Infants experience multiple stressors in the neonatal intensive care unit (NICU), which may have adverse effects on brain development. Non‐invasive interventions that reduce stress may improve neurodevelopmental functioning.1 This pilot study assessed whether live music therapy, performed by a certified neonatal music therapist,2 had beneficial effects on neurodevelopment. It focused on a convenience sample of 10 preterm and two full‐term infants admitted to the NICU at the Beatrix Children's Hospital, University Medical Center Groningen, The Netherlands, between March and July 2019. The parents provided written informed consent and the study was approved by the Medical Ethical Committee of the University (2020/646).
The infants received approximately 15 min of music therapy, tailored to their behavioural state. Infants in quiet sleep received calmly played music entrained to their breathing patterns. If they were in active sleep, quiet wakefulness or active states, the improvisation focused on muscle tension and breathing patterns. The musical interventions comprised an acoustic guitar, Ocean disc and voice. These were based on the Rhythm, Breath and Lullaby method, which aims to simulate womb sounds and reduce discomfort and stress.3 An Ocean disc is a round drum with metal balls that produces sounds as the therapist moves. When the guitar was used, the therapist used “Song of Kin” which employs parent‐selected songs modified to resemble a lullaby or the lullaby Twinkle Twinkle Little Star.3
We video recorded the infants in the supine position, for about 15–30 min, and provided the music therapy 1 h later. This process was repeated 1 h after the music therapy finished. The recordings were scored using the General Movement Optimality Score (GMOS), which is one of the most reliable methods for assessing early neurodevelopmental functioning.4 The assessors were blinded to whether the recording was before or after the therapy. The GMOS scores the characteristics of general movements separately for the neck and trunk, upper extremities and lower extremities and ranges from 5 to 42 points, with higher scores indicating better movement.4 We tested differences in the GMOS before and after therapy using the Wilcoxon signed rank test for paired data. Univariable linear regression analyses were performed to determine whether gestational age, being born small for gestational age, sex, postnatal age at recording and respiratory support were associated with change in (=delta) GMOS. The analyses were performed using SPSS for Windows version 26 (IBM Corp). p < 0.05 was considered statistically significant.
The 12 infants had a median gestational age of 26.7 weeks and interquartile range (IQR) of 26.5–28.7. They were videotaped at a median postnatal age of 31.9 weeks (IQR 30.9–36.9). We analysed a total of 32 recordings, 16 before and 16 after music therapy, because two infants were recorded twice and one was recorded three times. Of these, three had normal scores and 29 had abnormal scores: 28 had poor repertoire movements and one had cramped synchronised movements. The median GMOS before therapy was 21.0 (IQR 17.3–25.8) compared with 28.0 (IQR 23.5–32.3) after (p < 0.001). The median delta GMOS was 7.0 (IQR 4–8.8) and the mean was 5.8 ± 4.8. Of the 16 paired recordings, 13 improved in GMOS after music therapy, two deteriorated and one remained the same (Figure 1). We did not find any associations between the participants’ characteristics and delta GMOS, apart from respiratory support. Children receiving continuous positive airway pressure (CPAP) had a lower delta GMOS (B −6.86, 95% CI −11.16 to −2.56, p = 0.004) than infants with low‐ or high‐flow nasal cannulas or without respiratory support.
FIGURE 1.
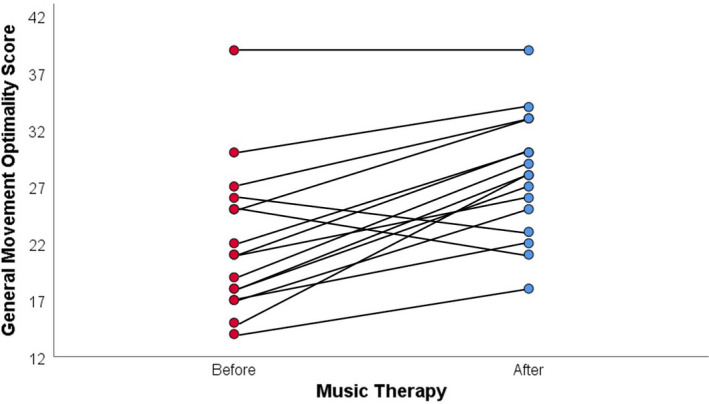
General Movement Optimality Score before and after music therapy.
There was a substantial improvement in GMOS after the music intervention and the only association between delta GMOS and the patient characteristics was respiratory support. We believe this improvement showed that the therapy was effective. It is important to consider why the GMOS scores deteriorated in two of the sessions. We previously reported on how feasible this musical intervention was for extremely and very preterm infants, and how well they tolerated it, and we did not observe overstimulation.5 One of the two infants with deteriorating scores was recorded three times. The first recording showed deteriorating scores, but improved scores in the subsequent recordings. Unfortunately, the other infant with deteriorating scores was not videotaped multiple times. It may be that music therapy improves neurological functioning over multiple sessions, which evidently requires further study. Respiratory support, particularly nasal CPAP, may interfere with improvements in delta GMOS. Smaller improvements during CPAP may reflect a lower physiologically stable state, with less energy to process music therapy, and interference from the device's background noise.
Music therapy may provide a beneficial non‐pharmacological and non‐invasive intervention during NICU stays. Further large‐scale studies are needed on any associations with improved neurodevelopment. Studies on longer term exposure would also be interesting, as the infant that was videotaped three times showed a clear improvement from the first to third session. We concluded that music therapy may be beneficial for infants’ neurodevelopment and could contribute to developmental care in NICUs.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Aucott S, Donohue PK, Atkins E, Allen MC. Neurodevelopmental care in the NICU. Ment Retard Dev Disabil Res Rev. 2002;8(4):298‐308. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DE, Patel AD. Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: might music have an impact? Dev Med Child Neurol. 2018;60(3):256‐266. [DOI] [PubMed] [Google Scholar]
- 3.Loewy J. NICU music therapy: song of kin as critical lullaby in research and practice. Ann N Y Acad Sci. 2015;1337(1):178‐185. [DOI] [PubMed] [Google Scholar]
- 4.Einspieler C, Marschik PB, Pansy J, et al. The general movement optimality score: a detailed assessment of general movements during preterm and term age. Dev Med Child Neurol. 2016;58(4):361‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dokkum NH, Jaschke AC, Ravensbergen A‐G, et al. Feasibility of live‐performed music therapy for extremely and very preterm infants in a tertiary NICU. Front Pediatr. 2020;8:581372. [DOI] [PMC free article] [PubMed] [Google Scholar]


