ABSTRACT
Background and Objective
The sensory cell somata in the DRG contain all equipment necessary for extensive GABAergic signaling and are able to release GABA upon depolarization. With this study, we hypothesize that pain relief induced by conventional dorsal root ganglion stimulation (Con‐DRGS) in animals with experimental painful diabetic peripheral neuropathy is related to the release of GABA from DRG neurons. With use of quantitative immunocytochemistry, we hypothesize DRGS to result in a decreased intensity of intracellular GABA‐immunostaining in DRG somata.
Materials and Methods
Female Sprague‐Dawley rats (n = 31) were injected with streptozotocin (STZ) in order to induce Diabetes Mellitus. Animals that developed neuropathic pain after four weeks (Von Frey) were implanted with a unilateral DRGS device at L4 (n = 14). Animals were then stimulated for 30 min with Con‐DRGS (20 Hz, pulse width = 0.2 msec, amplitude = 67% of motor threshold, n = 8) or Sham‐DRGS (n = 6), while pain behavior (von Frey) was measured. DRGs were then collected and immunostained for GABA, and a relation to size of sensory cell soma diameter (small: 12–26 μm, assumed to be C‐fiber related sensory neurons; medium: 26–40 μm, assumed to be Aδ related sensory neurons; and large: 40–54 μm, assumed to be Aβ related sensory neurons) was made.
Results
DRGS treated animals showed significant reductions in STZ‐induced mechanical hypersensitivity. No significant differences in GABA immunostaining intensity per sensory neuron cell soma type (small‐, medium‐, or large‐sized) were noted in DRGs of stimulated (Con‐DRGS) animals versus Sham animals. No differences in GABA immunostaining intensity per sensory cell soma type in ipsi‐ as compared to contralateral DRGs were observed.
Conclusion
Con‐DRGS does not affect the average intracellular GABA immunofluorescence staining intensity in DRG sensory neurons of those animals which showed significant pain reduction. Similarly, no soma size related changes in intracellular GABA immunofluorescence were observed following Con‐DRGS.
Keywords: Dorsal root ganglion stimulation, GABA, neuromodulation, neuropathic pain, painful diabetic peripheral neuropathy
INTRODUCTION
Spinal cord stimulation (SCS) is a recognized treatment option for patients with intractable chronic neuropathic pain (1, 2). SCS was first utilized in 1967 by Shealy and colleagues (3) two years after the publication of the “Gate‐control” theory by Melzack and Wall (4). According to the Gate Control Theory, antidromic stimulation of the non‐nociceptive Aβ‐fibers located in the dorsal column of the spinal cord can close the “spinal gate” by inhibiting incoming signals entering the spinal cord from the periphery via nociceptive C‐ and Aδ‐fibers. The neurotransmitter γ‐aminobutyric acid (GABA) is thought to be a key molecule in this process (5, 6, 7). A study by Janssen et al. found intracellular GABA levels to be increased in the dorsal horn (DH) of the spinal cord following peripheral nerve injury (8). It was later shown that conventional (Con‐)SCS decreased the intracellular GABA concentration in the DH of the spinal cord in this same neuropathic pain model (9), as well as multiple studies demonstrating increased extracellular GABA levels, as measured by microdialysis, following Con‐SCS (10, 11, 12). In addition, the application of a GABAB receptor antagonist can transiently abolish the SCS‐induced effects in neuropathic rats (10), and application of subeffective doses of Baclofen (a GABAB receptor agonist) was shown to turn SCS nonresponders into SCS responders both in rats (13) as well as humans (14). Thus, spinal GABA release seems to play a pivotal role in the analgesic mechanism of action (MoA) of Con‐SCS of the dorsal columns.
Dorsal root ganglion stimulation (DRGS) is a promising novel addition to the field of neuromodulation, with advantages over SCS in terms of a smaller charge requirement, better dermatomal coverage, and efficacy in the treatment of complex regional pain syndrome (15, 16, 17, 18). Over the years, an increasing amount of literature has been published on the analgesic properties of DRGS, including both clinical (19) and rodent studies (20, 21, 22, 23, 24, 25). Whereas SCS is thought to only recruit Aβ‐fibers, DRGS can theoretically stimulate not only Aβ‐, but also C‐ and Aδ‐fibers due to the unique properties of the DRG (26). The somata of all peripheral fibers (Aβ‐, C‐, and Aδ‐fibers) reside in the DRG, and the DRG has shown to be surprisingly tolerant for trauma following lead insertion (27). These different types of sensory DRG neurons have different properties, both in terms of electrophysiological responses as well as morphology. Whereas the touch‐affiliated Aβ‐fibers have thick, myelinated fibers with a large cell soma (40–54 μm) and high conduction velocity, the nociceptive Aδ‐fibers (thinly myelinated, medium‐sized cell soma: 26–40 μm, medium conduction velocity) and C‐fibers (unmyelinated, small‐sized cell soma: 12–26 μm, low conduction velocity) have their own distinct electrophysiological and morphological properties (28). To date, the MoA of DRGS remains largely unknown. It was recently shown that DRGS with conventional settings (Con‐DRGS) does not decrease intracellular levels of GABA immunoreactive staining in the DH of the spinal cord in rats with painful diabetic polyneuropathy (PDPN) (29). From this, it is concluded that Con‐DRGS, in contrast to Con‐SCS, is not likely to depend on GABA release in the DH of the spinal cord. In this context it is interesting that Du et al. reported the presence of an extensive GABAergic communication network between sensory neuron somata inside the DRG itself (30). The authors showed that sensory neurons in the DRG express major proteins required for GABA synthesis and release, and that these neurons are capable of releasing GABA upon depolarization. From this it was postulated that the GABAergic system in the DRG itself may act as a second gate, in addition to the aforementioned gate control theory (or first gate) in the spinal DH.
In the present study, we therefore hypothesized that electrical stimulation of the DRG with conventional settings (Con‐DRGS) decreases intracellular GABA immunoreactivity in DRG somata of those animals which showed reduced mechanical hypersensitivity. To investigate this, we quantitatively assessed the intensity of local intracellular GABA immunoreactivity in the L4 DRG of Con‐DRGS or sham treated PDPN animals.
MATERIALS AND METHODS
Animals
This study was performed in 31 female Sprague‐Dawley rats (Charles River, Maastricht, The Netherlands), with an average body weight of 160–220 g at the start of the experiment. Animals were socially housed in a climate‐controlled room at a 12/12 reversed day‐night cycle in transparent polycarbonate cages, with ad libitum access to food and drinking water. The experiments described in this study were approved by the Animal Care Committee of the Maastricht University Medical Centre (under project license 2017–022), and experiments were performed in accordance with the guidelines of the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU).
Induction of Diabetes Mellitus
Before streptozotocin (STZ) injections, animals were weighed and fasted overnight. 65 mg/kg freshly dissolved STZ in 0.9% NaCl was then intraperitoneally injected to induce Diabetes Mellitus (DM). Six days following STZ injection, blood glucose levels of the animals were measured using an Accu‐Chek Aviva® glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Only rats with a blood glucose level of ≥15 mmol/L were considered diabetic and included in the study (31, 32, 33, 34, 35).
Assessment of Mechanical Hypersensitivity
Pain behavior was assessed by applying Von Frey monofilaments (bending forces 0.6, 1.2, 2.0, 3.6, 5.5, 8.5, 15.1, and 28.84 g) to the plantar surface of the hind paws of the animals at baseline (before STZ injection) and four weeks following STZ injection. Rats were individually placed in plastic cages with a mesh floor, after which they were allowed to habituate to the testing environment for 15 min. The 50% withdrawal threshold (WT) was then calculated based on the up‐down method as previously described (36). A cutoff of 28.84 g was used in order to prevent tissue damage. Calculated 50% WT values were then multiplied by 10,000 and logarithmically transformed to obtain a linear scale and account for Weber's law (37). Only animals with a decrease of ≥0.2 of the log10 (10,000 × 50% WT) unit were considered to have mechanical hypersensitivity and were treated with DRGS.
Dorsal Root Ganglion Stimulation
A bipolar, custom‐made DRGS lead was implanted as previously described (20, 21, 22, 23, 27). Briefly, a paravertebral incision was made, and the intravertebral foramen of L4 was exposed. The foramen was then opened, and both the anode and cathode of the electrode were inserted into the foramen in order to stimulate the L4 DRG. The electrode was then secured to the transverse process caudal to the L4 foramen using a small screw and steel wire. The external connectors of the electrode were then tunneled under the skin via the neck of the animal, and the incision was closed in layers.
Animals were externally stimulated for 30 min with Con‐DRGS (20 Hz, 0.2 msec pulse width, amplitude at 67% of the motor threshold [MT]) or Sham‐DRGS (amplitude at 0% MT) using a Proclaim implantable pulse generator (Abbott, Inc., TX, USA) in the first week following DRGS implantation (31, 32). The MT was measured before stimulation onset using a frequency of 2 Hz, and pulse width of 0.2 msec. The MT was defined as the current inducing contractions of the lower trunk or hind limb. In order to test the effect of Con‐DRGS on pain behavior, Von Frey measurements were performed just before, and at 30 min following onset of Con‐DRGS. Following 30 min of DRGS, animals were immediately anesthetized with pentobarbital (100 mg/kg) and transcardially perfused with 15% picric acid and 4% paraformaldehyde in 0.2 M phosphate buffer saline (PBS; pH 7.6).
Tissue Preparation
Following transcardial perfusion, both the ipsi‐ and contralateral L4 DRG were extracted as previously described (38). Tissue was then stored in PBS with 1% azide until further use. DRGs were pre‐embedded in a 50:50 mix of 4% agar/5% gelatin in order to prevent tissue shrinking, followed by paraffin embedding. Tissue was serially sectioned using a microtome at a thickness of 4 μm, after which each out of 10 sections were mounted on glass slides (each 40 μm). The glass slides containing the tissue were then incubated overnight at a temperature of 37°C, after which they were stored at room temperature until further use.
Immunohistochemical GABA Staining
Sections were first deparaffinized using a sequence of 2x 15 min xylene, 2x 5 min 100% ethanol, 2x 5 min 96% ethanol, and 2x 70% ethanol. Slides were then washed 10 min with Tris‐buffered saline (TBS, 0.1 M, pH 7.6) including 0.3% Triton X‐100 (TBS‐T), 10 min TBS, and 10 min TBS‐T. Next, sections were blocked for one hour using 2% normal donkey serum (Sigma‐Aldrich, Zwijndrecht, The Netherlands, D9663) diluted in TBS‐T. Slides were then incubated overnight with a rabbit anti‐GABA polyclonal antibody (1:1000 diluted in TBS‐T; Sigma‐Aldrich, Zwijndrecht, The Netherlands, A2052). After rinsing unbound primary antibody in TBS (3x 10 min), slides were incubated with the far‐red secondary antibody Alexa‐Fluor 647 donkey anti‐rabbit IgG (1:100 diluted in TBS‐T; Invitrogen, Breda, The Netherlands) for two hours. Slides were then again washed in TBS (3x 10 min), after which slides were incubated with Hoechst (1:1000 in TBS) for 15 min in order to visualize nuclei. Last, slides were washed 3x 10 min in TBS, and then cover slipped with TBS/glycerol (80%/20%).
Quantitative Immunocytochemical Analysis
Following the GABA‐staining protocol, immunostained sections were observed under a Disk Scanning Unit (DSU) microscope (Olympus, Tokyo, Japan). First, photomicrographs were taken of the DRG sections (three sections per DRG) using Micromanager Software (Ron Vale's Laboratory, UCSF, San Francisco, CA, USA). Images were then merged together using Adobe Photoshop (Adobe, Inc., San Jose, CA, USA), and somata were outlined. Only somata with a visible nucleus were included in the analysis, which, in combination with the fact that only one section in every 40 μm (1:10) was mounted on a glass slide, should make sure that no cells were measured twice. Average grayscale values at the 647 channel were analyzed per cell soma using ImageJ software (National Institutes of Health [NIH] and the Laboratory for Optical and Computational Instrumentation [LOCI], University of Wisconsin, USA), and the corresponding diameter per soma was measured and noted. Average gray values per treatment group were then calculated, as well as the average gray values per cell diameter per treatment group. Three diameter ranges were chosen based on literature: 12–26 μm (small‐sized soma, assumed to be C‐fiber related sensory neurons), 26–40 μm (medium‐sized soma, assumed to be Aδ related sensory neurons), and 50–54 μm (large‐sized soma, assumed to be Aβ related sensory neurons) (28). The investigator was blinded for the condition of the tissue and animals throughout the whole experiment.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). For statistical analysis, Von Frey data were logarithmically transformed to account for Weber's Law and obtain a linear scale (37). For comparisons between pre‐STZ WTs and preimplant WTs, and analysis of the effect of DRGS on WTs, a one‐way analysis of variance (ANOVA) was performed, followed by Tukey's multiple comparison test. For the comparison of MTs between groups, an unpaired t‐test was used. For comparisons of grayscale values between treatments (DRGS vs. Sham‐DRGS), and between ipsilateral and contralateral, one‐way and two‐way ANOVAs were used, followed by a Tukey's multiple comparisons test. A p value <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software version 8.4.3 (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Flowchart of Animals
Twenty‐six out of 31 rats that were injected with STZ developed DM within one week (84%; blood glucose level > 15 mmol/L). No animals required additional insulin treatment (blood glucose ≥31.4 mmol/L). Out of the 26 diabetic rats, 14 animals developed PDPN after four weeks (54%; ≥0.2 decrease in log10 (10,000 × 50% WT) on von Frey when compared to the pre‐STZ injection baseline) and were subsequently implanted with a unilateral DRGS device at the L4 lumbar level in week 5. The remaining 12 rats were excluded from the study and sacrificed.
Behavior
Development of PDPN
The mean log10 (10,000 × 50% WT) of the 14 implanted animals dropped from 5.3 ± 0.05 g (ipsilateral hind paw) and 5.1 ± 0.05 g (contralateral hind paw) at pre‐STZ‐baseline to 4.8 ± 0.06 g (ipsilateral hind paw; p < 0.0001 compared to baseline) and 4.8 ± 0.07 g (contralateral hind paw; p < 0.01 compared to baseline) at preimplantation (four weeks after STZ injection). No differences between the ipsi‐ and contralateral hind paw were noted at either the pre‐STZ baseline (p = 0.20) or the preimplantation measurement (p > 0.99) (Fig. 1a).
Figure 1.
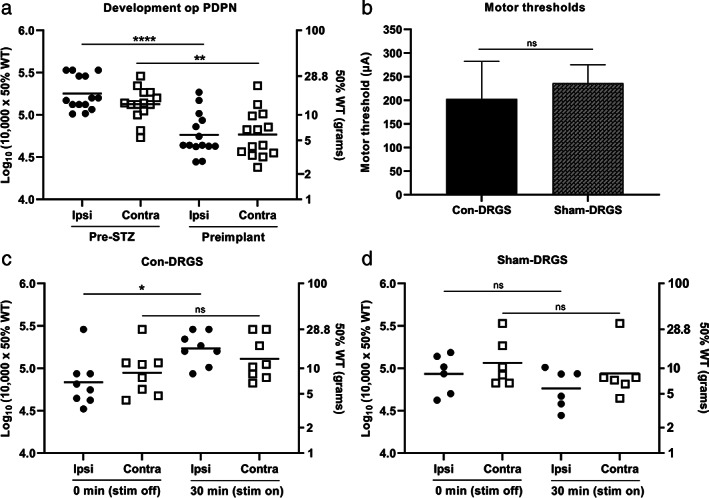
a. Development PDPN over time in all implanted rats (n = 14). Animals developed profound STZ‐induced mechanical hypersensitivity in both the ipsilateral and contralateral hind paw. b. No significant differences were observed in terms of average MTs between the Con‐DRGS and Sham‐DRGS group. MT was assessed using a frequency of 2 Hz, and pulse width of 0.2 msec. c. The effect of 30 min of Con‐DRGS (20 Hz) on mechanical hypersensitivity values of both the ipsilateral (stimulated) and contralateral (unstimulated) hind paw (n = 8). Con‐DRGS attenuated STZ‐induced mechanical hypersensitivity in the ipsilateral, but not contralateral hind paw. d. No significant effect was observed of Sham‐DRGS on mechanical hypersensitivity values of both the ipsi‐ and contralateral hind paw (n = 6). *p < 0.05; **p < 0.01; ****p < 0.0001; ns, not significant.
Dorsal Root Ganglion Stimulation
No significant differences in terms of MTs were observed between the Con‐DRGS and Sham‐DRGS group, indicating correct and stable implantation of the DRGS lead in both arms (DRGS: 202 ± 79 μA, Sham‐DRGS: 236 ± 39 μA; p = 0.74) (Fig. 1b).
Con‐DRGS significantly attenuated STZ‐induced mechanical hypersensitivity in the ipsilateral (stimulated) hind paw. 6/8 animals (75%) were classified as responders to Con‐DRGS (≥0.2 increase in log10 (10,000 × 50% WT) on Von Frey when compared to the stim off measurement). Average ipsilateral Log10 (10,000 × 50% WT) values were 4.8 ± 0.10 g at baseline (before Con‐DRGS onset), and significantly increased to 5.2 ± 0.07 g after 30 min of Con‐DRGS (p < 0.05). Contralateral log10 (10,000 × 50% WT) values also increased from 4.9 ± 0.10 g at baseline (before Con‐DRGS onset) to 5.1 ± 0.09 g after 30 min of Con‐DRGS, albeit not significant (p > 0.05). No significant differences were observed between the ipsi‐and contralateral hind paw at either baseline (stim off; p = 0.27) or the 30 min time point (stim on; p = 0.75) (Fig. 1c).
No effect of Sham‐DRGS was observed on either the ipsilateral or contralateral hind paw. Average ipsilateral log10 (10,000 × 50% WT) values were 4.9 ± 0.09 g at baseline (before Sham‐DRGS onset), and 4.8 ± 0.08 g after 30 min of Sham‐DRGS (p = 0.11). Contralateral log10 (10,000 × 50% WT) values were 5.0 ± 0.11 at baseline (before Sham‐DRGS onset), and 4.9 ± 0.12 g after 30 min of Sham‐DRGS (p = 0.25). No significant differences were observed between the ipsi‐and contralateral hind paw at either baseline (stim off; p = 0.69) or the 30 min time point (stim on; p = 0.44) (Fig. 1d).
Immunohistochemical GABA Staining
Average Gray Values
Anti‐GABA immunostained sections showed a strong and specific GABA immunoreactivity (Fig. 2a). No significant intragroup differences (ipsi vs. contra) in terms of average gray values were observed in either the Con‐DRGS (p = 0.99) or Sham‐DRGS group (p = 0.71). Along the same lines, no significant intergroup differences (Con‐DRGS vs. Sham‐DRGS) were observed in neither the ipsilateral DRG (p = 0.99) nor the contralateral DRG (p = 0.64) (Fig. 2b).
Figure 2.
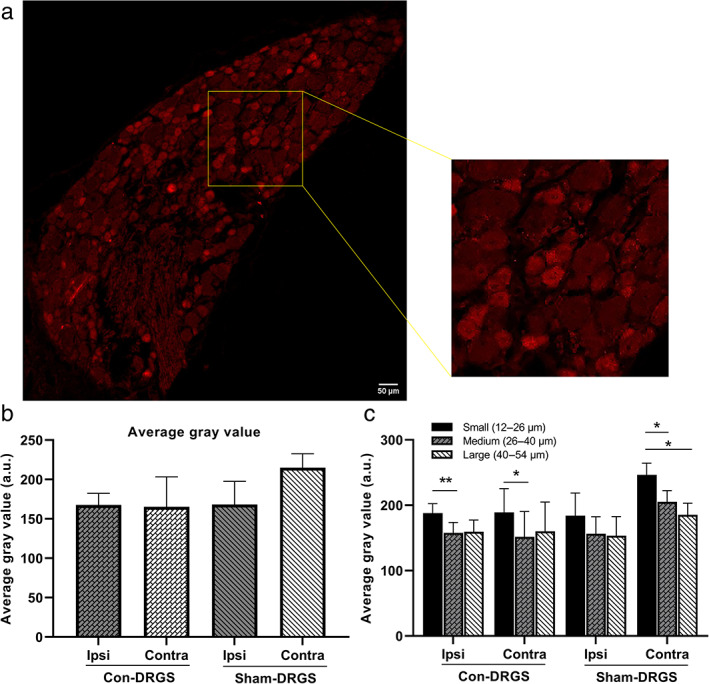
a. Representative photomicrograph of anti‐GABA immunostaining. Note the difference in staining intensity between the large and small diameter somata. b. Comparison of average gray values in the ipsi‐ and contralateral DRG of both the Con‐DRGS and Sham‐DRGS treated group. No differences were observed between ipsi‐ and contralateral DRGs in both the Con‐DRGS and Sham‐DRGS group, nor were differences observed between the Con‐DRGS and Sham‐DRGS group in both the ipsi‐ and contralateral DRG. c. Comparison of average gray values based on soma diameter. Significant differences were observed in intensity of GABA‐immunoreactivity between the small‐sized as compared to the medium‐ and large‐sized soma. No differences were observed between ipsi‐ and contralateral DRGs in both the Con‐DRGS and Sham‐DRGS group for all three soma diameter ranges, nor were differences observed between the Con‐DRGS and Sham‐DRGS group in both the ipsi‐ and contralateral DRG for all three soma diameter ranges. *p < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
Average Gray Values per Soma Diameter
We then tested whether there was a difference in the average gray value of small‐ (12–26 μm), medium‐ (26–40 μm), and large‐sized (40–54 μm) somata, and whether or not Con‐DRGS specifically acts on one of these ranges of soma diameter. An overall significant effect of diameter was noted (p < 0.0001). A significant difference also was noted between the gray values of the small versus medium (Con‐DRGS ipsi, p < 0.01; Con‐DRGS contra, p < 0.05; Sham‐DRGS contra, p < 0.05) and small‐ versus large‐sized somata (Sham‐DRGS contra, p < 0.05). A trend also was observed between the medium‐ versus large‐sized somata in the Sham‐DRGS contra DRG sections (p = 0.09) (Fig. 2c).
No intragroup differences (ipsi vs. contra) in terms of gray values were observed in both the Con‐DRGS and Sham‐DRGS group for all three soma diameter ranges (Con‐DRGS: small, p = 0.99; medium, p = 0.99; large, p = 0.99. Sham‐DRGS: small, p = 0.44; medium, p = 0.46; large, p = 0.79). Along these lines, no intergroup differences (Con‐DRGS vs. Sham‐DRGS) in terms of gray values were observed in the ipsilateral and contralateral DRG for all soma diameter ranges (ipsilateral: small, p = 0.99; medium, p = 0.99; large, p = 0.99. Contralateral: small, p = 0.53; medium, p = 0.61; large, p = 0.95) (Fig. 2c).
DISCUSSION
The present study showed that Con‐DRGS does not alter the average intracellular GABA immunofluorescence staining intensity in DRG sensory neurons. Similarly, no cell size specific effect was noted as the intensity of GABA‐immunoreactivity in cell somata of small (12–26 μm), medium (26–40 μm), or large DRG sensory neurons (40–54 μm) was similar between Con‐DRGS and Sham‐DRGS treated animals. At the same time, animals did show marked reductions in STZ‐induced mechanical hypersensitivity values after 30 min of stimulation. These reductions in pain behavior are comparable to those obtained previously in the same diabetic rat model (21, 31, 32, 39), as well as in rat models of tibial nerve injury (25, 27), collagen‐induced rheumatoid arthritis (24), and intra‐articular knee monosodium iodoacetate‐induced osteoarthritis (25).
The role of GABA as a key player in the mechanism underlying Con‐SCS of the dorsal columns has been well established over the years. The general consensus is that, in accordance with the Gate Control Theory (4), Con‐SCS antidromically stimulates the non‐nociceptive Aβ‐fibers, and thereby releases GABA from inhibitory interneurons in the DH of the spinal cord. This is represented by a decrease in intracellular GABA immunoreactivity in the DH of the spinal cord directly following stimulation (9, 40), and an increase in extracellular GABA levels (10, 11, 12). Moreover, the analgesic properties of Con‐SCS can be blocked with GABAB receptor antagonists (10, 40), while they can be improved with GABAB receptor agonists (13, 14).
In theory, this same principle may hold true also for DRGS. As all the somata of the Aβ‐fibers reside in the DRG, one could speculate that DRGS activates these Aβ‐fibers, and thereby induces the release of GABA in the spinal cord dorsal horn, similarly to what happens in Con‐SCS of the dorsal columns. Indeed, a computational analysis performed by Graham et al. found Con‐DRGS to modulate Aβ‐fiber, but not C‐fiber activity (41), which could theoretically lead to the occurrence of pain gating mechanisms in the spinal cord DH. However, a study by Koetsier et al., using the same PDPN model as used in the present study, found that Con‐DRGS does not affect the intensity of GABA‐immunoreactivity staining in the DH, and from this concluded that Con‐DRGS is not likely to release GABA in the DH of the spinal cord (42). Based on these observations, it is unlikely that Con‐DRGS relies on GABAergic pain gating mechanisms within the DH of the spinal cord.
Con‐DRGS differentiates itself from Con‐SCS by having access to the somata of all three types of sensory fibers: Aδ, Aβ, and C‐type soma. Along these lines, another computational study found that DRGS in fact does modulate C‐fiber activity, and that DRGS can amplify local T‐junction filtering, thereby blocking afferent signaling (43). The latter finding is consistent with multiple electrophysiological studies (26, 44), and might indicate that a more local effect is responsible for the analgesic properties of DRGS.
A recent study by Du and colleagues reported the presence of an extensive, local, GABAergic network in the DRG itself, as DRG cells expressed all the major proteins required for GABA synthesis and release (30). They also reported that DRG neurons of all sizes (small DRG neurons assumed to be C‐fiber related; medium DRG neurons assumed to be Aδ related; large DRG neurons assumed to be Aβ related) are capable of releasing GABA upon depolarization, and that GABA can produce a net inhibitory effect at the T‐junction in small diameter cells (30). Based on these findings, we hypothesized that Con‐DRGS induces the release of GABA from DRG somata for its analgesic action which would result in decreased intensity of intracellular GABA‐immunoreactivity. Clearly, we were not able to demonstrate any differences between Con‐DRGS treated animals and Sham‐DRGS treated animals in terms of average GABA immunofluorescence, nor were differences observed between the ipsilateral and contralateral DRG of (Sham‐)DRGS treated animals. Furthermore, as it is possible that specific soma types behave differently in response to Con‐DRGS, something that is impossible to detect by only measuring the average immunofluorescence per condition, we also looked into the effect of Con‐DRGS on groups of somata based on three ranges of diameter: 12–26, 26–40, and 40–54 μm (28). In general, the small‐ and medium‐sized somas are more likely to include nociceptive cells (C‐type and Aδ‐type, respectively), whereas the large‐sized soma are more likely to include non‐nociceptive cells (Aβ‐type). In this context, the small nociceptive DRG somata (12–26 μm) were found to express significantly more GABA as compared to the medium‐ and large‐sized somata in most groups, suggesting the importance of GABA in (slow) nociceptive signaling. Interestingly, the medium‐sized nociceptive cells (26–40 μm; assumed to be Aδ somata) showed the same GABA staining intensity as the non‐nociceptive Aβ somata. Nevertheless, no significant effects were observed between Con‐DRGS and Sham‐DRGS for all three types of DRG somata (small, medium, large), nor were ipsi‐ versus contralateral differences observed. This indicates that Con‐DRGS does not act by reducing intracellular GABA concentrations in either nociceptive (small‐ and medium‐sized somata) or non‐nociceptive cells (large‐sized somata).
Although it is tempting to strictly classify the small, medium, and large cells as C‐type soma, Aδ‐type soma, and Aβ‐type soma, respectively, one should be cautious in doing so, as C, Aδ, and Aβ‐type in rat DRG cells are known to superpose the soma diameter (28). Furthermore, Lee et al. showed that the soma size of these intermediate cells does not correlate with conduction velocity as these cells appear with both myelinated as well as unmyelinated axons (45). Moreover, the GABA immunoreactivity was analyzed in a 2D plane, which makes it possible that some somata that were classified as being medium‐ or even small‐sized, might in fact be larger cells due to the unknown depth of the Z‐axis. Future studies might therefore be undertaken including double labeling of GABA with C‐, Aδ‐, and/or Aβ‐specific antibodies, as well as 3D visualizations of DRG cell somata.
From these results, it seems very well possible that Con‐DRGS acts via totally different, GABA independent, mechanisms. As mentioned earlier, there is some evidence suggesting the ability of DRGS to inhibit the T‐junction electrophysiologically (26, 44). Furthermore, a functional magnetic resonance imaging study showed that DRGS can attenuate BOLD signals in pain‐affiliated brain regions, such as the contralateral ventral posterolateral and ventral posteromedial thalamic nuclei, and cortical S1 and S2 (46). Last, it was recently suggested that DRGS, especially at low frequencies (<20 Hz), might induce DH inhibition via the activation of low threshold mechanoreceptors and the activation the body's own opioid system via the release of endorphins and dynorphins (47).
In conclusion, under the conditions tested, we found no evidence for a local, GABA‐mediated MoA of Con‐DRGS and suggest a GABA independent mechanism to be involved in pain relief. The future research agenda should include more specific classification of nociceptive and non‐nociceptive neurons in the DRG, as well as 3D visualizations of the DRG cell somata.
Authorship Statement
Glenn Franken performed the experiments, analyzed the data, and wrote the manuscript. Perla Douven helped performing the experiments and analyzing the data. Elbert A.J. Joosten and Glenn Franken conceived and designed the experiment. Jacques Debets performed the DRGS implantations. All authors have approved the final version of the manuscript.
Comment
This is another example of the excellent preclinical work performed by this group working to elucidate the mechanisms underlying DRG‐S. This research paper underscores prior works demonstrating a separate and distinct mechanism of action for DRG‐S as compared to SCS, and the limited role of GABA plays in DRG‐S.
The time, effort, skill, and commitment from these scientists to put together such a project deserves praise from all those involved with neuromodulation.
Kenneth Chapman, MD
New York, NY USA
Acknowledgement
The authors would like to thank Hellen Steinbusch for helping with the sectioning of the tissue and her advice regarding the immunocytochemical GABA staining.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: None.
Conflict of Interest: The authors reported no conflict of interest.
REFERENCES
- 1.Medical Advisory Secretariat . Spinal cord stimulation for neuropathic pain: an evidence‐based analysis. Ont Health Technol Assess Ser 2005;5:1–78. [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar K, Abbas M, Rizvi S. The use of spinal cord stimulation in pain management. Pain Manag 2012;2:125–134. [DOI] [PubMed] [Google Scholar]
- 3.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967;46:489–491. [PubMed] [Google Scholar]
- 4.Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 5.Vallejo R, Bradley K, Kapural L. Spinal cord stimulation in chronic pain: mode of action. Spine 2017;42:S53–S60. [DOI] [PubMed] [Google Scholar]
- 6.Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep 2012;16:217–225. [DOI] [PubMed] [Google Scholar]
- 7.Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract 2018;18:1048–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen SP, Truin M, Van Kleef M, Joosten EA. Differential GABAergic disinhibition during the development of painful peripheral neuropathy. Neuroscience 2011;184:183–194. [DOI] [PubMed] [Google Scholar]
- 9.Janssen SP, Gerard S, Raijmakers ME, Truin M, Van Kleef M, Joosten EA. Decreased intracellular GABA levels contribute to spinal cord stimulation‐induced analgesia in rats suffering from painful peripheral neuropathy: the role of KCC2 and GABAA receptor‐mediated inhibition. Neurochem Int 2012;60:21–30. [DOI] [PubMed] [Google Scholar]
- 10.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1997;73:87–95. [DOI] [PubMed] [Google Scholar]
- 11.Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma‐aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery 1996;39:367–374. [DOI] [PubMed] [Google Scholar]
- 12.Linderoth B, Stiller CO, Gunasekera L, O'Connor WT, Ungerstedt U, Brodin E. Gamma‐aminobutyric acid is released in the dorsal horn by electrical y: an in vivo microdialysis study in the rat. Neurosurgery 1994;34:484–488. [DOI] [PubMed] [Google Scholar]
- 13.Cui JG, Meyerson BA, Sollevi A, Linderoth B. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABA(B) and adenosine receptor activation. Neurosci Lett 1998;247:183–186. [DOI] [PubMed] [Google Scholar]
- 14.Lind G, Schechtmann G, Winter J, Meyerson BA, Linderoth B. Baclofen‐enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: long‐term outcome of a pilot study. Eur J Pain 2008;12:132–136. [DOI] [PubMed] [Google Scholar]
- 15.Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman KB, Yousef TA, Vissers KC, van Helmond N, Stanton‐Hicks MD. Very low frequencies maintain pain relief from dorsal root ganglion stimulation: an evaluation of dorsal root ganglion neurostimulation frequency tapering. Neuromodulation 2020; e‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 2015;18:24–32. [DOI] [PubMed] [Google Scholar]
- 18.Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med 2016;41:511–519. [DOI] [PubMed] [Google Scholar]
- 19.Vuka I, Marcius T, Dosenovic S et al. Neuromodulation with electrical field stimulation of dorsal root ganglion in various pain syndromes: a systematic review with focus on participant selection. J Pain Res 2019;12:803–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koetsier E, Franken G, Debets J et al. Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther 2019;25:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koetsier E, Franken G, Debets J et al. Dorsal root ganglion stimulation in experimental painful diabetic polyneuropathy: delayed wash‐out of pain relief after low‐frequency (1Hz) stimulation. Neuromodulation 2020;23:177–184. [DOI] [PubMed] [Google Scholar]
- 22.Franken G, Debets J, Joosten EAJ. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation 2020;23:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs. conventional stimulation paradigm. Neuromodulation 2019;22:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation 2018;21:247–253. [DOI] [PubMed] [Google Scholar]
- 25.Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal root ganglion stimulation alleviates pain‐related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology 2020;133:408–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 2013;16:304–311. [DOI] [PubMed] [Google Scholar]
- 27.Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain 2016;17:1349–1358. [DOI] [PubMed] [Google Scholar]
- 28.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol 1996;76:1924–1941. [DOI] [PubMed] [Google Scholar]
- 29.Koetsier E, Franken G, Debets J et al. Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther 2020;26:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du X, Hao H, Yang Y et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 2017;127:1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs. conventional stimulation paradigm. Neuromodulation 2018;22:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franken G, Debets J, EAJ J. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation 2019;23:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluijms WA, van Kleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain 2013;17:1338–1346. [DOI] [PubMed] [Google Scholar]
- 34.van Beek M, Hermes D, Honig WM et al. Long‐term spinal cord stimulation alleviates mechanical hypersensitivity and increases peripheral cutaneous blood perfusion in experimental painful diabetic polyneuropathy. Neuromodulation 2018;21:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Beek M, van Kleef M, Linderoth B, van Kuijk SM, Honig WM, Joosten EA. Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: delayed effect of high‐frequency stimulation. Eur J Pain 2017;21:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 37.Mills C, Leblond D, Joshi S et al. Estimating efficacy and drug ED50's using von Frey thresholds: impact of Weber's law and log transformation. J Pain 2012;13:519–523. [DOI] [PubMed] [Google Scholar]
- 38.Sleigh JN, Weir GA, Schiavo G. A simple, step‐by‐step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res Notes 2016;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koetsier E, Franken G, Debets J et al. Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther 2018;25:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meuwissen KPV, de Vries LE, Gu JW, Zhang TC, Joosten EAJ. Burst and tonic spinal cord stimulation both activate spinal GABAergic mechanisms to attenuate pain in a rat model of chronic neuropathic pain. Pain Pract 2020;20:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham RD, Bruns TM, Duan B, Lempka SF. Dorsal root ganglion stimulation for chronic pain modulates Aβ‐fiber activity but not C‐fiber activity: a computational modeling study. Clin Neurophysiol 2019;130:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koetsier E, Franken G, Debets J et al. Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther 2019;26:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kent AR, Min X, Hogan QH, Kramer JM. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation 2018;21:234–246. [DOI] [PubMed] [Google Scholar]
- 44.Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain 2020;161:2872–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol 1986;243:335–346. [DOI] [PubMed] [Google Scholar]
- 46.Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: insights into a possible mechanism for analgesia. Neuroimage 2017;147:10–18. [DOI] [PubMed] [Google Scholar]
- 47.Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton‐Hicks MD. The pathways and processes underlying spinal transmission of low back pain: observations from dorsal root ganglion stimulation treatment. Neuromodulation 2020; e‐pub ahead of print. [DOI] [PubMed] [Google Scholar]


