Abstract
Resistance (R) genes usually compete in a coevolutionary arms race with reciprocal effectors to confer strain‐specific resistance to pathogens or herbivorous insects. Here, we investigate the specificity of SLI1, a recently identified R gene in Arabidopsis that encodes a small heat shock‐like protein involved in resistance to Myzus persicae aphids. In a panel with several aphid and whitefly species, SLI1 compromised reproductive rates of three species: the tobacco aphid M. persicae nicotianae, the cabbage aphid Brevicoryne brassicae and the cabbage whitefly Aleyrodes proletella. Electrical penetration graph recording of aphid behaviour, revealed shorter salivations and a 3‐to‐5‐fold increase in phloem feeding on sli1 loss‐of‐function plants. The mustard aphid Lipaphis erysimi and Bemisia tabaci whitefly were not affected by SLI1. Unlike the other two aphid species, L. erysimi exhibited repetitive salivations preceding successful phloem feeding, indicating a role of salivary effectors in overcoming SLI1‐mediated resistance. Microscopic characterization showed that SLI1 proteins localize in the sieve tubes of virtually all above‐ and below‐ground tissues and co‐localize with the aphid stylet tip after penetration of the sieve element plasma membrane. These observations reveal an unconventional R gene that escapes the paradigm of strain specificity and confers broad‐spectrum quantitative resistance to phloem‐feeding insects.
Keywords: aphids, phloem, plant resistance, R genes, whiteflies
Short abstract
We found that the Arabidopsis R gene SLI1 is not strain specific, but affects a wide range of phloem‐feeding insects, including two aphid and a whitefly species, via a phloem‐located mechanism that co‐localizes with the insect stylet tip.
1. INTRODUCTION
Aphids (Aphididae) are herbivores that live almost exclusively on phloem sap. To exploit plant sap, their hemipteran ancestors evolved piercing‐sucking mouthparts around 370 million years ago (Misof et al., 2014). Nowadays, an average aphid stylet bundle contains a 1 μm wide food canal and a salivary duct that unite in the tip of the stylet (Forbes, 1977). Because phloem sap is under strong osmotic pressure in the plant, the food canal is mainly used for passive uptake of sap (Will & van Bel, 2006), whereas the salivary duct is used for two kinds of secretions: a gelling saliva that is released during probing of the apoplast, and watery saliva that is secreted both extra‐ and intracellularly (Moreno et al., 2011). Both types of saliva can contain effectors that overrule the plant defence system, such as cell‐wall‐degrading enzymes and Ca2+‐binding proteins that suppress wound responses in the plant (Bos et al., 2010; Mutti et al., 2008; Will & Vilcinskas, 2015). Saliva may also contain viruses, which is a reason why aphids represent a great threat to agriculture. Aphids transmit an estimated 50% of all insect‐vectored plant viruses (James & Perry, 2004), depending on the virus, either during brief probes or after longer contact and more salivation (Zitter, 1977). Crop yields are furthermore compromised due to the asexual reproduction strategy that results in exponential increases in aphid populations and mold growth on honeydew excrements (Minks & Harrewijn, 1989). As systemic insecticides have negative impacts on non‐target organisms (Calvo‐Agudo et al., 2019), host‐plant resistance is a focal point in new, sustainable pest management strategies. To realize an effective suppression of aphid pests, more insight is required into the genetic and physiological basis underlying plant resistance mechanisms.
Resistance genes, so‐called ‘R genes’, play a central role in plant immune responses to microbial pathogens, nematodes and herbivorous insects. Classically, the term R gene is used for genes with a function in effector‐triggered immunity (Kaloshian, 2004; Keith & Mitchell‐Olds, 2013). They involve proteins on the in‐ and outside of the cell that can (help to) recognize a specific attacker and result in a signalling cascade to mount an immune response that may lead to absolute or quantitative resistance (Hammond‐Kosack & Jones, 1997). Five classes of R genes were originally described by Dangl and Jones (2001), all having different combinations of structural protein motifs, such as leucine‐rich repeats (LRRs) or nucleotide‐binding sites (NBS). Compatibility between a receptor and ligand is highly specific and tightly connected to the amino acid structure that shapes the properties of the binding domain. R genes therefore engage in gene‐for‐gene interactions, where they compete with complementary effectors in a coevolutionary arms race to recognize effectors or to escape recognition, respectively (Flor, 1971; Jones & Dangl, 2006; Keith & Mitchell‐Olds, 2013). The continuous adaptation to pathogen virulence is a major drive for gene diversification. Overall, these characteristics make R genes effective in mounting tailor‐made immune responses against a narrow range of attackers.
Although more than 300 R genes have been cloned in the last 30 years, the majority accounts for microbial pathogens, and supposedly around 10 to 20 for herbivorous insects (Kourelis & van der Hoorn, 2018). Most of the currently known R genes or R loci that affect aphids encode LRR domains, which are involved in perceiving an attacker through the binding with effectors and cofactors (Aylwin & Ramnik, 2011). Examples are the Mi1.2 gene in tomato that confers resistance to the aphid Macrosiphum euphorbiae (Rossi et al., 1998), the Vat gene in melon that confers resistance to Aphis gossypii (Dogimont, Chovelon, Pauquet, Boualem, & Bendahmane, 2014), and the Rmprp‐1 locus in pepper that is associated with resistance to certain Myzus persicae populations (Sun et al., 2019). Only the recently identified EDR1‐like, a Raf‐like kinase, lacks an LRR domain and interacts with the M. persicae effector CathB3 in tobacco (Guo et al., 2020). After coining the term R genes, it was acknowledged that several resistance genes were not covered by the defined five classes (Martin, Bogdanove, & Sessa, 2003). Based on new insights, some studies suggested to open up the R gene dictionary to genes with yet unknown mechanisms, other protein motifs, or involved in recognition of more conserved pathogen‐associated molecular patterns (PAMPs) (Keith & Mitchell‐Olds, 2013; Kourelis & van der Hoorn, 2018). In such a broader framework, more R genes against aphids can be considered, including the Nr locus in lettuce that conveys absolute resistance to Nasonovia ribisnigri biotype Nr:0 via an unknown mechanism (Reinink & Dieleman, 1989), PAD4 that infers antixenosis and antibiosis against Myzus persicae in Arabidopsis and tomato (Louis & Shah, 2015), and ADF3, encoding an actin‐binding protein, that affects M. persicae feeding (Mondal et al., 2018). While the characterization of R genes against aphids is in progress, most mechanisms seem to follow the strain‐specific nature of typical R genes and provide, as far as currently known, resistance to only one aphid species or a certain population or biotype of aphids.
In this study, we investigated the pathogenic specificity of the R gene SIEVE ELEMENT‐LINING CHAPERONE1 (SLI1) in Arabidopsis. SLI1 falls into the category of unconventional R genes, being a small heat shock‐like (hsp20) protein with a mechanism that remains to be characterized. SLI1 was identified in a large population screen of natural Arabidopsis accessions, where genome‐wide association mapping revealed an association between M. persicae feeding behaviour and a mutation in a small heat shock‐like (hsp20‐like) gene, which was discovered to have a dual function in mild heat‐stress tolerance and aphid resistance (Kloth et al., 2017). At 26°C, when SLI1 expression was two‐fold higher than at 20°C, M. persicae populations were reduced to 50% and aphids suffered from shorter phloem‐feeding events and reduced sap‐ingestion rates on the Col‐0 accession compared to loss‐of‐function mutants. The gene was named SLI1 based on its localization in the sieve element margins where it co‐localized with endoplasmic reticulum‐like structures. Its homologue, RTM2, restricts the long‐distance transport of tobacco etch potyvirus (TEV) (Chisholm, Parra, Anderberg, & Carrington, 2001) and has been classified as an R gene for ‘loss of susceptibility’ with unknown mechanism (Kourelis & van der Hoorn, 2018). As the involvement of hsp20‐like proteins is uncommon in biotic stress resistance, we investigated whether SLI1 may deviate from the inherent strain specificity of classical R genes. Interestingly, we found that SLI1 is equally effective against a different subspecies, M. persicae nicotianae, and confers quantitative resistance to another aphid and a whitefly species. With new information on the co‐localization of SLI1 proteins with aphid stylets, we describe an R gene that is involved in broad‐spectrum resistance to several phloem‐feeding insects.
2. MATERIALS AND METHODS
2.1. Plants and insects
For Arabidopsis, Arabidopsis thaliana L. (Heynh.) Col‐0 accession (CS60000), homozygous T‐DNA lines sli1‐1 (SALK_027475) and sli1‐2 (SAIL_1269_C01), and a pSLI1:EYFP:SLI1 reporter line in the Col‐0 (CS60000) background were used from Kloth et al. (2017). Seeds were cold stratified for 72 hr at 4°C before they were sown into pots (6‐cm diameter) with Arabidopsis potting soil (Lentse Potgrond). Plants were grown in a climate room at 26 ± 1°C during the day and 23 ± 0.5°C during the night, 50 to 70% relative humidity (RH), and an 8:16 light (L):dark (D) photoperiod. Plants were treated with Entonem (Koppert Biological Systems) once a week to control sciarid flies. Green peach aphids, Myzus persicae (Sulzer), tobacco aphids, Myzus persicae nicotianae (Blackman) and mustard aphids, Lipaphis erysimi (Kaltenbach) were reared on radish, Raphanus sativus (L.). Cabbage aphids, Brevicoryne brassicae (L.), were reared on Brussels sprout plants, Brassica oleracea (L.). Aphid rearing was maintained at 21 ± 0.5°C, 50 to 70% RH and a 16:8 L:D photoperiod. Cabbage whiteflies, Aleyrodes proletella (L.), were reared on Brussels sprout plants at 22 to 24°C and silverleaf whiteflies, Bemisia tabaci (Gennadius) biotype B, were reared on cucumber plants, Cucumis sativus (L.), at 26 ± 0.5°C, 50 to 70% RH and 10‐hr:14‐hr L:D.
2.2. Population development of phloem feeders
Life history parameters of the above‐mentioned herbivores were monitored on the wild type, Col‐0 and sli1‐1 and sli1‐2 knockout mutants at 26 ± 1°C, when SLI1 transcription is relatively high in Col‐0 (Kloth et al., 2017) and at 50 to 70% relative humidity and 8:16 L:D photoperiod. The herbivores were subjected to a no‐choice situation, as described below. For aphids, 3.5‐week‐old plants were infested with one neonate between 1 and 24‐hr old. Individual plants were placed atop an inverted Petri dish lid within a tray and separated via a soap‐water barrier to prevent between‐plant movements of aphids. Developmental rate was determined from day 4 (L. erysimi) or day 6 (M. persicae nicotianae and B. brassicae) onwards, by recording the first aphid offspring three times per day using magnification glasses (3×). Aphids with wing development were removed and excluded from the experiment. The total number of M. persicae nicotianae and B. brassicae offspring was counted 14 days post infestation (dpi) on three subsequent days. Due to the rapid development and reproduction of L. erysimi (up to 300 aphids in 2 weeks), the reproduction of these aphids was assessed earlier, at 10 dpi, on two subsequent days. The three plant lines were equally represented on each counting day, unless plants had to be removed because of missing aphids, or aphids with wing development. For whiteflies, a mature leaf of each 4.5‐week‐old plant was infested with five female adults in a clip cage of 2.5 cm in diameter. After an infestation time (t) of 5 days (B. tabaci) or 7 days (A. proletella), the clip cage was removed and the number of eggs (e) and surviving adults (m) and dead adults (d) were all counted on 1 day. Oviposition rate (OR) was calculated as OR = e/(m + 0.5*d)/t, according to the methodology of Broekgaarden, Riviere, Steenhuis, and del sol Cuenca M., Kos M., and Vosman B. (2012). In all experiments, plant lines were positioned alternately in each tray to avoid any potential environmental bias within the growth chamber. Data analyses were performed in R (R‐Core‐Team, 2017). Data distributions were tested with a Shapiro–Wilk test and a Levene's test for homogeneity of variances using the R package ‘car’ version 3.0.10 (Fox & Weisberg, 2019). Normally distributed data were subjected to one‐way ANOVA tests. Data not complying to a normal distribution were analysed with non‐parametric Kruskal‐Wallis or Mann Whitney U tests, or, in the case of proportional data, with χ 2 tests. Violin plots were produced with the R package ‘vioplot’ version 0.3.5 (Adler & Kelly, 2020).
2.3. Electrical penetration graph recording
Feeding behaviour of M. persicae nicotianae, B. brassicae and L. erysimi was studied with an electrical penetration graph (EPG) recording on 4.5‐week‐old Arabidopsis plants (Col‐0, sli1‐1 and sli1‐2). For this method, a thin gold wire was attached to the dorsum of the insect, thereby making the plant and insect part of an electrical circuit (Tjallingii, 1988). Direct currents were used according to the methodology of ten Broeke, Dicke, and van Loon (2013). One day before the experiment, alate adult aphids were collected and transferred to a Col‐0 Arabidopsis plant to habituate to Arabidopsis. Just before the EPG recording, a thin gold wire of 18 μm diameter and 1.5 ± 0.5 cm length was gently attached to the dorsum of the aphid using water‐based silver glue. Brevicoryne brassicae aphids were first gently brushed with a soft brush to remove the waxy layer on the dorsum. Each wired aphid was transferred to a unique plant, and all three plant lines (i.e., Col‐0, sli1‐1 and sli1‐2) were represented in each recording. Electrical signals associated with stylet activities were recorded for 8 hr with Direct Current Giga‐8 systems (http://www.epgsystems.eu), annotated with EPG Stylet software (http://www.epgsystems.eu) and further processed in R (R‐Core‐Team, 2017). Waveforms that did not occur, were considered as missing data for the total duration, mean duration and latency variables. Events that were interrupted by the end of the recording were included in all calculations. For summary variables in Table 1, Mann–Whitney U and χ 2 tests were performed. For in‐depth analysis of individual probes in Figure 3, probes were selected with the following sequence of events: non probing > pathway > (repetitive potential drops >) phloem salivation > phloem feeding. Of these, the total duration of the pathway, including repetitive potential drops, was square‐root transformed, and total duration of phloem feeding was log transformed to obtain normally distributed data. Because some probes originated from the same aphid/plant individual, a linear mixed model was performed with aphid species as fixed effect and aphid/plant individual as a random effect to correct for any bias between individuals, using the R package ‘nlme’ version 3.1.131.1 (Pinheiro, Bates, DebRoy, Sarkar, & Team, 2018).
TABLE 1.
Parameters of aphid feeding behaviour (mean ± SE) during 8‐hr electrical penetration graph recordings
| Behaviour | Myzus persicae nicotianae | Brevicoryne brassicae | Lipaphis erysimi | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Col‐0 | sli1‐1 | sli1‐2 | Col‐0 | sli1‐1 | sli1‐2 | Col‐0 | sli1‐1 | sli1‐2 | ||||||||
| Pathway | NP | 157 ± 19 | 58 ± 13 | *** | 95 ± 22 | * | 97 ± 11 | 55 ± 10 | * | 103 ± 14 | ns | 62 ± 11 | 74 ± 15 | ns | 77 ± 17 | ns |
| # | 45 ± 4.3 | 14 ± 2.4 | *** | 20 ± 4.4 | ** | 43 ± 6.4 | 24 ± 3.3 | * | 35 ± 6.4 | ns | 35 ± 4.7 | 29 ± 4.7 | ns | 38 ± 7.4 | ns | |
| # short | 31 ± 4.5 | 7.6 ± 2 | *** | 15 ± 3.5 | ** | 25 ± 5.9 | 13 ± 2.2 | ns | 21 ± 4.9 | ns | 26 ± 4.4 | 21 ± 4.4 | ns | 29 ± 7.1 | ns | |
| Total | 182 ± 14 | 88 ± 16 | *** | 82 ± 11 | *** | 268 ± 16 | 166 ± 23 | ** | 177 ± 16 | *** | 133 ± 16 | 110 ± 14 | ns | 122 ± 12 | ns | |
| Total Rpd | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 90 ± 1114 | 67 ± 10 | ns | 78 ± 10 | ns | |||||
| Mean Rpd | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 21 ± 314 | 18 ± 3 | ns | 24 ± 2.8 | ns | |||||
| Salivation (E1) | Lat | 145 ± 24 | 94 ± 18 | ns | 154 ± 27 | ns | 258 ± 27 | 147 ± 25 | ** | 179 ± 3214 | ns | 171 ± 2414 | 168 ± 28 | ns | 152 ± 23 | ns |
| # | 9.1 ± 1.2 | 4.1 ± 0.7 | ** | 2.5 ± 0.4 | *** | 5.5 ± 0.8 | 3.5 ± 0.5 | ns | 2.8 ± 0.5 | * | 3.5 ± 0.6 | 3.1 ± 0.6 | ns | 2.9 ± 0.4 | ns | |
| Total | 9.8 ± 1.6 | 4.5 ± 0.9 | ** | 2.1 ± 0.4 | *** | 17 ± 1.8 | 9.5 ± 2.3 | ** | 7.2 ± 1.114 | *** | 4 ± 114 | 3 ± 0.4 | ns | 3.8 ± 0.7 | ns | |
| Mean | 1.1 ± 0.1 | 1.1 ± 0.2 | ns | 1 ± 0.2 | ns | 4.1 ± 0.9 | 2.5 ± 0.3 | ns | 3.2 ± 0.914 | ns | 1.2 ± 0.214 | 1 ± 0.1 | ns | 1.3 ± 0.1 | ns | |
| Max | 3.5 ± 0.7 | 2 ± 0.4 | ns | 1.3 ± 0.2 | ** | 6.6 ± 1 | 4.1 ± 0.6 | * | 3.7 ± 0.914 | ** | 1.6 ± 0.414 | 1.3 ± 0.1 | ns | 1.8 ± 0.3 | ns | |
| Prop (%) | 8.1 ± 1.6 | 1.4 ± 0.4 | *** | 1 ± 0.3 | *** | 16 ± 3.7 | 3.9 ± 1.2 | ns | 11 ± 5.114 | ns | 4.8 ± 2.614 | 1.5 ± 0.2 | ns | 3.9 ± 1.6 | ns | |
| Aphids sin | 40% | 13% | ns | 7% | ns | 93% | 73% | ns | 53% | * | 7% | 0% | ns | 13% | ns | |
| # sin | 0.7 ± 0.3 | 0.2 ± 0.1 | ns | 0.1 ± 0.1 | * | 2.1 ± 0.4 | 1.1 ± 0.2 | ns | 0.7 ± 0.2 | ** | 0.1 ± 0.1 | 0 ± 0 | ns | 0.1 ± 0.1 | ns | |
| Phloem (E2) | Aphids | 100% | 100% | 100% | 73% | 100% | ns | 93% | ns | 100% | 100% | 100% | ||||
| # | 6.3 ± 0.8 | 3.5 ± 0.6 | ** | 2.2 ± 0.4 | *** | 2.4 ± 0.5 | 1.9 ± 0.3 | ns | 1.7 ± 0.3 | ns | 3 ± 0.5 | 2.8 ± 0.5 | ns | 2.4 ± 0.3 | ns | |
| Total | 111 ± 18 | 322 ± 22 | *** | 300 ± 31 | *** | 40 ± 1311 | 212 ± 27 | *** | 164 ± 3014 | * | 175 ± 2014 | 210 ± 24 | ns | 182 ± 20 | ns | |
| Mean | 22 ± 4.9 | 131 ± 21 | *** | 186 ± 36 | *** | 12 ± 3.611 | 138 ± 25 | *** | 126 ± 3014 | ** | 85 ± 1914 | 98 ± 20 | ns | 94 ± 16 | ns | |
| Max | 61 ± 11 | 274 ± 26 | *** | 284 ± 32 | *** | 22 ± 8.411 | 200 ± 27 | *** | 144 ± 2914 | ** | 126 ± 1814 | 155 ± 21 | ns | 137 ± 19 | ns | |
| E2 sust | Aphids | 100% | 100% | 100% | 47% | 100% | ** | 80% | ns | 93% | 100% | ns | 93% | ns | ||
| # | 2.6 ± 0.3 | 1.9 ± 0.3 | ns | 1.1 ± 0.1 | *** | 0.6 ± 0.2 | 1.3 ± 0.1 | ** | 1 ± 0.2 | ns | 2 ± 0.3 | 2.1 ± 0.3 | ns | 1.9 ± 0.3 | ns | |
| Total | 96 ± 17 | 318 ± 23 | *** | 296 ± 31 | *** | 50 ± 157 | 210 ± 27 | ** | 190 ± 2812 | ** | 185 ± 1714 | 208 ± 24 | ns | 193 ± 1814 | ns | |
| F | Aphids | 40% | 13% | ns | 7% | ns | 53% | 47% | ns | 53% | ns | 53% | 47% | ns | 53% | ns |
| Total | 31 ± 9.96 | 60 ± 162 | ns | 12.91 | ns | 76 ± 178 | 82 ± 7.67 | ns | 59 ± 208 | ns | 42 ± 178 | 34 ± 8.77 | ns | 33 ± 7.18 | ns | |
| G | Aphids | 20% | 0% | ns | 7% | ns | 33% | 0% | ns | 40% | ns | 7% | 0% | ns | 0% | ns |
| Total | 36 ± 213 | ‐ | 6.71 | ns | 89 ± 285 | ‐ | 21 ± 116 | ns | 101 | ‐ | ‐ | |||||
Note: NP = non probing, Rpd = total duration repetitive potential drops (min), E1 = salivation in phloem, E2 = phloem ingestion, E2 sust = sustained phloem ingestion events >10 min, F = penetration difficulties in the cell wall, G = xylem ingestion, Lat = latency from the first probe of the recording to the first occurrence (min), short = pathway probes <3 min, Total = total duration (min), Mean = mean duration (min), Max = maximum duration (min), Prop = proportion of time spent on salivation relative to the total phloem phase, Aphids = percentage of aphids performing the behaviour, sin = single salivations that are not directly preceded or followed by phloem ingestion. Behaviours that were not performed by an aphid, were treated as missing values for the calculation of Lat, Total, Mean, Max, and Prop. Data show mean ± SE, n = 15 unless differently mentioned in superscript. Behaviours that were not performed on a plant line are annotated with ‘‐’, proportional variables (%) were tested with a χ 2 test (only when represented by both performing and non‐performing aphids), other variables were tested with Mann–Whitney U pairwise comparisons between Col‐0 and its respective mutant (*p < .05, **p < .01, ***p < .001).
FIGURE 3.

Repetitive potential drops and the effects on phloem feeding on Col‐0. (a) ‘Normal’ potential drop (pd) and (b) repetitive potential drop (R‐pd) of Lipaphis erysimi. (c) Electropenetrographs from Myzus persicae nicotianae (upper), Brevicoryne brassicae (middle), and L. erysimi aphids (lower) on Col‐0. After the start of probing (black triangle), aphids penetrated the cell wall matrix and occasionally showed punctures of cell membranes (pd's) followed by a approximately 5 s intracellular phase (pathway phase, red boxplots). Only L. erysimi showed repetitive potential drops (R‐pd's) with a long, approximately 20 s, intracellular phase (blue boxplot). When the phloem was reached, aphids secreted watery saliva (not shown in boxplot) and started phloem feeding (yellow boxplots). Lipaphis erysimi required two‐fold more time to reach the phloem phase than the other two aphid species (p = .0087) but fed longer from the phloem (p = .000, linear mixed model with aphid/plant individual as random effect, probes as biological replicates, including only those probing events with the following sequence of events: non‐probing > pathway > (repetitive potential drops >) phloem salivation > phloem feeding; M. persicae nicotianae n = 35 probes (14 aphids), B. brassicae n = 18 probes (10 aphids), L. erysimi n = 14 probes (10 aphids)). Boxplots represent median (thick line), interquartile range (box), 1.5‐fold interquartile range (whiskers) and extremes (open circles) [Colour figure can be viewed at wileyonlinelibrary.com]
2.4. Cryofixation and dissection of aphids
To investigate interactions between SLI1 proteins and the stylets of the aphids, cryofixation of phloem‐feeding aphids was performed according to the methodology of Walker and Medina‐Ortega (2012). In brief, aphids were attached to the EPG system as described above, and placed on the pSLI1:EYFP:SLI1 Arabidopsis reporter line. A mature leaf was secured with a plastic holder to enable positioning of the aphid on the abaxial side and rotation of the leaf without disrupting the plant. When the electropenetrographs indicated phloem feeding, 20 to 30 ml liquid nitrogen was poured on the adaxial side of the leaf and 1 to 2 teaspoons of finely crushed dry ice were placed on top of it. The petiole was cut and the holder with the leaf were transferred to a bed of dry ice. If the aphid had not already fallen off the leaf, half a teaspoon of liquid nitrogen was poured on it and a tweezer was used to break off the aphid body while preserving the stylets in planta. Leaf and holder were placed in a Petri dish in a Styrofoam box with dry ice and transferred to a −80°C freezer for storage. Before dissection, samples were thawed to −20°C overnight and gradually brought to room temperature in a Styrofoam box. Subsequently, the leaf was transferred and taped to a glass slide, adaxial side facing up. With a sharpened tungsten wire, the xylem was carefully removed by lifting, pulling and scraping until the sieve tubes and aphid stylets were exposed. To confirm stylet localization in a sieve tube, samples were stained with 0.1% aniline blue (VWR Chemicals) sodium phosphate solution (70 mM, pH 9) for 15 min, washed with sodium phosphate buffer (70 mM, pH 9) and directly transferred to the microscope for imaging. To assess ingestion of SLI1, adults and late instar M. persicae aphids were transferred to pSLI1:EYFP:SLI1 reporter plants and left to feed for 7 days. Subsequently, six adults were collected, dissected, mounted on a glass slide with distilled water and directly imaged on the confocal laser scanning microscope as described below.
2.5. Plant samples and dissection
Seedling roots of the pSLI1:EYFP:SLI1 reporter line were imaged in vivo. Seedlings were grown on agar plates with 1% (w/v) agar and 0.5× Murashige Skoog (MS) medium at 22°C and 8‐hr:16‐hr L:D photoperiod. Seedlings were incubated at room temperature in 1 μM Mitotracker Deep Red (Thermo Fisher Scientific) 0.5× MS solution for 24 hr. For imaging of above‐ground plant tissue in 5‐to‐6‐week‐old pSLI1:EYFP:SLI1 reporter lines, sieve tubes were exposed using two different dissection methods. For longitudinal sections in the leaf midvein, mature leaves were cut at the base of the petiole and taped to a glass slide with the abaxial side up. With a sharp razor blade, a longitudinal section was removed from the petiole, starting with a cut at the base and ending with an ultrathin slice near the start of the leaf blade. The exposed area was directly submerged in distilled water to prevent desiccation. For transverse sections of the petiole and sections of the inflorescence stem and apex, tissues were cast in agar (5%) and sliced in 80 to 120 μm sections using a vibratome (Leica VT 1000 S).
2.6. Microscopy
Confocal laser scanning microscopy was performed with a Zeiss LSM 780. EYFP in the pSLI1:EYFP:SLI1 reporter line was excited using a 514 nm laser and the emitted wavelengths were detected with a 517‐to‐598‐nm emission filter. A narrower emission filter of 517 to 540 nm was used for aphid‐infested leaf samples to avoid auto‐fluorescence of the stylets. To confirm that the stylet localized in a sieve tube, aniline blue‐stained sieve plates were visualized with a 405‐nm laser and a 420‐to‐500‐nm emission filter. Light microscopy images of aphid stylets in planta were taken with an AxioCam HRc digital camera (Zeiss) mounted on an Axioplan 2 light microscope (Zeiss).
3. RESULTS
3.1. SLI1 affects multiple phloem feeding species
As several R genes tend to confer strain‐specific resistance (Kaloshian, 2004), we tested SLI1 effectiveness on a panel of five phloem‐feeding insect species, consisting of three aphid and two whitefly species. To maximize the contrast between wild‐type and sli1 loss‐of‐function mutants, experiments were conducted at 26°C, a temperature with relatively high SLI1 expression (Kloth et al., 2017). Interestingly, two out of three aphid species and one out of two whitefly species showed a lower reproductive rate on Col‐0 than on sli1‐1 and sli1‐2 mutants (Figure 1a). The largest effects were observed for M. persicae nicotianae with an almost 50% lower reproductive rate and 10% extended developmental time from neonate to age of first reproduction on Col‐0 (Figure 1b). The relative difference in the number of offspring between Col‐0 and sli1 mutants was comparable for M. persicae nicotianae and the previously assessed main species M. persicae (p = .87, p = .77 for sli1‐1 and sli1‐2, respectively, Mann Whitney U tests) (Kloth et al., 2017), and shows that SLI1‐mediated resistance is not strain‐specific. SLI1 effectiveness was also found outside the M. persicae species complex. Smaller, but significant reductions in the number of offspring or eggs of at least 20% were observed on Col‐0 for the aphid B. brassicae and the whitefly A. proletella, respectively. The reproductive rate of many B. brassicae founder aphids on Col‐0 could not be recorded as they were lost more often due to dispersal or mortality (Figure 1c, Col: 41%, mutants: 5 to 14%). In contrast, SLI1 did not affect the reproductive rate of L. ersyimi aphids and B. tabaci whiteflies (L. erysimi p = .94; B. tabaci p = .09, one‐way ANOVA), although L. erysimi nymphs developed faster on sli1‐1 mutants than on Col‐0 (Figure 1b, sli1‐1: p = .01, sli1‐2: p = .07, Mann Whitney‐U test). Altogether, these bioassays show that SLI1 is involved in quantitative resistance to two aphid species and one whitefly species.
FIGURE 1.
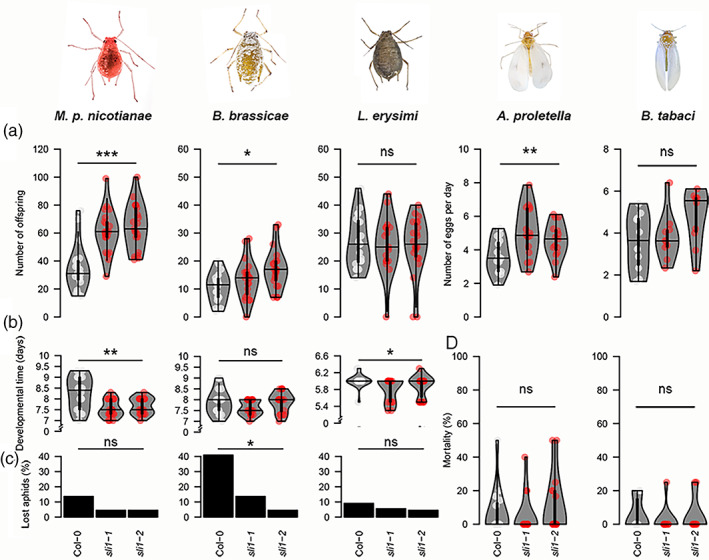
Aphid and whitefly performance on Col‐0 and sli1‐1 and sli1‐2 mutants. (a) Number of offspring per adult aphid of Myzus persicae nicotiana (Mpn), Brevicoryne brassicae (Bb) and Lipaphis erysimi (Le) and number of eggs per adult whitefly per day of Aleyrodes proletella (Ap) and Bemisia tabaci (Bt) (days post‐infestation: 14 days (M. persicae nicotianae and B. brassicae), 10 days (L .erysimi), 7 days (A. proletella), and 5 days (B. tabaci)). (b) Aphid development time from neonate to first reproduction. (c). Founder aphids lost to dispersal or mortality. (d) Mortality of whiteflies (* p < .05, ** p < .01, *** p < .001, one‐way ANOVA tests (reproductive rates), Kruskal Wallis tests (developmental time and mortality) and χ 2 tests (lost aphids), violin plots show interquartile range with kernel density distribution and median value (horizontal line), dots represent observations, in order of plant material (Col‐0, sli1‐1, sli1‐2): Mpn n = 20, 21, 21; Bb n = 18, 21, 21; Le n = 20, 17, 22; Ap n = 15, 15, 15; and Bt n = 10, 10, 10, insect photo's courtesy of Hans Smid)
3.2. Longer salivation and shorter phloem feeding
To understand the differences in SLI1‐mediated effects among species, the feeding behaviour of the two aphids, M. persicae nicotianae and B. brassicae, which were affected in reproductive rate, and the aphid L. erysimi that was unaffected by SLI1, was monitored. This was achieved via 8‐hr‐recordings with an electrical penetration graph (EPG) system, which delivered information on more than 50 behavioural variables. In general, aphids showed different degrees of adaptation to Col‐0 plants. Lipaphis ersyimi engaged in 4‐to‐7‐fold longer phloem feeding events on Col‐0 than M. persicae nicotinae and B. brassicae (p = .000, Kruskal Wallis test on mean duration of phloem ingestion, Table 1), and did not show any differences in feeding behaviour between Col‐0 and the two sli1 mutants, indicating that this aphid species is very well adapted to Arabidopsis as a host. Of the other two aphid species, B. brassicae individuals encountered most problems in both the pre‐phloem and phloem phase on Col‐0. They required more time from the first stylet‐plant contact until the start of their first phloem phase (p = .013, Mann Whitney U test on latency to first salivation, Table 1) and salivated longer in the phloem than M. persicae nicotianae (p = .000, Mann–Whitney U test on mean duration salivation, Table 1). On the sli1 mutants, both M. persicae nicotianae and B. brassicae performed better than on the Col‐0 wild type. There was less salivation in the phloem (Table 1) and a 3‐to‐5‐fold increase in phloem feeding duration compared to Col‐0 (Figure 2). As the establishment of phloem feeding was easier, aphids spent less time on the pathway phase in sli1 mutants (Table 1, total time pathway).
FIGURE 2.
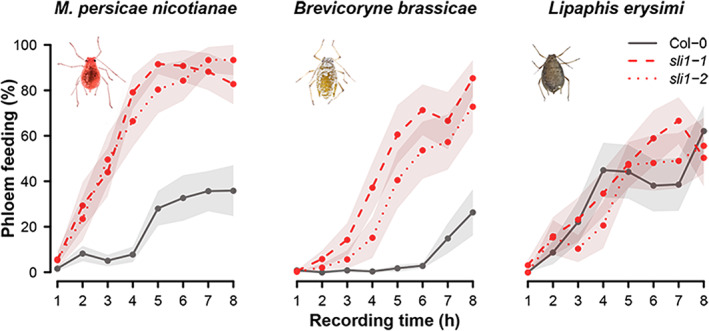
Phloem feeding by Myzus persicae nicotianae, Brevicoryne brassicae and Lipaphis erysimi on sli1‐1 and sli1‐2 knockout mutants and the Col‐0 wild type as percentage of total time (mean values with SE in shaded area, n = 15) [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Repetitive punctures precede successful phloem feeding
Unlike the other two aphid species, L. erysimi displayed so‐called ‘repetitive potential drops’ (R‐pd's) before each phloem feeding event. R‐pd's is the repeated puncturing of a cell membrane. For pea aphids, Acyrthosiphon pisum, and most likely for other aphid species as well, R‐pd's are repeated punctures of a sieve element or companion cell membrane, followed by a long intracellular phase that probably involves salivation and cell content ingestion (Tjallingii & Gabryś, 1999; Tjallingii, Garzo, & Fereres, 2010; Walker & Medina‐Ortega, 2012). Membrane punctures are not an unfamiliar phenomenon, as aphids puncture around 70% of the cells they encounter along their pathway to the phloem (Tjallingii & Hogen Esch, 1993). These punctures usually cause relatively short potential drops of 3 to 12 s (Powell, Pirone, & Hardie, 1995) with a low and inconsistent occurrence, while R‐pd's have a longer intracellular phase and a regular occurrence. In our study, only L. ersyimi aphids displayed R‐pd's. Each feeding event of L. ersyimi was preceded by a 15 to 40 min period of R‐pd's, in which the aphids alternated 20‐s lasting intracellular activities with 20‐s lasting extracellular phases (Figure 3). The intracellular phases of each R‐pd pattern increased in duration, until phloem feeding was initiated. Due to these R‐pd's, L. erysimi required almost double the time from the start of a probe until the onset of phloem feeding (39.5 ± 2.8 min) compared to M. persicae nicotianae (21.2 ± 2.1 min) and B. brassicae (22.1 ± 2.2 min) (Figure 3). Once phloem feeding was initiated, however, L. ersyimi aphids continued phloem sap ingestion 4‐to‐7‐fold longer than the other two aphid species and were no longer affected by SLI1 (Table 1, Figure 3, Table S1).
3.4. SLI1 localization in leaves and stems
Previous work showed that SLI1 proteins localize in sieve elements (Kloth et al., 2017). This evidence was, however, based on root images of young seedlings grown in agar and not in tissues exposed to aphids (Kloth et al., 2017). To determine the above‐ground presence of SLI1 proteins in mature, soil‐grown Arabidopsis, we used several fixation and dissection techniques to visualize fluorescent fusion proteins of SLI1 and aphid stylets. These images showed that SLI1 was present in leaves, petioles, inflorescence stems, and the apex of uninfested plants. Consistent with previous observations in seedling roots, SLI1 proteins localized to the parietal layer of sieve elements (Figure 4). There were no observations of SLI1 in companion cells or other cells adjacent to sieve tubes or elsewhere in the plant. Images of the seedling root tip showed strong EYFP signals in the region associated with phloem differentiation (Seo, Kim, & Lee, 2020) (Figure 4d), indicating that SLI1 expression and translation occurs in young sieve elements. As an ultimate challenge, co‐localization of SLI1 and the aphid stylets was assessed. For this, M. persicae aphids were connected to the EPG system, mounted on SLI1 fluorescent reporter lines, and cryofixed within 1 min after they had started phloem feeding. After careful dissection, aphid stylets in planta were retrieved (Figure 4e, f) and co‐localization of SLI1 proteins with the aphid stylet tip was observed (Figure 4g), illustrating that the aphid stylet encounters this phloem protein directly after plasma membrane puncture. From confocal images of aphid guts (Figure S1), we could not confirm ingestion of SLI1 proteins, although quenching of EYFP signal in the digestive tract may have occurred. Localization of SLI1 at or around the entrance of the food and salivary canal was, however, apparent and putatively plays a role in the impaired feeding behaviour of M. persicae, M. persicae nicotianae and B. brassicae.
FIGURE 4.
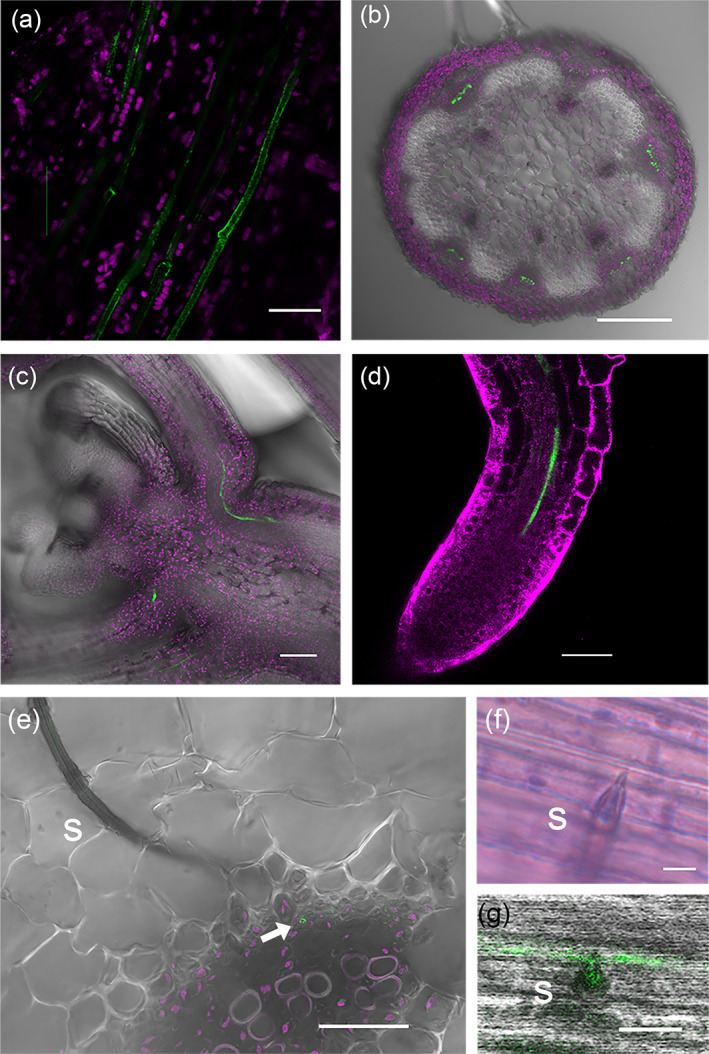
Localization of SLI1 proteins and Myzus persicae aphid stylets in Arabidopsis. YFP fusion proteins of SLI1 (in green) expressed under the native promoter in: (a) the petiole of a mature leaf, (b) the inflorescence stem, (c) the growth apex, and (d) the root tip. Cryofixation of aphid stylets in a mature leaf with: (e) upper part of stylets, (f) the stylet tip with maxillary stylets protruding into a sieve tube, and (g) co‐localization of the stylet tip with SLI1 proteins (green = pSLI1:YFP:SLI1, magenta = chlorophyll (a–c, e), Mitotracker Deep Red (d), s = stylets, arrow = sieve tube, images (a–e) and (g) confocal laser scanning microscopy, image (f) light microscopy, scale bars (a) 20 μm, (b) 250 μm, (c, d) 50 μm, (e) 25 μm, (f‐g) 5 μm) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
4.1. Conserved resistance to phloem‐feeding insects
In this study, we found that SLI1‐mediated resistance in Arabidopsis is not restricted to M. persicae aphids (Kloth et al., 2017), but is equally effective against the subspecies M. persicae nicotianae and has significant effects on B. brassicae aphids and A. proletella whiteflies. In the context of other characterized R genes, broad‐spectrum resistance is an uncommon phenomenon. Exceptions to this are the Vat gene that confers resistance to both an aphid species and several viruses, and the Mi1.2 gene that confers resistance to M. euphorbiae aphid and the B. tabaci whitefly (Dogimont et al., 2014; Nombela, Williamson, & Muniz, 2003; Rossi et al., 1998). SLI1 can now be added to these broad‐spectrum R genes as it confers resistance to more than one aphid species and a whitefly species. Broad‐spectrum resistance is generally an indication of a conserved mechanism, equivalent to general defences induced by conserved PAMPs, such as flagellin or chitin (Deng et al., 2020). While aphids are masters in manipulating their host (Åhman, Kim, & Zhu, 2019; Züst & Agrawal, 2016), it is somewhat surprising to find conserved plant resistance. Presumably low herbivore pressure on short‐lived Arabidopsis plants in combination with the quantitative nature of SLI1‐mediated resistance, may explain why it has escaped the arms race with some aphid effectors. In addition, SLI1 effects are more pronounced at the relatively high temperature of 26°C, which may not be representative for the temperate regions where Arabidopsis naturally occurs (Kloth et al., 2017). A conserved resistance mechanism is most likely induced by a conserved molecule, such as damage‐associated molecular patterns (DAMPs), chitin, or common salivary proteins. Shared salivary proteins among aphid species can originate from the aphids themselves or from their primary endosymbiont Buchnera aphidicola, which occurs in most Aphididae and has been reported for M. persicae, B. brassicae and the Lipaphis genus as well (Clark, Daniell, Wishart, Hubbard, & Karley, 2012; Nováková et al., 2013). The Buchnera protein GroEL is, for example, known to induce plant defences against several aphid species (Chaudhary, Atamian, Shen, Briggs, & Kaloshian, 2014).
4.2. Phloem‐localization and involvement in (a)biotic stresses
Our observations show that SLI1 proteins are confined to sieve tubes (Figure 4) and that they provide a phloem‐located protection against invaders of this specific tissue. It is, therefore, unlikely that SLI1 will confer resistance to chewing or other piercing‐sucking insects that do not primarily feed on the phloem. Further investigation would be relevant of putative effects on other phloem‐dependent organisms, such as cyst nematodes that tap into the phloem with syncytia (Absmanner, Stadler, & Hammes, 2013) or microorganisms that use the phloem for systemic spread. Viruses seem particularly interesting in view of RTM2, a member of the same hsp20‐like subclade as SLI1 (Bondino, Valle, & ten Have, 2012), which restricts long‐distance transport of several potyviruses (Chisholm et al., 2001). Functional overlap between SLI1 and RTM2 is possible, as both hsp20‐like proteins reside in the parietal layer of sieve tubes and protect the phloem sap from attackers (Kloth & Kormelink, 2020). The characterization of RTM2 and SLI1 sheds new light on a rather elusive group of hsp20‐like proteins and raises the question of where transcription and translation of these phloem proteins take place. Although companion cells are considered to be the main protein suppliers for the anucleate and ribosome‐devoid sieve tubes (White, 2013), we only observed SLI1 proteins in sieve tubes (Figure 4). The strong EYFP signals in the phloem differentiation zone of the root apical meristem (Figure 4d), therefore, suggest that translation occurs in immature sieve elements. How SLI1 protein turnover would take place in mature sieve elements, remains to be solved. Apart from its role in herbivory by phloem‐feeding insects, SLI1 is also involved in abiotic stress, as was illustrated by heat‐induced SLI1 transcription and reduced seed set under moderate heat stress in sli1 mutants (Kloth et al., 2017). This, together with the constitutive SLI1 protein levels in sieve tubes of above‐ and below‐ground tissues, indicate that SLI1 has a basic role in sieve tube maintenance and protection throughout the plant under several (a)biotic stresses.
4.3. Underlying mechanisms of SLI1‐mediated resistance
In view of the absence of classical R gene motifs, such as LRRs or NBS, and the lack of a transcriptional machinery in mature sieve tubes (van Bel, 2003), it is unlikely that SLI1 is required for a transcriptional defence response. The alpha‐crystallin domain rather suggests a chaperonin function in, for example, stabilizing or degrading other (resistance) proteins (Bakthisaran, Tangirala, & Rao, 2015; Van Ooijen et al., 2010). EPG recording of M. persicae nicotianae and B. brassicae confirmed that SLI1 impedes the uptake of phloem sap already 2 hr after the start of the recording when aphids initiated sustained phloem‐feeding on sli1 mutants (Figure 2). The co‐localization of SLI1 and the aphid stylet tip, directly after puncturing a sieve element membrane (Figure 4g), illustrates the interaction of SLI1 at the entrance of the food and salivary canal. These observations depict a constitutive resistance mechanism, where there is direct contact between the host protein and feeding apparatus of the aphid. One of the hypotheses about the underlying mechanism is a role in Ca2+‐triggered signal cascades. Injury or temperature shocks of a sieve element are known to result in a Ca2+ influx that can lead to phloem protein dispersion and the formation of protein coagulations (Knoblauch & Van Bel, 1998; Thorpe et al., 2010; Will, Furch, & Zimmermann, 2013). In the melon genotype, Cucumis melo TGR‐1551, Ca2+ triggered protein occlusions in both the food canal of cotton aphids, A. gossypii, and the attacked sieve tubes (Garzo et al., 2018; Peng & Walker, 2020). These occlusions were associated with increased salivation, shorter phloem‐feeding events, and reduced aphid reproduction, which are comparable to the SLI1 effects observed here. Although it is unknown whether Arabidopsis harbours a similar phloem protein machinery as melons, SLI1 could be involved in a phloem‐protein occlusion mechanism via the import and release of Ca2+ and phloem proteins into the sieve tube, or directly, via protein polymer formation. Alternative mechanisms include the sequestering and degradation of aphid effectors, callose deposition around the aphid stylet or the sieve plate, and the release of toxic metabolites. Further proteomic, metabolomic and antibody studies would be required to test these hypotheses.
4.4. Repeated potential drops and their role in overcoming resistance
Previous work showed that M. persicae could overcome SLI1‐mediated resistance by increased salivation (Kloth et al., 2017). Interestingly, we observed here that L. ersyimi aphids could successfully feed after performing R‐pd's, which is a repetitive cell puncturing behaviour (Figure 3). Tjallingii and Gabryś (1999) suggested that these punctures include salivations in the same companion cell or sieve element, which was confirmed by microscopic evidence of Walker and Medina‐Ortega (2012). To prepare a sieve tube for feeding by repetitive secretion of effectors, might be the ultimate solution for an aphid to tackle SLI1‐mediated resistance. After all, L. ersyimi aphids do not encounter negative effects of SLI1 during phloem feeding. Why certain aphid species show this behaviour and others not, is unknown, although it can be assumed that performing R‐pd's requires investment of time and protein‐rich saliva that may not always tip the cost–benefit balance. Host specificity plays a role as well, since R‐pd's were observed for B. brassicae on Sinapis alba (Tjallingii & Gabryś, 1999), but not in our study on Arabidopsis wild‐type and mutant lines. The latter suggests that unknown Arabidopsis host factors prevent the initiation of R‐pd's by B. brassicae. Examination of saliva composition and diversity in effectors between L. erysimi and the other aphid species will be relevant to further understand the specificity of SLI1‐mediated resistance.
Overall, SLI1 presents a remarkable example of an R gene involved in broad‐spectrum quantitative resistance to several phloem‐feeding insects. Whether it is triggered by conserved salivary components, chitin or Ca2+, and what the nature of the underlying mechanism is, remains to be elucidated. The identification of one aphid and one whitefly species that can overcome SLI1‐mediated resistance will be instrumental in follow‐up studies on effector biology. Nonetheless, the impact on three economically important pest species, M. persicae, B. brassicae and A. proletella, is an incentive to explore SLI1's potential as a resistance marker in plant breeding programmes.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Figure S1. Guts from Myzus persicae aphids that had been living on pSLI1:EYFP:SLI1 reporter plants. No EYFP signals were observed in any of the guts, including (a1‐2) an aphid head with attached guts, (b1‐2) foregut and midgut, (c1‐2) midgut and hindgut, and (d1‐2) hindgut (1: transmission, 2:517‐to‐540‐nm emission filter, excitation with a 514 nm laser, scale bars (a) 200 μm, (b–d) 50 μm).
Table S1. Linear mixed model on the duration of the pathway phase and subsequent phloem feeding by Myzus persicae nicotinae, Brevicoryne brassicae and Lipaphis erysimi aphids.
ACKNOWLEDGMENTS
We thank Pieter Rouweler, André Gidding, Frans van Aggelen, Quint Rusman and Alessia Vitiello for rearing the aphid species, Hans Smid for insect pictures (https://bugsinspace.nl/), and Gregory Walker for advice on cryofixation of aphid stylets. This work was supported by the Talent Programme Veni, project no. 16766, financed by the Dutch Research Council (NWO), and by Carl Tryggers Stiftelse för Vetenskaplig Forskning (CTS15:14).
Kloth KJ, Shah P, Broekgaarden C, Ström C, Albrectsen BR, Dicke M. SLI1 confers broad‐spectrum resistance to phloem‐feeding insects. Plant Cell Environ. 2021;44:2765–2776. 10.1111/pce.14064
Funding information Carl Tryggers Stiftelse för Vetenskaplig Forskning, Grant/Award Number: CTS15:14; Dutch Research Council (NWO), Grant/Award Number: 16766
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Absmanner, B., Stadler, R., & Hammes, U. Z. (2013). Phloem development in nematode‐induced feeding sites: The implications of auxin and cytokinin. Frontiers in Plant Science, 4, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler D. & Kelly S.T. (2020) Vioplot: Violin plot.
- Åhman, I., Kim, S.‐Y., & Zhu, L.‐H. (2019). Plant genes benefitting aphids—Potential for exploitation in resistance breeding. Frontiers in Plant Science, 10, 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylwin, N., & Ramnik, X. J. (2011). Leucine‐rich repeat (LRR) proteins: Integrators of pattern recognition and signaling in immunity. Autophagy, 7, 1082–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthisaran, R., Tangirala, R., & Rao, C. M. (2015). Small heat shock proteins: Role in cellular functions and pathology. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1854, 291–319. [DOI] [PubMed] [Google Scholar]
- Bondino, H. G., Valle, E. M., & ten Have, A. (2012). Evolution and functional diversification of the small heat shock protein/α‐crystallin family in higher plants. Planta, 235, 1299–1313. [DOI] [PubMed] [Google Scholar]
- Bos, J. I. B., Prince, D., Pitino, M., Maffei, M. E., Win, J., & Hogenhout, S. A. (2010). A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (Green Peach Aphid). PLoS Genetics, 6, e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekgaarden, C., Riviere, P., Steenhuis, G., & del sol Cuenca M., Kos M. & Vosman B. (2012). Phloem‐specific resistance in Brassica oleracea against the whitefly Aleyrodes proletella . Entomologia Experimentalis et Applicata, 142, 153–164. [Google Scholar]
- Calvo‐Agudo, M., González‐Cabrera, J., Picó, Y., Calatayud‐Vernich, P., Urbaneja, A., Dicke, M., & Tena, A. (2019). Neonicotinoids in excretion product of phloem‐feeding insects kill beneficial insects. Proceedings of the National Academy of Sciences of the United States of America, 116, 16817–16822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, R., Atamian, H. S., Shen, Z., Briggs, S. P., & Kaloshian, I. (2014). GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proceedings of the National Academy of Sciences of the United States of America, 111, 8919–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S. T., Parra, M. A., Anderberg, R. J., & Carrington, J. C. (2001). Arabidopsis RTM1 and RTM2 genes function in phloem to restrict long‐distance movement of Tobacco Etch Virus. Plant Physiology, 127, 1667–1675. [PMC free article] [PubMed] [Google Scholar]
- Clark, E. L., Daniell, T. J., Wishart, J., Hubbard, S. F., & Karley, A. J. (2012). How conserved are the bacterial communities associated with aphids? A detailed assessment of the Brevicoryne brassicae (Hemiptera: Aphididae) using 16S rDNA. Environmental Entomology, 41, 1386–1397. [DOI] [PubMed] [Google Scholar]
- Dangl, J. L., & Jones, J. D. G. (2001). Plant pathogens and integrated defence responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Deng, Y., Ning, Y., Yang, D.‐L., Zhai, K., Wang, G.‐L., & He, Z. (2020). Molecular basis of disease resistance and perspectives on breeding strategies for resistance improvement in crops. Molecular Plant, 13, 1402–1419. [DOI] [PubMed] [Google Scholar]
- Dogimont, C., Chovelon, V., Pauquet, J., Boualem, A., & Bendahmane, A. (2014). The Vat locus encodes for a CC‐NBS‐LRR protein that confers resistance to Aphis gossypii infestation and A. gossypii‐mediated virus resistance. The Plant Journal, 80, 993–1004. [DOI] [PubMed] [Google Scholar]
- Flor, H. H. (1971). Current status of the gene‐for‐gene concept. Annual Review of Phytopathology, 9, 275–296. [Google Scholar]
- Forbes, A. (1977). Mouthparts and feeding mechanism of aphids. In Harris K. F. & Maramorosch K. (Eds.), Aphids as virus vectors (pp. 83–103). London, England: Academic Press. [Google Scholar]
- Fox J. & Weisberg S. (2019) An R companion to applied regression.
- Garzo, E., Fernández‐Pascual, M., Morcillo, C., Fereres, A., Gómez‐Guillamón, M. L., & Tjallingii, W. F. (2018). Ultrastructure of compatible and incompatible interactions in phloem sieve elements during the stylet penetration by cotton aphids in melon. Insect Science, 25, 631–642. [DOI] [PubMed] [Google Scholar]
- Guo, H., Zhang, Y., Tong, J., Ge, P., Wang, Q., Zhao, Z., … Sun, Y. (2020). An aphid‐secreted salivary protease activates plant defense in phloem. Current Biology, 30, 1–11. [DOI] [PubMed] [Google Scholar]
- Hammond‐Kosack, K. E., & Jones, J. D. G. (1997). Plant disease resistance genes. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 575–607. [DOI] [PubMed] [Google Scholar]
- James, C. K. N., & Perry, K. L. (2004). Transmission of plant viruses by aphid vectors. Molecular Plant Pathology, 5, 505–511. [DOI] [PubMed] [Google Scholar]
- Jones, J. D. G., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kaloshian, I. (2004). Gene‐for‐gene disease resistance: Bridging insect pest and pathogen defense. Journal of Chemical Ecology, 30, 2419–2434. [DOI] [PubMed] [Google Scholar]
- Keith, R., & Mitchell‐Olds, T. (2013). Genetic variation for resistance to herbivores and plant pathogens: Hypotheses, mechanisms and evolutionary implications. Plant Pathology, 62, 122–132. [Google Scholar]
- Kloth, K. J., Busscher‐Lange, J., Wiegers, G. L., Kruijer, W., Buijs, G., Meyer, R. C., … Jongsma, M. A. (2017). SIEVE ELEMENT‐LINING CHAPERONE1 restricts aphid feeding on Arabidopsis during heat stress. The Plant Cell, 29, 2450–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth, K. J., & Kormelink, R. (2020). Defenses against virus and vector: A phloem‐biological perspective on RTM‐ and SLI1‐mediated resistance to potyviruses and aphids. Viruses, 12(2), 129. 10.3390/v12020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch, M., & Van Bel, A. J. E. (1998). Sieve tubes in action. The Plant Cell, 10, 35–50. [Google Scholar]
- Kourelis, J., & van der Hoorn, R. A. L. (2018). Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. The Plant Cell, 30, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, J., & Shah, J. (2015). Plant defence against aphids: The PAD4 signalling nexus. Journal of Experimental Botany, 66, 449–454. [DOI] [PubMed] [Google Scholar]
- Martin, G. B., Bogdanove, A. J., & Sessa, G. (2003). Understanding the functions of plant disease resistance proteins. Annual Review of Plant Biology, 54, 23–61. [DOI] [PubMed] [Google Scholar]
- Minks, A. K., & Harrewijn, P. (1989). World crop pests. Aphids. Their biology, natural enemies and control. Amsterdam, the Netherlands: Elsevier Science Publishers. [Google Scholar]
- Misof, B., Liu, S., Meusemann, K., Peters, R. S., Donath, A., Mayer, C., … Zhou, X. (2014). Phylogenomics resolves the timing and pattern of insect evolution. Science, 346, 763–767. [DOI] [PubMed] [Google Scholar]
- Mondal, H. A., Louis, J., Archer, L., Patel, M., Nalam, V. J., Sarowar, S., … Shah, J. (2018). Arabidopsis Actin‐depolymerizing factor3 is required for controlling aphid feeding from the phloem. Plant Physiology, 176, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, A., Garzo, E., Fernandez‐Mata, G., Kassem, M., Aranda, M. A., & Fereres, A. (2011). Aphids secrete watery saliva into plant tissues from the onset of stylet penetration. Entomologia Experimentalis et Applicata, 139, 145–153. [Google Scholar]
- Mutti, N. S., Louis, J., Pappan, L. K., Pappan, K., Begum, K., Chen, M.‐S., … Reeck, G. R. (2008). A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proceedings of the National Academy of Sciences of the United States of America, 105, 9965–9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela, G., Williamson, V. M., & Muniz, M. (2003). The root‐knot nematode resistance gene Mi‐1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci . Molecular Plant‐Microbe Interactions, 16, 645–649. [DOI] [PubMed] [Google Scholar]
- Nováková, E., Hypša, V., Klein, J., Foottit, R. G., von Dohlen, C. D., & Moran, N. A. (2013). Reconstructing the phylogeny of aphids (Hemiptera: Aphididae) using DNA of the obligate symbiont Buchnera aphidicola . Molecular Phylogenetics and Evolution, 68, 42–54. [DOI] [PubMed] [Google Scholar]
- Peng, H.‐C., & Walker, G. P. (2020). Sieve element occlusion provides resistance against Aphis gossypii in TGR‐1551 melons. Insect Science, 27, 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D. & Team R.C. (2018) Linear and nonlinear mixed effects models.
- Powell, G., Pirone, T., & Hardie, J. (1995). Aphid stylet activities during potyvirus acquisition from plants and an in vitro system that correlate with subsequent transmission. European Journal of Plant Pathology, 101, 411–420. [Google Scholar]
- R‐Core‐Team . (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reinink, K., & Dieleman, F. L. (1989). Comparison of sources of resistance to leaf aphids in lettuce (Lactuca sativa L.). Euphytica, 40, 21–29. [Google Scholar]
- Rossi, M., Goggin, F. L., Milligan, S. B., Kaloshian, I., Ullman, D. E., & Williamson, V. M. (1998). The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proceedings of the National Academy of Sciences of the United States of America, 95, 9750–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, M., Kim, H., & Lee, J.‐Y. (2020). Information on the move: Vascular tissue development in space and time during postembryonic root growth. Current Opinion in Plant Biology, 57, 110–117. [DOI] [PubMed] [Google Scholar]
- Sun, M., Voorrips, R. E., van't Westende, W., van Kaauwen, M., Visser, R. G. F., & Vosman, B. (2019). Aphid resistance in capsicum maps to a locus containing LRR‐RLK gene analogues. Theoretical and Applied Genetics, 133, 227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Broeke, C. J. M., Dicke, M., & van Loon, J. J. A. (2013). Performance and feeding behaviour of two biotypes of the black currant‐lettuce aphid, Nasonovia ribisnigri, on resistant and susceptible Lactuca sativa near‐isogenic lines. Bulletin of Entomological Research, 103, 511–521. [DOI] [PubMed] [Google Scholar]
- Thorpe, M. R., Furch, A. C. U., Minchin, P. E. H., Foller, J., Van Bel, A. J. E., & Hafke, J. B. (2010). Rapid cooling triggers forisome dispersion just before phloem transport stops. Plant, Cell and Environment, 33, 259–271. [DOI] [PubMed] [Google Scholar]
- Tjallingii, W. F., & Gabryś, B. (1999). Anomalous stylet punctures of phloem sieve elements by aphids. Entomologia Experimentalis et Applicata, 91, 97–103. [Google Scholar]
- Tjallingii, W. F., Garzo, E., & Fereres, A. (2010). New structure in cell puncture activities by aphid stylets: A dual‐mode EPG study. Entomologia Experimentalis et Applicata, 135, 193–207. [Google Scholar]
- Tjallingii, W. F., & Hogen Esch, T. C. N. (1993). Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiological Entomology, 18, 317–328. [Google Scholar]
- Tjallingii, W. F. C. N. (1988). Electrical recording of stylet penetration activities. In Minks A. K. & Harrewijn P. (Eds.), Aphids, their biology, natural enemies and control (pp. 95–108). Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- van Bel, A. J. E. (2003). The phloem, a miracle of ingenuity. Plant, Cell and Environment, 26, 125–149. [Google Scholar]
- Van Ooijen, G., Lukasik, E., Van Den Burg, H. A., Vossen, J. H., Cornelissen, B. J. C., & Takken, F. L. W. (2010). The small heat shock protein 20 RSI2 interacts with and is required for stability and function of tomato resistance protein I‐2. The Plant Journal, 63, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, G. P., & Medina‐Ortega, K. J. (2012). Penetration of faba bean sieve elements by pea aphid does not trigger forisome dispersal. Entomologia Experimentalis et Applicata, 144, 326–335. [Google Scholar]
- White, R. G. (2013). Cell biology of sieve element–companion cell complexes. In Thompson G. A. & Van Bel A. J. E. (Eds.), Phloem: Molecular cell biology, systemic communication, biotic interactions (pp. 8–29). Oxford, England: Wiley‐Blackwell. [Google Scholar]
- Will, T., Furch, A. C. U., & Zimmermann, M. R. (2013). How phloem‐feeding insects face the challenge of phloem‐located defenses. Frontiers in Plant Science, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will, T., & van Bel, A. J. E. (2006). Physical and chemical interactions between aphids and plants. Journal of Experimental Botany, 57, 729–737. [DOI] [PubMed] [Google Scholar]
- Will, T., & Vilcinskas, A. (2015). The structural sheath protein of aphids is required for phloem feeding. Insect Biochemistry and Molecular Biology, 57, 34–40. [DOI] [PubMed] [Google Scholar]
- Zitter, T. A. (1977). Epidemiology of aphid‐borne viruses. In Harris K. F. & Maramorosch K. (Eds.), Aphids as virus vectors (pp. 385–404). London, England: Academic Press. [Google Scholar]
- Züst, T., & Agrawal, A. A. (2016). Mechanisms and evolution of plant resistance to aphids. Nature Plants, 2, 15206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Guts from Myzus persicae aphids that had been living on pSLI1:EYFP:SLI1 reporter plants. No EYFP signals were observed in any of the guts, including (a1‐2) an aphid head with attached guts, (b1‐2) foregut and midgut, (c1‐2) midgut and hindgut, and (d1‐2) hindgut (1: transmission, 2:517‐to‐540‐nm emission filter, excitation with a 514 nm laser, scale bars (a) 200 μm, (b–d) 50 μm).
Table S1. Linear mixed model on the duration of the pathway phase and subsequent phloem feeding by Myzus persicae nicotinae, Brevicoryne brassicae and Lipaphis erysimi aphids.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


