Abstract
Volatile organic compounds (VOCs) are produced by soil‐borne microorganisms and play crucial roles in fungal interactions with plants and phytopathogens. Although VOCs have been characterized in Trichoderma spp., the mechanisms against phytopathogens strongly differ according to the strain and pathosystem. This study aimed at characterizing VOCs produced by three Trichoderma strains used as biofungicides and to investigate their effects against grapevine downy mildew (caused by Plasmopara viticola). A VOC‐mediated reduction of downy mildew severity was found in leaf disks treated with Trichoderma asperellum T34 (T34), T. harzianum T39 (T39), and T. atroviride SC1 (SC1) and 31 compounds were detected by head space‐solid phase microextraction gas chromatography–mass spectrometry. Among the Trichoderma VOCs annotated, α‐farnesene, cadinene, 1,3‐octadiene, 2‐pentylfuran, and 6‐pentyl‐2H‐pyran‐2‐one reduced downy mildew severity on grapevine leaf disks. In particular, 6‐pentyl‐2H‐pyran‐2‐one and 2‐pentylfuran increased the accumulation of callose and enhanced the modulation of defense‐related genes after P. viticola inoculation, indicating an induction of grapevine defense mechanisms. Moreover, 6‐pentyl‐2H‐pyran‐2‐one activated the hypersensitive response after P. viticola inoculation, possibly to reinforce the grapevine defense reaction. These results indicate that Trichoderma VOCs can induce grapevine resistance, and these molecules could be further applied to control grapevine downy mildew.
1. INTRODUCTION
Volatile organic compounds (VOCs) are small molecules with low molecular mass (100–500 Da), high vapor pressure, low boiling point, and a lipophilic character that readily evaporate and diffuse through heterogeneous mixtures of solids, liquids, and gasses, such as the gas‐ and water‐filled pores of the soil (Effmert et al., 2012; Schmidt et al., 2015). VOCs are produced by a large variety of organisms and play crucial roles in the communication among individuals of the same species and life forms of different kingdoms (Werner et al., 2016). In particular, soil‐borne microorganisms are prolific producers of VOCs, which play important roles in plant growth and defense against biotic or abiotic stress (Garbeva & Weisskopf, 2020; Li et al., 2016). Some VOCs produced by soil‐borne fungi can protect plants by direct growth inhibition of phytopathogens (Strobel et al., 2001) or by induction of plant resistance (Li et al., 2016; Werner et al., 2016). Plant resistance can be induced by diverse beneficial microbes, including plant growth‐promoting rhizobacteria and soil‐borne fungi (e.g., Trichoderma spp.), and it is generally associated with an enhanced defense reaction after pathogen inoculation, such as the deposition of callose‐rich papillae at the sites of pathogen infection (Segarra et al., 2009). This phenomenon, also known as priming effect, consists of a cost‐effective defense mechanism against pathogens, and its targeted activation was proposed as a promising tool for crop protection (Conrath et al., 2015; Martínez‐Medina et al., 2016).
Trichoderma spp. are among the most widespread soil microorganisms and have been widely used as biocontrol agents against numerous phytopathogens (Harman et al., 2004). Biocontrol mechanisms of Trichoderma spp. are based on induction of plant resistance, production of antimicrobial substances, lytic enzymes, and competition with other microorganisms for nutrients and/or space (Lorito et al., 2010). Moreover, Trichoderma spp. are known to produce VOCs (Crutcher et al., 2013; Guo et al., 2020; Hung et al., 2013; Wheatley et al., 1997) that play crucial roles in the inter‐kingdom communications and in the biocontrol mechanisms (Guo et al., 2019; Malmierca et al., 2015; Zhang et al., 2014). For example, VOCs produced by T. asperellum T1 induced lettuce resistance against leaf spot fungal pathogens (Corynespora cassiicola and Curvularia aeria) and increased the activity of the cell‐wall degrading enzymes (Wonglom et al., 2020). Likewise, VOCs produced by T. asperellum T34 and T. harzianum T78 primed Arabidopsis thaliana and tomato plants for enhanced expression of jasmonic acid (JA)‐dependent defense reactions against Botrytis cinerea (Martínez‐Medina et al., 2017). In particular, 6‐pentyl‐2H‐pyran‐2‐one was produced by T. atroviride P1 and reduced the disease severity on tomato and canola seedlings inoculated with B. cinerea and Leptosphaeria maculans, respectively (Vinale et al., 2008). The same compound was found in T. asperellum IsmT5 and induced resistance against B. cinerea and Alternaria brassicicola in A. thaliana (Kottb et al., 2015). Moreover, VOCs of T. virens strains (Tv29.8, Tv10.4, and Δppt1‐1 mutant) included a large number of terpenes (e.g., β‐caryophyllene, β‐elemene, germacrene D, τ‐cadinene, α‐amorphene, δ‐cadinene, and τ‐selinene) and they induced A. thaliana resistance against B. cinerea (Contreras‐Cornejo et al., 2014). Some Trichoderma VOCs also showed direct inhibitory effects against phytopathogens (Scarselletti & Faull, 1994; Wonglom et al., 2020; Zhang et al., 2014). For example, 6‐pentyl‐2H‐pyran‐2‐one (also known as 6‐pentyl‐α‐pyrone produced by T. harzianum IMI 288012, T. harzianum T23 and T. atroviride IMI 206040) inhibited the growth of Fusarium moniliforme (El‐Hasan et al., 2007) and Rhizoctonia solani (Cruz‐Magalhães et al., 2019; Scarselletti & Faull, 1994), suggesting broad‐spectrum activities and multiple modes of action of Trichoderma VOCs against phytopathogens. Likewise, VOCs produced by T. harzianum T‐E5 inhibited F. oxysporum growth, including lignocerane, nerolidol, and verticillol as the most abundant compounds (Zhang et al., 2014), suggesting that Trichoderma VOCs have great potential for controlling phytopathogens.
Three Trichoderma strains are well documented for their ability to control a broad spectrum of phytopathogens and are used as biofungicides, such as T. asperellum T34 (T34), T. atroviride SC1 (SC1), T. harzianum T39 (T39) (Cotxarrera et al., 2002; Elad et al., 1997; Pertot et al., 2008). In particular, T34 is known for the induction of systemic resistance in cucumber against Pseudomonas syringae pv. lachrymans (Segarra et al., 2007) and in A. thaliana against Hyaloperonospora arabidopsidis (Segarra et al., 2009) and B. cinerea (Martínez‐Medina et al., 2017). SC1 antagonized a broad range of grapevine (Vitis vinifera) root (e.g., Armillaria mellea) and shoot (e.g., Phaeomoniella chlamydospora and Phaeoacremonium aleophilum) pathogens (Longa et al., 2008; Pellegrini et al., 2014; Pertot et al., 2016), while T39 induced systemic resistance against Plasmopara viticola by the modulation of defense‐related genes and resistance processes (Banani et al., 2014; Perazzolli et al., 2011; Perazzolli et al., 2012). T39‐induced resistance was characterized by an enhanced accumulation of callose and reactive oxygen species (ROS) after P. viticola infection (Palmieri et al., 2012), and ROS are considered as key signals for hypersensitive response (HR) activation (Kortekamp & Zyprian, 2003). Plasmopara viticola is one of the most important phytopathogenic oomycetes (Kamoun et al., 2015) and the causal agent of grapevine downy mildew (Gessler et al., 2011). Plasmopara viticola is normally controlled by frequent applications of chemical fungicides (Buonassisi et al., 2017; Gessler et al., 2011), but more sustainable control strategies are needed because of negative impact of pesticides on human health and the environment, emerging pesticide resistance and stricter rules on levels of pesticide residues in agricultural products (Spring et al., 2018). Several studies have highlighted the importance of Trichoderma spp. as an alternative to chemical fungicides against grapevine pathogens (Zanotto & Morroni, 2016), but no information is available on the possible biocontrol mechanism mediated by Trichoderma VOCs against downy mildew. The aim of this study was to identify and annotate VOCs produced by T34, T39, and SC1 using head space‐solid phase microextraction gas chromatography–mass spectrometry (HS‐SPME/GC‐MS) analysis and to investigate their effects against P. viticola. The final goal was to better understand the contribution of Trichoderma VOCs to limit downy mildew infection and to provide more information on the VOC‐mediated effects of Trichoderma spp. against phytopathogens.
2. MATERIALS AND METHODS
2.1. Biological material and growth conditions
Vitis vinifera cultivar Pinot Noir (downy mildew‐susceptible) and V. riparia (downy mildew‐resistant) plants were grown in 2.5 L‐pots under greenhouse conditions at 25 ± 1°C with a photoperiod of 16 h light and relative humidity (RH) of 70 ± 10% as previously described (Perazzolli et al., 2012).
A P. viticola population was collected from an untreated vineyard in the Trentino region (northern Italy) and maintained by subsequent inoculations on Pinot Noir plants under greenhouse conditions, as described by Perazzolli et al. (2012). To obtain the P. viticola inoculum, plants with disease symptoms were incubated overnight in the dark at 95 ± 5% RH, and P. viticola sporangia were collected by washing the abaxial leaf surfaces, bearing sporulating lesions, with cold distilled water. The inoculum concentration was then adjusted to 2.5 × 105 sporangia ml−1 with a hemocytometer under a light microscope (LMD7000, Leica Microsystems).
The Trichoderma strains, T34 (Cotxarrera et al., 2002), T39 (Elad et al., 1997), and SC1 (Pertot et al., 2008), were grown on potato dextrose agar (PDA; Oxoid) for seven days in the dark at 25 ± 1°C. Conidia were scraped gently from the colony surface of each strain with a sterile loop and collected in a sterile 2 ml‐tube containing 1 ml of cold (4°C) sterile distilled water. The concentration of the conidial suspension was adjusted to 1 × 107 conidia ml−1 for the assessment of direct effects against downy on grapevine plants and to 1 × 104 conidia ml−1 for the headspace (HS) VOC analysis, assessment of VOC‐mediated effects and callose deposition assay, by counting with a hemocytometer under the light microscope (LMD7000 microscope, Leica Microsystems) (Banani et al., 2014).
2.2. Assessment of direct effects of Trichoderma strains against downy mildew on grapevine plants
Leaves of downy mildew‐susceptible plants grown under greenhouse conditions (25 ± 1°C with a photoperiod of 16 h light and 70 ± 10% RH) were sprayed with the conidial suspension of the respective Trichoderma strain (1 × 107 conidia ml−1) or treated with water (Control). The abaxial and adaxial surfaces of all leaves were treated three times (1, 2, and 3 days before pathogen inoculation) using a compressed air hand sprayer (20–30 ml for each plant) in order to maximize the Trichoderma effects against P. viticola (Perazzolli et al., 2008). One day after the last treatment, all leaves of each plant were inoculated with the P. viticola suspension (2.5 × 105 sporangia ml−1) using a compressed‐air hand sprayer as previously described (Perazzolli et al., 2012), and plants were incubated overnight in the dark at 25 ± 1°C with 95 ± 5% RH to allow P. viticola infection (Perazzolli et al., 2012). Inoculated plants were maintained under greenhouse conditions (25 ± 1°C with a photoperiod of 16 h light and 70 ± 10% RH) to allow pathogen development (Perazzolli et al., 2012). Six days post inoculation (6 dpi), plants were incubated overnight in the dark at 25 ± 1°C with 95 ± 5% RH to promote downy mildew sporulation (Perazzolli et al., 2012), and the disease severity of each leaf was assessed visually as the percentage of abaxial leaf area covered by P. viticola sporulation, according to the standard guidelines of the European and Mediterranean Plant Protection Organization (EPPO, 2001). The disease severity of each replicate (plant) was calculated as the average of the disease severity of all leaves (Perazzolli et al., 2008). The disease reduction (efficacy) was calculated for each replicate (plant) according to the following formula: (disease severity of control plants—disease severity of Trichoderma‐treated plants)/disease severity of control plants × 100. Five replicates (plants) were used for each treatment, and the experiment was carried out twice.
2.3. Head space analysis of volatile organic compounds from Trichoderma spp.
For the HS VOC analysis, 5 ml PDA were poured into sterile 20 ml‐HS vials (Supelco, Merck), and they were left open in a slanted position under a laminar flow for 2 h at room temperature to avoid condensation (Lazazzara et al., 2017). Each HS vial was inoculated with 20 μl of the conidial suspension of the respective Trichoderma strain (1 × 104 conidia ml−1) and left to dry under a laminar flow for 1 h at room temperature (Trichoderma‐inoculated). Each HS vial was closed with a sterile cotton plug and the rubber strap for aerobic cultivation of Trichoderma spp. without oxygen limitation (Stoppacher et al., 2010), and it was incubated at 25 ± 1°C in the dark for 48 or 72 h, as described by Crutcher et al. (2013). The volume (20 μl) and concentration (1 × 104 conidia ml−1) of the conidial suspension were optimized in a preliminary trial in order to allow the complete colonization of the PDA surface of the HS vial by the Trichoderma mycelium in 48 h at 25 ± 1°C (data not shown). The time point of 48 h was selected since the VOC production of Trichoderma spp. was maximum (Crutcher et al., 2013; Stoppacher et al., 2010). Two independent experiments were carried out (named first and second experiment hereafter), three and five biological replicates were analyzed for each Trichoderma strain and time point in the first and second experiment, respectively, due to space limitation in the auto‐sampler of the first experiment. For each experiment, three additional HS vials containing non‐inoculated PDA (Uninoculated) were used as controls in order to exclude VOCs released from the culture medium in the absence of Trichoderma spp. (Kluger et al., 2013).
VOCs produced by Trichoderma strains were measured using HS‐SPME/GC–MS analysis according to Crutcher et al. (2013). After 48 or 72 h cultivation, each HS vial was purged with synthetic air filtered through a 0.2 μm‐politetrafluoroetilene (PTFE) Midisat BV membrane filter (Sartorius) for 30 s, sealed with a sterilized 18 mm‐screw metal cap assembled with a 1.3 mm‐silicone/PTFE septum (Supelco, Merck) and incubated for 6 h at 25 ± 1°C to accumulate VOCs before analysis (Crutcher et al., 2013, Stoppacher et al., 2010). Each HS vial was then placed in an auto‐sampler (MPS2XL, Gerstel) and equilibrated for 15 min at 30°C. For VOC extraction, a polydimethylsiloxane/divinylbenzene fiber (PDMS/DVB 65 μm; Supelco, Merck) was inserted into the HS vial for 30 min at 30°C (Crutcher et al., 2013). The fiber was transferred to the Agilent 6890 N gas chromatograph coupled to a quadrupole mass spectrometer 5975B Mass Selective Detector (MSD; Agilent Technologies), and analytes were desorbed in splitless mode at 250°C for 2 min. A non‐polar HP‐5MS column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies) was operated at a constant flow of helium (1 ml min−1). The oven temperature consisted of 40°C (hold 2 min), 10°C min−1 to 200°C, 25°C min−1 to 260°C (hold 5 min) and the transfer line was set at 270°C. Analytes were ionized at 70 eV in the ion source at 230°C and detected in full scan (45–400 amu). Mixed alkane standard solutions for retention index (RI) calibration was included in the sample list to facilitate reliable compound annotation, and three SPME conditions were applied to obtain good peak shapes: (1) 1 μl alkane standard solution from C5 to C10, with 0.01 min at 90°C for both equilibration and extraction steps, (2) 10 μl alkane standard solution from C8 to C20, with 5 min equilibration and 45 min extraction both at 90°C and (3) 40 μl alkane standard solution from C21 to C40, with 30 min equilibration and 60 min extraction both at 120°C (Kluger et al., 2013).
Raw data were acquired with an Agilent MSD ChemStation (G1701EA E.02.00.493, Agilent Technologies), and the abundance of each VOC was calculated as the integrated peak area, expressed as counts per scan (cps), using the MetaboliteDetector software (version 3.020151231 Ra‐Linux) (Hiller et al., 2009). The mass deconvolution settings were as follows: peak threshold of 5, the minimum peak height of 5, deconvolution width (scans) of 1 and the required number of peaks set at 5. For compound annotation, deconvoluted mass spectra were compared with the NIST14 database (National Institute of Standards and Technology, http://www.nist.gov/) and with an in‐house library obtained with authentic reference standards (Crutcher et al., 2013). Compound annotation was achieved imposing a relative deviation of RI value lower than 2% from the reference value published in the literature (http://www.nist.gov/) and according to the highest mass spectrum similarity score, which was set higher than 70% after first successful annotation, in order to include low‐abundant substances or substances where the deconvolution process did not lead to a complete elimination of interfering mass signals (Weingart et al., 2012). Chromatograms of not annotated compounds were searched for different types of terpenes using extracted ion current (EIC) chromatograms at a mass/charge ratio (m/z) 136 for monoterpenes, m/z 202 and m/z 204 for sesquiterpenes, and m/z 272 for diterpenes (Crutcher et al., 2013). The deconvoluted mass spectra underneath these EIC peaks were inspected manually, and, in the case of typical terpene mass spectrum, the corresponding mass spectra and RI values were included in the data matrix and named as “unknown sesquiterpene” or “unknown diterpene.” Deconvoluted mass spectra different from that of terpenes were named “unknown compound” according to their mass spectra and RI values. VOCs were included in the data matrix only if their signal‐to‐noise ratio (S/N) was greater than 10 (Bu et al., 2016) for at least one time point and Trichoderma strain.
2.4. Standard solutions and pure volatile organic compounds
Alkane standard solutions from C8 to C20 (40 mg L−1 each in n‐hexane) and C21 to C40 (40 mg L−1 each in toluene) were purchased from Sigma‐Aldrich (Merck). A standard solution from C5 to C10 was prepared using pure substances in a ratio resulting in narrow and symmetric peak shapes as described by Weingart et al. (2012). VOCs were selected according to HS‐SPME/GC–MS results and pure VOCs were used in functional assays, such as 1,3‐octadiene (98%) purchased from ChemSampCo (Dallas, TX, USA); 2‐pentylfuran (98%) and 6‐pentyl‐2H‐pyran‐2‐one (96%) purchased from Sigma‐Aldrich (Merck); cadinene (85%, corresponding to a mixture of isomers, such as ɣ‐cadinene, ɣ‐muurolene and δ‐cadinene) purchased from BOC Sciences (Shirley), and α‐farnesene (corresponding to a mixture isomers, such as (Z,E)‐α‐farnesene, (E,Z)‐α‐farnesene, (E,E)‐α‐farnesene and (Z,Z)‐α‐farnesene) purchased from SAFC Supply Solutions.
2.5. Assessment of volatile‐mediated effects of Trichoderma strains and pure compounds against grapevine downy mildew
Leaf disks (18 mm diameter) were obtained from the greenhouse‐grown grapevine plants (from the fourth to the sixth node of downy mildew‐susceptible plants) with a cork borer, and they were placed onto two layers of wet sterilized filter paper in Petri dishes (90 mm diameter; five disks for each dish), with the abaxial surface uppermost as previously described (Lazazzara et al., 2018).
To analyze VOC‐mediated effects of Trichoderma spp. on P. viticola, 15 ml PDA was poured into Petri dishes (90 mm diameter), inoculated with 20 μl of the conidial suspension of the respective Trichoderma strain (1 × 104 conidia ml−1) and left to dry under a laminar flow for 1 h at room temperature. As control, dishes containing uninoculated PDA were used. The lid of each dish containing grapevine leaf disks was removed, and the dish was covered with the bottom of a Trichoderma‐inoculated or uninoculated dish (dish sandwich; Figure S1A). The dish sandwich was sealed with Parafilm (Bemis) and incubated at 25 ± 1°C in the dark for 48 h without physical contact with leaf tissues. Each leaf disk was inoculated with five drops (5 μl each) of a P. viticola suspension (2.5 × 105 sporangia ml−1; P. viticola‐inoculated), the dish sandwich was assembled with respective Trichoderma‐inoculated, or uninoculated, dish and sealed with Parafilm (Bemis). Dish sandwiches were incubated in the dark at 25 ± 1°C overnight, leaf disks were dried under a laminar hood, covered with a dish lid and incubated for 6 days under greenhouse conditions.
In order to verify that leaf disks were not contaminated by Trichoderma spp. conidia, control dish sandwiches were prepared. Briefly, the lid of each dish containing 15 ml PDA was removed, and the dish was covered with the bottom of a Trichoderma‐inoculated dish. The dish sandwich was sealed with Parafilm (Bemis) and incubated at 25 ± 1°C for 72 h. Each Trichoderma‐inoculated dish was replaced with a dish lid and the absence of Trichoderma spp. growth on PDA was verified 6 days after incubation at 25 ± 1°C under greenhouse conditions.
Functional assays of pure VOCs against P. viticola were carried out according to Lazazzara et al. (2018). Briefly, each pure VOC was diluted 10‐fold in dimethyl sulfoxide (DMSO; Sigma‐Aldrich, Merck) and serially diluted in distilled water to obtain the appropriate concentration for each treatment. The respective pure VOC was applied to a filter paper disk (Whatman, Merck) fixed on the lid (without physical contact with the leaf tissues) of a dish containing grapevine leaf disks (Figure S1B). Each VOC was applied at a concentration of 2.5, 5, 10, 15, 20, or 50 mg L−1 in air volume (VOC‐treated leaf disks), assuming the complete VOC evaporation from the filter paper, and DMSO was applied as control (0 mg L−1 in air volume of VOCs; Control leaf disks). Moreover, two blends of the two most efficient VOCs (2‐pentylfuran and 6‐pentyl‐2H‐pyran‐2‐one) were tested at a concentration of 5 or 10 mg L−1 in air volume for each compound. Dishes were sealed with Parafilm (Bemis) and incubated in the dark at 25 ± 1°C under greenhouse conditions for 24 h. Each leaf disk was inoculated with five drops (5 μl each) of a P. viticola suspension (2.5 × 105 sporangia ml−1; P. viticola‐inoculated) or with five drops (5 μl each) of distilled water (mock‐inoculated), the respective pure VOC, or VOC blend, was applied again to the filter paper disk in the appropriate concentration. Dishes were sealed with Parafilm (Bemis) and incubated in the dark at 25 ± 1°C overnight. Leaf disks were dried under a laminar hood and dishes were incubated for 6 days under greenhouse conditions.
The downy mildew disease severity was assessed on each leaf disk at 6 dpi as a percentage of the leaf disk surface covered by sporulation (EPPO, 2001), calculated as the sum of the five inoculum drops: 0%, no sporulation; 10%, scarce sporulation; 20%, dense sporulation (Lazazzara et al., 2018). The presence of phytotoxic effect was assessed visually by checking for discolouration, chlorosis, and whitening of leaf disks (EPPO, 2014). The disease severity of each replicate (dishes with five disks each) was calculated as an average of the disease severity of five leaf disks (Lazazzara et al., 2018), five replicates were analyzed for each treatment and the experiment (i.e., VOC‐mediated Trichoderma effects and functional assay of pure VOCs) was carried out twice.
2.6. Visualization of callose deposition and Plasmopara viticola structures by aniline blue staining
VOC‐treated and control leaf disks were collected before inoculation (0 dpi) and at 1, 2, and 6 dpi with P. viticola. Samples were stained with aniline blue as reported by Lazazzara et al. (2018) to visualize P. viticola structures and callose deposition (Palmieri et al., 2012). Briefly, leaf disks were incubated in 1 M KOH at 95°C for 15 min and stained with 0.05% aniline blue (Sigma‐Aldrich, Merck) in 0.067 M K2HPO4 at pH 8 for 15 min. Leaf disks were observed under a LMD7000 microscope (Leica Microsystem) using an A4 filter (BP 360–400 nm excitation, 400 nm dichroic mirror, and 470–400 nm emission). As control of callose deposition (Palmieri et al., 2012), leaf disks were sprayed with T39 conidia (1 × 104 conidia ml−1) and inoculated with P. viticola (2.5 × 105 sporangia ml−1) as described above. Five leaf disks were analyzed for each treatment and time point and the experiment was carried out twice.
2.7. Visualization of grapevine hypersensitive response by lactophenol‐trypan blue staining
VOC‐treated and control leaf disks were collected before inoculation (0 dpi) and at 1 and 6 dpi with P. viticola. Samples were stained with lactophenol‐trypan blue as reported by Roetschi et al. (2001) to visualize P. viticola structures and dead plant cells of HR (Keogh et al., 1980). VOC‐treated and control leaf disks were mock‐inoculated with water as an additional control, in order to verify the absence of cell death. For the staining, leaf disks were incubated in lactophenol‐trypan blue at 100°C for 2 min and washed with 2.5 g ml−1 chloral hydrate for 24 h. Leaf disks were transferred to microscope slides and observed using a light microscope (LMD7000 microscope, Leica Microsystem). As control of the HR activation (Brilli et al., 2018), leaf disk of the downy mildew‐resistant grapevine (V. riparia) were inoculated with P. viticola (2.5 × 105 sporangia ml−1) as described above. Five leaf disks were analyzed for each treatment and time point and the experiment was carried out twice.
2.8. RNA extraction and gene expression analysis
Plasmopara viticola‐inoculated and mock‐inoculated leaf disks (25 mm diameter) were collected at 6 dpi from VOC‐treated and control samples. This time point was chosen to maximize the grapevine defense reaction (Malacarne et al., 2011; Vrhovsek et al., 2012), according to the lactophenol‐trypan blue staining results. Leaf disks were reduced to 18 mm in diameter using a cork borer, in order to eliminate the outlying area where defense responses to injury can occur (Adrian et al., 2017). Samples were immediately frozen in liquid nitrogen, stored at −80°C and crushed using a mixer mill disruptor (MM200, Retsch) at 25 Hz for 45 s with sterile steel jars and beads refrigerated in liquid‐N2. Total RNA was extracted from 100 mg of ground sample using the Spectrum Plant total RNA kit (Sigma‐Aldrich, Merck) with an on‐column DNase treatment with the RNase‐Free DNase Set (Qiagen). RNA was quantified by Qubit RNA Broad Range Assay Kit (Thermo Fisher Scientific) and the first strand cDNA was synthesized from 0.5 μg of total RNA using Superscript III (Invitrogen, Thermo Fisher Scientific) and oligo‐dT primer. Genes encoding pathogenesis‐related protein 2 (PR2), osmotin 1 (OSM1), osmotin 2 (OSM2), and chitinase 3 (CHIT3) were used as markers of grapevine induced resistance (Banani et al., 2014; Perazzolli et al., 2011, 2012) and the HR‐related gene (HSR) was selected as a marker of grapevine cell death (Lakkis et al., 2019) (Table S1). Quantitative real‐time PCR (qPCR) reactions were carried out with Platinum SYBR Green qPCR SuperMix‐UDG (Invitrogen, Thermo Fisher Scientific) and specific primers (Table S1) using the Light Cycler 480 (Roche Diagnostics) as previously described (Perazzolli et al., 2012). Briefly, the PCR conditions were: 50°C for 2 min and 95°C for 2 min as initial steps, followed by 50 cycles at 95°C for 15 s and at 60°C for 1 min. Each sample was examined in three technical replicates and dissociation curves were analyzed to verify the specificity of each amplification reaction. The Light Cycler 480 SV 1.5.0 software (Roche) was used to extract Ct‐values based on the second derivative calculation and the LinReg software version 11.1 was used to calculate reaction efficiencies for each primer pair (Ruijter et al., 2009). The relative expression level (fold‐change) of each gene was calculated according to the Pfaffl equation (Pfaffl, 2001), using mock‐inoculated control leaf disks as the calibrator. The grapevine actin and VATP16 were used as housekeeping genes for data normalization, because their expression was not affected by P. viticola inoculation (Perazzolli et al., 2012; Polesani et al., 2010). Three replicates (dishes with five leaf disks each) were analyzed for each treatment and the gene expression profiles were confirmed in an independent experimental repetition.
2.9. Statistical analysis
VOC data were processed using an in‐house R‐script (R version 3.1.0). Each experimental repetition was analyzed separately, the Kruskal‐Wallis test (P ≤ 0.05) and a fold‐change in VOC abundance higher than 1.5 were set as criteria to identify VOCs with significant changes in abundance among the three Trichoderma strains for each time point. For each experiment, only VOCs which showed higher mean abundance in Trichoderma‐inoculated compared to uninoculated HS vials for at least one strain and time point were considered, according to the Kruskal‐Wallis test (P ≤ 0.05) with a fold‐change higher than 1.5.
Each experiment was carried out twice and disease severity data were analyzed using the Statistica 13.3 software (TIBCO Software Inc.). Each experimental repetition was analyzed separately and a Kruskal‐Wallis test was used to demonstrate equivalent results in the two experiments (P > 0.05, non‐significant differences between experimental repetitions). Data from the two experimental repetitions were pooled and a Kruskal‐Wallis test was used to detect significant differences among treatments (P ≤ 0.05). Fold change values of the gene expression analysis were transformed using the equation y = Log10 (fold change +1) (Casagrande et al., 2011) and the analysis of variance (anova) with the Fisher's test (P ≤ 0.05) was carried out to detect significant differences among treatments after validation of normal distribution (Kolmogorov–Smirnov test, P > 0.05) and variance homogeneity (Levene's test, P > 0.05) of the data.
3. RESULTS
3.1. Effect and annotation of Trichoderma spp. volatile organic compounds
Treatments with T34, SC1 or T39 conidia reduced downy mildew severity on susceptible grapevine plants compared to control plants (Figure 1A) with an efficacy of 72.1 ± 5.8%, 65.9 ± 9.2%, and 71.1 ± 4.4%, respectively (mean ± standard error values). Moreover, a VOC‐mediated reduction of downy mildew severity was found on leaf disks treated with T34, SC1, or T39 colonies in a dish sandwich without contact with the leaf tissues (Figure S1A), with an efficacy of 43.1 ± 4.9%, 46.2 ± 7.2%, and 31.6 ± 4.2%, respectively (Figure 1B). No Trichoderma colonies developed on control dish sandwich assembled with a dish containing sterilized PDA covered with the bottom of a Trichoderma‐inoculated dish (data not shown), confirming that no conidia dropped down and that only VOCs produced by Trichoderma spp. were involved in the reduction of downy mildew severity on leaf disks.
FIGURE 1.
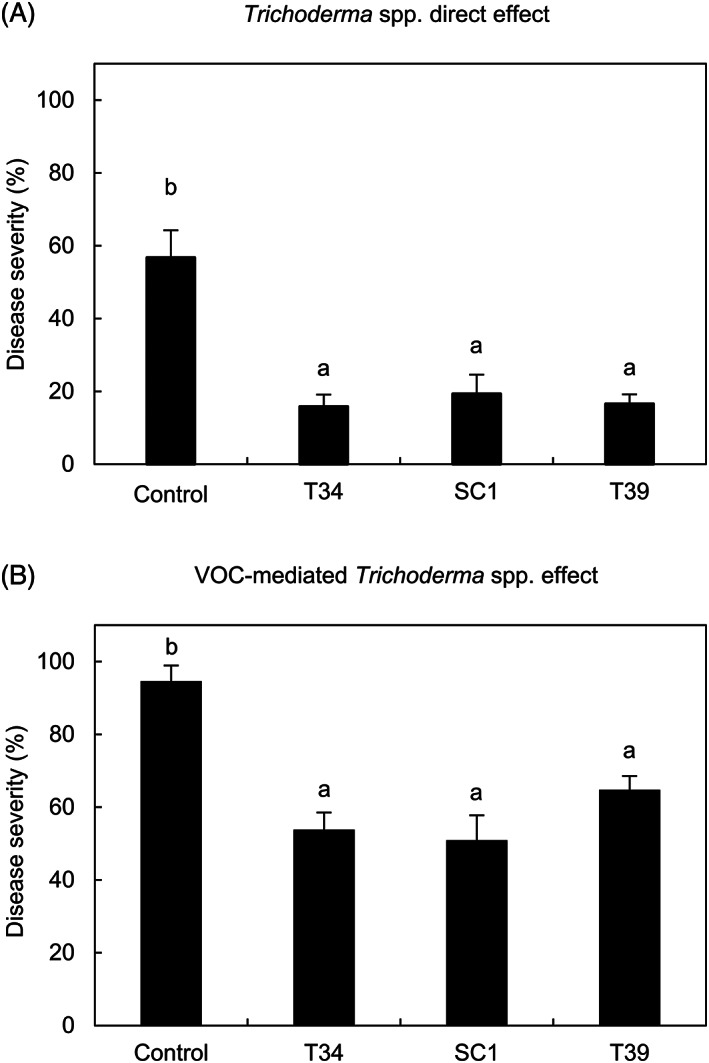
Effects of Trichoderma strains against downy mildew. Direct effects of Trichoderma strains (A) were assessed on greenhouse‐grown grapevine plants, sprayed with water (control) and with a conidial suspension of T. asperellum T34 (T34), T. atroviride SC1 (SC1) or T. harzianum T39 (T39). Volatile‐mediated effects of Trichoderma strains (B) were assessed on grapevine leaf disks, treated with uninoculated potato dextrose agar (PDA) dishes (control) and dishes with PDA‐grown T34, SC1 or T39 colonies without contact with leaf tissues. Downy mildew severity was assessed at 6 days post inoculation. Five replicates were assessed for each treatment and each experiment was carried out twice. The Kruskal‐Wallis test indicated no significant differences between the two experimental repetitions (P > 0.05) and data from the two experiments were pooled. The pooled mean and standard error values of 10 replicates from the two experiments are presented for each treatment. Different letters indicate significant differences among treatments according to the Kruskal‐Wallis test (P ≤ 0.05)
In order to characterize VOCs produced by the three Trichoderma strains, the HS‐SPME/GC–MS analysis was carried out at 48 or 72 h after incubation on PDA and a total of 26 and 21 VOCs were found in the first and second experiment, respectively (Figure 2; Figure S1B; Tables S2 and S3). Annotated Trichoderma VOCs include alkenes (e.g., 1,3‐octadiene), furanes (e.g., 2‐pentylfuran and 2‐n‐heptylfuran), ketones (e.g., 3‐octanone and 2‐undecanone), pyrones (lactones, e.g., 6‐pentyl‐2H‐pyran‐2‐one), and terpenes, such as monoterpenes (α‐phellandrene, α‐terpinene, limonene, γ‐terpinene, and β‐phellandrene) and sesquiterpenes ([Z,E]‐α‐farnesene, γ‐cadinene, γ‐muurolene, α‐curcumene, α‐zingiberene, trans‐β‐farnesene, germacrene A, β‐sesquiphellandrene, β‐himachalene, β‐bisabolene, and δ‐cadinene). Moreover, nine VOCs were annotated as three unknown sesquiterpenes (e.g., unknown sesquiterpene 1, 2, and 3), three unknown diterpenes (e.g., unknown diterpene 1, 2, and 3), and three unknown compounds (unknown compounds 1, 2, and 3), according to the mass spectrum and measured RI (Table S4). VOC emission profiles were mainly consistent in the two experiments and they differed according to the Trichoderma strain and time point (Figures 2 and S2). In particular, T39 produced a higher amount of VOCs compared to T34 and SC1, with 24 and 21 VOCs found in the first and second experiment, respectively. On the other hand, 15 and 9 compounds were found in the case of T34, while 9 and 7 compounds were found in the case of SC1 in the first and second experiment, respectively. Moreover, T39 produced a higher amount of terpenes ([Z,E]‐α‐farnesene, γ‐cadinene, γ‐muurolene, α‐curcumene, α‐phellandrene, α‐zingiberene, unknown diterpene 1, 2, and 3, and unknown sesquiterpene 1 and 2) compared to T34 ([Z,E]‐α‐farnesene, γ‐muurolene, α‐curcumene, α‐zingiberene, and unknown sesquiterpene 2) and SC1 (α‐zingiberene and unknown diterpene 3).
FIGURE 2.
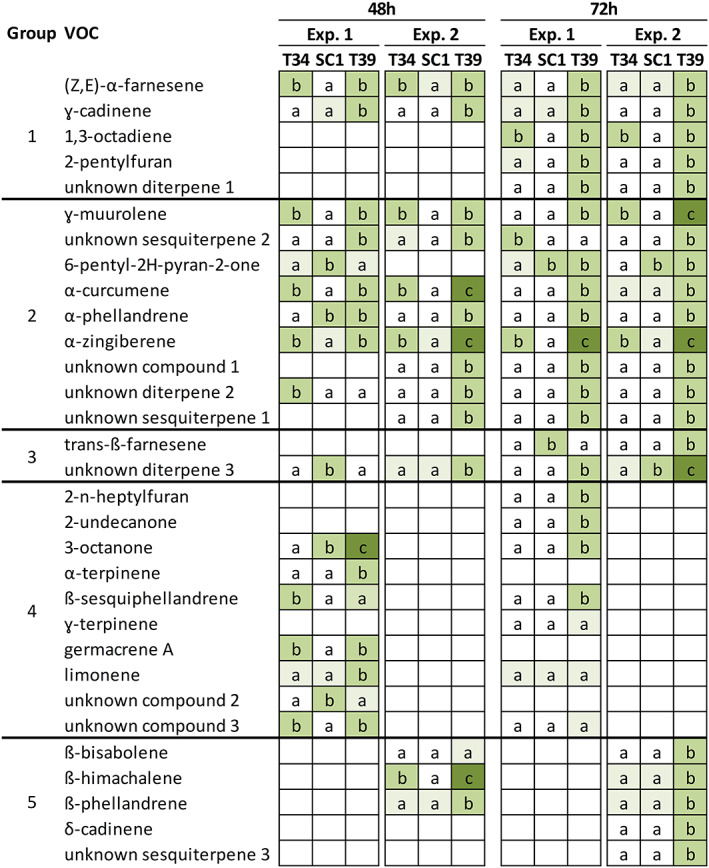
Volatile organic compounds (VOCs) produced by Trichoderma strains. VOCs analysis was carried out using head space‐solid phase microextraction gas chromatography–mass spectrometry (HS‐SPME/GC‐MS) for T. asperellum T34 (T34), T. atroviride SC1 (SC1) and T. harzianum T39 (T39) grown for 48 h or 72 h on potato dextrose agar (PDA) in two independent experiments (Exp. 1 and Exp. 2). Different letters and color gradients indicate significant differences in VOC abundance among the Trichoderma strains for each experiment and time point, according to the Kruskal‐Wallis test (P ≤ 0.05) with a fold change higher than 1.5 (Tables S2 and S3). The letter ‘a’ was assigned to low VOC abundance (light green cell) or VOC abundance below the limit of detection (white cell). Five metabolite groups were identified according to changes in abundance among the three Trichoderma strains: Consistent changes in the two experiments at both time points (group 1) or at one time point (group 2); different changes in the two experiments (group 3); detection in the first (group 4) or in the second (group 5) experiment
VOCs were divided into five metabolite groups according to changes in abundance among the three Trichoderma strains (Figure 2; Tables S2 and S3). The first metabolite group included three terpenes ([Z,E]‐α‐farnesene, γ‐cadinene, and unknown diterpene 1), 1,3‐octadiene, and 2‐pentylfuran, whose changes in abundance were consistent in both experiments and time points (Group 1). More specifically, the abundance of (Z,E)‐α‐farnesene was higher in T34 and T39 compared to SC1 at 48 h and in T39 compared to T34 and SC1 at 72 h in both experiments. The abundance of γ‐cadinene was higher in T39 compared to T34 and SC1 at both time points in both experiments. Moreover, 1,3‐octadiene was more abundant in T34 and T39 compared to SC1 at 72 h, 2‐pentylfuran and the unknown diterpene 1 were more abundant in T39 compared to T34 and SC1 at 72 h and these three VOCs were not found at 48 h in both experiments. The second metabolite group comprised nine VOCs, whose changes in abundance among the three Trichoderma strains were consistent in the two experiments at one time point (Group 2). At 48 h, γ‐muurolene was more abundant in T34 and T39 compared to SC1 and the unknown sesquiterpene 2 was more abundant in T39 compared to T34 and SC1 in both experiments. At 72 h, the abundance of 6‐pentyl‐2H‐pyran‐2‐one was higher in SC1 and T39 compared to T34, while that of α‐curcumene, α‐ phellandrene, α‐zingiberene, unknown compound 1, unknown diterpene 2, and uknown sesquiterpene 1 was higher in T39 compared to T34 and SC1 in both experiments. The abundance of two VOCs (trans‐β‐farnesene and unknown diterpene 3) differed in the two experiments (Group 3). Moreover, 10 VOCs (2‐n‐heptylfuran, 2‐undecanone, 3‐octanone, α‐terpinene, β‐sesquiphellandrene, γ‐terpinene, limonene, germacrene A, unknown compounds 2 and 3) and five VOCs (β‐bisabolene, β‐himachalene, β‐phellandrene, δ‐cadinene and unknown sesquiterpene 3) were detected only in the first (Group 4) or second (Group 5) experiment, respectively.
3.2. Efficacy of pure volatile organic compounds against downy mildew on grapevine leaf disks
Five VOCs were selected according to their consistent changes in abundance among the three Trichoderma strains in the two experiments and they were tested as a pure compound against P. viticola at different concentrations in air volume (Figure S1B). More specifically, (Z,E)‐α‐farnesene (α‐farnesene mixture of isomers), 1,3‐octadiene, and 2‐pentylfuran were selected, since their changes in abundance were consistent in both experiments and time points (Group 1). Moreover, ɣ‐cadinene and ɣ‐muurolene were tested as a mixture of isomers (namely cadinene) and 6‐pentyl‐2H‐pyran‐2‐one was used as pure compound, since their changes in abundance were consistent in the two experiments at one time point. The pure VOCs were tested against P. viticola and 2‐pentylfuran reduced disease severity at dosages of 5, 10, and 15 mg L−1 in air volume with no visible phytotoxic effects (Figure 3A). Likewise, 6‐pentyl‐2H‐pyran‐2‐one reduced downy mildew severity at dosages of 5 and 10 mg L−1 in air volume with no visible phytotoxic effects (Figure 3B). However, leaf disks treated with 20 mg L−1 in air volume of 2‐pentylfuran or with 15 and 20 mg L−1 in air volume of 6‐pentyl‐2H‐pyran‐2‐one showed phytotoxic effects.
FIGURE 3.
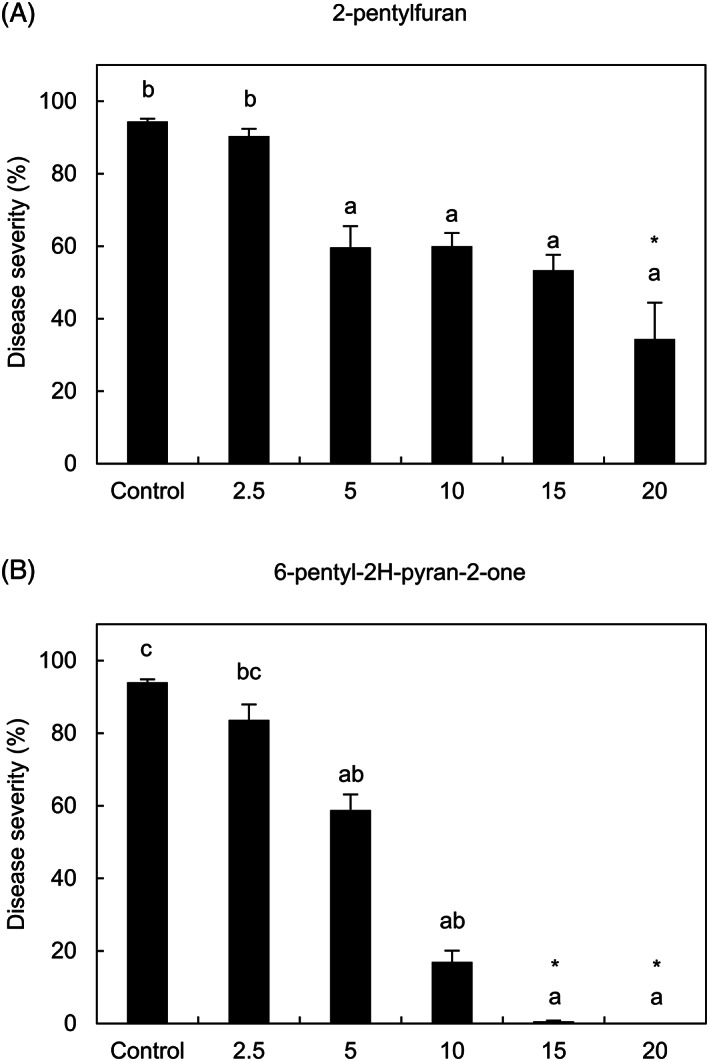
Effects of the most efficient pure volatile organic compounds (VOCs) against downy mildew. Grapevine leaf disks were treated with water (control), 2‐pentylfuran (A) or 6‐pentyl‐2H‐pyran‐2‐one (B) at 2.5, 5, 10, 15 and 20 mg L−1 in air volume and downy mildew severity was assessed at 6 days post inoculation. Five replicates (dishes with five disks each) were assessed for each treatment and the experiment was carried out twice. For each assay, the Kruskal‐Wallis test indicated no significant differences between the two experimental repetitions (p > 0.05) and data from the two experiments were pooled. The pooled mean and standard error values of 10 replicates from the two experiments are presented for each treatment. Different letters indicate significant differences among treatments according to the Kruskal‐Wallis test (P ≤ 0.05). Asterisks indicate phytotoxic effects on leaf disks
Leaf disks treated with 1,3‐octadiene, α‐farnesene, and cadinene at a concentration of 50 mg L−1 in air volume showed a reduction of downy mildew severity with an efficacy of 21.5 ± 4.6% (Figure 4A), 21.6 ± 4.4% (Figure 4B) and 18.4 ± 5.9% (Figure 4C), respectively. At a concentration of 20 mg L−1 in air volume, α‐farnesene reduced downy mildew severity, but 1,3‐octadiene and cadinene did not and these three VOCs were not further used in activity tests due to the low efficacy with high application dosages.
FIGURE 4.
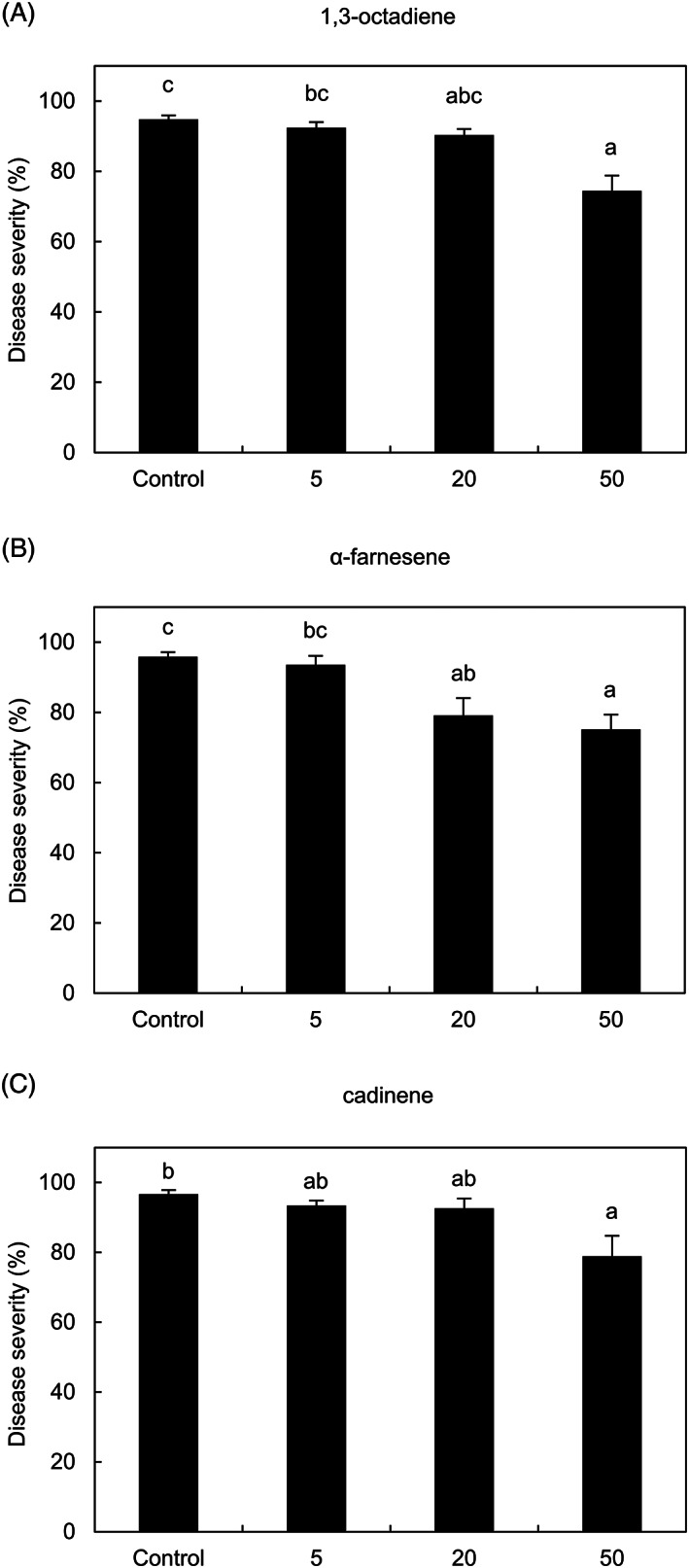
Effects of the less efficient pure volatile organic compounds (VOCs) against downy mildew. Grapevine leaf disks were treated with water (control), 1,3‐octadiene (A), α‐farnesene (B) or cadinene (C) at 5, 20 and 50 mg L−1 in air volume and downy mildew severity was assessed at 6 days post‐inoculation. Five replicates (dishes with five disks each) were assessed for each treatment and the experiment was carried out twice. For each assay, the Kruskal‐Wallis test indicated no significant differences between the two experimental repetitions (P > 0.05) and data from the two experiments were pooled. The pooled mean and standard error values of 10 replicates from the two experiments are presented for each treatment. Different letters indicate significant differences among treatments according to the Kruskal‐Wallis test (P ≤ 0.05)
Neither synergistic nor additive effects against downy mildew severity were observed with a blend of the two most efficient VOCs (2‐pentylfuran and 6‐pentyl‐2H‐pyran‐2‐one) at a concentration of 5 and 10 mg L−1 in air volume (Kruskal‐Wallis test, P > 0.05). In particular, the reduction of downy mildew severity on leaf disks treated with the blend of the two most efficient VOCs (77.2 ± 2.1%; at 10 mg L−1 in air volume for each compound) was comparable (Kruskal‐Wallis test P > 0.05) to that on leaf disks treated with 10 mg L−1 in air volume of pure 2‐penthylfuran (36.2 ± 3.8%) or 6‐pentyl‐2H‐pyran‐2‐one (82.1 ± 3.3%), therefore this blend was not further used in activity tests.
3.3. Effects of pure volatile organic compounds on callose deposition and hypersensitive response in grapevine leaf disks
Effects of the two most efficient VOCs were further characterized, using the lowest concentration at which the highest efficacy without visible phytotoxicity was observed (i.e., optimized concentration, namely 10 mg L−1 in air volume for 2‐pentylfuran and 6‐pentyl‐2H‐pyran‐2‐one). Aniline blue staining revealed no differences between VOC‐treated and control leaf disks before P. viticola inoculation (0 dpi, Figure 5A–C), as well as in leaf disks sprayed with T39 conidia (T39 conidia‐treated; Figure 5D). At 1 dpi, the pathogen had already penetrated the stomata of control leaf disks and substomatal vesicles were visible (Figure 5E). On the other hand, strong turquoise fluorescence was observed in the stomata of leaf disks treated with 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one at 1 dpi (Figure 5F,G), indicating intense callose deposition at infection sites, as found in T39 conidia‐treated leaf disks (Figure 5H). Thus, the number of zoospores that had successfully entered the stomata at 1 dpi was reduced in leaf disks treated with 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one. Elongated and branched hyphae were visible in control leaf disks at 2 dpi (Figure 5I), while hyphae were occasionally visible in leaf disks treated with 2‐pentylfuran (Figure 5J) or 6‐pentyl‐2H‐pyran‐2‐one (Figure 5K). Callose deposition was visible in leaf disks treated with 2‐pentylfuran (Figure 5J), 6‐pentyl‐2H‐pyran‐2‐one (Figure 5K), and T39 conidia at 2 dpi (Figure 5L). At 6 dpi, P. viticola mycelium had already spread to the parenchyma and produced sporangiophores in control leaf disks (Figure 5M,Q), while P. viticola sporulated areas were reduced in leaf disks treated with 2‐pentylfuran (Figure 5N,R), 6‐pentyl‐2H‐pyran‐2‐one (Figure 5O,S), and T39 conidia (Figure 5P,T).
FIGURE 5.
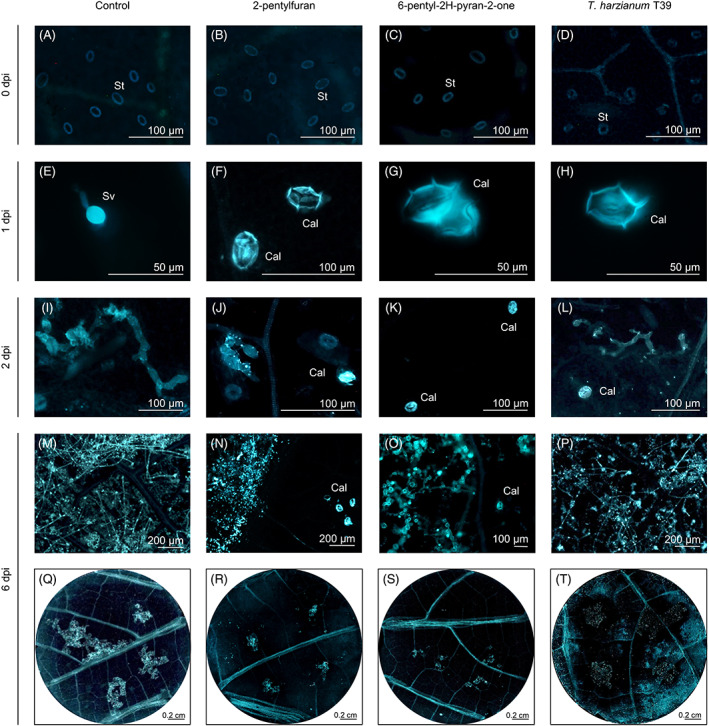
Effects of pure volatile organic compounds (VOCs) on callose deposition and downy mildew development. Grapevine leaf disks were treated with water (control) or with 10 mg L−1 in air volume of 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one. Callose deposition and Plasmopara viticola development were monitored before inoculation (0 dpi, A–D), at one (E–H), two (I–L) and six (M–T) days post inoculation (dpi) using aniline blue staining. As control of callose deposition, leaf disks were sprayed with Trichoderma harzianum T39 conidia and inoculated with P. viticola (Palmieri et al., 2012). The experiment was carried out twice and a representative leaf disk of 10 is shown for each treatment. Abbreviations: Cal, callose; Sv, substomatal P. viticola vesicle; St, stomata guard cells
Lactophenol‐trypan blue staining revealed no pathogen structures nor dead plant cells in control, VOC‐treated and V. riparia leaf disks at 0 dpi (Figure 6A–D) and 1 dpi (Figure 6E–H). At 6 dpi, blue areas corresponding to P. viticola mycelia confirmed the reduction of pathogen growth in 2‐pentylfuran‐treated (Figure 6J,N) compared to control (Figure 6I,M) leaf disks. Moreover, dark blue‐stained dead cells with no mycelial structures were found at P. viticola infection sites in 6‐pentyl‐2H‐pyran‐2‐one‐treated leaf disks at 6 dpi (Figure 6K,O), indicating HR activation, as found in downy mildew‐resistant (V. riparia) leaf disks at 6 dpi (Figure 6L,P). Conversely, no HR response was found in mock‐inoculated leaf disks treated with 6‐pentyl‐2H‐pyran‐2‐one or 2‐pentylfuran at 1 and 6 dpi (Figure S3).
FIGURE 6.
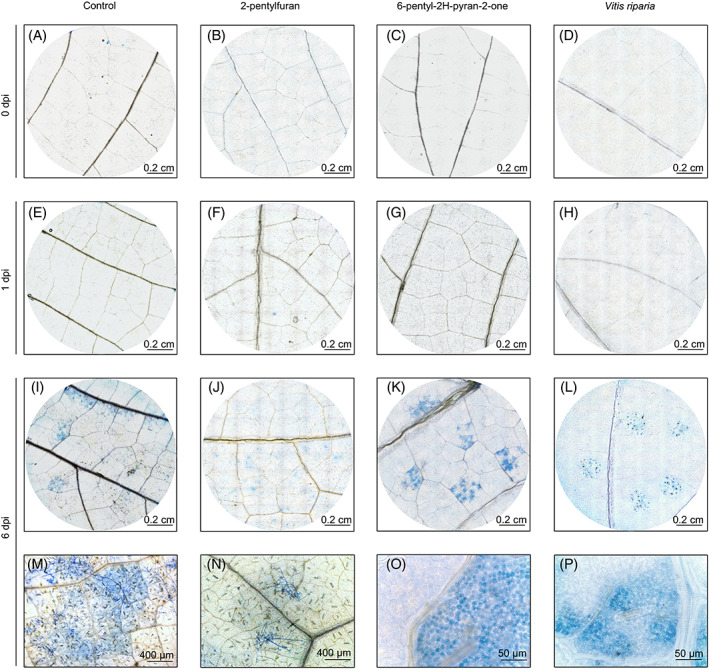
Effects of pure volatile organic compounds (VOCs) on grapevine hypersensitive response. Grapevine leaf disks were treated with water (control) or with 10 mg L−1 in air volume of 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one. Hypersensitive response and Plasmopara viticola development were monitored before inoculation (0 dpi, A–D), at one (E–H) and six (I–P) days post inoculation (dpi) using lactophenol‐trypan blue staining. As positive control, leaf disks of the downy mildew‐resistant grapevine (Vitis riparia) were inoculated with P. viticola (Brilli et al., 2018).The experiment was carried out twice and a representative leaf disk of 10 is shown for each treatment. Infected areas with P. viticola sporangiophores were visible in control (I and M) and 2‐pentylfuran‐treated (J and N) leaf disks at 6 dpi
3.4. Effects of pure volatile organic compounds on the modulation of defense‐related genes in grapevine leaf disks
Plasmopara viticola inoculation upregulated the expression of PR2, OSM1, OSM2, CHIT3, and HSR at 6 dpi in control leaf disks (Table 1). In mock‐inoculated leaf disks, the expression levels of the five defense‐related genes were not affected by 2‐pentylfuran, but they were induced by 6‐pentyl‐2H‐pyran‐2‐one. In P. viticola‐inoculated leaf disks, the expression levels of PR2, OSM2, and HSR were higher in 2‐pentylfuran‐treated compared to control leaf disks, as a reinforced modulation of defense‐related genes after pathogen inoculation. Likewise, the P. viticola‐dependent upregulation of PR2, OSM1, OSM2, CHIT3, and HSR was enhanced in 6‐pentyl‐2H‐pyran‐2‐one‐treated compared to control leaf disks.
TABLE 1.
Gene expression analysis of defense‐related genes in grapevine leaf disks treated with volatile organic compounds and inoculated with Plasmopara viticola
| Gene name | Abbreviation | Treatmenta | |||||
|---|---|---|---|---|---|---|---|
| Control | 2‐pentylfuran | 6‐pentyl‐2H‐pyran‐2‐one | |||||
| Mock | P. viticola | Mock | P. viticola | Mock | P. viticola | ||
| Pathogenesis‐related protein 2 | PR2 | 1.1 ± 0.4a | 6.7 ± 0.9b | 0.9 ± 0.0a | 10.6 ± 1.0c | 5.2 ± 0.4b | 14.4 ± 0.8d |
| Osmotin 1 | OSM1 | 1.0 ± 0.2a | 49.3 ± 9.8b | 7.7 ± 1.4a | 93.1 ± 39.2c | 73.5 ± 13.7b | 199.0 ± 2.3d |
| Osmotin 2 | OSM2 | 1.0 ± 0.2a | 5.9 ± 0.9b | 0.8 ± 0.1a | 7.7 ± 2.4b | 13.2 ± 1.0c | 40.6 ± 3.1d |
| Chitinase 3 | CHIT3 | 1.0 ± 0.1a | 3.1 ± 0.4b | 1.0 ± 0.2a | 5.2 ± 0.6c | 9.2 ± 1.4d | 12.5 ± 0.6e |
| HR‐related gene | HSR | 1.4 ± 0.7a | 400.4 ± 134.7c | 1.4 ± 0.3a | 340.4 ± 96.4c | 55.0 ± 7.7b | 1185.5 ± 84.4d |
Grapevine leaf disks (Vitis vinifera) were treated with water (Control) or with 10 mg L−1 in air volume of 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one, on a filter paper disk without contact with leaf tissues. Disks were inoculated with Plasmopara viticola or water (Mock) and the respective pure VOC was applied again to the filter paper disk. Leaf disks were collected 6 days post‐inoculation and the relative expression levels (fold change) were calculated respect to mock‐inoculated control leaf disks using actin as constitutive gene for normalization (Perazzolli et al., 2012) and comparable results were obtained with VATP16 gene. Mean and standard error values of three replicates (dishes with five leaf disks each) are presented for each treatment. For each gene, different letters indicate significant differences according to Fisher's test (P ≤ 0.05). Expression profiles were validated by an independent repetition of the experiment.
4. DISCUSSION
Strains belonging to T. asperellum, T. atroviride, and T. harzianum are known for their biocontrol activity against phytopathogens (Brunner et al., 2005; Inglis & Kawchuk, 2002; Nagaraju et al., 2012; Perazzolli et al., 2008; Segarra et al., 2009; Segarra et al., 2013) and grapevine VOCs are known for their inhibitory activity against downy mildew (Lazazzara et al., 2018), but no information is available on the VOC‐mediated effects of Trichoderma spp. against P. viticola. In this study, T34, T39, and SC1 conidia reduced downy mildew severity on grapevine plants and VOCs produced by the T34, T39, and SC1 colonies reduced downy mildew severity on grapevine leaf disks. Although VOC emission profiles differed according to the Trichoderma strain and time point, the VOC‐mediated disease reduction was comparable in T34‐, T39‐, and SC1‐treated leaf disks, suggesting possible synergistic interactions among VOCs (Strobel et al., 2001). In particular, 31 compounds were found in the HS‐SPME/GC–MS analysis of the three Trichoderma strains and they belong to the compound classes of alkenes (e.g., 1,3‐octadiene), furanes (e.g., 2‐pentylfuran and 2‐n‐heptylfuran), ketones (e.g., 3‐octanone and 2‐undecanone), pyrones (lactones, e.g., 6‐pentyl‐2H‐pyran‐2‐one), and terpenes, such as monoterpenes (e.g., α‐phellandrene, α‐terpinene, limonene, γ‐terpinene and β‐phellandrene) and sesquiterpenes (e.g., [Z,E]‐α‐farnesene, γ‐cadinene, γ‐muurolene, α‐curcumene, α‐zingiberene, trans‐β‐farnesene, germacrene A, β‐sesquiphellandrene, β‐himachalene, β‐bisabolene, and δ‐cadinene). In agreement with the previous literature (Contreras‐Cornejo et al., 2014; Crutcher et al., 2013; Guo et al., 2019; Nieto‐Jacobo et al., 2017; Sridharan et al., 2020; Stoppacher et al., 2010), terpenes dominated the VOC emission profiles of the three Trichoderma strains. In particular, T39 produced a higher amount of terpenes compared to T34 and SC1, such as (Z,E)‐α‐farnesene, γ‐cadinene, γ‐muurolene, α‐curcumene, α‐phellandrene, α‐zingiberene, unknown diterpene 1, 2, and 3, and unknown sesquiterpene 1 and 2. Strain‐specific VOCs were found and the unknown diterpene 1, unknown compound 1, and unknown sesquiterpene 1 were produced by T39 only, but further studies are required to fully elucidate the chemical structure and potential roles of these compounds. Moreover, VOC emission profiles of the three Trichoderma strains depended on the time point of sampling, corroborating that VOC production changed according to the incubation time and that it was possibly related to the developmental stage of Trichoderma spp. (Crutcher et al., 2013; Guo et al., 2020; Stoppacher et al., 2010). For example among VOCs with consistent changes in abundance in both experiments and time points, the abundance of (Z,E)‐α‐farnesene was higher in T34 and T39 compared to SC1 at 48 h and in T39 compared to T34 and SC1 at 72 h, while that of γ‐cadinene was higher in T39 compared to T34 and SC1 at both time points and that of 1,3‐octadiene was higher in T34 and T39 compared to SC1 at 72 h.
Functional assays reported in this study demonstrated that five VOCs (α‐farnesene, cadinene, 1,3‐octadiene, 2‐pentylfuran, and 6‐pentyl‐2H‐pyran‐2‐one) reduced downy mildew severity on leaf disks when applied in air volume without physical contact with the leaf tissues. In particular, two VOCs (6‐pentyl‐2H‐pyran‐2‐one and 2‐pentylfuran) strongly inhibited downy mildew severity with no synergistic or additive effects when used in a blend. Among them, 6‐pentyl‐2H‐pyran‐2‐one was more abundant in T39 and SC1 compared to T34 samples at 72 h and it was previously identified as a characteristic VOC of numerous Trichoderma spp. (Fadel et al., 2015; Leylaie & Zafari, 2018; Mutawila et al., 2016; Stoppacher et al., 2010). Moreover, 2‐pentylfuran was more abundant in T39 compared to T34 and SC1 samples at 72 h and it was previously found also in several Trichoderma spp. (Crutcher et al., 2013; Estrada‐Rivera et al., 2019; González‐Pérez et al., 2018; Lee et al., 2016; Nieto‐Jacobo et al., 2017; Stoppacher et al., 2010).
Different modes of action against phytopathogens have been attributed to VOCs, such as induction of plant resistance and direct inhibition of pathogen growth by absorption on cuticular waxes (Camacho‐Coronel et al., 2020; Quintana‐Rodriguez et al., 2015). T34 and T39 are well known inducers of systemic resistance in different plant species (Martínez‐Medina et al., 2017; Perazzolli et al., 2008; Segarra et al., 2007; Segarra et al., 2009). In particular, T39 has been demonstrated to induce grapevine resistance against P. viticola by enhanced callose deposition and modulation of defense‐related genes (Banani et al., 2014; Palmieri et al., 2012; Perazzolli et al., 2008, 2011; Perazzolli et al., 2012). In this study, the callose deposition was found at infection sites of leaf disks treated with 2‐pentylfuran or 6‐pentyl‐2H‐pyran‐2‐one, indicating that these Trichoderma VOCs induced grapevine resistance against P. viticola. The deposition of callose at the sites of pathogen infection is a key defense process against downy mildew (Gindro et al., 2003) that can be enhanced in susceptible grapevine genotypes by chemical resistance inducers, such as β‐aminobutiric acid (Hamiduzzaman et al., 2005), benzothiadiazole‐7‐carbothioic acid S‐methyl ester (Palmieri et al., 2012) and sulfated laminarin PS3 (Trouvelot et al., 2008). Furthermore, we found that 6‐pentyl‐2H‐pyran‐2‐one activated the HR at P. viticola infection sites, indicating the VOC‐mediated reinforcement of characteristic grapevine defense processes commonly activated in downy mildew‐resistant genotypes (Brilli et al., 2018; Gindro et al., 2003). In particular, both callose deposition and HR response were found only after P. viticola inoculation in leaf disks treated with 6‐pentyl‐2H‐pyran‐2‐one, suggesting a priming state activation for enhanced defense reaction upon pathogen infection. Likewise, the P. viticola‐dependent upregulation of defense‐related genes was enhanced by 2‐pentylfuran (PR2, OSM2, and HSR genes) and 6‐pentyl‐2H‐pyran‐2‐one (PR2, OSM1, OSM2, CHIT3, and HSR genes) treatment, as previously found in T39 conidia‐treated grapevine plants (Banani et al., 2014; Perazzolli et al., 2011, 2012). Previous studies showed that VOCs produced by T34 and T. harzianum T78 enhanced the JA‐dependent defenses of A. thaliana and tomato against B. cinerea (Martínez‐Medina et al., 2017). The ability of Trichoderma VOCs to induce plant resistance is known to be related to the upregulation of defense‐related genes, such as PR‐1 in 6‐pentyl‐2H‐pyran‐2‐one‐treated Brassica napus (Vinale et al., 2008), the activation of defense‐related enzyme, such as chitinase and β‐1,3‐glucanase of lettuce treated with T. asperellum T1 VOCs (Wonglom et al., 2020), and the accumulation of defense molecules, such as JA and ROS in A. thaliana treated with T. virens Tv29.8 VOCs (Contreras‐Cornejo et al., 2014). In this study, 6‐pentyl‐2H‐pyran‐2‐one induced the expression of grapevine defense genes in mock‐inoculated leaf disks (PR2, OSM1, OSM2, CHIT3 and HSR genes), suggesting the partial activation of some defense processes also in the absence of the pathogen. This Trichoderma VOC is known to induce resistance against B. cinerea and Alternaria brassicicola in A. thaliana (Kottb et al., 2015), against Erysiphe necator in V. vinifera (Pascale et al., 2017), against B. cinerea in tomato seedlings and against L. maculans in canola seedlings (Vinale et al., 2008), suggesting a broad spectrum activity against phytopathogens.
Since chemical profiles and functional properties of microbial VOCs differed according to the growth media (González‐Pérez et al., 2018; Lazazzara et al., 2017), further studies under natural conditions are required, in order to better evaluate the possible migration of VOCs produced by Trichoderma spp. to grapevine tissues and the reduction of downy mildew severity by plant resistance induction. Effects against P. viticola can be tested only in the presence of host tissues, due to the obligate biotrophic lifestyle of this pathogen. Thus, possible direct inhibitory effects of Trichoderma VOCs against P. viticola can also occur on leaf tissues. It was previously reported that some VOCs can be absorbed by the leaf cuticle and can persist on the leaf surface (Himanen et al., 2010), exerting direct inhibitory effects against fungal pathogens (Camacho‐Coronel et al., 2020, Quintana‐Rodriguez et al., 2015). For example, farnesene can be absorbed by plant cuticular wax layers and persist on plant leaves and to inhibit Colletotrichum lindemuthianum (Camacho‐Coronel et al., 2020, Quintana‐Rodriguez et al., 2015). Moreover, 6‐pentyl‐2H‐pyran‐2‐one is a well‐known compound with antifungal activity against F. moniliforme (El‐Hasan et al., 2007), R. solani (Cruz‐Magalhães et al., 2019; Scarselletti & Faull, 1994) and Sclerotinia sclerotiorum (Cruz‐Magalhães et al., 2019) and it could potentially inhibit P. viticola, as well. The antifungal activity of 6‐pentyl‐2H‐pyran‐2‐one has been proposed to be associated to its hydrophobic nature and the possible mechanical impediment to water absorption by the fungal cells due to the formation of a hydrorepellent film on the cell wall (Scarselletti & Faull, 1994). The same mechanism can be hypothesized against P. viticola and it might partially explain the phytotoxic effects observed at high dosages on tomato or oilseed rape seedlings (Vinale et al., 2008) and on grapevine leaf disks, indicating the importance of dose optimization and mode of action investigation for this compound. Likewise, the antifungal effects of 2‐pentylfuran were previously reported against Monilinia fructicola (Liu et al., 2018), S. sclerotiorum, and F. oxysporum (Wu et al., 2015), but further studies are required to clarify the mode of action of this compound and the less efficient VOCs (i.e., 1,3‐octadiene, α‐farnesene, and cadinene) against downy mildew.
5. CONCLUSIONS
VOCs emission profiles differed in the three Trichoderma strains tested and T39 produced higher amounts of terpenes compared to T34 and SC1, indicating genetic determinants of VOC production at strain level. Five Trichoderma VOCs (α‐farnesene, cadinene, 1,3‐octadiene, 2‐pentylfuran, and 6‐pentyl‐2H‐pyran‐2‐one) reduced downy mildew severity on grapevine leaf disks. In particular, 6‐pentyl‐2H‐pyran‐2‐one and 2‐pentylfuran enhanced the accumulation of callose and reinforced the upregulation of defense‐related genes after P. viticola inoculation, indicating the induction of grapevine resistance. Moreover, 6‐pentyl‐2H‐pyran‐2‐one upregulated the expression of defense‐related genes in mock‐inoculated leaf disks and activated HR after P. viticola inoculation, as possible reinforcement of the grapevine defense mechanisms against this pathogen. Thus, airborne signals produced by beneficial soil‐borne Trichoderma spp. can be perceived by plant tissues as possible mediators of fungus‐plant communications and as inducers of plant resistance. Although further transcriptomic and functional studies are required to shed light on the mode of action of Trichoderma VOCs in the induction of grapevine defense mechanisms against downy mildew, Trichoderma VOCs could open new opportunities to develop biofungicides from natural origin.
AUTHOR CONTRIBUTIONS
Valentina Lazazzara performed the experiments, analyzed the data and wrote the manuscript. Bianca Vicelli contributed to leaf disk experiments. Christoph Bueschl wrote the R scripts and analyzed the data. Alexandra Parich contributed to chemical analysis. Ilaria Pertot supervised the experiments and revised the manuscript. Rainer Schuhmacher coordinated chemical analysis and helped to draft the manuscript. Michele Perazzolli conceived the study, coordinated the experiments and wrote the manuscript.
Supporting information
Figure S1 Overview of the experimental design.
Figure S2. Total ion current chromatograms of volatile organic compound (VOC) profiles.
Figure S3. Effects of pure volatile organic compounds (VOCs) on mock‐inoculated leaf disks.
Table S1. Primer sequences of grapevine genes analyzed by quantitative real‐time PCR (qPCR).
Table S2. Volatile organic compounds (VOCs) produced by Trichoderma spp. in the first experiment.
Table S3. Volatile organic compounds (VOCs) produced by Trichoderma spp. in the second experiment.
Table S4. Deconvoluted mass spectra of unknown diterpenes, unknown sesquiterpenes and unknown compounds.
ACKNOWLEDGMENTS
This project has received funding from the Autonomous Province of Trento. The authors thank Yigal Elad (The Volcani Center, Israel) for providing Trichoderma harzianum T39 and Oscar Giovannini for technical support with greenhouse experiments.
Lazazzara V, Vicelli B, Bueschl C, et al. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense‐related processes against downy mildew. Physiologia Plantarum. 2021;172:1950–1965. 10.1111/ppl.13406
Edited by: H. Saitoh
Funding information Provincia Autonoma di Trento
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supporting information of this article.
REFERENCES
- Adrian, M., Lucio, M., Roullier‐Gall, C., Héloir, M.‐C., Trouvelot, S., Daire, X. et al. (2017) Metabolic fingerprint of PS3‐induced resistance of grapevine leaves against Plasmopara viticola revealed differences in elicitor‐triggered defenses. Frontiers in Plant Science, 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani, H., Roatti, B., Ezzahi, B., Giovannini, O., Gessler, G., Pertot, I. et al. (2014) Characterization of resistance mechanisms activated by Trichoderma harzianum T39 and benzothiadiazole to downy mildew in different grapevine cultivars. Plant Pathology, 63, 334–343. [Google Scholar]
- Brilli, M., Asquini, E., Moser, M., Bianchedi, P.L., Perazzolli, M. & Si‐Ammour, A. (2018) A multi‐omics study of the grapevine‐downy mildew (Plasmopara viticola) pathosystem unveils a complex protein coding‐ and noncoding‐based arms race during infection. Scientific Reports, 8, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner, K., Zeilinger, S., Ciliento, R., Woo, S.L., Lorito, M., Kubicek, C.P. et al. (2005) Improvement of thefungal biocontrol agent Trichoderma atroviride to enhance both antagonism and induction of plant systemic disease resistance. Applied and Environmental Microbiology, 71, 3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, Y., Feng, J., Sun, M., Zhou, C. & Luo, C. (2016) Facile and efficient poly(ethylene terephthalate) fibers‐in‐tube for online solid‐phase microextraction towards polycyclic aromatic hydrocarbons. Analytical and Bioanalytical Chemistry, 408, 4871–4882. [DOI] [PubMed] [Google Scholar]
- Buonassisi, D., Colombo, M., Migliaro, D., Dolzani, C., Peressotti, E., Mizzotti, C. et al. (2017) Breeding for grapevine downy mildew resistance: a review of “omics” approaches. Euphytica, 213, 103. [Google Scholar]
- Camacho‐Coronel, X., Molina‐Torres, J. & Heil, M. (2020) Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational aesistance: a proof of concept. Frontiers in Plant Science, 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande, K., Falginella, L., Castellarin, S.D., Testolin, R. & Di Gaspero, G. (2011) Defence responses in Rpv3‐dependent resistance to grapevine downy mildew. Planta, 234, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Conrath, U., Beckers, G.J.M., Langenbach, C.J.G. & Jaskiewicz, M.R. (2015) Priming for enhanced defense. Annual Review of Phytopathology, 53, 97–119. [DOI] [PubMed] [Google Scholar]
- Contreras‐Cornejo, H.A., Macías‐Rodríguez, L., Herrera‐Estrella, A. & López‐Bucio, J. (2014) The 4‐phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant and Soil, 379, 261–274. [Google Scholar]
- Cotxarrera, L., Trillas‐Gay, M.I., Steinberg, C. & Alabouvette, C. (2002) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biology and Biochemistry, 34, 467–476. [Google Scholar]
- Crutcher, F.K., Parich, A., Schuhmacher, R., Mukherjee, P.K., Zeilinger, S. & Kenerley, C.M. (2013) A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens . Fungal Genetics and Biology, 56, 67–77. [DOI] [PubMed] [Google Scholar]
- Cruz‐Magalhães, V., Nieto‐Jacobo, M.F., van Zijll de Jong, E., Rostás, M., Padilla‐Arizmendi, F., Kandula, D. et al. (2019) The NADPH oxidases Nox1 and Nox2 differentially regulate volatile organic compounds, fungistatic activity, plant growth promotion and nutrient assimilation in Trichoderma atroviride . Frontiers in Microbiology, 9, 3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effmert, U., Kalderás, J., Warnke, R. & Piechulla, B. (2012) Volatile mediated interactions between bacteria and fungi in the soil. Journal of Chemical Ecology, 38, 665–703. [DOI] [PubMed] [Google Scholar]
- Elad, Y., Zimand, G., Chet, I. (1997) Novel isolate of Trichoderma harzianum, T‐39, fungicidal compositions containing said isolate and use against B. cinerea and S. sclerotiorum. Patent: EP 0466133 B1.
- El‐Hasan, A., Walker, F., Schöne, J. & Buchenauer, H. (2007) Antagonistic effect of 6‐pentyl‐alpha‐pyrone produced by Trichoderma harzianum toward Fusarium moniliforme . Journal of Plant Diseases and Protection, 114, 62–68. [Google Scholar]
- EPPO . (2001) European and mediterranean plant protection organization. Guidelines for the efficacy evaluation of fungicides: Plasmopara viticola . EPPO Bulletin, 31, 313–317. [Google Scholar]
- EPPO . (2014) European and mediterranean plant protection organization. Efficacy evaluation of plant ptoducts: phytotoxicity assessment. EPPO Bulletin, 44, 265–273. [Google Scholar]
- Estrada‐Rivera, M., Rebolledo‐Prudencio, O.G., Pérez‐Robles, D.A., Rocha‐Medina, M.C., González‐López, M.C. & Casas‐Flores, S. (2019) Trichoderma histone deacetylase HDA‐2 modulates multiple responses in Arabidopsis . Plant Physiology, 179, 1343–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel, H.H.M., Mahmoud, M.G., Asker, M.M.S. & Lotfy, S.N. (2015) Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC‐107 in solid state fermentation on sugarcane bagasse. Electronic Journal of Biotechnology, 18, 5–9. [Google Scholar]
- Garbeva, P. & Weisskopf, L. (2020) Airborne medicine: bacterial volatiles and their influence on plant health. The New Phytologist, 226, 32–43. [DOI] [PubMed] [Google Scholar]
- Gessler, C., Pertot, I. & Perazzolli, M. (2011) Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathologia Mediterranea, 50, 3–44. [Google Scholar]
- Gindro, K., Pezet, R. & Viret, O. (2003) Histological study of the responses of two Vitis vinifera cultivars (resistant and susceptible) to Plasmopara viticola infections. Plant Physiology and Biochemistry, 41, 846–853. [Google Scholar]
- González‐Pérez, E., Ortega‐Amaro, M.A., Salazar‐Badillo, F.B., Bautista, E., Douterlungne, D. & Jiménez‐Bremont, J.F. (2018) The Arabidopsis‐Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Scientific Reports, 8, 16427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y., Ghirardo, A., Weber, B., Schnitzler, J.‐P., Benz, J.P. & Rosenkranz, M. (2019) Trichoderma species differ in their volatile profiles and in antagonism toward ectomycorrhiza Laccaria bicolor . Frontiers in Microbiology, 10, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y., Jud, W., Ghirardo, A., Antritter, F., Benz, J.P., Schnitzler, J.‐P. et al. (2020) Sniffing fungi – phenotyping of volatile chemical diversity in Trichoderma species. The New Phytologist, 227, 244–259. [DOI] [PubMed] [Google Scholar]
- Hamiduzzaman, M.M., Jakab, G., Barnavon, L., Neuhaus, J.‐M. & Mauch‐Mani, B. (2005) β‐aminobutyric acid‐induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Molecular Plant‐Microbe Interactions, 18, 819–829. [DOI] [PubMed] [Google Scholar]
- Harman, G.E., Howell, C.R., Viterbo, A., Chet, I. & Lorito, M. (2004) Trichoderma species – opportunistic, avirulent plant symbionts. Nature Reviews. Microbiology, 2, 43–56. [DOI] [PubMed] [Google Scholar]
- Hiller, K., Hangebrauk, J., Jäger, C., Spura, J., Schreiber, K. & Schomburg, D. (2009) MetaboliteDetector: comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Analytical Chemistry, 81, 3429–3439. [DOI] [PubMed] [Google Scholar]
- Himanen, S.J., Blande, J.D., Klemola, T., Pulkkinen, J., Heijari, J. & Holopainen, J.K. (2010) Birch (Betula spp.) leaves adsorb and re‐release volatiles specific to neighbouring plants – a mechanism for associational herbivore resistance? The New Phytologist, 186, 722–732. [DOI] [PubMed] [Google Scholar]
- Hung, R., Lee, S. & Bennett, J.W. (2013) Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecology, 6, 19–26. [Google Scholar]
- Inglis, G.D. & Kawchuk, L.M. (2002) Comparative degradation of oomycete, ascomycete, and basidiomycete cell walls by mycoparasitic and biocontrol fungi. Canadian Journal of Microbiology, 48, 60–70. [DOI] [PubMed] [Google Scholar]
- Kamoun, S., Furzer, O., Jones, J.D.G., Judelson, H.S., Ali, G.S., Dalio, R.J.D. et al. (2015) The top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology, 16, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh, R.C., Deverall, B.J. & McLeod, S. (1980) Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Transactions of the British Mycological Society, 74, 329–333. [Google Scholar]
- Kluger, B., Zeilinger, S., Wiesenberger, G., Schöfbeck, D. & Schuhmacher, R. (2013) Detection and identification of fungal microbial volatile organic compounds by HS‐SPME‐GC–MS. In: Gupta, V.K., Tuohy, M.G., Ayyachamy, M., Turner, K.M. & O'Donovan, A. (Eds.) Laboratory protocols in fungal biology: current methods in fungal biology. New York: Springer, pp. 455–465. [Google Scholar]
- Kortekamp, A. & Zyprian, E. (2003) Characterization of Plasmopara‐resistance in grapevine using in vitro plants. Journal of Plant Physiology, 160, 1393–1400. [DOI] [PubMed] [Google Scholar]
- Kottb, M., Gigolashvili, T., Großkinsky, D.K. & Piechulla, B. (2015) Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Frontiers in Microbiology, 6, 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkis, S., Trotel‐Aziz, P., Rabenoelina, F., Schwarzenberg, A., Nguema‐Ona, E., Clément, C. et al. (2019) Strengthening grapevine resistance by Pseudomonas fluorescens PTA‐CT2 relies on distinct defense pathways in susceptible and partially resistant genotypes to downy mildew and gray mold diseases. Frontiers in Plant Science, 10, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzara, V., Perazzolli, M., Pertot, I., Biasioli, F., Puopolo, G. & Cappellin, L. (2017) Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiological Research, 201, 52–62. [DOI] [PubMed] [Google Scholar]
- Lazazzara, V., Bueschl, C., Parich, A., Pertot, I., Schuhmacher, R. & Perazzolli, M. (2018) Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Scientific Reports, 8, 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Yap, M., Behringer, G., Hung, R. & Bennett, J.W. (2016) Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biology and Biotechnology, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leylaie, S. & Zafari, D. (2018) Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Frontiers in Microbiology, 9, 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., Alfiky, A., Vaughan, M.M. & Kang, S. (2016) Stop and smell the fungi: fungal volatile metabolites are overlooked signals involved in fungal interaction with plants. Fungal Biology Reviews, 30, 134–144. [Google Scholar]
- Liu, C., Yin, X., Wang, Q., Peng, Y., Ma, Y., Liu, P. et al. (2018) Antagonistic activities of volatiles produced by two Bacillus strains against Monilinia fructicola in peach fruit. Journal of the Science of Food and Agriculture, 98, 5756–5763. [DOI] [PubMed] [Google Scholar]
- Longa, C.M.O., Pertot, I. & Tosi, S. (2008) Ecophysiological requirements and survival of a Trichoderma atroviride isolate with biocontrol potential. Journal of Basic Microbiology, 48, 269–277. [DOI] [PubMed] [Google Scholar]
- Lorito, M., Woo, S.L., Harman, G.E. & Monte, E. (2010) Translational research on Trichoderma: from 'omics to the field. Annual Review of Phytopathology, 48, 395–417. [DOI] [PubMed] [Google Scholar]
- Malacarne, G., Vrhovsek, U., Zulini, L., Cestaro, A., Stefanini, M., Mattivi, F. et al. (2011) Resistance to Plasmopara viticola in a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biology, 11, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca, M.G., McCormick, S.P., Cardoza, R.E., Alexander, N.J., Monte, E. & Gutiérrez, S. (2015) Production of trichodiene by Trichoderma harzianum alters the perception of this biocontrol strain by plants and antagonized fungi. Environmental Microbiology, 17, 2628–2646. [DOI] [PubMed] [Google Scholar]
- Martínez‐Medina, A., Flors, V., Heil, M., Mauch‐Mani, B., Pieterse, C.M.J., Pozo, M.J. et al. (2016) Recognizing plant defense priming. Trends in Plant Science, 21, 818–822. [DOI] [PubMed] [Google Scholar]
- Martínez‐Medina, A., Van Wees, S.C.M. & Pieterse, C.M.J. (2017) Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid‐dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum . Plant, Cell & Environment, 40, 2691–2705. [DOI] [PubMed] [Google Scholar]
- Mutawila, C., Vinale, F., Halleen, F., Lorito, M. & Mostert, L. (2016) Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathology, 65, 104–113. [Google Scholar]
- Nagaraju, A., Sudisha, J., Murthy, S.M. & S‐i, I. (2012) Seed priming with Trichoderma harzianum isolates enhances plant growth and induces resistance against Plasmopara halstedii, an incitant of sunflower downy mildew disease. Australasian Plant Pathology, 41, 609–620. [Google Scholar]
- Nieto‐Jacobo, M.F., Steyaert, J.M., Salazar‐Badillo, F.B., Nguyen, D.V., Rostás, M., Braithwaite, M. et al. (2017) Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Science, 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, M.C., Perazzolli, M., Matafora, V., Moretto, M., Bachi, A. & Pertot, I. (2012) Proteomic analysis of grapevine resistance induced by Trichoderma harzianum T39 reveals specific defence pathways activated against downy mildew. Journal of Experimental Botany, 63, 6237–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale, A., Vinale, F., Manganiello, G., Nigro, M., Lanzuise, S., Ruocco, M. et al. (2017) Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Protection, 92, 176–181. [Google Scholar]
- Pellegrini, A., Prodorutti, D. & Pertot, I. (2014) Use of bark mulch pre‐inoculated with Trichoderma atroviride to control Armillaria root rot. Crop Protection, 64, 104–109. [Google Scholar]
- Perazzolli, M., Dagostin, S., Ferrari, A., Elad, Y. & Pertot, I. (2008) Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biological Control, 47, 228–234. [Google Scholar]
- Perazzolli, M., Roatti, B., Bozza, E. & Pertot, I. (2011) Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biological Control, 58, 74–82. [Google Scholar]
- Perazzolli, M., Moretto, M., Fontana, P., Ferrarini, A., Velasco, R., Moser, C. et al. (2012) Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genomics, 13, 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertot, I., Longa, C.M., Prodorutti, D., Michelon, L., Savazzini, F. (2008) Trichoderma atroviride SC1 for biocontrol of fungal disease in plants . Patent: WO/2009/116106.
- Pertot, I., Prodorutti, D., Colombini, A. & Pasini, L. (2016) Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl, 61, 257–267. [Google Scholar]
- Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real‐time RT–PCR. Nucleic Acids Research, 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesani, M., Bortesi, L., Ferrarini, A., Zamboni, A., Fasoli, M., Zadra, C. et al. (2010) General and species‐specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. riparia) grapevine species. BMC Genomics, 11, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana‐Rodriguez, E., Morales‐Vargas, A.T., Molina‐Torres, J., Ádame‐Alvarez, R.M., Acosta‐Gallegos, J.A. & Heil, M. (2015) Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum . Journal of Ecology, 103, 250–260. [Google Scholar]
- Roetschi, A., Si‐Ammour, A., Belbahri, L., Mauch, F. & Mauch‐Mani, B. (2001) Characterization of an Arabidopsis–Phytophthora pathosystem: resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. The Plant Journal, 28, 293–305. [DOI] [PubMed] [Google Scholar]
- Ruijter, J.M., Ramakers, C., Hoogaars, W.M.H., Karlen, Y., Bakker, O., van den Hoff, M.J.B. et al. (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research, 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarselletti, R. & Faull, J.L. (1994) In vitro activity of 6‐pentyl‐α‐pyrone, a metabolite of Trichoderma harzianum, in the inhibition of Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici . Mycological Research, 98, 1207–1209. [Google Scholar]
- Schmidt, R., Cordovez, V., de Boer, W., Raaijmakers, J. & Garbeva, P. (2015) Volatile affairs in microbial interactions. The ISME Journal, 9, 2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra, G., Casanova, E., Bellido, D., Odena, M.A., Oliveira, E. & Trillas, I. (2007) Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics, 7, 3943–3952. [DOI] [PubMed] [Google Scholar]
- Segarra, G., Van der Ent, S., Trillas, I. & Pieterse, C.M.J. (2009) MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biology, 11, 90–96. [DOI] [PubMed] [Google Scholar]
- Segarra, G., Avilés, M., Casanova, E., Borrero, C. & Trillas, I. (2013) Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathologia Mediterranea, 52, 77–83. [Google Scholar]
- Spring, O., Gomez‐Zeledon, J., Hadziabdic, D., Trigiano, R.N., Thines, M. & Lebeda, A. (2018) Biological characteristics and assessment of virulence diversity in pathosystems of economically important biotrophic oomycetes. Critical Reviews in Plant Sciences, 37, 439–495. [Google Scholar]
- Sridharan, A.P., Thankappan, S., Karthikeyan, G. & Uthandi, S. (2020) Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina . Microbiology Research, 236, 126436. [DOI] [PubMed] [Google Scholar]
- Stoppacher, N., Kluger, B., Zeilinger, S., Krska, R. & Schuhmacher, R. (2010) Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS‐SPME‐GC‐MS. Journal of Microbiological Methods, 81, 187–193. [DOI] [PubMed] [Google Scholar]
- Strobel, G.A., Dirkse, E., Sears, J. & Markworth, C. (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology, 147, 2943–2950. [DOI] [PubMed] [Google Scholar]
- Trouvelot, S., Varnier, A.‐L., Allègre, M., Mercier, L., Baillieul, F., Arnould, C. et al. (2008) A β‐1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR‐like cell death. Molecular Plant‐Microbe Interactions, 21, 232–243. [DOI] [PubMed] [Google Scholar]
- Vinale, F., Sivasithamparam, K., Ghisalberti, E.L., Marra, R., Barbetti, M.J., Li, H. et al. (2008) A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiological and Molecular Plant Pathology, 72, 80–86. [Google Scholar]
- Vrhovsek, U., Malacarne, G., Masuero, D., Zulini, L., Guella, G., Stefanini, M. et al. (2012) Profiling and accurate quantification of trans‐resveratrol, trans‐piceid, trans‐pterostilbene and 11 viniferins induced by Plasmopara viticola in partially resistant grapevine leaves. Australian Journal of Grape and Wine Research, 18, 11–19. [Google Scholar]
- Weingart, G., Kluger, B., Forneck, A., Krska, R. & Schuhmacher, R. (2012) Establishment and application of a metabolomics workflow for identification and profiling of volatiles from leaves of Vitis vinifera by HS‐SPME‐GC‐MS. Phytochemical Analysis, 23, 345–358. [DOI] [PubMed] [Google Scholar]
- Werner, S., Polle, A. & Brinkmann, N. (2016) Belowground communication: impacts of volatile organic compounds (VOCs) from soil fungi on other soil‐inhabiting organisms. Applied Microbiology and Biotechnology, 100, 8651–8665. [DOI] [PubMed] [Google Scholar]
- Wheatley, R., Hackett, C., Bruce, A. & Kundzewicz, A. (1997) Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. International Biodeterioration & Biodegradation, 39, 199–205. [Google Scholar]
- Wonglom, P., S‐i, I. & Sunpapao, A. (2020) Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecology, 43, 100867. [Google Scholar]
- Wu, Y., Yuan, J., Yaoyao, E., Raza, W., Shen, Q. & Huang, Q. (2015) Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. Journal of Basic Microbiology, 55, 1104–1117. [DOI] [PubMed] [Google Scholar]
- Zanotto, A. & Morroni, M. (2016) Major biocontrol studies and measures against fungal and oomycete pathogens of grapevine. In: Compant, S. & Mathieu, F. (Eds.) Biocontrol of major grapevine diseases: leading research. Oxfordshire: CAB International, pp. 1–34. [Google Scholar]
- Zhang, F., Yang, X., Ran, W. & Shen, Q. (2014) Fusarium oxysporum induces the production of proteins and volatile organic compounds by Trichoderma harzianum T‐E5. FEMS Microbiology Letters, 359, 116–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Overview of the experimental design.
Figure S2. Total ion current chromatograms of volatile organic compound (VOC) profiles.
Figure S3. Effects of pure volatile organic compounds (VOCs) on mock‐inoculated leaf disks.
Table S1. Primer sequences of grapevine genes analyzed by quantitative real‐time PCR (qPCR).
Table S2. Volatile organic compounds (VOCs) produced by Trichoderma spp. in the first experiment.
Table S3. Volatile organic compounds (VOCs) produced by Trichoderma spp. in the second experiment.
Table S4. Deconvoluted mass spectra of unknown diterpenes, unknown sesquiterpenes and unknown compounds.
Data Availability Statement
The data that supports the findings of this study are available in the supporting information of this article.


