Abstract
Objective
Month of birth (MOB) is associated with specified mental disorders (MDs). However, whether these relationships extend to all MDs remains unclear. We investigate the association using a population‐based cohort study and a meta‐analysis.
Methods
First, we examined patients with 34 DSM‐5‐classified MDs in the Taiwan national database. We estimated the relative risk ratios (RR) of each illness in each MOB relative to that in the general population and assessed the periodicity, with six further sensitivity analyses. Second, we searched PubMed, Embase, and Cochrane for related articles through 31 December 2020. We used a random‐effects model, pooled RRs with 95% confidence intervals of each MOB from the identified studies, and transformed them from MOB to relative age in a year or season.
Results
The cohort included 1,951,777 patients. Except for posttraumatic stress disorder, dissociative disorders, feeding/eating disorders, gender dysphoria, and paraphilic disorders, the other MDs had significant MOB periodicity. The meta‐analysis included 51 studies investigating 10 MDs. The youngest age at the start of school owing to MOB was associated with the highest RRs of intellectual disability (1.13), autism (1.05), attention‐deficit/hyperactivity disorder (1.13). Winter births had significant risks of schizophrenia (1.04), bipolar I disorder (1.02), and major depressive disorder (1.01), and autumn births had a significant risk of alcohol use disorder (1.02). No significant associations between season of birth and Alzheimer's disease, or eating disorders were found.
Conclusions
MOB is related to the risks of certain MDs. This finding provides a reference for future research on the etiology of MDs.
Keywords: mental disorder, meta‐analysis, month of birth, population‐based
Summations
Children who were relatively young when they start school had an increased risk of a diagnosis of intellectual disability, autism, and attention‐deficit/hyperactivity disorder.
Winter births had a high risk of schizophrenia, bipolar I disorder, and major depressive disorder, and autumn births had a high risk of alcohol use disorder
No specified season had a predominant risk of Alzheimer's disease, anorexia nervosa, and bulimia nervosa.
Limitations
The population‐based study and meta‐analyses focused on reports in which patients were assessed within the last 30 years, so they lacked epidemiological findings before 1990.
1. INTRODUCTION
Previous studies have provided evidence that month of birth (MOB) is associated with an increased risk of specific mental disorders (MDs), such as autism spectrum disorder (ASD),1 attention‐deficit/hyperactivity disorder (ADHD),2 schizophrenia,3 bipolar disorder,4 and depressive disorder.5 The influence of MOB on childhood‐related disorders may result from the cutoff dates for school enrollment.2 There is generally a 12‐month age span among students within a grade, and individuals who are relatively younger have poorer school performance and a higher risk of ADHD than their peers.6 In addition, seasons could be another factor affecting the onset of MDs, such as schizophrenia. Two meta‐analyses have shown a 3% to 7% higher rate among people born in the winter compared to those born in other seasons in both the Northern and Southern Hemispheres.7, 8 It has been suggested that meteorological factors (such as ambient temperature and bright sunshine) and various infectious agents,9, 10, 11 which are more prevalent in some months or seasons, result in differences in individual vulnerability during the perinatal period.12
The season or age effects due to MOB offer interesting clues regarding the impact of prenatal exposures13 and early life experiences at school6 on the risk of some MDs and may have an important influence on future health policy (eg, immature children may delay starting school to decrease the risk of ADHD14). However, whether the effect exists in other MDs remains uncertain. For example, evidence regarding this relationship in many illnesses classified in the newest Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM‐5), such as posttraumatic stress disorder, is limited.15 A comprehensive assessment of all main categories of MDs in the DSM‐5 is needed. Moreover, a recent study indicated that an association between MOB and depression was largely nonexistent.16 Therefore, it is useful to adopt a systematic approach to assess the current evidence regarding such associations in major MDs.
1.1. Aims of the Study
First, we aimed to use the Taiwan nationwide health insurance database from 1995 to 2013 to investigate the relationships between month of birth and the risks of the main mental disorders classified in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition. Second, we incorporated our findings with those of previous studies in recent decades investigating major mental disorders by performing a systematic review and meta‐analysis.
2. MATERIAL AND METHODS
2.1. Taiwan population‐based cohort study
Chang Gung Medical Foundation Institutional Review Board approved this project (201801250B0). This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Table S1).17 To comprehensively investigate the associations between MOB and MDs, we performed a population‐based cohort study using data from the Taiwan National Health Insurance (NHI) program. The NHI program was implemented in 1995 as the sole payer for healthcare services and covered 99% of Taiwan's population. In this study, the data of a cohort of individuals born between 1 January 1900 and 31 December 2013 were extracted from the National Health Insurance Research Database (NHIRD), which was derived from the reimbursement medical claims records of the NHI program. The NHIRD includes personal information, such gender, date of birth, place of residence, income status, and clinical diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification; ICD‐9‐CM).18
2.2. Assessment of individual birth months
We selected and matched patients with MDs and general population in the NHIRD by their MOBs. Data were analyzed according to each MOB (January to December); the months were also divided into four groups by birth quarter to denote the relative age within the grade cohort of children with childhood‐onset disorders6 (in Taiwan, level 1: September to November, level 2: December to February, level 3: March to May, and level 4: June to August; children in level 1 were the oldest, and those in level 4 were the youngest. Table S11 lists the school enrollment month in other countries, which varies across different areas) or by season for all other MDs (spring: March to May, summer: June to August, autumn: September to November, and winter: December to February).
2.3. Assessment of MDs
This cohort included all patients with a diagnosis of MD in the NHIRD. For each patient, the date of onset was defined as the first day of each DSM‐5 diagnosis of interest by a board‐certified psychiatrist based on clinical judgment and diagnostic interviews in outpatient or inpatient settings. Then, we calculated the age of onset of the patient. Besides, the items considered for the main classification in the DSM‐5 are more detailed than those in the ICD. For example, bipolar and depressive disorders, which are separate entities in the DSM‐5, are classified as mood disorders in the ICD.15 Hence, we examined a broad range of MDs according to their classifications in the DSM‐5. However, substance‐ or medication‐induced MDs and MDs due to another medical condition were classified as other independent disorders without further analysis. The major MDs (12 primary and 15 secondary diseases) are shown as follows, and all MDs (19 primary and 15 secondary diseases) with diagnostic codes are listed in Table S2:
Neurodevelopmental disorders (neurodevelopmental‐Ds), with separate analyses for intellectual disability, ASD, ADHD, and tic disorder;
Disruptive, impulse control, and conduct disorders (disruptive‐ICCDs);
Schizophrenia spectrum and other psychotic disorders (schizophrenia‐SOPDs), with a separate analysis for schizophrenia;
Bipolar and related disorders (bipolar‐RDs), with a separate analysis for bipolar I disorder (BID);
Depressive disorders (depressive‐Ds), with a separate analysis for major depressive disorder (MDD);
Anxiety disorders (anxiety‐Ds), with separate analyses for panic disorder and generalized anxiety disorder;
Obsessive‐compulsive and related disorders (obsessive‐CRDs), with a separate analysis for obsessive‐compulsive disorder (OCD);
Sleep‐wake disorders (sleep‐WDs);
Substance‐related and addictive disorders (substance‐RADs), with a separate analysis for alcohol use disorder (AUD);
Neurocognitive disorders (neurocognitive‐Ds), with a separate analysis for Alzheimer's disease;
Trauma‐ and stressor‐related disorders (trauma‐SRDs), with a separate analysis for posttraumatic stress disorder (PTSD);
Feeding and eating disorders (feeding‐EDs), with separate analyses for anorexia nervosa and bulimia nervosa.
2.4. Statistical methods and sensitivity analyses
For each MD, individuals were observed from 1 January 1995 to the onset of the outcome or 31 December 2013. To assess the presence of an MOB effect on each MD, we performed Walter & Elwood's (W&E) tests to estimate the within‐year fluctuations with 12‐month periodicity.19 The W&E tests examine seasonality by assessing the amplitude of the seasonal variation and the time at which the maximum occurs in an expected simple harmonic fluctuation according to the general population and whether the distribution of patients follows a simple seasonal curve. Moreover, we calculated the ratio of the relative risk (RR, the distribution of MOBs in the patients relative to those in the general population). An RR greater than 1 suggests an increased risk of the target disorder in those born within a particular month. All analyses were conducted with SAS version 9.4 and R version 3.6.1 (package season 20). Due to the use of multiple tests for the various MDs, we considered the estimates statistically significant if the P‐value was less than 0.0025, thus approximating a Bonferroni correction.
We performed 6 sensitivity analyses (SAs) to assess the robustness of our results and examine the influence of potential bias in our study. First, to improve the diagnostic stability and validity, we increased the thresholds for the inclusion criteria of diagnosis by psychiatrists to at least three times (SA1).21 Second, as different disorders may be comorbid, the same individual may have multiple MDs.22 We restricted the inclusion criteria to patients who had no more than one psychiatric comorbidity to reduce the effects of comorbidities (SA2). Third, to explore potential bias due to gender, we repeated the primary analysis in only the male group (SA3). Fourth, to investigate the effect of MOB on the onset age, we analyzed only the early‐onset group6; the early onset group comprised those for whom the age at onset of a specified psychiatric illness was below the average age of onset (SA4). Fifth, to avoid the potential influence of urbanization on our outcomes,12 we performed subgroup analyses of those living in highly urbanized areas (SA5). The urbanization levels in Taiwan were divided into 4 strata, with level 1 referring to the most urbanized. In this study, we selected level 1 and level 2 for the analysis. Sixth, the income status may have an effect similar to urbanization12; therefore, we selected patients with an income lower than the average for further analysis (SA6).
2.5. Systematic review and meta‐analysis
To compare the results of the Taiwan cohort to studies in other countries, we conducted a systematic review and meta‐analysis following the Meta‐analyses Of Observational Studies in Epidemiology (MOOSE) guidelines23 (Table S6), and the detailed information is provided in the eMethods. In brief, we identified potential studies by searching the PubMed, Embase, and Cochrane Central databases from their inception to 31 December 2020. We used the following inclusion criteria: 1) Cohort and case–control studies were included, but case series or reports, conference papers, protocols, and nonpeer‐reviewed articles were excluded; 2) studies conducted after 1990 were included to match our cohort period; 3) studies that enrolled patients with select disorders and excluded individuals with limited symptoms of disorders were included; 4) the outcome of the studies showed a complete 12‐month distribution, except for those investigating neurodevelopmental‐Ds, which reported 12‐month or 4‐level distributions; one level comprised three months, and level 1 was the first birth quarter of a school year in the country (eg, the patients in level 1 had birth dates from September to November in Taiwan and were the oldest in the grade cohort); 5) different studies with potentially duplicate participants were screened, and we included only the study with the largest sample size; and 6) if only one study investigated a specified MD, we did not perform a further meta‐analysis.
The risk of bias was evaluated by Hoy's risk of bias tool, which measures internal and external validity to assess the prevalence of studies concerning various health conditions with different designs.24 We adopted the random‐effects model for the meta‐analysis comparing the RRs of the same MOBs across different studies, which were expressed as RRs‐meta with 95% confidence intervals (CIs). For months in opposing seasons between the Northern and Southern Hemispheres, we matched the month in the country in the Southern Hemisphere to that in the Northern Hemisphere (eg, January in the Southern Hemisphere was matched to July in the Northern Hemisphere). In addition, we converted the MOBs into corresponding levels or seasons for further analysis. A Chi‐square (X 2) test was used to evaluate the differences in the birth month, season, or level. We also assessed the heterogeneity among studies using Cochran's Q test and the I‐square test,25 and publication bias was assessed in more than 10 included studies through funnel plots and Egger's test.26 All analyses were performed with Comprehensive Meta‐Analysis software version 3.
3. RESULTS
3.1. Characteristics of population‐based cohort and meta‐analysis
This Taiwanese cohort included a total of 29 074 024 persons, including 1 951 777 patients with any MD who were followed from 1995 through 2013. The selection process (Figure S1) and demographic data of the included patients (Table S3) are shown in the Supplement. Table 1 lists the RRs of major psychiatric disorders by month (whole spectrum of MDs is shown in Table S4). In general, these MDs can be classified into three categories by the pattern of birth distribution, including childhood‐onset disorders (Figure 1), MDs with significant periodicity (Figure 2, eFigure 2), and MDs without significant periodicity (Figure 3, Figure S3).
TABLE 1.
Relative risk ratio for mental disorders from 1995 to 2013 categorized by month of birth
| Mental disordersa | General population and RR by birth month | p (W&E)b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | ||
| General population |
2 637 653 9.07% |
2 323 757 7.99% |
2 394 240 8.24% |
2 217 977 7.63% |
2 226 393 7.66% |
2 184 808 7.52% |
2 302 963 7.92% |
2 498 922 8.60% |
2 488 654 8.56% |
2 702 221 9.29% |
2 575 805 8.86% |
2 520 631 8.67% |
‐ |
| Neurodevelopmental disorders | 0.908 | 0.929 | 0.979 | 1.040 | 1.082 | 1.140 | 1.203 | 1.205 | 0.876 | 0.882 | 0.901 | 0.914 | < 0.001 |
| Intellectual disability | 0.933 | 0.960 | 0.967 | 1.019 | 1.038 | 1.079 | 1.159 | 1.166 | 0.931 | 0.924 | 0.926 | 0.935 | < 0.001 |
| Autism spectrum disorder | 0.910 | 0.917 | 1.030 | 1.044 | 1.072 | 1.138 | 1.153 | 1.102 | 0.909 | 0.887 | 0.958 | 0.932 | < 0.001 |
| Attention‐deficit/hyperactivity disorder | 0.872 | 0.908 | 0.981 | 1.063 | 1.123 | 1.198 | 1.285 | 1.274 | 0.811 | 0.833 | 0.854 | 0.883 | < 0.001 |
| Tic disorder | 0.974 | 0.979 | 1.024 | 1.039 | 1.070 | 1.088 | 1.105 | 1.069 | 0.917 | 0.923 | 0.936 | 0.916 | < 0.001 |
| Disruptive, impulse control, and conduct disorder | 0.924 | 0.957 | 0.974 | 0.990 | 1.067 | 1.109 | 1.165 | 1.161 | 0.900 | 0.877 | 0.980 | 0.942 | < 0.001 |
| Schizophrenia spectrum and other psychotic disorders | 1.046 | 1.035 | 1.006 | 0.967 | 0.960 | 0.953 | 0.976 | 0.992 | 0.996 | 1.012 | 1.021 | 1.018 | < 0.001 |
| Schizophrenia | 1.056 | 1.026 | 1.009 | 0.967 | 0.957 | 0.944 | 0.992 | 1.004 | 1.008 | 1.010 | 1.002 | 1.007 | < 0.001 |
| Bipolar and related disorders | 0.999 | 1.000 | 0.995 | 0.966 | 0.968 | 0.986 | 0.980 | 1.017 | 1.009 | 1.018 | 1.052 | 0.996 | < 0.001 |
| Bipolar I disorder | 0.995 | 1.002 | 0.989 | 0.964 | 0.966 | 0.998 | 0.985 | 1.018 | 1.003 | 1.018 | 1.054 | 0.996 | < 0.001 |
| Depressive disorders | 1.016 | 1.021 | 0.996 | 0.965 | 0.952 | 0.951 | 0.970 | 0.998 | 1.027 | 1.023 | 1.043 | 1.017 | < 0.001 |
| Major depressive disorder | 1.015 | 1.019 | 0.996 | 0.960 | 0.953 | 0.951 | 0.975 | 1.000 | 1.025 | 1.029 | 1.038 | 1.020 | < 0.001 |
| Anxiety disorders | 1.019 | 1.021 | 0.996 | 0.964 | 0.951 | 0.955 | 0.974 | 1.007 | 1.019 | 1.017 | 1.039 | 1.019 | < 0.001 |
| Panic disorder | 1.001 | 1.013 | 0.968 | 0.949 | 0.926 | 0.947 | 0.964 | 1.020 | 1.034 | 1.039 | 1.070 | 1.038 | < 0.001 |
| Generalized anxiety disorder | 1.014 | 1.023 | 0.998 | 0.960 | 0.941 | 0.931 | 0.949 | 1.001 | 1.034 | 1.034 | 1.059 | 1.029 | < 0.001 |
| Obsessive‐compulsive and related disorders | 1.008 | 1.002 | 0.982 | 0.964 | 0.947 | 0.940 | 0.967 | 1.006 | 1.038 | 1.048 | 1.062 | 1.011 | < 0.001 |
| Obsessive‐compulsive disorder | 0.998 | 0.985 | 0.975 | 0.949 | 0.947 | 0.940 | 0.962 | 1.002 | 1.042 | 1.057 | 1.084 | 1.026 | < 0.001 |
| Sleep‐wake disorders | 1.026 | 1.027 | 0.999 | 0.961 | 0.951 | 0.950 | 0.969 | 0.999 | 1.023 | 1.017 | 1.041 | 1.017 | < 0.001 |
| Substance‐related and addictive disorders | 0.995 | 0.991 | 0.987 | 0.973 | 0.966 | 0.988 | 1.005 | 1.049 | 1.017 | 1.008 | 1.019 | 0.993 | < 0.001 |
| Alcohol use disorder | 0.997 | 0.999 | 0.990 | 0.980 | 0.958 | 0.980 | 0.999 | 1.039 | 1.015 | 1.013 | 1.025 | 0.994 | < 0.001 |
| Neurocognitive disorders | 1.086 | 1.066 | 0.999 | 0.932 | 0.959 | 0.928 | 0.943 | 0.967 | 1.049 | 1.006 | 1.026 | 1.012 | < 0.001 |
| Alzheimer's disease | 1.079 | 1.071 | 0.987 | 0.937 | 0.967 | 0.940 | 0.961 | 0.931 | 1.073 | 1.004 | 1.016 | 1.011 | < 0.001 |
| Trauma‐ and stressor‐related disorders | 0.975 | 0.976 | 0.989 | 0.977 | 0.966 | 1.000 | 1.026 | 1.041 | 1.007 | 1.010 | 1.019 | 1.007 | < 0.001 |
| Posttraumatic stress disorder | 0.930 | 0.957 | 0.944 | 1.002 | 1.014 | 1.050 | 0.980 | 1.058 | 1.024 | 1.024 | 1.014 | 1.007 | 0.009 |
| Feeding and eating disorders | 0.983 | 0.980 | 0.979 | 0.957 | 0.969 | 1.045 | 1.020 | 1.038 | 1.031 | 1.038 | 0.976 | 0.979 | 0.006 |
| Anorexia nervosa | 1.057 | 1.006 | 0.889 | 0.932 | 0.981 | 1.012 | 0.886 | 1.029 | 1.041 | 1.072 | 1.097 | 0.967 | 0.042 |
| Bulimia nervosa | 0.999 | 0.946 | 0.961 | 0.902 | 0.982 | 1.046 | 1.011 | 1.064 | 1.043 | 1.049 | 0.976 | 1.006 | 0.003 |
Includes persons born from 1 January 1900, through 31 December 2013, and followed from 1 January 1995, through 31 December 2013. Data are expressed as general population (N, %) and the ratio of the relative risk of patients to that of the general population (RR). The gray background color indicates a RR ≥1.
Walter & Elwood's (W&E) test results for differences between months and 12‐month periodicity in patients with specified mental disorders, respectively. Bold type indicates statistical significance (p < 0.0025) in the W&E test.
FIGURE 1.
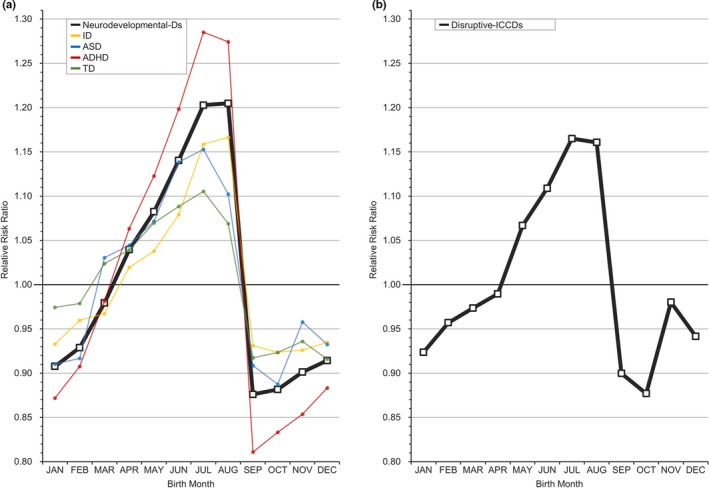
Relative risk ratio for childhood‐onset disorders by month. A, Neurodevelopmental‐Ds = neurodevelopmental disorders; ID = intellectual disability; ASD = autism spectrum disorder; ADHD = attention‐deficit/hyperactivity disorder; TD = tic disorder. B, Disruptive‐ICCDs = disruptive, impulse control, and conduct disorders [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
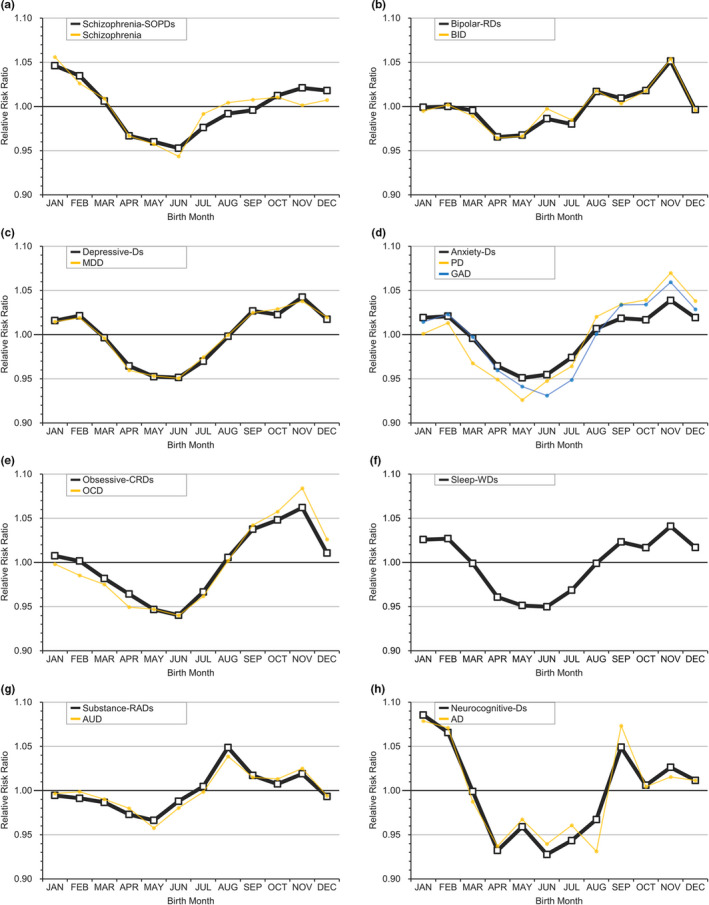
Relative risk ratio for mental disorders with significant periodicity by month. A, Schizophrenia‐SOPDs = schizophrenia spectrum and other psychotic disorders. B, Bipolar‐RDs = bipolar and related disorders; BID = bipolar I disorder. C, Depressive‐Ds = depressive disorders; MDD = major depressive disorder. D, Anxiety‐Ds = anxiety disorders; PD = panic disorder; GAD = generalized anxiety disorder. E, Obsessive‐CRDs = obsessive‐compulsive and related disorders; OCD = obsessive‐compulsive disorder. F, Sleep‐WDs = sleep‐wake disorders. G, Substance‐RADs = substance‐related and addictive disorders; AUD = alcohol use disorder. H, Neurocognitive‐Ds = neurocognitive disorders; AD = Alzheimer's disease [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
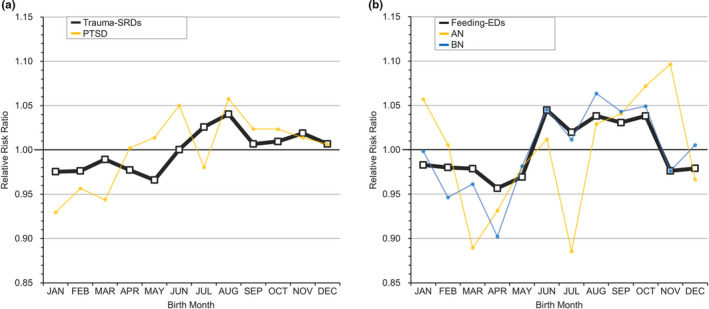
Relative risk ratio for mental disorders without significant periodicity by month. A, Trauma‐SRDs = trauma‐ and stressor‐related disorders; PTSD = posttraumatic stress disorder. B, Feeding‐EDs = feeding and eating disorders; AN = anorexia nervosa; BN = bulimia nervosa [Colour figure can be viewed at wileyonlinelibrary.com]
In a further systematic review, we identified 10 disorders (intellectual disability, ASD, ADHD, schizophrenia, BID, MDD, AUD, Alzheimer's disease, anorexia nervosa, and bulimia nervosa) in 51 articles conducted in 25 countries involving 1 539 811 patients.1, 3, 5, 6, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 The meta‐analytic results of our findings and included studies are summarized in Table 2. Similar to the previous 3 categories in the cohort, relatively young children have a higher risk of childhood‐onset disorders (intellectual disability, ASD, and ADHD); people born in the winter or autumn have a significantly higher risk of schizophrenia, BID, MDD, AUD, and Alzheimer's disease than those born in other seasons; and the risk of anorexia nervosa and bulimia nervosa does not apparently differ by any birth month or season. Heterogeneity varied across different MDs, and Egger's tests of ADHD and schizophrenia (≥ 10 included studies) did not identify any risk of publication bias. Further detailed information concerning the meta‐analysis is provided in the eResults, including the selection flowchart, exclusion reasons, characteristics and bias of the included studies, school enrollment month in all countries, heterogeneity results, forest plots, and funnel plots (Tables S7‐S12, Figures S7‐S19).
TABLE 2.
Meta‐analysis of relative risk ratio for mental disorders categorized by level of relative age, month of birth, or season of birth
| Mental disordersa | Classification | RR‐M (95% CI) by relative age levelc | p (X2 )b | |||
|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | |||
| Intellectual disability | Age | 0.922 (0.892–0.952) | 0.941 (0.924–0.959) | 1.016 (0.982–1.051) | 1.129 (1.080–1.180) | < 0.001 |
| Autism spectrum disorder | Age | 0.955 (0.937–0.973) | 0.980 (0.952–1.008) | 1.020 (0.996–1.045) | 1.046 (1.008–1.085) | < 0.001 |
| Attention‐deficit/hyperactivity disorder | Age | 0.869 (0.848–0.891) | 0.933 (0.909–0.956) | 1.067 (1.045–1.090) | 1.127 (1.092–1.162) | < 0.001 |
| RR‐M (95% CI) by birth month and birth season | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March | April Spring | May | June | July Summer | August | September | October Autumn | November | December | January Winter | February | ||||
| Schizophrenia | Month | 1.019 (0.979–1.060) | 0.992 (0.965–1.020) | 0.994 (0.973–1.016) | 0.973 (0.945–1.002) | 0.987 (0.965–1.010) | 0.965 (0.941–0.991) | 0.967 (0.942–0.993) | 0.981 (0.969–0.994) | 0.987 (0.965–1.010) | 1.046 (1.014–1.078) | 1.055 (1.033–1.077) | 1.007 (0.963–1.052) | < 0.001 | |
| Season | 1.002 (0.984–1.021) | 0.974 (0.960–0.989) | 0.978 (0.966–0.990) | 1.038 (1.019–1.057) | < 0.001 | ||||||||||
| Bipolar I disorder | Month | 1.031 (0.976–1.089) | 0.972 (0.946–1.000) | 0.983 (0.940–1.027) | 0.990(0.961–1.019) | 0.983(0.957–1.010) | 0.968 (0.919–1.019) | 0.977 (0.937–1.019) | 1.007 (0.981–1.033) | 1.022 (0.982–1.063) | 1.026 (0.977–1.077) | 1.039 (0.990–1.090) | 1.013 (0.986–1.041) | 0.149 | |
| Season | 0.993 (0.970–1.016) | 0.986 (0.968–1.004) | 1.000 (0.978–1.023) | 1.020 (1.001–1.041) | 0.083 | ||||||||||
| Major depressive disorder | Month | 1.015(0.984–1.047) | 0.980(0.959–1.001) | 0.996(0.965–1.027) | 0.984(0.952–1.017) | 1.000(0.972–1.030) | 1.004 (0.987–1.021) | 1.008 (0.993–1.024) | 1.005 (0.986–1.025) | 0.996 (0.966–1.027) | 1.010 (1.000–1.021) | 1.014 (1.004–1.025) | 1.006 (0.995–1.017) | 0.355 | |
| Season | 0.994 (0.980–1.009) | 0.993 (0.978–1.009) | 1.005 (0.993–1.016) | 1.010 (1.004–1.017) | 0.075 | ||||||||||
| Alcohol use disorder | Month | 0.993(0.969–1.017) | 0.973(0.914–1.034) | 0.989(0.924–1.057) | 0.984(0.959–1.009) | 1.001(0.977–1.026) | 1.027 (0.977–1.080) | 1.012 (0.989–1.036) | 1.013 (0.991–1.036) | 1.023 (1.00002–1.047) | 0.998 (0.974–1.021) | 0.995 (0.973–1.018) | 0.996 (0.972–1.021) | 0.518 | |
| Season | 0.980 (0.961–0.999) | 1.009 (0.985–1.032) | 1.016 (1.003–1.030) | 0.996 (0.983–1.010) | 0.014 | ||||||||||
| Alzheimer's disease | Month | 0.987(0.961–1.013) | 0.994(0.922–1.072) | 0.973(0.909–1.042) | 0.969(0.917–1.023) | 0.994(0.954–1.037) | 0.965 (0.920–1.014) | 1.038 (1.001–1.075) | 0.998 (0.972–1.025) | 0.999 (0.972–1.026) | 0.988 (0.962–1.015) | 1.039 (0.975–1.107) | 1.042 (0.991–1.095) | 0.251 | |
| Season | 0.985 (0.961–1.010) | 0.978 (0.954–1.002) | 1.012 (0.993–1.031) | 1.023 (0.995–1.051) | 0.032 | ||||||||||
| Anorexia nervosa | Month | 0.943(0.845–1.053) | 0.988(0.883–1.106) | 1.031(0.924–1.150) | 1.002(0.896–1.120) | 0.939(0.839–1.051) | 1.003 (0.899–1.118) | 0.995 (0.892–1.109) | 1.027 (0.909–1.161) | 1.042 (0.934–1.163) | 1.002 (0.897–1.118) | 1.011 (0.885–1.156) | 1.016 (0.909–1.135) | 0.983 | |
| Season | 0.987 (0.926–1.052) | 0.981 (0.920–1.046) | 1.022 (0.960–1.088) | 1.012 (0.950–1.078) | 0.774 | ||||||||||
| Bulimia nervosa | Month | 0.957(0.874–1.047) | 0.937(0.853–1.030) | 0.967(0.882–1.061) | 1.046(0.954–1.148) | 1.009(0.921–1.106) | 1.052 (0.964–1.148) | 1.040 (0.953–1.135) | 1.045 (0.960–1.137) | 0.982 (0.899–1.073) | 0.990 (0.906–1.082) | 1.002 (0.919–1.092) | 0.969 (0.884–1.063) | 0.720 | |
| Season | 0.954 (0.904–1.006) | 1.036 (0.983–1.091) | 1.023 (0.973–1.075) | 0.988 (0.938–1.040) | 0.124 | ||||||||||
Includes our data and 51 articles involving 10 disorders. Data were expressed as the ratio of the relative risk of patients to that of the general population after meta‐analysis (RR‐M) with a 95% confidence interval (CI). March replaces January as the first‐month column. The gray background color indicates that the RR‐M was significantly higher than 1.
Chi‐square (X2 ) test for heterogeneity among different levels, months, and seasons in patients with specified mental disorders. Bold type indicates statistical significance (p < 0.05) in the X2 test.
Relative age within the school year was determined by birth month and categorized into four 3‐month levels. Children in level 1 were the oldest, and those in level 4 were the youngest.
3.2. Childhood‐onset disorders
Figure 1 reveals the RRs of childhood‐onset disorders in this study (neurodevelopmental‐Ds, including intellectual disability, ASD, ADHD, and tic disorders, and disruptive‐ICCDs). The pattern of risk distribution was A‐shaped, with a highly pronounced drop between August and September. Moreover, six SAs of the diseases further showed similar distributions (Figure S4). When the MOB was converted to the relative age level in the school year, Taiwanese individuals born in level 1 (September to November, RR range: 0.81–0.98) were the oldest and had lower risks of childhood‐onset disorders than those born in level 4 (June to August, RR range: 1.07 to 1.29) as shown in Table 1. A similar finding was found in the current meta‐analysis of intellectual disability, ASD, and ADHD (Table 2), with a notably increased risk from level 1 to level 4 (intellectual disability, RR 0.92 [95% CI, 0.89 to 0.95] to 1.13 [1.08 to 1.18]; ASD, 0.96 [0.94 to 0.97] to 1.05 [1.01 to 1.09]; ADHD, 0.87 [0.85 to 0.89] to 1.13 [1.09 to 1.16]).
3.3. MDs with significant periodicity
Most major MDs in the DSM‐5 had apparent periodicity in risk by MOB (Table 1) as follows: schizophrenia‐SOPDs, bipolar‐RDs, depressive‐Ds, anxiety‐Ds, obsessive‐CRDs, sleep‐WDs, substance‐RADs, and neurocognitive‐Ds. The risk distributions of these disorders slightly differed, but their overall RRs showed an approximate U‐shaped pattern, with the lowest risk between March and July (mainly in the spring to summer) and the highest risk between August and February (mostly in the autumn or winter) as shown in Figure 2. In the 6 SAs of these disorders, the seasonality of some illnesses was attenuated (Table S5). Notable attenuations were observed in SA2 (fewer psychiatric comorbidities) and SA4 (early‐onset). In SA2, some diseases did not have obvious periodicity after limiting the patients to only those with one comorbidity, such as bipolar‐RDs, obsessive‐CRDs, and substance‐RADs. In SA4, a pattern similar to the A‐shaped distribution of the childhood‐onset disorders existed for many early‐onset MDs, such as schizophrenia‐SOPDs, bipolar‐RDs, depressive‐Ds, and anxiety‐Ds (Figure S5).
Regarding the meta‐analysis of these diseases (Table 2), significantly high RRs were observed in December and January for schizophrenia (1.05 [1.01 to 1.08] and 1.06 [1.03 to 1.08]), January for MDD (1.01 [1.004 to 1.03]), November for AUD (1.02 [1.00002 to 1.05]), and September for Alzheimer's disease (1.04 [1.001 to 1.08]). When MOB was converted to season, the RRs of schizophrenia, MDD, and AUD remained consistently high in their relative season (schizophrenia in the winter, 1.04 [1.02 to 1.06]; MDD in the winter, 1.01 [1.004 to 1.02]; AUD in the autumn, 1.02 [1.003 to 1.03]). Although the RR of Alzheimer's disease in the autumn did not reach significance, the RR in autumn‐winter was greater than 1 compared to that in spring‐summer. Moreover, BID had a relatively high RR between October and March (1.01 to 1.04) and a significantly high RR in the winter (1.02, [1.00 to 1.04]).
3.4. MDs without significant periodicity
The birth distributions of patients with PTSD (a component of trauma‐SRDs), feeding‐EDs, dissociative disorders, gender dysphoria, and paraphilic disorders lacked remarkable periodicity (W&E) (Table 1, Table S4). Regarding the sensitivity tests, the seasonality in all aforementioned groups, except for trauma‐SRDs, almost did not reach statistical significance (Table S5). Moreover, the findings of the meta‐analysis of anorexia nervosa and bulimia nervosa (components of feeding‐EDs) did not show significantly higher or lower RRs in any month or season (Table 2).
4. DISCUSSION
We used the Taiwanese national health insurance database and meta‐analyses to perform the first comprehensive assessment of the associations between MOBs and risks of MDs. We observed that starting school at a relatively younger age than peers was associated with increases in the prevalence of neurodevelopmental‐Ds, disruptive‐ICCDs, and many early‐onset MDs (findings from SA4). Especially for intellectual disability, ASD, and ADHD, our meta‐analyses suggested that the risk increased with younger age. Second, we found that the RRs in different MOBs exhibited 12‐month periodicity for schizophrenia‐SOPDs, bipolar‐RDs, depressive‐Ds, anxiety‐Ds, obsessive‐CRDs, sleep‐WDs, substance‐RADs, and neurocognitive‐Ds. The current meta‐analyses revealed significantly high RRs for schizophrenia, BID, and MDD in the winter and AUD in the autumn. Third, the MOB and RR of PTSD and feeding‐EDs had no notable periodicity in the cohort study, and the meta‐analysis of anorexia nervosa and bulimia nervosa showed no significant difference in any MOB.
Recent evidence demonstrated that the youngest children in their respective classes had the highest risks of being diagnosed with intellectual disability and ADHD.2, 6 Our study supports this phenomenon and extends the principle to other disorders (ASD, tic disorder, and disruptive‐ICCDs). We also conducted a meta‐analysis of those with intellectual disability, ASD, and ADHD, which strengthened the graded associations considering the oldest and youngest individuals. Compared with the RRs of peers in the same grade, the elevated RRs in younger individuals may result from relative physiological immaturity,6 potentially resulting in overdiagnosis in relatively younger children and underdiagnosis in relatively older children with intellectual disability, ASD, and ADHD. Furthermore, the study revealed an A‐shaped pattern, with a highly pronounced decrease between August (youngest) and September (oldest) in these childhood‐onset disorders. This pattern was also observed in some early‐onset MDs (SA4) in our study, which revealed increased RRs of psychotic disorders, mood disorders, and anxiety disorders among those born between June and August (relatively young at the start of school) as shown in Figure S5. Regarding the above findings of these disorders in childhood, attention should be paid to the impact of education policies on children's early life experience at school.
Most other MDs in the DSM‐5 also had significant 12‐month periodicity in Taiwan. These diseases subtly revealed a similar pattern, with the highest risk in individuals born in autumn‐winter. In the meta‐analyses, we found obvious risks of schizophrenia, BID, and MDD associated with winter births and AUD associated with autumn births. Many potential factors can explain seasonality.74 For example, in schizophrenia, one factor is low prenatal vitamin D due to low sunlight exposure in the winter,10 which is associated with prenatal changes in brain structures and functions, including altered dopaminergic functioning.75 Another factor is seasonal infection during the prenatal period, such as specified Viruses, Chlamydia, or Toxoplasma, which produce neurological damage before birth.12, 76 These mechanisms can influence a fetus's brain development and identify seasons in which mothers are highly susceptible during pregnancy. Moreover, our findings reveal a similar seasonal association in many MDs, which may result from a high proportion of comorbidity in MDs. First, a Danish population‐based study has shown that comorbidity in MDs was pervasive.22 This finding may indicate that some common factors affect clusters of MDs or that overlapping symptoms exist. Second, the SA2 (psychiatric comorbidities ≤1) in our study was a negative example (Table S5). The periodicity of some MDs, such as OCD and AUD, was attenuated when we restricted the analysis to patients who had no more than one psychiatric comorbidity. Taken together, current evidence suggests that people born in the same month have a similar biological vulnerability to a specific cluster of MDs or psychiatric symptoms. Future research concerning the pathogenesis of psychiatric illness by MOB should investigate clusters of disorders rather than single diseases to identify the common factors.
The relationships between MOBs and the RRs of the remaining illnesses were inconsistent (trauma‐SRDs and PTSD) or not significant (feeding‐EDs, including anorexia nervosa and bulimia nervosa). This study showed significant seasonality in trauma‐SRDs but not PTSD, which may be a consequence of the PTSD definition in the DSM‐5. A prerequisite for PTSD is that the individual must be exposed to or threatened with death, serious injury, or sexual violence.15 Hence, the occurrence of a major traumatic event may be independent of the effect of the MOB. Regarding the spectrum of feeding‐EDs, no 12‐month periodicity was observed in our cohort, and the meta‐analysis further indicated that anorexia nervosa and bulimia nervosa had no MOB or season difference. Additionally, dissociative disorders, gender disorders, and paraphilic disorders (Table S3‐S5) were not significantly associated with MOB. A previous report indicated that at least 4500 subjects are needed to obtain statistical significance in assessments of monthly distributions,77 which may be a reason.
Although our results show that MOB plays a role in the risk of some MDs, the magnitude of these effects does not seem to be large enough to influence parents’ decisions regarding the timing of pregnancy. For example, MOBs associated with most MDs show a high risk between September and January, but the birth number in the general population is still higher than the theoretical average birth rate (8.4%, normal proportion per month) as shown in Table 1. Furthermore, there are several limitations to our study. First, this study was subject to the usual limitations of a retrospective analysis of reimbursement data. Although we attempted to examine potential bias from observable baseline characteristics, unobserved confounders, such as the severity of the symptoms,67 family history,3 and prenatal exposures (vitamin or infection),75, 76 were lacking in the current study. Moreover, although we conducted a sensitivity analysis (SA1, the inclusion criteria of diagnosis to at least three times),21 the diagnosis of databases mainly relies on clinical judgment, which may include the variability of different doctors. Second, given prior work, this study only investigated the association between MDs and MOBs, including relative age or season.2, 7, 8 Our study did not determine the causes of the patterns of the associations.78 Further, this study matched the month in the country in the Southern Hemisphere to that in the Northern Hemisphere. Therefore, our research does not assess the impact of certain global environmental events that are not related to the Hemisphere‐dependent seasons. Third, to gain updated information and match our study period from 1995 to 2013, the meta‐analyses focused on reports in which patients were assessed within the last 30 years. Hence, we lacked epidemiological findings before 1990 and the most recent data. The experience with major national disaster or epidemic during the study period was not considered in the analyses.
According to nationwide data, MOB was significantly associated with the risk of most MDs classified in the DSM‐5. In the current meta‐analyses, children who were relatively young when they started school had an increased risk of a diagnosis of intellectual disability, ASD, and ADHD. Those born in the winter had a high risk of schizophrenia, BID, and MDD, and those born in the autumn had a high risk of AUD. Our study provides references for future research interests in the etiology of MDs, such as early life experience in school or prenatal exposure.
CONFLICT OF INTEREST
The authors report no potential conflicts of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors had contacted Professor Damiaan Denys, Holger J. Sørensen, Kenneth D. Gadow, Li‐Ching Lee, L. Stephen Miller, Mary Regina Boland, Preben Bo Mortensen, and Yeates‐Frederikx to request original data. The authors would like to thank the Biostatistics Center at the Kaohsiung Chang Gung Memorial Hospital, and the Health Information and Epidemiology Laboratory at the Chiayi Chang Gung Memorial Hospital for the assistance in data analysis. C‐WH, P‐TT, Y‐KT, P‐YL, C‐FH, L‐JW, and H‐YK contributed to study conception and data interpretation. C‐WH drafted the manuscript. C‐WH, L‐JW, C‐SL, Y‐YH, and Y‐HY contributed to data collection and analysis. All authors were involved in critically revising the article for important intellectual content and gave final approval of the version to be published. C‐WH, L‐JW, and H‐YK were responsible for supervision of the register‐based study. P‐TT, Y‐KT, and P‐YL were responsible for supervision of the meta‐analysis. The population‐based study is supported by the Chang Gung Memorial Hospital Research Project (CFRPG8H0261). The meta‐analysis is supported by the Ministry of Science and Technology, Taiwan (MOST 109‐2314‐B‐182A‐009‐MY2). The funding sources had no role in the design of the study.
Liang‐Jen Wang, Hung‐Yu Kao contributed equally as corresponding authors.
Funding information
Chang Gung Memorial Hospital Research Project Kaohsiung Chang Gung Memorial Hospital, Grant Numbers: CFRPG8H0261. Ministry of Science and Technology, Taiwan. Grant Numbers: 109‐2314‐B‐182A‐009‐MY2.
Contributor Information
Liang‐Jen Wang, Email: wangliangjen@gmail.com.
Hung‐Yu Kao, Email: hykao@mail.ncku.edu.tw.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available but can be accessed with permission from the National Health Insurance Administration, Ministry of Health and Welfare in Taiwan.
REFERENCES
- 1.Lee BK, Gross R, Francis RW, et al. Birth seasonality and risk of autism spectrum disorder. Eur J Epidemiol. 2019;34:785‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layton TJ, Barnett ML, Hicks TR, Jena AB. Attention deficit‐hyperactivity disorder and month of school enrollment. N Engl J Med. 2018;379:2122‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603‐608. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchiya KJ, Byrne M, Mortensen PB. Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord. 2003;5:231‐242. [DOI] [PubMed] [Google Scholar]
- 5.Disanto G, Morahan JM, Lacey MV, et al. Seasonal distribution of psychiatric births in England. PLoS One. 2012;7:e34866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Root A, Brown JP, Forbes HJ, et al. Association of relative age in the school year with diagnosis of intellectual disability, attention‐deficit/hyperactivity disorder, and depression. JAMA pediatrics. 2019;173(11):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G, Welham J, Chant D, Torrey EF, Mcgrath J. A systematic review and meta‐analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587‐593. [DOI] [PubMed] [Google Scholar]
- 8.Mcgrath JJ, Welham JL. Season of birth and schizophrenia: a systematic review and meta‐analysis of data from the Southern Hemisphere. Schizophr Res. 1999;35:237‐242. [DOI] [PubMed] [Google Scholar]
- 9.Tochigi M, Okazaki Y, Kato N, Sasaki T. What causes seasonality of birth in schizophrenia? Neurosci Res. 2004;48:1‐11. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JY, Ko JS, Chen RY, Ng EM. Meta‐regression analysis using latitude as moderator of paternal age related schizophrenia risk: high ambient temperature induced de novo mutations or is it related to the cold? Schizophr Res. 2008;99:71‐76. [DOI] [PubMed] [Google Scholar]
- 11.Mcgrath J, Selten JP, Chant D. Long‐term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration–data from Australia and the Netherlands. Schizophr Res. 2002;54:199‐212. [DOI] [PubMed] [Google Scholar]
- 12.Radua J, Ramella‐Cravaro V, Ioannidis JPA, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiat. 2018;17:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Segre G, Estradé A, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta‐analysis. The lancet Psychiatry. 2020;7:399‐410. [DOI] [PubMed] [Google Scholar]
- 14.Whitely M, Raven M, Timimi S, et al. Attention deficit hyperactivity disorder late birthdate effect common in both high and low prescribing international jurisdictions: a systematic review. J Child Psychol Psychiatry. 2019;60:380‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington, DC: American Psychiatric Pub.: American Psychiatric Association; 2013. [Google Scholar]
- 16.Schnittker J. Season of birth and depression in adulthood: Revisiting historical forerunner evidence for in‐utero effects. SSM Popul Health. 2018;4:307‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CW, Lee SY, Yang YH, Wang LJ. Brand‐name antidepressants outperform their generic counterparts in preventing hospitalization for depression: The real‐world evidence from Taiwan. Int J Neuropsychopharmacol. 2020;23(10):653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter SD, Elwood JM. A test for seasonality of events with a variable population at risk. Br J Prev Soc Med. 1975;29:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett AG, Baker PJ, Dobson AJ. season: Analysing Seasonal Data R Functions. 2020.
- 21.Cheng CM, Chang WH, Chen MH, et al. Co‐aggregation of major psychiatric disorders in individuals with first‐degree relatives with schizophrenia: a nationwide population‐based study. Mol Psychiatry. 2018;23:1756‐1763. [DOI] [PubMed] [Google Scholar]
- 22.Plana‐Ripoll O, Pedersen CB, Holtz Y, et al. Exploring comorbidity within mental disorders among a danish national population. JAMA Psychiatry. 2019;76(3):259‐270. 10.1001/jamapsychiatry.2018.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 24.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934‐939. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitiello B, Hill JL, Molchan SE, Martinez RA, Martinson HJ, Sunderland T. Lack of seasonal variation in the births of patients with dementia of the Alzheimer type. Psychiatry Res. 1991;39:21‐24. [DOI] [PubMed] [Google Scholar]
- 28.Torrey EF, Rawlings RR, Ennis JM, Merrill DD, Flores DS. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder and stillbirths. Schizophr Res. 1996;21:141‐149. [DOI] [PubMed] [Google Scholar]
- 29.Vezina H, Houde L, Charbonneau H, et al. Season of birth and Alzheimer's disease: a population‐based study in Saguenay‐Lac‐St‐Jean/Quebec (IMAGE Project). Psychol Med. 1996;26:143‐149. [DOI] [PubMed] [Google Scholar]
- 30.Balestrieri M, Rucci P, Nicolaou S. Gender‐specific decline and seasonality of births in operationally defined schizophrenics in Italy. Schizophr Res. 1997;27:73‐81. [DOI] [PubMed] [Google Scholar]
- 31.Jones IH, Hay DA, Kirkby KC, Daniels BA, Mowry BJ. Season of birth and schizophrenia in Tasmania. Aust N Z J Psychiatry. 1997;31:57‐61. [DOI] [PubMed] [Google Scholar]
- 32.Kunugi H, Nanko S, Watanabe H, Sekiba K, Kazamatsuri H. Season of birth of chronic alcoholics. J Psychiatr Res. 1998;32:321‐323. [DOI] [PubMed] [Google Scholar]
- 33.Landau EC, Cicchetti DV, Klin A, Volkmar FR. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29:385‐393. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg AE, Newlin DB. Season of birth and substance abuse: findings from a large national sample. Alcohol Clin Exp Res. 2000;24:774‐780. [PubMed] [Google Scholar]
- 35.Mino Y, Oshima I, Okagami K. Seasonality of birth in patients with mood disorders in Japan. J Affect Disord. 2000;59:41‐46. [DOI] [PubMed] [Google Scholar]
- 36.Morgan JF, Lacey JH. Season of birth and bulimia nervosa. Int J Eat Disord. 2000;27:452‐458. [DOI] [PubMed] [Google Scholar]
- 37.Parker G, Mahendran R, Koh ES, Machin D. Season of birth in schizophrenia: no latitude at the equator. Br J Psychiat. 2000;176:68‐71. [DOI] [PubMed] [Google Scholar]
- 38.Selten JP, Van Der Graaf Y, Dijkgraaf M, Edlinger M, Kahn R. Seasonality of schizophrenia and stillbirths in The Netherlands. Schizophr Res. 2000;44:105‐111. [DOI] [PubMed] [Google Scholar]
- 39.Suvisaari JM, Haukka JK, Tanskanen AJ, Lönnqvist JK. Decreasing seasonal variation of births in schizophrenia. Psychol Med. 2000;30:315‐324. [DOI] [PubMed] [Google Scholar]
- 40.De Messias EL, Cordeiro NF, Sampaio JJ, Bartko JJ, Kirkpatrick B. Schizophrenia and season of birth in a tropical region: relationship to rainfall. Schizophr Res. 2001;48:227‐234. [DOI] [PubMed] [Google Scholar]
- 41.Morgan VA, Jablensky AV, Castle DJ. Season of birth in schizophrenia and affective psychoses in Western Australia 1916–61. Acta Psychiatr Scand. 2001;104:138‐147. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz‐Delgado J, Pérez‐Rincón H, Nicolini H, et al. Season of birth in schizophrenia in the mexico city area: Comparison with general population. Biol Rhythm Res. 2003;34:485‐492. [Google Scholar]
- 43.Penas‐Lledo EM, Rodriguez Santos L, Vaz Leal FJ, Waller G. Pattern of birth in restrictive and bulimic eating disorders. Eat Behav. 2003;3:325‐328. [DOI] [PubMed] [Google Scholar]
- 44.Carrion‐Baralt JR, Fuentes‐Rivera Z, Schmeidler J, Silverman JM. A case‐control study of the seasonality effects on schizophrenic births on a tropical island. Schizophr Res. 2004;71:145‐153. [DOI] [PubMed] [Google Scholar]
- 45.Frazee J, Russell M, Chicota C, et al. Seasonality of birth in Alzheimer's disease. J Orthomol Med. 2004;19:162‐166. [Google Scholar]
- 46.Bembenek A, Kociuba L. Seasonality of birth in schizophrenia patients in Poland. Schizophr Res. 2005;75:447‐448. [DOI] [PubMed] [Google Scholar]
- 47.Bersani G, Pucci D, Gherardelli S, et al. Excess in the spring and deficit in the autumn in birth rates of male schizophrenic patients in Italy: potential role of perinatal risk factors. J Matern Fetal Neonatal Med. 2006;19:425‐431. [DOI] [PubMed] [Google Scholar]
- 48.Jordaan E, Niehaus DJH, Koen L, Seller C, Mbanga I, Emsley RA. Season of birth, age and negative symptoms in a Xhosa schizophrenia sample from the Southern Hemisphere. Aust N Zealand J Psychiat. 2006;40:698‐703. [DOI] [PubMed] [Google Scholar]
- 49.Messias E, Mourao C, Maia J, et al. Season of birth and schizophrenia in Northeast Brazil: relationship to rainfall. J Nerv Ment Dis. 2006;194:870‐873. [DOI] [PubMed] [Google Scholar]
- 50.Mino Y, Oshima I. Seasonality of birth in patients with schizophrenia in Japan. Psychiatry Clin Neurosci. 2006;60:249‐252. [DOI] [PubMed] [Google Scholar]
- 51.Atladottir HO, Parner ET, Schendel D, Dalsgaard S, Thomsen PH, Thorsen P. Variation in incidence of neurodevelopmental disorders with season of birth. Epidemiology. 2007;18:240‐245. [DOI] [PubMed] [Google Scholar]
- 52.Button E, Aldridge S. Season of birth and eating disorders: patterns across diagnoses in a specialized eating disorders service. Int J Eat Disord. 2007;40:468‐471. [DOI] [PubMed] [Google Scholar]
- 53.Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res. 2010;3:185‐190. [DOI] [PubMed] [Google Scholar]
- 54.Janík P, Novotný V, Kešický D. Month of birth as the schizophrenia risk factor. Ceska Slov Psychiatr. 2010;106:15‐21. [Google Scholar]
- 55.Disanto G, Handel AE, Para AE, Ramagopalan SV, Handunnetthi L. Season of birth and anorexia nervosa. Br J Psychiat. 2011;198:404‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zerbo O, Iosif AM, Delwiche L, Walker C, Hertz‐Picciotto I. Month of conception and risk of autism. Epidemiology. 2011;22:469‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrow RL, Garland EJ, Wright JM, Maclure M, Taylor S, Dormuth CR. Influence of relative age on diagnosis and treatment of attention‐deficit/hyperactivity disorder in children. CMAJ. 2012;184:755‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vellisca MY, Latorre JI, Santed MA, Reales JM. Absence of seasonal pattern of birth in patients with anorexia nervosa. Int J Eat Disord. 2013;46:86‐88. [DOI] [PubMed] [Google Scholar]
- 59.Vellisca MY, Latorre JI, Santed MA, Reales JM, Orejudo S, Cañete M. Lack of pattern of birth in patients with bulimia nervosa. Int J Eating Dis. 2013;46:690‐692. [DOI] [PubMed] [Google Scholar]
- 60.Winje E, Torgalsbøen A‐K, Brunborg C, Lask B. Season of birth bias and bulimia nervosa–results from a multi‐centre collaboration. Eur Eat Disord Rev. 2013;21:170‐174. [DOI] [PubMed] [Google Scholar]
- 61.Winje E, Torgalsboen AK, Brunborg C, Lask B. Season of birth bias and anorexia nervosa: results from an international collaboration. Int J Eat Disord. 2013;46:340‐345. [DOI] [PubMed] [Google Scholar]
- 62.Halldner L, Tillander A, Lundholm C, et al. Relative immaturity and ADHD: findings from nationwide registers, parent‐ and self‐reports. J Child Psychol Psychiatry. 2014;55:897‐904. [DOI] [PubMed] [Google Scholar]
- 63.Park SC, Sakong JK, Koo BH, et al. Potential relationship between season of birth and clinical characteristics in major depressive disorder in Koreans: Results from the CRESCEND study. Yonsei Med J. 2016;57:784‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwandt H, Wuppermann A. The youngest get the pill: ADHD misdiagnosis in Germany, its regional correlates and international comparison. Labour Economics. 2016;43:72‐86. [Google Scholar]
- 65.Tolppanen A‐M, Ahonen R, Koponen M, et al. Month and season of birth as a risk factor for Alzheimer's Disease: A nationwide nested case‐control study. J Prev Med Public Health. 2016;49:134‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlstad O, Furu K, Stoltenberg C, Haberg SE, Bakken IJ. ADHD treatment and diagnosis in relation to children's birth month: Nationwide cohort study from Norway. Scand J Public Health. 2017;45:343‐349. [DOI] [PubMed] [Google Scholar]
- 67.Kim JS, Park CM, Choi JA, et al. The association between season of birth, age at onset, and clozapine use in schizophrenia. Acta Psychiatr Scand. 2017;136:445‐454. [DOI] [PubMed] [Google Scholar]
- 68.Whitely M, Lester L, Phillimore J, Robinson S. Influence of birth month on the probability of Western Australian children being treated for ADHD. Med J Aust. 2017;206:85. [DOI] [PubMed] [Google Scholar]
- 69.Boland MR, Parhi P, Li L, et al. Uncovering exposures responsible for birth season ‐ disease effects: a global study. J Am Med Inform Assoc. 2018;25:275‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao Q, Liu L, Li H‐M, et al. Interaction between season of birth and COMT Val158Met (rs4680) in ADHD in a large sample of Chinese Han Participants. J Attention Dis. 2018;22:886‐895. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira FR, De Paula GC, De Carvalho RJV, Ribeiro‐Barbosa ER, Spini VBMG. Impact of season of birth on psychiatric disorder susceptibility and drug abuse incidence in a population from the Köppen Tropical Savanna Region of Brazil. Neuropsychobiology. 2020;79(2):131–140. 10.1159/000503069 [DOI] [PubMed] [Google Scholar]
- 72.Karlsson H, Dal H, Gardner RM, Torrey EF, Dalman C. Birth month and later diagnosis of schizophrenia. A population‐based cohort study in Sweden. J Psychiatr Res. 2019;116:1‐6. [DOI] [PubMed] [Google Scholar]
- 73.Szoke A, Pignon B, Schurhoff F. Schizophrenia risk factors in exceptional achievers: a re‐analysis of a 60‐year‐old database. Sci Rep. 2019;9:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown AS. The environment and susceptibility to schizophrenia. Prog Neurogibol. 2011;93:23‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mcgrath JJ, Burne TH, Feron F, Mackay‐Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10‐year update. Schizophr Bull. 2010;36:1073‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arias I, Sorlozano A, Villegas E, et al. Infectious agents associated with schizophrenia: a meta‐analysis. Schizophr Res. 2012;136:128‐136. [DOI] [PubMed] [Google Scholar]
- 77.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28:1‐38. [DOI] [PubMed] [Google Scholar]
- 78.Valente MJ, Pelham WE, Smyth H, Mackinnon DP. Confounding in statistical mediation analysis: What it is and how to address it. J Couns Psychol. 2017;64:659‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are not publicly available but can be accessed with permission from the National Health Insurance Administration, Ministry of Health and Welfare in Taiwan.


