Abstract
We report outcomes of 60 patients with steroid-refractory (SR)-aGVHD treated with pentostatin. Almost half (47%) of patients had grade 4 GVHD - 22% had stage 3-4 liver GVHD and 51% had stage 3-4 lower gastrointestinal tract (LGI) GVHD. Patients received a median of 3 courses (range, 1-9) of pentostatin. Day 28 overall response rate (ORR) was 33% (n=20) (complete response 18% (n=11), partial response 15% (n=9)). Non-relapse mortality was 72% (95% confidence interval (CI) 61-84%) and overall survival (OS) was 21% (95% CI 12- 32%) at 18 months. On univariate analysis, age >60 years (HR 1.9, 95% CI 1.01-3.7, p=0.045) and presence of liver GVHD (HR 1.9, 95% CI 1.9, 95% CI 1.5-3.3, p=0.03) were significant predictors of poor OS while patients with LGI GVHD had superior OS than those without (HR 0.4, 95% CI 0.2-0.8, p=0.01). On stratified analysis, patients <60 years with isolated LGI GVHD had the best outcomes with an ORR of 48% and OS of 42% at 18 months. Among older patients, OS was 14% in those with isolated LGI aGVHD and 0% in others. Pentostatin remains a viable treatment option for SR-aGVHD, especially in patients 60 years or younger with isolated LGI involvement.
INTRODUCTION
The most common and serious complication of allogeneic hematopoietic stem cell transplant (HSCT) is acute graft versus host disease (aGVHD). Typically manifesting within the first 100 days following HSCT, multiple organs can be affected including the skin, gastrointestinal tract, and/or liver. Systemic steroids such as prednisone or methylprednisolone at a dose of 1-2 mg/kg ideal body weight are offered as initial therapy for patients with grades II-IV aGVHD.1 However, only approximately one-third to half of these patients will respond to initial steroid therapy depending on the grade and organs involved.2-6 Roughly half of the patients who do not respond to initial steroid therapy respond to second-line therapy but their mortality rates approximate 70%.6
For patients with steroid-refractory (SR) aGVHD, various additional immunosuppressive medications have been tried with no therapy of proven superiority over others.6 Pentostatin, a potent adenosine deaminase (ADA) inhibitor has also been studied for GVHD prevention, therapy of newly diagnosed aGVHD, and SR- acute and chronic GVHD.7-11 However, previous reports on the role of pentostatin in SR-aGVHD have been limited by short term follow-up and small sample sizes.10, 12-15
Although patients with SR-aGVHD generally have dismal outcomes, long-term survival does occur in select cases. In this study, we sought to determine the factors associated with improved responses, early mortality and long term survival after pentostatin use in patients with SR-aGVHD.
METHODS
Patient Selection
All patients who received pentostatin for the treatment of SR-aGVHD from January 2006 to December 2014 at M.D. Anderson Cancer Center (MDACC) were reviewed. Criteria for inclusion in this analysis included receiving at least one dose of pentostatin as second- or third-line treatment following progression or failure to respond to initial therapy with steroids. Patients who received pentostatin solely for GVHD prevention (prophylaxis study) or on a clinical trial for initial therapy of aGVHD were excluded from this analysis.
Variables of Interest and Data Source
Patient characteristics were retrieved from the departmental database and verified by chart review, including age, gender, indication for transplant, conditioning regimen, graft source, GVHD prophylaxis regimen, aGVHD start date, organ stage, overall grade and preceding and concurrent GVHD therapy at time of administration of pentostatin.
Objectives
Our objectives in this analysis were to (a) determine response rate to pentostatin received in the second or third line setting following failure to respond to frontline steroids for aGVHD, (b) evaluate overall survival (OS) and non-relapse mortality (NRM), and (c) determine predictors of response, early mortality and OS.
Treatment Regimen
All patients received pentostatin at a dose of at 1.5 mg/m2 on days 1-3 (defined as one course), repeated every two weeks as indicated. Upon initiation of pentostatin, patients were usually continued on systemic steroids, usually at a tapered dose as clinically warranted, as well as concomitant GVHD therapy which they were already receiving. These agents included tacrolimus, cyclosporine, mycophenolate mofetil (MMF), extracorporeal photopheresis (ECP) or their combination.
Response Assessment
All patients underwent pathological evaluation for the diagnosis of GVHD. In case of discrepancy between pathological and clinical diagnosis, clinical criteria were used. Acute GVHD was graded per Glucksberg criteria16 and refers to clinical (not pathological) grading and/or staging. Steroid refractory aGVHD was defined as progression of aGVHD after 72 hours of initiation of steroids or no improvement after 7 days. The response to pentostatin was determined at 28 and 56 days from the first day of administration of pentostatin. Complete response (CR) was defined as complete resolution in all organs without development of new organ involvement. A partial response (PR) was defined as improvement in one GVHD organ by one GVHD stage without worsening in any additional organs. A mixed response was defined as improvement (PR or CR) in one organ with progression in a second organ by at least one clinical stage. No response was defined as no improvement or worsening in any organ. Progression was defined as worsening by one stage in one or more organs without improvement in any additional organ or requiring new, additional agents to control GVHD. Overall response rate (ORR) was defined as CR plus PR. Patients who died or had disease progression before day 28 were considered non-responders.
Statistical Analysis
Descriptive analyses were performed to summarize clinical and demographic characteristics of the patient population. The cumulative incidence of NRM was determined using relapse or death in relapse as competing risks. Actuarial OS was estimated using the Kaplan-Meier method. Cox’s proportional hazards regression analysis was used on univariate and multivariate analysis to evaluate predictors of survival after the initiation of pentostatin. Competing risk regression analysis (considering progression of underlying malignancy before day 28 as competing risk) was used to evaluate predictors of day 28 response after the initiation of pentostatin. Factors that were significant on univariate analysis were considered in multivariate analysis. Statistical significance was set at the 0.05 level. Statistical analyses were performed using primarily STATA 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp L). All outcomes were measured from the first day of administration of pentostatin.
RESULTS
Patient Characteristics
A total of 60 patients received pentostatin as second (n=22; 36.7%) or third line (n=38; 63.3%) treatment for SR-aGVHD. Table 1 demonstrates baseline characteristics for the study population. Median age at the time of start of pentostatin was 52 years (range, 2-70). Two patients were younger than 18 years and about a quarter (n=14) were over the age of 60 years. A majority of patients (63%) received myeloablative conditioning and granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood progenitor cells as the graft source (77%). Tacrolimus and methotrexate was the most common GVHD prophylaxis regimen used (75%), followed by tacrolimus plus MMF (22%). The median time to start of pentostatin was 69 days post-transplant (range, 27-595) and 15 days (range, 4-172) from the start of steroids. Patients received a median of 3 cycles of pentostatin (range, 1-9). All patients received additional concurrent GVHD therapy at the start of pentostatin, with tacrolimus (n=28) and tacrolimus plus MMF (n=21) being the most common drugs. Nine patients were also on ECP. A majority of patients had lower gastrointestinal tract (LGI) GVHD (80%, n=47) and liver was involved in 44% (n=26), which was stage 3-4 in half of the cases. Consequently, 82% (n=49) of patients had an overall grade 3-4 GVHD. Approximately half (n=31) of the population had an infection at the time of pentostatin initiation, including viral infections (n=16) such as BK cystitis, HHV-6, adenovirus and cytomegalovirus reactivation/disease, fungal sinusitis/pneumonia (n=2), bacterial infections (n=7) and others. Two patients were in the Intensive Care Unit when pentostatin was first administered, detailing the severity of illness in this patient population. The median follow-up in survivors was 19 months (range 7-77).
Table 1.
Baseline characteristics at first dose of pentostatin.
| N=60 | (%) | |
|---|---|---|
| Age at pentostatin, median (range), in years | 52 (2-70) | |
| Gender mismatched | ||
| Female-to-male | 25 | 41.7% |
| Diagnosis | ||
| AML/MDS | 14 | 23% |
| ALL | 13 | 22% |
| Lymphoma | 14 | 23% |
| CLL | 10 | 17% |
| Other | 9 | 15% |
| Graft source | ||
| Cord Blood | 4 | 7% |
| Peripheral Blood | 46 | 77% |
| Bone Marrow | 10 | 17% |
| Donor type | ||
| HLA-matched related | 19 | 31.7% |
| HLA-matched unrelated | 35 | 58.3% |
| Haploidentical | 3 | 5% |
| Cord blood | 3 | 5% |
| Preparative Regimen Intensity | ||
| Non-myeloablative | 22 | 37% |
| Myeloablative | 38 | 63% |
| GVHD prophylaxis | ||
| Tacrolimus/Methotrexate | 45 | 75% |
| Tacrolimus/MMF +/− PT Cy | 13 | 21.7% |
| PT Cy | 2 | 3.3% |
| Prophylactic in vivo T cell depletion | ||
| Anti-thymocyte globulin | 30 | 50% |
| Alemtuzumab | 5 | 8.33% |
| Time to pentostatin after transplant, median days | 69 | |
| (range) | (27-595) | |
| Time to pentostatin after first line Steroid, median days | 15 | |
| (range) | (4-172) | |
| Pentostatin Line of Therapy | ||
| Second | 22 | 37% |
| Third | 38 | 63% |
| 2ndline treatment (in patients who received pentostatin as 3rd line) | ||
| MMF | 17 | 45% |
| Photopheresis | 19 | 50% |
| Infliximab | 1 | 3% |
| Cyclosporine | 1 | 3% |
| Cycles of Pentostatin, median | 3 | |
| (range) | (1-9) | |
| Concurrent GVHD Therapy | ||
| Tacrolimus | 28 | 46% |
| Tacrolimus/MMF | 21 | 35% |
| Cyclosporine | 1 | 2% |
| Cyclosporine/MMF | 1 | 2% |
| Tacrolimus/Photopheresis | 9 | 15% |
| GVHD overall grade at time of initiation of pentostatin | ||
| 2 | 11 | 18% |
| 3 | 21 | 35% |
| 4 | 28 | 47% |
| Skin GVHD Stage | N=12 | |
| 1-2 | 9 | 15% |
| 3 | 3 | 5% |
| UGI GVHD Stage | N=27 | |
| 1 | 27 | 45% |
| LGI GVHD Stage | N=47 | |
| 1-2 | 16 | 27% |
| 3-4 | 31 | 51% |
| Liver GVHD Stage | N=26 | |
| 1-2 | 13 | 22% |
| 3-4 | 13 | 22% |
| GVHD organ combination | ||
| LGI only | 28 | 46.7% |
| Liver only | 10 | 16.7% |
| LGI + Liver | 10 | 16.7% |
| LGI + Liver + Skin | 6 | 10% |
| Skin + LGI | 4 | 6.7% |
| Skin + Liver | 1 | 1.7% |
| Skin only | 1 | 1.7% |
| Follow-up among survivors, median (range), months | 19 (7-77) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; HLA, Human Leucocyte Antigen; LGI, Lower Gastrointestinal tract; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; PT Cy, Post-transplant cyclophosphamide; UGI Upper Gastrointestinal tract.
Start of pentostatin within 10 days of steroids improves responses
A total of 24 (40%) patients died (n=22) before day 28 and were considered non-responders; 2 patients had progression of the underlying malignancy (competing risk) and were considered non-evaluable. In evaluable patients, ORR at day 28 was 33.3% (n=20) including CR in 11 patients (18.3%) and PR in 9 (15%) [Table 2]. In univariate analysis assessing the predictors of overall response at day 28, we found that initiation of pentostatin within 10 days following start of steroids was the only significant factor (Hazard ratio (HR) 2.2, 95% confidence interval (CI) 1.2-4.3, p=0.02). The ORR was 50% in those who received pentostatin within 10 days of steroids (n=20) as compared to 25% in those who were started on pentostatin more than 10 days after the start of steroids (n=40). As time to start of pentostatin was the only significant factor in univariate analysis, multivariate analysis was not performed.
Table 2.
Responses to Pentostatin at day 28 and 56.
| Day 28 | Day 56 | ||||||
|---|---|---|---|---|---|---|---|
| N=60 | (%) | N= 60 | (%) | ||||
| Complete Response | 11 | 18% | 16 | 27% | |||
| Partial Response | 9 | 15% | 3 | 5% | |||
| No Response | 11 | 18% | 4 | 7% | |||
| Progression | 5 | 9% | 6 | 10% | |||
| Died prior to index day | 22 | 37% | 31 | 52% | |||
| Progression of malignancy | 2 | 3% | 0 | 0% | |||
| Day 56 response | |||||||
| Day 28 response |
Complete
Response |
Partial
Response |
No response | Progression | N.E. | Total | |
| Complete Response | 9 | 1 | 0 | 1 | 0 | 11 | |
| Partial Response | 3 | 2 | 3 | 1 | 0 | 9 | |
| No response | 4 | 0 | 1 | 2 | 4 | 11 | |
| Progression | 0 | 0 | 0 | 2 | 3 | 5 | |
| N.E. | 0 | 0 | 0 | 0 | 24 | 24 | |
| Total | 16 | 3 | 4 | 6 | 31 | 60 | |
N.E., non-evaluable
Overall survival is poor and non-relapse mortality is high in patients with SR-aGVHD
The univariate estimates of NRM were 37% (95% CI 26-51) at day 30, 63% (95% CI 52-77) at 6 months and 72% (95% CI, 61-84) at 18 months. The univariate estimates of OS were 62% (95% CI 48- 73%) at day 30, 30% (95% CI 19-42) at 6 months and 21% (95% CI 12- 32%) at 18 months. Only 10 patients were alive at the end of the study period. Major causes of death included aGVHD (n= 23), chronic GVHD (n=16), infection (n=4), recurrence or persistent of underlying malignancy (n=4), second malignancy (n=1), graft rejection or failure (n=1), and multi-organ failure (n=1).
Age <60 years and presence of LGI GVHD are significant predictors of survival after pentostatin
Next, we explored factors associated with a risk of early mortality after starting pentostatin and factors associated with long-term survival. In univariate analysis, two factors that emerged as significant predictors of OS at day 30 [Supplemental table 1] and at 18 months [Table 3] were age and the type of organ involved. Patients older than 60 years had significantly poor OS compared with younger patients (HR 1.9, 95% CI 1.01-3.7, p=0.045, at 18 months). [Fig 1] Day 28 ORR and OS at 18-months in patients with isolated LGI GVHD (43% and 35%, respectively) were similar to that in patients with liver plus LGI GVHD (50% and 20%, respectively), while patients with isolated liver GVHD (10% and 10%, respectively) or any other GVHD (17% and 8%, respectively) had inferior responses and survival. [Table 4].
Table 3.
Univariate analysis for overall survival at 18 months.
| n | Hazard Ratio | 95% confidence interval |
P-value | |
|---|---|---|---|---|
| First line steroids to Pentostatin, days | ||||
| <=10 days | 20 | Reference | ||
| >10 days | 40 | 1.1 | 0.6-2.0 | 0.8 |
| Pentostatin used as | ||||
| second line therapy | 22 | Reference | ||
| third line therapy | 38 | 0.9 | 0.5-1.7 | 0.8 |
| Age at Pentostatin | ||||
| <=60 years | 46 | Reference | ||
| >60 years | 14 | 1.9 | 1.01-3.7 | 0.045 |
| Female-to-male gender Mismatch | ||||
| No | 35 | Reference | ||
| Yes | 25 | 0.7 | 0.2-1.9 | 0.4 |
| Donor type | ||||
| HLA-matched related | 19 | 0.7 | 0.2-2.2 | 0.5 |
| HLA-matched unrelated | 35 | |||
| Haploidentical | 3 | |||
| Cord blood | 3 | |||
| Graft source | ||||
| Bone marrow | 10 | Reference | ||
| Peripheral blood | 46 | 1.3 | 0.6-2.9 | 0.5 |
| Cord blood | 4 | Excluded | ||
| Conditioning | ||||
| Myeloablative | 38 | Reference | ||
| Non-myeloablative | 22 | 1.4 | 0.8-2.6 | 0.2 |
| Overall grade (at diagnosis) | ||||
| Grade 2 | 11 | Reference | ||
| Grade 3-4 | 49 | 1.9 | 0.9-4.3 | 0.1 |
| GVHD organs | ||||
| Skin (Reference no skin) | 12 | 2 | 1.02-4.1 | 0.04 |
| UGI (Reference no UGI) | 27 | 0.95 | 0.5-1.7 | 0.9 |
| LGI (Reference no LGI) | 47 | 0.4 | 0.2-0.8 | 0.01 |
| Liver (Reference no liver) | 26 | 1.9 | 1.05-3.3 | 0.03 |
| GVHD organ combination | ||||
| LGI only | 28 | Reference | ||
| Liver only | 10 | 3.3 | 1.4-7.3 | 0.004 |
| LGI + Liver | 10 | 1.4 | 0.6-3.3 | 0.4 |
| LGI + Liver + Skin | 6 | 3.1 | 1.02-9.6 | 0.05 |
| Skin + LGI | 4 | 2.5 | 0.96-6.3 | 0.06 |
| Skin only | 1 | Excluded | ||
| Skin + Liver | 1 | Excluded |
Figure 1:
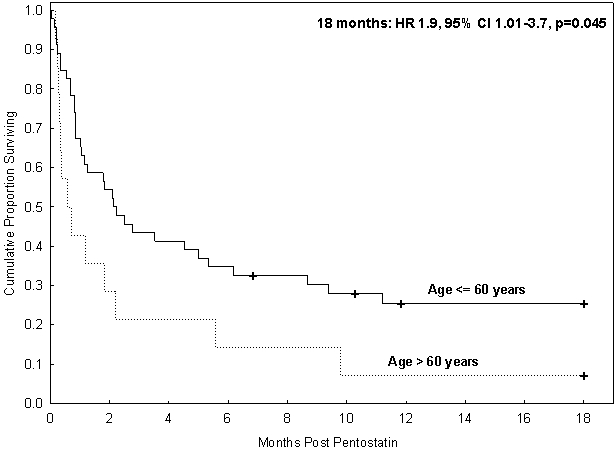
Overall survival by age
Table 4:
Day 28 overall response rate, overall survival at day 30 and overall survival at 18 months by risk factors
| N | Day 28 | Day 30 | 18 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % CR/PR | P-value | % OS | HR | 95% CI | P-value | % OS | HR | 95% CI | P-value | ||
| ALL PATIENTS | |||||||||||
| LGI only | 28 | 43% | 0.01 $ | 75% | Reference | 35% | Reference | ||||
| Liver + LGI | 10 | 50% | 60% | 2.1 | 0.6-7.1 | 0.2 | 20% | 1.4 | 0.6-3.2 | 0.4 | |
| Liver only | 10 | 10% | 30% | 4.9 | 1.7-14 | 0.003 | 10% | 3.3 | 1.5-7.4 | 0.004 | |
| All others * | 12 | 17% | 58% | 1.7 | 0.5-5.5 | 0.3 | 8% | 2.8 | 1.3-5.9 | 0.009 | |
| YOUNGER PATIENTS ONLY (Age ≤60 years) | |||||||||||
| LGI only | 21 | 48% | 0.04 $ | 86% | Reference | 42% | Reference | ||||
| Liver + LGI | 8 | 50% | 62% | 3.6 | 0.7-18 | 0.1 | 25% | 1.6 | 0.6-4.4 | 0.3 | |
| Liver only | 7 | 14% | 43% | 6.0 | 1.3-27 | 0.02 | 14% | 2.9 | 1.1-8 | 0.03 | |
| All Others ** | 10 | 20% | 50% | 4.2 | 0.9-18 | 0.05 | 0% | 3.8 | 1.6-9.3 | 0.003 | |
| OLDER PATIENTS ONLY (Age ≥ 60 years) | |||||||||||
| LGI only | 7 | 29% | 0.50 | 43% | 0.7 | 0.2-2.9 | 0.7 | 14% | 0.6 | 0.2-1.8 | 0.4 |
| All Others *** | 7 | 14% | 43% | 0% | |||||||
Abbreviations: CI, confidence interval; CR, complete response; HR, hazard ratio; LGI, lower gastrointestinal tract; PR, partial response
includes skin only (n=1), liver + skin (n=1), LGI + skin (n=4), liver + LGI + skin (n=6)
includes liver + skin (n=1), LGI + skin (n=4), liver + LGI + skin (n=5)
includes skin only (n=1), liver only (n=3), liver + LGI (n=2), liver + LGI + skin (n=1)
p-value denotes difference between “LGI only or Liver + LGI” versus other groups.
As age and the type of organ involved were the only factors that predicted survival, we performed further analyses stratified by these two factors. In patients 60 years or younger, OS at 18 months was 42% in those with isolated LGI GVHD, 25% in patients with liver plus LGI GVHD and 14% in those with isolated liver GVHD. There were no survivors with any other organ combination at 18 months. [Table 4, Fig 2a] Among older patients, 14% of patients with isolated LGI GVHD were alive at 18 months compared with 0% with any other GVHD. [Table 4, Fig 2b]
Figure 2:
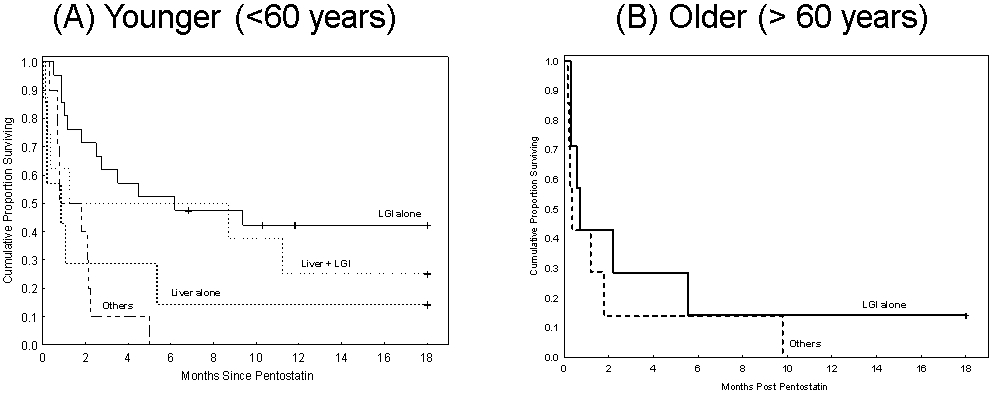
Overall survival stratified by age and GVHD organ
Causes of death by age group are summarized in supplemental table 2. Organ toxicities and infections observed at day 56, which may be related to GVHD and/or treatment, are summarized in supplemental table 3.
DISCUSSION
We present the largest retrospective series of patients who received pentostatin for SR-aGVHD. Our findings suggest that there is a specific subset of patients that benefits from pentostatin while a specific group is destined for early mortality following its use. Younger patients (age ≥ 60 years) with isolated LGI GVHD (day 28 ORR 48%, OS 42% at 18 months) or (to a lesser extent) liver plus LGI GVHD (day 28 ORR 50%, OS 25% at 18 months) appear to benefit the most from pentostatin. In contrast, patients older than 60 years uniformly had poor survival, suggesting pentostatin should be used with caution in this population. It is likely that co-morbidities, frailty and the toxicities of prior GVHD therapies (namely steroids) in older aged patients make it challenging for positive outcomes. Even in those older patients who responded (29% of those with isolated lower GI) survival was poor (14% OS at 18 months). We also found that although early initiation of pentostatin within 10 days of steroids improved ORR, it did not impact survival. Response to treatment with early initiation of therapy and superior outcomes in younger patients may be related to the biology of the disease and the host characteristics rather than pentostatin, as is true for most GVHD therapies. Moreover, survival was not influenced by the overall GVHD grade, the type of conditioning, graft source or if pentostatin was used as a second line or third line agent, highlighting the general poor prognosis of patients who fail to respond to steroids.
Given the dismal prognosis of patients with SR–aGVHD, multiple strategies either alone or in combination have been explored in several distinct series in the past, including the use of ATG,17-22 IL-2 receptor antagonists (daclizumab, basiliximab, inolimomab),23-32 denileukin diftitox,7, 33, 34 TNF-α receptor antagonists (infliximab, etanercept), 31, 35-40 alemtuzumab,41-45 sirolimus,46, 47 ECP, 48-53 MMF 7, 54-57 and pentostatin 9, 11, 13-15, 58 among others. In a comprehensive recent review, Martin el at 6 described outcomes after various drugs used for SR–aGVHD and provided benchmarks to assess response and survival for future trials. Combining 25 to 29 different studies, the authors reported an aggregated CR rate of 32%, an aggregated ORR of 58% and an estimated 6 months weighted average survival of 49%.6 Of note, none of these studies used pentostatin.
The role of pentostatin as a treatment for SR-aGVHD in adults was evaluated in 5 studies [Table 5]. These studies vary remarkably not only in the patient population, but also in terms of the dose and the number of pentostatin cycles used, additional therapies for GVHD, continuation of steroids, definition of SR–aGVHD and the assessment of response. For instance, two studies that reported a CR rate of 64-70% assessed “best response”,10, 12 while other studies that assessed either day 28 response or durable response lasting 4 weeks reported CR of 13-33% and PR of 13-17%.13-15 Nevertheless, one common denominator across all studies was an extremely poor prognosis of these patients with a median survival of less than 3 months. In our study, where almost half (47%) of patients had an overall grade 4 SR-aGVHD, 44% had liver involvement, over 50% had documented infection at the start of pentostatin and a majority (63%) received pentostatin as a third line treatment, we observed modest responses with CR rate of 20%, CR/PR rate of 33% and OS of 30% (95% CI 19-42) at 6 months.
Table 5:
Studies evaluating the role of pentostatin in adults with steroid refractory acute GVHD
| Reference | Sampl e size |
Dose of pentostatin |
Number of cycles |
Liver GVHD |
Median time from GVHD to pentostati n use, days (range) |
Median time from steroid initiation to pentostatin use, days (range) |
Response assessment |
Complete response |
Partial response |
Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Bolaños-Meade et al | 23 | 1 mg/m2 (n= 10), 1.5 mg/m2 (n= 5), 2 mg/m2 (n= 7) 3 mg/m2 (n=1). |
1 (74%) 2 (26%) |
10 (43%) | 34 (5–140) | - | Best response assessed weekly till day 28 | 64% | 30% | Median 85 days from therapy |
| Pidala et al | 12 | 1 mg/m2 (n=2) 1.5 mg/m2 (n=2) 2 mg/m2 (n=3) 4 mg/m2 (n=5) |
2 (range; 1-8) | 5 (42%) | - | 42 (13–213) | durable, sustained for 4 weeks | 33% | 17% | Median 1.4 months from therapy |
| Alam et al. | 15 | 1.4 mg/m2 | 1 (7%) 2 (20%) 3 (73%) |
6 (40%) | 33 (7–292) | - | durable, sustained for 4 weeks | 13% | 20% | Median 82 days from SCT |
| Klein et al. | 23 | 1 mg/m2 | 1 (57%) 2 (22%) 3 (17%) 4 (4%) |
5 (22%) | - | - | Best response assessed weekly till day 28 | 70% | 13% | Median 85 days from therapy |
| Schmitt el at | 24 | 1 mg/m2 | 1 (67%) 2 (23%) |
6 (25%) | 78 (5-211) | 10 (3-42) | Day 28 | 21% (in GI GVHD symptoms) | 17% “VGPR” (in GI GVHD symptoms) | 6 months OS 25%; 2 year OS 17% |
| Current study | 60 | 1.5 mg/m2 | 3 (range; 1–9) | 26 (44%) | 69 (27–5 95) | 15 (4–172) | Day 28 | 18% | 15% | Median OS: 55 days |
Pentostatin inhibits cellular ADA which is an essential enzyme for the metabolism of purines. As this mechanism of action is distinct from that of other GVHD drugs, pentostatin offers a rationale non-overlapping treatment strategy for patients with SR-aGVHD. However, it is highly myelo- and lympho- suppressive, has high rates of infection and NRM. One of the main reasons for dismal prognosis in these patients is high risk of infections due to significant T- lymphopenia caused by pentostatin. However, in our study, despite the presence of infection in over half of our patients at the start of pentostatin, only 4 patients had an infection-related mortality. This is harmonious with pre-clinical data suggesting that although pentostatin can interfere with the induction of new humoral responses (after exposure to drug), it may actually enhance already established humoral responses (before exposure to drug). This can also be explained by the differential impact of pentostatin on various immune subsets –relative sparing and increased activity of natural killer (NK) cells, helper T cells, antigen presenting B-cells and increased phagocytic activity of macrophages that may improve outcomes of certain infections.59-63 As such, presence of an active infection at the start of pentostatin should not be considered as an absolute contraindication to its use. Yet, exposure to any immunosuppressive agent in already notably immunocompromised GVHD patients does carry significant risk of infections and aggressive monitoring and treatment of infections is of paramount importance.
We acknowledge limitations of our study most of which are inherent in its retrospective nature. As anticipated in the absence of established standard of care, patients received different lines of rescue therapies before or with pentostatin. Patients with LGI plus liver GVHD had better responses (but not survival) than those with isolated liver GVHD. This finding is rather perplexing and cannot be explained from this exploratory analysis, but may possibly be related to differential mechanism of action of pentostatin on different organs. Also, given the complicated course of patients with SR-aGVHD who are commonly affected by multiple complications that contribute to NRM, it is difficult to detach the side-effects of GVHD treatment from that of GVHD itself. Moreover, despite being the largest analysis of the use if pentostatin for SR-aGVHD, this is a single center experience. Evaluating patients across multiple institutions that employ pentostatin in this setting would further elucidate characteristics and outcomes in this setting.
CONCLUSION
In summary, patients younger than 60 years with isolated LGI aGVHD had the best outcomes with pentostatin for SR-aGVHD. However, the overall long term outcome of these patients remains dismal. All patients should be encouraged to participate in clinical trials assessing the role of novel agents for treatment of SR-aGVHD. Many of these drugs have shown encouraging results in preliminary reports and are under active investigation, such as the JAK1/2 inhibitor ruxolitinib,64 (ClinicalTrials.gov Identifier NCT02953678, NCT02913261), monoclonal antibody against CD26 on CD4+ T lymphocytes - Begelomab (NCT02411084), monoclonal antibody against integrin α4β7 (LPAM-1, lymphocyte Peyer's patch adhesion molecule 1) - Vedolizumab (NCT02993783), ultra-low dose IL-2 (NCT00529035) and the infusion of mesenchymal stem cells (NCT00603330, NCT02770430), to name a few. In the absence of clinical trial, pentostatin remains as one of the therapeutic options especially in patients 60 years old or younger with steroid-refractory isolated LGI aGVHD.
Supplementary Material
Footnotes
DISCLOSURES
The authors have no relevant conflicts of interest
REFERENCES
- 1.Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood 2009; 113(13): 2888–2894. doi: 10.1182/blood-2008-07-168401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant 2002; 8(7): 387–394. [DOI] [PubMed] [Google Scholar]
- 3.Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation 1991; 51(6): 1197–1203. [DOI] [PubMed] [Google Scholar]
- 4.Hings IM, Severson R, Filipovich AH, Blazar BR, Kersey JH, Ramsay NK et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation 1994; 58(4): 437–442. [DOI] [PubMed] [Google Scholar]
- 5.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood 1991; 77(8): 1821–1828. [PubMed] [Google Scholar]
- 6.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant 2012; 18(8): 1150–1163. doi: 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 2009; 114(3): 511–517. doi: 10.1182/blood-2009-03-212290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar S, Andersson BS, Couriel D, Munsell MF, Fernandez-Vina M, Jones RB et al. Prophylaxis of graft-versus-host disease in unrelated donor transplantation with pentostatin, tacrolimus, and mini-methotrexate: a phase I/II controlled, adaptively randomized study. J. Clin. Oncol 2011; 29(3): 294–302. doi: 10.1200/JCO.2010.30.6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsohn DA, Chen AR, Zahurak M, Piantadosi S, Anders V, Bolanos-Meade J et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J. Clin. Oncol 2007; 25(27): 4255–4261. doi: 10.1200/JCO.2007.10.8456 [DOI] [PubMed] [Google Scholar]
- 10.Bolaños-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC et al. Pentostatin in Steroid-Refractory Acute Graft-Versus-Host Disease. J. Clin. Oncol 2005; 23(12): 2661–2668. doi: 10.1200/jco.2005.06.130 [DOI] [PubMed] [Google Scholar]
- 11.Jacobsohn DA, Gilman AL, Rademaker A, Browning B, Grimley M, Lehmann L et al. Evaluation of pentostatin in corticosteroid-refractory chronic graft-versus-host disease in children: a Pediatric Blood and Marrow Transplant Consortium study. Blood 2009; 114(20): 4354–4360. doi: 10.1182/blood-2009-05-224840 [DOI] [PubMed] [Google Scholar]
- 12.Klein S MS, Bug G, Wassmann B, Martin H. Long-Term follow up of a phase II study of pentostatin in patients with steroid refractory acute intestinal graft-versus-host disease. Bone Marrow Transplant. 2008; 41(Suppl 1): S222 (abstract 773). [Google Scholar]
- 13.Pidala J, Kim J, Roman-Diaz J, Shapiro J, Nishihori T, Bookout R et al. Pentostatin as rescue therapy for glucocorticoid-refractory acute and chronic graft-versus-host disease. Ann. Transplant 2010; 15(4): 21–29. [PubMed] [Google Scholar]
- 14.Schmitt T, Luft T, Hegenbart U, Tran TH, Ho AD, Dreger P. Pentostatin for treatment of steroid-refractory acute GVHD: a retrospective single-center analysis. Bone Marrow Transplant. 2011; 46(4): 580–585. doi: 10.1038/bmt.2010.146 [DOI] [PubMed] [Google Scholar]
- 15.Alam N, Atenafu EG, Tse G, Viswabandya A, Gupta V, Kim D et al. Limited benefit of pentostatin salvage therapy for steroid-refractory grade III-IV acute graft-versus-host disease. Clin. Transplant 2013; 27(6): 930–937. doi: 10.1111/ctr.12268 [DOI] [PubMed] [Google Scholar]
- 16.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18(4): 295–304. [DOI] [PubMed] [Google Scholar]
- 17.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol. Blood Marrow Transplant 2002; 8(3): 155–160. doi: 10.1053/bbmt.2002.v8.pm11939605 [DOI] [PubMed] [Google Scholar]
- 18.MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NK et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol. Blood Marrow Transplant 2002; 8(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 19.Van Lint MT, Milone G, Leotta S, Uderzo C, Scime R, Dallorso S et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood 2006; 107(10): 4177–4181. doi: 10.1182/blood-2005-12-4851 [DOI] [PubMed] [Google Scholar]
- 20.Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001; 27(10): 1059–1064. doi: 10.1038/sj.bmt.1703032 [DOI] [PubMed] [Google Scholar]
- 21.Roy J, McGlave PB, Filipovich AH, Miller WJ, Blazar BR, Ramsay NK et al. Acute graft-versus-host disease following unrelated donor marrow transplantation: failure of conventional therapy. Bone Marrow Transplant. 1992; 10(1): 77–82. [PubMed] [Google Scholar]
- 22.Macmillan ML, Couriel D, Weisdorf DJ, Schwab G, Havrilla N, Fleming TR et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood 2007; 109(6): 2657–2662. doi: 10.1182/blood-2006-08-013995 [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood 2000; 95(1): 83–89. [PubMed] [Google Scholar]
- 24.Massenkeil G, Rackwitz S, Genvresse I, Rosen O, Dorken B, Arnold R. Basiliximab is well tolerated and effective in the treatment of steroid-refractory acute graft-versus-host disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002; 30(12): 899–903. doi: 10.1038/sj.bmt.1703737 [DOI] [PubMed] [Google Scholar]
- 25.Nadeau M, Perreault S, Seropian S, Foss F, Isufi I, Cooper DL. The use of basiliximab-infliximab combination for the treatment of severe gastrointestinal acute GvHD. Bone Marrow Transplant. 2016; 51(2): 273–276. doi: 10.1038/bmt.2015.247 [DOI] [PubMed] [Google Scholar]
- 26.Perales MA, Ishill N, Lomazow WA, Weinstock DM, Papadopoulos EB, Dastigir H et al. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transplant. 2007; 40(5): 481–486. doi: 10.1038/sj.bmt.1705762 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Hieber M, Fietz T, Knauf W, Uharek L, Hopfenmuller W, Thiel E et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br. J. Haematol 2005; 130(4): 568–574. doi: 10.1111/j.1365-2141.2005.05631.x [DOI] [PubMed] [Google Scholar]
- 28.Socie G, Vigouroux S, Yakoub-Agha I, Bay JO, Furst S, Bilger K et al. A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD. Blood 2017; 129(5): 643–649. doi: 10.1182/blood-2016-09-738625 [DOI] [PubMed] [Google Scholar]
- 29.Wang JZ, Liu KY, Xu LP, Liu DH, Han W, Chen H et al. Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation. Transplant. Proc 2011; 43(5): 1928–1933. doi: 10.1016/j.transproceed.2011.03.044 [DOI] [PubMed] [Google Scholar]
- 30.Willenbacher W, Basara N, Blau IW, Fauser AA, Kiehl MG. Treatment of steroid refractory acute and chronic graft-versus-host disease with daclizumab. Br. J. Haematol 2001; 112(3): 820–823. [DOI] [PubMed] [Google Scholar]
- 31.Rao K, Rao A, Karlsson H, Jagani M, Veys P, Amrolia PJ. Improved survival and preserved antiviral responses after combination therapy with daclizumab and infliximab in steroid-refractory graft-versus-host disease. J. Pediatr. Hematol. Oncol 2009; 31(6): 456–461. doi: 10.1097/MPH.0b013e31819daf60 [DOI] [PubMed] [Google Scholar]
- 32.Bay JO, Dhedin N, Goerner M, Vannier JP, Marie-Cardine A, Stamatoullas A et al. Inolimomab in steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: retrospective analysis and comparison with other interleukin-2 receptor antibodies. Transplantation 2005; 80(6): 782–788. [DOI] [PubMed] [Google Scholar]
- 33.Shaughnessy PJ, Bachier C, Grimley M, Freytes CO, Callander NS, Essell JH et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol. Blood Marrow Transplant 2005; 11(3): 188–193. doi: 10.1016/j.bbmt.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 34.Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood 2004; 104(4): 1224–1226. doi: 10.1182/blood-2004-01-0028 [DOI] [PubMed] [Google Scholar]
- 35.van Groningen LF, Liefferink AM, de Haan AF, Schaap NP, Donnelly JP, Blijlevens NM et al. Combination Therapy with Inolimomab and Etanercept for Severe Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant 2016; 22(1): 179–182. doi: 10.1016/j.bbmt.2015.08.039 [DOI] [PubMed] [Google Scholar]
- 36.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol 2007; 82(1): 45–52. doi: 10.1002/ajh.20752 [DOI] [PubMed] [Google Scholar]
- 37.Patriarca F, Sperotto A, Damiani D, Morreale G, Bonifazi F, Olivieri A et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica 2004; 89(11): 1352–1359. [PubMed] [Google Scholar]
- 38.Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood 2004; 104(3): 649–654. doi: 10.1182/blood-2003-12-4241 [DOI] [PubMed] [Google Scholar]
- 39.Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: a case series. Am. J. Hematol 2003; 74(2): 119–124. doi: 10.1002/ajh.10392 [DOI] [PubMed] [Google Scholar]
- 40.Kennedy GA, Butler J, Western R, Morton J, Durrant S, Hill GR. Combination antithymocyte globulin and soluble TNFalpha inhibitor (etanercept) +/− mycophenolate mofetil for treatment of steroid refractory acute graft-versus-host disease. Bone Marrow Transplant. 2006; 37(12): 1143–1147. doi: 10.1038/sj.bmt.1705380 [DOI] [PubMed] [Google Scholar]
- 41.Meunier M, Bulabois CE, Thiebaut-Bertrand A, Itzykson R, Carre M, Carras S et al. Alemtuzumab for severe steroid-refractory gastrointestinal acute graft-versus-host disease. Biol. Blood Marrow Transplant 2014; 20(9): 1451–1454. doi: 10.1016/j.bbmt.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 42.Khandelwal P, Lawrence J, Filipovich AH, Davies SM, Bleesing JJ, Jordan MB et al. The successful use of alemtuzumab for treatment of steroid-refractory acute graft-versus-host disease in pediatric patients. Pediatr. Transplant 2014; 18(1): 94–102. doi: 10.1111/petr.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schub N, Gunther A, Schrauder A, Claviez A, Ehlert C, Gramatzki M et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011; 46(1): 143–147. doi: 10.1038/bmt.2010.68 [DOI] [PubMed] [Google Scholar]
- 44.Martinez C, Solano C, Ferra C, Sampol A, Valcarcel D, Perez-Simon JA et al. Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol. Blood Marrow Transplant 2009; 15(5): 639–642. doi: 10.1016/j.bbmt.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, Gonzalez-Llano O, Gutierrez-Aguirre H, Cantu-Rodriguez O et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol. Blood Marrow Transplant 2008; 14(1): 10–15. doi: 10.1016/j.bbmt.2007.08.052 [DOI] [PubMed] [Google Scholar]
- 46.Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation 2001; 72(12): 1924–1929. [DOI] [PubMed] [Google Scholar]
- 47.Hoda D, Pidala J, Salgado-Vila N, Kim J, Perkins J, Bookout R et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010; 45(8): 1347–1351. doi: 10.1038/bmt.2009.343 [DOI] [PubMed] [Google Scholar]
- 48.Pierelli L, Perseghin P, Marchetti M, Messina C, Perotti C, Mazzoni A et al. Extracorporeal photopheresis for the treatment of acute and chronic graft-versus-host disease in adults and children: best practice recommendations from an Italian Society of Hemapheresis and Cell Manipulation (SIdEM) and Italian Group for Bone Marrow Transplantation (GITMO) consensus process. Transfusion (Paris) 2013; 53(10): 2340–2352. doi: 10.1111/trf.12059 [DOI] [PubMed] [Google Scholar]
- 49.Kitko CL, Braun T, Couriel DR, Choi SW, Connelly J, Hoffmann S et al. Combination Therapy for Graft-versus-Host Disease Prophylaxis with Etanercept and Extracorporeal Photopheresis: Results of a Phase II Clinical Trial. Biol. Blood Marrow Transplant 2016; 22(5): 862–868. doi: 10.1016/j.bbmt.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malagola M, Cancelli V, Skert C, Leali PF, Ferrari E, Tiburzi A et al. Extracorporeal Photopheresis for Treatment of Acute and Chronic Graft Versus Host Disease: An Italian Multicentric Retrospective Analysis on 94 Patients on Behalf of the Gruppo Italiano Trapianto di Midollo Osseo. Transplantation 2016; 100(12): e147–e155. doi: 10.1097/TP.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 51.Abu-Dalle I, Reljic T, Nishihori T, Antar A, Bazarbachi A, Djulbegovic B et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol. Blood Marrow Transplant 2014; 20(11): 1677–1686. doi: 10.1016/j.bbmt.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 52.Jagasia M, Greinix H, Robin M, Das-Gupta E, Jacobs R, Savani BN et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol. Blood Marrow Transplant 2013; 19(7): 1129–1133. doi: 10.1016/j.bbmt.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 53.Perfetti P, Carlier P, Strada P, Gualandi F, Occhini D, Van Lint MT et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008; 42(9): 609–617. doi: 10.1038/bmt.2008.221 [DOI] [PubMed] [Google Scholar]
- 54.Pidala J, Kim J, Perkins J, Field T, Fernandez H, Perez L et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant. 2010; 45(5): 919–924. doi: 10.1038/bmt.2009.252 [DOI] [PubMed] [Google Scholar]
- 55.Furlong T, Martin P, Flowers ME, Carnevale-Schianca F, Yatscoff R, Chauncey T et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009; 44(11): 739–748. doi: 10.1038/bmt.2009.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann. Hematol 2005; 84(10): 681–685. doi: 10.1007/s00277-005-1070-0 [DOI] [PubMed] [Google Scholar]
- 57.Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur. J. Haematol 2004; 73(1): 56–61. doi: 10.1111/j.1600-0609.2004.00247.x [DOI] [PubMed] [Google Scholar]
- 58.Bolanos-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J. Clin. Oncol 2005; 23(12): 2661–2668. doi: 10.1200/JCO.2005.06.130 [DOI] [PubMed] [Google Scholar]
- 59.Thaler J, Grunewald K, Gattringer C, Ho AD, Weyrer K, Dietze O et al. Long-term follow-up of patients with hairy cell leukaemia treated with pentostatin: lymphocyte subpopulations and residual bone marrow infiltration. Br. J. Haematol 1993; 84(1): 75–82. [DOI] [PubMed] [Google Scholar]
- 60.Bagasra O, Howeedy A, Pomerantz RJ. Adenosine-deaminase-associated immunodeficiency. I. Differential sensitivities of lymphocyte subpopulations exposed to 2-deoxycoformycin in vivo. Clin. Exp. Immunol 1992; 88(3): 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraut EH, Neff JC, Bouroncle BA, Gochnour D, Grever MR. Immunosuppressive effects of pentostatin. J. Clin. Oncol 1990; 8(5): 848–855. doi: 10.1200/JCO.1990.8.5.848 [DOI] [PubMed] [Google Scholar]
- 62.Luebke RW, Lawson LD, Rogers RR, Riddle MM, Rowe DG, Smialowicz RJ. Selective immunotoxic effects in mice treated with the adenosine deaminase inhibitor 2'-deoxycoformycin. Immunopharmacology 1987; 13(1): 25–35. [DOI] [PubMed] [Google Scholar]
- 63.Nicholson JK, Gordon DS, McDougal JS. Inhibition of adenosine deaminase leads to enhanced antibody responses in the mouse. Cell. Immunol 1983; 79(2): 320–333. [DOI] [PubMed] [Google Scholar]
- 64.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015; 29(10): 2062–2068. doi: 10.1038/leu.2015.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


