Abstract
CDKL5 deficiency disorder (CDD) is an X‐linked pharmacoresistant neurogenetic disorder characterized by global developmental delays and uncontrolled seizures. Fenfluramine (FFA), an antiseizure medication (ASM) indicated for treating convulsive seizures in Dravet syndrome, was assessed in six patients (five female; 83%) with CDD whose seizures had failed 5–12 ASMs or therapies. Median age at enrollment was 6.5 years (range: 2–26 years). Mean FFA treatment duration was 5.3 months (range: 2–9 months) at 0.4 mg/kg/day (n = 2) or 0.7 mg/kg/day (n = 4; maximum: 26 mg/day). One patient had valproate added for myoclonic seizures. The ASM regimens of all other patients were stable. Among five patients with tonic‐clonic seizures, FFA treatment resulted in a median 90% reduction in frequency (range: 86%–100%). Tonic seizure frequency was reduced by 50%–60% in two patients with this seizure type. One patient experienced fewer myoclonic seizures; one patient first developed myoclonic seizures on FFA, which were controlled with valproate. Adverse events were reported in two patients. The patient with added valproate experienced lethargy; one patient had decreased appetite and flatus. No patient developed valvular heart disease or pulmonary arterial hypertension. Our preliminary results suggest that FFA may be a promising ASM for CDD. Randomized clinical trials are warranted.
Keywords: CDKL5 deficiency disorder, epilepsy, fenfluramine
1. INTRODUCTION
CDKL5 deficiency disorder (CDD) is an X‐linked disorder resulting from mutations in the CDKL5 gene, which encodes a kinase involved in synaptic plasticity, glutaminergic signaling, and dendrite formation.1, 2 Girls are ~4‐fold more often affected, but boys are more severely affected. The incidence is ~1 per 50 000 births.1 CDD typically presents in the first 3 months of life with treatment‐resistant epilepsy (TRE) and hypotonia followed by global developmental delays and cortical visual impairment.1, 2 Infantile spasms and other generalized or mixed generalized/focal epilepsies may be the initial seizure type, with evolution to multiple seizure types that often straddle or fail to conform to standard classifications. Seizures often respond initially but recur, and most children have daily seizures despite multiple antiseizure medication (ASM) regimens.1, 2
Fenfluramine (FFA) enhances serotonin release, positively modulates sigma‐1 receptors,3 and has potent, durable efficacy in treating convulsive seizures in Dravet syndrome and drop seizures in Lennox‐Gastaut syndrome,4, 5 with approval for Dravet syndrome by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Long‐term open‐label extension studies and the Belgium experience demonstrated durable reduction in convulsive seizure frequency for up to 30 years in patients with Dravet syndrome, with no observations of pulmonary arterial hypertension or valvular heart disease in any patient at any time.6, 7
We studied FFA in patients with CDD and treatment‐resistant epilepsy.
2. MATERIALS AND METHODS
This open‐label, investigator‐initiated trial was performed at the NYU Epilepsy Center and was designed to enroll up to 10 patients (NCT03861871). Inclusion criteria included confirmed pathogenic CDKL5 mutation and clinical diagnosis of CDD, ages 2–35 years inclusive, ≥4 convulsive seizures lasting ≥3 s (tonic‐clonic, tonic, atonic, clonic, focal motor) during the 4‐week baseline period, and therapy with ≥1 ASM with stable doses of ASMs, dietary therapies, or vagus nerve stimulation settings for ≥4 weeks before screening and expected stability throughout the study.
Patients were titrated to effect and were treated with FFA for ≥14 weeks, followed by a long‐term follow‐up phase. FFA was administered twice daily as an oral solution of FFA hydrochloride containing 2.2 mg/mL FFA. The primary outcome was median monthly convulsive seizure frequency on seizure diary. Secondary outcomes included caregiver ratings on the Clinical Global Impression of Improvement (CGI‐I) scale, a 7‐point Likert scale; Quality of Life in Childhood Epilepsy (QOLCE), a 91‐item survey assessing five functional domains on a 5‐point Likert scale; and the Pediatric Quality of Life Inventory (PedsQL), a 23‐item survey assessing five functional domains in children and adolescents. Echocardiography were performed at baseline before treatment with FFA and then 6 weeks after FFA therapy was initiated. We did not assess fine motor skills, stereotypies, or eye contact.
Descriptive statistics included medians, means, ranges, and standard deviations for continuous variables, and frequencies and percentages for categorical variables. The study was approved by the NYU Langone Medical Center Institutional Review Board.
3. RESULTS
Patient characteristics are presented in Table 1. Six children with CDD with TRE were enrolled; five were female. Age at enrollment ranged from 2–26 years (median: 6.5 years). Patients had failed 5–12 ASMs. All patients' epilepsy therapies were stable except for one patient who had valproate added while on FFA treatment for myoclonic seizures. Doses were titrated to effect, and patients were treated for ≥14 weeks (2 months) at the maintenance dose. Mean treatment duration was 5.3 months (range: 2–9 months). The maximum dose of 0.7 mg/kg/day (maximum daily dose: 26 mg/day) was reached in four patients and 0.4 mg/kg/day (maximum and maintenance dose) in two patients.
TABLE 1.
Patient characteristics
| Patient | Age at diagnosis | CDKL5 pathogenic Variant | Prior ASMa | ASM at FFA initiationb | Predominant seizure type, BL (seizure history)c | CGI | QOLCE | PedsQL | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Post FFA | BL | Post FFA | BL | Post FFA | ||||||
| 1 | 2 months | p.Glu449LeufsX38 | Levetiracetam, oxcarbazepine, pentobarbital, topiramate | Diazepam, valproate |
TC (AA, MC) |
Much Impr | Fair | Very Good | 200 | 425 |
| 2 | 3 months | p.Arg550Ter | Ataluren, cannabidiol, clobazam, levetiracetam, lamotrigine, lorcaserin, valproate | Diazepam, midazolam, perampanel, quetiapine, zonisamide |
A (MC, TC) |
Slt Worse |
Very Good | Fair | 500 | 450 |
| 3 | 6 weeks | p.Glu416ValfsX2 | Clonazepam, levetiracetam, topiramate | Clobazam, diazepam, valproate, vigabatrin |
TC (A, ES) |
Much Impr | Good | Good | 650 | 1100 |
| 4 | 6 weeks | p.His127Tyr | Acetazolamide, cannabidiol, ezogabine, felbamate, lorcaserin, lacosamide, phenytoin, prednisone, primidone, rufinamide, topiramate, vigabatrin | Clonazepam, diazepam, midazolam, valproate |
T (A, MC, TC, TA) |
Much Impr | Fair | Very Good | 450 | 800 |
| 5 | 5 weeks | p.Arg558ThrfsTer9 | Clonazepam, lacosamide, levetiracetam, lorazepam, phenobarbital, phenytoin, vigabatrin | Cannabidiol, clobazam, midazolam, perampanel, valproate |
T (A, AA, TA, FWMC, MC, TC) |
Slt Impr | Poor | Fair | NC | NC |
| 6 | 7 months | Xp22.2p22.13 | Brivaracetam, clobazam, felbamate, lacosamide, levetiracetam, perampanel, prednisone, topiramate, valproate | Lorazepam, cannabidiol, clonazepam, midazolam |
TC (AA) |
Much Impr | Poor | Fair | NC | NC |
| Median (range) |
7 (5‐28) |
— |
6.5 (5‐12) |
3.5 (2‐5) |
3 (2‐7) |
— | — | — | — | — |
Seizure types: A, atonic; AA, atypical absence; ES, epileptic spasm; FWMC, focal impaired with motor components; MC, myoclonic; T, tonic; TA, typical absence; TC, tonic‐clonic.
Abbreviations: ASM, antiseizure medication; BL, baseline; CGI, clinical global impression; FFA, fenfluramine; Impr, improved; N/A, not applicable; NC, not calculated (N/A listed too many times); QOLCE, Quality of Life Childhood Epilepsy; Slt, slightly; VNS, vagus nerve stimulation.
Patient 2 was also on ketogenic diet as a prior ASM.
Patient 1 was also on ketogenic diet at FFA initiation; Patient 4 was also on VNS at FFA initiation.
Nonseizure symptoms at baseline in ≥2 patients: constipation (n = 4), hypotonia (n = 3), sleep issues (n = 3), tiredness (n = 3), unsteadiness (n = 3), headaches (n = 2), kidney stones (n = 2), and scoliosis (n = 2).
Among five patients with generalized tonic‐clonic seizures (GTCS), there was a median 90% (range: 86%–100%) reduction in GTCS (Figure 1A). Among two patients with tonic seizures, there was a median 55% (range: 50%–60%) reduction in tonic seizures (Figure 1B). The only patient with myoclonic seizures had a 71.4% reduction. One additional patient developed new‐onset myoclonic seizures while on FFA, and valproate was added, which resulted in reduced myoclonic seizures.
FIGURE 1.
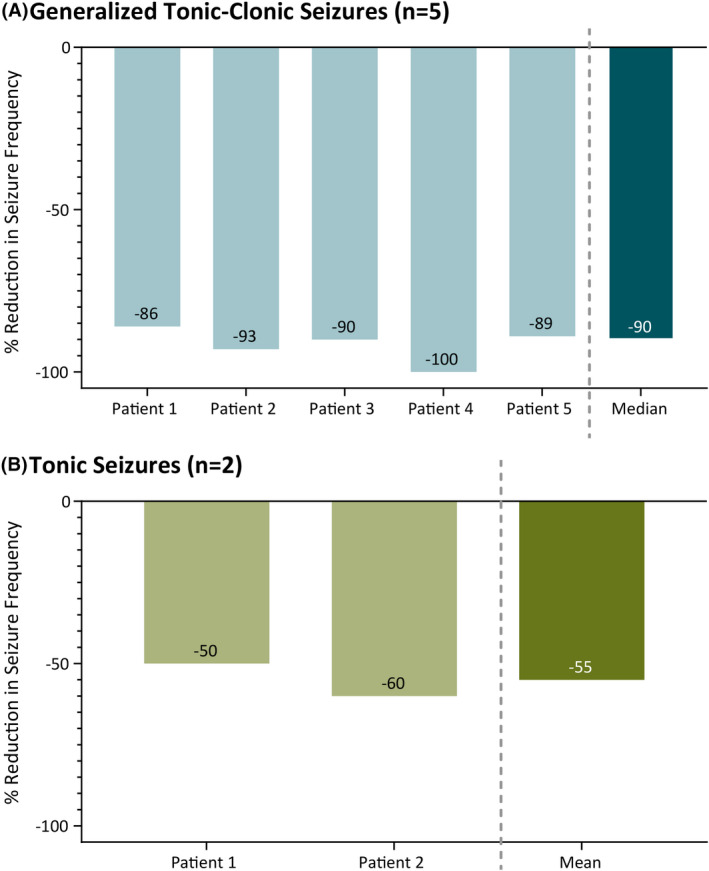
Reduction in (A) generalized tonic‐clonic seizures and (B) tonic seizures after fenfluramine
Treatment‐emergent adverse events were reported in two patients. One had decreased appetite and flatus, and the other had lethargy after valproate was added. Decreased appetite resolved after 20 days, flatus resolved after 5 months, and lethargy persisted with valproate. No patient developed signs or symptoms of valvular heart disease or pulmonary arterial hypertension.
Secondary outcomes improved after FFA treatment in most patients. Most caregivers (4/6; 67%) rated patients as having clinically meaningful improvement overall on the CGI‐I scale (“Much Improved” or greater). Four patients (67%) showed overall improvement on the QOLCE, and half (3/6; 50%) showed improvement on the PedsQL. Individual patient scores showed consistent improvement, no change, or worsening across all three metrics.
4. DISCUSSION
FFA was a safe and effective ASM in these six patients with CDD. FFA, with its novel mechanism of action involving both serotonergic and sigma‐1 activity,3 may be a promising ASM treatment option to achieve durable clinically meaningful seizure frequency reduction in patients with CDD. Our preliminary results suggest that FFA is very effective in controlling GTCS and is effective in controlling tonic seizures in CDD patients. All five patients with GTCS had previously been on 5–12 ASMs, often in three to four medication combinations, without achieving comparable efficacy in seizure control. We only counted seizures with motor activity lasting 3 seconds or longer, and likely included some of the hypermotor–tonic spasm seizures within the tonic group but did not include isolated epileptic spasms. Because myoclonic seizures are brief and difficult to accurately count, we planned not to include these. Although we had planned to recruit 10 subjects, enrollment stalled after six subjects were enrolled, and given the positive data, we decided to halt enrollment, as a randomized, placebo‐controlled clinical trial with FFA was planned and is currently being initiated in this population.
CONFLICT OF INTEREST
OD: Research funding, Novartis, PTC Therapeutics, Zogenix; Equity interest, Rettco, Pairnomix, Tilray, and Egg Rock Holdings; LK, DP: No disclosures. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The studies described in this article were funded by an unrestricted grant from Zogenix, Inc. (Emeryville, CA, USA). Professional medical writing and editing were provided to the authors by Danielle Ippolito, PhD, and Dolores Matthews, ELS, of PharmaWrite, LLC (Princeton, NJ, USA), and were paid for by Zogenix. [Correction added on 17 May, 2021, after first online publication: The acknowledgement section has been modified.]
REFERENCES
- 1.Jakimiec M, Paprocka J, Smigiel R. CDKL5 deficiency disorder—a complex epileptic encephalopathy. Brain Sci. 2020;10(2):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson HE, Demarest ST, Pestana‐Knight EM, Swanson LC, Iqbal S, Lal D, et al. Cyclin‐dependent kinase‐like 5 deficiency disorder: clinical review. Pediatr Neurol. 2019;97:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma‐1 receptors. Epilepsy Behav. 2020;105:e106989. [DOI] [PubMed] [Google Scholar]
- 4.Balagura G, Cacciatore M, Grasso EA, Striano P, Verrotti A. Fenfluramine for the treatment of Dravet syndrome and Lennox‐Gastaut syndrome. CNS Drugs. 2020;34(10):1001–7. [DOI] [PubMed] [Google Scholar]
- 5.Lagae L, Sullivan J, Knupp K, Laux L, Polster T, Nikanorova M, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2020;394(10216):2243–54. [DOI] [PubMed] [Google Scholar]
- 6.Müller A, Helbig I, Jansen C, Bast T, Guerrini R, Jähn J, et al. Retrospective evaluation of low long‐term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5‐related epilepsy. Eur J Paediatr Neurol. 2016;20(1):147–51. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan J, Scheffer IE, Lagae L, Nabbout R, Pringsheim M, Talwar D, et al. Fenfluramine HCl (Fintepla®) provides long‐term clinically meaningful reduction in seizure frequency: analysis of an ongoing open‐label extension study. Epilepsia. 2020;61(11):2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


