Abstract
Marine reserves are a key tool for the conservation of marine biodiversity, yet only ~2.5% of the world's oceans are protected. The integration of marine reserves into connected networks representing all habitats has been encouraged by international agreements, yet the benefits of this design has not been tested empirically. Australia has one of the largest systems of marine reserves, providing a rare opportunity to assess how connectivity influences conservation success. An Australia‐wide dataset was collected using baited remote underwater video systems deployed across a depth range from 0 to 100 m to assess the effectiveness of marine reserves for protecting teleosts subject to commercial and recreational fishing. A meta‐analytical comparison of 73 fished species within 91 marine reserves found that, on average, marine reserves had 28% greater abundance and 53% greater biomass of fished species compared to adjacent areas open to fishing. However, benefits of protection were not observed across all reserves (heterogeneity), so full subsets generalized additive modelling was used to consider factors that influence marine reserve effectiveness, including distance‐based and ecological metrics of connectivity among reserves. Our results suggest that increased connectivity and depth improve the aforementioned marine reserve benefits and that these factors should be considered to optimize such benefits over time. We provide important guidance on factors to consider when implementing marine reserves for the purpose of increasing the abundance and size of fished species, given the expected increase in coverage globally. We show that marine reserves that are highly protected (no‐take) and designed to optimize connectivity, size and depth range can provide an effective conservation strategy for fished species in temperate and tropical waters within an overarching marine biodiversity conservation framework.
Keywords: fully protected areas, marine conservation, marine protected areas, marine reserve design, marine reserve effectiveness, meta‐analysis, sanctuaries
An Australia‐wide dataset was collected using baited remote underwater video systems deployed across a depth range from 0 to 100 m to assess the effectiveness of marine reserves for protecting fished species. A meta‐analytical comparison of 73 fished species within 91 marine reserves found that, on average, marine reserves had 28% greater abundance and 53% greater biomass of fished species compared to adjacent areas open to fishing. We show that marine reserves that are highly protected (no‐take) and designed to optimize connectivity, size and depth range can provide an effective conservation strategy for fished species in temperate and tropical waters.

1. INTRODUCTION
Marine reserves exist across the world's oceans with the broad objective of contributing to the conservation of marine biodiversity through the exclusion of extractive activities such as fishing. Some of the most tangible measures of ecological changes within marine reserves are increased abundance, size and biomass of fished species (Lester et al., 2009), which make up an important component of marine biodiversity. The protection of large‐bodied individuals, with higher fecundity may result in significant larval supply (Marshall et al., 2019) or spillover of adult fish (Di Lorenzo et al., 2016, 2020), hence providing benefits to adjacent fished areas. However, while the parties to the convention on biological diversity agreed to protect 10% of their coastal and marine waters by 2020, only 2.5% of the global ocean is presently within highly protected marine reserves (Sala et al., 2018), with striking differences across sea basins (Claudet et al., 2020).
Previous meta‐analyses over continental to global scales have demonstrated that the conservation benefits of marine reserves depend on the taxa considered and increase with reserve size, age, level of protection, enforcement and management effectiveness (Bergseth et al., 2015; Claudet et al., 2008, 2010; Di Lorenzo et al., 2020; Edgar et al., 2014; Gill et al., 2017; Lester et al., 2009; Mosquera et al., 2000; Zupan et al., 2018). While empirical evidence is limited (Grorud‐Colvert et al., 2014; Harrison et al., 2020), theory suggests that the ability of marine reserves to improve ecosystem resilience and benefit ecosystem services, such as fisheries, will depend on the extent to which reserves are connected (Ballantine, 2014; Botsford et al., 2009). Some studies recognize connectivity (i.e. demographic links among assemblages via the dispersal of individuals as adults, juvenile and larvae) as a key component in the design of marine reserve networks (Álvarez‐Romero et al., 2018; Tittensor et al., 2019), while others suggest it is less important (Costello & Connor, 2019). An empirical assessment of connectivity between reserves, at an appropriately large spatial scale, is therefore required. Similarly, the extent of fishing adjacent to marine reserves combined with distal social drivers can have a strong effect on local fish assemblages and should be considered in any large‐scale assessment of marine reserves. For example, Cinner et al. (2018), found that fish biomass within marine reserves, declines with increasing human impacts outside of reserves, and areas with moderate human impacts show the greatest differences in fish biomass.
The influence of social, ecological and design factors on marine reserve effectiveness has also differed among studies. For example, Halpern (2003) found that reserve size did not influence fish abundance within reserves, yet subsequent empirical studies and meta‐analyses found that large reserves are more effective in promoting biomass and abundance (Claudet et al., 2008; Edgar et al., 2014; Malcolm et al., 2016). Differences among studies may be due to data being sourced from different social and ecological systems such that the range of marine reserve sizes differs among studies, or over‐representation of significant results in the published literature compared to null results (Graham et al., 2011). In addition, meta‐analyses often pool data collected using different methods, yet this may not always be appropriate (Cresswell et al., 2019). Species‐level measures of fish size and abundance can vary considerably among methods (Murphy & Jenkins, 2010) and as such may influence the ability to detect spatial differences in fishes (Goetze et al., 2015).
One way to avoid the confounding effects of different methodologies in meta‐analyses is to only consider data collected by a single technique. Baited remote underwater video systems (BRUVs) are now commonly used for monitoring and research, providing non‐extractive, spatially extensive data for the assessment of the relative abundance of fishes and, when stereo systems are used, length and biomass. This method is especially adept at detecting mobile predatory species (Goetze et al., 2015; Harvey et al., 2018; Watson et al., 2005), many of which are targeted by fishers. Moreover, BRUVs have repeatedly recorded the direct effects of fishing on the abundance, biomass and/or size of targeted species (Goetze et al., 2011; Langlois et al., 2012; Malcolm et al., 2007) and are more likely to detect differences in fish abundance between marine reserves and fished areas than underwater visual census (UVC) due to bait increasing the proportion of predatory species surveyed (Goetze et al., 2015; Willis & Babcock, 2000). BRUVs are, however, limited to relative estimates of abundance/biomass due to variation in the bait plume (which prevents the calculation of a definitive sampling area; Harvey et al., 2007) and have a limited ability to survey cryptic species (Watson et al., 2005). There is also the potential for changes to fish behaviour when they approach baited cameras, which can influence abundance estimates (Dunlop et al., 2015), although this is most problematic in downward facing BRUVs which have a limited field of view (Coghlan et al., 2017; Cundy et al., 2017). Conversely, most broad‐scale assessments of marine reserve have used UVC to survey reef fishes, which limits these studies to shallow waters (e.g. a mean depth of 7.5 m in Edgar et al., 2018). Like BRUVs, UVC is also subject to biases. For example, divers can cause behavioural responses of fish, which in some locations has exaggerated the effectiveness of marine reserves (Gray et al., 2016; Januchowski‐Hartley et al., 2015; Lindfield, Harvey, et al., 2014). Importantly, BRUVs are a remote technique, removing the biases associated with divers and enabling assessments of marine reserves over a much greater depth range (Whitmarsh et al., 2017), and are being used to study fisheries‐targeted communities globally (MacNeil et al., 2020).
In Australia, BRUVs have been used extensively over a range of depths and habitats to answer a broad range of ecological questions, including those related to marine reserves (Harvey et al., 2021). Both state and federal governments have aimed to establish a comprehensive, adequate and representative system of marine reserves (Kenchington, 2016), providing a unique opportunity to assess a broad range of marine reserves. Here, we make use of a national‐scale BRUVs dataset to empirically assess a snapshot of the effect that Australian marine reserves have had on the abundance and biomass of fishes over a broad depth‐range. We use meta‐analyses to investigate how relative measures of fish abundance and biomass vary among these reserves. Uniquely, our analysis considers the influence of depth over ranges previously not considered (0–100 m) in similar studies (Edgar et al., 2018), as well as the role of ecological and distance‐based connectivity of marine reserves. The inclusion of these factors, with others known to affect the abundance or biomass of fish within marine reserves, enables a comprehensive assessment of which are most important when designing marine reserve networks.
2. METHODS
2.1. Selection criteria and data evaluation
Australia has one of the largest systems of marine reserves, with more than 400 reserves (CAPAD, 2020), covering an area close to 1 million km2 (mpatlas.org 2020). However, most bioregions across Australia still have less than 10% of their waters within marine reserves (Roberts et al., 2018). The size range of marine reserves in Australia is also extensive, ranging from <0.1 to >100,000 km2 (Department of Agriculture, Water and the Environment 2020). We utilized a national dataset compiled in GlobalArchive (https://globalarchive.org/) consisting of both single camera and stereo‐BRUVs deployments, covering the Australian continental shelf, across six states and five marine regions. Survey data were collected and analysed using standard operating procedures for BRUVs (Langlois et al., 2020). Bait type (pilchards), the method of recording abundance (MaxN; calculated as the maximum number of individuals of a given species present in a single video frame; Priede et al., 1994) and taxonomic resolution (species level where possible), were consistent across 19,260 BRUVs replicates, deployed between 2004 and 2017. MaxN is a conservative estimate of relative abundance that has become the standard metric for BRUVs as it avoids double counting of individuals (Langlois et al., 2020) and tracks absolute abundance well for predatory species which are often observed in lower abundance (MacNeil et al., 2020). There were differences across studies in the quantity of bait, cameras used, single versus stereo systems and separation distance (discussed in detail in Harvey et al., 2021), however, these factors did not vary for each marine reserve/control pair and log‐ratio effect sizes were used to account for between study variation (described below).
The following criteria were used to select data that were suitable for assessing the effectiveness of marine reserves; (1) BRUVs collected data on demersal fishes with at least two replicates inside and two replicates outside of a zone designated as a sanctuary, marine national park, conservation park/area or no‐take following Australian nomenclature used in different state and commonwealth waters (herein referred to as marine reserves). While this includes some partially protected areas that allow fishing within a proportion of their boundaries (e.g. fishing from shore or trolling for pelagic species), all were designated as no‐take for demersal fishing from a boat and the extent of fishing regulations were taken into account as a covariate (see regulation classification below); (2) sampled during daylight hours at depths less than 100 m, (3) relative abundance data (MaxN) was available for fished species and (4) only the most recent inside/outside assessment was used if temporal sampling had occurred, unless a dataset had greater replication and was completed less than 2 years earlier. In this case the study with greater replication was used. (5) Paired inside/outside assessments were only taken from the same field campaign (so were completed at the same time of year).
To ensure the appropriate controls were assigned for each marine reserve, the spatial layout of data was overlayed on satellite imagery with reserve boundaries and the closest sites across similar broad‐scale geography (e.g. exposure/distance from shore) either side of each reserve were assigned as controls. This was done in consultation with the researchers that had originally designed each study and three studies were deemed to have inadequately sampled the marine reserve (e.g. had <4 replicate BRUVs deployed in marine reserves >100 km2) so were removed from the analysis (Data S1). A total of 91 individual marine reserves were included after selection criteria and data evaluation, representing ~25% of marine reserves across Australia (CAPAD, 2020; Supp I).
2.2. Response variables
Species were classified as ‘fished’ if they were retained as food fish by either recreational or commercial fishers in Australia, using the expert knowledge of fisheries managers across each state (listed in Data S2). Non‐targeted species (classified as all remaining species) were also examined to provide a control for fished species. Relative abundance data were available for all 91 marine reserves; however, length data were only collected in studies using stereo‐BRUVs, hence relative biomass data were available for 69 marine reserves.
2.3. Meta‐analysis
The average abundance and biomass (based on MaxN) was calculated per replicate inside and outside of each marine reserve. Effect sizes were modelled as log‐ratios to quantify differences in the average abundance and biomass (of both fished and non‐target species) inside relative to outside for each marine reserve. In cases where fish were absent either inside or outside the reserve (i.e. zero values), one individual fish and the average (mean) weight of an individual fish was added to one replicate (inside and outside the reserve) to allow calculation of the log ratio for abundance and biomass, following Thiault et al. (2019). Effect sizes were calculated as follows:
where Em , i is the log response ratio for each marine reserve i based on the metric m (abundance or biomass) and and are the mean of each metric m in protected (P) and fished (F) areas respectively.
Variance of the effect sizes were calculated as:
where is the variance associated with the effect size Em,i , σi is the standard deviations associated with the mean, ni is the number of replicates, and the means for the protected (P) and fished areas (F).
We then used a mixed‐effects weighted meta‐analysis where weights of each individual effect size incorporate these variances as follows:
where wm,i is the weight associated to each effect Em,i , vEm,i is the within study variance for each marine reserve i using the metric m and vm,a is the among‐study variance across marine reserves for each metric. The among‐study variance was obtained using the generalized equation reported in Hedges and Pigott (2004). Confidence intervals for group and overall effect sizes were derived from a Student's t statistic. The among‐study variance was calculated using the restricted maximum likelihood estimator with the metafor package (Viechtbauer, 2010) in the statistical program R (R Core Team, 2018). Log‐ratio effect sizes were converted back to percentage differences in text to assist with the interpretation of magnitudes.
2.4. Habitat analysis
To ensure studies had sampled comparable habitat and depths inside compared to outside of each marine reserve, information on the mean relief, depth and percentage composition of biotic reef was collected following the procedures outlined in (Langlois et al., 2020). Paired t‐tests compared the means for each of these factors inside/outside of each marine reserve. For assessments with a significant difference in habitat or depth inside compared to outside the reserve (p < 0.05), outlying replicates were removed until no significant differences were found (p > 0.05). As a result, the habitat sampled was balanced inside versus outside and these variables were not considered as covariates when modelling.
2.5. Factors influencing marine reserve protection
For each marine reserve, we collated information on the size (total area in km2) and age based on the time between active enforcement of the reserve regulations and sampling (CAPAD, 2020). Depth was calculated as the average depth of BRUVs deployed within the marine reserve. The influence of fishing pressure was assessed using a modification of the human gravity metric (Cinner et al., 2018). Gravity was calculated as the sum of the human population within a 200‐km radius of each marine reserve (using the LandScan 2011 human population grid) divided by the distance (km) from the marine reserve to the nearest town centre. Distance to boat ramp was calculated as the average distance (km) from the marine reserve to the nearest boat ramp. To determine the protection level and level of exploitation in each marine reserve and fished site, respectively, we used the regulation‐based classification system for marine protected areas of Horta e Costa et al. (2016). This system gives a score from one to eight based on the number and potential impact of different fishing gears, other human activities (e.g. aquaculture) and accessibility (e.g. no anchoring) on fishes and their environment. We used the difference in zone classification scores between each marine reserve and its corresponding control/fished areas as a measure of the level of protection afforded by each marine reserve (herein referred to as the regulation difference). Note we assume that the classification scores represent a linear relationship with the impact of restrictions, given this could not be calculated. Compliance was categorized into three levels by local park authorities or researchers with substantial experience working in the area: high (infrequent breaches of management rules), moderate (occasional breaches of management rules) and low (frequent breaches of management rules).
2.6. Connectivity
To explore the influence of ‘demographically significant’ connectivity based on a biophysical model of larval fish dispersal (Treml et al., 2012), we summarized the total relative in‐flow for each marine reserve location, from a recent study quantifying the ecological connectivity among Australia's MPA system (Roberts et al., 2020). In‐flow is a relative measure of connectivity representing the amount of incoming larvae into a destination site and does not include local retention (Young et al., 2020). Only protected patches were used to calculate connectivity, where an upstream connection was considered protected, if it contained a protected area (see Roberts et al., 2020 for details). This analysis was based on the ecological connectivity of wrasses (labridae) as they are relatively well represented in our fished species list (9 species; Data S2), while other species presented in Roberts et al. (2020) were not included in our analysis. However, the top models did not change when large‐bodied, long‐range dispersers were considered (Trevally), and ecological connectivity was absent from top models when small‐bodied, non‐targeted Damselfish were considered (Data S3).
A distance‐based connectivity metric was also calculated by summing the number of spatial connections a marine reserve has to all other marine reserves within a 50‐km radius. A 50‐km radius was chosen to empirically test if there are conservation benefits based on the recommendation made by Almany et al. (2009), to ensure that between‐reserve distance is ≤50 km. This distance provides a conservative estimate of a distance‐based connectivity that ensures zones are demographically connected for most fish species (Almany et al., 2009). For each marine reserve, potential connection points were spaced 1 km along the boundary, using a random starting position. Points that fell on a boundary attached to land were removed so that distance‐based connectivity was only assessed using boundary points connected by sea. The distance‐based connectivity of each marine reserve was calculated by summing the number of points that connected to other points belonging to neighbouring marine reserves within a 50‐km Euclidean distance radius (or vector). Vectors that intercepted land (e.g. a headland) were excluded from analysis. Analysis was completed using the ‘EucDistance’ function arcpy python library in ESRI ArcPro version 2.4, with planar coordinates within marine reserve boundary points (GDA94 Geoscience Australia Lambert projection; EPSG:3112). Figure 1a shows a conceptual diagram of how distance‐based connectivity was calculated and Figure 1b shows the distance‐based connectivity scores for the 91 marine reserves sampled. We also calculated distance‐based connectivity for all marine reserves within the 2018 Collaborative Australian Protected Area Database (CAPAD, 2020), to highlight gaps in the use of marine reserves across Australia (Data S4). Distance‐based connectivity of marine reserves was highest on the Great Barrier Reef, as there is a high density of marine reserves compared to other locations around Australia (Figure 1b). Clusters of relatively high distance‐based connectivity were also observed in the Ningaloo Marine Park, Western Australia, the Encounter Marine Park (Adelaide/Kangaroo Island) in South Australia and Moreton Bay Marine Park in Queensland. No distance‐based connectivity was observed between marine reserves in Victoria or Tasmania. There were also two significant gaps in use of marine reserves, one in the Northern Territory and northern Queensland (to the start of the Great Barrier Reef Marine Park) and the other across the southern coast from south‐west Western Australia to South Australia, including the Great Australian Bight (Data S4).
FIGURE 1.
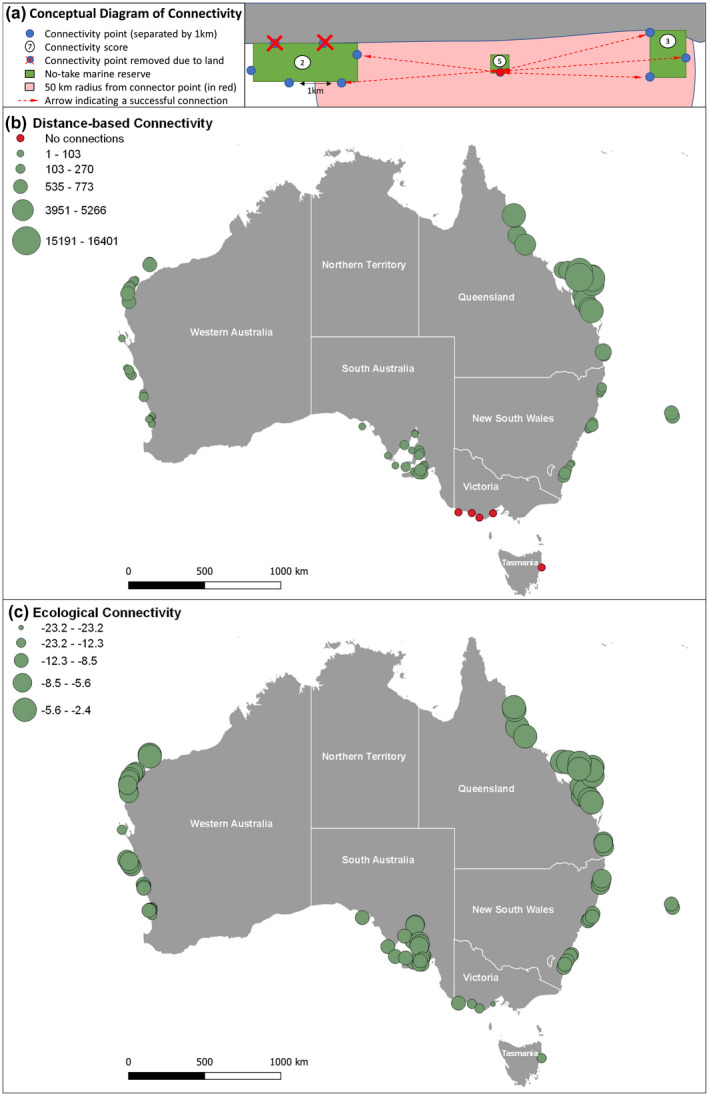
(a) A conceptual diagram showing how distance‐based connectivity was calculated for a small marine reserve in between two large reserves with land boundaries. (b) The resulting map of distance‐based connectivity marine reserves and (c) the ecological connectivity (based on wrasses) for the sampled marine reserves across Australia using Jenks natural breaks. R = 0.815 between distance‐based and ecological connectivity
The two measures of connectivity were termed; ecological connectivity (which was based on realistic oceanography, biology and habitat characteristics; Figure 1c) and distance‐based connectivity (which incorporated the spatial distance‐based connectivity of highly protected reserves based on an estimate of a suitable distance for demographic connectivity of fishes; Almany et al., 2009). Ecological connectivity was calculated independent of local larval retention and therefore the size of the focal marine reserve, while the distance‐based metric was dependent on the size of the focal marine reserve.
2.7. Models
The influence of marine reserve characteristics (size, age, compliance, ecological and distance‐based connectivity, depth, gravity, distance to boat ramp and regulation difference) and location covariates (marine region and state) on abundance and biomass effect sizes were investigated using weighted generalized additive mixed models (GAMMs; Lin & Zhang, 1999). The distribution of continuous predictors (depth, age, size, connectivity and gravity) was examined and transformed appropriately to ensure they were evenly distributed across their range. We examined the possibility of any spatial correlation in the data based on latitude and longitude using a variogram, which showed no evidence of spatial autocorrelation and therefore no spatial correlation structure was used in the models (Data S5). Any effects of State or Marine Region were included as potential fixed effects in models rather than random effects, as they were highly correlated with several continuous predictors (Data S6). A weighted full subsets method was used to fit models of all possible combinations up to a maximum of three variables (Fisher et al., 2018). To avoid multicollinearity issues, predictor variables with Pearson correlations (or an equivalent approximation) greater than 0.33 were not included in the same model (Data S6). The correlation cut‐off value was increased from the recommended value of 0.28 (based on Graham, 2003), to allow simultaneous inclusion of the covariates size and age which are known to influence marine reserve effectiveness (Claudet et al., 2008; Edgar et al., 2014). This represents a marginal increase to a very conservative cut‐off and is unlikely to cause issues with bias in parameter estimates. In all models the smoothing parameter was limited to a simple spline, allowing only monotonic relationships (k = 3). Model selection was based on Akaike's information criterion for small sample sizes (AICc; Akaike, 1998) and AICc weights (ωAICc; Burnham & Anderson, 2007). Models with AICc values that differ by less than two units show weak evidence for favouring one over the other (Burnham & Anderson, 2004; Raftery, 1995). The best models were therefore the ones within two AICc units of the lowest AICc values. The ωAICc, which represent probabilities or weights of evidence for each model, were used to facilitate interpretation of the best models. Relative support for each predictor variable was obtained by calculating the summed wAIC across all subsets of models containing that variable to obtain its relative importance which were plotted in R. Importance plots and P‐values derived with the GAM model summaries (Wood, 2013) were used to assess whether a significant relationship with effect sizes and covariates existed, which were subsequently plotted in R. Effect sizes were modelled with a Gaussian distribution using gam() in the mgcv package in R (Wood, 2011). The R language for statistical computing (R Core Team, 2018) was used for all data manipulation (dplyr, Wickham et al., 2018) and graphing (ggplot2, Wickham, 2009).
3. RESULTS
On average, Australian marine reserves had a 28% greater abundance and 53% greater biomass of fished species compared to areas open to fishing (Figure 2a,b). There were no effects of protection on the abundance or biomass of non‐targeted species. There was heterogeneity across effect sizes, suggesting considerable variation in the effectiveness of marine reserves across Australia. For the abundance of fished species across the 91 reserves studied, we observed 60 null (66%), 25 positive (27.5%) and six negative (6.5%) effect sizes (Figure 2c). Positive and negative effect sizes for abundance were detected in all states, except for Tasmania (where only one marine reserve was sampled). For biomass of fished species across the 69 reserves studied, we observed 46 null (66.5%), 20 positive (29%) and three negative (4.5%) effect sizes (Figure 2d). Positive effect sizes for biomass also occurred across all states; however, no negative effect sizes were observed in NSW or Victoria.
FIGURE 2.
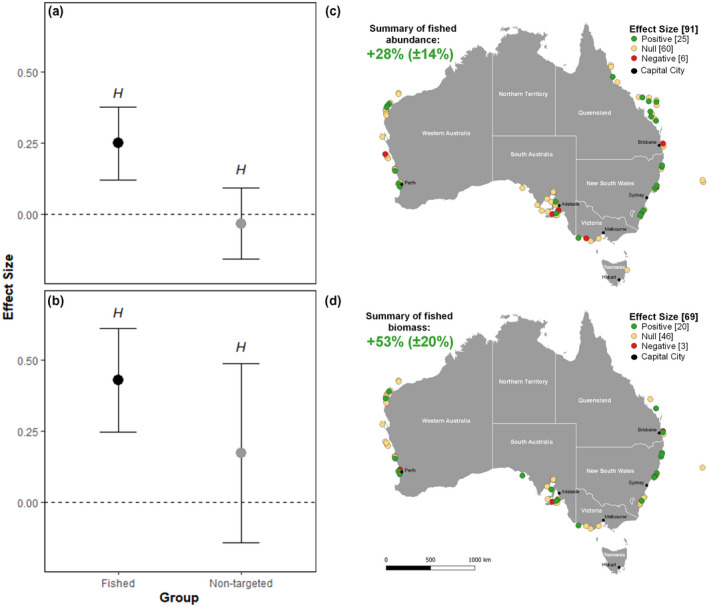
The log ratio effect sizes of (a) abundance and (b) biomass of fished and non‐targeted species inside/outside of marine reserves across Australia. Black dots represent significant results where the 95% confidence interval does not overlap zero. The superscript H indicates that significant heterogeneity (H < 0.05) was associated with the effect size. Effect sizes are converted back to percentages and the spatial extent shown for (c) abundance and (d) biomass of fished species only; green points represent a marine reserve with a significantly greater abundance or biomass of fished species; yellow a marine reserve where confidence levels overlapped zero and red where a significantly lower abundance was observed within marine reserve boundaries compared to nearby fished sites
The five most important variables for explaining variation in marine reserve effectiveness for fished abundance were age, size, regulation difference, distance‐based connectivity and depth (Figure 3). The five most important variables for explaining variation in marine reserve effectiveness for fished biomass were depth, regulation difference, age, ecological connectivity and distance‐based connectivity.
FIGURE 3.
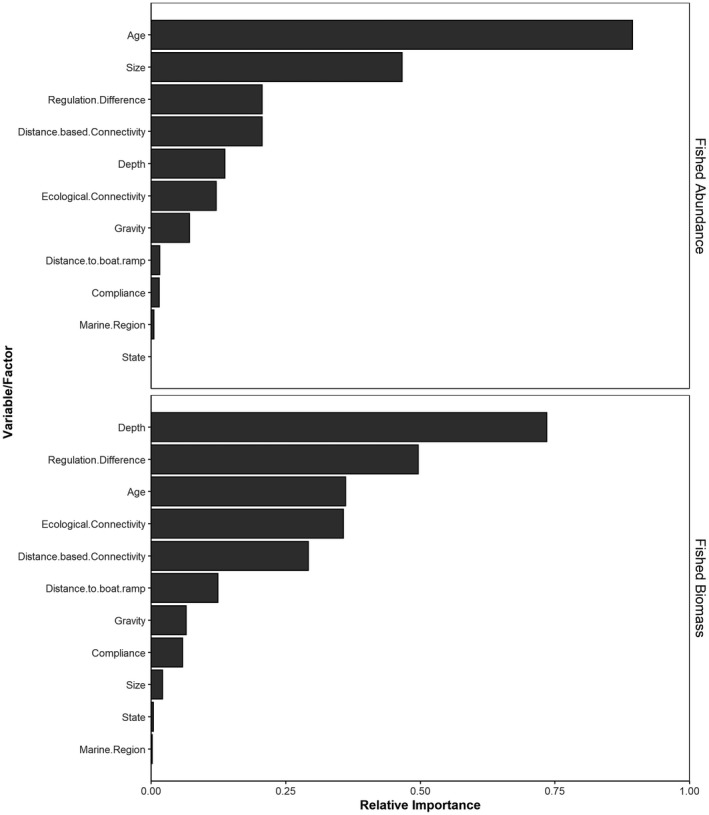
Importance scores for each explanatory variable in predicting the effectiveness of marine reserves to protect the abundance and biomass of fished species
All variables were considered in the full subset modelling and while the variance explained by top models was low (Figures 4a and 5a), suggesting factors not considered here and natural variation are contributing to unexplained variance, they explained a greater proportion of the variance than the null model for both abundance (ΔAICc = 9.9, ωAICc = 0.001) and biomass (ΔAICc = 6.7, ωAICc = 0.004). For fished abundance, there were three competing top models, with size in the first model, distance‐based connectivity and age in the second and ecological connectivity and age in the third model having a significant relationship with effect size (p < 0.05; Figure 4a). Abundance of fished species within marine reserves compared to fished areas (effect size) increased with increasing marine reserve size, distance‐based connectivity and ecological connectivity (Figure 4b,d,e). Effect size also increased with the age of marine reserves up to approximately 10 years, and then remained relatively stable (Figure 4c). The small number of marine reserves greater than 25 years old, provided little confidence in the interpretation of a decrease in effectiveness in older reserves.
FIGURE 4.
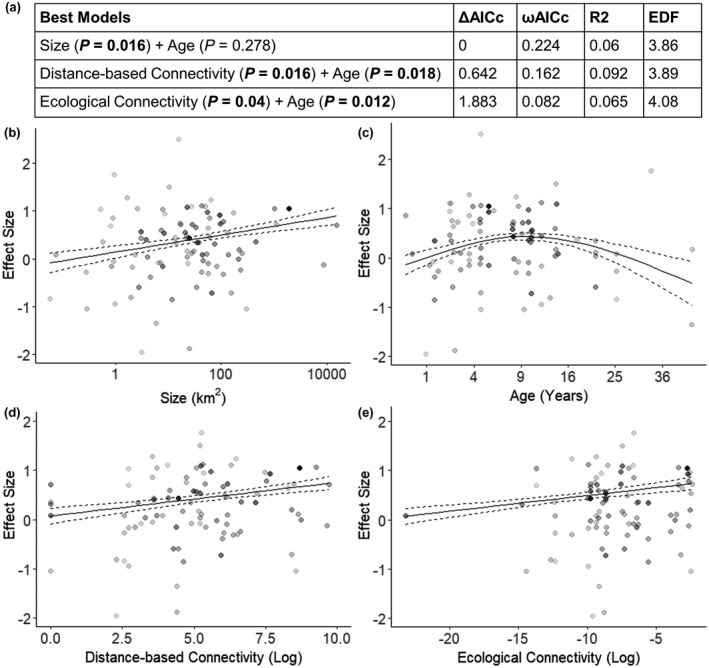
(a) Top models for explaining the effectiveness of marine reserves to increase the abundance of fished species. Difference between lowest reported corrected Akaike information criterion (ΔAICc), AIC weights (ωAICc), variance explained (R 2) and effective degrees of freedom (EDF) are reported for model comparison. The resulting relationships between (b) marine reserve size, (c) age of marine reserve in years, (d) distance‐based connectivity and (e) ecological connectivity, with log‐ratio effect sizes for fished abundance are shown. Darker dots represent effect sizes with a greater weighting based on the inverse of variance. Solid lines are fitted GAM curves, with dashed lines indicating standard error confidence bands
FIGURE 5.
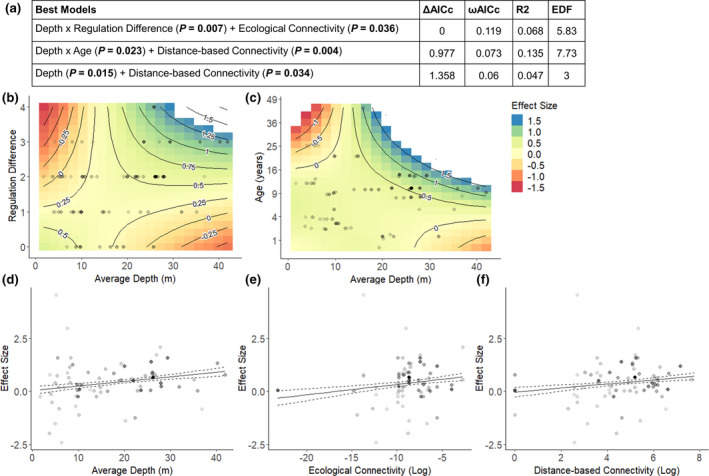
(a) Top models for explaining the effectiveness of marine reserves to increase biomass of fished species. Difference between lowest reported corrected Akaike information criterion (ΔAICc), AIC weights (ωAICc), variance explained (R 2) and effective degrees of freedom (EDF) are reported for model comparison. Relationship of log‐ratio effect sizes for fished biomass with (b) the interaction between depth and regulation difference (c) depth and age (d) marine reserve depth, (e) ecological connectivity and (f) distance‐based connectivity. The contours in plots (b) and (c) represent the predicted effect sizes based on the interactions. In plots (d‐f) the solid line is a fitted GAM curve and dashed line a standard error confidence band. For all plots darker dots represent effect sizes with a greater weighting based on the inverse of variance
For fished biomass there were three competing top models with all covariates having a significant impact on effect sizes (p < 0.05; Figure 5a). While the effect size for fished biomass increased with depth and greater variation in the effectiveness of marine reserves within shallow waters (<15 m) was observed (Figure 5d), this factor interacted with regulation difference and age in other top models. The interaction between regulation difference and depth, suggests that marine reserves needed to incorporate deeper waters and be highly protected (which results in a high regulation difference), to provide significant benefits for fished biomass (Figure 5b). An interaction between age and depth, suggests that marine reserves which incorporated deeper waters (>15 m), showed increased effect sizes as age increased, with most positive effect sizes occurring in older, deeper marine reserves (Figure 5c). Effect size also increased with increasing ecological and distance‐based connectivity, indicating the fished biomass within marine reserves compared to fished areas was greater for reserves with greater connectivity to other reserves (Figure 5e,f).
4. DISCUSSION
Our broad‐scale assessment of 91 marine reserves across Australia shows that both abundance and biomass of fished species is on average greater in marine reserves than in adjacent areas open to fishing. However, these benefits were not observed across all marine reserves, were greater in highly protected (no‐take) reserves and increased with size, age, connectivity, and the average depth of marine reserves. Although the positive relationship with age and benefits from full protection are well established (Claudet et al., 2008; Edgar et al., 2014), we demonstrate that these relationships can be complex with substantial influence from other emergent factors. We provide the first empirical evidence that both connectivity among marine reserves and the depths they cover influence the effects of their protection. Conceptually, marine reserves that are well connected with each other are thought to improve resilience to disturbance and ongoing stressors as the dispersal of eggs, larvae and adult fishes between boundaries is more likely and could contribute to maintaining populations (Almany et al., 2009; Álvarez‐Romero et al., 2018; Leis, 2003). The Great Barrier Reef and Ningaloo Marine Parks are two examples where marine reserves have been implemented as interconnected networks, with evidence of small networks in the former, generating a connectivity portfolio that can potentially replenish exploited fish stocks (Harrison et al., 2020).
We found that increased ecological and distance‐based connectivity among Australian marine reserves is associated with a higher abundance and biomass of fished species within their boundaries. It is possible that the positive relationships of abundance and biomass with connectivity are partly driven by increased capability to manage marine reserves that are closer together (e.g. increased enforcement and compliance due to public awareness; Edgar et al., 2018). However, compliance did not explain a significant proportion of the variance across marine reserves and both ecological and distance‐based connectivity metrics had a positive influence on effect size, suggesting that both ecological and marine reserve design factors are contributing to this result. While both metrics of connectivity were complementary with a relatively high correlation (Data S5), the distance‐based connectivity metric suggests that a maximum separation distance of 50 km between marine reserves (Almany et al., 2009) will provide increased conservation benefits for fishes. There is increasing evidence to suggest that simple distance‐based measures of connectivity can provide a useful tool for conservation and marine reserve planning at local scales (Abesamis et al., 2017; D’Aloia et al., 2015), and the metric presented here may provide a useful tool for countries/jurisdictions with limited in‐situ larval and oceanographic information, that is needed for modelling ecological connectivity.
By utilizing a nationwide BRUVs dataset, we were able to elicit complicated interactions with marine reserve effectiveness and depth, demonstrating the ability of this method to examine marine reserves over depth ranges to at least 40 m (Knott et al., 2021), and with the potential to explore broader depth ranges as data become available. We found differences in biomass between marine reserves and fished areas across depths, suggesting it is important to incorporate a broad depth range within marine reserve boundaries where possible. This contrasts with other studies that found a greater response to protection in shallower depths compared to deeper areas, attributed to the stronger fishing pressure in shallow water and depth refugia (Claudet et al., 2011; Goetze et al., 2011; Lindfield, McIlwain, et al., 2014). This is likely due to the increased occurrence of larger fished species at greater depths. Interestingly, the relationship between marine reserve effect on fish biomass over time (age) was influenced by depth. Consequently, when deeper areas are not incorporated within marine reserve boundaries, benefits for the biomass of fished species may be reduced, even for very old reserves. This might be explained by the relatively large depth range of many commonly fished species in Australia (e.g. pink snapper from 1 to 200 m; Paulin, 1990), as well as ontogenetic shifts, whereby larger individuals move into greater depths as they get larger and older, partly driven by the tendency for harvest of larger individuals to first occur in shallow, more accessible waters (Frank et al., 2018). These results may suggest a depth refuge effect in some marine reserves, where species most vulnerable to fishing are uncommon in shallower waters, and do not participate in the response to protection in this depth range (<20 m). The effect of depth refugia may be even greater in areas where the absence of fishing technologies (sonar, electric reels) inhibits fishing efficiency in deeper waters. There was also a high proportion of marine reserves with lower levels of protection in shallow waters (e.g. shore fishing in some marine reserves). This may be contributing to the interaction between the regulation difference and depth, where marine reserves that were highly protected and greater than 20 m deep resulted in the greatest differences in fished biomass. As mentioned above many exploited species exhibit broad depth ranges across and along the continental shelf around Australia. Indeed, most of the commercial harvest and some recreational capture of many of these species, lie outside of the depth ranges of the marine reserves considered herein. As such, the marine reserves considered here may not contribute directly to the conservation benefits (i.e. increased abundance and biomass) of many exploited species. Nevertheless, marine reserves that exhibit a broad depth range are more likely to have increased levels of biomass and abundance that benefit the conservation of marine biodiversity. Moreover, high levels of biomass and abundance of species targeted by fishers (artisanal, commercial and recreational) in marine reserves offer a range of non‐extractive benefits and related business opportunities such as tourism and diving.
While the benefits of marine reserves to fished species were consistent with other broad‐scale syntheses of marine reserves (Edgar et al., 2014; Lester et al., 2009; Molloy et al., 2009), the magnitude of our results (28% > abundance and 53% > biomass) were generally lower than global assessments (Lester et al., 2009; Molloy et al., 2009). This may be explained by the broader range of fished species that were considered here, which are subject to varying levels of fishing pressure and/or the broader depth range that was examined. However, overestimation of marine reserve effectiveness has been observed when using diver‐based methodologies in areas where spearfishing is common (Gray et al., 2016; Januchowski‐Hartley et al., 2015; Lindfield, Harvey, et al., 2014). Australia is also considered above the global average for effective fisheries management (Mora et al., 2009), and the gravity of human impacts are generally low (Cinner et al., 2018), resulting in lower rates of exploitation in areas open to fishing. Indeed, effective fisheries management across Australia contributes to robust populations of some predators (e.g. reef sharks; MacNeil et al., 2020), and it is likely that these management arrangements are contributing to the presence of equivocal effects from some of the marine reserves surveyed. Regardless, we observed no effect of marine reserves on non‐target species, suggesting the results for fished species are likely driven by the increased fishing pressure outside of marine reserves in the areas surveyed.
A stronger effect of marine reserve protection was observed for biomass when compared to the abundance of fished species, suggesting that marine reserves across Australia potentially benefit large‐bodied fishes. Size‐related responses of populations to protection from fishing are common (McClanahan et al., 2007, 2019; Russ et al., 2005) and are generally regarded as a more sensitive metric compared to abundance (Goetze et al., 2017; Nash & Graham, 2016). This is due to the preferential targeting of large individuals by fishers (Birkeland & Dayton, 2005), resulting in greater impacts to biomass than abundance. Our results also demonstrate the value of stereo‐BRUVs when monitoring, which provide an accurate measure of fish length that can be converted to biomass (Harvey et al., 2002, 2007; Langlois et al., 2012). The build‐up of fish biomass within marine reserves across Australia demonstrates how this strategy of protection of large‐bodied fish can provide benefits in terms of increased fecundity and therefore spill over of larvae/eggs into fished areas (Abesamis & Russ, 2005; Evans & Russ, 2004; Evans et al., 2008; McClanahan & Mangi, 2000). However, to have a positive effect at the population scale, such reserves would have to be extensive. It is important to consider the spatial scale of the marine reserves in comparison to the area under effective fisheries management, as there are numerous harvest control measures that may be applied and marine reserves are just one tool that have an increasingly important role, particularly in countries where governance is less effective. We also observed variation in the importance of covariates between metrics, with the size of marine reserves included in top models for targeted abundance (in line with the literature; Claudet et al., 2008; Edgar et al., 2014), but not for biomass. This may be partly driven by a lack of biomass data in some of the largest marine reserves surveyed (e.g. in the Great Barrier Reef) and the greater influence of other factors (e.g. depth, connectivity and age). Size and distance‐based connectivity also shared ~55% and 35% of their variance for abundance and biomass respectively, due to size being incorporated within the distance‐based connectivity metric, suggesting that this measure of connectivity is likely explaining variation due to size as well as proximity to other marine reserves.
Despite compliance with marine reserve rules and regulations being considered one of the most important drivers of conservation success globally (Edgar et al., 2014; Guidetti et al., 2008; McClanahan et al., 2009), we found compliance was a poor predictor of effect sizes for fish abundance or biomass. Our results reflect a relatively high level of marine reserve and fisheries management across Australia compared to other countries, where reserves may vary from ‘paper parks’ (offering little protection in the water) to strictly enforced no‐entry zones (Costello & Ballantine, 2015). Although this is an endorsement of current management strategies in some parts of Australia, a large proportion of marine reserves (~66%) provided no discernible benefits to fished species and some less than areas open to fishing (~5%), which suggests improvement of enforcement and compliance may be required. However, this result is also explained by the extensive size range of marine reserves across Australia (<0.1 to >100,000 km2; Department of Agriculture, Water and the Environment 2020) and the need for marine reserves to be large and deep enough to provide significant benefits to fished species (Claudet et al., 2008; Edgar et al., 2014). Similarly, distance to boat ramp and gravity were not included in top models or regarded as important variables for explaining variation in effect sizes of marine reserves. This contrasts with global studies where conservation success is typically dependent on the gravity of human impacts (Cinner et al., 2016, 2018). Australia is dominated by reefs with lower levels of gravity compared to other countries, so the range of human impacts considered here was likely not large enough to have a significant impact on marine reserve effectiveness. Moreover, recreational and commercial fishing can be high in rural locations where resident human populations are low (e.g. the Gascoyne region of Western Australia; Ryan et al., 2019), information that may not be captured by the Gravity measure. Finally, our top models explained a small proportion of the overall variance in effect size, although still above the average for ecological meta‐analyses (Møller & Jennions, 2002). Nonetheless large amounts of unexplained variance in effect size suggests factors not considered here (e.g. management effectiveness; Gill et al., 2017 and the life‐history and ecological characteristics of taxa considered; Claudet et al., 2010) are likely contributing to the ability of marine reserves to provide conservation benefits.
While marine reserves that preclude fishing are relatively common within multi‐use marine parks in Australia, a large proportion of coastal waters (~70%) are not incorporated into marine parks (Roberts et al., 2018). We identified two large spatial gaps in the presence of marine parks and marine reserves within state waters, from (1) the Northern Territory to Northern Queensland and (2) the southern coast of Western Australia and the Great Australian Bight. Notably the gap on the south coast of Australia covers a large proportion of the Great Southern Reef, which has been identified as a global biodiversity hotspot and provides extensive economic benefits to the tourism and fisheries industries (Bennett et al., 2016). We also identified no distance‐based connectivity between marine reserves within Victoria and Tasmania, suggesting the potential for enhancement of existing management strategies and network designs. Roberts et al. (2020) made a similar suggestion and found that Australia's marine reserve system is not functioning as a connected network due to breaks in the connectivity of reef habitat. It will be important to consider natural breaks in connectivity when planning for marine reserves networks (e.g. lack of habitat suitable for fished species), given it may not be practical or beneficial to implement marine reserves separated by less than 50 km (as recommended here) in these circumstances. We also found that state and marine region did not influence the success of marine reserves, with positive effect sizes observed across all major states (apart from Tasmania where only one reserve was sampled) suggesting that this conservation strategy can be successful in both tropical and temperate waters.
By using a national database of BRUVs to comprehensively assess marine reserves across Australia, we demonstrate that they provide significant benefits to fished species and we identify factors that can improve marine reserve design and management globally. We provide new insights to marine reserve design that suggests depth and connectivity are important factors for achieving conservation gains. Although the benefits of marine reserves generally increase with age, this effect was not common in shallow waters, supporting the recommendation that marine reserves should be representative of a broad range of habitats across depths (Ballantine, 2014). Similarly, marine reserves that are connected provide benefits that may extend to an entire ecosystem over time. Ongoing implementation and enhancement of comprehensive networks of marine reserves will, however, depend on effective engagement and consultation with all stakeholders on socio‐economic and access issues. This will be especially important given increasing anthropogenic pressures and competition for the use of resources. We demonstrate that marine reserves provide an effective conservation strategy for temperate and tropical fished species within an overarching marine biodiversity conservation framework, provided they are highly protected (no‐take) and have been designed to optimize connectivity, size and cover a large depth range.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to the Great Barrier Reef Marine Park Authority compliance team, Parks Victoria, Fisheries NSW managers, operations, compliance and research staff for information on compliance and Brooke Gibbons for data cleaning and management. We would also like to thank Mike Cappo for his contribution to the BRUV workshop and foundation work on BRUVs. We acknowledge funding for the workshop from Curtin University, a Community of Practice grant from the Australian Institute of Marine Science and from the Global FinPrint Project funded by Paul G Allen Philanthropies. We thank everyone who contributed to the data in GlobalArchive, which was supported by the Australian Research Data Commons (ARDC) and synthesis work was supported through the ARDC’s Marine Research Data Cloud and Australian Data Partnerships projects. Researchers Tim Langlois, Neville Barrett, Jacquomo Monk and Alan Jordan were supported by the Marine Biodiversity Hub through funding from the Australian Government's National Environmental Science Program. We acknowledge the Traditional Owners of the land and sea country where this research was conducted and pay respects to Elders past, present and future. Thank you to Tiffany Tailor for the design of the infographic and Juliet Corley for fish images. The authors have no conflicts of interest associated with this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abesamis, R. A., & Russ, G. R. (2005). Density‐dependent spillover from a marine reserve: Long‐term evidence. Ecological Applications, 15, 1798–1812. [Google Scholar]
- Abesamis, R. A., Saenz‐Agudelo, P., Berumen, M. L., Bode, M., Jadloc, C. R. L., Solera, L. A., Villanoy, C. L., Bernardo, L. P. C., Alcala, A. C., & Russ, G. R. (2017). Reef‐fish larval dispersal patterns validate no‐take marine reserve network connectivity that links human communities. Coral Reefs, 36(3), 791–801. [Google Scholar]
- Akaike, H. (1998). Information theory and an extension of the maximum likelihood principle. In Parzen E., Tanabe K., & Kitagawa G. (Eds.), Selected papers of Hirotugu Akaike (pp. 199–213). Springer. [Google Scholar]
- Almany, G. R., Connolly, S. R., Heath, D. D., Hogan, J. D., Jones, G. P., McCook, L. J., Mills, M., Pressey, R. L., & Williamson, D. H. (2009). Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs, 28, 339–351. [Google Scholar]
- Álvarez‐Romero, J. G., Munguía‐Vega, A., Beger, M., Mar Mancha‐Cisneros, M., Suárez‐Castillo, A. N., Gurney, G. G., Pressey, R. L., Gerber, L. R., Morzaria‐Luna, H. N., Reyes‐Bonilla, H., Adams, V. M., Kolb, M., Graham, E. M., VanDerWal, J., Castillo‐López, A., Hinojosa‐Arango, G., Petatán‐Ramírez, D., Moreno‐Baez, M., Godínez‐Reyes, C. R., & Torre, J. (2018). Designing connected marine reserves in the face of global warming. Global Change Biology, 24, e671–e691. [DOI] [PubMed] [Google Scholar]
- Ballantine, B. (2014). Fifty years on: Lessons from marine reserves in New Zealand and principles for a worldwide network. Biological Conservation, 176, 297–307. [Google Scholar]
- Bennett, S., Wernberg, T., Connell, S. D., Hobday, A. J., Johnson, C. R., & Poloczanska, E. S. (2016). The “Great Southern Reef”: Social, ecological and economic value of Australia’s neglected kelp forests. Marine and Freshwater Research, 67, 47–56. [Google Scholar]
- Bergseth, B. J., Russ, G. R., & Cinner, J. E. (2015). Measuring and monitoring compliance in no‐take marine reserves. Fish and Fisheries, 16, 240–258. [Google Scholar]
- Birkeland, C., & Dayton, P. K. (2005). The importance in fishery management of leaving the big ones. Trends in Ecology & Evolution, 20, 356–358. [DOI] [PubMed] [Google Scholar]
- Botsford, L. W., Brumbaugh, D. R., Grimes, C., Kellner, J. B., Largier, J., O’Farrell, M. R., Ralston, S., Soulanille, E., & Wespestad, V. (2009). Connectivity, sustainability, and yield: Bridging the gap between conventional fisheries management and marine protected areas. Review in Fish Biology and Fisheries, 19, 69–95. [Google Scholar]
- Burnham, K. P., & Anderson, D. R. (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261–304. [Google Scholar]
- Burnham, K. P., & Anderson, D. R. (2007). Model selection and multimodel inference: A practical information‐theoretic approach. Springer Science & Business Media. [Google Scholar]
- CAPAD . (2020). Collaborative Australian protected area database. https://www.environment.gov.au/land/nrs/science/capad [Google Scholar]
- Cinner, J. E., Huchery, C., MacNeil, M. A., Graham, N. A. J., McClanahan, T. R., Maina, J., Maire, E., Kittinger, J. N., Hicks, C. C., Mora, C., Allison, E. H., D’Agata, S., Hoey, A., Feary, D. A., Crowder, L., Williams, I. D., Kulbicki, M., Vigliola, L., Wantiez, L., … Mouillot, D. (2016). Bright spots among the world’s coral reefs. Nature, 535, 416–419. [DOI] [PubMed] [Google Scholar]
- Cinner, J. E., Maire, E., Huchery, C., MacNeil, M. A., Graham, N. A. J., Mora, C., McClanahan, T. R., Barnes, M. L., Kittinger, J. N., Hicks, C. C., D’Agata, S., Hoey, A. S., Gurney, G. G., Feary, D. A., Williams, I. D., Kulbicki, M., Vigliola, L., Wantiez, L., Edgar, G. J., … Mouillot, D. (2018). Gravity of human impacts mediates coral reef conservation gains. Proceedings of the National Academy of Sciences of the United States of America, 115, E6116–E6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudet, J., García‐Charton, J. A., & Lenfant, P. (2011). Combined effects of levels of protection and environmental variables at different spatial resolutions on fish assemblages in a marine protected area: Species‐habitat relations and MPAs. Conservation Biology, 25, 105–114. [DOI] [PubMed] [Google Scholar]
- Claudet, J., Loiseau, C., Sostres, M., & Zupan, M. (2020). Underprotected marine protected areas in a global biodiversity hotspot. One Earth, 2, 380–384. [Google Scholar]
- Claudet, J., Osenberg, C. W., Benedetti‐Cecchi, L., Domenici, P., García‐Charton, J.‐A., Pérez‐Ruzafa, Á., Badalamenti, F., Bayle‐Sempere, J., Brito, A., Bulleri, F., Culioli, J.‐M., Dimech, M., Falcón, J. M., Guala, I., Milazzo, M., Sánchez‐Meca, J., Somerfield, P. J., Stobart, B., Vandeperre, F., … Planes, S. (2008). Marine reserves: Size and age do matter. Ecology Letters, 11, 481–489. [DOI] [PubMed] [Google Scholar]
- Claudet, J., Osenberg, C. W., Domenici, P., Badalamenti, F., Milazzo, M., Falcón, J. M., Bertocci, I., Benedetti‐Cecchi, L., García‐Charton, J.‐A., Goñi, R., Borg, J. A., Forcada, A., de Lucia, G. A., Pérez‐Ruzafa, Á., Afonso, P., Brito, A., Guala, I., Diréach, L. L., Sanchez‐Jerez, P., … Planes, S. (2010). Marine reserves: Fish life history and ecological traits matter. Ecological Applications, 20, 830–839. [DOI] [PubMed] [Google Scholar]
- Coghlan, A. R., McLean, D. L., Harvey, E. S., & Langlois, T. J. (2017). Does fish behaviour bias abundance and length information collected by baited underwater video? Journal of Experimental Marine Biology and Ecology, 497, 143–151. [Google Scholar]
- Costello, M. J., & Ballantine, B. (2015). Biodiversity conservation should focus on no‐take marine reserves: 94% of marine protected areas allow fishing. Trends in Ecology & Evolution, 30, 507–509. [DOI] [PubMed] [Google Scholar]
- Costello, M. J., & Connor, D. W. (2019). Connectivity is generally not important for marine reserve planning. Trends in Ecology & Evolution, 34, 686–688. [DOI] [PubMed] [Google Scholar]
- Cresswell, A. K., Langlois, T. J., Wilson, S. K., Claudet, J., Thomson, D. P., Renton, M., Fulton, C. J., Fisher, R., Vanderklift, M. A., Babcock, R. C., Stuart‐Smith, R. D., Haywood, M. D. E., Depczynski, M., Westera, M., Ayling, A. M., Fitzpatrick, B., Halford, A. R., McLean, D. L., Pillans, R. D., … Holmes, T. H. (2019). Disentangling the response of fishes to recreational fishing over 30 years within a fringing coral reef reserve network. Biological Conservation, 237, 514–524. [Google Scholar]
- Cundy, M. E., Santana‐Garcon, J., Ferguson, A. M., Fairclough, D. V., Jennings, P., & Harvey, E. S. (2017). Baited remote underwater stereo‐video outperforms baited downward‐facing single‐video for assessments of fish diversity, abundance and size composition. Journal of Experimental Marine Biology and Ecology, 497, 19–32. [Google Scholar]
- D’Aloia, C. C., Bogdanowicz, S. M., Francis, R. K., Majoris, J. E., Harrison, R. G., & Buston, P. M. (2015). Patterns, causes, and consequences of marine larval dispersal. Proceedings of the National Academy of Sciences of the United States of America, 112(45), 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo, M., Claudet, J., & Guidetti, P. (2016). Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. Journal for Nature Conservation, 32, 62–66. [Google Scholar]
- Di Lorenzo, M., Guidetti, P., Di Franco, A., Calò, A., & Claudet, J. (2020). Assessing spillover from marine protected areas and its drivers: A meta‐analytical approach. Fish and Fisheries, 15, 1798. [Google Scholar]
- Dunlop, K. M., Marian Scott, E., Parsons, D., & Bailey, D. M. (2015). Do agonistic behaviours bias baited remote underwater video surveys of fish? Marine Ecology, 36, 810–818. [Google Scholar]
- Edgar, G. J., Stuart‐Smith, R. D., Willis, T. J., Kininmonth, S., Baker, S. C., Banks, S., Barrett, N. S., Becerro, M. A., Bernard, A. T. F., Berkhout, J., Buxton, C. D., Campbell, S. J., Cooper, A. T., Davey, M., Edgar, S. C., Försterra, G., Galván, D. E., Irigoyen, A. J., Kushner, D. J., … Thomson, R. J. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature, 506, 216–220. [DOI] [PubMed] [Google Scholar]
- Edgar, G. J., Ward, T. J., & Stuart‐Smith, R. D. (2018). Rapid declines across Australian fishery stocks indicate global sustainability targets will not be achieved without an expanded network of “no‐fishing” reserves. Aquatic Conservation: Marine and Freshwater Ecosystems, 28, 1337–1350. [Google Scholar]
- Evans, R. D., & Russ, G. R. (2004). Larger biomass of targeted reef fish in no‐take marine reserves on the Great Barrier Reef, Australia. Aquatic Conservation: Marine and Freshwater Ecosystems, 14, 505–519. [Google Scholar]
- Evans, R. D., Russ, G. R., & Kritzer, J. P. (2008). Batch fecundity of Lutjanus carponotatus (Lutjanidae) and implications of no‐take marine reserves on the Great Barrier Reef, Australia. Coral Reefs, 27, 179–189. [Google Scholar]
- Fisher, R., Wilson, S. K., Sin, T. M., Lee, A. C., & Langlois, T. J. (2018). A simple function for full‐subsets multiple regression in ecology with R. Ecology and Evolution, 8, 6104–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, K. T., Petrie, B., Leggett, W. C., & Boyce, D. G. (2018). Exploitation drives an ontogenetic‐like deepening in marine fish. Proceedings of the National Academy of Sciences of the United States of America, 115, 6422–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, D. A., Mascia, M. B., Ahmadia, G. N., Glew, L., Lester, S. E., Barnes, M., Craigie, I., Darling, E. S., Free, C. M., Geldmann, J., Holst, S., Jensen, O. P., White, A. T., Basurto, X., Coad, L., Gates, R. D., Guannel, G., Mumby, P. J., Thomas, H., … Fox, H. E. (2017). Capacity shortfalls hinder the performance of marine protected areas globally. Nature, 543, 665–669. [DOI] [PubMed] [Google Scholar]
- Goetze, J. S., Januchowski‐Hartley, F. A., Claudet, J., Langlois, T. J., Wilson, S. K., & Jupiter, S. D. (2017). Fish wariness is a more sensitive indicator to changes in fishing pressure than abundance, length or biomass. Ecological Applications, 27, 1178–1189. [DOI] [PubMed] [Google Scholar]
- Goetze, J. S., Jupiter, S. D., Langlois, T. J., Wilson, S. K., Harvey, E. S., Bond, T., & Naisilisili, W. (2015). Diver operated video most accurately detects the impacts of fishing within periodically harvested closures. Journal of Experimental Marine Biology and Ecology, 462, 74–82. [Google Scholar]
- Goetze, J. S., Langlois, T. J., Egli, D. P., & Harvey, E. S. (2011). Evidence of artisanal fishing impacts and depth refuge in assemblages of Fijian reef fish. Coral Reefs, 30, 507–517. [Google Scholar]
- Graham, M. H. (2003). Confronting multicollinearity in ecological multiple regression. Ecology, 84, 2809–2815. [Google Scholar]
- Graham, N. A. J., Ainsworth, T. D., Baird, A. H., Ban, N. C., Bay, L. K., Cinner, J. E., De Freitas, D. M., Diaz‐Pulido, G., Dornelas, M., Dunn, S. R., Fidelman, P. I. J., Foret, S., Good, T. C., Kool, J., Mallela, Jennie, Penin, L., Pratchett, M. S., & Williamson, D. H. (2011). From microbes to people: Tractable benefits of no‐take areas for coral reefs. Oceanography and Marine Biology‐an Annual Review, 49, 105. [Google Scholar]
- Gray, A. E., Williams, I. D., Stamoulis, K. A., Boland, R. C., Lino, K. C., Hauk, B. B., Leonard, J. C., Rooney, J. J., Asher, J. M., Lopes, Jr., K. H., & Kosaki, R. K. (2016). Comparison of reef fish survey data gathered by open and closed circuit SCUBA divers reveals differences in areas with higher fishing pressure. PLoS One, 11, e0167724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grorud‐Colvert, K., Claudet, J., Tissot, B. N., Caselle, J. E., Carr, M. H., Day, J. C., Friedlander, A. M., Lester, S. E., de Loma, T. L., Malone, D., & Walsh, W. J. (2014). Marine protected area networks: Assessing whether the whole is greater than the sum of its parts. PLoS One, 9, e102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti, P., Milazzo, M., Bussotti, S., Molinari, A., Murenu, M., Pais, A., Spanò, N., Balzano, R., Agardy, T., Boero, F., Carrada, G., Cattaneo‐Vietti, R., Cau, A., Chemello, R., Greco, S., Manganaro, A., Notarbartolo di Sciara, G., Russo, G. F., & Tunesi, L. (2008). Italian marine reserve effectiveness: Does enforcement matter? Biological Conservation, 141, 699–709. [Google Scholar]
- Halpern, B. S. (2003). The impact of marine reserves: Do reserves work and does reserve size matter? Ecological Applications, 13, 117–137. [Google Scholar]
- Harrison, H. B., Bode, M., Williamson, D. H., Berumen, M. L., & Jones, G. P. (2020). A connectivity portfolio effect stabilizes marine reserve performance. Proceedings of the National Academy of Sciences of the United States of America, 117(41), 25595–25600. 10.1073/pnas.1920580117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, E. S., Cappo, M., Butler, J., Hall, N., & Kendrick, G. (2007). Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Marine Ecology Progress Series, 350, 245–254. [Google Scholar]
- Harvey, E., Fletcher, D., & Shortis, M. (2002). Estimation of reef fish length by divers and by stereo‐video: A first comparison of the accuracy and precision in the field on living fish under operational conditions. Fisheries Research, 57, 255–265. [Google Scholar]
- Harvey, E. S., McLean, D. L., Goetze, J. S., Saunders, B. J., Langlois, T. J., Monk, J., Barrett, N., Wilson, S. K., Holmes, T. H., Ierodiaconou, D., Jordan, A. R., Meekan, M. G., Malcolm, H. A., Heupel, M. R., Harasti, D., Huveneers, C., Knott, N. A., Fairclough, D. V., Currey‐Randall, L. M., … Newman, S. J. (2021). The BRUVs workshop – An Australia‐wide synthesis of baited remote underwater video data to answer broad‐scale ecological questions about fish, sharks and rays. Marine Policy, 127, 104430. [Google Scholar]
- Harvey, E. S., Santana‐Garcon, J. S., Goetze, J. S., Saunders, B. J., & Cappo, M. (2018). The use of stationary underwater video for sampling sharks. In Carrier J. C., Heithaus M. R., & Simpfendorfer C. A. (Eds.), Shark research: Emerging technologies and applications for the field and laboratory (pp. 111–132). CRC Press. [Google Scholar]
- Hedges, L. V., & Pigott, T. D. (2004). The power of statistical tests for moderators in meta‐analysis. Psychological Methods, 9(4), 426–445. [DOI] [PubMed] [Google Scholar]
- Horta e Costa, B., Claudet, J., Franco, G., Erzini, K., Caro, A., & Gonçalves, E. J. (2016). A regulation‐based classification system for Marine Protected Areas (MPAs). Marine Policy, 72, 192–198. [Google Scholar]
- Januchowski‐Hartley, F. A., Graham, N. A. J., Cinner, J. E., & Russ, G. R. (2015). Local fishing influences coral reef fish behavior inside protected areas of the Indo‐Pacific. Biological Conservation, 182, 8–12. [Google Scholar]
- Kenchington, R. (2016). The evolution of marine conservation and marine protected areas in Australia. In Fitzsimons J. & Wescott G. (Eds.), Big, bold and blue: Lessons from Australia’s marine protected areas (pp. 29–42). CSIRO. [Google Scholar]
- Knott, N. A., Williams, J., Harasti, D., Malcolm, H. A., Coleman, M. A., Kelaher, B. P., Rees, M. J., Schultz, A., & Jordan, A. (2021). A coherent, representative, and bioregional marine reserve network shows consistent change in rocky reef fish assemblages. Ecosphere, 12(4). 10.1002/ecs2.3447 [DOI] [Google Scholar]
- Langlois, T., Goetze, J., Bond, T., Monk, J., Abesamis, R. A., Asher, J., Barrett, N., Bernard, A. T. F., Bouchet, P. J., Birt, M. J., Cappo, M., Currey‐Randall, L. M., Driessen, D., Fairclough, D. V., Fullwood, L. A. F., Gibbons, B. A., Harasti, D., Heupel, M. R., Hicks, J., … Harvey, E. S. (2020). A field and video annotation guide for baited remote underwater stereo‐video surveys of demersal fish assemblages. Methods in Ecology and Evolution, 11(11), 1401–1409. 10.1111/2041-210X.13470 [DOI] [Google Scholar]
- Langlois, T. J., Harvey, E. S., & Meeuwig, J. J. (2012). Strong direct and inconsistent indirect effects of fishing found using stereo‐video: Testing indicators from fisheries closures. Ecological Indicators, 23, 524–534. [Google Scholar]
- Leis, J. M. (2003). What does larval fish biology tell us about the design and efficacy of Marine Protected Areas. In Beumer J. P., Grant A., & Smith D. C. (Eds.), Aquatic Protected Areas: What works best and how do we know? Proceedings of the World Congress on Aquatic Protected Areas, Cairns, August 2002 (pp. 170–180). Australian Society for Fish Biology. [Google Scholar]
- Lester, S., Halpern, B., Grorud‐Colvert, K., Lubchenco, J., Ruttenberg, B., Gaines, S., Airamé, S., & Warner, R. (2009). Biological effects within no‐take marine reserves: A global synthesis. Marine Ecology Progress Series, 384, 33–46. [Google Scholar]
- Lin, X., & Zhang, D. (1999). Inference in generalized additive mixed models by using smoothing splines. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 61, 381–400. [Google Scholar]
- Lindfield, S. J., Harvey, E. S., McIlwain, J. L., & Halford, A. R. (2014). Silent fish surveys: Bubble‐free diving highlights inaccuracies associated with SCUBA‐based surveys in heavily fished areas. Methods in Ecology and Evolution, 5, 1061–1069. [Google Scholar]
- Lindfield, S. J., McIlwain, J. L., & Harvey, E. S. (2014). Depth refuge and the impacts of SCUBA spearfishing on coral reef fishes. PLoS One, 9, e92628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil, M. A., Chapman, D. D., Heupel, M., Simpfendorfer, C. A., Heithaus, M., Meekan, M., Harvey, E., Goetze, J., Kiszka, J., Bond, M. E., Currey‐Randall, L. M., Speed, C. W., Sherman, C. S., Rees, M. J., Udyawer, V., Flowers, K. I., Clementi, G., Valentin‐Albanese, J., Gorham, T., … Cinner, J. E. (2020). Global status and conservation potential of reef sharks. Nature, 583(7818), 801–806. [DOI] [PubMed] [Google Scholar]
- Malcolm, H. A., Gladstone, W., Lindfield, S., Wraith, J., & Lynch, T. P. (2007). Spatial and temporal variation in reef fish assemblages of marine parks in New South Wales, Australia‐baited video observations. Marine Ecology Progress Series, 350, 277–290. 10.3354/meps07195 [DOI] [Google Scholar]
- Malcolm, H. A., Jordan, A., Creese, R. G., & Knott, N. A. (2016). Size and age are important factors for marine sanctuaries: Evidence from a decade of systematic sampling in a subtropical marine park. Aquatic Conservation: Marine and Freshwater Ecosystems, 26, 1090–1106. [Google Scholar]
- Marshall, D. J., Gaines, S., Warner, R., Barneche, D. R., & Bode, M. (2019). Underestimating the benefits of marine protected areas for the replenishment of fished populations. Frontiers in Ecology and the Environment, 17, 407–413. [Google Scholar]
- McClanahan, T. R., Graham, N. A. J., Calnan, J. M., & MacNeil, M. A. (2007). Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecological Applications, 17, 1055–1067. [DOI] [PubMed] [Google Scholar]
- McClanahan, T. R., Graham, N. A. J., Wilson, S., Letourner, Y., & Fisher, R. (2009). Effects of fisheries closure size, age, and history of compliance on coral reef fish communities in the western Indian Ocean. Marine Ecology Progress Series, 396, 99–109. [Google Scholar]
- McClanahan, T. R., & Mangi, S. (2000). Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecological Applications, 10, 1792–1805. [Google Scholar]
- McClanahan, T. R., Schroeder, R. E., Friedlander, A. M., Vigliola, L., Wantiez, L., Caselle, J. E., Graham, N. A. J., Wilson, S., Edgar, G. J., Stuart‐Smith, R. D., Oddenyo, R. M., & Cinner, J. E. (2019). Global baselines and benchmarks for fish biomass: comparing remote reefs and fisheries closures. Marine Ecology Progress Series, 612, 167–192. [Google Scholar]
- Møller, A., & Jennions, M. D. (2002). How much variance can be explained by ecologists and evolutionary biologists? Oecologia, 132(4), 492–500. [DOI] [PubMed] [Google Scholar]
- Molloy, P. P., McLean, I. B., & Côté, I. M. (2009). Effects of marine reserve age on fish populations: A global meta‐analysis. Journal of Applied Ecology, 46, 743–751. [Google Scholar]
- Mora, C., Myers, R. A., Coll, M., Libralato, S., Pitcher, T. J., Sumaila, R. U., Zeller, D., Watson, R., Gaston, K. J., & Worm, B. (2009). Management effectiveness of the world’s marine fisheries. PLoS Biology, 7, e1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera, I., Côté, I. M., Jennings, S., & Reynolds, J. D. (2000). Conservation benefits of marine reserves for fish populations. Animal Conservation, 3, 321–332. [Google Scholar]
- Murphy, H. M., & Jenkins, G. P. (2010). Observational methods used in marine spatial monitoring of fishes and associated habitats: A review. Marine and Freshwater Research, 61, 236–252. [Google Scholar]
- Nash, K. L., & Graham, N. A. J. (2016). Ecological indicators for coral reef fisheries management. Fish and Fisheries, 17, 1029–1054. [Google Scholar]
- Paulin, C. D. (1990). Pagrus auratus, a new combination for the species known as “snapper” in Australasian waters (Pisces: Sparidae). New Zealand Journal of Marine and Freshwater Research, 24, 259–265. [Google Scholar]
- Priede, I. G., Bagley, P. M., Smith, A., Creasey, S., & Merrett, N. R. (1994). Scavenging deep demersal fishes of the Porcupine Seabight, north‐east Atlantic: Observations by baited camera, trap and trawl. Journal of the Marine Biological Association of the United Kingdom, 74(3), 481–498. [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Raftery, A. E. (1995). Bayesian model selection in social research. Sociological Methodology, 25, 111–163. [Google Scholar]
- Roberts, K. E., Cook, C. N., Beher, J., & Treml, E. A. (2020). Assessing the current state of ecological connectivity in a large marine protected area system. Conservation Biology, 35(2), 699–710. 10.1111/cobi.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, K. E., Valkan, R. S., & Cook, C. N. (2018). Measuring progress in marine protection: A new set of metrics to evaluate the strength of marine protected area networks. Biological Conservation, 219, 20–27. [Google Scholar]
- Russ, G. R., Stockwell, B., & Alcala, A. C. (2005). Inferring versus measuring rates of recovery in no‐take marine reserves. Marine Ecology Progress Series, 292, 1–12. [Google Scholar]
- Ryan, K. L., Hall, N. G., Lai, E. K., Smallwood, C. B., Tate, A., Taylor, S. M., & Wise, B. S. (2019). Statewide survey of boat‐based recreational fishing in Western Australia 2017/18 (Fisheries Research Report No. 297). Department of Primary Industries and Regional Development, Western Australia.
- Sala, E., Lubchenco, J., Grorud‐Colvert, K., Novelli, C., Roberts, C., & Sumaila, U. R. (2018). Assessing real progress towards effective ocean protection. Marine Policy, 91, 11–13. [Google Scholar]
- Thiault, L., Kernaléguen, L., Osenberg, C. W., Lison de Loma, T., Chancerelle, Y., Siu, G., & Claudet, J. (2019). Ecological evaluation of a marine protected area network: A progressive‐change BACIPS approach. Ecosphere, 10, e02576. [Google Scholar]
- Tittensor, D. P., Beger, M., Boerder, K., Boyce, D. G., Cavanagh, R. D., Cosandey‐Godin, A., Crespo, G. O., Dunn, D. C., Ghiffary, W., Grant, S. M., Hannah, L., Halpin, P. N., Harfoot, M., Heaslip, S. G., Jeffery, N. W., Kingston, N., Lotze, H. K., McGowan, J., McLeod, E., … Worm, B. (2019). Integrating climate adaptation and biodiversity conservation in the global ocean. Science Advances, 5, eaay9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treml, E. A., Roberts, J. J., Chao, Y., Halpin, P. N., Possingham, H. P., & Riginos, C. (2012). Reproductive output and duration of the pelagic larval stage determine seascape‐wide connectivity of marine populations. Integrative and Comparative Biology, 52(4), 525–537. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analysis in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Watson, D. L., Harvey, E. S., Anderson, M. J., & Kendrick, G. A. (2005). A comparison of temperate reef fish assemblages recorded by three underwater stereo‐video techniques. Marine Biology, 148, 415–425. [Google Scholar]
- Whitmarsh, S. K., Fairweather, P. G., & Huveneers, C. (2017). What is Big BRUVver up to? Methods and uses of baited underwater video. Review in Fish Biology and Fisheries, 27, 53–73. [Google Scholar]
- Wickham, H. (2009). ggplot2: Elegant graphics for data analysis. Springer‐Verlag. [Google Scholar]
- Wickham, H., François, R., Henry, L., & Müller, K. (2018). dplyr: A grammar of data manipulation. R package version 0.7.6. https://CRAN.R‐project.org/package=dplyr
- Willis, T. J., & Babcock, R. C. (2000). A baited underwater video system for the determination of relative density of carnivorous reef fish. Marine and Freshwater Research, 51, 755–763. [Google Scholar]
- Wood, S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 73(1), 3–36. 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
- Wood, S. N. (2013). On p‐values for smooth components of an extended generalized additive model. Biometrika, 100(1), 221–228. [Google Scholar]
- Young, M. A., Treml, E. A., Beher, J., Fredle, M., Gorfine, H., Miller, A. D., Swearer, S. E., & Ierodiaconou, D. (2020). Using species distribution models to assess the long‐term impacts of changing oceanographic conditions on abalone density in south east Australia. Ecography, 43(7), 1052–1064. [Google Scholar]
- Zupan, M., Fragkopoulou, E., Claudet, J., Erzini, K., Horta e Costa, B., & Gonçalves, E. J. (2018). Marine partially protected areas: Drivers of ecological effectiveness. Frontiers in Ecology and the Environment, 16, 381–387. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


