Abstract
Wilson’s disease (WD) is a rare inherited impaired copper metabolism with diverse clinical pictures dominated by hepatic and neurologic manifestations. We report the case of a 14-year-old female patient who attended the Department of Neuropsychiatry at Ali Abad Teaching Hospital, Kabul, Afghanistan, with generalized tonic-clonic seizure and cerebellar dysfunction. The patient was initially diagnosed as encephalitis and epilepsy and finally diagnosed with WD based on the clinical and laboratory findings. After 6 months of follow-up, the patient showed substantial clinical recovery.
Keywords: Wilson’s disease, copper accumulation, KF rings, seizure, cerebellar dysfunction
Introduction
Wilson’s disease (WD) is an infrequent autosomal recessive disturbance of copper metabolism which happens as a result of mutations in the copper-transporting ATP7B gene.1 Increased free copper levels in different organs cause injury to them, with broad-ranging clinical manifestations dominated by signs of liver and brain injury, especially of the basal ganglia and cerebellum.2 Abnormal movements such as tremor, dystonia, bradykinesia, and chorea, accompanied with difficulty swallowing, difficulty speaking and poor articulation, and excessive salivation are the chief neurologic manifestations.3 Apart from movement disorders, neurologic signs of WD are uncommon and include neuropathy, autonomic system dysfunction, headaches, and epilepsy.3 Epilepsy may be one of the earliest presentations of WD, sometimes years before other neurological symptoms appear.4 The frequency and importance of epilepsy in WD patients were first described by Dening et al in 1988.4 In this study, the authors found seizures in 6.2% of 200 patients (in England) — 10 times more than the frequency of seizures in general, healthy population.3 Neurologic manifestations of WD are revealed at an older age compared to hepatic manifestations. They are commonly found in patients with misdiagnosed hepatic disease, in those with asymptomatic hepatic impairment, in the case of non-adherence to de-coppering therapy, or with treatment failure.5 In view of its diverse presentations, misdiagnosis is not unusual.6
Case Presentation
A 14-year-old female patient came to the Department of Neuropsychiatry at Ali Abad Teaching Hospital, Kabul, Afghanistan with generalized tonic-clonic seizure, ataxia, intention tremor, and disturbance of gait and speech. During investigation, it was noted that she had suffered from seizure attacks for one year. It was accompanied by ataxia, intention tremor, and disturbances of gait and speech in the last nine months. The symptoms had started insidiously and progressed gradually over time. The situation had worsened in the last month to the extent that she was not able to perform her daily activities without support. Throughout this period, she had been hospitalized under various conditions, including encephalitis and epilepsy, and had taken various medicines without any improvement.
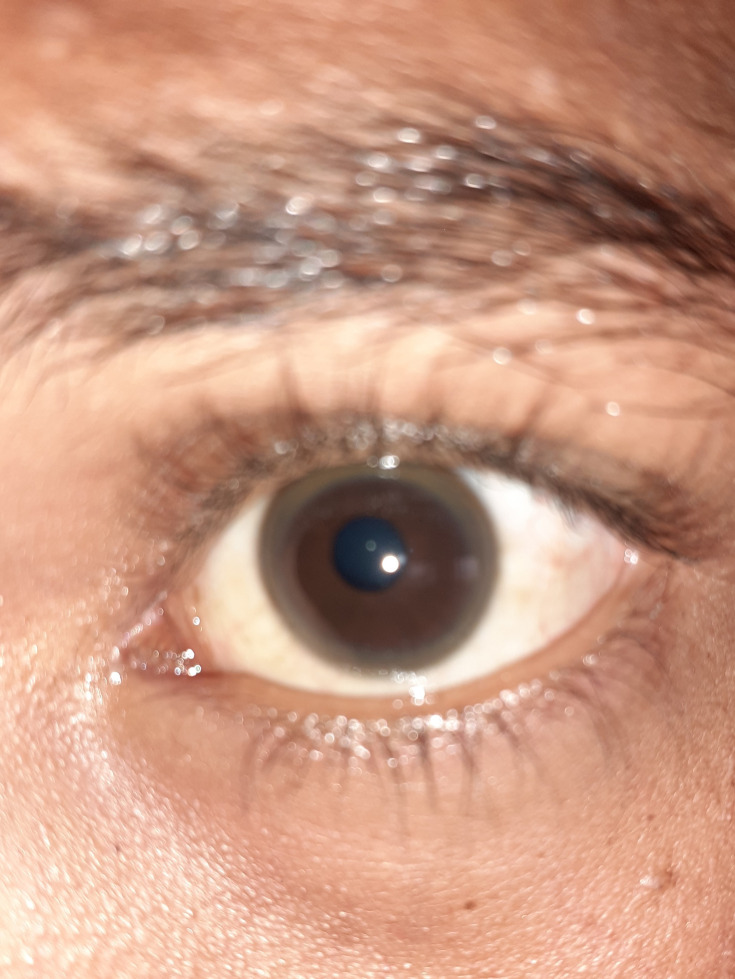
Nothing significant was found in her family history. On general physical examination, the patient was alert, cooperative, and oriented. No abnormal finding was noted in other systems except for a dark ring in the cornea of both eyes as shown in Figure 1. On neurologic examination, ataxia, intention and resting tremor, dysarthria, tandem gait, dysmetria, and abnormal finger to finger test were present.
Figure 1.
Kayser–Fleischer ring seen in the patient’s left cornea.
Lab investigations including complete blood count, liver function test, and renal function test were within normal ranges. Hepatitis B surface antigen and hepatitis C virus antibody were negative. Based on the presence of the dark ring in both eyes, the diagnosis of WD was suspected; therefore, further evaluation was conducted. Abdominal sonography revealed coarse echotexture of liver and cholelithiasis. Slit lamp examination confirmed the presence of Kayser–Fleischer (KF) ring in both eyes. Serum ceruloplasmin level was 0.10 g/L. Magnetic resonance imaging (MRI) which was done one year before showed mesial temporal sclerosis in the left temporal lobe. The patient’s guardian was reluctant to do a follow-up MRI due to financial restriction.
Clinical characteristics, serum ceruloplasmin (0.10 g/L), and the KF rings on slit lamp examination were used to make the diagnosis of WD. According to the Ferenci score, a patient with a score of more than 4 is diagnosed as WD.7
After confirmation of diagnosis, the treatment regimen was planned as follows: restriction of copper-rich foods, such as liver, mushroom, cocoa, chocolate, nuts, and shellfish; penicillamine 250 mg once daily initially and increased up to 1.5 g/day in divided doses gradually; zinc acetate 150 mg daily in three divided doses; pyridoxine 20 mg daily; levetiracetam 500 mg in two divided doses and increased in steps of 250 mg every two weeks according to the patient’s response; and propranolol 10 mg three times per day. Six months after treatment, the patient had shown remarkable improvement, ie, was relieved of seizure attacks and was able to do her daily activities without assistance. The patient’s parent provided written informed consent for the case details to be published.
Discussion
WD is an infrequent, genetic disorder of copper metabolism, in which the causative gene, ATP7B, results in dysfunction of the ATP7B transporter necessary for biliary excretion of copper and incorporation of copper into ceruloplasmin.8 More than 500 mutations have been identified in the ATP7B gene thus far. Even though there is some evidence that truncating mutations may be accompanied with earlier onset than missense mutations, and patients with frameshift mutations may be more likely to develop neurologic symptoms, individual gene mutations have not been found to accompany various manifestations of WD. Studies have reported cases of monozygotic twins with WD who were phenotypically discordant. This finding demonstrates that environmental and epigenetic factors might play a significant role in WD, at least in part.9 WD usually starts with a clinically silent period, during which copper accumulation in liver results in subclinical hepatitis and progresses toward liver cirrhosis and appearance of neuropsychiatric manifestations.1 Changes in behavior, decline in academic performance, or inability to carry out activities that need strong hand–eye coordination may be found in pediatric patients insidiously in the early course of disease. Hand-writing may become worse, leading to cramped, small handwriting (micrographia) as seen in Parkinson’s disease. Tremor, incoordination, excessive salivation, dysarthria, dystonia, and spasticity are all prevalent neurologic manifestations of WD. Transfer dysphagia may occur as a result of pseudobulbar palsy, with the risk of aspiration in severe cases. Dysautonomia, migraine headaches, and sleeplessness may also be present, although seizures are uncommon.10
Rarely, epileptic seizures can be the presenting symptom of WD, which can happen at any stage of the disease and might be more usual after initiation of anti-coppering treatment.11,12 Seizures can affect 14.5% of patients with neurologic WD, particularly those who have cortical, subcortical, or cerebellar involvement on MRI.13 Various types of seizures such as grand mal, focal, or absence can be present.14 The types of seizures seen in WD patients do not differ significantly from those observed in general population surveys,4 although there is an increase of focal motor seizures in WD.4 Diseases characterized by involuntary and paroxysmal movements can mimic epilepsy. Detecting the presence or absence of seizures can be challenging in such conditions.4 Epilepsy related to WD appears to have a better outcome than epilepsy in the general population.4 The prognosis of epilepsy appears to be influenced more by WD therapy than by anticonvulsants.4 Treatment of such epilepsy cases is conventional like other forms, but treatment of Wilson disease must include penicillamine or trientine.14 When choosing antiepileptic medications for WD patients, their possible hepatotoxicity should be considered (as such, treatment with valproate should be avoided).3,4 Cerebellar signs and symptoms are rarely clinically relevant and are not found alone. On examination, frank limb ataxia is unusual. Cerebellar signs, apart from limb dysmetria such as overshoot dysmetria of the eyes and extremities, or ataxic dysarthria can be seen.15
WD is diagnosed based on some clinical and biochemical tests. Age at onset between 5 and 40 years, detectable KF rings, and decreased serum ceruloplasmin are the classic manifestations of WD.16 KF rings caused by copper accumulation in Descemet’s membrane, a pathognomonic sign, may be detectable in the eyes, either directly or on slit lamp examination.17
Initial presentations of WD are easily ignored and often diagnosed incorrectly as hepatitis, cirrhosis, splenomegaly, or encephalitis, leading to postponed therapy.18 The average time between development of symptoms and diagnosis is about two years. This delay is longer in neurologic than hepatic presentations, according to Kim et al (44 vs 14 months).18 A delay of 30 years has also been reported.19 Providing timely diagnosis and proper management may prevent irreversible organ damage.18 Pharmacotherapy of WD focuses on de-coppering through chelators and/or zinc to decrease the intestinal absorption of copper.8 Drug therapy in WD must be lifelong as abnormal copper deposition cannot be controlled by a lower copper diet.11 In patients with neurological features, clinical improvement begins 5–6 months after beginning de-coppering treatment, and most patients finally demonstrate significant improvement.8 Paradoxically, the neurological symptoms are said to be aggravated with penicillamine. This may be ascribed to mobilization of copper from liver and elevations of unbound copper levels which result in deteriorating of neurological manifestations. Some studies have reported an initial neurological worsening in 30–75% of patients after penicillamine treatment. However, this has been denied by some other reports.20 The neurologic symptoms ameliorated in our patient after treatment with penicillamine.
WD being an inherited disorder, it is highly recommended to carry out familial screening in WD patients. The American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of Liver (EASL) suggest screening the first-degree relatives of a proband. First-degree relatives not only include the siblings of a proband, but also the children and parents.21 Our patient presented with unusual features such as generalized tonic-clonic epilepsy and predominant cerebellar findings. Asymptomatic liver changes were noted on abdominal sonography. She was misdiagnosed as encephalitis and epilepsy for one year. After following WD treatment for 6 months, she had noticeable improvements. This case highlights the importance of suspecting WD in young patients presenting with unknown isolated neurological signs such as seizure or cerebellar dysfunction. Eye examination, either directly or by slit lamp, and abdominal sonography (even in the absence of clinical evidence of hepatic involvement) in patients with movement disorders can help to diagnose life-threatening diseases. Likewise, on-time diagnosis and management of WD could help patients enjoy a healthy lifestyle during the rest of their lives.
Funding Statement
There is no funding to report.
Ethics
This report was approved by the Ethics Committee of the Department of Neuropsychiatry, Kabul University of Medical Sciences under protocol no. 2020-130.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Güngör Ş, Selimoğlu MA, Varol Fİ, et al. Pediatric Wilson’s disease: findings in different presentations. A cross-sectional study. Sao Paulo Med J. 2018;136(4):304–309. doi: 10.1590/1516-3180.2018.0210230718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Žigrai M, Vyskočil M, Tóthová A, et al. Late-onset Wilson’s disease. Front Med. 2020;7(26). doi: 10.3389/fmed.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Członkowska A, Litwin T, Chabik G. Wilson disease: neurologic features. Handb Clin Neurol. 2017;142:101–119. [DOI] [PubMed] [Google Scholar]
- 4.Dening TR, Berrios GE, Walshe JM. Wilson’s disease and epilepsy. Brain. 1988;111(Pt 5):1139–1155. doi: 10.1093/brain/111.5.1139 [DOI] [PubMed] [Google Scholar]
- 5.Dusek P, Litwin T, Członkowska A. Neurologic impairment in Wilson disease. Ann Transl Med. 2019;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Luan J, Zhou X, et al. Epidemiology, diagnosis, and treatment of Wilson’s disease. Intractable Rare Dis Res. 2017;6(4):249–255. doi: 10.5582/irdr.2017.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litwin T, et al. Psychiatric manifestations in Wilson’s disease: possibilities and difficulties for treatment. Ther Advan Psychopharmacol. 2018;8:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kathawala M, Hirschfield GM. Insights into the management of Wilson’s disease. Therap Adv Gastroenterol. 2017;10(11):889–905. doi: 10.1177/1756283X17731520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulligan C, Bronstein JM. Wilson disease: an overview and approach to management. Neurol Clin. 2020;38(2):417–432. doi: 10.1016/j.ncl.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 10.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089–2111. doi: 10.1002/hep.22261 [DOI] [PubMed] [Google Scholar]
- 11.Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–113. doi: 10.1016/S1474-4422(14)70190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao C, Colangelo T, Dhanekula RK, et al. A rare case of wilson disease in a 72-year-old patient. ACG Case Rep J. 2019;6(3):e00024. doi: 10.14309/crj.0000000000000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalita J, Misra UK, Kumar V, et al. Predictors of seizure in Wilson disease: a clinico-radiological and biomarkers study. Neurotoxicology. 2019;71:87–92. doi: 10.1016/j.neuro.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Walshe JM. Wilson disease. In: Andermann F, Guerrini R, Shorvon SD, editors. The Causes of Epilepsy: Common and Uncommon Causes in Adults and Children. Cambridge: Cambridge University Press; 2011:249–251. [Google Scholar]
- 15.Lorincz M. Neurologic Wilson’s disease. Ann N Y Acad Sci. 2010;1184:173–187. doi: 10.1111/j.1749-6632.2009.05109.x [DOI] [PubMed] [Google Scholar]
- 16.Cao C, Colangelo T, Dhanekula RK, et al. A rare case of Wilson disease in a 72-year-old patient. ACG Case Rep J. 2019;6(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradeepkumar S, Rudrappa R, Rajakumar S. Neuro Wilson’s – an Alien Presentation. Ann Int Med Dental Res. 2016;3. [Google Scholar]
- 18.Kim M-K, Lee K, Woo H-Y, et al. Late diagnosis of wilson disease, initially presenting as cerebellar atrophy mimicking spinocerebellar ataxia, by multigene panel testing. Ann Lab Med. 2020;40(6):500–503. doi: 10.3343/alm.2020.40.6.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shribman S, Warner TT, Dooley JS. Clinical presentations of Wilson disease. Ann Transl Med. 2019;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur H, Kaur K, Sharma N, Kumar K. Wilson's disease: a case report. International Journal of Contemporary Medical Research. 2019;6(7):G42–44. [Google Scholar]
- 21.Woimant F, Djebrani-Oussedik N, Poujois A. New tools for Wilson’s disease diagnosis: exchangeable copper fraction. Ann Transl Med. 2019;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]