Abstract
The diagnostic challenges associated with type 2 myocardial infarction (T2MI) evolve around an extensive evidence base. T2MI is a type of MI that occurs secondary to ischemia due to increased demand or decreased oxygen supply. This classification has been used for the last 5 years, yet there is little understanding of the characteristics and clinical outcomes. According to a survey, T2M1 can be caused mainly by different factors such as anemia (31%), sepsis (24%), and arrhythmia (17%). Other associated factors, such as age and gender, also play a part in the disease. The pathology behind T2MI is the rise and fall of cardiac troponin values with at least one value above the 99 percentile and evidence of an imbalance unrelated to coronary thrombosis. The diagnosis of the condition is evidence-based backed up with imaging techniques. The treatment of T2MI may involve blood pressure management, administration of blood products, heart rate control, and respiratory support. Depending on the clinical presentation, coronary evaluations can be used to assess the likelihood of coronary artery disease (CAD). If indicated, the MI guidelines may apply to CAD. If it shows, the MI guidelines may use electrocardiography findings of ST-segment elevation myocardial infarction (STEMI) or non-STEMI. However, the absence of CAD indicates that the benefits of cardiovascular risk reduction strategies with T2MI remain uncertain.
Keywords: Arrhythmia, cardiac troponins, coronary heart disease, electrocardiographic, myocardial infarction, type 2 myocardial infarction
Introduction
In the late 19th century, advancements in diagnostic techniques demonstrated a possible relationship between myocardial infarction (MI) and a coronary artery's thrombotic occlusion. Despite the improvement, it was not until the early 20th century that a connection between the emergence of a thrombus in a coronary artery and its associated clinical features was described. Despite these landmark observations, a considerable time elapsed before general clinical acceptance was gained in part as a result of the autopsy study that showed no thrombi in the coronary arteries of 31% of deceased patients with an MI. The clinical presentation was referred to as coronary thrombosis, although the use of the term “MI” ultimately prevailed.[1]
The fundamental was further refined by the Global MI Task Force, which led to the global definition of MI Consensus in 2007. This also led to the introduction of a novel MI classification system with five subcategories. The development of highly sensitive assays for markers of myocardial injury resulted in a further revision of the diagnostic methodology necessary for patients who are undergoing cardiac surgery or coronary procedures. Studies have indicated that myocardial injury defined by an increased cardiac troponin (cTn) value is regularly encountered clinically and is associated with a bad prognosis.
To establish a diagnosis of MI, guidelines in addition to abnormal biomarkers are required. Nonischemic myocardial injury can arise secondary to cardiac conditions like myocarditis or correlate with noncardiac conditions like renal failure. Therefore, for patients with elevated cTn values, physicians must distinguish whether patients have experienced a nonischemic myocardial injury or any other type of MI subtypes. In the absence of evidence to support the presence of myocardial ischemia, the diagnosis should be a myocardial injury. This diagnosis can be changed if the subsequent evaluation indicates the criteria for MI.
In other instances, myocardial cell death can result from a mismatch between blood supply to the myocardium and myocardial oxygen demand in the absence of an acute atherothrombotic plaque disruption. Conditions that can either increase myocardial oxygen demand, such as severe anemia, hypertension, aortic valvular disease, or decrease myocardial oxygen supply, such as tachyarrhythmias, hypoxic respiratory failure, also leads to myocardial cell injury and death. Myocardial injury in this context is referred to as type 2 MI (T2MI), which is not due to acute atherothrombotic plaque disruption. The 5'prevalence of MI is more common in women than in men, with some research studies pointing out the relationship between estrogen and MI.[3]
In most cases, it is usually tricky clinically to make such a distinction. A recent study reported that despite a universal definition for T2MI, there is no validated, reproducible definition in clinical research and practice, making the diagnosis mostly subjective. The broad definition of MI has been attributed to the terms ST-segment elevation myocardial infarction (STEMI) and non-STEMI to T2MI, failure of researchers to report “altered variables,” and ambiguous cases that meet both diagnostic criteria for T1MI and T2MI.[2,3,4,5]
Epidemiology of Type 2 Myocardial Infarction
The epidemiologic data of T2MI are limited. Only a few studies have established its incidence in hospitalized patients and depend mainly on the definition applied to T2MI. Saaby et al. concluded that 26% of the 533 patients diagnosed with MI (8.4% of the newly admitted patients,4,499 had T2MI.[3,6] The low prevalence of T2MI may reflect the location, cardiac, or medical intensive care unit (ICU).[5] They analyzed patients admitted to the ICU in 73 Swedish hospitals, whereas T1MI made up 88.5% of the acute myocardial infarction patients. The frequency of T2MI is the opposite of the cases of ischemic coronary systems.[7]
Etiology and Pathology
Among the five types of myocardial infraction introduced since 2007, T2MI has been established to result from an imbalance between oxygen demand and consumption by the heart, especially in the absence of plaque rupture or recent revascularization. T2MI presents with coronary endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, hypotension, hypertension, anemia, respiratory failure, and conditions that reduce oxygen supply to the heart.[8] As compared to T1MI, the sequel to a plaque rupture, usually in arterial sclerosis scenery or ulceration, fissuring, or dissection, may result in acute myocardial necrosis. T3MI attributes to sudden cardiac death, T4MI to percutaneous coronary intervention (PCI) or stent thrombosis, and T5MI to coronary artery bypass surgery.[4,9]
As earlier stated, the etiology and pathology of the imbalance between oxygen demand/supply/consumption by the myocardium are ascribed to several variables. Three factors that influence myocardial oxygen demands include systolic wall tension, contractility, and heart rate. Hence, any pathology that affects these factors may shift the oxygen consumption equilibrium of the myocardium. Similarly, the myocardial oxygen supply depends on the coronary blood flow and oxygen-carrying capacity, which in turn can be affected by coronary spasm, embolism, endothelial dysfunction, shock, and anemia.[10] This is summarized in Figure 1.
Figure 1.
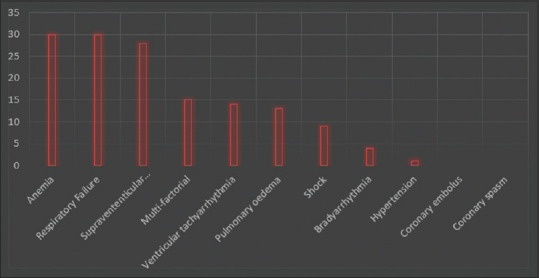
Mechanisms underlying myocardial oxygen demand/supply imbalance leading to type 2 myocardial infarction (n = 144)[17]
According to research, anemia, supraventricular tachyarrhythmias, and respiratory failures were the three most common mechanisms leading to about 20% of T2MI.[2,11]
Figure 1 analyses mechanisms underlying myocardial oxygen demand/supply imbalance leading to T2MI (n = 144).[12]
Diagnosis and Prognosis
Biochemical approach for diagnosing myocardial infarction and injury
cTnI and cTnT are the desired biomarkers recommended to prove and disregard myocardial injury, defining MI and each particular subtype of MI. Detection of a rise and fall of cTn values is essential as the first component alongside other clinical evaluation elements to determine acute MI diagnosis. It should be appreciated that the biomarkers' release depends on the blood flow, which has significant variability in the time to a peak value (velocity) when a changing pattern is observed.[4]
For high-sensitivity (hs)-cTn tests, biological variation assessment is also essential. In most studies, natural variation and conjoint analytical are in the range of 50%–60%. For that reason, this percentage has been recommended for use when initial baseline values are 99th percentile upper reference limit (URL).[13] However, for individuals with a developing amount more significant than the 99th percentile URL, a lesser level of change during serial measurements is vital to achieving improved clinical sensitivity (compared with inceptive values 99th percentile URL). Thus, an expert consensus group has suggested the following changes more significant than 20% used in this situation. Absolute changes are assay dependent but emerge superior to relative percent changes with hs-cTn tests, and in some studies, this is predominantly the case when the initial value increased.[14]
The use of non-hs-cTn assays (10% coefficient of variation [CV] at the 99th percentile URL) makes the diagnosis of a significant serial change extra tricky but does not result in a false positive. Assays with CVs of 10%–20% are acceptable for clinical use.[15] If a cTn assay is not accessible, the best alternative is CK-MB measured by a mass assay. As with cTn, increased creatine kinase (CK)-MB value can be defined as a measurement more significant than the 99th percentile URL. The 99th percentile URL has been identified as the decision level for the determination of MI. Sex-specific CK-MB values should be employed.[11]
Analytical issues for cardiac troponins
The diagnostic sensitivity (limit of detection [LoD]) of cTn and cTnIT assays varies 10-fold. Since tests are not standardized, values from one assay cannot be directly compared with those from other assays.[4]
Furthermore, costs are different between assay generations, and changes can occur when the same assay reagents are measured on various instruments. Physicians must learn about their local assay and look for standardized tests when they have questions concerning analytical problems.[12,16]
The latest guidelines accommodate all tests, whether hs-cTn, conventional cTn, or point-of-care (POC) cTn. While hscTn assays measure relatively low values and minor documented increases above the 99th percentile URL, many conventional and POC cTn tests do not usually indicate small increasing values within the reference interval. This leads to significant differences in the frequency of events based solely on the cTn assay.[17] These differences are magnified when multiples of the 99th percentile URL are used. IFCC supports the concept that hs-cTn assays are differentiated from conventional or POC cTn assays potentially by measures of cTn values beyond the assay's LoD in 50% of healthy individuals. This concept provides a rough approximate of assay sensitivity.[18]
Electrocardiographic diagnosis of myocardial infarction
Electrocardiography (ECG) is an essential part of the diagnostic workup of patients with suspected MI. It should be acquired and interpreted immediately (within 10 min) after the first medical contact. Prehospital ECGs reduce the time to diagnosis and treatment. They can facilitate the triage of STEMI patients to hospitals with PCI facilities within the recommended time interval (120 min from STEMI diagnosis). Acute myocardial ischemia is usually associated with dynamic ECG waveform changes, and serial ECG possession can provide critical information.[19] Figure 2 shows the ECG waveforms.
Figure 2.
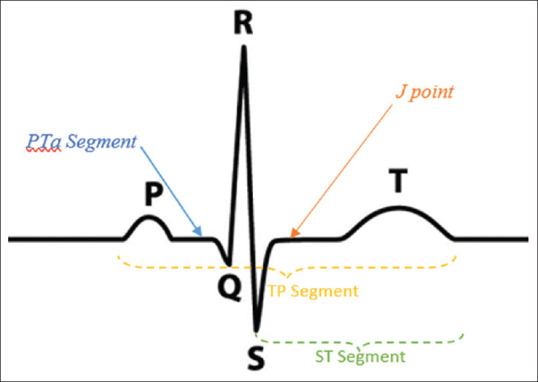
The electrocardiography waveforms and segment: PQ segment represents the signal from the SA node to the AV node. The Q wave is produced when the AV nodes releases signals that moves through the interventricular septum. Q, R, and S waves together are referred to as QRS complex which represents the electrical forces produced by ventricular depolarization. R wave is the positive deflection after the P wave. S wave is produced when the basal parts of the ventricles are depolarized
Figure 2 shows the ECG waveforms and segment: PQ segment represents the SA node signal to the AV node. The Q wave is produced when the AV nodes release signals that move through the interventricular septum. Q, R, and S waves together are referred to as QRS complex, representing the electrical forces produced by ventricular depolarization. R wave is the positive deflection after the P wave. S wave is created when the basal parts of the ventricles are depolarized. ST segment marks the time for the ventricles to pump the blood to the lung and body, while the TP segment is used as a baseline reference to determine whether the ST segment is elevated or depressed.
Reperfusion is mostly associated with a significant and prompt reduction in ST-segment elevation. More profound T wave inversions or ST-segment shifts involving several leads/territories are associated with an increased degree of myocardial ischemia and a worse prognosis. An ST-segment depression 1 mm in all six leads, in addition to ST-segment elevation in lead V1 or leads aVR and blood circulation compromise, is suggestive of several vascular diseases or left primary disease. Pathologic Q waves elevate the risk associated with the prognosis.[20,21]
The ECG singularly is often insufficient to diagnose acute myocardial ischemia or infarction since ST deviation is also observed in other conditions, such as acute pericarditis, left bundle branch block (LBBB), left ventricular hypertrophy (LVH), Brugada syndrome, and Takotsubo cardiomyopathy (TTS) with an initial repolarization pattern.[14] A prior ECG is often useful in distinguishing a new MI from a chronic MI finding, but this should not delay treatment consideration. Prolonged new convex ST-segment elevation is mainly related to reciprocal ST-segment depression, which commonly reflects acute coronary occlusion and results in myocardial injury accompanied by necrosis.[23]
Figure 3 represents a reciprocal ECG image.[15]
Figure 3.
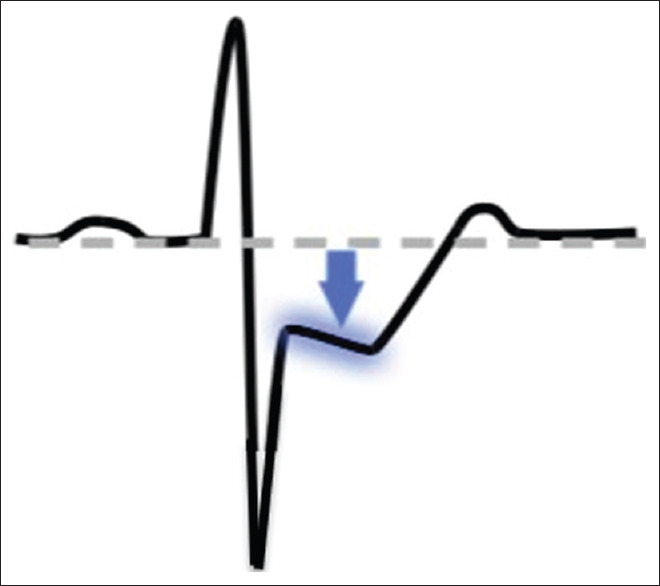
Reciprocal electrocardiography image[14]
Reciprocal ECG changes usually help to differentiate STEMI from pericarditis or initial repolarization changes. As in cardiomyopathy, Q waves can also occur due to myocardial fibrosis in the absence of coronary artery disease (CAD). Some of the initial manifestations of myocardial ischemia are typical T wave and ST-segment changes.[18,22]
Elevated hyperacute T wave amplitude, with prominent symmetrical T waves in at least two contiguous leads, is an initial sign that may precede the elevation of the ST segment. In general, the development of new Q waves is evidence of myocardial necrosis, which begins minutes/hours after the myocardial insult.[20,24] Transient Q waves are observed during acute ischemia or acute MI episodes, and successful reperfusion occurs rarely. ST-segment–T wave (ST-T) criteria indicate acute myocardial ischemia that might or might not lead to MI.[25]
The J-point (junction between ST-segment onset and QRS termination) is used to determine the magnitude of the ST-segment shift, with the beginning of the QRS serving as the reference point. In patients with a fixed baseline, the TP segment is a more accurate method to evaluate the magnitude of ST-segment shift and differentiating pericarditis (depression) from acute myocardial ischemia. Tachycardia and baseline shift are usually in the acute setting and can make this determination difficult. QRS onset is recommended as the reference point for J-point resolution.[13]
Conditions that confound the electrocardiographic diagnosis of myocardial infarction
A QRS complex observed in lead V1 is usually standard. A Q wave >0.03 s and <0.25 of the R wave amplitude in lead III is typically normal if the frontal QRS axis is within-30 and 0. A Q wave can also be typical in aVL with the condition that the frontal QRS axis is within 60–90.[15] Septal Q waves are minor nonpathological Q waves <0.03 s and <0.25 of the R wave amplitude within leads I, aVL, aVF, and V4–V6. Preexcitation, TTS, cardiac amyloidosis, LBBB, cardiomyopathy, left anterior hemiblock, LVH, right ventricular hypertrophy, myocarditis, acute cor pulmonale, or hyperkalemia may have some level of correlation with Q waves or QS complexes in the absence of MI.[26]
Figure 4 represents ECG diagnosis of myocardial ischemia with ST-T wave abnormalities, usually observed with different pathological cardiac conditions.
Figure 4.
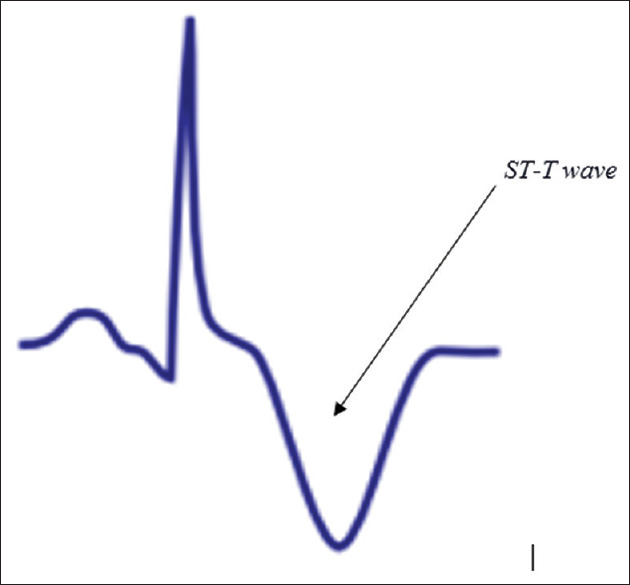
Electrocardiography diagnosis of myocardial ischemia with ST-T wave abnormalities which are usually observed with different pathological cardiac conditions
Conduction disturbances and pacemakers
The diagnosis of MI is difficult in the presence of conduction disturbances associated with ST-T wave changes since the conduction disturbance itself maybe heart rate dependent. Comparison to a preadmission ECG may help determine if the conduction deficiency or ST-T wave changes are new, considering no delay to the treatment period. Ischemic symptoms and presumed new right bundle branch block or LBBB that is not rate related are associated with an adverse prognosis.[12]
The pathophysiological mechanism is by causing ischemic myocardial injury in the presence of a mismatch between oxygen demand and supply classified as T2MI. By definition, acute atherothrombotic plaque obstruction is not a characteristic of T2MI.[27] Patients with stable known or assumed CAD with an acute stressor like severe gastrointestinal bleed or a sustained tachyarrhythmia with myocardial ischemia can result in myocardial injury and a T2MI. These effects result from insufficient blood flow to the ischemic myocardium to meet the stressor's elevated myocardial oxygen demand.[26]
Prospective evaluations of the essentials of CAD with T2MI using consistent definitions and approaches are needed. The frequency of ST-segment elevation in T2MI varies from 3% to 24%. In most cases, coronary embolism caused by thrombi, calcium or vegetation from the atria or ventricles, or acute aortic dissection may lead to a T2MI. Unconstrained coronary artery dissection with or without intramural hematoma is another nonatherosclerotic condition that can occur, especially in young women.[28]
Imaging techniques
Noninvasive imaging plays several roles in patients with known or suspected MI. The underlying rationale is that structural myocardial hypoperfusion and ischemia causes a cascade of events involving myocardial dysfunction, healing by fibrosis, and cell death. Essential imaging parameters include myocardial perfusion, myocardial thickness, thickening, motion, myocyte viability, and the effects of myocyte loss observed in radio-opaque contrast agents identifying myocardial fibrosis or scar.[24]
Frequently used imaging techniques in acute and prior occurring MI is echocardiography, myocardial perfusion scintigraphy using single-photon emission computed tomography (SPECT) or positron emission tomography (PET), cardiovascular magnetic resonance (CMR), and possibly computed tomography (CT). While the various techniques have overlaps in their application, they can also determine myocardial perfusion, viability, and function to an increased or decreased extent.[25] The radionuclide technique can provide a direct evaluation of myocyte viability due to the tracers' inherent properties. Other technologies offer an indirect assessment of myocardial viability, as the response to dobutamine by EKG and enlarged extracellular space secondary to myocyte loss by CMR or CT.[24]
Echocardiography
Echocardiography's strength is the combined cardiac structure and function evaluation, specifically myocardial thickness, thickening/thinning, and motion. Regional wall motion abnormalities induced by ischemia may be detected by echocardiography almost immediately after onset when >20% transmural myocardial thickness is affected. New cardiac defects without an alternative etiology reinforce MI's diagnosis when cTn values indicate an increasing and decreasing pattern. EKG also allows observation of noncoronary cardiac pathologies known to cause chest pain, for example, acute pericarditis, acute aortic stenosis, and hypertrophic cardiomyopathy, among others.[17]
Radionuclide imaging
Different radionuclide tracers allow viable myocytes to be imaged directly, including the SPECT tracers 201TI chloride, 99mTc sestamibi, tetrofosmin, and the PET tracers 18F 2-fluorodeoxyglucose and 82Rb. A strength of radionuclide techniques is that they are usually the only available methods for evaluating viability directly.[29] Although, the comparatively low resolution of the images limits them for diagnosing the smallest areas of MI. Phantom studies indicate that myocyte loss of as little as 4% of the myocardium may be detected, matching with 5–10 g of muscle structure – ECG-gated imaging yields a reliable evaluation of myocardial motion, thickening, and global function. Evolving radionuclide techniques for the assessment of MI involve imaging of sympathetic innervation using 123I-labeled meta-iodobenzylguanidine. Diagnostic imaging of matrix metalloproteinase activation in ventricular remodeling.[30]
Cardiac magnetic resonance imaging
The high tissue resolution and contrast of CMR provides an accurate evaluation of myocardial structure and function. However, less commonly used in the acute setting, it has similar capabilities to echocardiography in suspected MI. Paramagnetic contrast agents may be used to assess myocardial perfusion and the increase in extracellular space that is related to the fibrosis of prior MI (diagnosed by late gadolinium enhancement-CMR [LGE-CMR]).[21] These techniques used in establishing acute MI and localized delay in contrast enhancement can detect even minor subendocardial MI areas, thought to be as little as 1 g.
Computed tomography coronary angiography
Infarcted myocardium is initially visible as an essential area of decreased LV myocardial enhancement, but later, imaging indicates hyperenhancement as with LGE-CMR. This finding is clinically significant because contrast-enhanced CT is performed for suspected aortic dissection and pulmonary embolism, conditions with clinical features that project with acute MI. Similarly, CT evaluation of myocardial perfusion is technically feasible but not widely applied.[26] CT coronary angiography (CTCA) can be used to diagnose CAD in patients with an acute coronary syndrome in the emergency department or chest pain unit, specifically in low-to-intermediate-risk patients with normal cTn at presentation. The only randomized trial in these individuals that involves both hs-cTn and CTCA found that imaging did not decrease the length of stay in the hospital.[30]
Applying imaging in acute myocardial infarction
Imaging techniques may be useful in diagnosing acute MI because of the ability to identify wall motion abnormalities or loss of viable myocardium in the presence of increased cardiac biomarker values. Illustration of new loss of myocardial viability in the absence of nonischemic causes reinforces MI's diagnosis.[19,25,31]
Standard function practically excludes significant MI, but a minor MI cannot be ruled out. Thus, imaging techniques are useful for the initial trial and discharge of patients with suspected MI. However, if biomarkers have been studied at appropriate times and are healthy, this excludes acute MI and takes precedence over the imaging standards. Abnormal regional myocardial motion and thickening can be caused by acute MI or by one or more of different other conditions, including prior infarction, acute ischemia, hibernation, or stunning.[26]
Nonischemic conditions such as inflammatory or infiltrative diseases, and cardiomyopathy, can also lead to structural loss of viable myocardium or functional abnormality. The positive predictive value of imaging is not high unless these conditions are avoided, and a new anomaly is analyzed or increases in the setting of other characteristics of acute MI.[27,32]
Treatment and Management of Type 2 Myocardial Infarction
Different studies have shown that the treatment and management of T2MI remain unstable. It is vital to note that patients with T2MI receive lesser prescriptions for preventative therapies than those with T1MI. Notably, the factors responsible for T2MI can be used to treat acute settings by replacing the effects of those factors or reversing them.[27,33]
However, a lack of evidence over using conventional cardiovascular treatments or therapies still poses a challenge toward a standardized methodology in handling T2MI. It is indeed possible that patients might not benefit from using cardiovascular protective agents. It is indeed possible that patients might not benefit from using cardiovascular protective agents, such as beta blockers, statin drugs when given before their being diagnosed. such as beta blockers, statin drugs.[34]
Troponin assays and their extensive usage clinically have helped increase a certain level of awareness toward diagnoses of T2MI. These assays have helped draw more consciousness to the poor prognosis and management of T2MI.[30,35] The Wake-Up T2MI Registry, a retrospective cohort study investigating patients with T2MI, acute myocardial injury, and chronic myocardial injury, mainly generates better treatment designs and management of T2MI.29
Managing T2MI has still proven to be an issue considering poor prognosis and difficulty providing an early diagnosis. Adequately addressed MI would lead to providing better patient care and excellent quality of life. It would also drop mortality rates and misdiagnosis.[26]
Authors’ Overview
Cardiovascular disease is a global health issue, and prevalence is increasing in the developing world. The effects and burden of CAD in populations are of vital importance. Changing clinical understanding, criteria, and biomarkers add challenges to our knowledge and the possibility to improve the health of the public.[1,2,7,10] For clinicians, the definition of MI has essential and immediate therapeutic implications. Epidemiologists see the data as a retrospective, so consistent case definitions are vital for comparisons and trend analysis. The standards illustrated in this report are suitable for epidemiology studies and the international classification of diseases.[8,22,36]
In countries with little economic resources, cardiac biomarkers and imaging techniques cannot be accessible except in a few centers, and even the choice of ECG recordings can be lacking. MI's diagnosis can be established by pathological Q waves when the only information available in clinical history, ECG with absent or incomplete cardiac biomarkers.[8,25,37] Using the outlined standards, the diagnosis of MI requires integrating clinical discovery, ECG, laboratories, diagnostic imaging, and pathological findings overtime when the suspected event unfolds.[13,15,37]
Modern health-care systems are frequently using electronic medical records where medical information is entered, curated, and available for retrieval for a later date. This evolution offers the edge of a modern electronic database that is useful for a variety of purposes. The various purposes, including scientific discovery and quality improvement in medical care, carry the challenges of sifting through different locations and formats where key data elements for verifying MI diagnosis are located.[26] The use of the electronic medical record as an epidemiological and research tool is likely to require efforts to confirm an acute MI diagnosis's accuracy rather than accepting the coded diagnoses used for administrative and billing purposes. Such an attempt to create a data-based phenotype of MI will require input from experts in implementation science to translate into the routine practice of health-care delivery and documentation.[13,25,26]
With the evolution of biomarker tests used to support MI's diagnosis, a consistent approach must be used to construct the data-based phenotype of MI to make comparisons across institutions and track epidemiological trends reliably. Ideally, the information given should include the assay used to analyze MI, the 99th percentile of the URL, and the full sequence of values obtained to discern an increase and decrease in biomarker levels.[26]
The diagnostic challenges associated with T2MI are global health issues with the rising prevalence in the developing world. The evolution of various modalities in the early diagnosis of T2MI has helped decrease the mortality rate in developed and developing counties.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ryan TJ, Bauman WB, Kennedy JW, Kereiakes DJ, Iii SBK, Mccallister BD, et al. Guidelines for Percutaneous Transluminal Coronary Angioplasty Transluminal Coronary Angioplasty ) Circulation. 1990;88(6):2987–3002. doi: 10.1161/01.cir.88.6.2987. [DOI] [PubMed] [Google Scholar]
- 2.August VI, Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, Ill SRK, et al. Guidelines for Percutaneous Transluminal Coronary Angioplasty A Report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transl. J Am Coll Cardiol. 1988;12(2):529–45. [PubMed] [Google Scholar]
- 3.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC / AHA Practice Guidelines ACC / AHA Guideline Update for the Management of Patients With Unstable Angina and Non – ST-Segment Elevation Myocardial Infarction 2002 : Summary Article. Circulation. 2002;6083:1893–900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 4.Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Ryan TJ, Antman EM, Brooks NH, Califf RM, Hillis LD, Russell RO, et al. 1999 update: ACC / AHA guidelines for the management of patients with acute myocardial infarction COMMITTEE MEMBERS. J Am Coll Cardiol. 1999;34:890–911. doi: 10.1016/s0735-1097(99)00351-4. [DOI] [PubMed] [Google Scholar]
- 6.Saaby L, Poulsen TS, Hosbond S, Larsen TB, Pyndt Diederichsen AC, Hallas J, Thygesen K, Mickley H. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. [Epub 2013 Jul 12];Am J Med. 2013 Sep;126(9):789–97. doi: 10.1016/j.amjmed.2013.02.029. doi: 10.1016/j.amjmed.2013.02.029. PMID: 23856021. [DOI] [PubMed] [Google Scholar]
- 7.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC / AHA Guideline ACC / AHA / ASE 2003 Guideline Update for the Clinical Application of Echocardiography : Summary Article A Report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines ( ACC / AHA / ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography ) Committee Members. J Am Coll Cardiol. 2003;42(5):954–70. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 8.Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kupersmith J, et al. Journal of the American College of Cardiology. Vol. 36. Elsevier Masson SAS; 2000. ACC / AHA PRACTICE GUIDELINES ACC / AHA Guidelines for the Management of Patients With Unstable Angina and Non – ST-Segment Elevation Myocardial Infarction A Report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines ( Committee on the Management of Patients With Unstable Angina ) TASK FORCE MEMBERS; pp. 970–1062. [DOI] [PubMed] [Google Scholar]
- 9.Smith SC, Dove JT, Jacobs AK, Kennedy JW. ACC / AHA PRACTICE GUIDELINES ACC / AHA Guidelines for Percutaneous Coronary Intervention ( Revision of the 1993 PTCA Guidelines )— Executive Summary A Report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines ( Committee to Revise committee members table of contents class i. j Am Coll Cardiol. 2001;37(8):2215–38. doi: 10.1016/s0735-1097(01)01344-4. [DOI] [PubMed] [Google Scholar]
- 10.Eagle KI, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, et al. ACC / AHA PRACTICE GUIDELINES ACC / AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery – Executive Summary committee members task force members table of contents. J Am Coll Cardiol. 2002;39(3):542–53. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Fihn SD, et al. ACC / AHA guideline ACC / AHA 2002 guideline update for the management of patients with chronic stable angina – Summary article. J Am Coll Cardiol. 2003;41:159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann E, Dormandy JA, Ds C, Charbonnel B, Massi-benedetti M, Moules IK, et al. The Effect of Pioglitazone on Recurrent Myocardial Infarction in 2, 445 Patients With Type 2 Diabetes and Previous Myocardial Infarction Results From the PROactive ( PROactive 05 ) Study. J Am Coll Cardiol. 2007;49(17):1772–80. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Gara PTO, Kushner FG, Ascheim DD, Casey DE, Chung MK, Lemos JA De, et al. 2013 ACCF / AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2013;61(4):78–140. [Google Scholar]
- 14.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC / AHA GUIDELINE ACC / AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery : Executive Summary. J Am Coll Cardiol. 2007;50(17):1707–32. doi: 10.1016/j.jacc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Scalon PJ, Faxon DP, Eagle KIMA, Legako RD, Leon DF, Murray JA, et al. Journal of the American College of Cardiology. Vol. 33. Elsevier Masson SAS; 1999. ACC / AHA practice guidelines acc / aha guidelines for coronary angiography committee members task force members; pp. 1756–1824. [DOI] [PubMed] [Google Scholar]
- 16.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer J V, Berman DS, et al. ACC / AHA Guideline ACC / AHA / ASNC Guidelines for the Clinical Use of Cardiac Radionuclide Imaging Executive Summary A Report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines ( ACC / AHA / ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging ) Committee Members. J Am Coll Cardiol. 2003;42(7):1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Boersma E, Poland AB, Ferna F, Dalby S, Denmark K, Widimsky P, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Hear Journaleart J. 2007;28(1):1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 18.Califf RM, Hochman JS, Steward DE. ACC / AHA PRACTICE GUIDELINES ACC / AHA 2002 Guideline Update for the Management of Patients With Unstable Angina and Non – ST-Segment Elevation Myocardial Infarction — Summary Article American Heart Association Task Force on Practice Guidelines ( Committee on the Management of Patients With Unstable Angina ) TASK FORCE MEMBERS Class I. J Am Coll Cardiol. 2002;40(7):1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 19.From M, Heart C, With D, Subjects D, Prior W, Infarction M. Mortal ity from coronary he art disease in subjects with and with out type 2 diabetes mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N engl j med. 1998;339(4):229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 20.Leape LL, Brennan TA, Laird NAN, Ph D, Lawthers ANNG, Sc D, et al. The nature of adverse events in hospitalized patients Results of the Harvard Medical Practice Study II fecting the quality of care has grown. Curiously, how- paratively little attention from either perspective. But an important objective for those conc. N Engl J Med. 1991;324(6):377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 21.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 22.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, et al. ACC / AHA / NASPE Practice Guidelines ACC / AHA / NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices – Summary Article. J Am Coll Cardiol. 2002;40(9):1703–19. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 23.Graham DJ, Ouellet Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, Denmark KT, et al. Universal Definition of Myocardial Infarction. Circulation. 2012;Vol. 126:2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care †. Eur Hear J. 2010;31(1):1–206. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 26.McAllister DA, Maclay JD, Mills NL, Leitch A, Reid P, Carruthers R, et al. hospitalisation for exacerbation of COPD. Eur Respir. 2012;39(5):1097–103. doi: 10.1183/09031936.00124811. [DOI] [PubMed] [Google Scholar]
- 27.Transplantation L, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. 2016 ACC / AHA / HFSA Focused Update on New Pharmacological Therapy for Heart Failure : An Update of the 2013 ACCF / AHA Guideline for the Management of Heart Failure. JOU RNAL AMER I CAN Coll Cardiol Y. 2016;68(13):1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, et al. PRACTICE GUIDELINE : FULL TEXT ACC / AHA / HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. JAC. 2008;51(21):e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Tayeh O, Ettori F. Vascular access and angiographic lesion morphology in elective percutaneous coronary intervention. Egypt Hear J. 2014;66(1):27–33. [Google Scholar]
- 30.Investigation O. Bezafibrate for the Secondary Prevention of Myocardial Infarction in Patients With Metabolic Syndrome. Arch Intern Med. 2005;165(1):1154–60. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 31.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF / AHA / SCAI Guideline for Percutaneous Coronary Intervention : Executive Summary. JAC. 2011;58(24):2550–83. [Google Scholar]
- 32.Eagle KIMA, Guyton RA, Gardner TJ, Gott JP, Herrmann HC, Marlow RA, et al. Journal of the American College of Cardiology. Vol. 34. Elsevier Masson SAS; 1999. ACC / AHA GUIDELINES FOR CABG SURGERY ACC / AHA Guidelines for Coronary Artery Bypass Graft Surgery American Heart Association Task Force on Practice Guidelines ( Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery ) COMMITTEE MEMBERS TASK FORCE MEMBERS; pp. 1262–1347. [DOI] [PubMed] [Google Scholar]
- 33.Thygesen K, Alpert JS, White HD, Kristian C, Denmark T, New HDW, et al. Universal Definition of Myocardial Infarction. J Am Coll Cardiol. 2007;50(22):2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Kang S, Burke AP, Virmani R. Combined IVUS and NIRS Detection of Fibroatheromas. JACC Cardiovasc Imaing. 2015;8(2):184–94. doi: 10.1016/j.jcmg.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Hamm CW, Ravkilde J, Gerhardt W, Jørgensen P, Peheim E, Ljungdahl L, et al. The prognostic value of serum troponin T in unstable Angina. N Engl J Med. 1992;327:146–50. doi: 10.1056/NEJM199207163270302. [DOI] [PubMed] [Google Scholar]
- 36.Gettes LS, Hlatky MA, Newby LK, Schoenfeld MH. ACC / AHA / HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities : Executive Summary. JAC. 2008;51(21):2085–105. [Google Scholar]
- 37.Epstein AE, Dimarco JP, Ellenbogen KA, Iii NAME, Freedman RA, Gettes LS, et al. 2012 ACCF / AHA / HRS Focused Update Incorporated Into the ACCF / AHA / HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. JAC. 2013;61(3):e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]


