Abstract
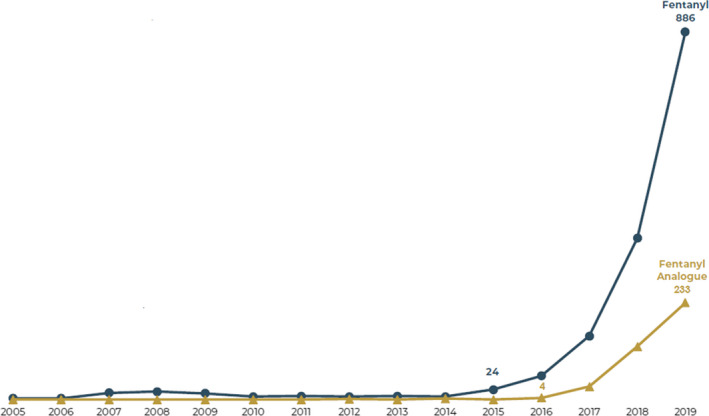
Fentanyl is now the primary driver of the current opioid crisis. Fentanyl and its analogues are subject to the Controlled Substances Act of 1970, the Controlled Substances Analogue Enforcement Act of 1986 (Federal Analogue Act), state laws, international treaties, and the laws of foreign countries. The appearance of novel psychoactive substances led to further legislative developments in scheduling. New fentanyl analogues proliferated in a manner previously unseen since about 2016. Overdose deaths of these fentanyl analogues prompted the Drug Enforcement Administration to reactively emergency schedule each new fentanyl analogue as it appeared. The international community also acted. Finally, on February 6, 2018, a proactive temporary (emergency) class‐wide scheduling of fentanyl‐related substances was implemented based upon the fentanyl core structure to save lives. This action spurred a similar action in China. Fentanyl analogues fell dramatically in the marketplace, despite further increases in fentanyl itself. Congress temporarily extended this scheduling, but it will soon expire. Opposition to permanent class‐wide was lodged due to concerns over law enforcement overreach, inadequate Health and Human Services input, and hindrance of research. This paper reaffirms the importance of a class‐based scheduling strategy while also arguing for increased research of schedule I controlled substances.
Keywords: fentanyl analogues, fentanyl‐related substances, scheduling, class‐wide scheduling, temporary scheduling, Controlled Substances Act
Highlights.
The DEA schedules drugs under the Controlled Substances Act.
Novel fentanyl analogues have been reactively scheduled through temporary emergency scheduling.
Proactive class‐wide scheduling of fentanyl‐related substances was instituted at home and abroad.
Class‐wide scheduling successfully reduced the emergence of new fentanyl analogues.
Current federal class‐wide scheduling of fentanyl‐related substances is set to expire.
1. INTRODUCTION
On December 29, 2017, the Drug Enforcement Administration (DEA) published a notice of intent to temporarily schedule fentanyl‐related substances (FRS), whether in existence or yet undiscovered, as schedule I substances under the Controlled Substances Act. This chemical structural class‐based approach appears, among other actions, to have effectively stopped the further development and production of new fentanyl analogues intended for the illicit drug market. The U.S. Congress extended the DEA’s temporary scheduling of fentanyl‐related substances to May 6, 2021. Congress should consider making such scheduling permanent rather than allowing the scheduling to expire. This article provides context for intelligent deliberation of such a change.
2. FENTANYL IN THE OPIOID CRISIS
The misuse and abuse of opiates continues to be a major crisis in America, and fentanyl is the primary driver [1]. Approximately 80% of overdose deaths involved opioids, and three quarters of these opioid overdose deaths involved illicitly manufactured fentanyls [2]. The current crisis began two to three decades ago with an increase of prescription opioid abuse. An increase in opioid‐dependent individuals and the subsequent demand for pharmaceutical opioids fueled a second wave of the opioid epidemic: a transition to heroin abuse. Illicit drug suppliers successfully exploited this demand by supplying inexpensive and higher purity heroin to the opioid‐dependent population. A third wave of the opioid epidemic began in 2012–2013 with the re‐introduction of synthetic opioids, particularly illicitly manufactured fentanyl [3, 4]. Fentanyl and fentanyl analogues, distributed in pure powder form, in powder mixtures, and as counterfeit pills, began to supplant heroin use in certain regions of the country around 2015 [5, 6]. By 2017, fentanyl and fentanyl analogues caused over 20,000 overdose deaths in the United States, accounting for half of all opioid‐related deaths and eclipsing all other overdose deaths [7].
Despite the rise of the coronavirus pandemic, drug overdose deaths have continued to increase, following a slight decrease in 2018 [8]. In the first half of 2020, the Miami‐Dade County Medical Examiner Department saw a 59% increase in the number of cases with illicitly manufactured fentanyls present compared to the first half of 2019. Fentanyl seizures were also up 59% in New York in 2020 [9]. Unfortunately, there is reason to believe that the number of overdoses may continue to escalate [10].
3. FENTANYL—HISTORICAL ORIGINS AND DEVELOPMENT
Fentanyl [11, 12, 13, 14], a schedule II substance under the CSA (21 U.S.C. § 812 Schedule II (b)(6)), has been illegally imported and has caused extensive mortality in the United States. In spite of this fact, it has a history of legitimate development and medical application. In 1953, Dr. Paul Janssen founded Janssen Pharmaceutica in Belgium [15]. Dr. Janssen recognized that the six member ring with a tertiary amine structure common to both morphine and meperidine was likely responsible for their analgesic properties. He also believed that greater lipid solubility would enhance the potency of a new drug. Janssen produced dozens of phenoperidine derivatives in the 1950s and then fentanyl in 1959. Janssen considered fentanyl to be useful only as an intravenous analgesic because the compound was rapidly destroyed after oral administration. Fentanyl was first used as intravenous analgesic in Europe in 1963 and in the United States in 1968. In 1968, McNeil Laboratories introduced fentanyl citrate in the United States under the tradename Sublimaze for anesthesia [16]. Lazanda, a fentanyl nasal spray, was approved by the Food and Drug Administration (FDA) for breakthrough pain in hospitalized cancer patients tolerant to opioids in 1968. Subsequently, high‐dose intravenous fentanyl was found to be useful for anesthesia. Intravenous fentanyl garnered FDA approval in 1972 for use during anesthesia and the peri‐operative period. Alza developed the transdermal fentanyl patch for chronic pain in the 1980s and Duragesic patches were approved by the FDA in 1990. Anesta introduced a transmucosal buccal lozenge (lollipop) which was approved in 1993 as an in‐hospital premedication for surgery. However, it was not successfully marketed until 1998 by the successor company, Cephalon, for quick‐acting relief of chronic breakthrough pain in or outside the hospital. Soon, numerous companies began selling fentanyl products. In addition to nasal sprays, newer products have been developed for buccal administration in 2006 and sublingual administration in 2011.
Fentanyl is a narcotic opioid that has legitimate medical uses for anesthesia and analgesia, but was recognized from the outset as having a high potential for abuse. Fentanyl is a μ‐opioid receptor agonist that produces euphoric effects and can serve as a direct pharmacological substitute for heroin in opioid‐dependent individuals. Like other narcotic opioids, fentanyl may cause profound and dangerous respiratory and central nervous depression, as well as miosis and constipation. Fentanyl is approximately 50–100 times more potent than morphine and 30–50 times more potent than heroin. The potency of fentanyl and of many fentanyl analogues causes extreme respiratory depression upon ingestion. It can produce immediate death in many circumstances, and in many cases, overdose victims require multiple administrations of the reversal agent naloxone following application.
The misuse and abuse of fentanyl citrate dates back to the 1980s. Fentanyl abuse was initially a result of diversion from the regulated, closed system of distribution tracked by the DEA. Abuse of transdermal patches began shortly after their introduction in the 1990s. While non‐medical use of fentanyl transdermal patches often caused death when abused, the relative lack of availability of diverted transdermal patches and fentanyl substances limited the usage and attendant mortality.
Although an opioid narcotic, fentanyl is not derived from opium, but is instead chemically synthesized in the laboratory. The original Janssen method of production, patented in 1964 (U.S. Patent US3141823A), is a process that uses N‐benzyl piperidone as a starting material. An alternative synthetic method, commonly referred to as the Siegfried method, was published [17, 18] and later shared on the internet. This method uses N‐phenethyl‐4‐piperidone (NPP) and 4‐anilino‐N‐phenethylpiperidine (4‐ANPP) as key precursors and was used in clandestine laboratories to illicitly synthesize fentanyl in the early 2000s.
4. DRUG CONTROL AND SCHEDULING
The U.S. federal government was not involved with drug enforcement until the 20th Century.[19] The FDA was established in 1906 after Upton Sinclair published his novel, The Jungle, in 1906, describing the appalling practices of the Chicago stockyards. The FDA was tasked to regulate not only food, but also the manufacture and distribution of drugs. The Harrison Narcotics Act of 1914 required registration of importers, manufacturers, and distributors of cocaine and opium. The 1951 Boggs Act and the 1956 Narcotic Control Act increased penalties for drug offenses.
Enforcement was primarily through local law enforcement until the Prohibition Era when the Federal Bureau of Narcotics (FBN) was established within the Department of the Treasury. The Federal Bureau of Narcotics lacked resources and gravitas due to inadequate support and funding in the period of recovery after the Great Depression. In response to a President's Commission on Narcotic and Drug Abuse, Congress promulgated the FDA Bureau of Drug Abuse Control (BDAC) in 1966, which allowed for rehabilitation and civil commitment of certain drug addicts. In 1968, the FBN and BDAC were merged into a Bureau of Narcotics and Dangerous Drugs (BNDD) and transferred to the Department of Justice (DOJ). In 1973, the BNDD was renamed the DEA.
Approximately two hundred drug laws were consolidated into the Controlled Substance Act (CSA, 21 U.S.C. § 801, et. seq.), which was enacted in 1970 (Pub. L. 91‐513) and fulfills the U. S. commitment to international drug control treaties [20, 21, 22]. The act begins with the acknowledgment that “Many of the drugs included within this title have a useful and legitimate medical purpose and are necessary to maintain the health and general welfare of the American people.” The CSA establishes a closed system of distribution for controlled substances in the sense that the distribution is restricted and can be tracked from source to user—everyone who handles controlled substances must be registered, assigned a unique DEA number, and must maintain complete and accurate inventories and records of transactions [23].
The CSA establishes a five‐tier schedule of drugs, based upon an eight‐factor analysis:
Its actual or relative potential for abuse.
Scientific evidence of its pharmacological effect, if known.
The state of current scientific knowledge regarding the drug or other substance.
Its history and current pattern of abuse.
The scope, duration, and significance of abuse.
What, if any, risk there is to the public health.
Its psychic or physiological dependence liability.
Whether the substance is an immediate precursor of a substance already controlled under this subchapter.
Schedule I drugs have no accepted medical use as determined by the U.S. FDA, a high potential for abuse, and cannot be safely prescribed, but other scheduled drugs may be prescribed by an appropriate medical provider and dispensed by a pharmacist. Changes to the drug scheduling can be initiated by the DEA, the FDA, or by petition and executed by the DEA in consultation with the FDA. Immediate precursors to an already scheduled drug can also be placed on the same schedule or higher schedule. The DEA implements and enforces the CSA.
The CSA (§ 802(14)) defines “controlled substance” as, “a drug or other substance, or immediate precursor, included in schedule I, II, III, IV, or V of part B of this subchapter. The term does not include distilled spirits, wine, malt beverages, or tobacco, as those terms are defined or used in subtitle E of the Internal Revenue Code of 1986.”
It seems apparent that when the CSA was originally promulgated, the drafters had individual drugs in mind, rather than entire classes of drugs, but did include language for derivatives of schedules I, II, and III. Specifically, § 812(c)(a) listed an initial 42 substances as schedule I and stated, “Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation.” Congress has since modified the schedules to include classes and groups of substances, anabolic steroids, and synthetic cannabinoids, respectively.
Fentanyl is a schedule II substance because it has a high potential for abuse, has a currently accepted medical use in treatment in the United States, and abuse of the drug or other substances may lead to severe psychological or physical dependence. The CSA refers to fentanyl by its chemical name, N‐phenyl‐N‐[1‐(2‐phenylethyl)‐4‐piperidinyl] propanamide (see, e.g., 21 U.S.C. §841(b)(1)(A)(vi) (2012). In response to the illicit manufacturing and trafficking of fentanyl analogues, Congress provided the temporary scheduling authority in 1984 and the Federal Analogue Act in 1986.
5. INTERNATIONAL CONTROL
Section “d” of the CSA acknowledges the additional ability to schedule drugs based upon international treaties, conventions, and protocols. In fact, the CSA was promulgated in large part to fulfill treaty obligations. Currently, three major international drug control treaties are in force [24, 25, 26, 27]. The U.S. Secretary of State is to notify the U.S. Attorney General of international scheduling efforts for federal scheduling consideration.
The Single Convention on Narcotic Drugs of 1961 [28, 29] was promulgated to consolidate, replace, and update nine predecessor treaties. Earlier treaties that dealt with opium, coca, and derivatives such as morphine, heroin, and cocaine; the Single Convention also included new synthetic opioids and cannabis. Moreover, the Single Convention established a mechanism to schedule drugs apart from including specific drugs in treaties, thus allowing a more responsive and uniform listing. Scheduling responsibility was given to the United Nations (UN) Economic and Social Council's Commission on Narcotic Drugs (CND) based upon recommendations and findings of the World Health Organization (WHO) and the International Narcotics Control Board (INCB) established as an independent, quasi‐judicial expert body for monitoring and supporting government compliance with the international drug control treaties. The UN Office on Drugs and Crime (UNODC) is an operational agency that monitors global drug abuse and assists law enforcement efforts. The treaty depends upon the laws and machinery of individual participating countries; the UN has worked with countries to achieve a remarkable worldwide uniformity of laws. The Single Convention went into effect in 1964, and the treaty went into force in 1975. There are currently 186 state parties, including the United States as of 1972.
The Convention on Psychotropic Substances of 1971 [30] supplements the Single Convention to establish comparable control of stimulants, depressants, and hallucinogens not covered by the Single Convention. The treaty went into effect in 1976. There are currently 184 state parties, including the United States as of 1980.
The United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 [31, 32] enhances international enforcement efforts, including strengthening provisions against money laundering and authorizing asset forfeiture. Moreover, it establishes a system of drug precursor regulation, listing 23 substances based upon the list of European Union‐controlled precursors. The treaty became effective as of 1990. There are currently 191 states are members, including the United States as of 1990.
Fentanyl, itself, has been internationally controlled, under the 1961 Single Convention, since 1964 and several fentanyl analogues and precursors have been brought under international control [33].
6. THE EMERGENCE OF FENTANYL ANALOGUES
There are two types of drug analogues. A chemical analogue has a similar structure to that of another compound, while a functional analogue is a compound with a very different structure yet has similar physical, biochemical, or pharmacological properties [34]. A chemical derivative is a modification of a drug's chemical structure. Positional isomers are molecules composed of the same atomic elements (compounds with the same molecular formula but variations in the position of structural elements), and stereoisomers are two chemicals with the same structural elements but with differences in orientation (similar to your left and right hands). The definitions of the CSA (§ 802(14)) define “isomer” as “the optical isomer, except as used in schedule I(c) and schedule II(a)(4). As used in schedule I(c), the term "isomer" means any optical, positional, or geometric isomer. In schedule II(a)(4), the term "isomer" means any optical or geometric isomer.”
The CSA (§ 802) gives a legal definition for “controlled substance analogue” as “a substance—(i) the chemical structure of which is substantially similar to the chemical structure of a controlled substance in schedule I or II; (ii) which has a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II; or (iii) with respect to a particular person, which such person represents or intends to have a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II. …”.
Not long after the development of fentanyl, other prescription fentanyl analogues became available, such as carfentanil, sufentanil, and thiafentanil. As part of the FDA drug approval process, they were scheduled and made available for medical use. Other fentanyl analogues have no currently recognized medical use. Collectively they are called fentanyl‐related substances (FRS). Kemp Chester noted that the U.S. Department of Homeland Security has estimated that there are about 3,024 potential fentanyl derivatives that might be synthesized [35].
A chemical analogue is technically a different chemical than the controlled substance that it resembles; hence, regulations specific to that substance may not apply to its analogues. Thus, a non‐pharmaceutical manufacturer can design and legally produce a chemical analogue of a controlled substance, despite a similar structure and pharmacologic function. This is done precisely to evade traditional chemical‐specific regulations. The term “designer drug” was coined in 1984 and popularly applied to such chemical analogues, but the preferred technical term is a “novel psychoactive substance” (NPS) [36, 37].
Beginning in 1979, deaths began to occur in Orange County, California from “China White,” that was eventually determined to be alpha‐methylfentanyl, a simple chemical analogue of fentanyl and twice as potent as fentanyl [38, 39]. Because the analogue was not specifically listed on the DEA's list of restricted drugs, it was legal and continued to be a legal substance until 1982 when it was classified as a schedule I drug. In 1984, alpha‐methyl acetylfentanyl (contaminated with the acryl analogue) and 3‐methylfentanyl appeared among overdose deaths. The fentanyl analogues largely disappeared by 1985. The decline may have been attributable to the arrests in 1985 and 1986 of organic chemists from legitimate East Coast laboratories. The outbreak resulted in 110 fentanyl‐related overdose deaths primarily in California from over 10 illicit fentanyl analogues. The outbreak made it clear that synthetic chemical analogues would be a significant challenge going forward.
7. TEMPORARY SCHEDULING
The Dangerous Drug Diversion Act of 1984, part of the sweeping Comprehensive Crime Control Act of 1984 (P.L. 98‐473), amended the CSA to, among other things, create a mechanism for temporary control of a substance as schedule I “to avoid an imminent hazard to public safety” (21 U.S.C. § 811 (h)). Thus, the Attorney General was given the emergency authority to ban an uncontrolled substance with no accepted medical use that was being abused and was a risk to public health while formal scheduling procedures were conducted. This temporary scheduling was considered an interim measure while further information on the eight‐factor analysis was gathered by the FDA for permanent scheduling. Originally, this temporary scheduling lasted for one year with a possible extension of up to six months, but these times were doubled in 2012 allowing for a maximum period of three years.
Temporary scheduling of synthetic drugs has been and continues to be a key tool in combating new synthetic drugs as they emerge and interdicting these substances at ports of entry [40]. When NPSs appear and medical examiner and coroner offices report overdose deaths, this information can then be used as the finding of the “imminent hazard to public safety” for the emergency declaration. The result of this process has been a cat‐and‐mouse or whack‐a‐mole game between law enforcement and illicit drug manufacturers, in which new analogues are created as old analogues become regulated. Law enforcement and regulators played catch‐up while many recreational drug users died.
According to Amanda Liskamm of the DOJ has reported that during the first 25 years, from 1985 to 2010, the DEA utilized this temporary scheduling authority 13 times to control 25 substances, but from March 11, 2011, through January 28, 2020, the DEA utilized this authority on 24 occasions to place 74 synthetic drugs into schedule I [41, 42].
8. CONTROLLED SUBSTANCES ANALOGUE ACT (THE FEDERAL ANALOGUE ACT)
Meanwhile, in response to the highly publicized development of Parkinson's disease caused by an impurity during the intentional synthesis of a non‐fentanyl morphine analogue [43], the U.S. Congress passed the Controlled Substances Analogue Act of 1986 (The Federal Analogue Act, P.L. 99‐570), which amended the CSA (21 U.S.C. § 813) to prohibit innovative drugs that are not yet listed as controlled substances [44, 45]. The Federal Analogue Act provides that if a jury finds that a drug that is “substantially similar” to a controlled substance listed in schedule I or II, a “controlled substance analogue,” is to be treated as a schedule I substance, if intended for human consumption. Exempted from treatment under the analogue provision is substances subject to an Investigational New Drug Approval. Since such analogues are not developed for medicinal purposes, they are not controlled, but instead outlawed as schedule I substances. Specifically:
A controlled substance analogue shall, to the extent intended for human consumption, be treated, for the purposes of any Federal law as a controlled substance in schedule I.
A “controlled substance analogue” is defined in the statute (§ 802(32(A))):
-
“(32) (A) Except as provided in subparagraph (C), the term ‘controlled substance analogue’ means a substance—
the chemical structure of which is substantially similar to the chemical structure of a controlled substance in schedule I or II;
which has a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II; or
with respect to a particular person, which person represents or intends to have a stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II.
…
-
Such term does not include—
a controlled substance;
any substance for which there is an approved new drug application; with respect to a particular person any substance, if an exemption is in effect for investigational use, for that person, under section 505 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 355) to the extent conduct with respect to such substance is pursuant to such exemption; or
any substance to the extent not intended for human consumption before such an exemption takes effect with respect to that substance.”
Thus, the legislation would seem to read that the NPS be substantially similar in chemical structure to a controlled substance, substantially similar in function to a controlled substance, or intended for use or represented by a distributor or dealer to be used as an illicit recreational drug rather than for medicinal purposes and that the key question is what constitutes substantial similarity.
In United States v. Forbes, 806 F. Supp. 232 (D. Colo. 1992), the federal district court was confronted with the question of whether alpha‐ethyltryptamine (AET) was substantially similar to dimethyl tryptamine (DMT) and diethyl tryptamine (DET), both schedule I substances (Figure 1). The Forbes court interpreted the Analogue Act to require parts A(i) and either A(ii) or A(iii) and specifically rejected the government's position that only one of the three legs of Section A be met. The court found no scientific consensus on whether AET's chemical structure is substantially similar to DMT or DET and noted that even some of the government's experts recognized the diversity of opinion on this point. Although they share a structural family root, the tryptamine family, AET is a primary amine while DMT and DET are tertiary amines and AET cannot be derived from DMT or DET molecules. Although they all produce some degree of hallucinogenic and stimulant activity, it was argued that AET does not have an effect on the central nervous system that is substantially similar to DMT or DET and in fact, it affects the central nervous system through different mechanisms. The federal court judge concluded, “I hold that the definition of controlled substance analogue as applied to AET under the unique facts here is unconstitutionally vague. Without doubt, it provides neither fair warning nor effective safeguards against arbitrary enforcement.”
FIGURE 1.
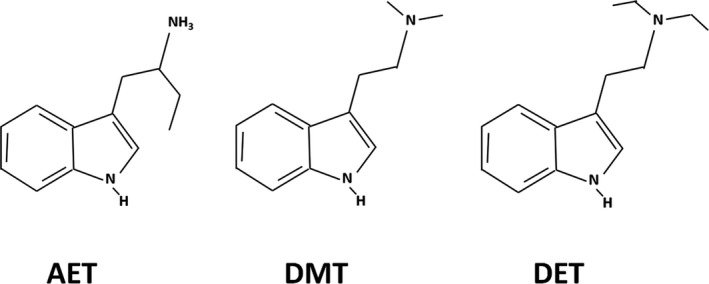
Chemical structures of AET, DMT, and DET
In United States v. Washam, 312 F.3d 926 (2002), the eighth judicial circuit considered whether 1,4‐butanediol (BD) was a controlled substance analogue of the rave dance and date‐rape drug gamma‐hydroxybutyric acid (GHB), a schedule I depressant (Figure 2). The Washam court upheld the Forbes interpretation of the statute to require a two‐prong test to find a substance to be a controlled substance analogue. Although BD and GHB contain different functional groups, the court ruled that they had a substantially similar chemical structure as they “are both linear compounds containing four carbons and that there is only one difference between the substances on one side of their molecules” and that BD is converted into GHB in the body and thus produces the same physiological effects. The court rejected the argument that the Federal Analogue Act was unconstitutionally vague in this case on the grounds that the defendant's actions in concealing her activities and lying to DEA agents showed that she knew her actions were illegal, and furthermore that “…a person of common intelligence has sufficient notice under the statute that 1,4‐Butanediol is a controlled substance analogue.” Thus, the court concluded, “The Analogue Statute is not void for vagueness as applied to 1,4‐Butanediol because Congress provided adequate notice of the proscribed conduct and prevented arbitrary enforcement through the terms of the statute.”
FIGURE 2.
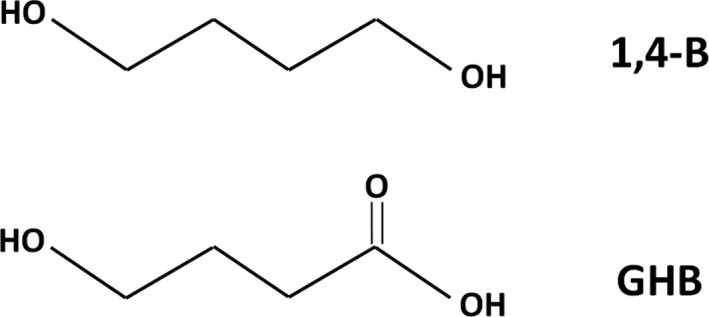
Chemical structures of BD and GHB
The reading of these two cases suggests that the statute must be tested on a case‐by‐case basis to determine whether substantial similarity can be determined or not. Moreover, these cases clearly illustrate the difficulty in trying to prosecute cases under the Federal Analogue Act. Although fentanyl analogues for the most part share a common chemical structural backbone, whether their structure is substantially similar, under the analogue law, is often debated by subject matter experts in a court of law. Studies to determine whether each of these analogues has a similar or greater stimulant, depressant, or hallucinogenic effect on the central nervous system rarely exist, nor are they required, creating the need for experts to provide and justify their opinions in court.
9. THE EMERGENCE OF NON‐FENTANYL NPS
The first synthetic cannabinoids (“spice” and “K2”) were reported in 2008 and 2009, but an expanding array of other synthetic cannabinoids followed. Synthetic cathinones (“bath salts”) began to appear as well. The Office of National Drug Policy (ONDCP) recognized these compounds as the new synthetic drug threat, but did not include any fentanyl analogues [46]. Prior to 2010, synthetic cannabinoids were not controlled by any State or at the Federal level. The Synthetic Drug Abuse Prevention Act of 2012 (P.L. 112‐144) permanently scheduled 26 synthetic cannabinoids and cathinones as schedule I. Specifically, it defined “cannabimimetic agents” to include any substance that is a cannabinoid receptor type 1 (CB1 receptor) agonist as demonstrated by binding studies and functional assays within any of the following [five] structural groupings…” and then added fifteen specific cannabinoids and ten specific cathinones and phenethylamines. This act also doubled the maximum period of time that the DEA can administratively schedule substances under its emergency scheduling authority to a possible three years. The act was criticized as out‐of‐date as it was passed because it did not cover all possible analogues [47, 48].
10. THE RE‐EMERGENCE OF FENTANYL ANALOGUES
Increases in illicit fentanyl began in 2014 and in illicit fentanyl analogues since 2016 and both have dramatically escalated since (Figure 3). The illicit fentanyl and FRS were often compressed into counterfeit pills or mixed with heroin, and later also in pure form. A constantly evolving list of fentanyl analogues began to appear, such as acetyl fentanyl, furanyl fentanyl, and cyclopropyl fentanyl, as well as other novel synthetic opioids, such as U‐47700 [49, 50, 51]. For a period of time, novel drugs would appear at a rate of approximately one every two weeks. As new unscheduled fentanyl analogues appeared on the market, deaths would follow [52, 53].
FIGURE 3.
Number of federal fentanyl and fentanyl analogue trafficking offenders over time prior to the Class‐wide FRS scheduling (Tennyson KM, Ray CS, Maass KT. Fentanyl and fentanyl analogues: federal trends and trafficking patterns. U.S. Sentencing Commission. Jan, 2021. p. 3. https://www.ussc.gov/research/research‐reports/fentanyland‐fentanyl‐analogues‐federal‐trends‐and‐trafficking‐patterns (accessed February 2, 2021) [Colour figure can be viewed at wileyonlinelibrary.com]
Standard toxicology testing such as gas chromatography mass spectrometry (GC‐MS) and liquid chromatography tandem mass spectrometry (LC‐MS/MS) targets certain known drugs and will miss novel substances. Detection and identification of novel compounds are aided by non‐targeted testing with newer instrumentation (high‐resolution time‐of‐flight mass spectrometry (HR‐TOF‐MS) that is unavailable in many laboratories. Thus, particularly early on, these fentanyl analogues were under‐reported [54].
These new fentanyl analogues would often appear in very pure pharmaceutical grade. Most FRS was manufactured in China and transported through the mail or through Mexican and Canadian distributors [55, 56, 57].
From 1981 to 2017, 19 different fentanyl analogues along with two other synthetic opiates, AH7921 and U‐47700 were scheduled by the DEA. At present, there are nearly three dozen FRS specifically scheduled (Table 1) [58].
TABLE 1.
Current list of specifically scheduled fentanyl‐related substances (DEA “Orange Book” https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf (accessed February 2, 2021)
| Listed as schedule I | Listed as schedule I | Listed as schedule II |
|---|---|---|
| acetyl fentanyl | β‐hydroxyfentanyl | alfentanyl |
| acetyl‐α‐methylfentanyl | β‐hydroxy−3‐methylfentanyl | carfentanil |
| acryl fentanyl | β‐hydroxythiofentanyl | fentanyl |
| butyryl fentanyl | isobutyryl fentanyl | norfentanyl |
| ρ‐chlororisobutyryl fentanyl | methoxyacetyl fentanyl | remifentanil |
| crotonyl fentanyl | ρ‐methoxybutyryl fentanyl | sufentanil |
| cyclopentyl fentanyl | α‐methylfentanyl | thiafentanil |
| cyclopropyl fentanyl | 3‐methylfentanyl | |
| ρ‐fluorobutyryl fentanyl | α‐methylthiofentanyl | |
| 2‐fluorofentanyl | 3‐methylthiofentanyl | |
| ρ‐fluorofentanyl | ocfentanil | |
| 4‐fluoroisobutyryl fentanyl | tetrahydrofuranyl fentanyl | |
| furanyl fentanyl | thiofentanyl | |
| valeryl fentanyl |
11. INTERNATIONAL CONTROL OF FENTANYL ANALOGUES
Although the presence of fentanyl and fentanyl analogues was a problem that occurred predominantly in the United States, the international community also mobilized a response [59]. For example, in 2017, China scheduled carfentanil, and seven other fentanyl‐related substances. The fentanyl analogues sufentanil, alfentanil, and remifentanil are United Nations schedule I drugs, but are schedule II in the United States. Successful control of individual analogues is reflected in the marketplace (Figure 4).
FIGURE 4.
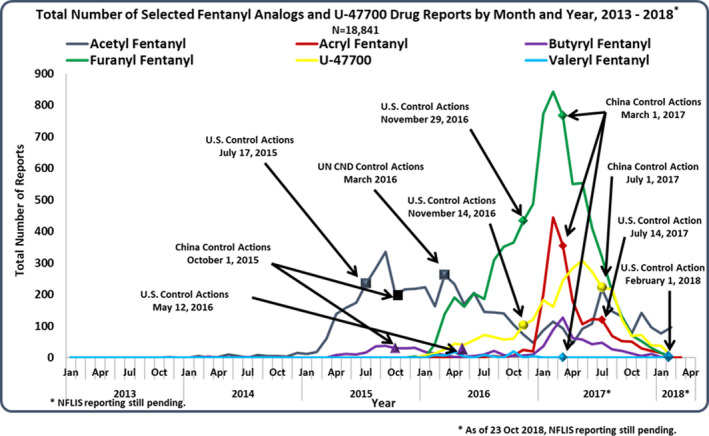
Marketplace responses to specific U.S. and China fentanyl analogue control actions prior to the class‐wide FRS scheduling (DEA Drug and Chemical Evaluation section) [Colour figure can be viewed at wileyonlinelibrary.com]
12. STATE SCHEDULING
States have drug laws to enable state and local drug interdiction efforts. In fact, the majority of drug crimes are handled at the state and local level. The states have long followed the drug scheduling of the federal government and, in fact, many if not most have a rule in place requiring their law to immediately comply with federal drug law changes. The power to schedule compounds variously falls to the State Attorney General, the Board of Pharmacy, or a Controlled Substances Board that advises the legislature.
However, localized outbreaks sparked state‐level responses [60, 61, 62]. For example, Ohio experienced a localized outbreak of acryl fentanyl in 2017 with 1213 reports, while neighboring states only reported a small fraction of that level, and many states west of the Mississippi River had no reports. In that same year, Kentucky experienced a dramatic increase in cyclopropyl fentanyl cases with neighboring states not experiencing the same effect. Moreover, given the historic delays in federal fentanyl analogue scheduling and facing mounting overdose deaths, state legislatures began looking for ways in which they could become proactive, rather than merely reacting to individual drugs as they appear in the marketplace.
The National Alliance for Model State Drug Laws (NAMSDL) first promulgated its Model Scheduling New/Novel Psychoactive Substances Act in 2014 and its third edition in 2019 [63]. Section IX (after DEA’s Class Control Action) covers fentanyl derivatives. It schedules derivatives of a 4–anilidopiperidine core structure, but specifically excludes Alfentanil, Carfentanil, Fentanyl, and Sufentanil and specifically includes 23 fentanyl analogues.
Beginning in the mid‐2010s, states did, in fact, begin to promulgate laws based upon the chemical structure of drug families and included in these laws the chemical substitutions that could be made to proactively incorporate them into their scheduling. A determination of functional similarity was not required. Arkansas took the unique step of adding a sixth schedule for drugs that do not properly fit into other schedules [64]. In so doing, these states got in front of the federal government's response. Specifically, chemical structural scheduling was adopted in Arizona, Florida, Georgia, Kentucky, North Dakota, New York, and Pennsylvania to control “fentanyl derivatives.” Different control mechanisms were employed by each legislature, but each state law defined a fentanyl core structure and the substitutions to that structure that remain controlled. In addition to defining the controlled structure and substitutions, Florida and North Dakota also list more than 20 individual analogues as examples to facilitate interpretation.
13. FEDERAL CORE STRUCTURE SCHEDULING
The DEA issued a press release November 9, 2017, indicating that they intended to take immediate action to emergency schedule all fentanyl analogues based upon core structure of the chemical derivative so that it would no longer be necessary to wait to see if the analogue would cause deaths [65]. Prior to this announcement, the DEA had consulted with the Department of Health and Human Services (HHS), which responded that they had no objection, although they did not produce an eight‐factor analysis as a class [66]. This proactive proposal was formally announced in the Federal Register on December 29, 2017 [67]. A question arose as to whether the CSA permitted class scheduling because it spoke in the singular terms “controlled substance” and “analogue,” and not in the plural. Nonetheless, the final rule was issued in the Federal Register on February 6, 2018 [68].
This class‐wide FRS scheduling was considered a novel approach, but, as noted above, cathinone scheduling based upon chemical structure had been legislated and legislation similar to the federal FRS scheduling had been advocated by NAMSDL and enacted in several states.
The DEA defines fentanyl analogues based on structural modifications to five specific regions of the molecule [69]. These include the following: (A) the phenyl portion, (B) the phenethyl group, (C) the piperidine ring, (D) the aniline ring, and (E) the N‐propionyl group (Figure 5).
FIGURE 5.
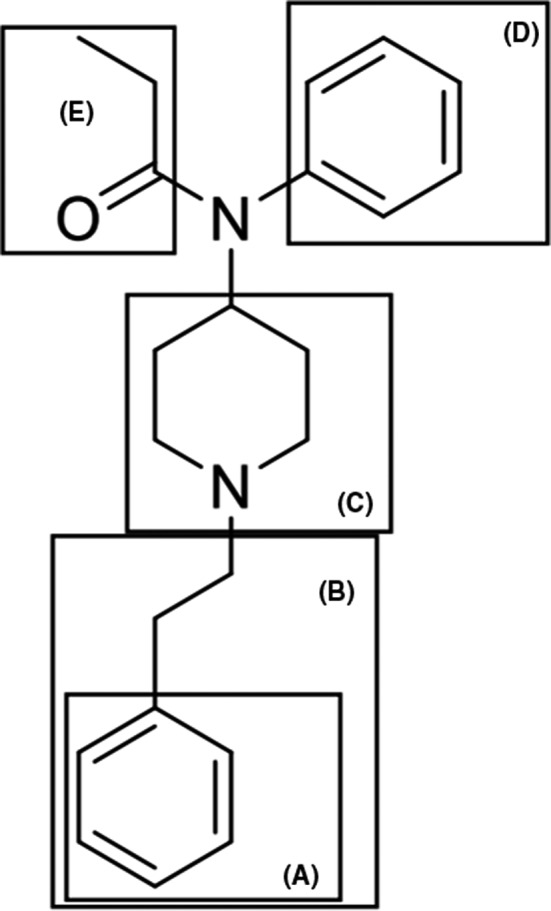
Regions of the chemical structure of fentanyl described in the definition of a fentanyl‐related Substance (Fentanyl‐related substances, DEA, May 2020. https://www.deadiversion.usdoj.gov/drug_chem_info/frs.pdf (accessed February 2, 2021)) [Colour figure can be viewed at wileyonlinelibrary.com]
Despite this class scheduling effort, the DEA continues to individually schedule FRS during the period of temporary class control. Core structure scheduling has been adopted by several states and by China, forbidding the manufacture of fentanyl analogues. In 2018, the appearance of new fentanyl analogues died away. Thus, this scheduling effort has been highly effective.
The 2018 amendments to the U.S. Sentencing Commission guidelines define “fentanyl analogue” as “any substance (including any salt, isomer, or salt of isomer thereof), whether a controlled substance or not, that has a chemical structure that is similar to fentanyl (N‐phenyl‐N‐[1‐(2‐phenylethyl)‐4‐piperidyl] propanamide)” [70]. The Sentencing Commission declares that because the CSA defines “analogue,” the sentencing guidelines definition may exclude scheduled substances for fentanyl analogues. Gerbasi notes that this “clarification is particularly important given the DEA’s temporary scheduling of fentanyl related substances” [71].
The DEA’s temporary class‐wide scheduling was a catalyst to international counterparts elsewhere. On April 1, 2019, China announced the class‐wide control of fentanyl‐related substances, effective May 1, 2019 [72]. Temporary core structure scheduling coupled with the Chinese scheduling action directly resulted in a substantial drop in Chinese‐origin FRS encountered in the United States by 2019 [73]. The downturn in FRS is reflected in the NFLIS (Figure 6) and medical examiner data (Tables 2 and 3).
FIGURE 6.
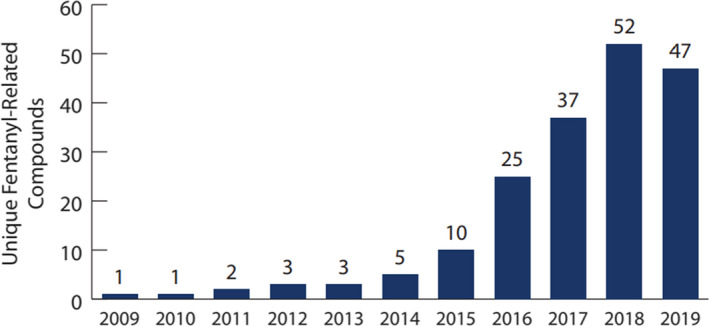
Number of unique fentanyl‐related compounds in NFLIS‐Drug in 2009‐2019, documenting the beginnings of the decline in FRS by number of different substances after temporary class‐wide scheduling (Tracking Fentanyl and Fentanyl‐Related Compounds Reported in NFLIS‐Drug, by State: 2018‐2019. Dec 2020. NFLIS, DEA. https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLISDrugSpecialRelease‐Fentanyl‐FentanylSubstancesStateMaps‐2018‐2019.pdf (accessed February 2, 2021))
TABLE 2.
Appearance of fentanyl and FRS in Miami‐Dade Medical Examiner decedents by year (mixtures reflected in the total) (courtesy of Liz Zaney)
| Year | Fentanyl cases | Fentanyl analogue cases | Total Cases containing either Fentanyl and/or Fentanyl Analogues |
|---|---|---|---|
| 2014 | 13 | 0 | 13 |
| 2015 | 83 | 13 | 86 |
| 2016 | 162 | 172 | 281 |
| 2017 | 162 | 194 | 251 |
| 2018 | 153 | 96 | 178 |
| 2019 | 247 | 130 | 257 |
| 2020 | 310 | 43 | 316 |
| 2021* | 12 | 1 | 12 |
For deaths through January 11, 2021.
TABLE 3.
Appearance of specific FRS in Miami‐Dade Medical Examiner decedents by year (courtesy of Liz Zaney)
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021* | |
|---|---|---|---|---|---|---|---|
| B‐Hydroxythiofentanyl | 8 | 1 | |||||
| Acetylfentanyl | 5 | 12 | 7 | 29 | 87 | 33 | 1 |
| Valerylfentanyl | 17 | 1 | |||||
| Carfentanil | 135 | 70 | 5 | 1 | 2 | ||
| Para‐fluoroisobutyrylfentanyl | 27 | 28 | 3 | 4 | |||
| Furanylfentanyl | 41 | 24 | 3 | 1 | |||
| Cyclopropyfentanyl | 89 | 47 | 2 | ||||
| Methoxyacetyl fentanyl | 30 | 34 | 26 | 4 | |||
| Other FRS | 1 | 3 | 1 | 17 | 6 | 1 |
For deaths through January 11, 2021.
14. ALTERNATIVE SCHEDULING SCHEMES
Scheduling under the Controlled Substances Act of 1970 is based upon an investigation of individual substances. The Federal Analogue Act of 1986 outlaws chemicals that are chemically and functionally (or represented to be) “substantially similar” to schedule I and II substances. The Synthetic Drug Abuse Prevention Act of 2012 controls “cannabimimetic agents” within specified structural classes and was based on receptor binding studies and functional assays. The scheduling of FRS in 2017 was based upon a structural definition from the scientific and patent literature. Thus, the federal government has attempted to move from reactive individual substance scheduling to some type of proactive class scheduling [74].
Congress could modify the Federal Analogue Act to drop the functional similarity requirement, such that a drug need only be chemically similar. Such would still beg the question of how to determine “substantial similarity.” Such structural similarity could be quantitatively determined using a computational medicinal scoring metric, such as the Tanimoto coefficient (Tc), and a threshold set in legislation [75, 76, 77, 78]. This approach would be similar to Canada's control of fentanyl derivatives. Instead of looking to the overall chemical similarity, scheduling could focus on chemical similarity to the structural portion or portions of the molecule responsible for the pharmacological effect of the molecule. This is the so‐called pharmacophore moiety. The pharmacophore and its derivatives could be chemically defined, or computational methods could score the similarity to the parent drug [79]. In 2014, Ohio enacted legislation applying the “Pharmacophore Rule” to synthetic cathinones, cannabinoids, and opioids. The pharmacophore for fentanyl‐related agents is a five‐, six‐, or seven‐membered ring containing a nitrogen, with a polar group and an aryl or aryl substitution attached to the ring for binding to the μ‐opioid receptor.
Alternatively, the Federal Analogue Act could emphasize functional similarity based upon biomedical studies. However, studies of pharmacologic effects on human beings cannot always be ethically conducted. Unlike a drug trafficker, the safety of a substance must first be investigated prior to feeding to humans. Congress should require any substance intended for human consumption, first be approved by the FDA for safety. Nonetheless, in vitro binding studies and functional assays of cell cultures or animals might be conducted [80, 81, 82, 83]. These policies may also consider the neurobiology of drug use and dependence.
15. EXPIRATION OF THE FEDERAL CORE STRUCTURE SCHEDULING
The fentanyl core structure scheduling was set to expire as of February 6, 2020 [84]. It is unclear that the DEA can create class scheduling without further authorizing legislation due to the use of singular terms in the CSA and also that class scheduling precludes adequate input on the safety and medical uses of the various substances included in the class. All previous class scheduling had occurred through legislation.
A hearing entitled “The Countdown: Fentanyl Analogues and the Expiring Emergency Scheduling Order” was held by the Senate Committee on the Judiciary, Chaired by Senator Lindsey Graham (R‐SC), on June 4, 2019 [85]. Kemp Chester (ONDCP), Amanda Liskamm (DOJ), and Greg Cherundolo (DEA) testified in favor of making the core structure scheduling permanent.
The DOJ testimony described the effectiveness of the temporary class‐wide scheduling:
The positive impacts in the 15 months since implementation are significant. The class control has substantially slowed the rate at which new fentanyl‐related substances are introduced to, and are encountered in, the illicit market. Prior to this action, DEA observed a rapid and continuous emergence of new fentanyl‐like substances each time it scheduled a fentanyl‐like substance. Under the temporary emergency scheduling order, there is little incentive for drug trafficking organizations to invent new substances in the fentanyl family for the purpose of evading DEA’s control. Specifically, DEA laboratories have not encountered any new fentanyl analogue substances in the first quarter Fiscal Year (FY) 2019 and is currently analyzing data for the second quarter of FY 2019. DEA’s experience under the relatively short temporary scheduling regime is proof of concept that class‐wide scheduling of fentanyl‐related substances produces solid law enforcement results, while it has a positive impact on the application process [86].
Several letters were written against the proposed permanency of FRS class scheduling, including from the Human Rights Watch coalition [87], the College of Problems of Drug Dependence (CPDD), the Friends of NIDA, and the American Psychological Association [88]. They argued that class‐wide scheduling provides for overly broad DEA powers, perpetuates a failed “war on drugs,” and will result in harsher penalties. They claimed that such scheduling precludes input from HHS. The Human Rights Watch letter pointed to Congressman Charlie Dent (R‐PA)’s 2015 introduction of a bill to schedule more than 300 synthetic fentanyl analogues as schedule I, but was ultimately reduced to 22 substances “after scientists could reach agreement that this small subset met schedule I’s criteria.” Critics also argued that class‐wide scheduling will limit biomedical research, may adversely affect public health, and could dissuade young investigators from potentially promising research pathways.
A second hearing entitled "Fentanyl Analogues: Perspectives on Classwide Scheduling" was held by the Subcommittee on Crime, Terrorism, and Homeland Security on House Judiciary Committee, Chaired by Karen Bass (D‐CA), on January 28, 2020 [89]. Admiral Brett Giroir (HHS), Amanda Liskamm (DOJ), and Donald Holman (VA) spoke in favor of making the core structure scheduling permanent; Dr. Daniel Ciccarone (UC San Francisco) and Kevin Butler (Public Defender from Alabama) spoke against such scheduling; and Dr. Sandra Comer (Columbia University, CPDD) spoke to research needs.
At the January 2020 hearing, Liskamm testified to the continued effectiveness of the temporary class‐wide scheduling:
The Chinese scheduling action, coupled with the DEA’s regulatory authority, enacted on February 6, 2018, which placed all non‐scheduled fentanyl‐related substances in Schedule I temporarily, on an emergency basis, for two years, has resulted in a significant decrease in direct Chinese‐origin fentanyl‐related substances being encountered in the United States since Fiscal Year 2019 [90].
The positive impacts in the two years since implementation are significant. Since 2018, there has been a significant decline in law enforcement reports to the National Forensic Laboratory Information System (NFLIS) of substances structurally related to fentanyl, including those captured under the February 2018 class control temporary order. In the 24 months preceding the temporary order (February 2016 through January 2018), there were more than 17,500 reports of these substances to NFLIS, excluding those controlled prior to 2016. Conversely, since the temporary class control (February 2018 through December 2019), and as of January 7, 2020, there were fewer than 8,800 reports to NFLIS for substances structurally related to fentanyl, a 50 percent reduction. It should be noted that NFLIS reporting is still on‐ going for 2019. The DEA attributes this significant decline to the series of control actions in recent years, culminating in the February 2018 class control [91].
By overwhelming majorities in the House and Senate, within days of the expiration date, Congress passed the Temporary Reauthorization and Study of the Emergency Scheduling of Fentanyl Analogues Act (P.L. 116‐114) to keep the temporary ban of all fentanyl analogues [92]. Other bills, such as the Stopping Overdoses of Fentanyl Analogues Act (SOFA) had been considered [93]. Nonetheless, the criticisms lodged prevented the temporary scheduling to become permanent, but instead merely extended the scheduling for another 15 months and mandating the topic be studied. The new expiration date is May 6, 2021.
Had the class control lapsed, reactive individual scheduling of fentanyl analogues would continue. International controls would have also stayed in place, including those stimulated by the class control. In particular, a continuation of the class‐wide ban in China would be key since China had been the predominant source of fentanyl analogues. Thus, despite the apparent immediate success of the fentanyl core structure scheduling, this lapse might not have been strongly felt. Although the fentanyl analogues have declined, the selling of fentanyl itself has continued largely unabated, even escalating, most recently from Mexican drug cartels [94].
Congress tasked the Government Accountability Office (GAO), but not National Institute on Drug Abuse (NIDA) or FDA, to report on the effectiveness and ramifications of the temporary class control of FRS; this report was not publicly available as this article was written.
It is significant that during the temporary scheduling and during the Congressional deliberations that the pharmaceutical industry did not express concern that the scheduling prevented the development of new medicines. As noted by registered researchers, no pharmaceutical companies are currently conducting studies on FRS per the registration requirement discussed below. The majority of researchers are registered in detection method development and are DEA contractors studying the pharmacological effects of FRS to inform policy decision and drug strategies.
16. RESEARCH
The most specific criticism of class‐wide fentanyl scheduling is that it will inhibit research on FRS. Research on fentanyl analogues is useful to understand their physiologic and pharmacologic effects, but moreover in attempts to find treatments. Such research may allow discovery of naloxone‐like reversal agents sufficiently potent to treat fentanyl analogue overdoses. Minor chemical modifications of agonists may be the key to changing a powerful opioid agonist into a powerful antagonist. New and promising avenues of research for treating addiction, such as the development of monoclonal antibodies against fentanyl and its analogues, should continue. This need for research is recognized by all sides.
Research on schedule I substances is not precluded—as is sometimes falsely believed. The CSA does contain language that authorizes pre‐clinical and clinical research—including schedule I substances. Schedule I substance research is conducted on a substance‐by‐substance basis under schedule I research registration, which has been seen as burdensome and inhibitory [95, 96]. Thus, claims that class scheduling will hinder research may have some merit, but this must be balanced against the number of deaths. Recent efforts have been made to facilitate such research [97, 98]. Dr. Sandra Comer in her testimony at the second hearing made specific suggestions for further relief [99].
Critics of the extension of fentanyl core structure scheduling have suggested that scheduling as schedule II, rather than schedule I, would allow clinical research but still permit significant control. This is not plausible, for a substance to be placed under schedule II, it must have an approved medical use. Moreover, lesser control would certainly eventuate in more overdose deaths. Even under a class‐wide scheduling regime, individual substances may be removed or moved to a different schedule upon the recommendation from HHS or upon approval for medical use by U.S. FDA.
The temporary reauthorization act did seem to recognize that the legislation might contain language to facilitate research on FRS when they tasked GAO to:
(7) evaluate the processes used to obtain or modify Federal authorization to conduct research with fentanyl‐related substances, including by— (A) identifying opportunities to reduce unnecessary burdens on persons seeking to research fentanyl‐related substances; (B) identifying opportunities to reduce any redundancies in the responsibilities of Federal agencies; (C) identifying opportunities to reduce any inefficiencies related to the processes used to obtain or modify Federal authorization to conduct research with fentanyl‐related substances; (D) identifying opportunities to improve the protocol review and approval process conducted by Federal agencies; and (E) evaluating the degree, if any, to which establishing processes to obtain or modify a Federal authorization to conduct research with a fentanyl‐related substance that are separate from the applicable processes for other schedule I controlled substances could exacerbate burdens or lead to confusion among persons seeking to research fentanyl‐related substances or other schedule I controlled substances.
Not only should the burden of research restrictions be eased, but Congress should increase the amount of research support in this area. It is challenging to study the proliferation of NPS and has resulted in a relative dearth of scientific literature upon which physicians in emergency rooms, forensic pathologists in morgues, and prosecutors in court can interpret and document the effects of a given level of an NPS. Congress could create NPS research centers that could produce a body of available scientific literature and a cadre of scientists with deep knowledge and understanding of these novel substances. These scientists should have diverse backgrounds, including physicians, pharmacologists, and toxicologists. There is currently both a general lack of scientific literature and a dearth of such professionals, but the need is evident.
17. CONCLUSION
The decrease in the availability of fentanyl analogues in the illicit drug market suggests that the fentanyl core structure scheduling has been an effective strategy. Lapse of such scheduling may reverse the gains and permanent scheduling legislation should continue to facilitate these positive effects. Such a strategy of class‐wide scheduling should be considered for other substances. Class scheduling control allows for these substances to be interdicted at our borders and seized outside the closed system of manufacture, distribution, and research as established by the CSA. In fact, the CSA and the Federal Analogue Act should be revisited generally and perhaps updated to current needs. Further, Congress may consider specific measures to facilitate and support research of FRS and NPS, more broadly. More research is needed to create a body of publicly available scientific literature and to produce a cadre of experts on the pharmacodynamics and cognitive and physical effects of these novel psychoactive substances. Regardless, if nothing else, a proactive approach to FRS scheduling, rather than the previous retroactive drug‐by‐drug scheduling, is needed to save lives.
REFERENCES
- 1.2019 national drug threat assessment. DEA. Dec 2019. https://www.dea.gov/sites/default/files/2020‐01/2019‐NDTA‐final‐01‐14‐2020_Low_Web‐DIR‐007‐20_2019.pdf. Accessed 2 Feb 2021.
- 2.O’Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital signs: Characteristics of drug overdose deaths involving opioids and stimulants—24 states and the District of Columbia, January‐June 2019. MMWR. 2020;69(35):1189–97. 10.15585/mmwr.mm6935a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Understanding the epidemic. CDC. https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed 2 Feb 2021.
- 4.Overdose death rates. NIDA. https://www.drugabuse.gov/drug‐topics/trends‐statistics/overdose‐death‐rates. Accessed 2 Feb 2021.
- 5.Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 2018;67(9):1–14. [PubMed] [Google Scholar]
- 6.Frank RG, Pollack HA. Addressing the fentanyl threat to public health. N Eng J Med. 2017;376:605–7. 10.1056/NEJMp1615145. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM. Deaths involving fentanyl, fentanyl analogs, and U‐47700—10 states, July‐December 2016. MMWR. 2017;66(43):1197–202. 10.15585/mmwr.mm6643e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid‐involved overdose deaths—United States, 2017–2018. MMWR. 2020;69(11):290–7. 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amid pandemic, NY seizures of fentanyl and meth surge. Associated Press. 2021 Jan 26. https://apnews.com/article/pandemics‐new‐york‐synthetic‐opioids‐opioids‐coronavirus‐pandemic‐02b52fce24241dcd7b25497a550d74e6. Accessed 2 Feb 2021.
- 10.Pardo B, Taylor J, Caulkins JP, Kilmer B, Reuter P, Stein BD. The future of fentanyl and other synthetic opioids. Rand. 2019. Accessed 2 Feb 2021 https://www.rand.org/content/dam/rand/pubs/research_reports/RR3100/RR3117/RAND_RR3117.pdf.
- 11.Burns SM, Cunningham CW, Mercer SL. DARK classics in chemical neuroscience: Fentanyl. ACS Chem Neurosci. 2018;9:2428–37. 10.1021/acschemneuro.8b00174. [DOI] [PubMed] [Google Scholar]
- 12.Poklis A. Fentanyl: A review for clinical and analytical toxicologists. J Toxicol Clin Toxicol. 1995;33(5):439–47. 10.3109/15563659509013752. [DOI] [PubMed] [Google Scholar]
- 13.Fentanyl . Pub Chem, compound summary. https://pubchem.ncbi.nlm.nih.gov/compound/Fentanyl. Accessed 2 Feb 2021.
- 14.Fentanyl . DrugBank Online, Accession Number DB00813. https://go.drugbank.com/drugs/DB00813. Accessed 2 Feb 2021.
- 15.Stanley TH. The fentanyl story. J Pain. 2014;15(12):12–5. 10.1016/j.jpain.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 16.McNeil Laboratories, Inc . Fentanyl (sublimaze). Clin Pharmacol Ther. 1968;9(5):704–6. 10.1002/cpt196895704. [DOI] [PubMed] [Google Scholar]
- 17.Jonczyk A, Jawdosiuk J, Makosza M. Searching for a new method of synthesizing the analgesic "fentanyl". Przem. Chem. 1978;57:131–4. [Google Scholar]
- 18.Zee SH, Wang WK. A new process for the synthesis of fentanyl. J Chin Chem Soc. 1980;27:147–9. [Google Scholar]
- 19.Sacco L. Drug enforcement in the United States: history, policy, and trends. Congressional Research Service. R43749. Oct. 2, 2014. https://fas.org/sgp/crs/misc/R43749.pdf. Accessed 2 Feb 2021.
- 20.Lampe JR. The Controlled Substances Act (CSA): A legal overview for the 116th Congress. Congressional Research Service. R45948. Oct. 9, 2019. https://fas.org/sgp/crs/misc/R45948.pdf. Accessed 2 Feb 2021.
- 21.Spillane JF. Debating the controlled substances act. Drug Alcohol Depend. 2004;76(1):17–29. 10.1016/j.drugalcdep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Kreit A. Controlled substances, uncontrolled law. Albany Govt L Rev. 2013;6:332–58. [Google Scholar]
- 23.Drugs of abuse: A DEA resource guide. DEA. 2020. ed. p. 11. https://www.dea.gov/sites/default/files/2020‐04/Drugs%20of%20Abuse%202020‐Web%20Version‐508%20compliant‐4‐24‐20_0.pdf. Accessed 2 Feb 2021.
- 24.The international drug control conventions. UNODC. https://www.unodc.org/documents/commissions/CND/Int_Drug_Control_Conventions/Ebook/The_International_Drug_Control_Conventions_E.pdf. Accessed 2 Feb 2021.
- 25.Armenta A, Jelsma M. The UN drug control conventions: A primer. Transnational Institute. 2015. https://idpc.net/publications/2015/10/the‐un‐drug‐control‐conventions‐a‐primer. Accessed 2 Feb 2021.
- 26.McAllister WB. The global political economy of scheduling: The international–historical context of the Controlled Substances Act. Drug Alcohol Depend. 2004;76(1):3–8. 10.1016/j.drugalcdep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Fazey SJ. The Commission on Narcotic Drugs and the United Nations International Drug Control Programme: Politics, policies and prospect for change. Intl J Drug Policy. 2003;14(2):155–69. 10.1016/S0955-3959(03)00004-5. [DOI] [Google Scholar]
- 28.Lande A. The single convention on narcotic drugs, 1961. Intl Org. 1962;16(4):776–97. [Google Scholar]
- 29.Bewley‐Taylor D, Jelsma M. Regime change: Re‐visiting the 1961 Single Convention on Narcotic Drugs. Int J Drug Policy. 2012;23:72–81. 10.1016/j.drugpo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Khan I. Convention on Psychotropic Substances, 1971: The role and responsibilities of the World Health Organization. Progress Neuro‐Psychopharmacol. 1979;3(1–3):11–4. 10.1016/0364-7722(79)90064-X. [DOI] [PubMed] [Google Scholar]
- 31.Gurule J. The 1988 U.N. Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances—A ten year perspective: Is international cooperation merely illusory. Fordham Intl L J. 1998;22(1):74–121. [Google Scholar]
- 32.Stewart DP. Internationalizing the war on drugs: The UN convention against illicit traffic in narcotic drugs and psychotropic substances. Denver J Intl Law Policy. 1990;18(3):387–404. [Google Scholar]
- 33.Substances under international control. WHO. https://www.who.int/medicines/areas/quality_safety/sub_Int_control/en/. Accessed 2 Feb 2021.
- 34.Johnson M, Maggiora GM, editors. Concepts and applications of molecular similarity. New York, NY: John Willey & Sons; 1990. p. 1–393. [Google Scholar]
- 35.Chester KL (ONDCP). Written testimony to the Senate Committee on the Judiciary. June 4, 2019. p. 2. https://www.judiciary.senate.gov/imo/media/doc/Chester%20Testimony.pdf. Accessed 2 Feb 2021.
- 36.King LA, Kieman AT. A brief history of ‘new psychoactive substances’. Drug Test Analysis. 2011;3:401–3. 10.1002/dta.319. [DOI] [PubMed] [Google Scholar]
- 37.Corazza O, Roman‐Urrestarazu A, editors. Handbook of novel psychoactive substances: What clinicians should know about NPS. New York, NY: Routledge; 2018. p. 1–408. [Google Scholar]
- 38.Henderson GL. Designer drugs: Past history and future prospects. J Forensic Sci. 1988;33(2):569–75. 10.1520/JFS11976J. [DOI] [PubMed] [Google Scholar]
- 39.Jones TJ, Jones TS, Krzywicki L, Maginnis J, Jones NL, Reid M, et al. Nonpharmaceutical fentanyl‐related deaths—Multiple states, April 2005‐March 2007. CDC. MMWR. 2008;57(29):793–6. [PubMed] [Google Scholar]
- 40.Sacco LN, Finklea K. Synthetic drugs: Overview and issues for Congress. Congressional Research Service. R42066. Updated May 3, 2016. p. 8–9, 12–3. https://fas.org/sgp/crs/misc/R42066.pdf. Accessed 2 Feb 2021.
- 41.Liskamm A (DOJ). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 2. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐LiskammA‐20200128.pdf. Accessed 2 Feb 2021.
- 42.DEA diversion control federal register notices. https://www.deadiversion.usdoj.gov/fed_regs/index.html. Accessed 2 Feb 2021.
- 43.Langston JW. The MPTP story. J Parkinsons Dis. 2017;7(Suppl 1):S11–S19. 10.3233/JPD-179006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown KE. Stranger than fiction: Modern designer drugs and the Federal Controlled Substances Analogue Act. Ariz St L J. 2015;47(2):449–74. [Google Scholar]
- 45.Kau G. Flashback to the Federal Analog Act of 1986: Mixing rules and standards in the cauldron. U Penn L Rev. 2008;156(4):1077–115. 10.2307/40041401. [DOI] [Google Scholar]
- 46.Synthetic drugs (a.k.a. K2, spice, bath salts, etc.). ONDCP. https://obamawhitehouse.archives.gov/ondcp/ondcp‐fact‐sheets/synthetic‐drugs‐k2‐spice‐bath‐salts#:~:text=The%20Synthetic%20Drug%20Abuse%20Prevention,Controlled%20Substances%20Act%20(CSA). Accessed 2 Feb 2021.
- 47.Ethridge E. Lawmakers attempt to keep up with synthetic drugs. Roll Call. Oct. 28, 2013. https://www.rollcall.com/2013/10/28/lawmakers‐attempt‐to‐keep‐up‐with‐synthetic‐drugs/. Accessed 2 Feb 2021.
- 48.Keim B. New federal ban on synthetic drugs already obsolete. Wired. July 12, 2012. https://www.wired.com/2012/07/synthetic‐drug‐ban/. Accessed 2 Feb 2021.
- 49.Armenian P, Vo K, Barr‐Walker J, Lynch K. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology. 2018;134:121–32. 10.1016/j.neuropharm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki J, El‐Haddad S. A review: Fentanyl and non‐pharmaceutical fentanyls. Drug Alcohol Depend. 2016;171:107–16. 10.1021/acschemneuro.8b00150. [DOI] [PubMed] [Google Scholar]
- 51.Schueler HE. Emerging synthetic fentanyl analogs. Acad Forensic Pathol. 2017;7(1):36–40. 10.23907/2017.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichini S, Solimi R, Berretta P, Pacifici R, Busardo FP. Acute intoxications and fatalities from illicit fentanyl and analogues: An update. Ther Drug Monit. 2018;40:38–51. 10.1097/FTD.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 53.Jones CM, Einstein EB, Compton WM. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA. 2018;319(17):1819–21. 10.1001/jama.2018.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Errico S. Commentary. Fentanyl‐related death and the underreporting risk. J Forensic Leg Med. 2018;60:35–7. 10.1016/j.jflm.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Rosen LW, Lawrence SV. Illicit fentanyl, China’s role, and U.S. foreign policy options. IF10890, V.5. Congressional Research Service. Dec 21, 2018. https://fas.org/sgp/crs/row/IF10890.pdf. Accessed 2 Feb 2021.
- 56.Finklea K. Illicit drug flows and seizures in the United States: In Focus. IF 11279, v.2. Congressional Research Service. Aug 1, 2019. https://fas.org/sgp/crs/misc/IF11279.pdf. Accessed 2 Feb 2021.
- 57.Felbab‐Brown V. Fentanyl and geopolitics: controlling opioid supply from China. Brookings Institution. July 2020. https://www.brookings.edu/wp‐content/uploads/2020/07/8_Felbab‐Brown_China_final.pdf. Accessed 2 Feb 2021.
- 58.Lists of scheduling actions, controlled substances, regulated chemicals ["The Orange Book"]. DEA. Dec. 2020. https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf. Accessed 2 Feb 2021.
- 59.Narcotic drugs. INCB. https://www.incb.org/incb/en/narcotic‐drugs/index.html. Accessed 2 Feb 2021.
- 60.O’Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region—United States, 2006–2015. MMWR. 2017;66(34):897–903. 10.15585/mmwr.mm6634a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massey J, Kilkenny M, Batdorf S, Sanders SK, Ellison D, Halpin J, et al. Opioid overdose outbreak — West Virginia, August 2016. MMWR. 2017;66(37):975–80. 10.15585/mmwr.mm6637a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daniulaityte R, Juhascik MP, Strayer KE, Sizemore IE, Harshbarger KE, Antonides HM, et al. Overdose deaths related to fentanyl and its analogs—Ohio, January–February 2017. MMWR. 2017;66(34):904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Model Scheduling New/Novel Psychoactive Substances Act. 3rd ed. National Alliance for Model State Drug Laws, July 1, 2019. https://namsdl.org/wp‐content/uploads/Model‐Scheduling‐New‐Novel‐Psychoactive‐Substances‐Act‐3.pdf. Accessed 2 Feb 2021.
- 64.Norwood AP. When apples taste like oranges, you cannot judge a book by its cover: How to fight emerging synthetic "designer" drugs of abuse, 39 U. Ark Little Rock L Rev. 2017;39(2):323–47. [Google Scholar]
- 65.DOJ press release: Department of Justice announces significant tool in prosecuting opioid traffickers in emergency scheduling of all fentanyls. Nov. 9, 2017. https://www.dea.gov/press‐releases/2017/11/09/department‐justice‐announces‐significant‐tool‐prosecuting‐opioid. Accessed 2 Feb 2021.
- 66.Giroir BP (HHS). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 5. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐GiroirB‐20200128.pdf. Accessed 2 Feb 2021.
- 67.Lewis MJ, DEA . Schedules of controlled substances: Temporary placement of fentanyl‐related substances in schedule I. Doc. No. 2017‐28114. Fed. Reg. 2017;82(249):61700–3. [Google Scholar]
- 68.Lewis MJ, DEA . Schedules of controlled substances: temporary placement of fentanyl‐related substances in schedule I. Doc. No. 2018‐02319. Fed. Reg. 2018;83(25):5188–92. [PubMed] [Google Scholar]
- 69.Fentanyl‐related substances . DEA. May 2020. https://www.deadiversion.usdoj.gov/drug_chem_info/frs.pdf. Accessed 2 Feb 2021.
- 70.Amendments to the sentencing guidelines. United States Sentencing Commission. Apr 30, 2018. p. 20. https://www.ussc.gov/sites/default/files/pdf/amendment‐process/reader‐friendly‐amendments/20180430_RF.pdf. Accessed 2 Feb 2021.
- 71.Gerbasi JS. New amendments to the U.S. Sentencing Guidelines concerning fentanyl and fentanyl analogues. U.S. Att’ys Bull. 2018;66(4):41–5. [Google Scholar]
- 72.Liskamm A, Cherundolo G (DOJ). Written testimony to the Senate Committee on the Judiciary. Jun. 4, 2019. p. 2. https://www.judiciary.senate.gov/meetings/the‐countdown‐fentanyl‐analogues‐and‐the‐expiring‐emergency‐scheduling‐order. Accessed 2 Feb 2021.
- 73.Liskamm A (DOJ). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 2. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐LiskammA‐20200128.pdf. Accessed 2 Feb 2021.
- 74.Lampe JR. Scheduling of fentanyl analogues: the new legal landscape. Congressional Research Service. LSB10404. Feb. 7, 2020. https://crsreports.congress.gov/product/pdf/LSB/LSB10404. Accessed 2 Feb 2021.
- 75.Rogers DJ, Tanimoto TT. A computer program for classifying plants. Science. 1960;132(3434):1115–8. 10.1126/science.132.3434.1115. [DOI] [PubMed] [Google Scholar]
- 76.Nikolova N, Jaworska J. Approaches to measure chemical similarity – A review. QSAR Combin Sci. 2004;22(9–10):1006–26. 10.1002/qsar.200330831. [DOI] [Google Scholar]
- 77.Willett P, Barnard JM, Downs GM. Chemical similarity searching. J Chem Inf Comput Sci. 1998;38(6):983–96. 10.1021/ci9800211. [DOI] [Google Scholar]
- 78.Rester U. From virtuality to reality – Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective. Curr Opin Drug Discov Devel. 2008;11(4):559–68. [PubMed] [Google Scholar]
- 79.Andres GW, Worst TJ, Sprague JE. Designer drugs legislating for tomorrow through the use of class definitions and pharmacophore principles. In: Kowalski T, Sajewicz M, Sherma J, editors. Chromatographic techniques in the forensic analysis of designer drugs. Boca Raton, FL: CRC Press; 2018. p. 17–27. [Google Scholar]
- 80.Martin YC, Kofron JL, Traphagen LM. Do structurally similar molecules have similar biological activity? J Med Chem. 2002;45(19):4350–8. 10.1021/jm020155c. [DOI] [PubMed] [Google Scholar]
- 81.Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48(22):2165–71. 10.1016/0024-3205(91)90150-A. [DOI] [PubMed] [Google Scholar]
- 82.Volpe DA, Tobin GAM, Mellon RD, Katki AG, Parker RJ, Colatsky T, et al. Uniform assessment and ranking of opioid Mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385–90. 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical‐dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychol. 2006;73(1):90–9. 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinks SA, DEA . Correcting amendment. Schedules of controlled substances: temporary placement of fentanyl‐related substances in schedule I; Correction. Doc. No. 2020‐06984. Fed. Reg. 2020;85(70):20155. [Google Scholar]
- 85.The countdown: Fentanyl analogues and the expiring emergency scheduling order. Senate Committee on the Judiciary. June 4, 2019. https://www.judiciary.senate.gov/meetings/the‐countdown‐fentanyl‐analogues‐and‐the‐expiring‐emergency‐scheduling‐order. Accessed 2 Feb 2021.
- 86.Liskamm A (DOJ). Written testimony to the Senate Committee on the Judiciary. Jun. 4, 2019. p. 3. https://www.judiciary.senate.gov/meetings/the‐countdown‐fentanyl‐analogues‐and‐the‐expiring‐emergency‐scheduling‐order. Accessed 2 Feb 2021.
- 87.Human Rights Watch Coalition . Coalition Opposes S.1622 Stopping Overdoses of Fentanyl Analogues Act (SOFA). Jul 1, 2019. https://www.hrw.org/news/2019/07/03/coalition‐opposes‐s1622‐stopping‐overdoses‐fentanyl‐analogues‐act‐sofa. Accessed 2 Feb 2021.
- 88.Butler KL. Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 17, fn 82. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐ButlerK‐20200128.pdf. Accessed 2 Feb 2021.
- 89.Fentanyl analogues: Perspectives on classwide scheduling. House Judiciary Committee; Subcomittee on Crime, Terrorism, and Homeland Security. January 28, 2020. https://www.congress.gov/event/116th‐congress/house‐event/110392. Accessed 2 Feb 2021.
- 90.Liskamm A (DOJ). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 2. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐LiskammA‐20200128.pdf. Accessed 2 Feb 2021.
- 91.Liskamm A (DOJ). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 3. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐LiskammA‐20200128.pdf. Accessed 2 Feb 2021.
- 92.Public Law 116 – 114, Temporary Reauthorization and Study of the Emergency Scheduling of Fentanyl Analogues Act. https://www.govinfo.gov/app/details/PLAW‐116publ114. Accessed 2 Feb 2021.
- 93.Butler KL. Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 4, fn 14. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐ButlerK‐20200128.pdf. Accessed 2 Feb 2021.
- 94.Revealed: How Mexico’s Sinaloa cartel has created a global network to rule the fentanyl trade. The Guardian. 2020 Dec 8.
- 95.Bloomberg . ‘And then you die.’ Research on deadly opioid fentanyl blocked by federal stalemate. Fortune. 2018 April 19.
- 96.Giroir BP (HHS). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 6. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐GiroirB‐20200128.pdf. Accessed 2 Feb 2021.
- 97.DEA press release: DEA speeds up application process for research on schedule I drugs. Jan 18, 2018. https://www.dea.gov/press‐releases/2018/01/18/dea‐speeds‐application‐process‐research‐schedule‐i‐drugs. Accessed 2 Feb 2021.
- 98.Giroir BP (HHS). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 6–8. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐GiroirB‐20200128.pdf. Accessed 2 Feb 2021.
- 99.Comer SD (CPDD). Written testimony to the House Committee on the Judiciary, Subcommittee on Crime, Terrorism, and Homeland Security. Jan. 28, 2020. p. 6. https://www.congress.gov/116/meeting/house/110392/witnesses/HHRG‐116‐JU08‐Wstate‐ComerS‐20200128.pdf. Accessed 2 Feb 2021.


