Abstract
Pure intravascular growth of epithelioid hemangioma (EH) is exceptional. Herein, we report a series of 21 intravascular EHs, representing a potential serious diagnostic pitfall by mimicking malignant vascular neoplsms with epithelioid morphology. The tumors developed in 12 males and 4 females, aged from 11 to 71 years (mean age 40.2 yrs) with a predilection for the extremities (13 of 21, 61.9%), followed by the head and neck (8 of 21, 38.1%). Lesions ranged in size from 2 to 30 mm (mean size 13 mm). The most common presenting feature was a slowly growing nodule. Most neoplasms were solitary (13 of 16 patients, 81.2%) but three patients developed more than one intravascular EH (3 of 16, 18.8%). Treatment consisted of complete surgical excision and was generally curative. Follow-up was available for 13 lesions that had developed in 10 patients (range 4 to 72 months, mean 27.3 months). No recurrences or development of additional tumors were observed. All 21 lesions developed in subcutaneous veins. Two morphological patterns of intravascular epithelioid endothelial cell proliferation were observed: 1) a lobular capillary hemangioma-like proliferation with variable formation of open vascular lumina and 2) a solid proliferation generally lacking open vascular spaces. A lobular capillary hemangioma-like pattern was the sole pattern in 9 lesions, a mixed lobular hemangioma-like pattern and solid pattern in 8 and a pure solid pattern in 4 intravascular EHs. Mitotic activity in epithelioid endothelial cells ranged from 0 to 7 mitoses per 10 high-power field (mean 2.1 mitoses per 10 HPFs). Six lesions displayed brisk mitotic activity of 5 or more mitoses per 10 HPF (6 of 21, 28.5%). The number of mitoses was usually more prominent in areas with solid growth. Atypical mitoses were not observed. No intratumoral necroses were seen. Cytological atypia was mild (20 out of 21 cases). By immunohistochemistry, all tumors were positive for CD31 (14 out of 14) and ERG (5 out of 5). While all tested cases were FOS negative by immunohistochemistry (6 out of 6), one out of six cases (case 6) displayed FOSB nuclear positivity in about 30% of the lesional endothelial cells. Eight cases were analysed by FISH for the presence of FOS and FOSB gene rearrangements. While all cases were negative for FOSB rearrangements, a single case proved positive for FOS gene break-apart. In conclusion, intravascular growth of EH is not associated with adverse biological behavior. Solid intravascular proliferations of endothelial cells can mimic a malignant vascular tumor with epithelioid morphology. Nevertheless, intravascular EHs display mild cytological atypia coupled with low mitotic activity, and a lack of atypical mitoses, pronounced nuclear atypia, multilayering or tumor necrosis. Finally, the FOS gene is infrequently rearranged, and there are no FOSB gene abnormalities in this subset of EHs, suggesting a potential distinct pathogenesis than most classic EHs.
Keywords: epithelioid haemangioma, intravascular, skin, FOS, FOSB, gene rearrangement
Epithelioid hemangioma (EH) is a benign vascular tumor originally reported by Wells and Whimster in 1969 as subcutaneous angiolymphoid hyperplasia with eosinophilia.1 Various names have been used in the past for the same type of proliferation, including angiolymphoid hyperplasia with eosinophilia, inflammatory angiomatous nodule, pseudo or atypical pyogenic granuloma, papular angioplasia, subcutaneous angioblastic lymphoid hyperplasia with eosinophilia and lymphofolliculosis, intravenous atypical vascular proliferation and histiocytoid hemangioma, thereby reflecting uncertainties in its histogenesis and with regard to its neoplastic or reactive nature.2
EH has been reported at diverse anatomic locations, including visceral organs, bones, penis, arteries, heart, mucosal sites and colon.3–11 In the skin, tumors shows a striking predilection for the head and neck area (about 85% of cases), in particular periauricular skin and forehead and scalp. 2,3,12,13 Cutaneous EH presents as an erythematous plaque, papule or nodule, measuring in size from a few mm to several cm.2,3,12,13 Although EH is usually asymptomatic, presenting symptoms can occasionally include pruritus, pain, pulsation and spontaneous bleeding.2,12,13 About 80% of EHs are solitary lesions.14 However, multiple tumors can develop within the same anatomic area or at distant sites and can develop in an eruptive pattern.13,15 Lesions show a slight predilection for females, have a broad age distribution and most often develop in the 3rd to 4th decades of life.2,3 Peripheral eosinophilia and regional lymphadenopathy is observed in 10–20% of patients.1–3
Interestingly, over 60% of EHs are associated with focal intramural growth within an artery or vein.3 Much more infrequently, the tumor arises within the walls of larger arteries, including the brachial artery,16 ulnar artery,17,18 peripheral medium sized muscular artery of the forearm,19 temporal artery,20,21 popliteal artery22 and internal carotid artery23. Nevertheless, pure or predominantly intravascular occurrence is exceptional. The first three such examples were reported as ‘intravenous atypical vascular proliferation’ by Rosai and Akerman in 1974.24 Only a handful of intravascular EHs have been published since, as individual case reports.25,26
Intravascular EHs lacking extravascular tumor growth can pose significant diagnostic problems and represent a potential serious diagnostic pitfall by mimicking malignant vascular tumors with epithelioid morphology, especially when associated with a more solid proliferation of endothelial cells, e.g., epithelioid hemangioendothelioma or epithelioid angiosarcoma. We report herein a series of 21 pure intravascular HEs, occurring in 16 patients, analyse their salient morphological features and rearrangements of FOS and FOSB genes in a subset of the tumors and discuss differential diagnosis with a particular emphasis on intravascular epithelioid endothelial proliferations.
MATERIALS AND METHODS
Selection of cases
To be included in the study, EHs had to meet the following entry criteria: 1) localization in the dermis and/or subcutis, 2) endothelial cells should have a predominantly epithelioid morphology, representing more than 50% of the lesional cell population and 3) equal or more than 95% of the proliferation should be within the lumen of the vessel(s).
All the cases were retrieved from the consultation files of one of the authors (EC). Hematoxilin and eosin stained slides were reviewed independently by two of the authors (BL and EI). Particular emphasis was given to the following histological parameters: localization (dermis, subcutis), type of vessel(s) with intravascular proliferation (artery, vein), formation of primitive-appearing uncanalized (immature) vessels distinguished by solid areas of epithelioid endothelial cells with/without intracytoplasmic vacuoles, formation of mature vasculature characterized by open vascular spaces lined by epithelioid endothelial cells, mitotic activity (number of mitoses per 10 high power fields), nuclear atypia (absent/mild, moderate or severe) and inflammatory cell infiltrate (presence, composition, formation of lymphoid follicles). The vessels were considered arteries if an internal elastic lamina was present. Clinical information, including age, gender, site, size, symptoms, pre-operative duration, clinical diagnosis and follow-up data featuring the possible development of local recurrence(s) and disease outcome, were requested from the referring physicians in cases in which this information was missing. Descriptive statistical analysis was performed using the statistical programme SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, USA).
Immunohistochemical studies
Immunohistochemical staining was performed on 4μm-thick sections, cut from formalin-fixed and paraffin embedded tissue using a Ventana BenchMark XT automated stainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer’s protocol. The following antibodies were used: CD31 (monoclonal, clone JC70A, DAKO, dilution 1:15), ERG (monoclonal, clone 9FY, Biorare, dilution 1:40), smooth muscle actin (monoclonal, clone 1A4, Cell Marque, dilution 1:1000), desmin (monoclonal, clone D33, DAKO, dilution 1:20), H-caldesmon (monoclonal, clone h-CD, DAKO, dilution 1:50) and CK-MNF116 (monoclonal, clone MNF116, DAKO, dilution 1:100). In a selected number of cases with sufficient material, additional immunohistochemistry for FOS (polyclonal, Millipore, dilution 1:3000), FOSB (monoclonal, clone 5G4, Cell Signaling Technology, dilution 1:50), and CAMTA1 (polyclonal, clone NBP1–93620, Novas Biologicals, dilution 1:100) was performed retrospectively.
FISH analysis on FOS and FOSB gene re-arrangement
Eight cases with adequate material were analysed by FISH for the presence of FOS gene and FOSB gene rearrangements with break-apart custom-designed BAC probes, as described previously.27,28
RESULTS
Clinical features
The clinical data are summarized in Table 1. A total of 21 intravascular EHs occurring in 16 patients were studied. The tumor had developed in 12 males and 4 females, aged from 11 to 71 years (mean age 40.2 yrs). The size of the tumor at presentation ranged from 2 to 30 mm (n=11, mean size 13 mm). The majority of intravascular EHs arose on the extremities (13 out of 21, 61.9%), followed by the head and neck area (8 out of 21, 38.1%). The clinical history was available for 18 out of 21 lesions. The most common presenting feature was a slowly growing mass or nodule (n=17), which was specifically mentioned on two occasions to be painful. Rapid recent growth was observed in a single lesion. The preoperative duration ranged from 1 to 36 months (mean 7.7 months). Solitary lesions by far predominated (13 of 16 patients, 81.2%). Three patients developed more than one intravascular EH (3 of 16, 18.8%); two of them within the same anatomical area (ulnar site of the left hand – 3 lesions; right thumb/index finger and forearm – 3 lesions) and one at distant sites (right hand and left finger). The lesions within the same anatomical area developed synchronously. The patient (case 14) with a slowly growing lesion on the right hand developed a nodule on the left ring finger two months later. The clinical differential diagnoses were generally very broad, ranging from reactive conditions (cyst, ganglion, vasculitis) to neoplasms (lipoma, adnexal tumors, giant cell tumour, sarcoma). The possibility of vascular proliferation (malformation or haemangioma) was suggested in five instances (5 of 17, 29.4%).
Table 1.
Pure cutaneous intravascular epithelioid hemangioma, current series – clinical features and follow up
| No | Age | Sex | Site | Size (mm) | Symptoms | Clinical diagnosis | Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | 29 | F | POSTAURICULAR | 30 | NONE | VASCULAR MALFORMATION | NER at 12 mo |
| 2 | 53 | M | FOREHEAD | N/A | 1 YEAR STABLE MASS/NODULE | EPIDERMOID CYST | NER AT 13 MO |
| 3 | 24 | F | R ANKLE | N/A | 3 YRS NON TENDER NODULE | GANGLION | NER AT 18 MO |
| 4 | 66 | M | R WRIST | N/A | HYPERVASCULAR SOLID MASS/NODULE | SARCOMA | NEW CASE |
| 5 | 71 | M | L PREAURICULAR | 10 | N/A | N/A | NEW CASE |
| 6 | 48 | M | R MEDIAL CANTHUS | N/A | N/A | ?ADNEXAL TUMOR | NER AT 16 |
| 7 | 37 | M | R KNEE | 22 | PAINFUL TENDER NODULE | ?HAEMANGIOMA | NER AT 24 |
| 8 | 36 | F | L PALM | 10 | PAINFUL LESION | ?GANGLION | N/A |
| 9 | 34 | M | R FOREARM R THUMB R INDEX FINGER |
11 6 2 |
MULTIPLE LESIONS FROM 3 MONTHS | ?ADNEXAL TUMOR | NER AT 30 |
| 10 | 11 | M | L FOREARM | 8 | 3 MONTHS INTERMITTENTLY SWALLEN AND RED | ?LIPOMA, ?EPIDERMOID CYST, ?PILOMATRICOMA | NEW CASE |
| 11 | 51 | M | L EYEBROW | N/A | 4 WEEKS | ?INTRAVASCULAR TUMOR, ?HEMANGIOMA | NER AT 36 |
| 12 | 17 | M | POSTERIOR SCALP | 15 | 2.5 MONTHS | ?HAEMANGIOMA | NER AT 72 |
| 13 | 51 | M | NECK | 10 | 3 MONTHS RAPID GROWTH | ?CYST | NER AT 48 |
| 14 | 52 | M | R HAND L RING FINGER |
20 N/A |
SLOWLY GROWING LESION ON R HAND, FOLLOWED BY LESION ON L RING FINGER 2 MONTHS LATER | ?GIANT CELL TUMOR | NER AT 4 AND 2 |
| 15 | 20 | F | FOREHEAD | N/A | N/A | ?ARTERITIS | N/A |
| 16 | 44 | M | ULNAR L HAND | N/A | 4 WEEKS SLOW GROWING | N/A | N/A |
Legend: L: left, R: right, N/A: not available, NER: no evidence of recurrence, MO: months
Treatment in all cases consisted of surgical excision with free margins. A re-excision was performed in a single lesion due to incomplete primary excision (1 of 21, 4.7%). Follow-up was available for 13 lesions developing in 10 patients (range 4 to 72 months, mean 27.3 months). Three patients were too recent to obtain any relevant follow-up and follow-up data could not be obtained for an additional three patients. None of the patients developed local recurrence following complete surgical excision or developed additional lesions within the same anatomical areas or at distant sites during the follow-up period.
Histopathological features
Histopathological features are summarized in Table 2. All 21 proliferations developed in subcutaneous fatty tissue within a pre-existing vein. None of the tumors developed within an artery, since all of the vessels lacked convincing internal elastic lamina (also confirmed by van Gieson Weigert stain, data not shown). The vessel lumina were generally entirely obliterated by the vascular proliferation. In some instances, therefore, determining whether a proliferation was intravascular proved difficult on hematoxillin and eosin slides alone. Intravascular growth in such cases was confirmed by additional smooth muscle, desmin and/or H-caldesmon immunohistochemistry (see below). Minimal extravascular extension, amounting to less than 5% of the total endothelial cell proliferation, was noticed in 2 cases. In all of the proliferations, vascular channels were lined by a single layer of endothelial cells.
TABLE 2.
Pure cutaneous intravascular epithelioid hemangioma, current series – histological features
| No | DEPTH | TYPE OF VESSEL | GROWTH PATTERN | MITOTIC ACTIVITY (PER 10 HPH) | NUCLEAR ATYPIA | INTRALESIONAL INFLAMMATION | PERILESIONAL INFLAMMATION |
|---|---|---|---|---|---|---|---|
| 1 | SC | VEIN | CH-LIKE | 1 | MILD | MILD | MODERATE+GC |
| 2 | SC | VEIN | SOLID | 0 | MILD | MILD | MODERATE+GC |
| 3 | SC | VEIN | CH-LIKE SOLID (20%) | 1 | MILD | MILD | ABSENT |
| 4 | SC | VEIN | CH-LIKE SOLID (80%) | 7 | MILD | MILD | ABSENT |
| 5 | SC | VEIN | CH-LIKE | 5 | MILD | MILD | ABSENT |
| 6 | SC | VEIN | SOLID | 7 | MODERATE, FOCAL | MILD | ABSENT |
| 7 | SC | VEIN | SOLID | 1 | MILD | MILD | ABSENT |
| 8 | SC | VEIN | CH-LIKE SOLID (30%) | 5 | MILD | MODERATE | MODERATE+GC |
| 9 | SC SC SC |
VEIN VEIN VEIN |
CH-LIKE (PHOTO) CH-LIKE SOLID (60%) SOLID |
0 7 0 |
MILD MILD MILD |
MODERATE MODERATE MODERATE |
MODERATE+GC ABSENT ABSENT |
| 10 | SC | VEIN | CH-LIKE SOLID (30%) | 5 | MILD | MILD | ABSENT |
| 11 | SC | VEIN | CH-LIKE SOLID (80%) | 0 | MILD | MILD | MODERATE+GC |
| 12 | SC | VEIN | MATURE | 0 | MILD | MILD | ABSENT |
| 13 | SC | VEIN | CH-LIKE SOLID 20% | 1 | MILD | MILD | MODERATE+GC |
| 14 | SC SC |
VEIN VEIN |
CH-LIKE CH-LIKE |
1 1 |
MILD MILD |
MODERATE MODERATE |
ABSENT ABSENT |
| 15 | SC | VEIN | CH-LIKE | 0 | MILD | SEVERE | ABSENT |
| 16 | SC SC SC |
VEIN VEIN VEIN |
CH-LIKE CH-LIKE CH-LIKE |
1 1 1 |
MILD MILD MILD |
ABSENT ABSENT ABSENT |
ABSENT ABSENT ABSENT |
Legend: SC: subcutis, ch-like: capillary hemangioma-like, GC: germinal center
Two morphological patterns of intravascular epithelioid endothelial cell proliferation were observed: 1) a lobular capillary hemangioma-like proliferation with a variable formation of open vascular lumina and 2) a solid proliferation generally lacking open vascular spaces. Both patterns frequently co-existed within a single lesion, albeit in variable proportions. The first morphological pattern, the lobular capillary hemangioma-like pattern, consisted of a proliferation of capillary-sized vessels lined by a single layer of epithelioid endothelial cells, frequently arranged in a lobular, or vaguely lobular growth pattern. Although lumina formation was usually readily present, an admixture of well- and poorly-canalized capillaries was typical. The second morphological pattern was characterized by a solid proliferation of epithelial endothelial cells, without a lobular arrangement of capillaries and without the formation of open vascular spaces, thereby obscuring the vascular lineage of the proliferation.
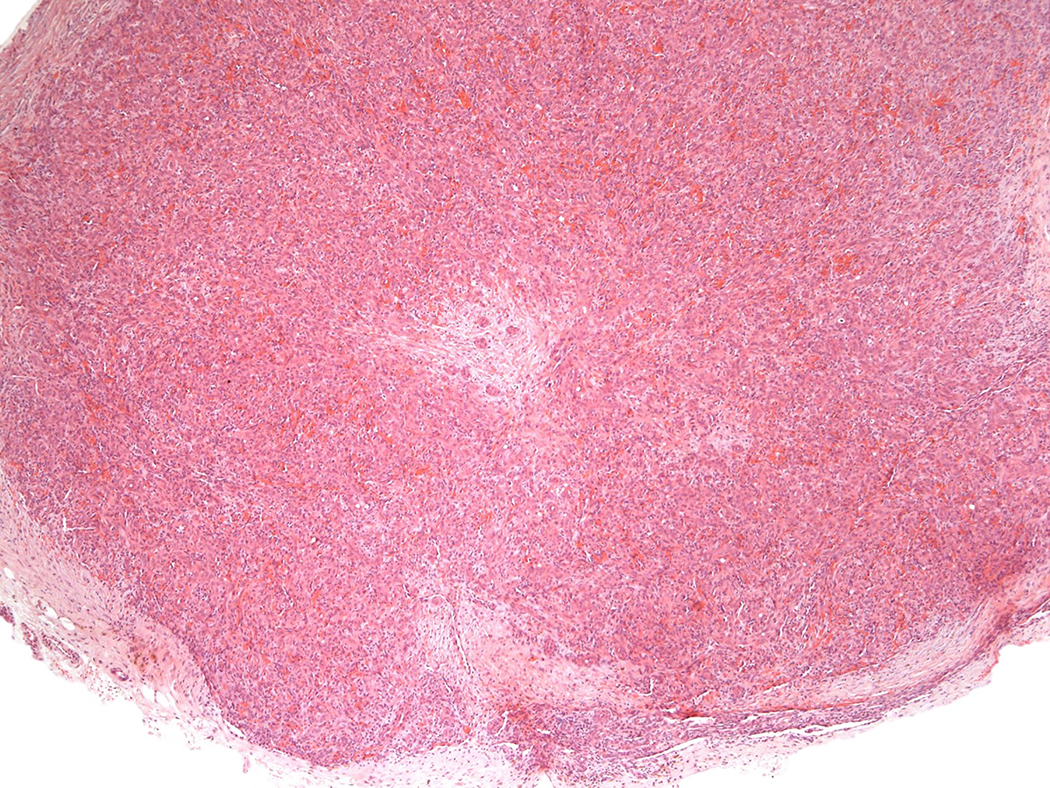
A lobular capillary hemangioma-like pattern was the sole pattern in 9 proliferations (9 of 21) (Figures 1A–C), a mixed lobular hemangioma-like pattern and solid pattern in 8 proliferations (8 of 21) (Figures 2A–D) and a pure solid pattern in four (4 out of 21) intravascular EHs (Figures 3A–B). A solid growth pattern was present in 11 intravascular EHs (11 out of 21, 47.6%); while in 8 of these proliferations, the solid component amounted to 30 to 80% of the total proliferation; four lesions were composed entirely of a solid arrangement of epithelioid endothelial cells.
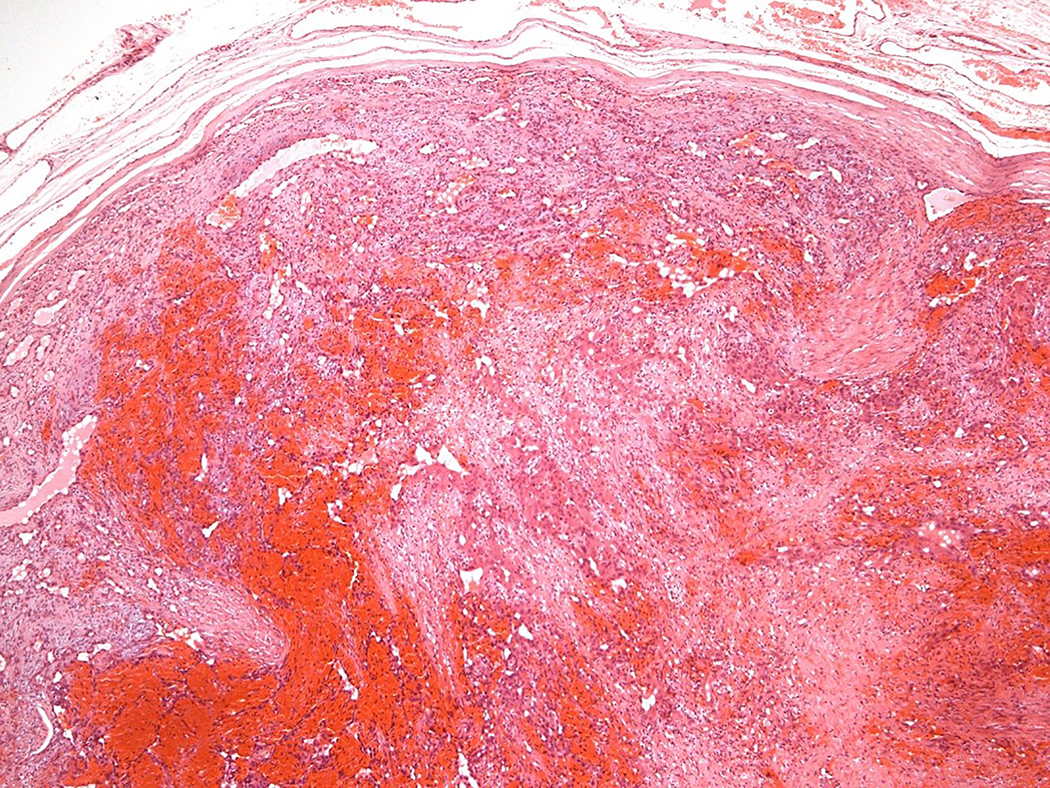
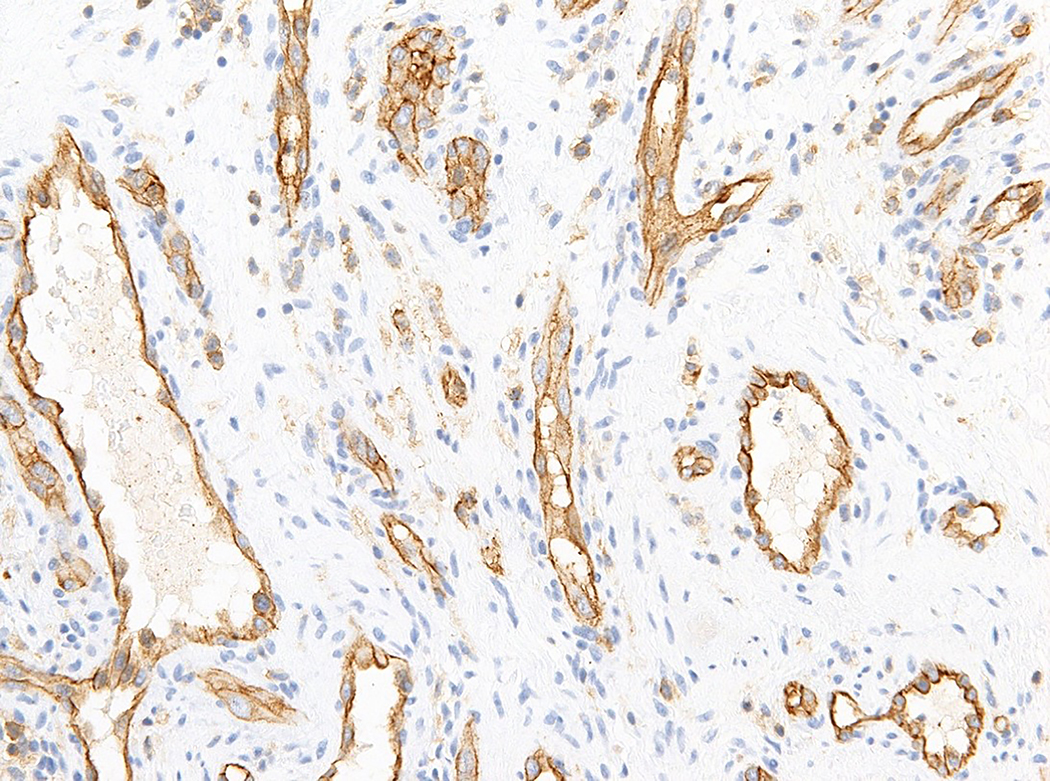
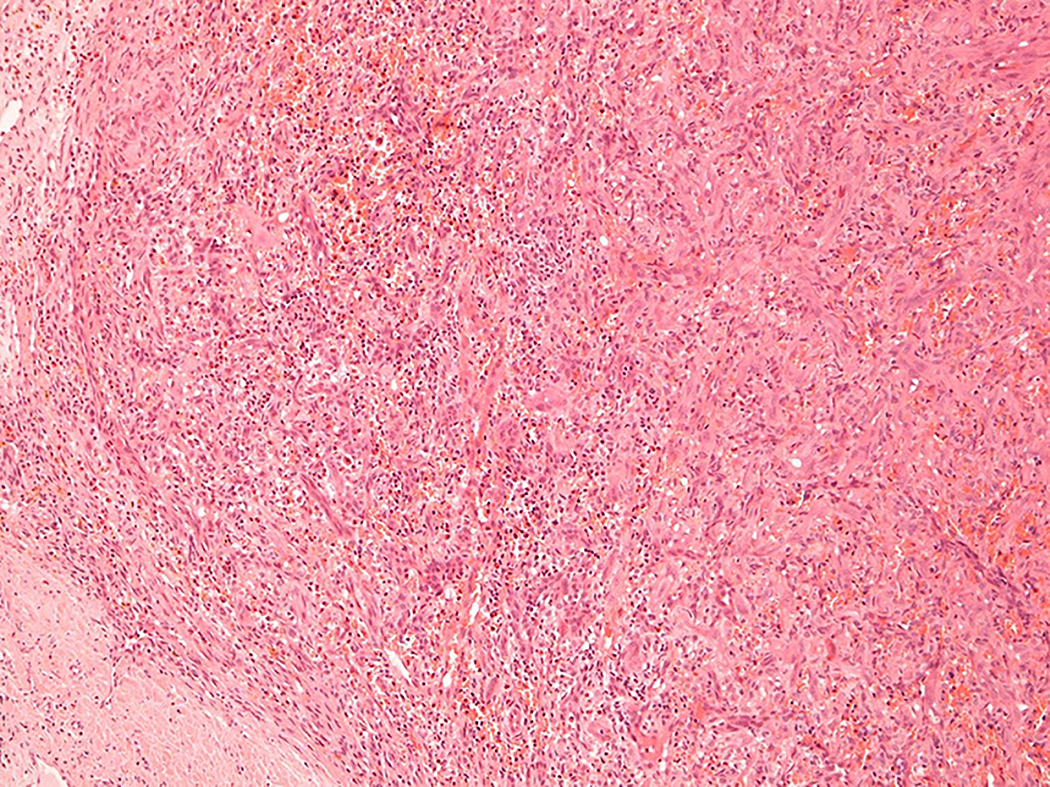
Figure 1.
Intravascular epithelioid hemangioma – capillary hemangioma type (case 12). A. the lumen of the vein is completely obliterated by vascular proliferation. B. Well-formed vascular channels are lined by a single layer of epithelioid endothelial cells. C. Mild nuclear atypia of endothelial cells is seen and there in no mitotic activity. D. Epithelioid endothelial cells are highlighted by CD31 immunohistochemistry.
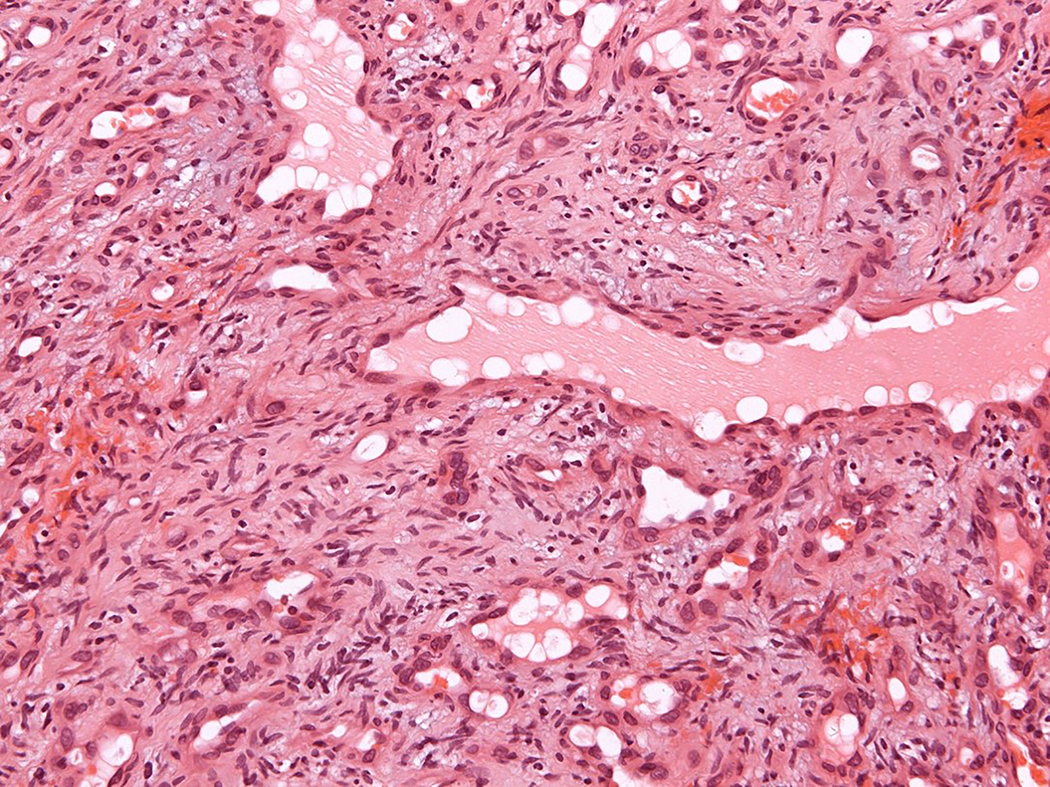
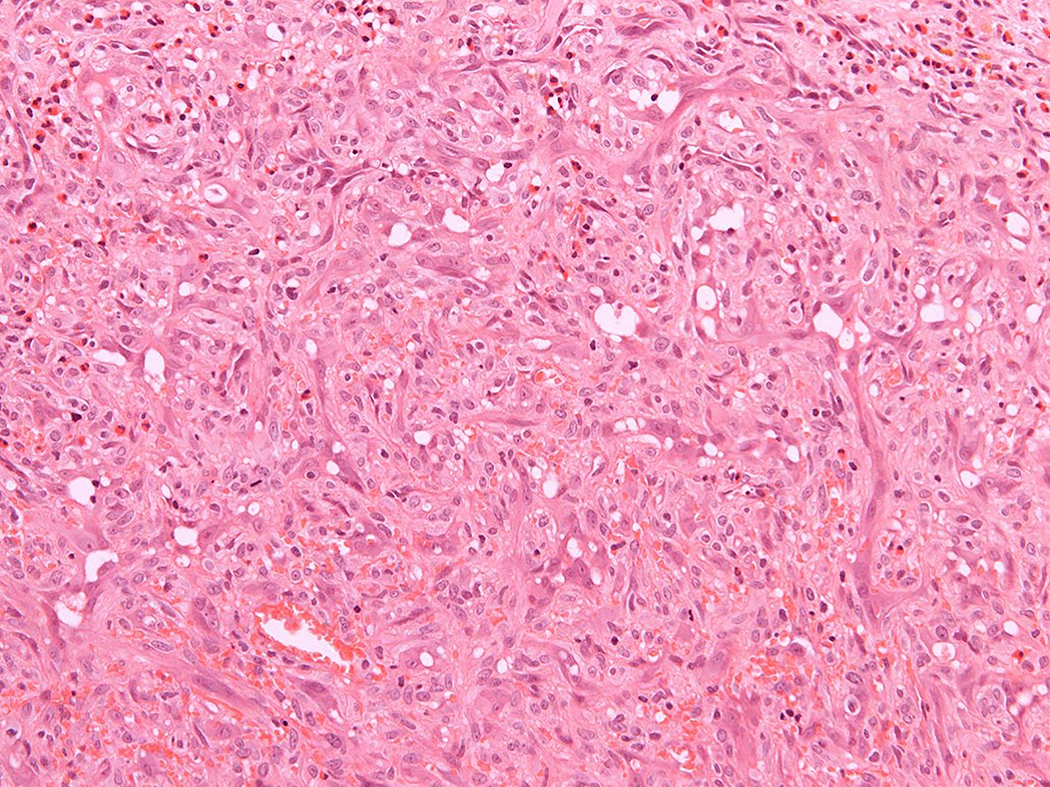
Figure 2.
Intravascular epithelioid hemangioma – mixed capillary hemangioma-like and solid type (case 9). A. Low-power magnification of an intravascular proliferation composed of capillary-type endothelial proliferation admixed with more solid areas. B. An admixtrue of well-formed vascular spaces lined with epithelioid endothelial cells and more solid areas with epithelioid endothelial cells lacking open-type vascular channels. C. Epithelioid endothelial cells in both display mild nuclear atypias and lack mitotic activity. Note intracytoplasmic vacuolisation, consistent with early lumina formation. D. This example features moderately intense mixed inflammatory cell infiltrate within the epithelioid endothelial cell proliferation.
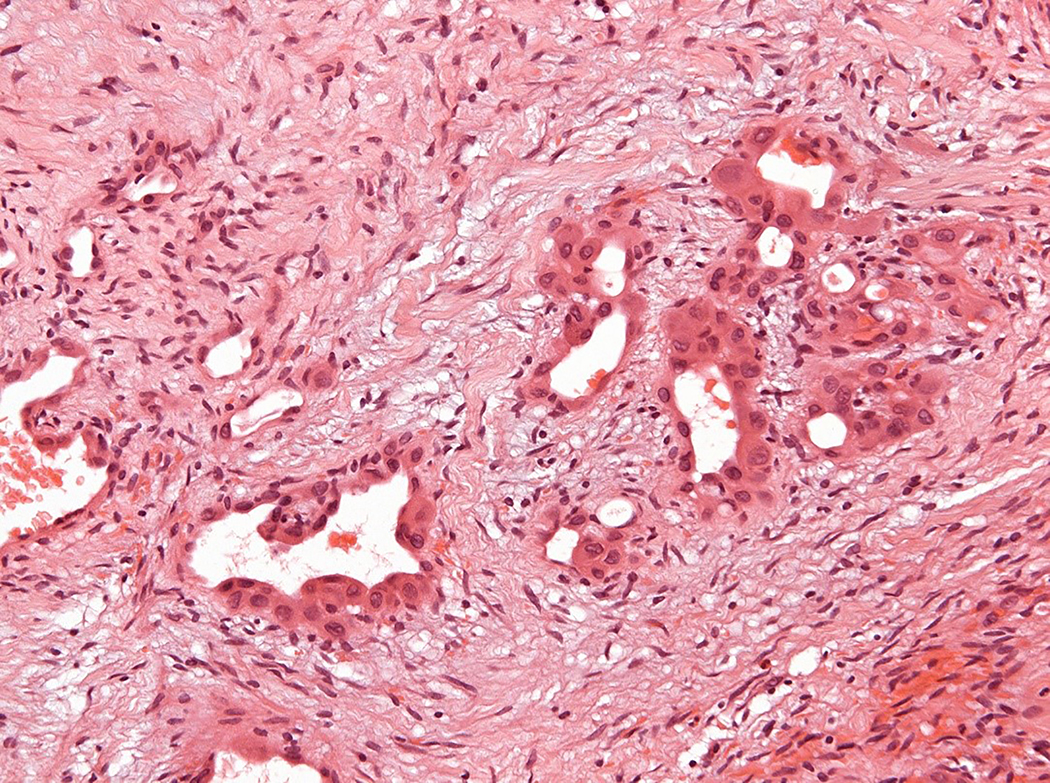
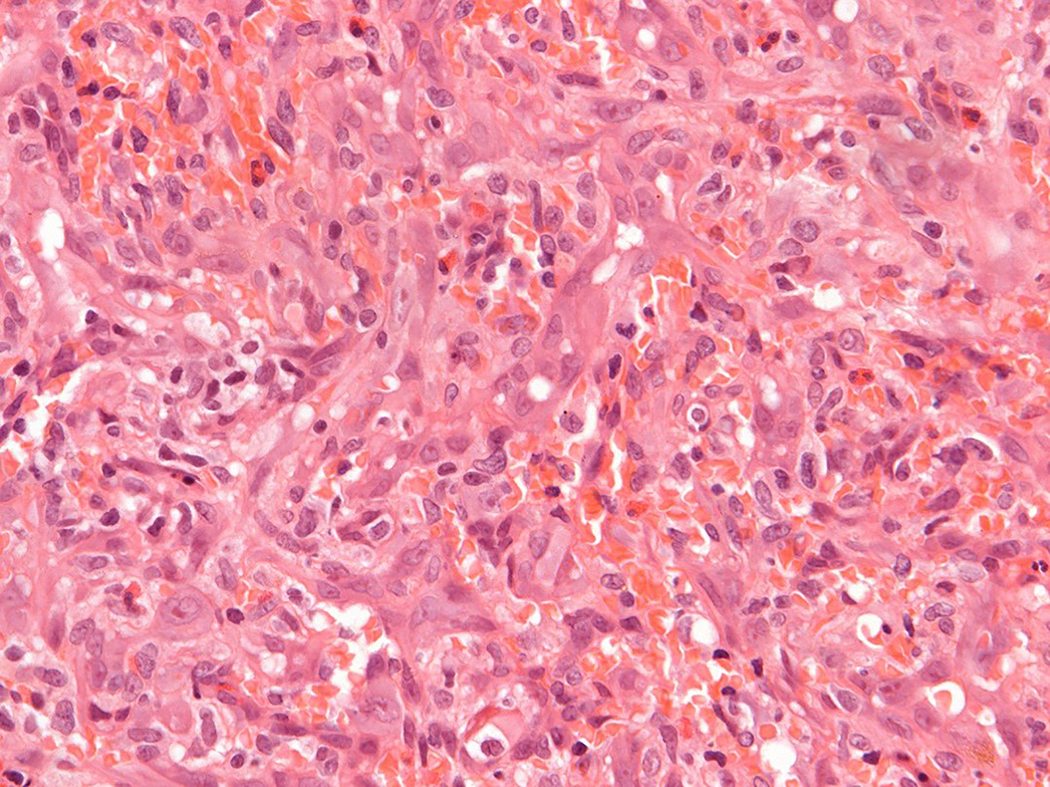
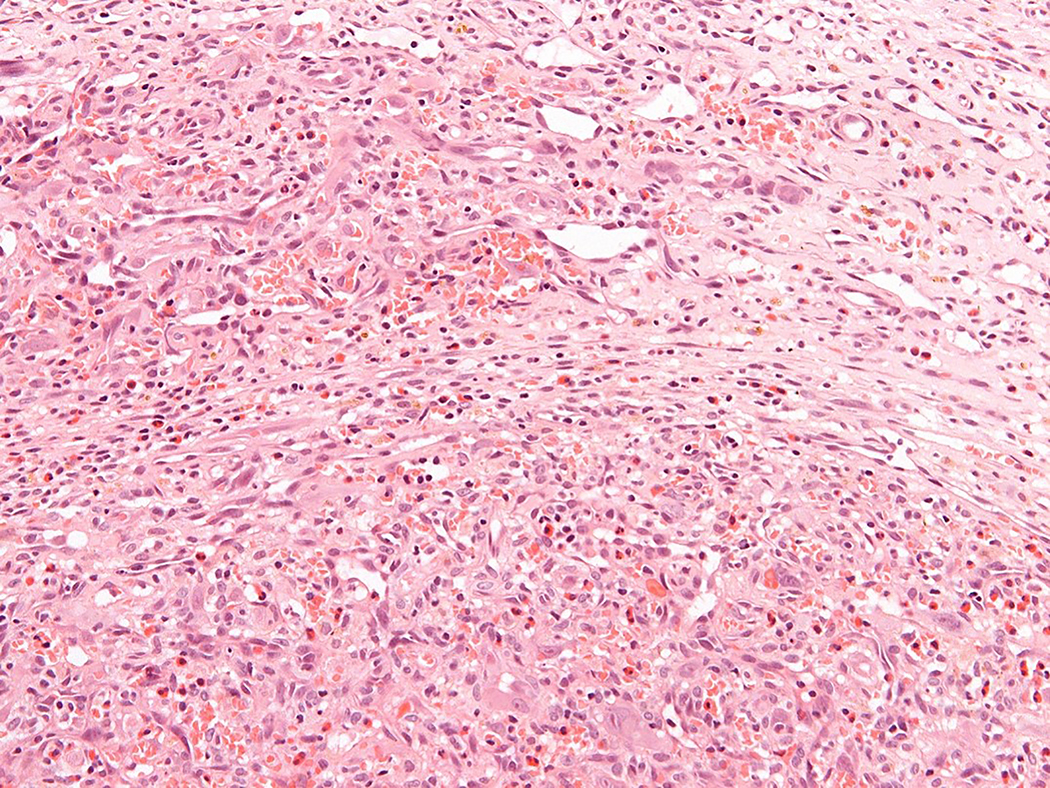
Figure 3.
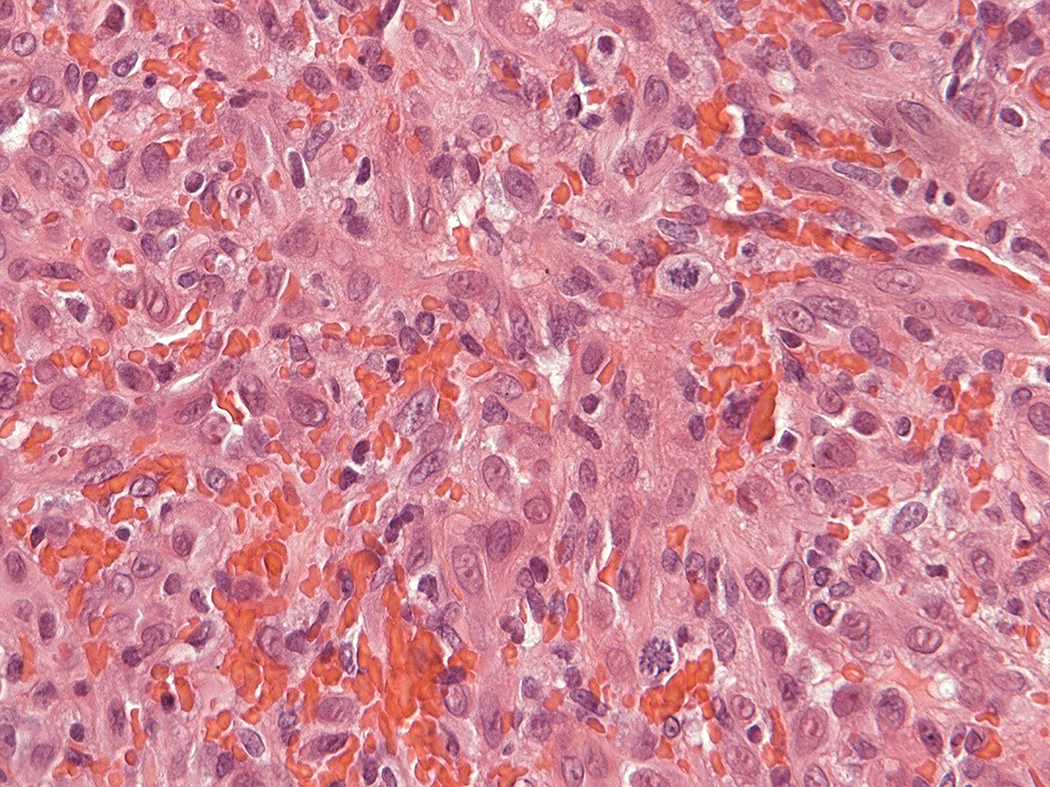
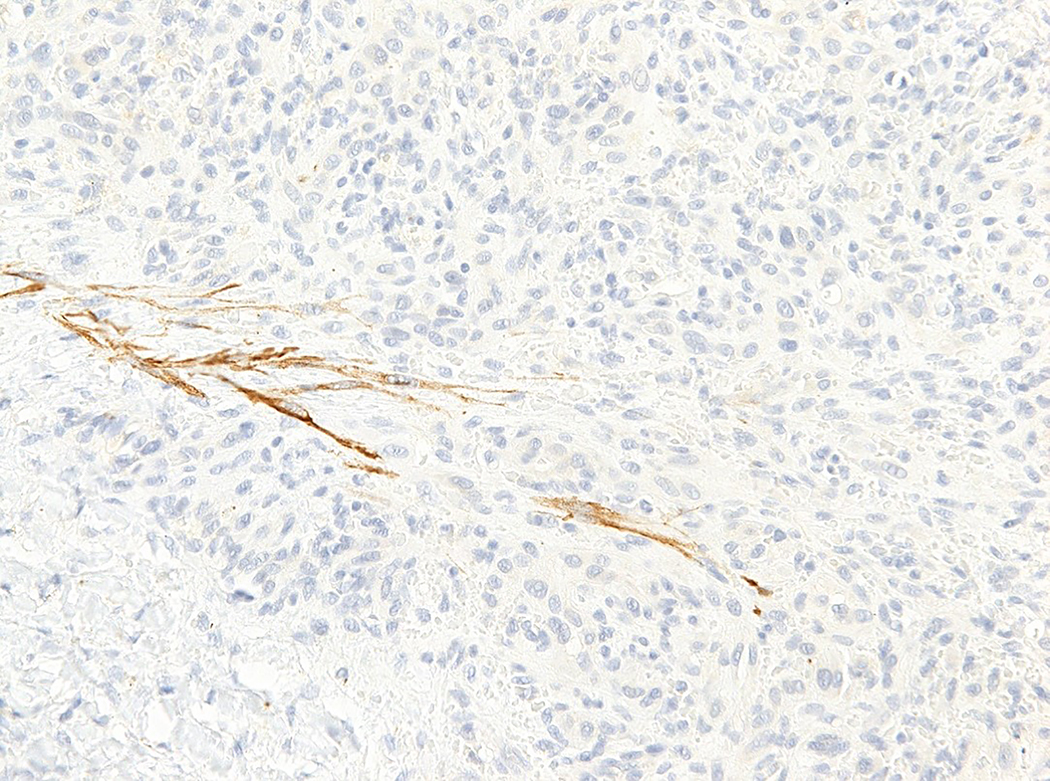
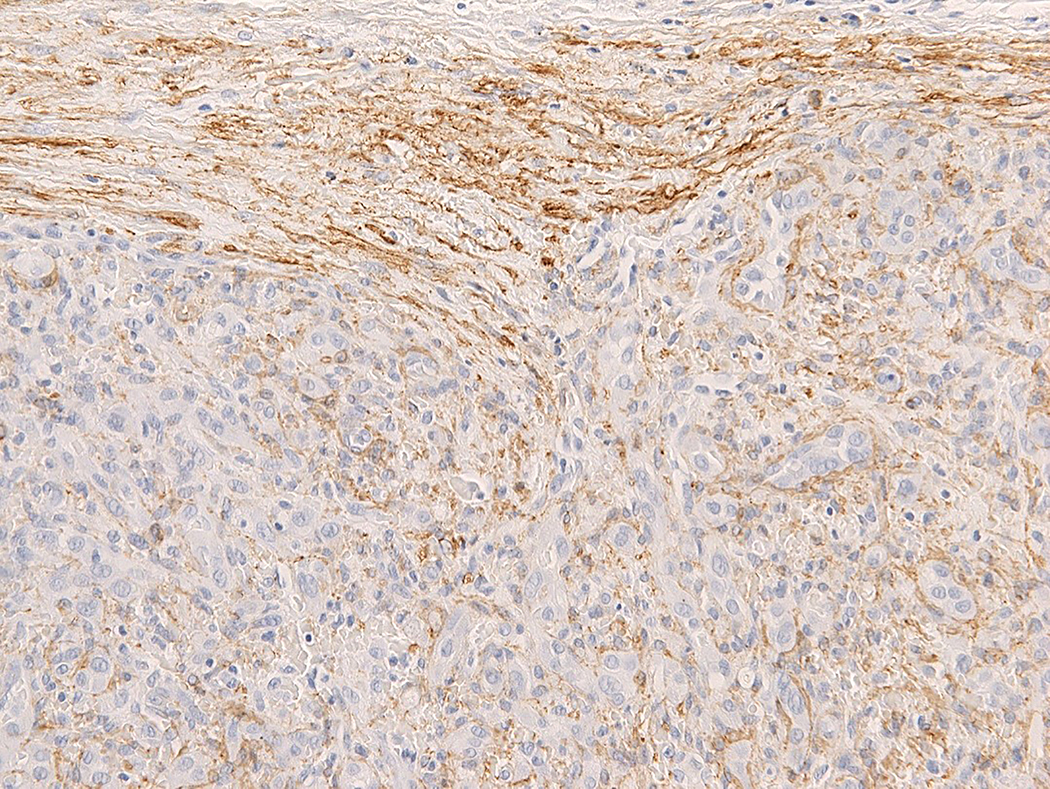
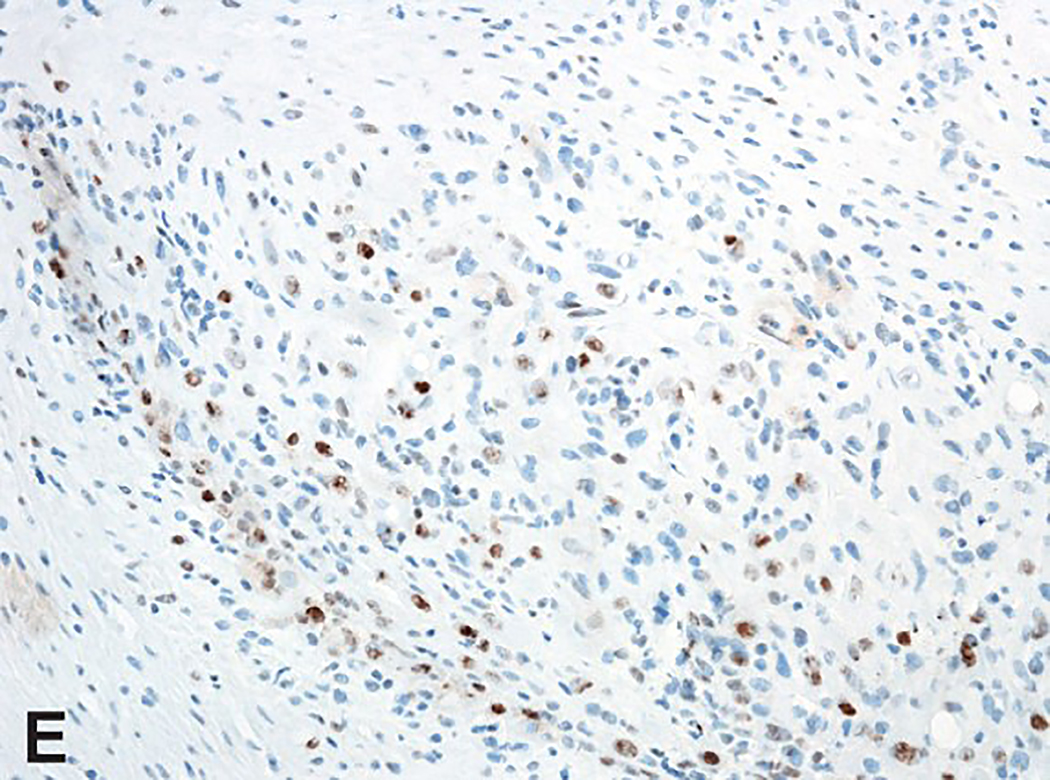
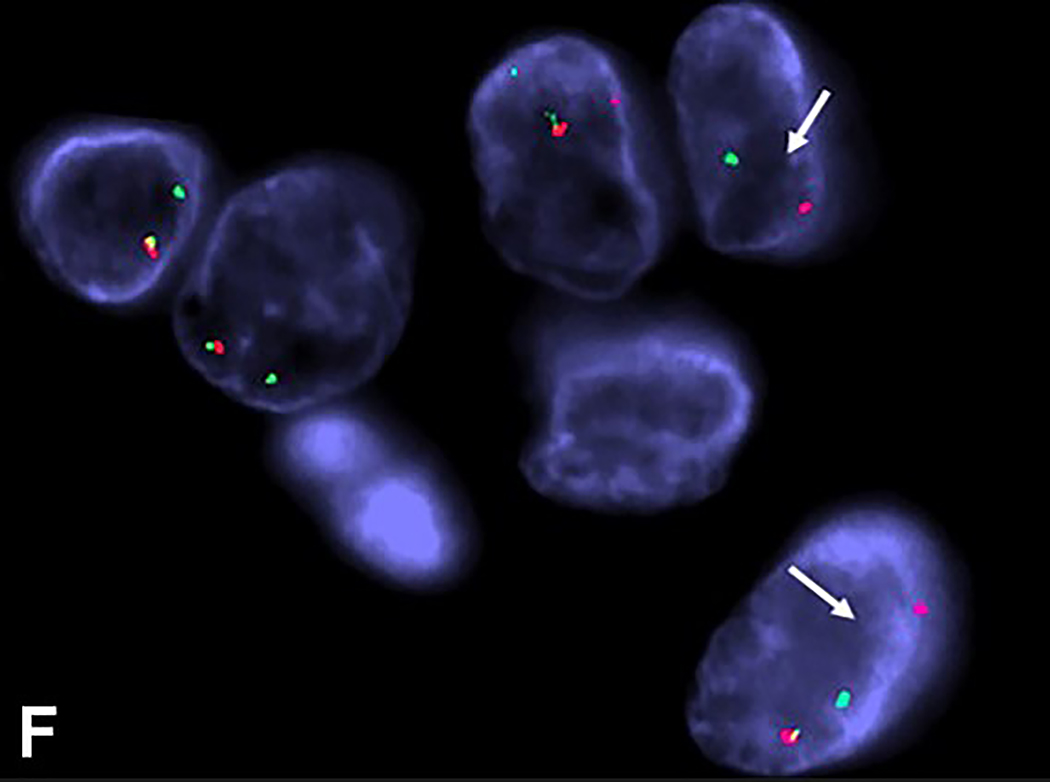
Intravascular epithelioid hemangioma – solid type (Case 6). A. Vascular differentiation of this intraluminal proliferation is not readily apparent at low-power magnification. B. Epithelioid endothelial cells display mild to moderate nuclear atypias. Note also brisk mitotic activity. C. Desmin immunohistochemistry can be discontinued or patchy due to vessel wall stretching. D. Immunohistochemistry for smooth muscle actin nicely demonstrated the wall of a distended vein. E. FOSB immunohistochemistry. About 30% of tumor cells display nuclear positivity for FOSB. Interestingly, this case lacked rearrangements of FOSB gene by FISH analysis. F. Case 6 was the only tumor that proved positive for FOS gene break-apart by FISH.
Epithelioid endothelial cells were characterized by a prominent, well-delineated eosinophilic cytoplasm and large single nucleus, with occasional prominent basophilic nucleus/nucleoli. Not uncommonly, intracytoplasmic vacuoles were seen, representing early stages of vascular lumina formation. Mitotic activity in epithelioid endothelial cells ranged from 0 to 7 mitoses per 10 high-power fields (mean 2.1 mitoses per 10 HPFs). Six lesions displayed brisk mitotic activity of 5 or more mitoses per 10 HPF (6 of 21, 28.5%). The number of mitoses was usually more prominent in areas with solid growth of epithelioid endothelial cells. Atypical mitoses were absent. No intratumoral necrosis was seen in any of the lesions. Epithelioid endothelial cells generally displayed mild and focal nuclear atypia (20 out of 21 cases). In a single case, nuclear atypia was moderate and associated with brisk mitotic activity (case 6, 7 mitoses per 10 HPFs). Prominent nuclear atypia of endothelial cells was not identified in any of the cases. In addition, the lesional endothelial cells lacked multilayering of vascular lumina.
The stroma in-between the vascular proliferations contained a variably abundant mixed inflammatory cell infiltrate, composed of eosinophils, lymphocytes, plasma cells and macrophages. The inflammatory infiltrate was absent in three lesions (3 out of 21), it was mild in 11 (11 out of 21), moderate in six (6 out 21) and prominent in a single case (1 out of 21). Extravasation of erythrocytes and deposition of hemosiderin pigment was additionally observed in all lesions. Prominent stromal hyalinization was present in a single case. In addition to an intralesional mixed inflammatory cell infiltrate, six lesions contained a mixed inflammatory cell infiltrate surrounding the affected vein (6 out of 21). The perilesional inflammatory cell infiltrate was moderately intense in five cases and prominent in one case. All of the cases with a perilesional mixed inflammatory cell infiltrate contained areas of lymphocytes with reactive germinal centers.
Immunohistochemistry
All tumors tested positive for CD31 (14 out of 14) (Figure 1D) and ERG (5 out of 5). Smooth muscle actin and desmin stains were performed mainly to highlight the presence of vessel wall. Staining for smooth muscle actin was usually diffuse and strong (14 out of 14), thereby nicely highlighting the vessel wall and intravascular origin of the tumor. In addition, staining for smooth muscle cells nicely highlighted the presence of pericytes surrounding each vascular channel. In proliferations with more solid morphology of epithelioid endothelial cells, a discontinuous pattern of staining was also observed. Although the predominant staining pattern for desmin was a diffuse and strong positivity of the vessel wall (11 out of 14), 2 cases displayed patchy or discontinued staining of the vessel wall (2 out of 14), reflecting expansion or stretching of the vessel wall by the tumor, with destruction of the protein (Figures 3C and 3D). In addition, one case lacked desmin positivity of the vessel wall (1 out of 14), which was nevertheless highlighted by H-caldesmon and smooth muscle actin positivity. All tumors were consistently negative for cytokeratin MNF116 (0 out of 14), and CAMTA1 (0 out of 2). Six cases had sufficient material for FOSB and FOS immunohistochemistry. While all tested cases were FOS negative (6 out of 6), one out of six cases (case 6) displayed FOSB nuclear positivity in about 30% of the lesional endothelial cells (Figure 3E).
FISH analysis for the presence of FOS and FOSB
Eight cases with adequate material were analysed by FISH for the presence of FOS and FOSB gene rearrangements. While all cases were negative for FOSB gene abnormalities, a single case of a 48-year-old male with a tumor on the right medial canthus (case 6) proved positive for FOS gene rearrangement (Figure 3F). The tumor was composed of a solid proliferation of epithelioid endothelial cells within the lumen of the vein, displaying mild diffuse nuclear atypia coupled with brisk mitotic activity, with 7 mitoses per 10 HPFs. Hwever, moderate or severe nuclear atypia was absent, there were no atypical mitoses and necrosis was absent.
DISCUSSION
Cutaneous intravascular EH shows a predilection for the limbs (62%), in particular the distal extremities, followed by the head and neck area (38%). This differs from classical EH, which arises most frequently in the head and neck area (about 85%).2,3,12,13 Cutaneous intravascular EH most commonly develops in the early 5th decade of life (range 11 to 71 years, mean 40.2 yrs), with a male predominance (3:1). The classical clinical presentation is that of a slowly growing non-painful nodule or mass, with a mean size of 13 mm (range from 2–30 mm). The pre-operative duration is usually short (range from 1 to 36 months, mean 7.7 months). The great majority of the tumors present as a solitary lesion (81%). Nevertheless, multiple tumors can develop either synchronously or metachronously within the same anatomical area, usually on distal extremities, following the path of the affected vessel, but also at distant and unrelated sites. In the present study, synchronous tumor development was typically observed within the same anatomical area, while a metachronous appearance of the tumor was characteristically seen affecting distant and unrelated anatomical sites. Complete excision of the tumor was generally curative. None of the cutaneous intravascular EHs recurred during the mean follow up of 27.3 months (range 4 to 72 months) after complete excision.
Histologically, cutaneous intravascular EH typically develops within the lumen of a subcutaneous vein, usually completely obliterating the lumen. Two patterns of intravascular proliferation of epithelioid endothelial cells are usually observed: 1) a lobular capillary hemangioma-like pattern and 2) a solid pattern characterized by proliferation of epithelioid endothelial cells, lacking the formation of open vascular. The two patterns often co-exist within the same tumor, albeit in different proportions. Epithelioid endothelial cells generally display a mild degree of nuclear atypia and low mitotic activity (mean number 2 per 10 high power fields). Rarely, brisk mitotic activity of five or more mitoses per 10 high power fields associated with focal moderate atypia of endothelial cells can be observed and is usually associated with a more solid pattern of tumor cell proliferation. However, atypical mitotic activity, severe nuclear atypia and necrosis of tumor cells are generally absent.
By immunohistochemistry, EH typically stains for markers of endothelial differentiation, including ERG, CD31 and CD34.6,15 Staining for factor VIII-related antigen can be variable,2 while D2–40 and CAMTA1 are generally negative.15 Alpha-smooth muscle actin delineates the presence of pericytes.15 In relation to intravascular EH, staining for smooth muscle actin and desmin may prove particularly useful in highlighting the presence of vessel wall. While staining of vessel walls with SMA is usually strong and diffuse, staining for desmin can be attenuated, disrupted or even absent, due to expansion and stretching of the vessel wall, with destruction of the protein. We observed attenuated and disrupted desmin staining in the vessel wall of two intravascular EHs (2 out of 14) and a complete absence of staining in a single case. Focal positivity for epithelial marker CK-AE1/AE3, limited to isolated cells or small groups of epithelioid endothelial cells, was detected in 75% of epithelioid hemangiomas occurring on the penis.6 Most recently, however, FOS-B nuclear positivity has been shown to be present in 75% (6 out of 8) of conventional EH, 10% (1 out of 10) of solid EHs and 100% (6 out of 6) of cases within the spectrum of angiolymphoid hyperplasia with eosinophilia.27 In another recent study, strong and diffuse FOS-B nuclear positivity was detected in 100% (13 out of 13) of the solid subtype of EHs presenting in an eruptive pattern.15 Furthermore, diffuse and strong nuclear FOSB positivity was detected in 100% (15 out of 15) of angiolymphoid hyperplasia with the eosinophilia spectrum of EH, while all cases of epithelioid hemangioendotheliomas and epithelioid angiosarcomas either lacked FOSB immunoreactivity, or were associated with focal and weak FOSB positivity in less than 5% of the nuclei.28 In the present study, only a single case of intravascular EH displayed nuclear positivity for FOSB, in about 30% of tumor cells, while all tumors lacked FOS immunoreactivity.
At the molecular level, recurrent FOS gene rearrangements, characterized by t(19;19)(q13.2;q13.2) or interstitial del19(q13.2–3), resulting in ZFP36-FOSB gene fusion and, less often t(3;19)(q25;q12), resulting in WWTR1-FOSB gene fusion, have been detected in about 30% of EHs across different locations.29,30 Interestingly, about two thirds of EH harbouring this translocation were of a solid subtype characterized histologically by moderate nuclear atypia, moderate mitotic activity and areas of necrosis.29 FOS gene rearrangements are most commonly found in EH occurring in the bone and penis,29–31 while EH occurring in the skin infrequently display this abnormality.29,30 Of note, all cases of angiolymphoid hyperplasia with the eosinophilia subtype of EH lacked FOS gene rearrangements.30 The discordance between the lack of FOSB gene fusions and FOSB immunoreactivity might reflect a translocation-independent mechanism of FOSB gene upregulation.28
In the present study, all 8 tested cases lacked FOSB gene rearrangement by FISH analysis. However, a single case proved positive for FOS gene break-apart by FISH. Furthermore, the same case displayed nuclear FOSB positivity in about 30% of the lesional cells, but was negative for FOS by immunohistochemistry. Interestingly, as originally also reported by Antonescu et al,29 cutaneous intravascular EH with FOS gene rearrangement consisted of a solid pattern of intravascular endothelial cell proliferation and was associated with brisk mitotic activity (7 mitoses per 10 HPFs), focal moderate nuclear pleomorphism and mild intralesional mixed inflammatory cell infiltrate.
The main differential diagnosis of intravascular EH is with cutaneous epithelioid angiomatous nodule and with malignant vascular tumors with an epithelioid morphology, including epithelioid hemangioenothelioma and epithelioid angiosarcoma.
Cutaneous epithelioid angiomatous nodule has significant morphological overlap with epithelioid hemangioma, and indeed, it is regarded by some authorities to represent a morphological spectrum of the same entity.32 Cutaneous epithelioid angiomatous nodule is characterized by well-delineated usually unilobular proliferation of epithelioid endothelial cells with uniform to mildy atypical nuclei localized in the superficial dermis.33 Solid proliferation of epithelioid endothelial cells generally predominates, nevertheless, open vascular spaces are typically seen towards the periphery of the proliferation.33 In addition, cutaneous epithelioid angiomatous nodule lacks intravascular growth.
Occurrence from, or involvement of a medium-sized or large vein has been reported in roughly two thirds of epitheliod hemangioendothelioma arising in deep soft tissue.34 However, this phenomenon appears to be uncommon in the pure cutaneous variants of the tumor.35 In contrast to intravascular EH, epithelioid hemangioendothelioma lacks lobular or multilobular growth and is characterized histologically by an infiltrative proliferation of epithelioid endothelial cells arranged in cords and strands, displaying intracytoplasmic lumina, so-called blister cells, embedded in a chondro-myxoid of collagenized stoma.35,36 The majority (over 90%) of epithelioid hemangioendotheliomas are associated with a reciprocal translocation t(1;3)(p36;q25) resulting in WWT1-CAMTA1 fusion, leading to overexpression of CAMTA1 protein.37 Confirmation of nuclear CAMTA1 positivity is an important diagnostic tool for epithelioid hemangioendothelioma. Two recent studies have confirmed CAMTA1 antibody to be a highly sensitive and specific marker for epithelioid hemangioendothelioma.38,39 Although well-formed vascular channels are usually absent in classical epithelioid haemangioendothelioma,34,36 a subset of cases is characterized by focal vasoformation and more voluminous eosinophilic cytoplasm.40 This morphological variant appears to be seen more often, although not exclusively, in epithelioid hemangioendotheliomas harbouring t(X;11)( p11;q13), resulting in overexpression of TFE3.40
Intravascular epithelioid angiosarcoma of the skin can be distinguished from intravascular EH by being a high-grade tumor with an infiltrative destructive proliferation of epithelioid endothelial cells, displaying prominent cellular pleomorphism, mitotic activity and tumor necrosis.41
In conclusion, we report the largest series yet of 21 pure cutaneous intravascular EH, occurring in 16 patients. Intravascular growth of EH is not associated with adverse biological behaviour. Although more solid intravascular proliferations of endothelial cells can mimic a malignant vascular tumor with epithelioid morphology, intravascular EH generally display mild degrees of nuclear atypia, coupled with low mitotic activity, and lack atypical mitoses, pronounced nuclear atypia, multilayering or tumoral necrosis. Finally, the FOS gene is infrequently rearranged, and there are no abnormalities of the FOSB gene by FISH in cutaneous intravascular EH.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no conflict of interest.
References
- 1.Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol 1969;81:1–15. [DOI] [PubMed] [Google Scholar]
- 2.Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. J Am Acad Dermatol 1985;12:781–796. [DOI] [PubMed] [Google Scholar]
- 3.Fetsch JF, Weiss SW. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia). Modern Pathol 1991;4:449–445. [PubMed] [Google Scholar]
- 4.Nielsen GP, Sristava A, Kattapuram S, Deshpande V, O’Connell JX, Mangham CD, et al. Epithelioid hemangioma of bone revisited. S study of 50 cases. Am J Surg Pathol 2009;33:270–277. [DOI] [PubMed] [Google Scholar]
- 5.Errani C, Zhang L, Panicek DM, Healey JH, Antonescu CR. Epithelioid hemangioma of bone and soft tissue. Clin Ortop Relat Res 2012;470:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fetsch JF, Seterhenn IA, Miettinen M, Davis CJ. Epithelioid hemangioma on the penis. A clinicopathologic and immunohistochemical analysis of 19 cases, with specific reference to exuberant examples often confused with epithelioid hemangioendothelioma and epithelioid angiosarcoma. Am J Surg Pathol 2004;28:523–533. [DOI] [PubMed] [Google Scholar]
- 7.Abrahim MJ, Gregory ND, Chennupati SK. Epithelioid hemangioma of the internal carotid artery: a case report supporting the reactive pathogenesis hypothesis of this vascular tumor. Int J Pediatr Otorhinolaryngol 2014;78:1186–1189. [DOI] [PubMed] [Google Scholar]
- 8.Sun ZJ, Zhang L, Zhang WF, Alsharif MJ, Chen XM, Zhao YF. Epithelioid hemangioma in the oral mucosa: a clinicopathological study of seven cases and review of the literature. Oral Oncol 2006;42:441–447. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal A, Keluskar V. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) in the oral mucosa. Indian J dent Res 2012;23:271–274. [DOI] [PubMed] [Google Scholar]
- 10.Nonose R, Priolli DG, Cardinalli IA, Máximo FR, Galvão PS, Martinez CA. Epithelioid hemangioma of the colon: a case report. Sao Paulo Med J 2008;126:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado I, Chong A, Serrano A, Naranjo Ugalde AM, Pineda D, Savón L, et al. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) of the heart with peripheral eosinophilia and nephrotic syndrome. Int J Surg Pathol 2015;24:59–65. [DOI] [PubMed] [Google Scholar]
- 12.Guo R, Gavino ACP. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med 2015;139:683–686. [DOI] [PubMed] [Google Scholar]
- 13.Guinovart RM, Bassas-Vila J, Morell L, Ferrándiz C. Angiolymphoid hyperplasia with eosinophilia: a clinicopathologic study of 9 cases. Actas Dermosifiliogr 2014;105:e1–e6. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer I-M, Fletcher CDM. Recent advances in the diagnosis of soft tissue tumours. Pathology 2018;50:37–48. [DOI] [PubMed] [Google Scholar]
- 15.Llamas-Velasco M, Kempf W, Cota C, Fernández-Figueras MT, Lee J, Ferrara G, et al. Multiple eruptive epithelioid hemangiomas. A subset of cutaneous cellular epithelioid hemangioma with expression of FOS-B. Am J Surg Pathol 2019;43:26–34. [DOI] [PubMed] [Google Scholar]
- 16.Moira R, Giuseppe F, Riccardo V, Nicola R, Daniele B, Pierfrancesco C, et al. Epithelioid hemangioma of brachial artery: report of a case and review of the literature. Open Med (Wars) 2015;10:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker SR. Epithelioid hemangioma of the ulnar artery. Vascular 2010;18:307–308. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y-C, Chuang W-Y, Hu C-H, Cheung Y-C, Ng S-H, Lin G, et al. Ulnar arterial epithelioid hemangioma: a unique demonstration using time-resolved MR angiography. Int J Cardiol 2016;223:18–20. [DOI] [PubMed] [Google Scholar]
- 19.Amin A, Umashankar T, Dsouza CO. Intra-arterial angiolymphoid hyperplasia with eosinophilia: a rare case report of peripheral medium sized muscular artery involvement. J Clin Diagn Res 2015;9:ED16–ED17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura H, Kitamura H, Ito S, Kuwana N, Yutani C. Epithelioid hemangioma of the temporal artery clinically mimicking temporal arteritis. Pathol Int 1999;49:831–835. [DOI] [PubMed] [Google Scholar]
- 21.Chopra P, Handoo A, Parakh R. Epithelioid hemangioma of the temporal artery: a case report. Indian J Pathol Microbiol 2007;50:595–598. [PubMed] [Google Scholar]
- 22.Ghotbi R, Sotiriou A, Mlinaric M. Epithelioid hemangioma of the popliteal artery. Chirurg 1999;70:1494–1496. [DOI] [PubMed] [Google Scholar]
- 23.Abrahim MJ, Gregory ND, Chennupati SK. Epithelioid hemangioma of the internal carotid artery: a case report supporting the reactive pathogenesis hypothesis of this vascular tumor. Int J Pediatr Otorhinolaryngol 2014;78:1186–1189. [DOI] [PubMed] [Google Scholar]
- 24.Rosai J, Akerman LR. Intravenous atypical vascular proliferation. A cutaneous lesion simulating a malignant blood vessel tumor. Arch Dermatol 1974;109:714–717. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva-Peña A, de Diego Rodriguez E, Gómez Ortega JM, Hernández Castrillo A, López Rasinas G. Considerations about angiolymphoid hyperplasia with eosinophilia /ALHE) with regard to a case localized in the penis. Actas Urol Esp 2005;29:113–117. [DOI] [PubMed] [Google Scholar]
- 26.Chung SR, Tan JSW, Selvarajan S. Intravascular angiolymphoid hyperplasia with eosinophilia (ALHE) of the hand. J Hand Surg Eur Vol 2015;40:750–751. [DOI] [PubMed] [Google Scholar]
- 27.Hung YP, Fletcher CDM, Hornick JL. FOSB is a useful diagnostic marker for pseudomyogenic hemangioendothelioma. Am J Surg Pathol 2017;41:596–606. [DOI] [PubMed] [Google Scholar]
- 28.Ortins-Pina A, Llamas-Velasco M, Turpin S, Soares-de-Almeida L, Filipe P, Kutzner H. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol 2018;45:395–402. [DOI] [PubMed] [Google Scholar]
- 29.Antonescu CR, Chen H-W, Zhang L, Sung YS, Panicek D, Agaram NP, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer 2014;53:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S-C, Zhang L, Sung Y-S, Chen CL, Krausz T, Dickson BC, et al. Frequent FOS gene rearrangements in epithelioid hemangioma. A molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol 2015;39:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van IJzendoorn DG, de Jong D, Romagosa C, Picci P, Benassi MS, Gambarotti M, et al. Fusion events lead to truncation of FOS in epithelioid hemangioma of bone. Genes Chromosomes Cancer 2015;54:565–574. [DOI] [PubMed] [Google Scholar]
- 32.Sangüeza OP, Walsh SN, Sheehan DJ, Orland AF, Llombart B, Requena L. Cutaneous epithelioid angiomatous nodule: a case series and proposed classification. Am J Dermatopathol 2008;30:16–20. [DOI] [PubMed] [Google Scholar]
- 33.Brenn T, Fletcher CDM. Cutaneous epithelioid angiomatous nodule: a distinct lesion in the morphological spectrum of epithelioid vascular tumors. Am J Dermatopathol 2004; 26:14–21. [DOI] [PubMed] [Google Scholar]
- 34.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma. A vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970–981. [DOI] [PubMed] [Google Scholar]
- 35.Quante M, Patel NK, Hill S, Merchant W, Courtauld E, Newman P, et al. Epithelioid hemangioendothelioma presenting in the skin: a clinicopathologic study of eight cases. Am J Dermatopathol 1998;20:541–546. [DOI] [PubMed] [Google Scholar]
- 36.Mentzel T, Beham A, Calonje E, Katenkamp D, Fletcher CD. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol 1997;21:363–374. [DOI] [PubMed] [Google Scholar]
- 37.Errani C, Zhang L, Shao SY, Hajdu M, Singer S, Maki RG, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 2011;50:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibuya R, Matsuyama A, Shiba E, Harada H, Yabuki K, Hisaoka M. CAMTA1 is a useful immunohistochemical marker for diagnosing epithelioid hemangioendothelioma. Histopathology 2015;67:827–835. [DOI] [PubMed] [Google Scholar]
- 39.Doyle LA, Fletcher CDM, Hornick JL. Nuclear expression of CAMTA1 distinguishes epitheliod hemangioendothelioma from histologic mimics. Am J Surg Pathol 2016;40:94–102. [DOI] [PubMed] [Google Scholar]
- 40.Antonescu CR, Le Loarer F, Mosquera J-M, Sboner A, Zhang L, Chen CL, et al. Novel YAP-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013;52:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suchak R, Thway K, Zelger B, Fisher C, Calonje E. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study on 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas with predilection for the limbs. Am J Surg Pathol 2011;35:60–69. [DOI] [PubMed] [Google Scholar]