Abstract
Foundational therapy for heart failure and a reduced ejection fraction consists of a combination of an angiotensin receptor–neprilysin inhibitor, a beta‐blocker, a mineralocorticoid receptor antagonist and a sodium–glucose co‐transporter 2 (SGLT2) inhibitor. However, the conventional approach to the implementation is based on a historically‐driven sequence that is not strongly evidence‐based, typically requires ≥6 months, and frequently leads to major gaps in treatment. We propose a rapid sequencing strategy that is based on four principles. First, since drugs act rapidly to reduce morbidity and mortality, patients should be started on all four foundational treatments within 2–4 weeks. Second, since the efficacy of each foundational therapy is independent of treatment with the other drugs, priority can be determined by considerations of relative efficacy, safety and ease‐of‐use. Third, low starting doses of foundational drugs have substantial therapeutic benefits, and achievement of low doses of all four classes of drugs should take precedence over up‐titration to target doses. Fourth, since drugs can influence the tolerability of other foundational agents, sequencing can be based on whether agents started earlier can enhance the safety of agents started simultaneously or later in the sequence. We propose an accelerated three‐step approach, which consists of the simultaneous initiation of a beta‐blocker and an SGLT2 inhibitor, followed 1–2 weeks later by the initiation of sacubitril/valsartan, and 1–2 weeks later by a mineralocorticoid receptor antagonist. The latter two steps can be re‐ordered or compressed depending on patient circumstances. Rapid sequencing is a novel evidence‐based strategy that has the potential to dramatically improve the implementation of treatments that reduce the morbidity and mortality of patients with heart failure and a reduced ejection fraction.
Keywords: Angiotensin receptor–neprilysin inhibitor, Beta‐blocker, Mineralocorticoid receptor antagonist, Sodium–glucose co‐transporter 2 inhibitor, Rapid sequencing
Comparison of conventional and rapid sequencing approaches to the implementation of foundational drug treatments for heart failure and a reduced ejection fraction. Rapid sequencing involves simultaneous initiation of a beta‐blocker and sodium–glucose co‐transporter 2 (SGLT2) inhibitor, followed 1–2 weeks later by an angiotensin receptor–neprilysin inhibitor, and 1–2 weeks later, by a mineralocorticoid receptor antagonist. The ordering of Step 2 and 3 may be reversed in a patient with a borderline systolic blood pressure. Patients already receiving a conventional inhibitor of the renin–angiotensin system may be switched to sacubitril/valsartan and started on a mineralocorticoid receptor antagonist at the same time. Reproduced and adapted with permission from McMurray and Packer.3

Four classes of drugs have been shown to have a meaningful effect to reduce morbidity and mortality in patients with chronic heart failure and a reduced ejection fraction, and there is growing consensus that all four therapies should be given in combination to all patients who can tolerate treatment. These drugs include angiotensin receptor–neprilysin inhibitors, beta‐adrenergic receptor blockers, mineralocorticoid receptor antagonists, and sodium–glucose co‐transporter 2 (SGLT2) inhibitors. Combination treatment has a profound effect to change the natural history of heart failure.1
However, there is uncertainty as to how treatment with these foundational drugs should be implemented. The urgency of this question has been underscored by the realization that the vast majority of patients who would benefit from comprehensive therapy are receiving only one or two of the four classes of drugs, and that—even when four are prescribed—the implementation of combination treatments is often delayed for long periods of time.2 During this delay, incomplete or suboptimal therapeutic regimens result in unnecessary deaths, hospitalizations and progression of the underlying disease process.
We have recently proposed a novel rapid‐sequencing strategy that strives to achieve treatment with all foundational drugs within 4 weeks of initiation of treatment (Graphical Abstract).3 In this paper, we review and critically analyse the compelling body of clinical trial evidence that supports our strategy.
Clinical trial evidence relevant to the sequencing of treatments for heart failure
What drugs represent foundational therapy for heart failure?
We consider a drug to represent a foundational therapy for patients with heart failure and a reduced ejection fraction if the treatment has been shown in large‐scale clinical trials to (i) reduce cardiovascular death and/or all‐cause mortality; and (ii) has a major effect to reduce the risk of hospitalizations for heart failure. To meet these prerequisites, these benefits should have been demonstrated in a statistically persuasive manner, i.e. that the reduction in risk for these endpoints should have been shown in more than one large‐scale trial and/or that the statistical strength of evidence in a single trial be equivalent to that of two or more trials. Typically, this would require a P‐value for the treatment effect in a single trial to be less than 0.00125.3 Replication of a treatment effect on morbidity and mortality is commonly achieved with different members of a drug class and may be achieved in different (and complementary) therapeutic settings.
Based on these criteria, the following drug classes are considered to represent foundational treatments for heart failure and a reduced ejection fraction.
Angiotensin receptor–neprilysin inhibition
Two large‐scale trials with enalapril showed that interference with the renin–angiotensin system reduces mortality in patients with heart failure and a reduced ejection fraction.4, 5 This approach has been supported by trials with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients with heart failure and/or left ventricular systolic dysfunction following an acute myocardial infarction.6, 7, 8 Subsequently, the PARADIGM‐HF trial established the superiority of sacubitril/valsartan over enalapril, thus establishing the importance of simultaneous inhibition of neprilysin when interfering the renin–angiotensin system.9 When compared with a conventional inhibitor of the renin–angiotensin system, the magnitude of the incremental risk reduction with sacubitril/valsartan was 20% for cardiovascular mortality and 20% for hospitalizations for heart failure, with both effects being demonstrated at statistically persuasive level of evidence (P < 0.00001).10 When compared with placebo, the estimated benefits of sacubitril/valsartan are a 30–35% reduction in cardiovascular death and a 45–50% reduction in hospitalizations for heart failure.11
Beta‐adrenergic receptor blockers
Three trials (CIBIS II, MERIT‐HF and COPERNICUS) demonstrated that certain beta‐blockers reduce the risk of all‐cause mortality and cardiovascular death by 30–35%, with a particularly marked (nearly 50%) decrease in the risk of sudden cardiac death; this benefit is accompanied by a 30% decrease in the risk of hospitalization for heart failure.12, 13, 14, 15, 16 These favourable effects have been demonstrated in patients with mild, moderate and severe heart failure and have been statistically persuasive within each trial. The evidence supports the use of bisoprolol, carvedilol and metoprolol succinate, in contrast to other members of the drug class. These findings are supported by other large‐scale trials in patients with heart failure and a reduced ejection fraction and in trials of survivors of a myocardial infarction with left ventricular systolic dysfunction or heart failure.17, 18, 19, 20
Mineralocorticoid receptor antagonists
Two trials (RALES and EMPHASIS‐HF) demonstrated a 25–30% reduction in the risk of all‐cause mortality and cardiovascular death, which was accompanied by a 35–40% decrease in the risk of hospitalization for heart failure.21, 22 These benefits have been demonstrated with both eplerenone and spironolactone and in patients with mild as well as moderate‐to‐severe symptoms. These findings are supported by the findings of favourable effects of mineralocorticoid receptor antagonists in other large‐scale trials in patients with post‐infarction left ventricular dysfunction and in diabetes‐related chronic kidney disease.23, 24
Sodium–glucose co‐transporter 2 inhibitors
Two trials (DAPA‐HF and EMPEROR‐Reduced) demonstrated a 30% reduction in the risk of hospitalizations for heart failure when patients were treated with dapagliflozin or empagliflozin.25, 26 A benefit of similar magnitude has been seen with different inhibitors of SGLT2 in large‐scale trials of patients with type 2 diabetes, including those who have heart failure or are at increased risk of serious heart failure events.27, 28, 29, 30 In addition, SGLT2 inhibitors have been shown to reduce the risk of cardiovascular death or all‐cause mortality in trials of patients with chronic heart failure, type 2 diabetes and chronic kidney disease25, 31, 32; this benefit on survival has been supported by a meta‐analysis of trials in patients with heart failure and a reduced ejection fraction and a meta‐analysis of trials in patients with diabetes.33, 34
For purposes of this paper, the following interventions are not considered foundational therapy, even though they may be valuable options for selected patients with heart failure and a reduced ejection fraction.
-
Certain cardiac devices or cardiovascular procedures (e.g. implantable cardioverter‐defibrillators, cardiac resynchronization therapy and transcatheter mitral valve repair) have been shown to reduce both morbidity and mortality in selected patients with heart failure and a reduced ejection fraction.35, 36, 37, 38, 39 However, with the advent of comprehensive neurohormonal blockade (particularly with the use of beta‐blockers), the risk of sudden death in patients with heart failure and left ventricular systolic dysfunction has declined considerably,40 and neurohormonal antagonists can ameliorate the severity of functional mitral regurgitation without the need for structural repair.41, 42 Consequently, device therapies are typically recommended after all appropriate drug therapies have been prescribed and optimized.43 When these prerequisites have been fulfilled, cardiac devices are appropriate for selected patients (e.g. those with left bundle branch block or with residual severe mitral regurgitation), but these typically represent a relatively small proportion of patients with heart failure and a reduced ejection fraction. In addition, the expense of these devices may limit their availability to many patients and in many geographical regions with constrained healthcare resources.
Prevention of sudden death in patients with left ventricular systolic dysfunction is of paramount importance, but the available evidence indicates that its pathogenesis may involve mechanisms that often do not involve a sustained ventricular tachyarrhythmia, and thus, may not be amenable to treatment with an implantable cardioverter‐defibrillator.44, 45, 46, 47 Importantly, a reduction in the risk of sudden death underlies many of the survival benefits of angiotensin receptor–neprilysin inhibitors, beta‐blockers, mineralocorticoid receptor antagonists and SGLT2 inhibitors13, 14, 27, 46, 47, 48, 49; in contrast, conventional inhibitors of the renin–angiotensin system may not prevent sudden death.50 Foundational drugs can inhibit neurohormonally mediated ventricular tachyarrhythmias, and they may also mitigate the development of adverse remodelling that can cause sudden deaths that are related to the rapid cascading failure of myocardial microadaptations, which becomes manifest as asystole, electromechanical dissociation, or a terminal bradyarrythmia.45, 46, 51 The duality of the mechanisms leading to sudden cardiac death likely explain why implantable cardioverter‐defibrillators do not prevent many sudden deaths44, 52 and why neurohormonal antagonists can prevent sudden death even in patients who have an implantable cardioverter‐defibrillator in place.48, 51
Certain pharmacological interventions have been shown to be effective in the management of patients with heart failure and a reduced ejection fraction, but the magnitude of the overall treatment effects has been modest; the strength of evidence (as reflected by the level of statistical significance) has not been persuasive; and reported benefits may be confined to specific subgroups. In large‐scale trials, vericiguat, omecamtiv mecarbil, ivabradine and digoxin have been shown to reduce the combined risk of cardiovascular death and hospitalization for heart failure, but the relative risk reduction for many of these agents has been less than 10–20%, with no overall benefit on cardiovascular death.53, 54, 55, 56 Furthermore, the strength of evidence—even for the combined risk of morbidity and mortality—has often not been compelling (i.e. a P‐value of 0.01 to 0.05 in a single trial), and occasionally, other trials in patients with left ventricular systolic dysfunction have yielded non‐confirmatory results.57 Because any favourable effect may be confined to subgroups,53, 54, 55 these agents—along with the combination of hydralazine and isosorbide dinitrate58—may be appropriate for use in selected patients.
Do the benefits of foundational drugs depend on background therapy?
Assuming that there are four foundational pharmacological treatments for patients with heart failure and a reduced ejection fraction, it is important to ask if the efficacy of each treatment is influenced (favourably or unfavourably) by co‐administration of the other therapies. Are angiotensin receptor–neprilysin inhibitors effective only in patients receiving beta‐blockers? Are the benefits of SGLT2 inhibitors evident only if patients are receiving mineralocorticoid receptor antagonists? It is understood that each drug class was tested in patients already receiving certain background therapy; however, the fact that background therapy was present does not imply that the background therapy was needed for the study medication to have demonstrated efficacy in a large‐scale trial.
It is therefore noteworthy that the totality of evidence from randomized controlled trials strongly indicates that background therapy does not influence the response to each of the foundational drug classes (Table 1).59 In general, for patients with heart failure and a reduced ejection fraction, beta‐blockers were tested in patients already receiving conventional inhibitors of the renin–angiotensin system.12, 13, 14 However, in the post‐infarction setting, the converse was true, i.e. angiotensin‐converting enzyme inhibitors were typically tested in patients already receiving beta‐blockers.6, 7 In patients with left ventricular dysfunction or heart failure following a myocardial infarction, beta‐blockers were shown to reduce mortality in trials with and without background therapy with an angiotensin‐converting enzyme inhibitor.18, 20 Furthermore, the first trial with a mineralocorticoid receptor antagonist (RALES) tested spironolactone in patients who were largely not receiving a beta‐blocker,21 but the second trial of the drug class (EMPHASIS‐HF) tested eplerenone in a setting where most patients were receiving a beta‐blocker as background therapy22; the benefits reported in the two trials were similar. Enalapril was effective in reducing mortality in a trial in which >50% of the patients received spironolactone and in a trial where potassium‐sparing diuretics were prescribed to <10%.4, 5 In the large‐scale trials with sacubitril/valsartan and with SGLT2 inhibitors, the favourable effects of both drug treatments were similar whether patients were or were not receiving a mineralocorticoid receptor antagonist.11, 25, 26 Moreover, SGLT2 inhibitors were effective in reducing hospitalizations for heart failure in patients with type 2 diabetes (with a magnitude similar to that seen in patients with heart failure and a reduced ejection fraction), but most of the patients in these trials were not receiving spironolactone or eplerenone.27, 28, 29 Finally, in the two trials of SGLT2 inhibitors in heart failure with a reduced ejection fraction, background therapy with a neprilysin inhibitor did not minimize the benefits of treatment with dapagliflozin and empagliflozin.33, 60 Background therapy has influenced the effects of the study medication only when the treatments unequivocally interfered with the same pathophysiological pathway (i.e. angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers and renin inhibitors).61, 62
Table 1.
Influence of background therapy on effects of foundational drugs on major outcomes

|
ACE, angiotensin‐converting enzyme; CI, confidence interval; LV, left ventricular; SGLT2, sodium–glucose co‐transporter 2.
The lack of a meaningful interaction with respect to the efficacy of the four foundational treatments for heart failure and a reduced ejection fraction has two important implications for the rational use of these drugs in the clinical setting. First, the lack of an interaction with respect to efficacy strongly supports the common belief that each drug class acts to mitigate the progression of the cardiomyopathic process by a distinct pathophysiological mechanism.59 Angiotensin receptor blockade, beta‐adrenergic receptor blockers and mineralocorticoid receptor antagonists interfere with the deleterious actions of angiotensin II, norepinephrine and aldosterone, respectively. Neprilysin inhibition potentiates the actions of endogenous peptides that mitigate adverse ventricular remodelling,63 and the favourable effects of SGLT2 inhibitors on cardiac remodelling may be related to their actions to augment nutrient deprivation signalling, resulting in favourable effects on cardiomyocyte function and survival.64, 65 Second, the lack of an interaction with respect to efficacy implies that decisions regarding the optimal sequencing of drugs need not depend on the precise order in which these drugs were tested in clinical trials. Drugs with therapeutic advantages and improved tolerability and ease‐of‐use should be prioritized even if they were introduced into clinical practice only recently. This principle is already widely practiced; e.g. even though most patients who participated in trials of angiotensin‐converting enzyme inhibitors and beta‐blockers were receiving digoxin as background therapy,4, 5, 12, 13, 14 physicians do not currently consider the use of cardiac glycosides to be a prerequisite for the use of drugs that interfere with the renin–angiotensin or sympathetic nervous systems.
One trial (CIBIS III) was specifically designed to determine if initiation of treatment with a beta‐blocker differed meaningfully from initiation of treatment with an angiotensin‐converting enzyme inhibitor.66, 67 A total of 1010 patients with heart failure and a reduced ejection fraction were randomized to bisoprolol 10 mg daily or enalapril 20 mg daily for 6 months, followed by combined therapy thereafter; the primary endpoint was all‐cause mortality and all‐cause hospitalization. There were 65 deaths and 151 hospitalizations for any reason in the bisoprolol‐first group, and 73 deaths and 159 hospitalizations for any reason in the enalapril‐first group. Patients treated with a beta‐blocker were less likely to die suddenly, but were more likely to experience early non‐fatal worsening of heart failure early following initiation of treatment. At 1 year, mortality was approximately 30% lower in the patients who had been first started on bisoprolol (P = 0.065). The results of the trial provide direct support for the premise that a beta‐blocker can be used successfully as the first neurohormonal antagonist for the treatment of heart failure and a reduced ejection fraction, a situation akin to the use of beta‐blockers before angiotensin‐converting enzyme inhibitors in the post‐infarction setting.6, 7
Does the safety of each drug depend on background therapy?
In contrast to the lack of an interaction with respect to measures of efficacy, there is considerable evidence that background therapy can influence the safety and tolerability of foundational drugs for heart failure and a reduced ejection fraction.
The use of diuretics exerts a significant effect on the safety of neurohormonal antagonists. Treatment with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers is frequently accompanied by early hypotension as well as meaningful worsening of renal function.68 These effects are related to inhibition of the systemic and intrarenal effects of angiotensin II, and are particularly notable in patients receiving high doses or longer‐acting agents.69, 70 Yet, the severity of both reactions can be ameliorated by a decrease in the dose of concomitantly administered diuretics.68, 71 A reduction in the dose of diuretics may also ameliorate the hypotensive response to angiotensin receptor–neprilysin inhibition.72 Conversely, initiation of treatment with a beta‐blocker is often accompanied by fluid retention, which can lead to worsening heart failure during 1–2 weeks, particularly in patients with advanced symptoms.66, 73 Intensification of concomitantly administered diuretics—either prior to initiation of treatment or expeditiously in response to increases in body weight—can mitigate the early increase in heart failure events. Accordingly, even though the COPERNICUS trial enrolled patients with severe heart failure, a protocol directive to intensify diuretics to ensure clinical euvolaemia prevented the early increased risk of worsening heart failure, an adverse event that was seen in the large‐scale beta‐blocker trials that did not mandate a pre‐emptive diuretic intensification strategy.12, 13, 14, 66
Initiation of treatment with an angiotensin receptor blocker, a neprilysin inhibitor and carvedilol can produce meaningful decreases in systolic blood pressure, which can be symptomatic in some patients. This reaction typically dissipates with repeated exposure to the same dose, even in the absence of changes in concomitant therapy, presumably as a result of circulatory adaptions.73 For these reasons, treatment with these drugs is typically initiated in low doses, and doses are increased when the tolerability of prescribed doses has been established in individual patients. Although it can be hypothesized that sacubitril/valsartan might produce greater decreases in blood pressure in patients treated with carvedilol, there is little clinical experience to support this concern in patients whose blood pressure has been stable while receiving long‐term treatment with carvedilol.
Patients with heart failure receiving neurohormonal antagonists are monitored to ensure that serum electrolytes and renal function are not adversely affected to a clinically meaningful degree. Worsening renal function is commonly seen with the use of conventional inhibitors of the renin–angiotensin system and the use of mineralocorticoid receptor antagonists71, 74; the decline in glomerular filtration rate with an angiotensin‐converting enzyme inhibitor can be lessened if neprilysin inhibition is added to angiotensin receptor blockade.75 Long‐term treatment with a SGLT2 inhibitor has favourable effects to slow the progressive decline in glomerular filtration rate in patients with heart failure26, 33, 76, 77; accordingly, this class of drugs may facilitate the utilization of angiotensin receptor blockers or mineralocorticoid receptor antagonists, whose appropriate use might be discouraged by the advent of worsening renal function. Similarly, hyperkalaemia can complicate the use of mineralocorticoid receptor antagonists, but this risk appears to be reduced by concomitant treatment with a neprilysin inhibitor or SGLT2 inhibitor.78, 79 As a result of these favourable interactions, the effects of sacubitril/valsartan and an SGLT2 inhibitor to mitigate the development of renal insufficiency and hyperkalaemia argues for their pre‐emptive use in patients who are destined to be treated with a mineralocorticoid receptor antagonist.
What are the early effects of starting doses of foundational therapy?
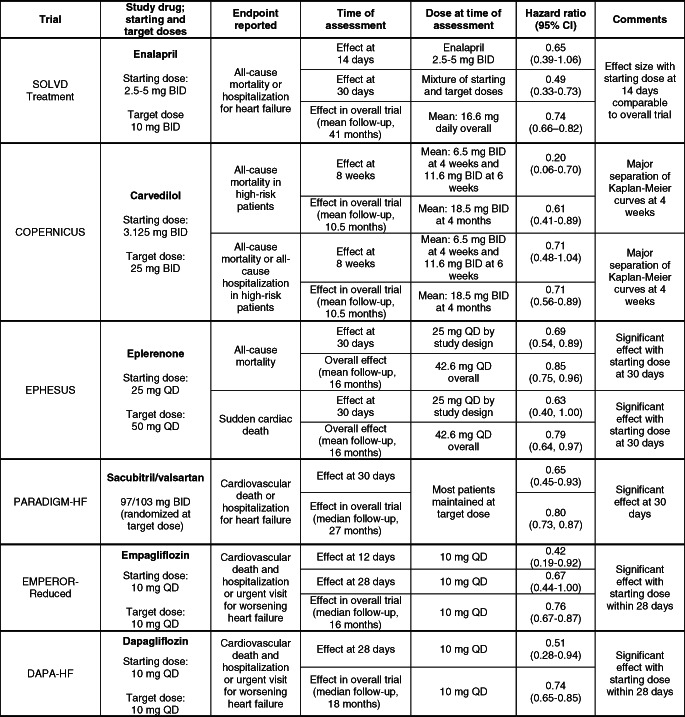
In most large‐scale clinical trials of drugs for heart failure, double‐blind treatment was typically initiated at low doses, and if tolerated, the dose of double‐blind therapy was increased at specified intervals until a target dose was achieved. This design feature has allowed investigators to estimate the magnitude of the early treatment effects of low doses and to compare these estimates to the effect seen with target doses administered during the total duration of double‐blind treatment (Table 2).
Table 2.
Early effects of foundational treatments on major outcomes in large‐scale trials
|
BID, twice daily; CI, confidence interval; QD, once daily.
The findings of the analyses of the early effects of low doses are highly instructive. In the SOLVD Treatment trial, low starting doses of enalapril yielded favourable effects on the combined risk of death and hospitalization for heart failure during the first 2–4 weeks of treatment which were comparable to those seen with target doses of enalapril during the duration of the trial.80 In the COPERNICUS trial, low starting and sub‐target doses of carvedilol produced favourable effects to reduce the risk of death or hospitalization for any reason, and these benefits became apparent at 30 days of randomization.81 In the EPHESUS trial with eplerenone, low starting doses of the mineralocorticoid receptor antagonists reduced the risk of death within the first 4 weeks of treatment.82 In all three trials, the magnitude of the benefit seen very early following initiation of therapy was comparable to or greater than that seen in the trial overall, even though the dose of the study medication had not yet been increased to the target dose specified in the protocol.
These observations suggest that low starting doses of neurohormonal antagonists achieve most of the benefit expected from the use of target doses. Large‐scale trials that were specifically designed to compare the long‐term efficacy of low starting doses and high target doses have largely confirmed this hypothesis. In the ATLAS trial with lisinopril and in the HEAAL trial with losartan,69, 83 patients were randomized to very high doses or very low doses of a conventional inhibitor of the renin–angiotensin system. In both trials, high target doses did not provide a survival benefit, and the incremental benefit of high doses on the primary endpoint of both studies was modest. However, as compared with low starting doses, the use of high doses was accompanied by more hypotension, renal insufficiency and hyperkalaemia. In the EMPHASIS‐HF trial, the use of low doses of eplerenone in patients with impaired renal function yielded effects on morbidity and mortality similar to those seen with higher doses of the drug in patients with preserved renal function.84 Limited data with carvedilol in a small parallel‐group trial also support the premise that much of the benefit with high target doses can be achieved with low starting doses.85 Observational data from the PARADIGM‐HF trial indicate that patients down‐titrated to low starting doses of sacubitril/valsartan achieved benefits comparable to those maintained at target doses of the drug.86
These findings, taken collectively, strongly suggest that the initiation of treatment with a low dose of a new drug class is likely to yield benefits on serious adverse heart failure outcomes that are greater than those that can be achieved by up‐titrating the dose of an existing therapy. Importantly, this conclusion is highly consistent with the design of all of the landmark heart failure trials, which enrolled and randomized patients to placebo or a new treatment, even though most participants were not receiving the highest tolerated doses of previously established foundational treatments.10
The analyses of the early effects of treatment in the large‐scale randomized trials with angiotensin‐converting enzyme inhibitors, beta‐blockers and mineralocorticoid receptor antagonists not only support the efficacy of low starting doses, but these findings also support the general conclusion that the benefits of foundational drugs are seen very rapidly following initiation of treatment. In addition to the early benefits seen in the SOLVD Treatment, COPERNICUS and EPHESUS trials (noted above), it is noteworthy that, in the PARADIGM‐HF trial with sacubitril/valsartan, the benefit of neprilysin inhibition to reduce the risk of hospitalization for heart failure was large and was statistically significant within 30 days following randomization.87 Similarly, the benefit of SGLT2 inhibitors to reduce the risk of hospitalization for heart failure was clinically meaningful and statistically significant within the first 2–4 weeks following randomization88; crucially, the starting doses and the target doses of SGLT2 inhibitors are identical. Trials using higher doses of SGLT2 inhibitors in patients with type 2 diabetes have not shown incremental benefits beyond those achieved with lower doses.31
These observations—derived from large‐scale randomized controlled trials with all four foundational drug classes for heart failure and a reduced ejection fraction—indicate that meaningful benefits accrue rapidly within a few weeks of initiating treatment, supporting the conclusion that delays in initiating treatment are inevitably accompanied by unnecessary death and hospitalization for heart failure. Importantly, a large proportion of these serious adverse outcomes can be prevented by the use of low or starting doses of angiotensin receptor–neprilysin inhibitors, beta‐blockers, mineralocorticoid receptor antagonists and SGLT2 inhibitors.
Conventional and novel sequencing strategies
Limitations of a conventional sequencing strategy
The conventional approach to the implementation of foundational therapy for heart failure and a reduced ejection fraction is based on two principles. First, physicians should initiate treatment with classes of drugs in the historical order in which the drugs were tested in clinical trials. Accordingly, patients should be started on a conventional inhibitor of the renin–angiotensin system, then initiated on treatment with a beta‐blocker and then a mineralocorticoid receptor antagonist, then switched to sacubitril/valsartan, and then started on an SGLT2 inhibitor. Second, practitioners should up‐titrate treatment with each drug to the highest tolerated dose and ensure tolerability with each drug class before moving onto the next drug class.3
Physicians may be surprised to learn that this conventional approach is not strongly evidence‐based, since (as noted earlier) large‐scale randomized controlled trials that demonstrated the efficacy of each new treatment typically enrolled patients who were not receiving the highest tolerated doses of previously established therapies. Furthermore, the conventional sequencing of drug treatment gives priority to drugs that were developed decades ago, even if drugs developed more recently proved to have meaningful advantages (in terms of efficacy, safety or ease‐of‐use) over earlier treatments. Physicians who believe that clinical practice should recapitulate the conditions of clinical trials should take note of the fact that the use of digoxin as background therapy has declined meaningfully over the years, even though most patients enrolled in the landmark trials with angiotensin‐converting enzyme inhibitors and beta‐blockers were treated with cardiac glycosides.
Most importantly, the conventional sequencing and up‐titration of drug treatments based on historical development requires a dedicated practitioner coupled with a strong patient–physician relationship that is capable of supporting numerous patient visits spaced over a period of 6 months or more. These prerequisites are distinctly uncommon in clinical practice, explaining why only a small fraction of patients with heart failure and a reduced ejection fraction are receiving all four foundational treatments in doses that are considered to be therapeutically effective.2
Development of a new rapid sequencing strategy
We propose a new sequencing strategy that is based on four key findings from large‐scale randomized controlled trials. First, since treatments act to reduce morbidity and mortality within 4 weeks of initiating therapy, patients should be started on all four foundational drug classes as rapidly as possibly, e.g. within 4 weeks. Second, since the efficacy of each foundational drug class is independent of treatment with the other drug classes, the historical sequence of drug development can be discarded, and the priority of drugs can be determined by considerations of relative efficacy, safety and ease‐of‐use. Third, low starting doses of foundational drugs have meaningful therapeutic benefits, and achievement of low starting doses of all four foundational drugs should take precedence over up‐titration of any individual drug class to target doses. Fourth, since drugs used for the treatment of heart failure can influence the tolerability of other foundational agents, proper sequencing can be based on whether agents started earlier can enhance the safety of agents started simultaneously or later in the sequence.
For all of the reasons enumerated above, we propose an accelerated three‐step approach, based on the assumptions that (i) patients have been stabilized with the use of diuretics to achieve clinical euvolaemia; and (ii) patients are not already receiving one or more of the foundational drugs for heart failure or for conditions that preceded the development of heart failure (e.g. hypertension, myocardial ischaemic disease, or type 2 diabetes).
Step 1. Simultaneous initiation of a beta‐blocker and an SGLT2 inhibitor
Based on the evidence from randomized controlled trials, which of the foundational agents should be initiated as the first step? In our view, the most serious and unexpected complication of heart failure and a reduced ejection fraction is sudden cardiac death, and the most effective and rapidly‐acting means of preventing such death is beta‐adrenergic blockade, presumably because beta‐blockers interfere with catecholamine‐related ventricular tachyarrhythmias.12, 13 Additionally, among the four foundational drug classes, beta‐blockers are most likely to exert favourable effects on left ventricular remodelling during long‐term treatment, yielding important recovery of a depressed ejection fraction.89, 90 However, for beta‐blockers to be initiated safely (regardless of their position in the sequence), physicians should ensure that patients are clinically euvolaemic before the start of treatment and that concurrently administered diuretics are promptly intensified if fluid retention (as evidenced by weight gain) occurs. Failure to do so may explain why initiation of beta‐blockade can result in early worsening of heart failure, which can be avoided (even in patients with severe symptoms) if clinical euvolaemia is achieved and maintained.14, 66
Because of the predilection of beta‐blockers to cause fluid retention, we favour a strategy where beta‐blockers are combined with an SGLT2 inhibitor as the first step in drug sequencing. As a result of their actions on sodium and glucose transport in the proximal renal tubule, SGLT2 inhibitors promote a short‐term osmotic diuresis,91, 92 which may contribute to the established ability of this drug class to reduce worsening heart failure events, a benefit that is seen rapidly following initiation of treatment.88 This early effect may help to minimize the risks of fluid retention that can be seen following initiation of treatment with a beta‐blocker. SGLT2 inhibitors also exert a striking nephroprotective effect, as evidenced by a slowing of the decline in glomerular filtration rate during long‐term therapy.76, 77 This favourable effect on kidney function is especially important since several other foundational drugs (e.g. angiotensin receptor blockers and mineralocorticoid receptor antagonists) often act to worsen renal function in patients with heart failure and a reduced ejection fraction.71, 74 Importantly, treatment with a beta‐blocker and an SGLT2 inhibitor does not require specialized safety monitoring or laboratory testing. As a result, the ability of patients to tolerate a combination of a beta‐blocker and an SGLT2 inhibitor can generally be fully established within 2 weeks of starting outpatient treatment with both classes of drugs.
Step 2. Initiation of an angiotensin receptor–neprilysin inhibitor
Sacubitril/valsartan has a rapid and profound effect to lower natriuretic peptides, a biomarker of cardiac wall stress, which may portend a beneficial effect on cardiac remodelling.93 When compared with placebo, the magnitude of the survival effect of sacubitril/valsartan is comparable with that seen with beta‐blockers (including a reduction in sudden death), but it is additive to the benefits of beta‐blockers.11 Adding a neprilysin inhibitor to an angiotensin receptor blocker greatly enhances the therapeutic effects of a conventional inhibitor of the renin–angiotensin system, while minimizing the possibility that inhibition of the renin–angiotensin system can worsen renal function.75 Furthermore, neprilysin inhibition can prevent the excess hyperkalaemia that can be seen with a mineralocorticoid receptor antagonist.78 The major concern of initiating treatment with sacubitril/valsartan is symptomatic hypotension, which can be minimized by the use of low doses followed by gradual up‐titration and/or by a reduction in the dose of concomitantly administered diuretics.72, 94 If hypotension is troublesome (i.e. when the pre‐treatment systolic blood pressure is <100 mmHg), it may be preferable to initiate treatment and establish the tolerability of valsartan alone, before switching patients to an angiotensin receptor–neprilysin inhibitor.94, 95
Step 3. Initiation of a mineralocorticoid receptor antagonist
Spironolactone and eplerenone reduce morbidity and mortality (including a benefit on sudden cardiac death), and major advantages of these drugs include once‐daily dosing, minimal up‐titration and a modest effect on blood pressure96; however, treatment with mineralocorticoid receptor antagonists can worsen renal function and produce hyperkalaemia.74, 97 Therefore, when starting a mineralocorticoid receptor antagonist, it seems advisable to have patients treated with a neprilysin inhibitor and an SGLT2 inhibitor, since these two drugs may mitigate the effect of spironolactone and eplerenone to worsen azotaemia and increase serum potassium, and thereby, increase the likelihood that patients can be maintained on long‐term treatment with a mineralocorticoid receptor antagonist.98
It is important to note that the sequencing of Step 2 and Step 3 can be appropriately modified under certain circumstances. For example, treatment with spironolactone or eplerenone may be considered as Step 2 and initiated before sacubitril/valsartan if the patient seems unlikely to tolerate the hypotensive effects of angiotensin receptor–neprilysin inhibition, e.g. patients with a systolic blood pressure <100 mmHg. Additionally, if a patient is already receiving treatment with a conventional inhibitor of the renin–angiotensin system, it is appealing to consider switching to sacubitril/valsartan and initiating treatment with a mineralocorticoid receptor antagonist at the same time. Simultaneous inhibition of the actions of neprilysin and aldosterone is not expected to produce any meaningful adverse interaction. By contrast, we would be reluctant to start sacubitril/valsartan and a mineralocorticoid receptor antagonist simultaneously in a patient who is not already receiving an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, since the combination can result in worsening renal function or hyperkalaemia unless the tolerability of a conventional inhibitor of the renin–angiotensin system has been firmly established in an individual patient.
Once all four foundational drugs have been initiated within 4 weeks, physicians can then increase the dose of each drug towards the target doses used in clinical trials, as tolerated by the patient. Such up‐titration may be particularly important with respect to the use of beta‐blockers. In the COMET trial, up‐titration of carvedilol to target doses provided incremental benefits with respect to all‐cause mortality when compared with medium doses of metoprolol tartrate; interestingly, this benefit could not be predicted by the achievement of a target heart rate, since the difference in resting heart rate between the carvedilol and metoprolol groups in that trial was only 1 bpm.19, 99
Alternative rapid sequencing strategies
Our proposal is one of many possible approaches to the rapid sequencing of foundational therapy for heart failure and a reduced ejection fraction. In many circumstances, it may be possible to maintain the order of our steps, but to achieve them more quickly; conceivably, in some patients, it may be possible to initiate all four classes of drugs within 4 days rather than 4 weeks. This possibility is particularly achievable if patients were already receiving one or more of the foundational drug classes, which were prescribed for heart failure or for a concurrent disorder. It is also reasonable to propose different sequences, e.g. to consider sacubitril/valsartan to represent a first step, either alone or in combination with an SGLT2 inhibitor, to be followed by simultaneous treatment with a beta‐blocker and a mineralocorticoid receptor antagonist as Step 2.100 However, we believe that it may not be very useful to debate the advantages and disadvantages of subtle differences in various rapid sequencing strategies. In the final analysis, if physicians achieve the goal of implementing four foundational drug classes within a few days or weeks, then the specific ordering of drug treatments should not matter very much.
Conclusions
The findings of large‐scale randomized controlled trials indicate that (i) the efficacy of each foundational drug is independent of treatment with other drugs; (ii) low starting doses of foundational drugs have substantial therapeutic benefits, and achievement of low starting doses of all four drug classes should take precedence over up‐titration of any individual drug class to target doses; (iii) treatments act to reduce morbidity and mortality very rapidly, i.e. within 4 weeks of initiating therapy; and (iv) certain drugs can influence the tolerability of other foundational agents, and thus, proper sequencing can enhance the safety of agents started later in the sequence. These observations have led us to propose a novel evidence‐based rapid sequencing strategy, which achieves initiation of all four foundational drug classes within 4 weeks. Alternative rapid sequencing strategies can be proposed, and even simultaneous initiation (as is recommended for patients with an acute myocardial infarction) may also be reasonable in selected patients. Regardless of the specific approach, rapid sequencing has the potential to dramatically improve the adoption and effective implementation of treatments that reduce the morbidity and mortality of patients with heart failure and a reduced ejection fraction.
Conflict of interest: M.P. and J.J.V.McM. have been the principal investigators of many of the trials described in this manuscript and have received financial support from the companies that sponsored these trials.
References
- 1.Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJ, Solomon SD. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121–128. [DOI] [PubMed] [Google Scholar]
- 2.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence‐based medicine. Circulation 2020;143:875–877. [DOI] [PubMed] [Google Scholar]
- 4.CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 7.Køber L, Torp‐Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 1995;333:1670–1676. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 10.Packer M. Kicking the tyres of a heart failure trial: physician response to the approval of sacubitril/valsartan in the USA. Eur J Heart Fail 2016;18:1211–1219. [DOI] [PubMed] [Google Scholar]
- 11.McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, Rizkala A, Shi V, Rouleau J, Solomon S, Swedberg K, Zile MR, Andersen K, Arango JL, Arnold M, Bĕlohlávek J, Böhm M, Boytsov S, Burgess L, Cabrera W, Chen CH, Erglis A, Fu M, Gomez E, Gonzalez A, Hagege AA, Katova T, Kiatchoosakun S, Kim KS, Bayram E, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires F, Refsgaard J, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire I, Starling RC, Vinereanu D, Teerlink JR, Wong R; PARADIGM‐HF Committees and Investigators . A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J 2015;36:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 13.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 14.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann‐Zalan I, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group . Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 16.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT‐HF). MERIT‐HF Study Group. JAMA 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 17.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 18.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 19.Poole‐Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp‐Pedersen C, Scherhag A, Skene A; Carvedilol Or Metoprolol European Trial Investigators . Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7–13. [DOI] [PubMed] [Google Scholar]
- 20.Chadda K, Goldstein S, Byington R, Curb JD. Effect of propranolol after acute myocardial infarction in patients with congestive heart failure. Circulation 1986;73:503–510. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 22.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 23.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 24.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO‐DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 26.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 27.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME trial. Eur Heart J 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 29.Furtado RH, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Ruff CT, Nicolau JC, Gause‐Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 2019;139:2516–2527. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 32.Heerspink HJ, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JF, McMurray JJ, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 34.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 35.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 36.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 37.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 38.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 39.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ; COAPT Investigators . Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 40.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JG, Dargie HJ, Granger CB, Kjekshus J, Køber L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJ. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 41.Packer M, Grayburn PA. Neurohormonal and transcatheter repair strategies for proportionate and disproportionate functional mitral regurgitation in heart failure. JACC Heart Fail 2019;7:518–521. [DOI] [PubMed] [Google Scholar]
- 42.Packer M, Grayburn PA. Contrasting effects of pharmacological, procedural, and surgical interventions on proportionate and disproportionate functional mitral regurgitation in chronic heart failure. Circulation 2019;140:779–789. [DOI] [PubMed] [Google Scholar]
- 43.Grayburn PA, Packer M, Sannino A, Stone GW. Disproportionate secondary mitral regurgitation: myths, misconceptions and clinical implications. Heart 2020. Nov 24. 10.1136/heartjnl-2020-316992 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44.Packer M. Neurohormonal antagonists are preferred to an implantable cardioverter‐defibrillator in preventing sudden death in heart failure. JACC Heart Fail 2019;7:902–906. [DOI] [PubMed] [Google Scholar]
- 45.Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J 2020;41:1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Packer M. Nonarrhythmic sudden cardiac death in chronic heart failure – a preventable event? JAMA Cardiol 2019;4:721–722. [DOI] [PubMed] [Google Scholar]
- 47.Packer M. Compelling first‐line drug and device therapies for the prevention of sudden death in patients with chronic heart failure and a reduced ejection fraction who are candidates for an implantable cardioverter‐defibrillator. Circ Arrhythm Electrophysiol 2019;12:e007430. [DOI] [PubMed] [Google Scholar]
- 48.Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin‐receptor‐neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 2015;36:1990–1997. [DOI] [PubMed] [Google Scholar]
- 49.Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJ, Van Veldhuisen DJ, Pitt B, Zannad F. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left‐ventricular systolic dysfunction: an individual patient‐level meta‐analysis of three randomized‐controlled trials. Clin Res Cardiol 2019;108:477–486. [DOI] [PubMed] [Google Scholar]
- 50.Al‐Gobari M, Al‐Aqeel S, Gueyffier F, Burnand B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: an overview of systematic reviews. BMJ Open 2018;8:e021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packer M. Major reduction in the risk of sudden cardiac death in patients with chronic heart failure with the use of drug and device combinations that favourably affect left ventricular structure. Eur J Heart Fail 2019;21:823–826. [DOI] [PubMed] [Google Scholar]
- 52.Poole JE, Olshansky B, Mark DB, Anderson J, Johnson G, Hellkamp AS, Davidson‐Ray L, Fishbein DP, Boineau RE, Anstrom KJ, Reinhall PG, Packer DL, Lee KL, Bardy GH; SCD‐HeFT Investigators . Long‐term outcomes of implantable cardioverter‐defibrillator therapy in the SCD‐HeFT. J Am Coll Cardiol 2020;76:405–415. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CS, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 54.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 55.Teerlink JR, Diaz R, Felker GM, McMurray JJ, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JG, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE; GALACTIC‐HF Investigators . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021;384:105–116. [DOI] [PubMed] [Google Scholar]
- 56.Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 57.Fox K, Ford I, Steg PG, Tendera M, Ferrari R; BEAUTIFUL Investigators . Ivabradine for patients with stable coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;372:807–816. [DOI] [PubMed] [Google Scholar]
- 58.Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN; African‐American Heart Failure Trial Investigators . Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–2057. [DOI] [PubMed] [Google Scholar]
- 59.Packer M. Are the benefits of SGLT2 inhibitors in heart failure and a reduced ejection fraction influenced by background therapy? Expectations and realities of a new standard of care. Eur Heart J 2020;41:2393–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Bruner‐La Rocha HP, Janssens S, Tsutsui H, Zhang J, Zannad F, Brueckmann M, Jamal W, Cotton D, Iwata T, Schnee J, Zannad F; EMPEROR‐Reduced Trial Committees and Investigators . Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR‐Reduced trial. Eur Heart J 2021;42:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM; Valsartan in Acute Myocardial Infarction Trial Investigators . Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 62.McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, Shao Q, Tarnesby G, Massie BM; ATMOSPHERE Committees Investigators . Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- 63.Packer M, McMurray JJ. Importance of endogenous compensatory vasoactive peptides in broadening the effects of inhibitors of the renin‐angiotensin system for the treatment of heart failure. Lancet 2017;389:1831–1840. [DOI] [PubMed] [Google Scholar]
- 64.Lee MM, Brooksbank KJ, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJ, Jhund PS, Petrie MC, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation 2020;143:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation 2020;141:2095–2105. [DOI] [PubMed] [Google Scholar]
- 66.Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, Ponikowski P, Skene A, van de Ven L, Verkenne P, Lechat P; CIBIS III Investigators . Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation 2005;112:2426–2435. [DOI] [PubMed] [Google Scholar]
- 67.Krum H, van Veldhuisen DJ, Funck‐Brentano C, Vanoli E, Silke B, Erdmann E, Follath F, Ponikowski P, Goulder M, Meyer W, Lechat P, Willenheimer R; CIBIS III Investigators . Effect on mode of death of heart failure treatment started with bisoprolol followed by enalapril, compared to the opposite order: results of the randomized CIBIS III trial. Cardiovasc Ther 2011;29:89–98. [DOI] [PubMed] [Google Scholar]
- 68.Packer M, Kessler PD, Gottlieb SS. Adverse effects of converting‐enzyme inhibition in patients with severe congestive heart failure: pathophysiology and management. Postgrad Med J 1986;62 Suppl 1:179–182. [PubMed] [Google Scholar]
- 69.Packer M, Poole‐Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin‐converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 70.Packer M, Lee WH, Yushak M, Medina N. Comparison of captopril and enalapril in patients with severe chronic heart failure. N Engl J Med 1986;315:847–853. [DOI] [PubMed] [Google Scholar]
- 71.Packer M, Lee WH, Medina N, Yushak M, Kessler PD. Functional renal insufficiency during long‐term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med 1987;106:346–354. [DOI] [PubMed] [Google Scholar]
- 72.Pharithi RB, Ferre‐Vallverdu M, Maisel AS, O'Connell E, Walshe M, Sweeney C, Barton J, McDonald K, O'Hare D, Watson C, Gallagher J, Ledwidge M, McDonald K. Sacubitril‐valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail 2020;7:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sackner‐Bernstein JD. Use of carvedilol in chronic heart failure: challenges in therapeutic management. Prog Cardiovasc Dis 1998;41:53–58. [DOI] [PubMed] [Google Scholar]
- 74.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD; RALES Investigators . Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012;60:2082–2089. [DOI] [PubMed] [Google Scholar]
- 75.Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJ. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail 2018;6:489–498. [DOI] [PubMed] [Google Scholar]
- 76.Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkely B, O'Meara E, Schou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJ. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA‐HF. Circulation 2021;143:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, Schnee J, Wanner C, Packer M. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from the EMPEROR‐Reduced trial. Circulation 2021;143:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol 2017;2:79–85. [DOI] [PubMed] [Google Scholar]
- 79.Kristensen SL, Docherty KF, Jhund PS, Bengtsson O, Demets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD, McMurray JJ. Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: a secondary analysis DAPA‐HF. Eur Heart J 2020;41 (Suppl 2):939 (abstr). [Google Scholar]
- 80.Lam PH, Packer M, Fonarow GC, Faselis C, Allman RM, Morgan CJ, Singh SN, Pitt B, Ahmed A. Early effects of starting doses of enalapril in patients with chronic heart failure in the SOLVD Treatment trial. Am J Med 2020;133:e25–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krum H, Roecker EB, Mohacsi P, Rouleau JL, Tendera M, Coats AJ, Katus HA, Fowler MB, Packer M; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group . Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS study. JAMA 2003;289:712–718. [DOI] [PubMed] [Google Scholar]
- 82.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, Vincent J; EPHESUS Investigators . Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005;46:425–431. [DOI] [PubMed] [Google Scholar]
- 83.Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, Poole‐Wilson PA; HEAAL Investigators . Effects of high‐dose versus low‐dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double‐blind trial. Lancet 2009;374:1840–1848. [DOI] [PubMed] [Google Scholar]
- 84.Ferreira JP, Abreu P, McMurray JJ, van Veldhuisen DJ, Swedberg K, Pocock SJ, Vincent J, Lins K, Rossignol P, Pitt B, Zannad F. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS‐HF trial. Eur J Heart Fail 2019;21:345–351. [DOI] [PubMed] [Google Scholar]
- 85.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807–2816. [DOI] [PubMed] [Google Scholar]
- 86.Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD; Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) Investigators . Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bělohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez‐Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC; PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 88.Packer M, Anker SD, Butler J, Filippatos GS, Ferreira JP, Pocock S, Carson PE, Anand IS, Doehner W, Haass M, Komajda M, Miller AB, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F; EMPEROR‐Reduced Trial Committees and Investigators . Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR‐reduced trial. Circulation 2021;143:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park CS, Park JJ, Mebazaa A, Oh IY, Park HA, Cho HJ, Lee HY, Kim KH, Yoo BS, Kang SM, Baek SH, Jeon ES, Kim JJ, Cho MC, Chae SC, Oh BH, Choi DJ. Characteristics, outcomes, and treatment of heart failure with improved ejection fraction. J Am Heart Assoc 2019;8:e011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Packer M, Antonopoulos GV, Berlin JA, Chittams J, Konstam MA, Udelson JE. Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta‐analysis. Am Heart J 2001;141:899–907. [DOI] [PubMed] [Google Scholar]
- 91.Scholtes RA, Muskiet MH, van Baar MJ, Hesp AC, Greasley PJ, Karlsson C, Hammarstedt A, Arya N, van Raalte DH, Heerspink HJ. Natriuretic effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT trial. Diabetes Care 2021;44:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boorsma EM, Beusekamp JC, Ter Maaten JM, Figarska SM, Danser AH, van Veldhuisen DJ, van der Meer P, Heerspink HJL, Damman K, Voors AA. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail 2021;23:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD; PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;322:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Senni M, McMurray JJ, Wachter R, McIntyre HF, Anand IS, Duino V, Sarkar A, Shi V, Charney A. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018;20:491–500. [DOI] [PubMed] [Google Scholar]
- 95.Vardeny O, Claggett B, Kachadourian J, Pearson SM, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJ, Solomon SD. Incidence, predictors, and outcomes associated with hypotensive episodes among heart failure patients receiving sacubitril/valsartan or enalapril: the PARADIGM‐HF Trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail 2018;11:e004745. [DOI] [PubMed] [Google Scholar]
- 96.Serenelli M, Jackson A, Dewan P, Jhund PS, Petrie MC, Rossignol P, Campo G, Pitt B, Zannad F, Ferreira JP, McMurray JJ. Mineralocorticoid receptor antagonists, blood pressure, and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2020;8:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, Barany P, Jernberg T, Lund LH, Carrero JJ. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018;20:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferreira JP, Zannad F, Pocock SJ, Anker S, Butler J, Filippatos G, Brueckmann M, Jamal W, Steubl D, Schueler E, Packer M. Interplay of mineralocorticoid receptor antagonists and empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR‐Reduced trial. J Am Coll Cardiol 2021;77:1397–1407. [DOI] [PubMed] [Google Scholar]
- 99.Packer M. Does a target dose or a target heart rate matter to outcomes when prescribing beta‐blockers to patients with chronic heart failure? Circ Cardiovasc Qual Outcomes 2018;11:e004605. [DOI] [PubMed] [Google Scholar]
- 100.Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:772–810. [DOI] [PubMed] [Google Scholar]


