeding; Tables 1 and 2) between the rivaroxaban‐ and warfarin‐treated patients. Secondary analysis of major bleeding by sites (ie, gastrointestinal, intracranial, other major bleeding) were similar (Table 2).50
Future studies with larger sample sizes and longer follow‐up periods should be conducted to help to strengthen the health care communities understanding of the effectiveness and safety of rivaroxaban in patients with advanced/ESRD.
As with all studies, there are inherent limitations that are worth noting for real‐world evidence studies. Multiple biases, for example, misclassification, confounding, and sampling bias, are possible when conducting nonrandomized studies and may impact a study's internal validity.41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 Residual confounding because of unobserved or unmeasured covariates is also possible.41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Administrative claims and EHR studies cannot confirm if patients took their medication as prescribed.41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Several of the real‐world studies summarized above lacked laboratory data and therefore could not calculate eGFR to confirm renal dysfunction ICD diagnosis codes in both treatment cohorts as well as INR time in the therapeutic range for the patients treated with warfarin.41, 44, 45, 47, 49
Discussion
It is well established that both intrinsic (ie, genetic, physiologic, pathophysiologic) and extrinsic (ie, environment, concomitant medications and supplements) factors may impact a person's response to an intervention. These factors are typically assessed during phase 1 drug development in small healthy adult participant trials, at which time, if a significant signal of drug variability is observed, then those factors may be further explored in later phases 2 and 3 clinical trials and/or through population PK modeling. If the drug in question relies heavily on hepatic metabolism or renal elimination, studies conducted in individuals with various degrees of hepatic or renal disease are then conducted to determine the impact of the impairment on the pharmacology of the drug.
All direct oral anticoagulants currently marketed display some degree of renal elimination, with rivaroxaban and apixaban on the lower side with 36% and 27% eliminated, respectively. The changes in rivaroxaban PK and PD parameters associated with various degrees of renal impairment (from mild impairment to ESRD/dialysis) were established during clinical drug development. It was observed that as renal function declined, so did the renal clearance of the drug. This led to an increase in systemic rivaroxaban concentrations of 44%, 52%, 64%, and 56% in those with mild renal impairment, moderate renal impairment, severe renal impairment, and ESRD with intermittent dialysis, respectively.
Interestingly, participants with ESRD/dialysis have an increase in systemic exposure similar to that in those with moderate to severe renal impairment, suggesting a limit may be reached by the time someone has developed severe renal impairment, a trend also observed with the direct oral anticoagulant apixaban. The changes observed in these trials may be a consequence of the decline in active drug transport via permeability glycoprotein (P‐gp) in the kidneys, which helps to clear rivaroxaban. As such, the decline in these P‐gp transporters may reach a maximum before there is a complete loss of passive filtration. This is an important concept, as the ratio of active transport to passive filtration of unchanged rivaroxaban observed in development was about 3:1, indicating the relative predominance of active renal secretion in elimination of this drug.
Changes in exposure observed in these initial trials further supported the design of phase 3 RCT studies. For example, the effects of renal function were incorporated into the ROCKET‐AF trial that assessed the prevention of stroke and systemic embolism in nonvalvular AF patients. Although patients with either normal renal function (CrCl ≥80 mL/min) or mild renal impairment (CrCl ≥ 50‐79 mL/min) received a 20‐mg dose of rivaroxaban once a day, those with moderate renal impairment (CrCl 30 to 49 mL/min) received a dose reduction of 15 mg once a day. Upon completion of enrollment, 2950 (∼21%) of the patients were diagnosed at baseline with moderate‐renal impairment, a far greater number of patients than other currently marketed direct oral anticoagulants. Although those with renal impairment had higher rates of stroke and bleeding, it was irrespective of the study treatments assessed, and rivaroxaban displayed efficacy and safety similar to warfarin, the standard of care in the trial.
Although patients with an initial CrCl < 30 mL/min were excluded from the trial, the US Food and Drug Administration (FDA) allowed for dosing down to dialysis in nonvalvular AF patients based on exposure matching. That is, the changes in exposure observed in participants with either severe RI or on dialysis from the phase 1 trials were essentially the same as those values observed in nonvalvular AF patients with moderate renal impairment enrolled in the phase 3 ROCKET‐AF trial.
This was an important labeling change, not only to the rivaroxaban label, but to the other DOAC labels as well. Considering the similarity in study designs (for both clinical pharmacology and phase 3 studies) and the data obtained across the direct oral anticoagulants, the FDA went through a process of label harmonization regarding the renally impaired nonvalvular AF population. The result is that the direct oral anticoagulant labels now have similar recommendations for patients diagnosed with severe renal impairment or ESRD/dialysis with nonvalvular fibrillation. Specifically, for rivaroxaban, this harmonization led to further label modifications for other indications (VTE prophylaxis and treatment) when additional efficacy, safety and pharmacology data became available from later trials.
Although large randomized efficacy and safety clinical trials specifically conducted in patients with various degrees of renal impairment would be the preferred standard, such trials are notoriously difficult to enroll, conduct, and complete. Nor is it feasible to conduct a dedicated phase 3 clinical trial in every possible subpopulation. Hence, researchers and physicians rely on the abundance of real‐world evidence data available once the drug has been marketed. Although real‐world evidence data have inherent limitations and are not meant to replace the rigor of RCTs, the wealth and importance of EHR data should not be ignored or dismissed. These studies continue to inform the medical community of the effectiveness and safety of rivaroxaban in renally impaired patients, a message that is consistent with the results of the pivotal RCTs.
Conclusions
Based on the available evidence, which includes, pharmacological, efficacy, and safety data from pivotal RCTs and effectiveness and safety data from real‐world evidence studies, rivaroxaban appears to be a viable option for when anticoagulation is needed in patients who have CVD and renal impairment. However, as with any therapy, the benefits and risks of intervention should be carefully assessed and balanced. Patients treated with rivaroxaban for several of its approved indications should have their kidney function assessed prior to and during continued therapy to ensure their treatment is consistent with the current drug label.
Author Contributions
V.A., S.K., and K.T.M. contributed to the conceptualization and methodology of the study, data curation and formal analysis, and the writing, review, and editing of this manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
V.A., S.K., and K.T.M. are employed by Janssen Pharmaceuticals Inc. The development of this article was funded by Janssen Pharmaceuticals Inc.
Funding
Financial support for this publication was provided by Janssen Pharmaceuticals Inc.
Acknowledgments
Not Applicable.
References
- 1.United States Renal Data System . Chapter 4: Cardiovascular Disease in Patients with CKD. Ann Arbor, MI: United States Renal Data System; 2020. [Google Scholar]
- 2.Fujii H, Kono K, Nishi S.Characteristics of coronary artery disease in chronic kidney disease. Clin Exp Nephrol. 2019;23(6):725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125‐e151. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. CHEST guideline and expert panel report. Chest. 2016;149(2):315‐352. [DOI] [PubMed] [Google Scholar]
- 5.Lewis S, Glen J, Dawoud D, et al. Venous thromboembolism prophylaxis strategies for people undergoing elective total knee replacement: a systematic review and network meta‐analysis. Lancet. 2019;6(10):e530‐e539. [DOI] [PubMed] [Google Scholar]
- 6.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 7.Gerhard‐Herman MD, Gornick H.L, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):e71‐e126. [DOI] [PubMed] [Google Scholar]
- 8.PRADAXA® (Dabigatran Etexilate Mesylate) [Prescribing Information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2019. [Google Scholar]
- 9.SAVAYSA® (Edoxaban) [Prescribing Information]. Tokyo Japan: Daiichi Sankyo Co., LTD; 2020. [Google Scholar]
- 10.XARELTO® (Rivaroxaban) [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2020. [Google Scholar]
- 11.ELIQUIS® (Apixaban) [Prescribing Information]. Princeton, NJ: Bristol‐Myers Squibb Company; 2019. [Google Scholar]
- 12.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). 2010; Guidance for Industry Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling.
- 13.Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703‐712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias C, Moore KT, Murphy J, et al. Pharmacokinetics, pharmacodynamics, and safety of single‐dose rivaroxaban in chronic hemodialysis. Am J Nephol. 2016;43(4):229‐236. [DOI] [PubMed] [Google Scholar]
- 15.De Vriese AS, Caluwe R, Bailleul E, et al. Dose‐finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 16.Moore KT, Vaidyanathan S, Natarajan J. et al. An open‐label study to estimate the effect of steady‐state erythromycin on the pharmacokinetics, pharmacodynamics, and safety of a single dose of rivaroxaban in subjects with renal impairment and normal renal function. J Clin Pharmacol. 2014;54(12):1407‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203‐216. [DOI] [PubMed] [Google Scholar]
- 18.Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once‐ and twice‐daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100(3):453‐461. [PubMed] [Google Scholar]
- 19.Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep‐vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;504(10):675‐686. [DOI] [PubMed] [Google Scholar]
- 20.Girgis IG, Patel MR, Peters GR, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non‐valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014;54(8):917‐927. [DOI] [PubMed] [Google Scholar]
- 21.Xu XS, Moore K, Burton P, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74(1):86‐97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor xa inhibitor–in healthy subjects. Int J Clin Pharmacol Ther. 2007;45(6):335‐344. [DOI] [PubMed] [Google Scholar]
- 23.Willmann S, Coboeken K, Kapsa S, et al. Applications of physiologically based pharmacokinetic modeling of rivaroxaban‐renal and hepatic impairment and drug‐drug interaction potential. J Clin Pharmacol. 2021;61(5):656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenbagamurthi P, Heffner W, Thomas D, Krook M. An in vitro analysis of the effects of phosphate binders on the dissolution profile of rivaroxaban tablets. RRJPPS. 2017;6(4):12‐16. [Google Scholar]
- 25.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. [DOI] [PubMed] [Google Scholar]
- 26.Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and‐ systemic embolims with rivaroxaban compared with warfarin in patients with non‐valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387‐2394 [DOI] [PubMed] [Google Scholar]
- 27.Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On‐treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37‐47 [DOI] [PubMed] [Google Scholar]
- 28.Lindner SM, Fordyce CB, Hellkamp AS, et al. Treatment consistency across levels of baseline renal function with rivaroxaban or warfarin: a ROCKET AF (Rivaroxaban Once‐Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) analysis. Circulation. 2017;135(10):1001‐1003. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765‐2775. [DOI] [PubMed] [Google Scholar]
- 30.Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet. 2008;372(9632):31‐39. [DOI] [PubMed] [Google Scholar]
- 31.Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776‐2786. [DOI] [PubMed] [Google Scholar]
- 32.Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomized trial. Lancet. 2009;373(9676):1673‐1680. [DOI] [PubMed] [Google Scholar]
- 33.Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105(3):444‐453. [DOI] [PubMed] [Google Scholar]
- 34.The EINSTEIN, Investigators . Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499‐2510. [DOI] [PubMed] [Google Scholar]
- 35.The EINSTEIN, Investigators . Buller HR, Prins MH, Lensing AWA, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287‐1297. [DOI] [PubMed] [Google Scholar]
- 36.Bauersachs RM, Lensing AWA, Prins MH, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J. 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitz JI, Lensing AWA, Prins MH, Bauersachs R, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211‐1222. [DOI] [PubMed] [Google Scholar]
- 38.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 39.Fox KA, Eikelboom JW, Shestakovska O, et al. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction. J Am Coll Cardiol. 2019;73(18):2243‐2250. [DOI] [PubMed] [Google Scholar]
- 40.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994‐2004. [DOI] [PubMed] [Google Scholar]
- 41.Harel Z, Mamdani M, Juurlink DN, et al. Novel oral anticoagulants and the risk of major hemorrhage in elderly patients with chronic kidney disease: a nested Case‐Control Study. Can J Cardiol. 2016;32(8):986.e17‐e22. [DOI] [PubMed] [Google Scholar]
- 42.Weir MR, Berger JS, Ashton V, et al. Impact of renal function on ischemic stroke and major bleeding rates in nonvalvular atrial fibrillation patients treated with warfarin or rivaroxaban: a retrospective cohort study using real‐world evidence. Curr Med Res Opin. 2017;33(10):1891‐1900. [DOI] [PubMed] [Google Scholar]
- 43.Weir MR, Haskell L, Berger JS, et al. Evaluation of clinical outcomes among nonvalvular atrial fibrillation patients treated with rivaroxaban or warfarin, stratified by renal function. Clin Nephrol. 2018;89(5):314‐329 [DOI] [PubMed] [Google Scholar]
- 44.Vaitsiakhovich T, Coleman CI, Kleinjung F, et al. Clinical outcomes among nonvalvular atrial fibrillation patients with renal dysfunction treated with warfarin or reduced dose rivaroxaban. Eur Stroke J. 2019;4:180. https://www.morressier.com/article/clinical-outcomes-among-nonvalvular-atrial-fibrillation-patients-renal-dysfunction-treatedwarfarin-reduced-dose-rivaroxaban/5cb58ce9c668520010b55f01 [Google Scholar]
- 45.Nielsen PB, Skjøth F, Søgaard M, et al. Effectiveness and safety of reduced dose non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Lullo L, Tripepi G, Ronco C, et al. Safety and effectiveness of rivaroxaban and warfarin in moderate‐to‐advanced CKD: real world data. J Nephrol. 2018;31(5):751‐756. [DOI] [PubMed] [Google Scholar]
- 47.Chan YH, Lee HF, See LC, et al. Effectiveness and safety of four direct oral anticoagulants in asian patients with nonvalvular atrial fibrillation. Chest. 2019;156(3):529‐543. [DOI] [PubMed] [Google Scholar]
- 48.Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman CI, Kreutz R, Sood NA, et al. Rivaroxaban versus Warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132(9):1078‐1083. [DOI] [PubMed] [Google Scholar]
- 50.Weir MR, Ashton V, Moore KT, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV‐V chronic kidney disease. Am Heart J. 2020;223:3‐11. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi S, Salmon JW, Kong SX, et al. Administrative databases and outcomes assessment: an overview of issues and potential utility. J Manag Care Pharmacy. 1999;5(3):215‐222. [Google Scholar]


