Abstract
Secondary adrenal insufficiency (AI) occurs as the result of any process that disrupts normal hypothalamic and/or anterior pituitary function and causes a decrease in the secretion of steroid hormones from the adrenal cortex. The most common cause of secondary AI is exogenous corticosteroid therapy administered at supraphysiologic dosages for ≥ 1 month. AI caused by oral corticosteroids (OCS) is not well‐recognized or commonly diagnosed but is often associated with reduced well‐being and can be life‐threatening in the event of an adrenal crisis. Corticosteroid use is common in respiratory diseases, and asthma is a representative condition that illustrates the potential challenges and opportunities related to corticosteroid‐sparing therapies. For individuals with severe asthma (approximately 5%–10% of all cases), reduction or elimination of maintenance OCS without loss of control can now be accomplished with biologic therapies targeting inflammatory mediators. However, the optimal strategy to ensure early identification and treatment of AI and safe OCS withdrawal in routine clinical practice remains to be defined. Many studies with biologics have involved short evaluation periods and small sample sizes; in addition, cautious approaches to OCS tapering in studies with a placebo arm, coupled with inconsistent monitoring for AI, have contributed to the lack of clarity. If the goal is to greatly reduce and, where possible, eliminate long‐term OCS use in severe asthma through the increasing adoption of biologic treatments, there is an urgent need for clinical trials that address both the speed of OCS withdrawal and how to monitor for AI.
Keywords: adrenal insufficiency, asthma, endocrinology, glucocorticoids, respiratory medicine
Introduction
The advent of modern disease‐modifying biologic therapies for the treatment of a diverse array of medical conditions, including rheumatoid arthritis, inflammatory bowel disease and asthma, offers the prospect of significantly reducing the need for long‐term systemic corticosteroid therapy and its attendant adverse effects (AEs). In the field of asthma, treatments targeting inflammatory mediators such as immunoglobulin E (IgE) (omalizumab), interleukin (IL)‐4 and IL‐13 (dupilumab), and IL‐5 (mepolizumab, reslizumab) or its receptor (benralizumab) have proved particularly effective in delivering disease control for severe asthma [1, 2, 3]. Thus, although corticosteroids retain an important role in the management of acute exacerbations, the long‐held belief that a subgroup of patients with the most severe disease will inevitably require maintenance therapy with oral corticosteroids (OCS) is being increasingly questioned.
Important challenges remain, however, since the move away from long‐term OCS therapy needs to be delivered effectively and safely. For example, many patients who might benefit from biologic therapies remain unknown to specialist asthma services and continue to receive treatment with OCS at dosages that put them at significant risk of adverse sequelae (e.g. hyperglycaemia/diabetes mellitus, hypertension, dyslipidaemia, premature cardiovascular disease [CVD], osteopenia/osteoporosis and cataracts) [4, 5]. Equally important, for those successfully treated with OCS‐sparing biologics, there remains the issue of how best to safely wean off long‐term corticosteroid therapy. Secondary adrenal insufficiency (AI) following exposure to supraphysiologic exogenous corticosteroids is common, yet under‐recognized [6]. Too rapid weaning and/or inappropriate cessation of physiologic equivalent dosages in those with hypothalamic–pituitary–adrenal (HPA) axis suppression is often associated with impaired well‐being and may be life‐threatening in the event of an adrenal crisis [7].
Here, we consider why AI should be an important consideration for any asthma patient transitioning from long‐term OCS therapy in the era of biologics and we examine the available evidence to guide safe OCS withdrawal.
Normal HPA axis function
The adrenal cortex secretes mineralocorticoids (e.g. aldosterone), glucocorticoids (e.g. cortisol) and sex steroids (e.g. dehydroepiandrosterone) from 3 distinct layers: outer zona glomerulosa (ZG), middle zona fasciculata (ZF) and inner zona reticularis (ZR), respectively (Fig. 1a). Mineralocorticoid production and release are governed by the renin–angiotensin(–aldosterone) system (RAS/RAAS) (Fig. 1b); in contrast, glucocorticoid and adrenal sex steroid secretion are under the control of hypothalamic corticotropin‐releasing hormone (CRH) and pituitary adrenocorticotropic hormone (ACTH) (Fig. 1c). ACTH activation of the melanocortin 2 receptor (MC2R) – a G protein‐coupled receptor – on cells of the ZF, stimulates production and release of several glucocorticoids including cortisol, the primary ligand for the glucocorticoid receptor (GR), which is a member of the steroid nuclear receptor superfamily. ACTH is a key determinant of adrenal gland growth and maturation, and cortisol is required to maintain normal blood pressure and blood glucose concentrations, along with other aspects of homeostasis [8]. In addition to mediating pleiotropic effects in multiple target tissues, cortisol, operating through a classical negative feedback loop, inhibits CRH and ACTH release (Fig. 1c) [8, 9, 10, 11, 12]. Consistent with this, exogenous corticosteroids can suppress HPA axis function and, if sustained, the resultant lack of endogenous ACTH leads to adrenocortical hypoplasia or atrophy [8, 9, 10, 19].
Fig. 1.
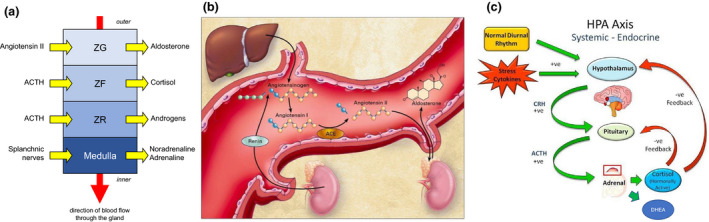
Schematic representation of the key pathways regulating secretion of cortisol and aldosterone. (a) Adrenal structure/function correlations: the zona glomerulosa (ZG), zona fasciculata (ZF) and zona reticularis (ZF) secrete aldosterone, cortisol and adrenal androgens respectively, whilst the innermost medulla is the source of catecholamines. © Jolly E, Fry A, Chaudhry A, eds. Training in Medicine (Oxford Specialty Training: Training In). 1st ed. 2016. Oxford University Press. Reproduced with permission of the Licensor through PLSclear. (b) Aldosterone synthesis and release are governed principally by the renin–angiotensin system (RAS). From New England Journal of Medicine, Weber KT. N Engl J Med. 2001;345:1689–1697. © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (c) In contrast, cortisol and adrenal sex steroids are under the control of the hypothalamus and pituitary gland, which together comprise the hypothalamic–pituitary–adrenal (HPA) axis. © CC BY NC ND. Hardy RS et al. Swiss Med Wkly. 2012;142:w13650. https://smw.ch/about‐us#c335.
Adrenal insufficiency
Epidemiology and aetiology
AI occurs when there is failure of normal adrenocortical function. Primary AI (often referred to by the eponym Addison’s disease) is characterized by glucocorticoid, mineralocorticoid and adrenal sex steroid insufficiency and occurs relatively infrequently, with an estimated prevalence of 82 to 144 per million people [7, 20]. Autoimmune disease is the most frequent cause of primary AI, but tuberculosis, fungal infections and human immunodeficiency virus can also lead to AI and are more commonly seen in developing countries [21]. Other causes are shown in Table 1. Secondary AI, in which there is glucocorticoid insufficiency but the preservation of mineralocorticoid production, occurs more frequently than primary AI, with an estimated prevalence of 150 to 280 per million people [20]. Secondary AI may complicate any process (i.e. disease, injury or drug) that disrupts normal hypothalamic and/or anterior pituitary function to cause a decrease in the secretion of corticosteroid hormones from the adrenal cortex (Table 1) [17].
Table 1.
Causes of adrenal insufficiency (AI)
| Primary AI |
|
| Secondary AI |
|
AI, adrenal insufficiency; CMV, cytomegalovirus; HIV, human immunodeficiency virus; MC2R, melanocortin 2 receptor; TB, tuberculosis.
Adrenal metastases are relatively common with certain primary tumours (e.g. renal, bronchogenic, breast), but clinically relevant AI is only rarely observed.
Drugs that interfere with the activity of CYP3A4 can exert profound effects for those receiving exogenous glucocorticoid therapy. For example, treatment with drugs that induce CYP3A4 leading to increased glucocorticoid metabolism can predispose to hypoadrenalism for patients receiving fixed dosages of exogenous glucocorticoids (and in whom the HPA axis is suppressed), whereas in those patients receiving treatment with CYP3A4 inhibitors, iatrogenic Cushing syndrome can develop because of impaired metabolism of even lower dosages of exogenous glucocorticoids, which leads to hypoadrenalism if the CYP3A4 inhibitor is discontinued and the remaining exogenous glucocorticoids are insufficient to compensate for HPA axis suppression.
Some cancer immunotherapy agents may predispose to the development of autoimmune adrenalitis.
The diagnosis of primary adrenal insufficiency in a young man mandates consideration of a rare but important cause – adrenoleukodystrophy (ALD) – especially when there are features to suggest associated neurologic dysfunction. ALD is an X‐linked disorder caused by pathogenic variants in the ABCD1 gene (ATP‐binding cassette subfamily D member 1), which encodes an ABC transporter. This is critical for the uptake of very‐long‐chain fatty acids into the peroxisome, with subsequent β‐oxidation and breakdown. Accumulation of very‐long‐chain fatty acids in the central nervous system, adrenal cortex and the Leydig cells of the testes leads to the well‐recognized clinical features of ALD.
Exogenous corticosteroid therapy is a common, but poorly recognized, cause of adrenal insufficiency.
The drug class most often associated with secondary AI is exogenous corticosteroids when used at supraphysiologic dosages for 3 to 4 weeks or longer [6, 9, 15, 17, 20, 22]. Surprisingly, however, AI caused by OCS is still not well‐recognized or commonly diagnosed, despite being potentially one of the most dangerous AEs of OCS therapy and despite the evidence confirming that AI is common [6, 15]. The risk of inducing AI with other routes of administration (e.g. higher dosages of inhaled corticosteroids) should also not be discounted [23, 24, 25]; indeed, topical, inhaled, intranasal, oral and injectable forms all pose a risk of AI [9, 17, 20, 22]. Moreover, many patients are taking exogenous corticosteroids through more than one route (e.g. intranasal and/or topical preparations for co‐existent conditions such as nasal polyps or eczema, respectively) and the effects may be additive in terms of suppressing HPA axis function. Overall, the potential to induce AI as a consequence of exogenous corticosteroid use remains underappreciated by many clinicians [10, 13, 14].
Risk of adrenal insufficiency with exogenous corticosteroid usage
Suppression of the HPA axis may occur even with physiologic dose equivalents of exogenous corticosteroids, but is more commonly observed following treatment with supraphysiologic dosages [12, 13]. The higher the dosage and the longer the duration of treatment, the greater the risk of developing AI; however, systematic literature reviews have not confirmed a clear relationship between cumulative dosage or duration of corticosteroid therapy and AI [6, 15].
The risk of developing AI also has been linked with specific aspects of the treatment regimen; for example, an increased propensity to develop AI was observed for patients receiving split daily dosing, night‐time dosing and total daily dosages that exceed physiologic equivalents (e.g. ≥5 mg prednisone/prednisolone) and with the use of long‐acting (e.g. dexamethasone) and/or systemic corticosteroid preparations; in contrast, lower risk was associated with the use of low maintenance dosages (i.e. not exceeding physiologic equivalents), single doses, morning only and/or alternate‐day dosing, short‐acting corticosteroids (e.g. hydrocortisone) and topical application [26].
The risk of AI is also related to the pharmacokinetics/pharmacodynamics of the OCS used (e.g. bioavailability, protein‐binding affinities, plasma half‐life and metabolic inactivation and clearance) [13]. Repeat courses of OCS are also a risk factor for AI [18], and patients with a predisposition to HPA axis dysfunction as a consequence of other underlying conditions or medications may be particularly susceptible to AI caused by OCS [27]. Still, the risk of AI can be reduced, though not fully negated, by using the lowest effective dosage of OCS for the shortest amount of time [16].
Although the absolute risk of developing AI following a short course of OCS is often assumed to be low, clinically relevant HPA axis suppression may be substantially under‐recognized and under‐reported in clinical practice [9, 12, 19]. In a study by Schuetz and colleagues, a 14‐day course of systemic corticosteroids (40 mg methylprednisolone injection once followed by 13 days of 40 mg oral prednisone) led to HPA axis suppression (as gauged by response to the 1 µg corticotropin [synthetic ACTH1‐24] stimulation test) in 7 of 9 patients on the first day following commencement of corticosteroid treatment; this increased to 8 of 9 by the last day of therapy. Moreover, 21 days after discontinuation of treatment, 3 patients still had evidence of adrenal dysfunction. Although all 9 patients had an underlying diagnosis of chronic obstructive pulmonary disease and 5 were on inhaled corticosteroids at the time of admission, none had received systemic corticosteroid therapy in the preceding 6 months [12].
Perhaps even more compelling are the findings of Henzen and colleagues, who examined suppression and recovery of adrenal response (using 1 µg corticotropin) in 75 hospitalized patients who were receiving glucocorticoid treatment at an equivalent of at least 25 mg prednisone daily for the first time in their lives [28]. Overall, 41 (55%) patients were classified as having a normal adrenal response; the other 34 (45%) patients had stimulated plasma cortisol levels below the predefined cut‐off when tested between 24 h and 72 h after stopping glucocorticoid therapy. Those with a normal response had similar durations of treatment (median 8 days [range: 5–30]) as those with suppressed responses (median 10 days [range 5–30]), as well as similar median daily and cumulative dosages [28]. Although the majority of patients showed a full recovery within 2 weeks, 2 patients continued to demonstrate suboptimal responses and required longer‐term follow‐up.
Individual variability in corticosteroid action and metabolism (especially CYP3A4 activity) also influence the propensity to develop AI [9]. In a recent systematic review examining systemic glucocorticoid therapy and AI in adults, the median percentage of patients with AI was 37.4% (interquartile range: 13%–63%) [15], which could translate to a substantial number of patients at risk of AI since an estimated 2.5% of adults are receiving prescribed corticosteroids for immune‐related or inflammatory conditions at any given time [20, 21, 29]. In 1 year, 10 million new prescriptions for systemic corticosteroids were filled in the United States [20], whereas 8 million were filled in the United Kingdom [15].
HPA axis recovery following exogenous corticosteroid exposure
Recovery of HPA axis function is unpredictable [30], and there are little data that accurately describe an expected time course for the recovery of adrenal function [15, 31]. Following discontinuation of exogenous corticosteroids, HPA axis suppression may resolve quickly (within hours or days) or persist, in some cases for up to 1 year or longer [13]. Moreover, in some patients, endogenous cortisol secretion remains permanently suppressed [14]. Although even a single dose of corticosteroid can cause HPA axis suppression (this is the basis for the overnight dexamethasone suppression test used to screen for Cushing syndrome), recovery is usually rapid with no adverse clinical sequelae.
Longer courses (e.g. 4 weeks or more) have been linked with longer recovery times, [10] and, as a general rule, slow tapering after long‐term OCS usage is almost always necessary to support the recovery of normal HPA axis function [30]. In this context, complete HPA axis recovery is unlikely to be observed until at least 4 weeks after cessation of corticosteroids [10]. In a systematic review of systemic glucocorticoid therapy and AI in adults, 75% of patients had persistent AI 10 weeks after discontinuation of OCS (≥4 weeks of use), whereas 15% still had AI 3 years beyond discontinuation. Glucocorticoid exposure varied significantly across studies, but no obvious correlations were noted when AI cases were stratified by dosage or duration of corticosteroid use. Across all groups, 14% of patients who received a medium cumulative dosage (0.5–5.0 g) and 50% of those who received a high cumulative dosage (>5.0 g) developed AI [15].
Currently, there is little consensus as to the optimum approach for safely tapering corticosteroids [13, 16, 21, 22, 32, 33]. The decision of when and how to taper corticosteroids, and when and how to monitor for AI, is usually at the clinician’s discretion and based on signs and symptoms of disease control [32]. The fear of underlying AI may actually hinder a clinician’s confidence in tapering OCS [30]. Ideally, tapering should be individualized, with continuous monitoring of the disease and AEs [32].
Presentation of adrenal insufficiency
The clinical presentation of AI can vary widely, but often includes several nonspecific features [12, 16, 17, 19, 22, 34]. Signs and symptoms of AI include weakness, fatigue, malaise, gastrointestinal upset (nausea, vomiting, diarrhoea, abdominal pain), anorexia/weight loss, headache, fever, myalgia, arthralgia, psychiatric symptoms and poor linear growth and/or poor weight gain in children [16, 17, 21]. The mnemonic ‘HIGH STAKES’ can be used as an aide‐memoire for the signs, symptoms and other important clinical features of AI (Fig. 2).
Fig. 2.

Clinical features of adrenal insufficiency.
For patients with AI secondary to exogenous glucocorticoid exposure, symptoms often develop after complete (or near‐complete) corticosteroid withdrawal when the removal of exogenous glucocorticoid unmasks the lack of endogenous cortisol production [11, 35]. Abrupt discontinuation of OCS can cause AI to appear suddenly, especially when HPA suppression is marked. However, in those patients with partial suppression, clinical features of AI may be mild or even absent, only manifesting at times of acute stress [36]. If such an event occurs weeks or months after OCS withdrawal, the connection between HPA axis suppression and OCS use may not be made [12, 14, 17, 20, 37].
Importantly, therefore, even in patients with AI who appear to be relatively asymptomatic (as observed in some cases of secondary AI where there is preservation of mineralocorticoid production), rapid decompensation in response to an acute intercurrent stress (e.g. infection, trauma, other acute critical illness) can result in a life‐threatening adrenal crisis [8, 11, 12, 17, 19]. Even when this occurs, the nonspecific nature of many of the clinical features may mean that a diagnosis of hypoadrenalism is not made in a timely manner [8, 12, 14, 16, 19, 22]. Laboratory clues to the underlying diagnosis include hyponatraemia, hyperkalaemia (only in primary AI), hypoglycaemia, hypercalcaemia, neutropenia, eosinophilia, lymphocytosis and anaemia [38]. Vasopressor/inotrope‐resistant shock may be another important indicator of the presence of hypoadrenalism [12, 22]. The death rate for patients who experience unrecognized/inadequately treated adrenal crisis may reach approximately 6%; in contrast, with timely intervention symptoms usually resolve within 1 to 2 hours after parenteral corticosteroid administration [38].
Amongst patients with AI, intercurrent infection/sepsis is the most common factor precipitating the development of adrenal crisis. Recent surgery and advanced age are also recognized risk factors [39], and certain pre‐existing comorbidities have been noted frequently amongst patients with adrenal crisis, such as CVD, cancer, diabetes mellitus and hypothyroidism [39, 40]. Up to 6% to 8% of patients with AI have an adrenal crisis incident every year [15, 38]. Owing to the nonspecific presentation of AI, adrenal crisis may be the first presentation of hypoadrenalism [22].
Longer‐term consequences of AI include increased mortality due to CVD, infections, cancer and adrenal crisis [41, 42]; reduced bone mineral density and increased prevalence of osteopenia [43]; and poor health‐related quality of life [44].
Assessment of HPA axis function
Given the apparent lack of symptoms in some patients with AI and the nonspecific clinical features in others, a low threshold for screening for AI is required, especially in those with predisposing factors (including exogenous corticosteroid exposure) [12, 17, 19, 45]. This is supported by the findings of a systematic review and meta‐analysis in which 10 of 521 (1.9%) patients reported symptoms consistent with AI, but after formal endocrine testing, 98 (18.8%) patients met laboratory diagnostic thresholds for AI [6].
In general, most endocrinologists recommend that screening for AI is not indicated until a patient has been weaned to a physiologic dosage of corticosteroids, since, at higher dosages, the HPA axis remains suppressed and testing may not accurately reflect HPA axis reserve [26]. Several tests are available to assess the HPA axis, each with its merits and limitations.
9 Am serum cortisol
Although untimed random cortisol measurements are generally discouraged when assessing the HPA axis (reflecting the pulsatile and diurnal nature of cortisol secretion), serum cortisol measurements at 9 am may exclude or confirm the presence of hypoadrenalism [11, 12]. For example, a 9 am serum cortisol level < 80 nmol/L indicates AI [30], although in routine clinical practice values < 100 nmol/L are treated as indicative of complete AI until further testing can confirm or refute the diagnosis. In contrast, and in the absence of confounding factors (as noted below), a 9 am serum cortisol > 400 nmol/L (with many centres using even lower thresholds [e.g. >350 nmol/L]) effectively excludes hypocortisolism. Values falling between these thresholds are generally considered as indeterminate and may be resolved by repeat testing or progressing to dynamic testing. Of note, in a seriously ill patient for whom hypoadrenalism is being considered, a random sample should be collected (a low value in this context is virtually diagnostic of AI) and hydrocortisone commenced without delay, followed by urgent referral to endocrinology.
That most clinical laboratories measure total serum cortisol is important; elevated circulating levels of cortisol‐binding globulin (CBG), as observed in pregnancy or in women receiving oral oestrogen therapy, may result in apparently higher cortisol concentrations being reported, which in turn can mask true hypoadrenalism [46]. Similar falsely reassuring results may also be seen after synthetic ACTH stimulation [47]. Accordingly, a discussion with a specialist endocrine team is advisable if the assessment of adrenal reserve is being considered in this context.
Corticotropin (synthetic ACTH1‐24; tetracosactide; cosyntropin) stimulation test
Various forms of the corticotropin (short Synacthen®, Cortrosyn®) stimulation test exist. The short ACTH stimulation test is the most widely used to evaluate the integrity of the HPA axis [9, 10, 11, 48]. This test assesses adrenal cortical reserve during chronic ACTH deficiency [10] by directly stimulating the adrenal glands and infers the integrity of the HPA axis based on peak response after ACTH injection. A single dose (250 μg) of synthetic ACTH is administered; blood samples are drawn immediately before the test and at 30 (±60) minutes after ACTH injection to assess the rise in serum cortisol [10, 11, 48]. Without normal ACTH production, the adrenal glands atrophy and eventually demonstrate an attenuated or absent response even to direct stimulation by supraphysiologic ACTH. The value of the test can be further enhanced by performing it at 9 am, thus allowing the interpretation of both the unstimulated and peak cortisol responses.
Importantly, the short ACTH stimulation test should not be used in the acute phase of suspected secondary AI (e.g. within 6 weeks of pituitary surgery) owing to the risk of a false‐negative (i.e. inappropriately reassuring) result [10], particularly for a patient with secondary hypoadrenalism if the adrenal glands have not yet atrophied. If testing is carried out in this context, then the finding of a low baseline concentration of cortisol, but with apparent preserved peak cortisol response to synthetic ACTH, should raise concerns of the possible early phase of secondary AI. In recognition of this, the low‐dose (1 µg) short ACTH stimulation test has been proposed as a more sensitive test for the detection of secondary AI [10, 49], although the lack of a suitable preparation to allow accurate dosing has led some to call into question the reliability of such a test. The long (depot) ACTH stimulation test is now rarely used.
Other tests of HPA function
In certain cases, an insulin tolerance test or a glucagon stimulation test may also be used to assess HPA and adrenal function. However, insulin‐induced hypoglycaemia should only be performed in a specialist endocrine unit and is contraindicated in patients with known or suspected seizures, arrhythmias or ischaemic heart disease. Other specialist tests, including glucagon stimulation, are also best performed in conjunction with endocrinology [50]. Collection of plasma for ACTH measurement (to help distinguish primary [raised ACTH and low cortisol] from secondary [low/inappropriately normal ACTH and low cortisol] AI) requires specific sample handling and should be discussed with the endocrine laboratory.
Treatment of secondary adrenal insufficiency
If glucocorticoid‐induced AI is diagnosed, treatment should be initiated as soon as possible. In an acutely unwell patient, urgent rehydration and hydrocortisone administration are required [51]. For chronic AI, glucocorticoid replacement is indicated and should be administered in such a way as to replicate circadian cortisol production as much as possible [51]. Typically, hydrocortisone or prednisolone is used [52, 53]. Since the recovery of HPA axis function is possible, patients with AI consequent on exogenous glucocorticoid use should not automatically be assumed to require lifelong, chronic corticosteroid replacement; weaning/tapering may be feasible. In addition, in some patients, full corticosteroid replacement may only be necessary in emergencies or in situations of stress or illness [51, 54]. Patient and caregiver education is therefore vital in cases of AI; patients must understand their condition and how their needs for glucocorticoid replacement change because of stress, illness or trauma [51]. A key element of the safe management of AI is ensuring that the patient, their relatives and healthcare professionals are alert to the possibility of AI and the need to rapidly intervene to prevent deterioration. Accordingly, the use of ‘steroid cards’, emergency bracelets/necklaces and the provision of ‘steroid rescue packs’ containing additional oral and parenteral glucocorticoid are strongly recommended [7].
Asthma, oral corticosteroids and adrenal insufficiency
As stated, secondary AI can occur in many disease states and as a result of taking several different drugs. The use of corticosteroids, the most common drug‐related cause of AI, is often traceable to respiratory diseases, and asthma is a representative condition that can be used in determining OCS use and the potential challenges and opportunities related to corticosteroid‐sparing therapies. Asthma is a complex, multifactorial disease of airway inflammation that typically includes symptoms of wheezing, shortness of breath, chest tightness and cough; the airway inflammation is usually chronic, leading to expiratory airflow limitations [55].
Asthma is estimated to affect more than 350 million people around the world [56]. Severe asthma only affects a relatively small percentage of patients (approximately 5%–10%), but these individuals are more likely to develop asthma‐related symptoms and an increased risk of acute exacerbations than those with less severe forms of the disease [57, 58, 59, 60, 61]. The management of severe asthma is difficult, and many patients require treatment with high‐dosage inhaled corticosteroids plus a second controller, with or without systemic corticosteroids (i.e. OCS), to maintain disease control [62]. In addition to the use as maintenance control therapy, OCS are also used for the treatment of acute exacerbations [5, 16, 55]. As many as 30% to 45% of patients regularly use systemic corticosteroids to control asthma symptoms [58, 59, 60, 61, 63, 64]. However, corticosteroids only suppress type 2 (T2) inflammation, driven by IL‐4, IL‐5 and IL‐13, and primarily treat symptoms owing to this type of inflammation. In the absence of T2 inflammation, corticosteroids offer less improvement in symptoms or disease control whilst putting patients at risk of AEs [65].
Although OCS may offer asthma control for some patients, frequent or continuous OCS use is associated with systemic AEs and poor quality of life [55, 58, 59, 66]. Additionally, long‐term corticosteroid use in asthma is associated with increased healthcare resource utilization for related AEs, including physician visits, specialist visits, hospitalizations, emergency department visits and prescriptions [67, 68]. Patients with asthma who are exposed to corticosteroids have greater healthcare costs than those without asthma, and those with larger cumulative corticosteroid exposures have greater costs than those exposed to lower cumulative dosages. Overall, asthma confers substantial personal and societal costs and causes an economic burden of billions of dollars worldwide per annum [67, 69, 70, 71].
Prevalence of adrenal insufficiency in asthma
The true prevalence of AI in asthma is difficult to determine, in part, because of limited studies, as well as a shortage of reliable interstudy comparisons [13, 34]. In one meta‐analysis, 48.7% of patients were diagnosed with AI after OCS use. The population included patients who were using OCS for multiple immune‐related and inflammatory conditions, including respiratory, dermatologic and rheumatologic disorders; most patients had been taking OCS for between 1 month and 1 year, and most were taking a ‘medium dose’ of corticosteroids, designated as the range between the lower and upper limits of the recommended dosages for each corticosteroid [6]. When patients with asthma were examined separately, the same analysis observed that 11.1% of patients using corticosteroids had AI. Only 6.8% of patients taking solely inhaled corticosteroids had AI, but 43.7% of those individuals using OCS had AI. The risk of AI was greater with longer durations of OCS use for asthmatic patients (1.4% with short term [<1 month] use, 11.9% during the medium term [1 month–1 year] and 27.4% for the long term [≥1 year]) [6].
Unfortunately, there is no established dosage or duration of OCS that is determined to control asthma symptoms without causing AEs [60]. Experts are calling for ‘OCS stewardship’ to reduce the risks, including AI, related to OCS use [33]. To mitigate the potential for AEs, OCS use should be minimized by optimizing inhaled and biologic therapies for asthma [55, 61]. The availability of newer biologics has allowed for the advent of OCS‐sparing therapies in asthma, although the process by which long‐term corticosteroids should be tapered and/or discontinued has not been established. Consideration should be given to the risk of symptom recurrence and withdrawal‐induced AI [33, 72].
In 2020, a consensus statement on the use of OCS was issued by 131 practitioners of specialties who frequently use OCS (mostly pulmonologists, respiratory disease specialists and allergists). Of the participating experts, more than 95% concurred that, ultimately, the goal in asthma should be to avoid the use of OCS. When OCS are required, the dosage and duration should be minimized as much as possible [33]. Experts also agreed that individualized tapering to a minimally effective dosage (or complete discontinuation) should be attempted in all patients receiving OCS for maintenance therapy regardless of comorbid conditions [33]. For AI risk, the experts agreed that AI is insufficiently assessed and that patients on long‐term OCS therapy should be assessed for the risk and presence of AI. This includes patients receiving OCS dosages in excess of 2.0 g per year or more than 4 repeat courses of short‐term OCS in 1 year [33].
The Safest corticosteroids are the Fewest corticosteroids
For patients with severe, uncontrolled asthma with T2 inflammation, reduction or elimination of long‐term OCS, without loss of control, can be accomplished by using a biologic treatment directed towards inflammatory mediators [2, 65]. Omalizumab is a recombinant humanized monoclonal antibody that binds to IgE and decreases the expression of IgE receptors on inflammatory cells [73]; dupilumab is a fully human monoclonal antibody directed against the IL‐4 receptor that blocks both IL‐4 signalling and IL‐13 signalling and ultimately lessens inflammation [63]; mepolizumab and reslizumab are monoclonal antibodies that act as IL‐5 antagonists [57], effectively neutralizing IL‐5 [74] and selectively inhibiting inflammation in the airways [1, 71, 75]; and benralizumab is an IL‐5Rα‐directed cytolytic monoclonal antibody that directly binds to the IL‐5 receptor and activates antibody‐dependent cell‐mediated cytotoxicity, ultimately decreasing eosinophilic inflammation [1, 59, 76, 77, 78].
Biologics have led to decreased OCS use, reduced rates of exacerbations and improved symptom control [3, 73, 76], and they are approved as add‐on therapy for patients with severe, persistent asthma. Although the OCS‐sparing results of both clinical trials and real‐world analyses of biologics in asthma are promising, many studies used short evaluation periods and small sample sizes. In addition, cautious approaches were used to taper OCS, given the placebo‐controlled nature of some of these studies, and AI was not consistently monitored (Table 2) [57, 59, 63, 73, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88].
Table 2.
Studies of OCS‐sparing capabilities of biologic agents for severe asthma
| Drug | Study / authors | Intervention / description | Duration of study | AI assessment | Results | Notes / limitations |
|---|---|---|---|---|---|---|
| Omalizumab |
rhuMAb‐E25 Study Milgrom et al. [79] |
Randomized, double‐blind, placebo‐controlled multicentre trial | 20 weeks | Not assessed |
Patients receiving high‐dose anti‐IgE therapy achieved 50% reduction in daily OCS dosage and patients receiving low‐dose therapy achieved 65% reduction compared with no reduction in the placebo group 78% of patients in the high‐dose group and 57% in the low‐dose group achieved ≥ 50% reduction in OCS dosage compared with 33% of the placebo group 33% of patients in the high‐dose group and 43% in the low‐dose group eliminated OCS use compared with 17% of the placebo group |
Only 35 patients were receiving long‐term OCS at baseline |
| Omalizumab |
APEXII Niven et al. [73] |
Noninterventional, mixed‐methodology study that incorporated retrospective and prospective data to evaluate usage in normal clinical practice | Data collected 12 months pre‐ and postomalizumab initiation | Not assessed |
Average daily OCS dosage decreased by 1.61 mg (10.37 to 8.76 mg) in the ITT population 42.1% of patients eliminated OCS use or reduced the daily dosage by ≥ 20% 15.8% of patients eliminated OCS Mean number of exacerbations requiring hospitalization decreased from 1.66 to 0.69 and those requiring OCS dosage increases decreased from 4.58 to 2.53 in the ITT population |
Only 76 of 258 patients studied were receiving long‐term OCS at baseline Pre‐omalizumab data were collected retrospectively, leading to missing data |
| Omalizumab |
eXpeRience Registry Braunstahl et al. [80] |
Multinational, noninterventional, observational registry | 24 months | Not assessed |
Average daily OCS dosage decreased from 15.5 mg to 7.7 mg at month 12 and 5.8 mg at month 24 Proportion of patients receiving long‐term OCS decreased from 28.6% to 16.1% at month 12 and to 14.2% at month 24 Annualized mean asthma exacerbation rate decreased from 4.9 to 1.0 at month 12 and to 0.6 at month 24 |
Included 263 patients who were receiving long‐term OCS at baseline |
| Omalizumab | Molimard M, et al. [81] | Pooled analysis of real‐life experiences | 16 weeks | Not assessed |
30.1% of patients reduced daily OCS dosage 20.5% of patients eliminated daily OCS use The mean OCS dosage decreased from 19.0 mg to 12.0 mg The mean number of exacerbations decreased from 5.7 per year to 1.9 per year |
Included 166 patients who were receiving long‐term OCS Limited by lack of placebo comparison; effectiveness was only compared with previous year |
| Dupilumab |
LIBERTY ASTHMA VENTURE Rabe et al. [63] |
Randomized, double‐blind, placebo‐controlled, phase III trial | 24 weeks | Not assessed |
69% of patients in the treatment group reduced daily OCS dosage to < 5 mg compared with 33% of the placebo group Patients achieved 70.1% mean reduction in OCS dosage with dupilumab compared with 41.9% with placebo 52% of the dupilumab group eliminated OCS use compared with 29% of the placebo group Exacerbation rate was 59% lower in the dupilumab group than in the placebo group |
Included 101 patients in the treatment group and 102 in the placebo group |
| Dupilumab | Dupin et al. [82] | Retrospective, multicentre, real‐world analysis | 12 months | Not assessed |
Median OCS dosage decreased from 20 mg to 5 mg 78% of patients reduced OCS dosage by 50% or more 24% of patients eliminated OCS Number of annual exacerbations decreased from 4 to 1 |
Only 47 of 62 (76%) of patients were receiving long‐term OCS at baseline Uncontrolled, retrospective design |
| Mepolizumab |
SIRIUS Bel et al. [57] |
Randomized, double‐blind, placebo‐controlled phase III trial | 24 weeks | Patient report of symptoms of AI; OCS dosage was reduced according to symptoms of AI and asthma control |
23% of the mepolizumab group decreased OCS dosage by 90%–100% compared with 7% of the placebo group Median OCS dosage decreased by 50% in the mepolizumab group compared with 0% in the placebo group 14% of the mepolizumab group eliminated OCS compared with 8% of the placebo group Exacerbation rate in the mepolizumab group was 32% of the rate in the placebo group |
Included 69 patients in the mepolizumab group and 66 in the placebo group Limited by short duration and cautious OCS reduction strategy |
| Mepolizumab | Kavanagh et al. [83] | Real‐world analysis | 52 weeks | Patient report of symptoms |
Median daily OCS dosage decreased from 10 mg to 0 mg 75% of patients had a decrease in OCS dosage of at least 50% 57% of patients eliminated OCS Annual exacerbation rate decreased from 1.04 to 1.86 |
Only 68 patients were taking OCS at baseline |
| Mepolizumab | Caminati et al. [84] | Retrospective, real‐world analysis | Compared data from 12 months before to 6 months after mepolizumab initiation | Not assessed |
Proportion of patients on long‐term OCS decreased from 70.5% to 32.2% Median daily OCS dosage decreased from 5 mg to 0 mg |
Only 43 of 61 patients were receiving OCS therapy at baseline |
| Reslizumab | Bernstein et al. [85] | Randomized, double‐blind, placebo‐controlled phase III trial | 24 weeks | Not assessed |
No improvement in OCS use Exacerbation rate did not differ between the treatment group and the placebo group |
Included 236 patients in the reslizumab group and 232 in the placebo group |
| Reslizumab | Bernstein et al. [85] | Randomized, double‐blind, placebo‐controlled phase III trial | 52 weeks | Not assessed | No improvement in OCS use | Included 88 patients in the reslizumab group and 89 in the placebo group |
| Reslizumab | Ibrahim et al. [86] | Real‐world analysis | 2 years | Not assessed |
Patients taking long‐term OCS achieved a mean reduction in OCS dosage from 9.3 to 4.8 mg after 1 year and to 4.6 mg (50% of baseline) at 2 years 35.7% of patients eliminated OCS Patients had 79% reduction in exacerbations at 1 year and 88% at 2 years |
Only 26 patients included (only 54% were on long‐term OCS) |
| Benralizumab |
ZONDA Nair et al.[59] |
Randomized, double‐blind, placebo‐controlled phase III trial | 28 weeks | Not assessed |
Benralizumab led to median 75% reduction in OCS dosage compared with 25% for placebo 52% of patients receiving benralizumab every 4 weeks and 56% of patients receiving benralizumab every 8 weeks eliminated OCS compared with 19% receiving placebo Exacerbation rate was reduced by 55% with benralizumab every 4 weeks and by 70% with benralizumab every 8 weeks |
Included 72 patients in the benralizumab every 4 weeks group, 73 in the benralizumab every 8 weeks group and 75 in the placebo group Limited by short study period |
| Benralizumab | Pelaia et al. [87] | Real‐world analysis | 4 weeks | Not assessed | Mean daily OCS dosage decreased from 15.58 mg to 0 mg | Only included 13 patients |
| Benralizumab | Kavanagh et al. [88] | Real‐world analysis | 48 weeks | Not assessed |
Median OCS dosage decreased from 10 mg to 0 mg 51.4% of patients eliminated OCS 43.8% of patients were exacerbation‐free |
Included 130 patients |
| Benralizumab |
PONENTE Menzies‐Gow et al. [78] |
Open‐label, single‐arm, phase IIIb study | Variable, minimum 36 weeks | Evaluated when patients have been receiving a daily OCS dosage of 5 mg for 4 weeks; further OCS dosage reduction determined by the presence of AI and asthma control | Expected mid‐2021 |
Aiming to include 600 patients Patients follow a personalized OCS dosage reduction scheme Limited by lack of control group and open‐label design |
AI, adrenal insufficiency; ITT, intent to treat; OCS, oral corticosteroids.
The PONENTE trial, the results of which are expected in mid‐2021, seeks to address the gaps left by previous studies and answer key questions to guide real‐world clinical practice, including how quickly long‐term OCS can be tapered and how best to monitor for and manage AI after introducing benralizumab. PONENTE is the largest OCS‐sparing trial conducted to date, with a goal of including approximately 600 patients, and is the only one to directly address AI [78]. The PONENTE trial will outline a personalized, rapid tapering of supraphysiologic dosages of OCS based on OCS dosage and asthma control, as well as provide guidance on tapering OCS in the presence of suspected or confirmed AI. The maintenance phase is also substantially longer (24–32 weeks after the OCS reduction phase) than most other studies (4 weeks) [57, 59, 63]. This longer maintenance phase will detail the long‐term effectiveness and safety of benralizumab after OCS reduction or elimination [78].
There is currently a lack of clarity on the impact of high‐dosage topical glucocorticoids on the development of AI in patients with severe asthma irrespective of maintenance OCS use. By definition, all severe asthmatics are treated with high dosages of inhaled corticosteroids and a significant minority will be using topical nasal steroids for allergic rhinitis or chronic rhinosinusitis with nasal polyps. High‐dosage inhaled corticosteroids were an inclusion criterion for PONENTE, which will make it possible to analyse whether or not adrenal function can be recovered following OCS withdrawal despite the continuation of high‐dosage inhaled corticosteroids.
Conclusions and future direction
Despite the advent of targeted biologics and OCS drawbacks and disadvantages, corticosteroids continue to be used in asthma management. The goal is to find ways to greatly reduce or eliminate their long‐term use and to better balance the safety and effectiveness of asthma treatments [60].
There are gaps in knowledge and practice related to OCS stewardship. Systemic corticosteroids are the option of last resort in Global Initiative for Asthma (GINA) Step 5 treatment, and GINA recommends gradual decrease and/or discontinuation of OCS after patients achieve a good response to biologic therapies [33, 55]. Ideally, the role of OCS will continue to narrow as the use of novel therapies targeting specific pathways increases [33, 60].
In order to prevent both the systemic effects and the development of AI associated with long‐term OCS use, steroid‐sparing strategies are needed to advance safety amongst patients with severe, uncontrolled asthma [71, 77]. Treatments that directly target airway inflammation offer improved overall control of asthma and reduce OCS use [1, 77]. Biologic therapies greatly decrease the role of OCS and, in many cases, eliminate their need entirely.
Education is key to elevating awareness of optimal asthma control for patients, as well as steroid‐sparing strategies amongst clinicians [30]. Ongoing clinical trials are already changing clinical practice by guiding the tapering of OCS and the screening and management of AI after introducing biologics [78]. Importantly, substantial and long‐lasting modifications in prescribing patterns of OCS may well require changes to healthcare systems and collaborations amongst different specialties [30].
Conflict of interest
Mark Gurnell (MG), Liam G Heaney (LH), David Price (DP) and Andrew Menzies‐Gow (AMG) are steering committee members for the AstraZeneca PONENTE study. MG has received speakers’ fees, and LH, DP and AMG have received research funding, consulting fees and speakers’ fees from AstraZeneca. David Price has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals and Thermo Fisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals and Theravance; grants and unrestricted funding for investigator‐initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis and Thermo Fisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp, which develops adherence monitoring technology; is a peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. Andrew Menzies‐Gow has attended advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Sanofi and Teva; received speaker fees from AstraZeneca, Novartis, Roche and Teva; participated in research with AstraZeneca, for which his institution has been remunerated; attended international conferences with Teva; and made consultancy agreements with AstraZeneca, Sanofi and Vectura.
Acknowledgements
Jennifer Gibson, PharmD (Kay Square Scientific, Newtown Square, PA, USA), provided writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporating author feedback and manuscript submission. This support was funded by AstraZeneca.
Gurnell M, Heaney LG, Price D, Menzies‐Gow A (Addenbrooke’s Hospital, Cambridge; Queens University Belfast, Belfast, UK; Observational and Pragmatic Research Institute Pte Ltd, Singapore, Singapore; University of Aberdeen, Aberdeen; and Royal Brompton Hospital, London, UK). Long‐term corticosteroid use, adrenal insufficiency and the need for steroid‐sparing treatment in adult severe asthma (Review). J Intern Med 2021; 290: 240–256. 10.1111/joim.13273
References
- 1.Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68:158–66. [DOI] [PubMed] [Google Scholar]
- 2.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edris A, De Feyter A, Maes T, Joos G, Lahousse L, et al. Monoclonal antibodies in type 2 asthma: a systematic review and network meta‐analysis. Respir Res. 2019;20:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney J, Patterson CC, Menzies‐Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross‐sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–46. [DOI] [PubMed] [Google Scholar]
- 5.Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Jie JLZ, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long‐term observational study. J Asthma Allergy. 2018;11:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100:2171–80. [DOI] [PubMed] [Google Scholar]
- 7.Simpson H, Tomlinson J, Wass J, Dean J, Arlt W. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond). 2020;20:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmet A, Kim H, Spier S. Adrenal suppression: a practical guide to the screening and management of this under‐recognized complication of inhaled corticosteroid therapy. Allergy Asthma Clin Immunol. 2011;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods CP, Argese N, Chapman M, Boot C, Webster R, Dabhi V, et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur J Endocrinol. 2015;173:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes AK, Younes NK. Recovery of steroid induced adrenal insufficiency. Transl Pediatr. 2017;6:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton DD, Cotton BA. Cosyntropin as a diagnostic agent in the screening of patients for adrenocortical insufficiency. Clin Pharmacol. 2010;2:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuetz P, Christ‐Crain M, Schild U, Süess E, Facompre M, Baty F, et al. Effect of a 14‐day course of systemic corticosteroids on the hypothalamic‐pituitary‐adrenal‐axis in patients with acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis‐Hansen L, Hilsted L, et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24:714–20. [DOI] [PubMed] [Google Scholar]
- 14.Laugesen K, Petersen I, Sørensen HT, Jørgensen JOL. Clinical indicators of adrenal insufficiency following discontinuation of oral glucocorticoid therapy: a Danish population‐based self‐controlled case series analysis. PLoS One. 2019;14:e0212259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216–26. [DOI] [PubMed] [Google Scholar]
- 18.Barra CB, Fontes MJF, Cintra MTG, Cruz RC, Rocha JAG, Guimarães MCC, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras (1992). 2017;63:899–903. [DOI] [PubMed] [Google Scholar]
- 19.Ceccato F, Scaroni C. Central adrenal insufficiency: open issues regarding diagnosis and glucocorticoid treatment. Clin Chem Lab Med. 2019;57:1125–35. [DOI] [PubMed] [Google Scholar]
- 20.Chabre O, Goichot B, Zenaty D, Bertherat J. Group 1. Epidemiology of primary and secondary adrenal insufficiency: prevalence and incidence, acute adrenal insufficiency, long‐term morbidity and mortality. Ann Endocrinol (Paris). 2017;78:490–4. [DOI] [PubMed] [Google Scholar]
- 21.Pazderska A, Pearce SHS. Adrenal insufficiency – recognition and management. Clin Med. 2017;17:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puar THK, Stikkelbroeck NMML, Smans LCCJ, Zelissen PMJ, Hermus ARMM. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129:339.e1–9. [DOI] [PubMed] [Google Scholar]
- 23.Choi SI, Sim DW, Kim SH, Wui JW. Adrenal insufficiency associated with long‐term use of inhaled steroid in asthma. Allergy Asthma Immunol. 2017;1:66–72. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer KJ, Tata LJ, Smith CPJ, West J, Harrison TW, Tattersfield AE, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case‐control study. Thorax. 2006;61:405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sannarangappa V, Jalleh R. Inhaled corticosteroids and secondary adrenal insufficiency. Open Resp Med J. 2014;8(Suppl 1:M6):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves C, Robazzi TCV, Mendonça M. Withdrawal from glucocorticosteroid therapy: clinical practice recommendations. J Pediatr (Rio J). 2008;84:192–202. [DOI] [PubMed] [Google Scholar]
- 27.Borstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–39. [DOI] [PubMed] [Google Scholar]
- 28.Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short‐term, high‐dose glucocorticoid treatment. Lancet. 2000;355:542–5. [DOI] [PubMed] [Google Scholar]
- 29.Fardet L, Petersen I, Nazareth I. Monitoring patients on long‐term glucocorticoid therapy. Medicine. 2015;94:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung LP, Upham JW, Bardin PG, Hew A. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baek JHA, Kim SK, Jung JH, Hahm JR, Jung J. Recovery of adrenal function in patients with glucocorticoids induced secondary adrenal insufficiency. Endocrinol Metab (Seoul). 2016;31:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto S, Ten Brinke A, Roldaan AC, van Veen IH, Möller GM, Sont JK, et al. Internet‐based tapering of oral corticosteroids in severe asthma: a pragmatic randomized controlled trial. Thorax. 2011;66:514–20. [DOI] [PubMed] [Google Scholar]
- 33.Suehs C, Menzies‐Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma: a Delphi study. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202007-2721OC. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Nicholas MN, Li SK, Dytoc M. An approach to minimising risk of adrenal insufficiency when discontinuing oral glucocorticoids. J Cutan Med Surg. 2018;22:175–81. [DOI] [PubMed] [Google Scholar]
- 35.Oboni JB, Marques‐Vidal P, Pralong F, Waeber G. Predictive factors of adrenal insufficiency in patients admitted to acute medical wards: a case control study. BMC Endocr Disord. 2013;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vulto A, Bergthorsdottir R, van Faassen M, Kema IP, Johannsson G, van Beek AP. Residual endogenous corticosteroid production in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2019;91:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chanson P, Guignat L, Goichot B, Chabre O, Boustani DS, Reynaud R, et al. Group 2: Adrenal insufficiency: screening methods and confirmation of diagnosis. Ann Endocrinol (Paris). 2017;78:495–511. [DOI] [PubMed] [Google Scholar]
- 38.Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med. 2019;381:852–61. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaku M, Shinzawa M, Tanaka S, Kimachi K, Kawakami K. Clinical characteristics of adrenal crisis in adult population and without predisposing chronic adrenal insufficiency: a retrospective cohort study. BMC Endocr Disord. 2017;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smans LCCJ, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2016;84:17–22. [DOI] [PubMed] [Google Scholar]
- 41.Johannsson G, Falorni A, Skrtic S, Lennernäs H, Quinkler M, Monson JP, et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2015;82:2–11. [DOI] [PubMed] [Google Scholar]
- 42.Quinkler M, Ekman B, Zhang P, Isidori AM, Murray RD. Mortality data from the European adrenal insufficiency registry‐patient characterization and associations. Clin Endocrinol (Oxf). 2018;89:30–5. [DOI] [PubMed] [Google Scholar]
- 43.Ragnarsson O, Nystrom HF, Johannsson G. Glucocorticoid replacement therapy is independently associated with reduced bone mineral density in women with hypopituitarism. Clin Endocrinol (Oxf). 2012;76:246–52. [DOI] [PubMed] [Google Scholar]
- 44.Ragnarsson O, Mattsson AF, Monson JP, Filipsson Nyström H, Åkerblad A‐C, Kołtowska‐Häggström M, et al. The relationship between glucocorticoid replacement and quality of life in 2737 hypopituitary patients. Eur J Endocrinol. 2014;171:571–9. [DOI] [PubMed] [Google Scholar]
- 45.Ospina NS, Nofal AA, Bancos I, Javed A, Benkhadra K, Kapoor E, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2016;101:427–34. [DOI] [PubMed] [Google Scholar]
- 46.Dichtel LE, Schorr M, Loures de Assis C, Rao EM, Sims JK, Corey KE, et al. Plasma free cortisol in states of normal and altered binding globulins: implications for adrenal insufficiency diagnosis. J Clin Endocrinol Metab. 2019;104:4827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burt M, Mangelsdorf BL, Rogers A, Ho JT, Lewis JG, Inder WJ, et al. Free and total plasma cortisol measured by immunoassay and mass spectrometry following ACTH1–24 stimulation in the assessment of pituitary patients. J Clin Endocrinol Metab. 2013;98:1883–90. [DOI] [PubMed] [Google Scholar]
- 48.El‐Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, et al. Method‐specific serum cortisol responses to the adrenocorticotropin test: comparison of gas chromatography‐mass spectrometry and five automated immunoassays. Clin Endocrinol. 2013;78:673–90. [DOI] [PubMed] [Google Scholar]
- 49.Mongioi LM, Condorelli RA, Barbagallo F, Cannarella R, La Vignera S, Calogero AE, et al. Accuracy of the low‐dose ACTH stimulation test for adrenal insufficiency diagnosis: a reassessment of the cut‐off value. J Clin Med. 2019;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ach T, Yosra H, Jihen M, Abdelkarim Asma B, Maha K, Molka C, et al. Cortisol cut‐points for the glucagon stimulation test in the evaluation of hypothalamic pituitary adrenal axis. Endocr J. 2018;65:935–42. [DOI] [PubMed] [Google Scholar]
- 51.Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383:2152–67. [DOI] [PubMed] [Google Scholar]
- 52.Ekman B, Fitts D, Marelli C, Murray RD, Quinkler M, Zelissen PM. European Adrenal Insufficiency Registry (EU‐AIR): a comparative observational study of glucocorticoid replacement therapy. BMC Endocr Disord. 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iqbal K, Halsby K, Murray RD, Carroll PV, Petermann R. Glucocorticoid management of adrenal insufficiency in the United Kingdom: assessment using real‐world data. Endocr Connect. 2019;8:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grossman A, Johannsson G, Quinkler M, Zelissen P. Perspectives on the management of adrenal insufficiency: clinical insights from across Europe. Eur J Endocrinol. 2013;169:R165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2020. https://ginasthma.org/wp‐content/uploads/2020/06/GINA‐2020‐report_20_06_04‐1‐wms.pdf. Accessed June 21, 2020.
- 56.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2020: Online Appendix. https://ginasthma.org/wp‐content/uploads/2020/04/GINA‐2020‐Appendix_final‐wms.pdf. Accessed June 21, 2020.
- 57.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. SIRIUS Investigators. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–97. [DOI] [PubMed] [Google Scholar]
- 58.Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Acute and chronic systemic corticosteroid‐related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1488–95. [DOI] [PubMed] [Google Scholar]
- 59.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. ZONDA Trial Investigators. Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–58. [DOI] [PubMed] [Google Scholar]
- 60.Ramsahai JM, Wark PA. Appropriate use of oral corticosteroids for severe asthma. Med J Austr. 2018;209:S18–21. [DOI] [PubMed] [Google Scholar]
- 61.Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long‐term oral corticosteroid therapy and its side‐effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:1800703. [DOI] [PubMed] [Google Scholar]
- 62.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation, and treatment of severe asthma. Eur Respir J. 2014;43:343–73. [DOI] [PubMed] [Google Scholar]
- 63.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378:2475–85. [DOI] [PubMed] [Google Scholar]
- 64.Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies‐Gow AN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157:790–804. [DOI] [PubMed] [Google Scholar]
- 65.Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne). 2017;4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gamble J, Stevenson M, Heaney LG. A study of multi‐level intervention to improve non‐adherence in difficult to control asthma. Respir Med. 2011;105:1308–15. [DOI] [PubMed] [Google Scholar]
- 67.Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The costs of systemic corticosteroid‐induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voorham J, Xu X, Price DB, Golam S, Davis J, Zhi Jie Ling J, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buhl R, Humbert M, Bjermer L, Chanez P, Heaney LG, Pavord I, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49:1700634. [DOI] [PubMed] [Google Scholar]
- 70.Emma R, Morjaria JB, Fuochi V, Polosa R, Caruso M. Mepolizumab in the management of severe eosinophilic asthma in adults: current evidence and practical experience. Ther Adv Respir Dis. 2018;12:1753466618808490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380:651–9. [DOI] [PubMed] [Google Scholar]
- 72.Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching‐adjusted comparison of oral corticosteroid reduction in asthma: systematic review of biologics. Clin Exp Allergy. 2020;50:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicenter observational study (the APEX II study). BMJ Open. 2016;6:e011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo‐controlled study. Am J Respir Crit Care Med. 2011;184:1125–32. [DOI] [PubMed] [Google Scholar]
- 75.Nair P, Bardin P, Humbert M, Murphy KR, Hickey L, Garin M, et al. Efficacy of intravenous reslizumab in oral corticosteroid‐dependent asthma. J Allergy Clin Immunol Pract. 2020;8:555–64. [DOI] [PubMed] [Google Scholar]
- 76.Yancey SW, Keene ON, Albers FC, Ortega H, Bates S, Bleecker ER, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140:1509–18. [DOI] [PubMed] [Google Scholar]
- 77.Menzella F, Biava M, Bagnasco D, Galeone C, Simonazzi A, Ruggiero P, et al. Efficacy and steroid‐sparing effect of benralizumab: has it an advantage over its competitors? Drugs Context. 2019;8:212580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menzies‐Gow A, Corren J, Bel EH, Maspero J, Heaney LG, Gurnell M, et al. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial. ERJ Open Res. 2019;5:00009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti‐IgE antibody. N Engl J Med. 1999;341:1966–73. [DOI] [PubMed] [Google Scholar]
- 80.Braunstahl G‐J, Chlumsky J, Peachey G, Chen C‐W. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real‐world setting. Allergy Asthma Clin Immunol. 2013;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molimard M, Buhl R, Niven R, Le Gros V, Thielen A, Thirlwell J, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real‐life data. Respir Med. 2010;104:1381–5. [DOI] [PubMed] [Google Scholar]
- 82.Dupin C, Belhadi D, Guilleminault L, Gamez A‐S, Berger P, De Blay F, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real‐life French multi‐centre adult cohort. Clin Exp Allergy. 2020;50:789–98. [DOI] [PubMed] [Google Scholar]
- 83.Kavanagh JE, d’Ancona G, Elstad M, Green L, Fernandes M, Thomson L, et al. Real‐world effectiveness and the characteristics of a “super‐responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158:491–500. [DOI] [PubMed] [Google Scholar]
- 84.Caminati M, Cegolon L, Vianello A, Chieco Bianchi F, Festi G, Marchi MR, et al. Mepolizumab for severe eosinophilic asthma: a real‐world snapshot on clinical markers and timing of response. Expert Rev Respir Med. 2019;13:1205–12. [DOI] [PubMed] [Google Scholar]
- 85.Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed‐dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid‐dependent asthma: results from two phase 3, randomised, double‐blind, placebo‐controlled trials. Lancet Respir Med. 2020;8:461–74. [DOI] [PubMed] [Google Scholar]
- 86.Ibrahim H, O’Sullivan R, Casey D, Murphy J, MacSharry J, Plant BJ, et al. The effectiveness of reslizumab in severe asthma treatment: a real‐world experience. Respir Res. 2019;20:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pelaia C, Busceti MT, Vatrella A, Rago GF, Crimi C, Terracciano R, et al. Real‐life rapidity of benralizumab effects in patients with severe allergic eosinophilic asthma: assessment of blood eosinophils, symptom control, lung function, and oral corticosteroid intake after the first drug dose. Pulm Pharmacol Ther. 2019;58:101830. [DOI] [PubMed] [Google Scholar]
- 88.Kavanagh JE, Hearn AP, Dhariwal J, d'Ancona G, Douiri A, Roxas C, et al. Real world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159:496–506. [DOI] [PubMed] [Google Scholar]


