Abstract
Background
Chromoblastomycosis (CBM), represents one of the primary implantation mycoses caused by melanized fungi widely found in nature. It is characterized as a Neglected Tropical Disease (NTD) and mainly affects populations living in poverty with significant morbidity, including stigma and discrimination.
Methods and findings
In order to estimate the global burden of CBM, we retrospectively reviewed the published literature from 1914 to 2020. Over the 106-year period, a total of 7,740 patients with CBM were identified on all continents except Antarctica. Most of the cases were reported from South America (2,619 cases), followed by Africa (1,875 cases), Central America and Mexico (1,628 cases), Asia (1,390 cases), Oceania (168 cases), Europe (35 cases), and USA and Canada (25 cases). We described 4,022 (81.7%) male and 896 (18.3%) female patients, with the median age of 52.5 years. The average time between the onset of the first lesion and CBM diagnosis was 9.2 years (range between 1 month to 50 years). The main sites involved were the lower limbs (56.7%), followed by the upper limbs (19.9%), head and neck (2.9%), and trunk (2.4%). Itching and pain were reported by 21.5% and 11%, respectively. Malignant transformation was described in 22 cases. A total of 3,817 fungal isolates were cultured, being 3,089 (80.9%) Fonsecaea spp., 552 (14.5%) Cladophialophora spp., and 56 Phialophora spp. (1.5%).
Conclusions and significance
This review represents our current knowledge on the burden of CBM world-wide. The global incidence remains unclear and local epidemiological studies are required to improve these data, especially in Africa, Asia, and Latin America. The recognition of CBM as NTD emphasizes the need for public health efforts to promote support for all local governments interested in developing specific policies and actions for preventing, diagnosing and assisting patients.
Author summary
Chromoblastomycosis (CBM), represents one of the primary implantation mycoses caused by melanized fungi widely found in nature. It is characterized as a Neglected Tropical Disease and mainly affect populations living in poverty with significant morbidity, including stigma and discrimination. The global incidence of CBM remains unclear because this mycosis is not a mandatory notifiable disease and most of the literature consists of case reports or small series incompletely characterized. Although several authors suggest that the CBM global burden may be comparable to mycetoma, its geographic distribution and incidence rates in different endemic areas have never been widely characterized in the medical literature. We retrospectively conducted a comprehensive systematic review of all medical literature published between 1914 and 2020 to better characterize the prevalence rates, geographic distribution, and clinical aspects of CBM in all continents. All reviewed data were not a substitute for high quality epidemiological study or comprehensive surveillance but do provide an approximation of the burden by country. Information generated corroborate the WHO recognition of CBM as a NTD and provides helpful support for all local governments interested in developing specific policies and actions for preventing, diagnosing and assisting patients with CBM.
Introduction
Chromoblastomycosis (CBM), together with mycetoma, represents one of the primary implantation mycoses caused by melanized or black fungi widely found in nature that may infect agricultural workers after transcutaneous inoculation during their daily activities [1–4]. Chromoblastomycosis is primarily an occupational disease associated with a considerable social stigma and severe personal and family socioeconomic consequences [1,3,5,6]. It is mainly caused by Fonsecaea spp., followed by Cladophialophora, Phialophora, and Rhinocladiella. The genera Fonsecaea includes three closely related siblings represented by F. pedrosoi, F. monophora, and F. nubica. The genus Cladophilophora spp. contains two related siblings: C. carrionii that may be found in clinical samples and nature, whereas C. yegresii is exclusively found in the environment [1,7–12]. These agents present some peculiarities in terms of geographic distribution and ecological niches. The clinical manifestations and therapeutic response of the patients differs by infecting fungus.
CBM is nowadays characterized as a Neglected Tropical Disease (NTD) because (a) it mainly affects populations living in poverty causing significant morbidity and mortality–including stigma and discrimination; (b) it is mostly found in tropical and sub-tropical areas; (c) it may be controlled or eradicated by applying one or more of the five public health strategies adopted by the Department for Control of NTDs; (d) it has been neglected by research when it comes to developing new diagnostics, medicines, and other control tools [1,3–5]. The process of recognizing CBM as NTD began at the meeting held in São Luís, state of Maranhão, Brazil, in 2011, when the disease’s centenary was celebrated. After an application by the Global Action Fund for Fungal Infections with support from the governments of Brazil and Madagascar, World Health Organization (WHO) incorporated CBM into the NTD portfolio in category B in 2017, together with mycetoma and other deep mycoses [1,7].
In most endemic areas, health services do not have professionals trained in the early diagnosis and clinical management of CBM. Skills in skin biopsy, direct microscopy, histopathology with fingal stains fungal culture is often lacking. Effective antifungal treatment rarely included in universal health coverage and government insurance. Long term itraconazole at 400mg daily or variable terbinafine dose are not be available in many countries and is expensive and requires monitoring [1,3,6–9]. Patients are usually diagnosed after several years of clinical manifestations, and medication is unavailable or unaffordable, two factors that may increase the risk of sequelae and further social stigma [1,3]. In addition, CBM in some patients is complicated by continuous bacterial co-infection and later neoplastic transformation of the CBM lesions into epidermoid carcinoma may occur [1,3]
The global incidence of CBM remains unclear. Only a few population epidemiology studies have been done. This mycosis is not a mandatory notifiable disease and most of the literature consists of case reports or small series incompletely characterized. Although several authors suggest that the CBM global burden may be comparable to mycetoma, its geographic distribution and incidence rates in different endemic areas have never been widely characterized in the medical literature.
We conducted a comprehensive systematic review of all medical literature published between 1914 and 2020 to characterize better the prevalence rates and geographic distribution of CBM in all continents. Data generated in this paper corroborate the WHO recognition of CBM as a NTD and provides helpful support for all local governments interested in developing specific policies and actions for preventing, diagnosing, and assisting patients with CBM [1,5–7,13,14].
Methods
Our plan for literature review included the selection of all articles addressing the epidemiology of chromoblastomycosis in the world that were published in four different languages (English, Spanish, French and Portuguese) between 1914 and 2020 and listed in the PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Bireme (http://portal.revistas.bvs.br/) with access to "LILACS", "IBECS", "MEDLINE", "Cochrane Library" and "SciELO” databases. The terms used to select papers included "chromoblastomycosis", "chromomycosis", "neglected mycoses", "subcutaneous mycoses" or "implantation fungal infections". Letters to the editor and abstracts available published in congress or conferences were also searched and identified. The literature review was complemented by reviewing the reference lists of all studies selected to be sure that we did not miss any relevant references. Due to the large number of single case reports published in some highly endemic countries, papers from Mexico, Brazil, Venezuela, Colombia, Madagascar, India, China, Japan, and Australia were only included if they reported at least 5 patients. Review papers were selected only to find references to original papers to avoid case duplication [2,15]. All papers in this comprehensive review were able to meet the main diagnostic criteria of CBM: presence of dark pigmented and thick-walled muriform cells in a biological sample. Whenever there was doubt about this finding, the paper was excluded from the analysis.
Epidemiological and clinical data of all cases of CBM were collected using a standard clinical form. The variables that were systematically assessed along the literature review included year and country of publication, the number of cases, the period of cases collection, age, gender, history of cutaneous trauma and previous agricultural work, time from onset of symptoms to diagnosis (years), symptoms, clinical pattern of the lesions and severity of the disease, malignant transformation and clinical management (physical methods such as surgery, thermotherapy, laser therapy, and photodynamic therapy; antifungal drugs with itraconazole, terbinafine, iodide, flucytosine) [1,3].
To determine the prevalence rate of CBM in each country, we used the method described by Van de Sande [2]. The number of reported cases along each year in all countries was divided by the total population of each country in the selected period. Population data for each country in each collection period was extracted from the website www.indexmundi.com/facts/indicators/SP.POP.TOTL/compare#country=ma) [2]. As an example 71 cases of CBM were reported between 1978 and 1993 in Sri Lanka, with a mean of 4.73 cases/year. The average population of this country in this period was 16,283,921 inhabitants. In this case, the prevalence of CBM in Sri Lanka was defined as 0.29 cases per 1 million inhabitants.
Results
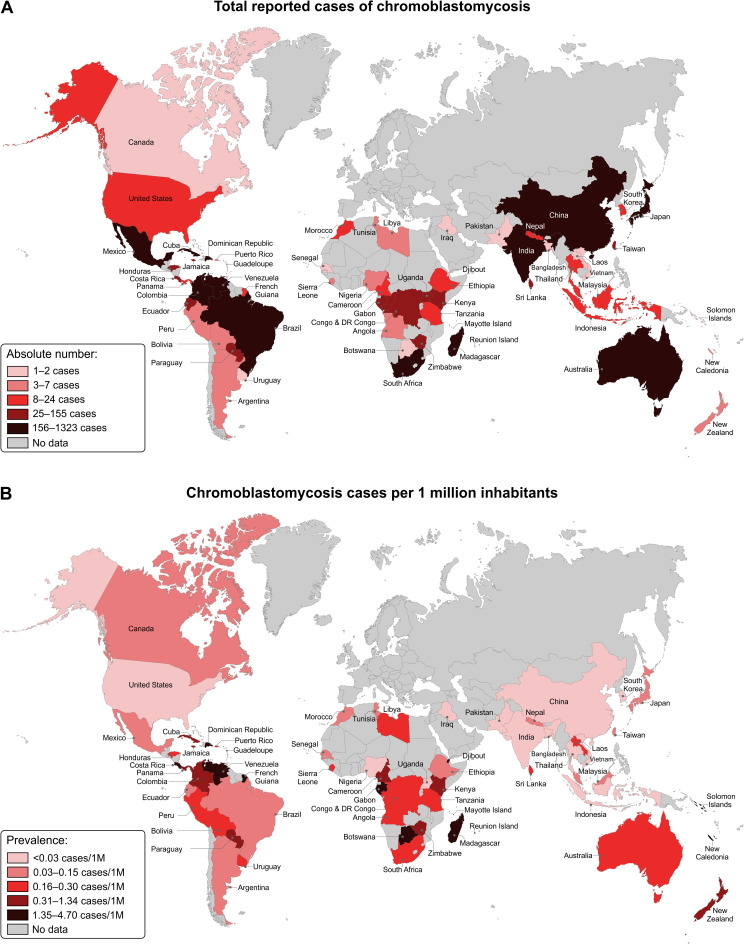
Our review identified a total of 208 articles that were published in English (119 articles), Spanish (42 articles), French (39 articles), and Portuguese (8 articles), accounting for 7,740 cases of CBM on all continents except Antarctica. The main characteristics of CBM are illustrated by countries and continents, as summarized in Tables 1 and S1. The worldwide distribution and prevalence of CBM cases are shown in Fig 1.
Table 1. Epidemiology and main clinical aspects of 7,740 cases of chromoblastomycosis documented in 5 different continents.
| Variables | South America (n = 2,619) | Central America, The Caribbean, and Mexico (n = 1,628) | Africa (n = 1,875) | Asia (n = 1,390) | Europe (n = 35) | USA and Canada (n = 25) | Oceania (n = 168) | Total (n = 7,740) |
|---|---|---|---|---|---|---|---|---|
| Age (years), (mean, range) | 57.1 (12y-93y) | 53.3 (9y-90y) | 47.9 (2y-73y) | 49.7 (7y-90y) | 60.9 (17y-85y) | 55.7 (19y-79y) | 53.8 (19y-91y) | 52.5 (2y-93y) |
| Male/Female n (%) | 1,237 (87.4%) / 178 (12.6%) | 802 (76.7%) /243 (23.3%) | 1,338 (83.6%) / 263 (16.4%) | 463 (71.6%) / 183 (28.4%) | 27 (77.2%) / 8 (22.8%) | 22 (88%) / 3 (12%) | 133 (88%) /18 (12%) | 4,022 (81.7%) / 896 (18.3%) |
| Rural occupation | 927 | 642 | 129 | 162 | 2 | 13 | 20 | 1,895 |
| History of trauma | 276 | 117 | 20 | 116 | 18 | 3 | 18 | 568 |
| Delay between onset and diagnosis (mean, years) | 10.8 (1mo - 50y) | 13.4 (2mo-28y) | 8.7 (4mo-31y) | 8.3 (1mo-40y) | 13.6 (3mo-31y) | 4.8 (2m-20y) | 8.02 (1mo-30y) | 9.2 (1mo-50y) |
| Sites of lesions | ||||||||
| Lower limbs | 1,021 | 472 | 1,340 | 308 | 16 | 6 | 34 | 3,197 |
| Upper limbs | 301 | 288 | 196 | 214 | 11 | 16 | 94 | 1,120 |
| Face, head, neck | 47 | 13 | 20 | 73 | 1 | 2 | 2 | 158 |
| Trunk | 54 | 42 | 32 | 50 | 2 | 0 | 0 | 180 |
| Unusual sites | 11 | 2 | 4 | 39 | 4 | 1 | 0 | 61 |
| Types of lesions | ||||||||
| Plaque | 248 | 39 | 153 | 109 | 4 | 11 | 1 | 565 |
| Verrucous | 329 | 162 | 131 | 76 | 0 | 7 | 5 | 710 |
| Tumoral | 87 | 6 | 558 | 9 | 1 | 0 | 2 | 663 |
| Nodular | 84 | 46 | 117 | 24 | 1 | 5 | 8 | 285 |
| Scarring | 37 | 1 | 32 | 2 | 1 | 0 | 2 | 75 |
| Ulcer | 47 | 7 | 27 | 16 | 1 | 7 | 0 | 105 |
| Etiologic agents | 864 | 703 | 1,406 | 750 | 30 | 21 | 43 | 3,817 |
| Fonsecaea spp. | 759 (87.9%) | 676 (96.2%) | 1,017 (72.3%) | 569 (75.8%) | 24 (80%) | 15 (71.5%) | 29 (67.4%) | 3,089 (80.9%) |
| Cladophialophora spp. | 82 (9.5%) | 11 (1.6%) | 376 (26.8%) | 68 (9%) | 1 (3.3%) | 0 | 14 (32.6%) | 552 (14.5%) |
| Phialophora spp. | 12 (1.4%) | 5 (0.7%) | 8 (0.6%) | 24 (3.2%) | 1 (3.3%) | 6 (28.5%) | 0 | 56 (1.5%) |
| Rhinocladiella spp. | 5 (0.6%) | 1 (0.1%) | 0 | 2 (0.3%) | 1 (3.3%) | 0 | 0 | 9 (0.2%) |
| Other and not identified agent | 6 (0.6%) | 10 (1.4%) | 4 (0.3%) | 87 (11.7%) | 3 (10.1%) | 0 | 0 | 110 (2.9%) |
Fig 1. Prevalence and absolute number of reported cases of chromoblastomycosis.
The world map was created, edited, and colored using the vector graphics editor Corel Draw X8. Public domain link to map base layer used in creating this figure:https://commons.wikimedia.org/wiki/File:BlankMap-World.svg.
Chromoblastomycosis in Central America, the Caribbean and Mexico
A total of 26 articles were identified which described 1,628 patients who were documented in Mexico (603 cases) [16], Dominican Republic (450 cases) [17], Cuba (319 cases) [18–25], Costa Rica (153 cases) [26–30], Honduras (52 cases) [31–34], Jamaica (31 cases) [35], Panama (8 cases) [36–39], Puerto Rico (7 cases) [40], and Guadalupe Islands (5 cases) [41]. Data from the Dominican Republic relies on a single publication from Isa-Isa, who mentioned 450 cases since 1966 without further details [17].
The authors described 802 male and 243 female patients, with Mexico the country with the lowest male: female ratio (1.95:1). They mention exposure to rural activities and history of trauma for 61.5% (642 out 1,043 cases) and 48% (117 out 244 cases), respectively. Histological findings were described for 269 (16.5%) patients. Cultures from the patient’s lesions allowed the isolation of 703 clinical isolates, being Fonsecaea spp. (676; 96.2%) the most frequent isolated agent with geographic distribution throughout Central America [16,19,20,30,35,40–42]. Cladophialophora spp. was found only in Mexico, Cuba, and Costa Rica [16,20,21,30]. A molecular identification study conducted in Mexico found only F. pedrosoi in all nine samples of Fonsecaea spp. isolated in culture [42]. The highest prevalence rates of CBM per 1 million inhabitants were observed in Costa Rica, Dominican Republic, Panama, and Guadalupe Island [16–41]. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in South America
A total of 51 articles described 2,619 patients that were reported in Venezuela (1,167 cases) [43–49], Brazil (1,143 cases) [50–70], Colombia (167 cases) [71–76], Paraguay (82 cases) [77–81], Ecuador (34 cases) [82,83], French Guiana (11 cases) [84–86], Peru (7 cases) [87–89], Argentina (4 cases) [90,91], Bolivia (3 cases) [92], and Uruguay (1 case) [93]. In Brazil, most cases came from the Amazon region (states of Pará, Rondonia, and Amazon), Maranhão state (northeast region), in addition to the Central-West Region (Mato Grosso) and South Regions (states of Paraná and Rio Grande do Sul) [50–54,56–58,62,68–70]. Venezuela is an important endemic area in South America, and the disease is found throughout the country, with the states of Falcón, Lara, and Zulia standing out [44,45,48]. The Falcón region is responsible for 55% of the cases described in the country [48]. Data from Peru, Bolivia, Argentina, and Uruguay are scarce, as most cases were divulgated only in local meetings and conferences or published in non-indexed publications [87–93]. Biagini et al. (1982) reported that from 1929 to 1982, 11 cases of the disease were recorded in Argentina. According to Ricardo Negroni (personal communication), there were 2 to 3 cases per year in most medical centers in Buenos Aires [91].
The authors described 1,237 male and 178 female patients. Venezuela, as mentioned in several publications, had the lowest male:female rate [43,44,47]. They mentioned the previous exposition to rural activities and history of trauma for 74.5% (927 out 1,244 cases) and 53.1% (276 out 520 cases), respectively. Histological findings were described for 555 (21.2%) patients. Cultures from the patient’s lesion allowed the isolation of 864 isolates. Fonsecaea spp. (759; 87.9%) was the most frequently found agent, followed by Cladophialophora spp. (82; 9.5%). The latter agent is typical of semi-arid rural areas of Venezuela and has been found sporadically in Brazil, Paraguay, Ecuador, and Peru [43–46,48,49,60,63,77,83,89]. The report by Richard-Yegres and Yegres accounted for 900 cases of CBM in Venezuela, with 490 (54.5%) in the state of Falcón, caused almost exclusively by C. carrionii [48]. Thus, the real number of cases caused by C. carrionii in Venezuela is underestimated in the indexed literature, specially in Falcón state, and it should be greater than 500. The highest prevalence rates of the disease per 1 million inhabitants were observed in Venezuela, French Guyana, Colombia, and Paraguay. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in Africa
A total of 65 articles were selected between 1947 and 2018 describing 1,875 patients distributed between Madagascar (1,323 cases) [94–99], South Africa (156 cases) [100–106], Republic of the Congo and Democratic Republic of the Congo (121 cases) [107–115], Gabon (64 cases) [116], Zimbabwe (35 cases) [117], Uganda (34 cases) [118], Kenya (33 cases) [119], Cameroon (23 cases) [120–123], Morocco (18 cases) [124–132], Tanzania (17 cases) [133,134], Ethiopia (14 cases) [135,136], Angola (7 cases) [115,137,138], Nigeria (5 cases) [138–141], Tunisia (5 cases) [142–146], Reunion Island (5 cases) [147–149], Libya (4 cases) [150–153], Comoro Island (4 cases) [154,155], Sierra Leone (3 cases) [156], Senegal (2 cases) [157,158], Botswana (1 case) [159] and Djibouti (1 case) [160]. There are descriptions of CBM in Chad and the Ivory Coast. Madagascar and other islands located in the Indian Ocean (Comoro and Reunion Islands) had the highest prevalence of the disease. Madagascar had 1,323 cases described in some case series published before the 1990s. New cases of CBM continue to be reported on the island, but a recent population survey has not been conducted. Data simulated by mathematical models suggest that Madagascar should have close to 2,745 cases spread throughout the country, many of them without diagnosis [161–164]. CBM is widely distributed in the central region of the continent (Democratic Republic of Congo and Republic of Congo—Congo Brazzaville), part of West Africa (Gabon), South Africa, and part of the east coast (Kenya and Tanzania) [105–116,119,133]. The disease is rarely described in desert areas.
The authors described 1,338 male and 263 female patients. Kenya, Ethiopia, Gabon, and Cameroon showed the highest male: female rates [116,119,122,135]. They mentioned the previous exposition to rural activities and history of trauma for 74.6% (129 out 173 cases) and 30.8% (20 out 65 cases), respectively. Histological findings were described for 854 (45.5%) patients. Cultures obtained from the patient’s lesion yielded 1,406 fungal agents, being Fonsecaea spp. the most frequently found etiological agent (1,017; 72.3%), followed by Cladophialophora spp. (376; 26.8%). Fonsecaea spp. is widely distributed throughout the African continent, while Cladophialophora spp. is found especially in the semi-arid zones of Madagascar (366 cases) and in other countries, such as Morocco, South Africa, Lybia, and Nigeria [99,100,102,110,112,113,116,122–124,127,130,131,155]. Phialophora spp. was also found in some African countries at a high frequency, such as Libya, Madagascar, Kenya, South Africa, Morocco, and Djibouti [95,104,119,130,132,150,153,160].
The highest prevalence rates of CBM per 1 million inhabitants were observed in Mayotte Island, Madagascar, Gabon, and Reunion Island. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in Asia
A total of 38 articles published between 1930 and 2019 were selected. They reported 1,390 patients distributed among China (589 cases) [165], Japan (450 cases) [166–169], India (169 cases) [170], Sri Lanka (71 cases) [171], Taiwan (33 cases) [172–175], Malaysia (20 cases) [176,177], Nepal (15 cases) [178,179], Thailand (14 cases) [180–183], Indonesia (13 cases) [184–187], South Korea (9 cases) [188–196], Pakistan (2 cases) [197,198], and Philippines, Bangladesh, Laos, Vietnam and Iraq each one with 1 case [199–202]. In India, the main provinces reporting cases of CBM were Kerala, Karnataka, Assam, Himachal Pradesh, and Maharashtra [170]. In Mainland China, most of the cases came from the southern provinces of Guangdong, Shandong, and Hebei [165].
The authors described 463 male and 183 female patients. Japan, Thailand, and Nepal the countries with the lowest male: female rates among patients with CBM [166–168,178,179,181,182]. They mentioned rural activities and a history of trauma for 20.3% (162 out 799 cases) and 14.3% (116 out 811 cases), respectively. Histological findings were described for 369 (26.5%) patients. Cultures from the patient’s lesion allowed the identification of 750 fungal pathogens, with Fonsecaea spp. the most frequent etiologic agent (569; 75.8%), followed by Cladophialophora spp. (68; 9%) and Phialophora spp. (24; 3.2%) [165,167–171,174]. Cladophialophora spp. was found only in China, India, Thailand, and Nepal [165,170,179,182]. Less common agents, such as Exophiala spp., Bipolaris spp., Rhinocladiella spp., and Rhytidhysteron spp. were reported, especially in Japan, India, Thailand, and South Korea [167–170,183,196]. Some authors describe the latter cases are doubtful because these genera do not belong to known agents of CBM and may concern misidentifications [1,3]. Some strains of Fonsecaea spp. (27 strains) were subjected to molecular identification, showing F. monophora in 21, F. pedrosoi in 5, and F. nubica in 1 case [165,175,201]. The highest prevalence rates of CBM per 1 million inhabitants were observed in Sri Lanka, Laos, Taiwan, Japan, Malaysia, and Nepal. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in Oceania
A total of 9 articles published between 1947 and 2013 were selected, describing 168 patients from Australia (158 cases) [203–208], New Caledonia (5 cases) [209], New Zealand (4 cases) [210], and Solomon Islands (1 case) [211]. There are some reports of patients who probably acquired the disease in Samoa and the Cook Islands [209–211]. In Australia, CBM occurred predominantly in rural areas of Queensland, northern New South Wales, and the Northern Territory, including the northern part of Western Australia [205,206,209].
The authors described 133 male and 18 female patients, exhibiting one of the highest male: female rates in all studies analyzed. They mentioned the previous rural activities or a history of trauma in 40.8% (20 out 49 cases) and 42.9% (18 out 42 cases), respectively. Histological findings were described for 23 (13.7%) patients. Cultures from the lesions yielded 43 fungal agents represented by Fonsecaea spp. (29; 67.5%) and Cladophialophora spp. (14; 32.5%). Cladophialophora spp. was found only in Australia. Fonsecaea spp. was widely distributed throughout Oceania, especially in the southeast coastal area of Queensland, Australia [203,205,208–211]. The highest prevalences of CBM per 1 million inhabitants were observed in New Caledonia and the Solomon Islands. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in Europe
Excluding two publications from Russia and Finland that were written in their native languages, which precludes our analysis of data, we were able to evaluate only 35 cases of CBM published on the European continent.
The 35 European cases (24 autochthonous) were documented in the following countries: Finland (9 cases), Poland (5 cases), United Kingdom (5 cases), Czech and Slovakia (3 cases), Germany (3 cases), France (3 cases), Ukraine (2 cases), Russia (1 case), Belgium (1 case), Spain (1 case), Portugal (1 case) and Netherlands (1 case). All cases of CBM diagnosed in the UK and Netherlands, in addition to 2 cases from Germany and 1 case from France, were imported [212–218].
The authors described 27 male and 8 female patients. They mentioned the previous exposition to rural activities and history of trauma for 66.6% (2 out 3 cases) and 56.3% (18 out 32 cases), respectively. Histological findings were described for all 35 (100%) patients. Cultures from the patient’s lesion yielded 30 fungal agents represented by Fonsecaea spp. (24; 80.1%), followed by Exophiala spp. (2; 6.7%), Phialophora spp. (1; 3.3%), Rhinocladiella spp. (1; 3.3%), and Cladophialophora spp. (1; 3.3%) [212,214–218]. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Chromoblastomycosis in the US and Canada
A total of 13 articles between 1915 and 2018 were selected, with 25 patients distributed between the United States (24 cases) and Canada (1 case) [219–231]. In the USA, most CBM cases were published before the 50s, and the disease is supposed to be rare nowadays. In the USA, most CBM reports came from Massachusetts (Boston), Texas, Missouri, Michigan, Georgia, Louisiana (New Orleans), and Pennsylvania (Philadelphia) [219–230]. The single case published in Canada was probably imported once the patient had a previous history of trauma in Sri Lanka [231].
The authors described 22 male and 3 female patients. They mentioned the previous exposition to rural activities and history of trauma for 56.5% (13 out 23 cases) and 100% (3 out 3 cases), respectively. Histological findings were described for 20 (57.1%) patients. Cultures from the patient’s lesion yielded 21 etiological agents, including Fonsecaea spp. (15; 71%), and Phialophora spp. (6; 29%). Cladophialophora spp. was not reported in any case from the USA [219–231]. The primary epidemiologic and clinical data of all CBM cases reported in the region were summarized in Tables 1 and S1.
Consolidated worldwide CBM data
A total of 7,740 cases of CBM was described in five continents. The authors described 4,022 (81.7%) male and 896 (18.3%) female patients. The median age was 52.5 years (range between 2–93 years), and the average time between the onset of the first lesion and CBM diagnosis was 9.2 years (range between 1 month to 50 years). The authors mentioned exposure to rural activities and history of trauma for 56.8% (1,895 out 3,334 cases) and 33.1% (568 out 1,717 cases), respectively. Histological findings were described for 2,125 (27.8%) patients.
The presence of immunosuppressive diseases at the time of diagnosis of CBM was reported in only 16 (0.2%) cases, with solid organ transplantation the most common condition (kidney, heart transplantation), followed by HIV infection, rheumatoid arthritis, systemic lupus erythematosus, bladder neoplasia, celiac disease, pernicious anemia, and non-Hodgkin lymphoma [66,68,92,148,170,229]. Concomitant infection diseases were reported in 19 cases of CBM, including mycetoma (5 cases), leprosy (5 cases), cutaneous filariasis (3 cases), paracoccidioidomycosis (2 cases), cutaneous histoplasmosis (1 case), syphilis (1 case), actinomycosis (1 case) and visceral leishmaniasis (1 case) [19,50,54,56,60,65,68,108,121,157,158,170].
CBM lesions were present in only one body segment in 1,313 out 1,472 cases (89.2%) and in more than one body segment in 159 out 1,472 cases (10.8%). Itching and pain were reported by 21.5% (281 out 1,309 cases) and 11% (145 out 1,313 cases), respectively.
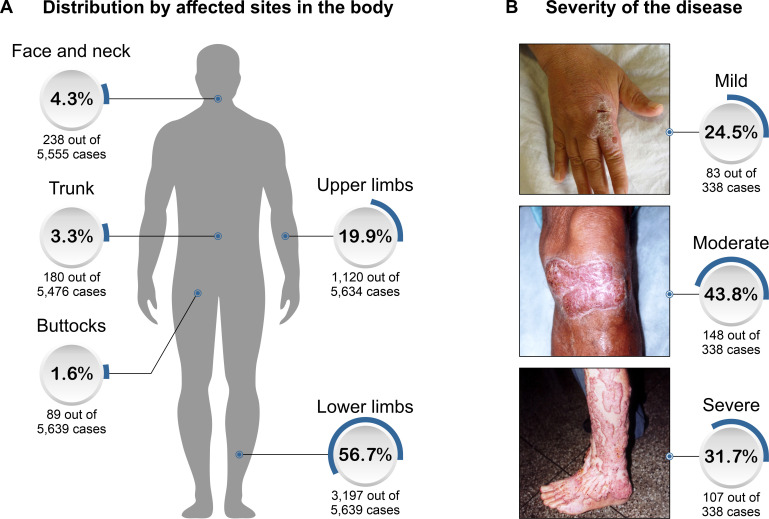
The main sites involved were the lower limbs (3,197 out 5,639 cases; 56.7%), followed by the upper limbs (1,120 out 5634 cases; 19.9%), head and neck (158 out 5,555 cases; 2.9%), trunk (180 out 7,568 cases; 2.4%), buttocks (89 cases) and unusual sites such as ear, breast, inguinal region (61 cases).
The main patterns of dermatological lesions were verrucous (710 cases), followed by tumorous (663 cases), plaque (565 cases), nodular (285 cases), ulcers (105 cases), and scarring (75 cases) lesions. Approximately 63.2% (470 out 743) of the patients had only 1 pattern of dermatologic lesions, while polymorphisms of lesions were observed in 36.8% (273 out 743) of patients. Regarding the severity of the disease by Carrión’s criteria, 83 out 338 patients (24.5%) had mild, 148 out 338 patients (43.8%) moderate, and 107 out 338 patients (31.7%) severe forms. Malignant transformation diagnosed by histopathology of CBM lesions was described in 22 cases. Except for one case of melanoma, all others were described as squamous and basal cell carcinomas. A total of 3,817 fungal isolates were cultured, being 3,089 (80.9%) Fonsecaea spp., 552 (14.5%) Cladophialophora spp., and 56 Phialophora spp. (1.5%). The primary epidemiologic and clinical data of all CBM cases reported in the world were summarized in Figs 1–3 and Tables 1 and S1.
Fig 3. The chromoblastomycosis lesion site.
A- The percentage of cases reported from a certain body site is shown. For lower limbs, lesions were described in 3,197 (56.7%) out of 5,639 patients; for upper limbs in 1,120 (19.9%) out of 5,634 patients; for the face and neck in 238 (4.3%) out of 5,555 patients; for the trunk in 180 (3.3%) out of 5,476 patients described; and finally for the buttocks in 89 (1.6%) out of 5,639 patients described. Unusual sites such as ear, breast, inguinal region were reported in 61 cases. B—Percentage of lesion severity.
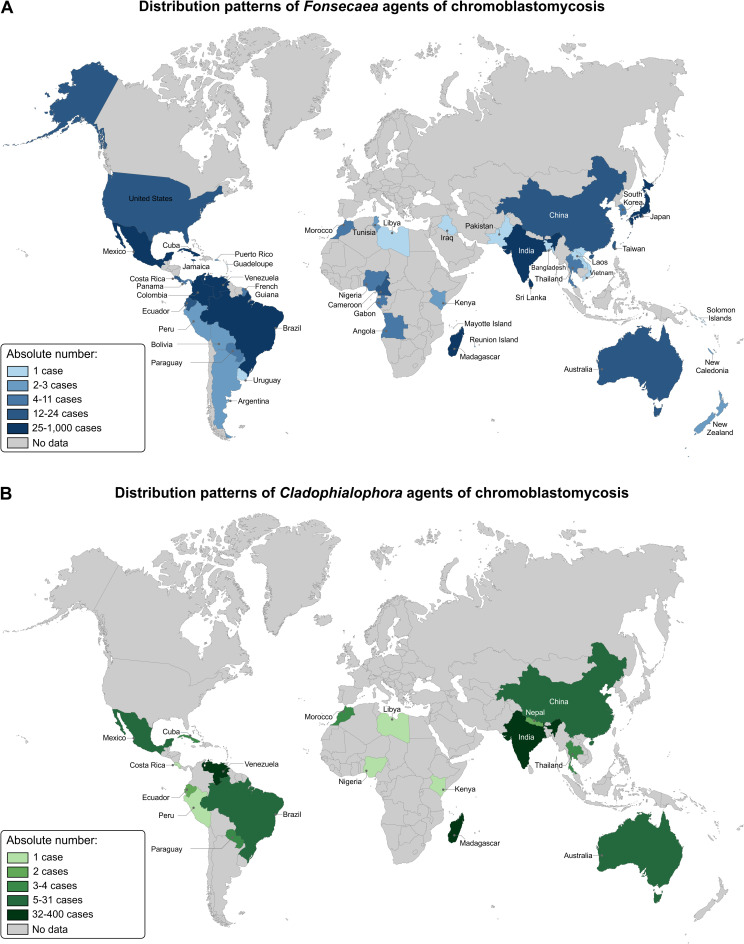
Fig 2. The global distribution of chromoblastomycosis agents.
The world map was created, edited, and colored using the vector graphics editor Corel Draw X8. Public domain link to map base layer used in creating this figure:https://commons.wikimedia.org/wiki/File:BlankMap-World.svg.
Data about the treatment of CBM were scarcely documented, with incomplete information in most papers. In this regard, the management of lesions solely by surgical excision or physical methods (cryotherapy, thermotherapy, and photodynamic therapy) was documented in 133 cases. Surgical debridement as adjuvant therapy was described in additional 191 cases [35,37,39,47,50,100,110,113,165,170,182,204,212]. Itraconazole was the antifungal therapy mostly used in all continents, being reported in 318 patients, followed by treatment with terbinafine that was used by 87 patients, especially in India, China, and Madagascar [18,65,67,68,70,77,99,165,170]. The use of fluconazole, ketoconazole, flucytosine, and systemic or intralesional amphotericin were only sporadically reported. Curiously, the administration of iodides as pharmacological therapy was described in 103 cases, and it was a common practice in India, Cuba, and Australia [19,170,204,212,228]. Unfortunately, clinical and laboratory follow up data were not available to check for the clinical response (complete or partial cure) in the mentioned articles. A total and partial cure were described in 302 and 188 cases, respectively. Amputation of the affected limb was observed in 14 cases [56,67,68,76,99,103,116,119,167,181].
Discussion
The true burden of CBM is not known. A lack of national surveillance systems checking for CBM in sentinel centers does not exist [1,2,232,233]. This paper represents the most comprehensive review of CBM cases published between 1914 and 2020, providing data to partially characterize the relative burden of this neglected implantation mycosis in different countries and the main clinical and mycological characteristics of the affected patients.
Our review showed that CBM has been widely described on all continents over the last eight decades and thrives in areas where access to adequate sanitation, clean water, and healthcare is limited. Regardless of the country considered, CBM is diagnosed in people who live in remote and rural areas and affects some of the world’s poorest and most marginalized communities, predominantly in Africa, Asia, and America [1,2,232]. Rural areas in developing countries highly endemic for CBM generally present high informal employment arrangements, low human development index, and lack of appropriate social protection systems for agriculture workers. In most countries, surveillance practices for personal protective equipment (PPE) in agriculture are unknown, and their use in rural areas is woefully inadequate and requires more attention. The lack of protective shoes, gloves, or garments associated with poor hygienic habits and insufficient nutrition may favor development of CBM after infection by implantation [1–7,14,234–236]. It is not known if there are other factors affecting the development of CBM in particular individuals or disease expression and severity. For example, an inability of toll like receptor 7 (TLR7) to recognize and respond appropriately to the causative fungi could underly the progressive nature of CBM in some patients [237].
Although the route of acquisition of CBM agents is by traumatic inoculation, most of the series did not track the source of infection once clinical manifestations as several years usually elapse after trauma when the patients and the lesion(s) grows very slowly. We were not capable to analyze data related to trauma or characterization of rural jobs as this information were not available in most papers [1,3,6,18,19,27,35,56,70,116,165,238,239].
Rarely CBM is described simultaneously with other NTDs (mycetoma, leprosy, filariasis and leishmaniasis). This occurrence reflects areas of co-endemicity, with common environmental exposure in populations under conditions of poverty [1,3,54,56,57,60,108,121].
Although the diagnosis of CBM does not rely on expensive and sophisticated laboratory tools, the disease remains neglected by all health systems, making the time of diagnosis too long (mean of 9 years). This aspect certainly impacts in morbidity, including disease progression, risk of superinfection and malignant transformation [8,56,68,99,167,175,240,241,242]. Considering the insidious progression of the fungal disease, patients continue their labor and social activities for many years before having the diagnosis made [1,3,5,48,176].
Notably, the disease is mostly observed in males probably due to different environmental exposition and possible protection of women by endogenous steroids [1,5]. This hypothesis needs further investigation and validation. However, in some countries the prevalence in women is high probably due to their involvement in agricultural activities [16,43,44,116,122,166–168,199]. Lower limbs are the most common site affected, although some countries frequently reported lesions in upper limbs due to local practice of carrying wood or other agricultural materials in arms or shoulders [16,19,20,165,180,182,204,205,228,229].
In the present review, we adopted the CBM Carrión classification for skin lesions considered the most consistent and comprehensive description of dermatological lesions with updated nomenclature [1,3,65,68]. As expected, warty lesions (29%), tumors (27%), infiltrative plaques (23%), and nodules (12%) were the most common pattern of CBM, but polymorphic lesions may also be found, especially in patients with a long history and chronic evolution of the process [52,56,65,67,68,99,165,169,175]. The main symptoms were itching (21%) and pain (11%), with local edema rarely reported by most authors [6,7,9,21,28,30]. Of note, the pattern of skin lesions is not linked to the etiological agent of CBM.
Some series have shown that secondary bacterial infections and lymphedema are concerns. Uncommonly, malignant transformation may occur, especially in patients with a long history of CBM diagnosis [1,3,54,67,68,76,99,103,116,119,167,240–242]. CBM progresses slowly, produces fibrotic changes and lymphatic stasis. Secondary recurrent bacterial infection exacerbates the involvement of lymphatic vessels, resembling elephantiasis. Severe forms of CBM disable and disfigure patients much more frequently than they kill, and are multifactorial [243,244]. Affected people live decades with disability, stigma and social withdrawal. Disability Adjusted Life-Years (DALY) lost due to CBM has not been comprehensively evaluated in endemic areas [1,3–5,7,14,15].
Laboratory diagnosis of CBM requires only the visualization of single or clustered muriform cells by direct mycological examination or histopathology. Most diagnoses in North American and European countries are provided by histological examination. In contrast, in Asian, African, and Latin American countries where CBM is endemic, less than 30% of all cases required biopsy for the diagnosis [8,16,25,49,51,56,68,99,169]. Direct mycological examination with potassium hydroxide solution of skin scrapings containing crusts or cellular debris is a fast and straightforward tool frequently useful in low-income countries to diagnose patients with CBM [56,68,99,170].
Although the different agents of CBM have certain ecological features, there is no apparent impact of the diversity of species on clinical manifestations or therapeutic management. Molecular characterization of different species has mostly been used to characterize this ecology and epidemiological aspects of the various etiological agents of CBM [1,11–13,238,239].
Fonsecaea spp. is the main genus causing CBM worldwide [16,18–25,50–56,68,99,116,165,169,170,189–191]. Molecular studies showed that Fonsecaea pedrosoi is the main species within this genera, and it is found practically in all countries where CBM has been reported. This species causes almost exclusively subcutaneous disease, with rare visceral involvement [11,12,16,68]. Disseminated forms of the disease have also been reported but without unambiguous muriform cells in tissue (44), and thus, they may be considered phaeohyphomycosis. Fonsecaea monophora is widely distributed, with high prevalence, especially in Asia and subtropical or temperate countries. F. monophora, together with F. pugnacius, can cause disseminated CBM or phaeohyphomycosis with visceral impairment [165,245,246]. Finally, Fonsecaea nubica is also widely distributed in Asia, but current studies showed that Madagascar might be the country with the highest number of CBM caused by this species [239].
The second main etiological agent of CBM is C. carrionii, with Venezuela, Madagascar, Australia, India, and China the countries most affected [44,45,48,49,99,165,170,203–208]. This agent is typically found in arid and semi-arid climates, with average yearly temperatures of 24°C, scarce rainfall (up to 600 annual mL) and is located at moderate altitude (up to 500 m) [43,48,99,208]. Finally, Phialophora spp. (P. verrucosa) is an uncommon agent, responsible for almost 30% of cases in the USA [166–170,219–221,228,230]. Rhinocladiella spp. is the etiologic agent of less than 1% of CBM cases [16,68,212].
Treatment of CBM is difficult, and several different therapeutic regimens have been tried, including physical methods. Most of small initial lesions in mild disease can be excised surgically, but clinical data and follow-up of these patients are incompletely characterized. CBM lesions are refractory, and healing is almost impossible to achieve, especially in its moderate to severe clinical presentations [19,21,22,30,52,95,99]. Although there are no randomized clinical trials to define the best choice for its treatment, itraconazole is the main antifungal drug used, specially 400 mg per day in moderate and severe cases, based on observational studies [65,66,68]. Terbinafine is the second most frequently used antifungal drug, specially in some countries as Madagascar and China, based on open and non-comparative clinical trials [163,240]. Voriconazole, posaconazole, and isavuconazole are used only in refractory disease [1,247,248]. Interestingly, some therapies have been abandoned, such as cholecalciferol, thiabendazole, intravenous amphotericin, ketoconazole, and topical 5-fluorouracil. Due to the low cost, potassium iodide has been used in some countries, especially in Cuba and India [19,23,170]. Adjuvant therapy for improve the cellular immune response with topical imiquimod or intramuscular glucan was used mostly in more severe and refractory cases [249–251].
Conclusions
Despite all limitations, our study provides a comprehensive review of clinical and therapeutic aspects of CBM and an estimate of the prevalence of the disease in each country. Our maps have shown CBM to be widespread in five different continents, specially in Latin America, Africa and Asia. Countries such as Madagascar, Gabon, Indian Ocean Islands (Comoro and Reunion), Costa Rica, Dominican Republic, Venezuela, French Guiane, and Island of Oceania (New Caledonia) are the countries with the highest incidence densities in the world. CBM in world is probably more common than expected. The disease especially affects men (81.7%), with an average delay of 9.2 years between onset and diagnosis. The mean age was 57.1 years (range 2–93 years), being the lower and upper limbs the most compromised sites. Verrucous, tumoral and plaque represent the main dermatological patterns. Fonsecaea spp. is the main etiological agent, being widely distributed on all continentes and responsible for more than 80% of cases. This review allows the understanding of a gap in epidemiological, diagnostic and therapeutic data. There is an urgent need to create and implement social protection policies for vulnerable populations and national programs for the diagnosis and treatment of the disease.
Supporting information
(DOCX)
Acknowledgments
We thank all the Brazilian Network of Melanized Fungal Infections and the Working Group of Chromoblastomycosis of ISHAM for the encouragement in the development of this work.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Queiroz-Telles F, de Hoog S, Santos DW, Salgado CG, Vicente VA, Bonifaz A et al. Chromoblastomycosis. Clin Microbiol Rev. 2017Jan;30(1):233–276. doi: 10.1128/CMR.00032-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van de Sande W. Global burden of human mycetoma: A systemic review and metanalisis. PLoS Negl. Trop. Dis. 2013;7doi: 10.1371/journal.pntd.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017Nov;17(11):e367–e377. doi: 10.1016/S1473-3099(17)30306-7 [DOI] [PubMed] [Google Scholar]
- 4.Watts C. Neglected tropical diseases: A DFID perspective. PLoS Negl Trop Dis. 2017Apr20;11(4):e0005492. doi: 10.1371/journal.pntd.0005492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S. World neglected tropical diseases day. PLoS Negl Trop Dis. 2020Jan29;14(1):e0007999. doi: 10.1371/journal.pntd.0007999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay R, Denning DW, Bonifaz A, Queiroz-Telles F, Beer K, Bustamante B et al. The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report. Trop Med Infect Dis. 2019Sep24;4(4). pii: E122. doi: 10.3390/tropicalmed4040122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GAFFI. Available from: https://www.gaffi.org/poor-farmers-fungal-skin-condition-gets-approval-from-who-as-neglected-after-lobbying-by-gaffi/
- 8.Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother. 2016Dec;71(12):3599–3606. doi: 10.1093/jac/dkw325 Epub 2016 Aug 10. . [DOI] [PubMed] [Google Scholar]
- 9.GAFFI. Available from: https://www.gaffi.org/antifungal-drug-maps/.
- 10.de Hoog GS, Attili-Angelis D, Vicente VA, Van Den Ende AH, QueirozTelles F. Molecular ecology and pathogenic potential of Fonsecaea species. Med Mycol. 2004;42:405–416. doi: 10.1080/13693780410001661464 [DOI] [PubMed] [Google Scholar]
- 11.Najafzadeh MJ, Gueidan C, Badali H, van den Ende AH, Xi L, de Hoog GS. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med Mycol. 2009;47:17–25. doi: 10.1080/13693780802527178 [DOI] [PubMed] [Google Scholar]
- 12.Najafzadeh MJ, Sun J, Vicente VA, Klaassen CH, Bonifaz A, Gerrits van den Ende AH et al. Molecular epidemiology of Fonsecaea species. Emerg Infect Dis. 2011Mar;17(3):464–9. doi: 10.3201/eid1703.100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Queiroz-Telles F, Santos DW. 2013. Challenges in the therapy of chromoblastomycosis. Mycopathologia 175:477–488. doi: 10.1007/s11046-013-9648-x [DOI] [PubMed] [Google Scholar]
- 14.WHO Department of Control of Neglected Tropical Diseases. Sustaining the drive to overcome the global impacto f neglected tropical diseases: second WHO reporto n neglected tropical diseases. Geneva: World Health Organization, 2013. Available from: https://www.who.int/neglected_diseases/9789241564540/en/ [Google Scholar]
- 15.Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). 2017;3(4):57.doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarrete MR, Arenas R, Estrada VFM, Diéguez CEA, Mayorga J, Bonifaz A et al. 2014. Cromoblastomicosis en México. Revisión de 603 casos en siete décadas. Dermatologia CMQ 12:87–93 [Google Scholar]
- 17.Maleck D, Amaya-Araujo M, Cochón M, Isa Isa R. Cromoblastomicosis facial y esporotricoide. A propósito de un caso. Revista Dominicana de Dermatologia. 2010; 37(1):28–30. [Google Scholar]
- 18.Larrondo Muguercia RJ, Gray Lovio O, Abreu Daniel A, Bonito Lovio D. Cromomicosis. Estudio de un decenio. Hospital Universitario Comandante Manuel Fajardo. 1996–2005. Folia Dermatol Cubana. 2009;3(2):1–7. [Google Scholar]
- 19.Pardo-Castello V, Leon R, Trespalacios F. Chromoblastomycosis in Cuba. Arch Dermatol Syphilograph. 1942;45:19–32. [Google Scholar]
- 20.Daniel Simón R, Moya Duque S, Abreu García M. Cromomicosis: Hongos dematiáceos que intervienen en su etiología. Rev Cubana Med. 1998;37(3), 136–140. [Google Scholar]
- 21.Pastrana Fundora F, Ramírez Albajés C, Naranjo Lorenzo M, Galiano Audivert O. Cromomicosis: quince años de incidencia / Chromomycosis: 15 years incidence. Rev Cuba Hig Epidemiol. 1989;27(3):285–92 [Google Scholar]
- 22.Díaz JGD, Taboas Gonzalez M, Dube A. Cromoblastomicosis en Cuba, estudio retrospectivo clínico y epidémico de 72 enfermos. Rev Cubana Med Trop. 1978;30(2):95–108. [PubMed] [Google Scholar]
- 23.Alfonso-Armenteros J. Micologia Medica. Micosis obervadas en Cuba. Ministério de Salud Publica. Capítulo XIX. Chromoblastomycosis (Cromomicosis–Dermatitis verrucosa). Editorial Científica.34 pp. 1965. [Google Scholar]
- 24.Olano SM, Rodriguez Gonzalez DP, D’escoubet EF, Leon GV, Perez AB. Cromomicosis, estudio de cinco años. Rev Cub Med Trop. 1984;36:102–109 [PubMed] [Google Scholar]
- 25.Manzur-Katrib J, Alvarez-Mesa M, Hernandez Z Bitor MA. Cromomicosis. Estúdio Retrospectivo de Junio de 1961 a Junio de 1978. Rev Cub Med Trop. 1979;31:217–224. [PubMed] [Google Scholar]
- 26.Romero A, Trejos A. La cromoblastomicosis en Costa Rica. Rev Biol Trop. 1953;1(2): 95–115. [Google Scholar]
- 27.Trejos A. La cromoblastomicosis como problema micológico. 1954. Tesis de grado para optar por el título de licenciatura. [Google Scholar]
- 28.Astorga E, Bonilla E, Martinez C, Mora W. Cromomicosis. Nuevos casos de Cromomicosis tratados con Anfotericina B y 5·Fluorcitosina en forma simultánea Revista Médica de Costa Rica XLVII. 1980;470:17–22. [Google Scholar]
- 29.Solano E. Cromomicosis. Acta Médica Cost. 1966;9(2):77–85 [Google Scholar]
- 30.Trejos LS, Víquez DJ. Cromoblastomicosis: Situación en Costa Rica. Revista Medica De Costa Rica Y Centroamerica LXXI. 2014;613:737–44. [Google Scholar]
- 31.Velásquez-Montoya X, Herrera-Guzmán M, Wilkinson-Oberti O, Jeremías-Soto R, Alger J. Micosis subcutánea y profunda en el hospital escuela, Tegucigalpa. Rev Med Post Unah. 2001;6(1):61–65. [Google Scholar]
- 32.Adan Cueva J. Cromoblastomicosis en Honduras. Revista Medica Hondureña.1956;24(4):112–117 [PubMed] [Google Scholar]
- 33.Corrales Padilla H. Cromoblastomicosis. Revista Medica Hondureña. 1955;23(4):1030–6. [PubMed] [Google Scholar]
- 34.Corrales Padilla H. Cromomicosis. Revista Medica Hondureña. 1970;38(2):55–63. [Google Scholar]
- 35.Bansal AS, Prabhakar P. Chromomycosis: a twenty-year analysis of histologically confirmed cases in Jamaica. Trop Geogr Med. 1989Jul;41(3):222–6 [PubMed] [Google Scholar]
- 36.Mugleston BJ, Usatine RP, Rosen T. Wide Morphologic Variability of Chromoblastomycosis in the Western Hemisphere. Skinmed. 2016Dec1;14(6):423–427. eCollection 2016. Available from: https://skinmedjournal.com/2016-issues/# [PubMed] [Google Scholar]
- 37.Calero C. Chromoblastomycosis in Panama; report of a new case and a new clinical form. Arch Derm Syphilol. 1948Feb;57(2):266–71. [PubMed] [Google Scholar]
- 38.Calero M. Chromoblastomycosis. Arch Derm Syphilol. 1946Sep;54:265–77. doi: 10.1001/archderm.1946.01510380012002 [DOI] [PubMed] [Google Scholar]
- 39.Snow JS, Wedding ES, Tomlinson WJ. Chromoblastomycosis: report of the first case observed in the canal zone. Arch Derm Syphilol. 1945; 51(2):90–93. [Google Scholar]
- 40.Carrión AL. Chromoblastomycosis in Puerto Rico. Puerto Rico J. Chromoblastomycosis in Puerto Rico. Puerto Rico J Pub Health and Trop Med. 1938;14:37. [Google Scholar]
- 41.Levang J, Muller P, Marreel A, Nicolas M, Puzenat E, Aubin F et al. Chromomycose en Guadeloupe. Annales de dermatologie et de vénéréologie (2008) 135, 111–115 [DOI] [PubMed] [Google Scholar]
- 42.Najafzadeh MJ, Sun J, Vicente VA, Klaassen CHW, Bonifaz A, Gerrits van den Ende AHG, et al. Molecular epidemiology of Fonsecaea species. Emerg Infect Dis 2011; 17(3): 464–469. doi: 10.3201/eid1703.100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barroeta S, Mejia de Alejos MA, Franco de Arias CM, Prado A, Zamora R. Cromomicosis en el estado Lara. Dermatol Venez. 1986;24(2/4):134–7 [Google Scholar]
- 44.Vargas Montiel H. Cromomicosis en el estado Zulia. Dermatologia Venezolana. 1982. 20(3):41–49. [Google Scholar]
- 45.Casas Rincon G. La Micología en el Estado Zulia. Kasmera. 1973;4:271–320 [Google Scholar]
- 46.Convit J, Borelli D, Albornoz R, Rodríguez G, Hómez J. Micetomas, Cromomicosis, Esporotricosis y Enfermedad de Jorge Lobo. Mycopathologia et Mycologia Applicata. 1961;15:394–407. doi: 10.1007/BF02136341 [DOI] [PubMed] [Google Scholar]
- 47.Saeb M, Arenas R. Cromomicosis: informe de cinco casos con énfasis histopatologico y terapéutico. Dermatologia Venezolana. 1999; 37(2): 46–50. [Google Scholar]
- 48.Richard-Yegres N, Yegres F. Cromomicosis: una endemia rural en la región noroccidental en Venezuela. Rev Cubana Med Trop. 2009;61(3):209–12. [Google Scholar]
- 49.Hómez J, Wenger F, Casas G. Cromoblastomicosis. Estudio de 50 casos observados en Maracaibo. Kasmera, 1 (3): 121–168, 1963. [Google Scholar]
- 50.Silva NN. Cromoblastomicose no Rio Grande do Sul. Anais Brasileiros de Dermatologia e Sifilografia. 1949;24(2):113–145. [Google Scholar]
- 51.Silva D. Estudo clínico-epidemiológico da micose de Lane e Pedroso (crocomicose ou cromoblastomicose) no Estado do Pará. An Bras Dermatol. 1957;32(4):121–130 [PubMed] [Google Scholar]
- 52.Bopp C. Cromoblastomicose. Contribuição ao Estudo de Alguns de seus Aspectos. Thesis for Cathedratic Professor of Dermatology of Universidade Federal do Rio Grande do Sul. 1959. pp 01–315. [Google Scholar]
- 53.Londero AT, Ramos CD. 1976. Chromomycosis: a clinical and mycologic study of thirty-five cases observed in the hinterland of Rio Grande do Sul, Brazil. Am J Trop Med Hyg 25:132–135. doi: 10.4269/ajtmh.1976.25.132 [DOI] [PubMed] [Google Scholar]
- 54.Cordeiro Azevedo P, Monteiro Leite J, Morais M. Considerações sobre a Cromomicose e sua frequencia no estado do Pará. Anais da Faculdade de Medicina e Cirurgia do Pará. 1952;1:1–33. [Google Scholar]
- 55.Correia RTM, Valente NYS, Criado PR, Martins JEC. Cromoblastomicose: relato de 27 casos e revisão da literatura. An Bras Dermatol. 2010Jul-Aug;85(4):448–54. doi: 10.1590/s0365-05962010000400005 [DOI] [PubMed] [Google Scholar]
- 56.Minotto R, Bernardi CD, Mallmann LF, Edelweiss MI, Scroferneker ML. Chromoblastomycosis: a review of 100 cases in the state of Rio Grande do Sul, Brazil. J Am Acad Dermatol. 2001;44(4):585–92. doi: 10.1067/mjd.2001.112220 [DOI] [PubMed] [Google Scholar]
- 57.Avelar-Pires C, Simoes-Quaresma JA, Moraes-de Macedo GM, Brasil-Xavier M, Cardoso-de Brito A. Revisiting the clinical and histopathological aspects of patients with chromoblastomycosis from the Brazilian Amazon region. Arch Med Res. 2013May;44(4):302–6 doi: 10.1016/j.arcmed.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 58.Talhari S, Cunha MG, Schettini AP, Talhari AC. Deep mycoses in Amazon region. Int J Dermatol. 1988Sep;27(7):481–4. doi: 10.1111/j.1365-4362.1988.tb00925.x [DOI] [PubMed] [Google Scholar]
- 59.Silva JP, de Souza W, Rozental S. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia. 1998–1999;143(3):171–5. [DOI] [PubMed] [Google Scholar]
- 60.Mouchalouat Mde F, Gutierrez Galhardo MC, Zancopé-Oliveira RM, Monteiro Fialho PC, de Oliveira Coelho JM, Silva Tavares PM et al. Chromoblastomycosis: a clinical and molecular study of 18 cases in Rio de Janeiro, Brazil. Int J Dermatol. 2011Aug;50(8):981–6. doi: 10.1111/j.1365-4632.2010.04729.x [DOI] [PubMed] [Google Scholar]
- 61.Pérez Herrera MA, Martinez GC, Machín Villafranca MC. Diagnóstico histopatológico de 98 casos de Micosis Profunda en el Hospital Dom Orione, Brasil. In: VI Congresso Virtual Hispanoamericano de Anatomia Patológica. 2004. Cited 10 january 2020. Available from: http://www.conganat.org/6congreso/index-261.htm [Google Scholar]
- 62.Matte SM, Lopes JO, Melo IS, Espadim LE, Pinto MS. Chromoblastomycosis in Rio Grande do Sul: a report of 12 cases. Rev Soc Bras Med Trop. 1997Jul-Aug;30(4):309–11. doi: 10.1590/s0037-86821997000400006 [DOI] [PubMed] [Google Scholar]
- 63.Marques GF, Barreto JÁ, Masuda PY, Wachholz PA, Sousa JMP. Perfil clínico e demográfico da cromoblastomicose em serviço de referência no centro-oeste do estado de São Paulo, Brasil. Anais Brasileiros de Dermatologia. 2015;90(1):143–145 doi: 10.1590/abd1806-4841.20142998e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mattedi MGS, Palhano Junior L, Coelho CC, Mattêde AF. Dermatite verrucosa cromoparasitária (cromomicose). Investigação de casos no estado do Espírito Santo. An Bras Dermatol. 1990;65(2):7074 [Google Scholar]
- 65.Queiroz-Telles F, Purim KS, Fillus JN. Itraconazole in the treament of chromoblastomycosis due to Fonsecaea pedrosoi. Int J Dermatol 1992;31:805–812 doi: 10.1111/j.1365-4362.1992.tb04252.x [DOI] [PubMed] [Google Scholar]
- 66.Queiróz AJR, Pereira Domingos F, Antônio JR. Chromoblastomycosis: clinical experience and review of literature. Int J Dermatol. 2018Nov;57(11):1351–1355 doi: 10.1111/ijd.14185 [DOI] [PubMed] [Google Scholar]
- 67.Silva Pacheco A. Cromomicose em Santa Catarina. Monografia de conclusão do curso de medicina. 2003. Cited 10 january 2020. Available from: https://repositorio.ufsc.br/bitstream/handle/123456789/114294/201442.pdf?sequence=1&isAllowed=y [Google Scholar]
- 68.Santos DWCL, Vicente VA, Weiss VA, de Hoog GS, Gomes RR, Batista EMM et al. Chromoblastomycosis in an Endemic Area of Brazil: A Clinical-Epidemiological Analysis and a Worldwide Haplotype Network. J Fungi (Basel). 2020Oct3;6(4):E204. doi: 10.3390/jof6040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Londero AT, Ramos CD. Cromoblastomicose no interior do Estado do Rio Grande do Sul. Anais Brasileiros de Dermatologia 64:155–58,1989. [Google Scholar]
- 70.de Andrade TS, de Almeida AMZ, Basano SA, Takagi EH, Szeszs MW, Melhem MSC et al. Chromoblastomycosis in the Amazon region, Brazil, caused by Fonsecaea pedrosoi, Fonsecaea nubica, and Rhinocladiella similis: Clinicopathology, susceptibility, and molecular identification. Med Mycol. 2020Feb1;58(2):172–180. doi: 10.1093/mmy/myz034 [DOI] [PubMed] [Google Scholar]
- 71.López H, Hurtado H, Correa E. Las micosis profundas en el Hospital de San Juan de Dios. Publicaciones del Hospital de San Juan de Dios (Cali). 1964;16:1–24. [Google Scholar]
- 72.Sánchez J. Micosis. Estudio etiológico de las diversas micosis admitidas em el Hospital de San Vicente de Paúl durante 1964 y 1965. Tesis de grado. Facultad de Medicina, Universidad de Antioquia, Medellín, pp. 68–85.
- 73.Duque O. Cromoblastomicosis. Revisión general y estudio de la enfermedad en Colombia. Ant Med. 1961;11:499–521. [Google Scholar]
- 74.Pena C. Cromoblastomicosis. Rev Fac Med Univ Nal. 1966;34:55–59. [PubMed] [Google Scholar]
- 75.Velasquez J, Restrepo A, Calle G. Cromomicosis. Experiencia de doce años. Acta Médica Colombiana. 1976;1(3):165–171. [Google Scholar]
- 76.Rocha H, Gutiérrez G. Cromomicosis: a propósito de 35 casos observados en el Hospital San Juan de Dios de Bogotá. Rev Fac Med. 1972;38(1):50–65. [Google Scholar]
- 77.Wattiez V, García J, Aquino N, Insaurralde S, Mendoza G, Celias L et al. Rev Virtual Soc Parag Med Int. 2017;4(2):27–33. [Google Scholar]
- 78.Rodríguez Mais M. Estudio clínico y epidemiológico de la paracoccidioidomicosis y otras micosis profundas. Anales de la Facultad de Ciencias Médicas de la UNA. 2004;37:9–19. [Google Scholar]
- 79.Canese A, Da Silva D. Hongos aislados durante el año 1972, en la Cátedra de Bacteriología y Parasitología de la Facultad de Medicina. Rev Parag Microb. 1973;8:53–6. [Google Scholar]
- 80.Canese A, Da Silva D. Micosis en el Paraguay. Rev Parag Microb. 1969; 4(1):12–4. [Google Scholar]
- 81.Maas LC. Aspectos Clínicos de las Micosis em el Paraguay. Thesis de Clinica Médica para la II Cátedra del Hospital Universitário. Asuncion, Paraguay. 1964. pp01-81.
- 82.Rodríguez M JD. Revisión Crítica de Investigaciones y literatura micológicas durante los años 1950–1960 em Ecuador. Mycopathologia et Mycologia Applicata. 1962;17:185–202. [Google Scholar]
- 83.Ronquillo TF, Acosta Y, Almeida R. Cromoblastomicosis en el Ecuador. Rev Med FCM-UCSG. 2015;19(4):246–251. [Google Scholar]
- 84.Silverie R, Ravisse P. On 2 cases of chromomycosis observed in French Guiana. Bull Soc Pathol Exot Filiales. 1962Sep-Oct;55:751–2. [PubMed] [Google Scholar]
- 85.Pradinaud R, Joly F, Basset M, Basset A, Grosshans E. Chromomycosis and Jorge Lobo disease in French Guiana. Bull Soc Pathol Exot Filiales. 1969Nov-Dec;62(6):1054–63. [PubMed] [Google Scholar]
- 86.Pradinaud R. Traitement de 6 chromomycoses par la 5 fluorocytosine em Guyane française. Nouv Presse Med. 1974;3(31):1955. [PubMed] [Google Scholar]
- 87.Ventura-Flores R, Failoc-Rojas V, Silva-Díaz H. Cromoblastomicosis: características clínicas y microbiológicas de una enfermedad desatendida. Rev Chilena Infectol 2017; 34 (4): 404–407 doi: 10.4067/s0716-10182017000400404 [DOI] [PubMed] [Google Scholar]
- 88.Galarza C. Enfoque de las micoses profundas em el Perú. Dermatol Peru. 1996;6(1 suppl):39S–40S. [Google Scholar]
- 89.Cavero J, Delgado V. Cromoblastomicosis por Cladosporium sp. Folia Dermatol. 2004;15(1):28–31. [Google Scholar]
- 90.Molina-Leguizamon EB, Casas JG, Perini GM. Cromomicosis de la nalga. Med Cut ILA. 1984;12:430–438. [PubMed] [Google Scholar]
- 91.Biagini RE, Maza AL, Abulafia J, Museli A. Cromomicosis. Arch Argent Dermat. 1982;32:93–100. [Google Scholar]
- 92.Escobar AY, Maldonando SE, Iriarte A, Rollano F. Revista Boliviana de Dermatologia. 2015;5(8):32–36. [Google Scholar]
- 93.Vignale B, Montero ED, Tost JFr, Sanjinés A. Cromoblastomicosis (Segundo caso descrito en el Uruguay). An Fac Med. 1955;40(3–4):87–92 [PubMed] [Google Scholar]
- 94.Brygoo ER. La chromoblastomycose à Madagascar. Sem Hop Paris. 1957;33:774–791. [PubMed] [Google Scholar]
- 95.Brygoo ER, Courdurier J, Meyer G. La chromoblastomycose à Madagascar. Arch Inst Pasteur Madagascar. 1958;26:11–22. [Google Scholar]
- 96.Brygoo ER, Destombes P. Epidémiologie de la chromoblastomycose humaine. Bull Inst Pasteur. 1976;74:219–243. [Google Scholar]
- 97.Brygoo ER, Segrétain G. Etude clinique, épidemiologique et mycologique de la chromoblastomycose à Madagascar. Bull Soc Path Exot. 1960;3:443–75. [Google Scholar]
- 98.Coulanges P, Locheron P. La chromomycose a Madagascar. Données épidemiologiques sur le foyer de plus importante actuellement connu dans le monde. Arch Inst Pasteur Madagascar. 1981;48(1)69–95. [PubMed] [Google Scholar]
- 99.Esterre P, Andriantsimahavandy A, Raharisolo C. Natural history of chromoblastomycosis in Madagascar and the Indian Ocean. Bull Soc Pathol Exot. 1997;90(5):312–7. [PubMed] [Google Scholar]
- 100.Simson FW. Chromoblastomycosis; some observations on the types of the disease in South Africa. Mycologia. 1946;38(4):432–449. [PubMed] [Google Scholar]
- 101.Friedlander J, Moss C. Chromoblastomycosis. S Afr Med J. 1949;23(36):736. [PubMed] [Google Scholar]
- 102.Bayles MA. Tropical Mycoses. Chemotherapy. 1992;38(Suppl 1):27–34. doi: 10.1159/000239050 [DOI] [PubMed] [Google Scholar]
- 103.Martin PM, Berson SD. Fungus diseases in Southern Africa. Mycopathol Mycol Appl. 1973;50(1):1–84. doi: 10.1007/BF02050005 [DOI] [PubMed] [Google Scholar]
- 104.Harwood-Nash DC. A case of chromoblastomycosis in a coloured male. S Afr Med J. 1962;36:647–50. [PubMed] [Google Scholar]
- 105.Findlay GH. Chromoblastomycosis caused by the Simson species of Hormodendrum. S Afr Med J. 1957;31(22):538–40. [PubMed] [Google Scholar]
- 106.Lurie HI. Fungal diseases in South Africa. S Afr Med J. 1955;29(8):186–8 [PubMed] [Google Scholar]
- 107.Ninane G. Rev Med Liege. 1956;11(21):601–3. [PubMed] [Google Scholar]
- 108.Banks IS, Palmieri JR, Lanoie L, Connor DH, Meyers WM. Chromomycosis in Zaire. Int J Dermatol. 1985;24(5):302–7. doi: 10.1111/j.1365-4362.1985.tb05789.x [DOI] [PubMed] [Google Scholar]
- 109.Destombes P, Ravisse P, Nazimoff O. Summary of deep mycoses established in 20 years of histopathology in the Institut Pasteur de Brazzaville. Bull Soc Pathol Exot Filiales. 1970;63(3):315–24. [PubMed] [Google Scholar]
- 110.Vanbreuseghem R, Vandepitte J, Thys A, Windey W. First case of chromoblastomycosis due to Phialophora pedrosoi in a Belgian Congo native. Ann Soc Belg Med Trop (1920). 1951;31(4):495–9. [PubMed] [Google Scholar]
- 111.Rasson G, Thys A. Second case of chromoblastomycosis in the Belgian Congo. Ann Soc Belg Med Trop (1920). 1951;31(5):547–50. [PubMed] [Google Scholar]
- 112.Defrenne P. Third case of chromoblastomycosis in the Belgian Congo. Ann Soc Belg Med Trop (1920). 1952;32(5):417–9. [PubMed] [Google Scholar]
- 113.Thys A, Courtois G, Vanbreuseghem R, Baker DH, Bertrand M, De Muyncke A, Limbos P, Verselder R et al. Nine new cases of chromoblastomycosis in the Belgian Congo; trial and failure of treatment by pentamidine. Ann Soc Belg Med Trop (1920). 1952;32(5):491–500. [PubMed] [Google Scholar]
- 114.Heuls J, Orio J. First case of chromoblastomycosis ever seen in French Equatorial Africa, with isolation of the causative organism. Bull Soc Pathol Exot Filiales. 1958;51(6):887–91. [PubMed] [Google Scholar]
- 115.Ricossé PJ, Guélain J, Boudon A, Ogrizek M. New cases of chromoblastomycosis: importances of anatomo-pathologic examinations. Bull Soc Pathol Exot Filiales. 1983;76(5):596–603. [PubMed] [Google Scholar]
- 116.Kombila M, Gomez de Diaz M, Richard-Lenoble D, Renders A, Walter P, Billiault X et al. Chromoblastomycosis in Gabon. Study of 64 cases. Sante. 1995;5(4):235–44 [PubMed] [Google Scholar]
- 117.Ross MD, Gelfand M. Deep fungal infections in Rhodesia—a 10-year survey of histological material. Part I. Cent Afr J Med. 1978Oct;24(10):208–12 [PubMed] [Google Scholar]
- 118.Kwizera R, Bongomin F, Lukande R. Deep fungal infections diagnosed by histology in Uganda: a 70-year retrospective study. Med Mycol. 2020Apr3. pii: myaa018. doi: 10.1093/mmy/myaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cameron HM, Gatei D, Bremner AD. The deep mycoses in Kenya: A histopathological study. 3. Chromomycosis. East Afr Med J. 1973;50(8):406–12. [PubMed] [Google Scholar]
- 120.Destombes P, Poirier A, Nazimoff O. Deep mycoses identified in 9 years’ histopathological practice in the Institut Pasteur du Cameroun. Bull Soc Pathol Exot Filiales. 1970;63(3):310–5. [PubMed] [Google Scholar]
- 121.Ravisse P, Mariat F, Destombes P. Association of chromoblastomycosis (Fonsecaea pedrosoi) and histoplasmosis (Histoplasma capsulatum) in South Cameroon. Bull Soc Pathol Exot Filiales. 1973;66(3):385–90. [PubMed] [Google Scholar]
- 122.Gamet A, Brottes H. Chromoblastomycosis in the Cameroons. Bull Soc Pathol Exot Filiales. 1963;56:117–9. [PubMed] [Google Scholar]
- 123.Campourcy A. Chromoblastomycose au Cameroun. Bull Soc Pathol Exot Filiales. 1947;40(7–8):252. [PubMed] [Google Scholar]
- 124.Samira Eddaoudi. Les mycoses profondes (à propos de 07 cas). 2016. Doctoral Thesis. 2016. Available from: http://scolarite.fmp-usmba.ac.ma/cdim/mediatheque/e_theses/239-16.pdf
- 125.Tlamcani Z, Figuigui S, Taghouti A, El Loudi S, Mernissi FZ. Chromoblastomycosis due to Cladosporium carrionii: case report. Moldovan Journal of Health Sciences. 2016;9:98–101 [Google Scholar]
- 126.Lassir A, Chiheb S, Azzouzi S, Benchikhi H. Chromomycoses intercostale. In: Congrès Maghrébin de Dermatologie, 3–4 November. 2006. Tunis.
- 127.Kawtar I, Salim G, Mariame M, Fatimazahra M, Imane T, Salma B et al. Sporotrichoid chromomycosis Dermatology Online Journal. 2013;19(11):3. Available from: https://escholarship.org/uc/item/913521rt. [PubMed] [Google Scholar]
- 128.lkhachinea Y, Elbenayea J, Er-Rami M, Sakkah A, Jakar A, Elhaouri M. Chromomycose cutanée étendue: efficacité de l’association terbinafine et cryothérapie. Annales de Dermatologie et de Vénéréologie. 2018; 145:512–515. doi: 10.1016/j.annder.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 129.Hali F, K. Khadir K, Zouhair K, Benchikhi H, Azzouzi S. Suppurations périnéofessières: étude étiologique de 60 cas. Annales de dermatologie et de vénéréologie. 2010;137:591–596. doi: 10.1016/j.annder.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 130.Labbardi W, Hali F, Moundib H, Baline K, Chiheb S. Chromomycose cutanée: trois nouveaux cas marocains. Annales de Dermatologie et de Vénéréologie. 2015;142(12):S625. [Google Scholar]
- 131.Belarbi F, Ouadi Z, Hocar O, Akhdari N, Amal S, Zoughari B et al. Chromomycose cutanée diffuse. Annales de Dermatologie et de Vénéréologie. 2016;143:S37–S38. doi: 10.1016/S0151-9638(18)30048-6 [DOI] [PubMed] [Google Scholar]
- 132.Radouane N, Hali F, Khadir K, Soussi M, Ouakadi A, Marouane S et al. Chromomycose cutanée diffuse à Phialophora verrucosa. Annales de dermatologie et de venereologie. 2013;140:197–201. doi: 10.1016/j.annder.2012.10.605 [DOI] [PubMed] [Google Scholar]
- 133.van Raalte JA, Venkataramaiah NR. Chromomycosis in Tanzania. Trop Geogr Med. 1982;34(1):39–42. [PubMed] [Google Scholar]
- 134.Savioli L, Bianco P. A case of chromomycosis from Pemba Island. J Trop Med Hyg. 1983Jun;86(3):109–11. [PubMed] [Google Scholar]
- 135.Olafsson J, Lindtjorn B, Beiske K. Chromomycosis: a report of three cases from Ethiopia. Olafsson J, Lindtjorn B, Beiske K. Ethiop Med J. 1981Jul;19(3):91–6. [PubMed] [Google Scholar]
- 136.Gimbel DC, Legesse TB. Dermatopathology practice in ethiopia. Gimbel DC, Legesse TB. Arch Pathol Lab Med. 2013Jun;137(6):798–804. doi: 10.5858/arpa.2012-0041-RA [DOI] [PubMed] [Google Scholar]
- 137.Gatti F, Renoirte R, Vanbreuseghem R. African histoplasmosis and chromoblastomycosis in Angolians. Ann Soc Belges Med Trop Parasitol Mycol. 1967;47(3):249–56. [PubMed] [Google Scholar]
- 138.Des Marchais J, Vanderick F. A case of chromoblastomycosis in Rwanda. Ann Soc Belges Med Trop Parasitol Mycol. 1970;50(2):205–9 [PubMed] [Google Scholar]
- 139.Jacyk WK, Lawande RV, Tulpule SS. Deep mycoses in West Africa: a report of 13 cases and review of the Nigerian literature. J Natl Med Assoc. 1981;73(3):251–6. [PMC free article] [PubMed] [Google Scholar]
- 140.Ive FA, Clark BM. Chromoblastomycosis in Nigeria. J Trop Med Hyg. 1966;69(8):184–6. [PubMed] [Google Scholar]
- 141.Grillo E, Mavura D, Jaén-Olasolo P. Chromoblastomycosis. Rev Clin Esp (Barc). 2014;214(3):e35. doi: 10.1016/j.rce.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 142.Fenniche S, Zaraa I, Benmously R, Marrak H, Debbiche A, Ayed MB et al. Chromomycosis: a new Tunisian case report. Int J Infect Dis. 2005;9(5):288–9. doi: 10.1016/j.ijid.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 143.Marrak H, Mnajaa N, Fenniche S, Fourati M, Zghal M, Chaker E et al. Chromomycose: a propos d’une observation. J Mycol Med 2003;13:37–9. [Google Scholar]
- 144.Ezzine-Sebaï N, Benmously R, Chaker BFE, Zermani R, Kamoun MR. Chromomycosis arising in a Tunisian man. Dermatol Online J. 2005;11(2):14. [PubMed] [Google Scholar]
- 145.Chaabane H, Mseddi M, Charfi S, Chaari I, Boudawara T, Turki H. Chromoblastomycosis: breast solitary lesion. Presse Med. 2015;44(7–8):842–3. doi: 10.1016/j.lpm.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 146.El Amine El Hadj O, Msakni I, Lamine F, Laabidi B, Bouzaiène A. Chromoblastomycosis: Report of a case from a non-endemic region. Our Dermatol Online. 2017;8(1):100–101. Available from: http://www.odermatol.com/odermatology/20171/27.Chromoblasto-HadjA.pdf [Google Scholar]
- 147.Martin De Mirandol P, Segretain G, Bataillard. A case of chromoblastomycosis in Réunion. Bull Soc Pathol Exot Filiales. 1958;51(6):884–7. [PubMed] [Google Scholar]
- 148.Thomas E, Bertolotti A, Barreau A, Klisnick J, Tournebize P, Borgherini G et al. From phaeohyphomycosis to disseminated chromoblastomycosis: A retrospective study of infections caused by dematiaceous fungi. Med Mal Infect. 2018;48(4):278–285. doi: 10.1016/j.medmal.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 149.Miquel P. Trois observations de chromoblastomycose à La Reunion. Médecine et Maladies Infectieuses. 1978;8:398–403. [Google Scholar]
- 150.Hofmann H, Choi SM, Wilsmann-Theis D, Horré R, de Hoog GS, Bieber T. Invasive chromoblastomycosis and sinusitis due to Phialophora verrucosa in a child from northern Africa. Mycoses. 2005;48(6):456–61. doi: 10.1111/j.1439-0507.2005.01150.x [DOI] [PubMed] [Google Scholar]
- 151.Bhaktaviziam C, Shafi M, Mehta MC, Bhaktaviziam CA. Chromoblastomycosis. Report of a case from Tripoli, Libya. Mycopathologia. 1983;82(2):111–3. doi: 10.1007/BF00437340 [DOI] [PubMed] [Google Scholar]
- 152.Zendah B, Elgaed K, Kavanagh K, Zorgani AA, Ellabib MS. Occurrence of Chromoblastomycosis due to Cladophialophora carrionii in a Libyan patient. The Libyan Journal of Infectious Diseases. 2009;3(1):65–68. [Google Scholar]
- 153.Siala E, Gastli M, Ben Abdallah R, Barbouche R, Zallaga N, Bouratbine A et al. Chromomucose récidivante du visage et des membres: a propos de 1er cas libyen. Médecine Tropicale 2006; 67:69–71. [PubMed] [Google Scholar]
- 154.Cateau E, Cante V, Garcia Hermoso D, Rodier MH. Case of Fonsecaea nubica chromoblastomycosis from the French territory of Mayotte. JMM Case Rep. 2014;1(4):e004218. doi: 10.1099/jmmcr.0.004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Laporte P. Chromomycosis in the Island of Mayotte (apropos of 3 case reports). Med Trop (Mars). 1990;50(2):195–6. [PubMed] [Google Scholar]
- 156.Bari AU, Khan MB. Pattern of skin infections in black Africans of Sierra Leone (West Africa). Indian Journal of Dermatology 2007; 52(1):361–368. [Google Scholar]
- 157.Develoux M, Dieng MT, Ndiaye B, Raphenon G, Lepers JP. Chromomycose à Exophiala spinifera en Afrique sahélienne. Ann Dermatol Venereol 2006;133:68–72. doi: 10.1016/s0151-9638(06)70849-3 [DOI] [PubMed] [Google Scholar]
- 158.Passeron T, Barberet P, Colbachini P, Hovette P, Lacour JP. Association d’un eumycétome et d’une chromomycose: une observation au Sénégal. Med Trop (Mars) 2003;63(6):614–6. [PubMed] [Google Scholar]
- 159.Merriweather AM, Weir A, Murray JF. S Afr Med J. 1972Nov4;46(44):1679–81. [PubMed] [Google Scholar]
- 160.Carteron B, Bruneau M, Morvan D, Rodhain-Rebourg F, Destombes P, Francq JR Human mycoses in the Republic of Djibouti. Bull Soc Pathol Exot Filiales. 1978Jan-Feb;71(1):63–70. [PubMed] [Google Scholar]
- 161.Denning DW, Razanamparany VR, Rakotoarivelo RA. The burden of serious fungal diseases in Madagascar. Special Issue:7th Trends in Medical Mycology, 9–12 October 2015, Lisbon, Portugal. Mycoses. 2015:58(suppl.4):132. [Google Scholar]
- 162.Rasamoelina T, Rakotozandrindrainy N, Raberahona M, Rapelanoro Rabenja F, Rakoto Andrianarivelo M, Andrianarison M et al. Chromoblastomycosis and sporotrichosis in Madagascar: epidemiology, molecular diagnostic and perspectives. Mycoses. 2015;58(S4):99–100.25590228 [Google Scholar]
- 163.Esterre P, Andriantsimahavandy A, Ramarcel ER, Pecarrere JL. Forty years of chromoblastomycosisin Madagascar: a review. Am J Trop Med Hyg 1996; 55: 45–47. doi: 10.4269/ajtmh.1996.55.45 [DOI] [PubMed] [Google Scholar]
- 164.Gaüzère BA, Aubry P. History of human epidemic and endemic diseases in the southwest Indian Ocean. Med Sante Trop. 2013May1;23(2):145–57. doi: 10.1684/mst.2013.0183 [DOI] [PubMed] [Google Scholar]
- 165.Lu S, Lu C, Zhang J, Hu Y, Li X, Xi L. Chromoblastomycosis in Mainland China: a systematic review on clinical characteristics. Mycopathologia. 2013;175(5–6):489–95 doi: 10.1007/s11046-012-9586-z [DOI] [PubMed] [Google Scholar]
- 166.Harada S, Fumiiri M, Honda M, Ueda T, Murakami M, Hattori S et al. Chromomycosis of the skin: report of five cases. Int J Dermatol. 1971;10(2):118–25. doi: 10.1111/j.1365-4362.1971.tb03721.x [DOI] [PubMed] [Google Scholar]
- 167.Fukushiro R. Chromomycosis in Japan. Int J Dermatol. 1983;22(4):221–9. doi: 10.1111/j.1365-4362.1983.tb03371.x [DOI] [PubMed] [Google Scholar]
- 168.Tanuma H, Hiramatsu M, Mukai H, Abe M, Kume H, Nishiyama S et al. Case report. A case of chromoblastomycosis effectively treated with terbinafine. Characteristics of chromoblastomycosis in the Kitasato region, Japan. Mycoses. 2000;43(1–2):79–83. doi: 10.1046/j.1439-0507.2000.00548.x [DOI] [PubMed] [Google Scholar]
- 169.Kondo M, Hiruma M, Nishioka Y, Mayuzumi N, Mochida K, Ikeda S et al. A case of chromomycosis caused by Fonsecaea pedrosoi and a review of reported cases of dematiaceous fungal infection in Japan. Mycoses. 2005;48(3):221–5 doi: 10.1111/j.1439-0507.2005.01089.x [DOI] [PubMed] [Google Scholar]
- 170.Agarwal R, Singh G, Ghosh A, Verma KK, Pandey M, Xess I. Chromoblastomycosis in India: Review of 169 cases. PLoS Negl Trop Dis. 2017Aug3;11(8):e0005534. doi: 10.1371/journal.pntd.0005534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Attapattu MC. Chromoblastomycosis—a clinical and mycological study of 71 cases from Sri Lanka. Mycopathologia. 1997;137(3):145–51. doi: 10.1023/a:1006819530825 [DOI] [PubMed] [Google Scholar]
- 172.Hsu Yung-Hsiang. Chromomycosis. Tzu Chi Medical Journal. 2009;21(1):89. [Google Scholar]
- 173.Huang S, Lee S, Lee C, Ho H, Chang L. Coexisting Chromoblastomycosis and Mycobacterium fortuitum Skin and Subcutaneous Infection. Coexisting Chromoblastomycosis and Mycobacterium fortuitum Skin and Subcutaneous Infection. Journal of Internal Medicine of Taiwan. 2008;19(4):365–370. [Google Scholar]
- 174.Yang SP, Liu CY, Cheng NC, Lee WS, Liu CE, Kuo BI. Successful treatment of subcutaneous mycoses with fluconazole: a report of two cases. Zhonghua Yi Xue Za Zhi (Taipei). 1995;56(6):432–5. [PubMed] [Google Scholar]
- 175.Yang CS, Chen CB, Lee YY, Yang CH, Chang YC, Chung WH et al. Chromoblastomycosis in Taiwan: A report of 30 cases and a review of the literature. Med Mycol. 2018;56(4):395–405. doi: 10.1093/mmy/myx075 [DOI] [PubMed] [Google Scholar]
- 176.Jayalakshmi P, Looi LM, Soo-Hoo TS. Chromoblastomycosis in Malaysia. Mycopathologia 109: 27–31, 1990. doi: 10.1007/BF00437003 [DOI] [PubMed] [Google Scholar]
- 177.Yap FB. Chromoblastomycosis in Sarawak, East malaysian Borneo. Trans R Soc Trop Med Hyg. 2010;104(2):168–9 doi: 10.1016/j.trstmh.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 178.Pradhan SV, Talwar OP, Ghosh A, Swami RM, Shiva Raj KC, Gupta S. Chromoblastomycosis in Nepal: a study of 13 cases. Indian J Dermatol Venereol Leprol. 2007May-Jun;73(3):176–8. doi: 10.4103/0378-6323.32741 [DOI] [PubMed] [Google Scholar]
- 179.Agarwalla A, Khanal B, Garg VK, Agrawal S, Jacob M, Rani S et al. Chromoblastomycosis: report of two cases from Nepal. J Dermatol. 2002May;29(5):315–9. doi: 10.1111/j.1346-8138.2002.tb00270.x [DOI] [PubMed] [Google Scholar]
- 180.McDaniel P, Walsh DS. Chromoblastomycosis in Western Thailand. Am J Trop Med Hyg. 2010;83(3):448. doi: 10.4269/ajtmh.2010.10-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ungpakorn R, Reangchainam S. Pulse itraconazole 400 mg daily in the treatment of chromoblastomycosis. Clin Exp Dermatol. 2006;31(2):245–7. doi: 10.1111/j.1365-2230.2005.02024.x [DOI] [PubMed] [Google Scholar]
- 182.Mahaisavariya P, Chaiprasert A, Sivayathorn A, Khemngern S. Deep fungal and higher bacterial skin infections in Thailand: clinical manifestations and treatment regimens. International Journal of Dermatology 1999, 38, 279–284. doi: 10.1046/j.1365-4362.1999.00681.x [DOI] [PubMed] [Google Scholar]
- 183.Kampirapap K, Reangchainam S, Ornpaew P, Tresukosol P. Chromoblastomycosis masquerading as dermatophytosis, with the description of a new opportunistic species. Southeast Asian J Trop Med Public Health. 2015Jan;46(1):105–9. [PubMed] [Google Scholar]
- 184.Bonne C. Sur la presence de la chromoblastomycose aux Indes Orientales Néerlandaises. Bull Soc Path Exot. 1930;23:765. [Google Scholar]
- 185.Haykal Ahmad, Kadir Dirmawati, Amin Safruddin. Chromoblastomycosis et causaphialophora verrucosa. Indonesian Journal of Dermatology and Venereology. 2013;1(4):66–72. [Google Scholar]
- 186.Yahya S, Widaty S, Miranda E, Bramono K, Islami AW. Subcutaneous mycosis at the Department of Dermatology and Venereology dr. Cipto Mangunkusumo National Hospital, Jakarta, 1989–2013. J Gen Pro DVI. 2016;1(2):36–43. [Google Scholar]
- 187.Harahap M, Nasution MA. Dermatomycoses in Indonesia. Int J Dermatol. 1984;23(4):273–4. doi: 10.1111/j.1365-4362.1984.tb01247.x [DOI] [PubMed] [Google Scholar]
- 188.Park SG, Oh SH, Suh SB, Lee KH, Chung KY. A case of chromoblastomycosis with an unusual clinical manifestation caused by Phialophora verrucosa on an unexposed area: treatment with a combination of amphotericin B and 5-flucytosine. Br J Dermatol. 2005;152(3):560–4. doi: 10.1111/j.1365-2133.2005.06424.x [DOI] [PubMed] [Google Scholar]
- 189.Kim DM, Hwang SM, Suh MK, Ha GY, Choi GS, Shin J et al. Chromoblastomycosis Caused by Fonsecaea pedrosoi. Ann Dermatol. 2011;23(3):369–74. doi: 10.5021/ad.2011.23.3.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ahn SK, Lee SN. A case of chromonycosis. Korean J Dermatol 1990;28:345–348. [Google Scholar]
- 191.Suh MK, Sung YO, Yoon KS, Ha GY, Kim JR. A case of chromoblastomycosis caused by Fonsecaea pedrosoi. Korean J Dermatol 1996;34:832–836. [Google Scholar]
- 192.Kim HU, Son GY, Ihm CW. A case of chromoblastomycosis showing a good response to itraconazole. Ann Dermatol 1997;9:51–54. [Google Scholar]
- 193.Kim SW, Oh SH, Choi SK, Lee YH, Yoon JH, Bang YJ et al. Chromoblastomycosis treated with occlusive dressing of amphotericin B cream. Korean J Med Mycol 2000;5: 144–149. [Google Scholar]
- 194.Kang NG, Suh MK, Park SG, Song KY, Kim TH. A case of chromomycosis showing ulcerative lesions on dorsa of hands. Korean J Dermatol 2002;40:174–177. [Google Scholar]
- 195.Lee CW, Sim SJ, Song KH, Kim KH. A case of chromoblastomycosis treated with terbinafine. Korean J Med Mycol 2003;8:26–29. [Google Scholar]
- 196.Jun JB, Park JY, Kim DW, Suh SB. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Korean J Med Mycol 2004;9:117–122. doi: 10.1080/13693780310001597700 [DOI] [PubMed] [Google Scholar]
- 197.Khan S, Khan M, Khan F, Ahmad Z, Zia-Ur-Rehman A. A Rare Case of Chromoblastomycosis in a 12-year-old boy. J Pak Med Assoc. 2019;69(9):1390–1393. [PubMed] [Google Scholar]
- 198.Hussain I, Rashid T, Haroon TS. Parenteral vitamin D(3) and oral terbinafine for refractory chromoblastomycosis. Br J Dermatol. 2002;146(4):704. doi: 10.1046/j.1365-2133.2002.04711.x [DOI] [PubMed] [Google Scholar]
- 199.Brun S, Zumelzu C, Hoanganh MB, Levy A, Garcia-Hermoso D, Laroche L et al. First case of chromoblastomycosis from Bangladesh. Med Mycol Case Rep. 2015;10:1–3. doi: 10.1016/j.mmcr.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Slesak G, Inthalad S, Strobel M, Marschal M, Hall M Jr, Newton PN. Chromoblastomycosis after a leech bite complicated by myiasis: a case report. BMC Infect Dis. 2011;11:14. doi: 10.1186/1471-2334-11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Le TA, Nguyen KL, Pham MH, Vi TT, Do NA. Case Report: A Case of Chromoblastomycosis Caused by Fonsecaea pedrosoi in Vietnam. Mycopathologia. 2019;184(1):115–119. doi: 10.1007/s11046-018-0284-3 [DOI] [PubMed] [Google Scholar]
- 202.Simuangco SA, Halde C. Chromoblastomycosis: first case in the Philippines. J Philipp Med Assoc. 1955;31(3):117–20. [PubMed] [Google Scholar]
- 203.Santos LD, Arianayagam S, Dwyer B, Lee KC, O’Kane G, Withnall K et al. Chromoblastomycosis: a retrospective study of six cases at the Royal Darwin Hospital from 1989 to 1994. Pathology. 1996;28(2):182–7. doi: 10.1080/00313029600169843 [DOI] [PubMed] [Google Scholar]
- 204.Leslie DF, Beardmore GL. Chromoblastomycosis in Queensland: a retrospective study of 13 cases at the Royal Brisbane Hospital. Australas J Dermatol. 1979;20(1):23–30. doi: 10.1111/j.1440-0960.1979.tb00120.x [DOI] [PubMed] [Google Scholar]
- 205.Weedon D, van Deurse M, Allison S, Rosendahl C. Chromoblastomycosis in Australia: an historical perspective. Pathology. 2013;45(5):489–91. doi: 10.1097/PAT.0b013e32836326a1 [DOI] [PubMed] [Google Scholar]
- 206.Barrack BB. Chromoblastomycosis in Queensland. Aust J Dermatol. 1952;1(4):207–13. doi: 10.1111/j.1440-0960.1952.tb01429.x [DOI] [PubMed] [Google Scholar]
- 207.Stevens FRT. Chromoblastomycosis. Med J Aust 1947; 2: 93. doi: 10.5694/j.1326-5377.1947.tb27572.x [DOI] [PubMed] [Google Scholar]
- 208.Powell RE. A survey of chromoblastomycosis in Queensland. Aust J Dermatol. 1952Oct;1(4):214–22. doi: 10.1111/j.1440-0960.1952.tb01430.x [DOI] [PubMed] [Google Scholar]
- 209.Huerre M, Ravisse P, Dubourdieu D, Morillon M, McCarthy S, Bobin P. Les mycoses profondes observées em Nuuvelle-Calédonie. Bull Soc Path Ex 1991; 84:247–256 [PubMed] [Google Scholar]
- 210.Woodgyer AJ, Bennetts GP, Rush-Munro FM. Four non-endemic New Zealand cases of chromoblastomycosis. Australas J Dermatol. 1992;33(3):169–76. doi: 10.1111/j.1440-0960.1992.tb00113.x [DOI] [PubMed] [Google Scholar]
- 211.Knox J, Marshall C. Med J Aust. 2012Sep17;197(6):350.doi: 10.5694/mja12.10006 [DOI] [PubMed] [Google Scholar]
- 212.Pindycka-Piaszczyńska M, Krzyściak P, Piaszczyński M, Cieślik S, Januszewski K, Izdebska-Straszak G et al. Chromoblastomycosis as an endemic disease in temperate Europe: first confirmed case and review of the literature. Eur J Clin Microbiol Infect Dis. 2014Mar;33(3):391–8 doi: 10.1007/s10096-013-1969-7 [DOI] [PubMed] [Google Scholar]
- 213.Sonck CE. Cromomicosis em Finlandia. Dermatol Rev Mex. 1975;19:189–193. [Google Scholar]
- 214.Menezes N, Varela P, Furtado A, Couceiro A, Calheiros I, Rosado L et al. Chromoblastomycosis associated with Fonsecaea pedrosoi in a carpenter handling exotic woods. Dermatol Online J. 2008;14(2):9. [PubMed] [Google Scholar]
- 215.Buot G, Bachmeyer C, Benazeraf C, Bourrat E, Beltzer-Garrely E, Binet O. Chromoblastomycosis: an unusual diagnosis in Europe. Acta Derm Venereol. 2005;85(3):259–60. doi: 10.1080/00015550410024508 [DOI] [PubMed] [Google Scholar]
- 216.Chopinaud M, Bonhomme J, Comoz F, Barreau M, Morice C, Verneuil L. Chromomycosis in metropolitan France. Ann Dermatol Venereol. 2014;141(5):396–8. doi: 10.1016/j.annder.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 217.Ouédraogo MS, Vignon-Pennamen MD, Battistella M, Levy A, Feuilhade de Chauvin M, Petit A. Chromomycosis acquired in a non-tropical area: A case report. Ann Dermatol Venereol. 2017Jun—Jul;144(6–7):438–442. doi: 10.1016/j.annder.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 218.Richarz NA, Jaka A, Fernández-Rivas G, Bassas J, Bielsa I, Ferrándiz C. First case of chronic cutaneous chromoblastomycosis by Rhinocladiella similis aquired in Europe. Clin Exp Dermatol. 2018;43(8):925–927. doi: 10.1111/ced.13659 [DOI] [PubMed] [Google Scholar]
- 219.Lane CG. A cutaneous lesion caused by a new fungus Phialophora verrucosa. The Journal of Cutaneous Diseases. 1915;33:1–8. [Google Scholar]
- 220.Wilson SJ, Hulsey S, Weidman FD. Chromoblastomycosis in Texas. Arch Derm Syphilol. 1933; 27(1):107–122. [Google Scholar]
- 221.Moore M, Mapother P. Chromomycosis of the face: report of a case and a study of the causative organism, Phialophora verrucosa. Arch Derm Syphilol. 1940; 41(1):42–54. [Google Scholar]
- 222.Weidman FD, Rosenthal LH. Chromoblastomycosis: a new and important blastomycosis in north america: report of a case in Philadelphia. Arch Derm Syphilol. 1941; 43(1):62–84. [Google Scholar]
- 223.Emmons CW, Hailey H, Hailey H. Chromoblastomycosis: report of the sixth case from continental United States. JAMA. 1941; 116(1):25–28. [Google Scholar]
- 224.Moore M, Cooper ZK, Weiss RS. JAMA. 1943; 122(18):1237–1243. [Google Scholar]
- 225.Binford CH, Hess G, Emmons CW. Chromoblastomycosis: report of a case from continental united states and discussion of the classification of the causative fungus. Arch Derm Syphilol. 1944; 49(6):398–402. [Google Scholar]
- 226.Barwasser NC. Chromoblastomycosis; thirteenth reported case in the United States. J Am Med Assoc. 1953;153(6):556. doi: 10.1001/jama.1953.02940230028006f [DOI] [PubMed] [Google Scholar]
- 227.French AJ, Russell SR. Chromoblastomycosis; report of first case recognized in Michigan, apparently contracted in South Carolina. AMA Arch Derm Syphilol. 1953;67(2):129–34. doi: 10.1001/archderm.1953.01540020007002 [DOI] [PubMed] [Google Scholar]
- 228.Howles JK, Kennedy CB, Garvin WH, Brueck JW, Buddingh GJ. Chromoblastomycosis: report of nine cases from a single area in Louisiana. AMA Arch Derm Syphilol. 1954;69(1):83–90. doi: 10.1001/archderm.1954.01540130085008 [DOI] [PubMed] [Google Scholar]
- 229.Mugleston BJ, Usatine RP, Rosen T. Wide Morphologic Variability of Chromoblastomycosis in the Western Hemisphere. Skinmed. 2016;14(6):423–427. eCollection 2016. Acesso https://skinmedjournal.com/2016-issues/# [PubMed] [Google Scholar]
- 230.Franco-Paredes C, Mathur S, Villamil-Gomez W, Henao-Martínez AF. A Man Who Harvest Peanuts With Verrucous Lesions in His Right Index Finger. Am J Med Sci. 2018;355(3):e7. doi: 10.1016/j.amjms.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 231.Burns RE. Chromoblastomycosis in a Canadian airman serving in Ceylon. Can Med Assoc J. 1950;63(6):595–6. [PMC free article] [PubMed] [Google Scholar]
- 232.Poverty Home—World Bank Group. In: The Wold Bank: Countries and Economies. Cited 26 april 2020 [Internet]. Available from: https://data.worldbank.org/country
- 233.Rural Population—World Bank Group. In: World Bank staff estimates based on the United Nations Populations Division’s World Urbanization Prospects: 2018 Revision. Cited 26 april 2020 [Internet]. Available from: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS
- 234.Santmyire A. The Effectiveness of a Multifocal Training to Improve the Treatment of Chromoblastomycosis in Rural Madagascar. J Health Care Poor Underserved. 2016;27(3):993–1010. doi: 10.1353/hpu.2016.0146 [DOI] [PubMed] [Google Scholar]
- 235.Al-Hamdani M, Salmo NA, Hadi AW. Chromomycosis of the skin: first case report in Iraq. Int J Dermatol. 1973Nov-Dec;12(6):354–7. doi: 10.1111/j.1365-4362.1973.tb00220.x [DOI] [PubMed] [Google Scholar]
- 236.Grandizio LC, Wagner B, Graham J, Klena JC. Upper Extremity Trauma Resulting From Agricultural Accidents: Mechanism and Severity for Patients With and Without Upper Extremity Injury. Hand (N Y). 2018;13(4):384–390. doi: 10.1177/1558944717715140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sousa MG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011May19;9(5):436–43. doi: 10.1016/j.chom.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gomes RR, Vicente VA, Azevedo CM, Salgado CG, da Silva MB, Queiroz-Telles F et al. Molecular Epidemiology of Agents of Human Chromoblastomycosis in Brazil with the Description of Two Novel Species. PLoS Negl Trop Dis. 2016;10(11):e0005102.doi: 10.1371/journal.pntd.0005102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rasamoelina T, Maubon D, Andrianarison M, Ranaivo I, Sendrasoa F, Rakotozandrindrainy N et al. Endemic Chromoblastomycosis Caused Predominantly by Fonsecaea nubica, Madagascar. Emerg Infect Dis. 2020;26(6):1201–1211. doi: 10.3201/eid2606.191498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Esterre P, Inzan CK, Ramarcel ER, Andriantsimahavandy A, Ratsioharana M, Pecarrere JL et al. Treatment of chromomycosis with terbinafine: preliminary results of an open pilot study. Br J Dermatol. 1996Jun;134Suppl 46:33–6. doi: 10.1111/j.1365-2133.1996.tb15658.x [DOI] [PubMed] [Google Scholar]
- 241.Marques SG, Bomfim MRQ, Azevedo CMPS, Martins CVB, Marques ACG, Gonçalves AG et al. Mixed secondary bacterial infection is associated with severe lesions of chromoblastomycosis in a neglected population from Brazil. Diagn Microbiol Infect Dis. 2019Oct;95(2):201–207. doi: 10.1016/j.diagmicrobio.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 242.Azevedo CM, Marques SG, Santos DW, Silva RR, Silva NF, Santos DA et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015May15;60(10):1500–4. doi: 10.1093/cid/civ104 Epub 2015 Feb 13. [DOI] [PubMed] [Google Scholar]
- 243.Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, Marques SG, Gonçalves AG, Vagner de Castro Lima Santos D et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005Apr;7(4):708–13. doi: 10.1016/j.micinf.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 244.Azevedo CD, Bruña-Romero O, Marques SG, Nascimento FR, Pinto MC, Silva LA et al. Association of IgG immunoglobulin and subclasses level with the severity of chromoblastomycosis due to Fonsecaea pedrosoi and therapeutic response to itraconazole. Eur J Clin Microbiol Infect Dis. 2014Oct;33(10):1791–7. doi: 10.1007/s10096-014-2138-3 [DOI] [PubMed] [Google Scholar]
- 245.Santos DW, Camargo LF, Gonçalves SS, Ogawa MM, Tomimori J, Enokihara MM et al. Melanized fungal infections in kidney transplant recipients: contributions to optimize clinical management. Clin Microbiol Infect. 2017May;23(5):333.e9–333.e14. doi: 10.1016/j.cmi.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 246.Ogawa MM, Peternelli MP, Enokihara MM, Nishikaku AS, Gonçalves SS, Tomimori J. Spectral Manifestation of Melanized Fungal Infections in Kidney Transplant Recipients: Report of Six Cases. Mycopathologia. 2016Jun;181(5–6):379–85. doi: 10.1007/s11046-016-0005-8 [DOI] [PubMed] [Google Scholar]
- 247.Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005Nov-Dec;47(6):339–46. doi: 10.1590/s0036-46652005000600006 [DOI] [PubMed] [Google Scholar]
- 248.Lima AM, Sacht GL, Paula LZ, Aseka GK, Goetz HS, Gheller MF et al. Response of chromoblastomycosis to voriconazole. An Bras Dermatol. 2016Sep-Oct;91(5):679–681. doi: 10.1590/abd1806-4841.20165142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.de Sousa Mda G, Belda W Jr, Spina R, Lota PR, Valente NS, Brown GD et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014Jun;58(12):1734–7. doi: 10.1093/cid/ciu168 Epub 2014 Mar 14. ; PMCID: PMC4036686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Belda W Jr, Criado PR, Passero LFD. Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod. J Dermatol. 2020Apr;47(4):409–412. doi: 10.1111/1346-8138.15225 Epub 2020 Jan 21. . [DOI] [PubMed] [Google Scholar]
- 251.Azevedo Cde M, Marques SG, Resende MA, Gonçalves AG, Santos DV, da Silva RR et al. The use of glucan as immunostimulant in the treatment of a severe case of chromoblastomycosis. Mycoses. 2008Jul;51(4):341–4. doi: 10.1111/j.1439-0507.2007.01485.x Epub 2008 Apr 28. . [DOI] [PubMed] [Google Scholar]