Abstract
Background:
Clinical discovery/staging of gastric cancer (GC) is crucial in designing the treatment strategies and largely decides GC patients’ survival. Endoscopic ultrasonography (EUS) and computed tomography (CT) are 2 useful GC diagnosis tools. High doses of radiation associated with CT make its use limited, while the process of EUS is stressful, time-consuming, and challenging. Transabdominal ultrasound (TAUS) is a promising candidate to address these shortcomings. This study aimed to meta-analyze the diagnostic accuracy and sensitivity of TAUS in discriminating between advanced and early GCs, as well as compare its utility with other imaging techniques.
Methods: Literature searches were conducted using PubMed, Web of Science, Embase, and Cochrane Library databases up to 2019. Data were analyzed using RevMan software (Cochrane Collaboration, Oxford, UK), and pooled estimates of accuracy, sensitivity, and other features were acquired. Seven papers were eventually selected for meta-analysis.
Results:
TAUS had distinct diagnostic efficacies for early and advanced GC patients. The accuracy and sensitivity were significantly higher in the advanced group. A high color Doppler vascularity index and a lesion larger than 1 cm were 2 features of advanced GC. Moreover, TAUS had a comparable (but slightly higher) accuracy than CT and EUS.
Conclusions:
TAUS is more accurate and sensitive in diagnosing advanced GC compared to early GC. More features of advanced GC are required to improve the recognition ability. At least, TAUS can be considered as a complementary imaging diagnostic tool to CT and EUS.
Keywords: computed tomography, endoscopic ultrasonography, gastric cancer, preoperative diagnosis, transabdominal ultrasound
1. Introduction
Gastric cancer (GC) is among the most malignant tumors and is associated with a poor prognosis.[1] The outcomes of patients with GC are primarily determined by the lesion status, distant metastases, and treatment time points. Timely discovery of the existing lesions is vital for GC treatment and survival. Endoscopic ultrasonography (EUS) and computed tomography (CT) are 2 useful tools in GC diagnosis. Especially, EUS is endorsed for GC preoperative staging, with a referential accuracy and sensitivity.[2–4] Some scholars even consider EUS as the gold standard technique in the loco-regional staging of gastric adenocarcinoma.[5] EUS provides an opportunity to achieve more insight into the gastric tumor process's loco-regional circumstances, bringing about a lot of benefits in determining the gross appearance of GC and providing a reliable assessment of the GC stage. However, the process of EUS is time-consuming and challenging and can be painful for GC patients. For this reason, quality control of EUS is needed, and efforts should be made to improve the compliance of patients.[6] Besides the inaccessibility in many clinical practices, published meta-analyses suggest that EUS performance cannot be considered optimal either for GC confirmation or for exclusion, especially for its ability to distinguish T stages and lymph node status.[7] CT is currently the most frequently used radiological tool for preoperative GC staging.[8] However, CT accuracy is high mainly in distant metastasis but relatively low for loco-regional staging.[9–12] A limited proportion of GC patients carrying locally advanced lesions can be identified preoperatively and benefit from this technique. This inadequacy can be addressed by EUS, which is considered accurate in the loco-regional staging. Moreover, CT induces radiation, which constrains its application. Additionally, for both CT and EUS, the performance of accuracy and sensitivity are still to be enhanced. Some articles have compared the results of these 2 modalities. For example, it is believed that EUS provides superior overall accuracy of T staging than CT. Still, the overall performance of EUS for N staging is inferior compared to CT (but no significant differences).[5] Together, EUS and CT have their specific disadvantages. Thereby, preoperative EUS and CT can be regarded as mutually complementary tools in preoperative gross classification.[5,13]
Based on current EUS and CT approaches, an ideal improvement is that: a non-invasive technique should be; non-radiative; convenient; and with performance not inferior to EUS and CT (or even superior). Based on these requirements, transabdominal ultrasound (TAUS) becomes a promising candidate. TAUS is useful in ascertaining the depth of cancer invasion.[14–16] Some studies suggest that the diagnostic value of TAUS is comparable to or even higher than EUS.[17] TAUS may have better performance in advanced-GC recognition compared to the early ones. Our present study aims to meta-analyze the diagnostic accuracy and sensitivity of TAUS in discriminating between advanced and early GCs, as well as comparing its usefulness with other imaging techniques.
2. Methods
2.1. Data sources
Since this study is a meta-analysis, an approval by the Ethical Committee was not required. Under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, relevant literature up to 2019 were searched in the following databases: PubMed, Embase, Cochrane Library, and Web of Science databases. The following phrases were used: “Transabdominal Ultrasound” or “TAUS” and “gastric cancer” or “gastric carcinoma.” The literature retrieval was performed by authors, and the abstract of any potential included reference was limited to English or Chinese. Some articles whose full texts were not available in online databases but considered worth analyzing based on the abstracts were acquired from the corresponding authors.
2.2. Inclusion and exclusion criteria
The screening process was performed as shown in Figure 1. The inclusion criteria were as follows: transabdominal ultrasonography in the diagnosis of GC; the patients’ data could be divided into 2 groups (advanced vs early according to tumor stage or ultrasonography techniques). The exclusion criteria were as follows: duplicate publications; not regarding the transabdominal ultrasonography or GC; conference abstracts, case reports, comments, reviews, or meta-analyses; non-clinical studies; insufficient data: no detailed data about our interested parameters, or no divisible groups in comparison.
Figure 1.
The study flow diagram of data search, inclusion, and exclusion.
Patients from different literature were grouped into: advanced or early as the routine clinical standard in cancer staging; TAUS or other imaging techniques.
2.3. Index extraction
All the data were expressed as dichotomous values, and the total cases and events were extracted for each group. Accuracy was defined as the proportion of tumors detected/staged by TAUS and agreed with the postoperative staging using histopathology. For accuracy comparison, the total cases referred to all the detected cases, and events referred to the corrected cases after histological verification. For sensitivity comparison, the total cases indicated all the real positive cases in histological verification, and events indicated detected ones in the corresponding group. For other comparisons, the total cases were all patients who had received the US detection, and events were the cases with features of specific indexes.
2.4. Statistical analysis
All data were analyzed with RevMan software version 5.3 (Cochrane Collaboration, Oxford, UK). Forest plots were presented after analysis, in which lines represented different estimates and confidence intervals, and boxes represented the weight of each study. The summary receiver operating characteristics curve was drawn by Meta-Disc (Version 1.4, Ramón y Cajal University Hospital). Briefly, data entry was performed based on the accuracy results of each study, especially true positive cases, false positive cases, false negative cases, and true negative cases. Then using the tool of analysis/plots, the SROC curve was drawn, as well as the AUC information. I2 test was performed to assess the heterogeneity between studies. When the heterogeneity between studies was P < .10 and I2 > 50%, it was considered as heterogeneous between the studies. The fixed-effect model was used when no significant heterogeneity existed; otherwise, a random-effect model was used.
3. Results
3.1. Enrolled studies
From all databases/libraries, we initially identified 30 papers that were potentially relevant to the search terms (PubMed: 18, Embase: 12, Cochrane: 0, and Web of Science: 0). Next, we removed 10 duplicate papers and 2 irrelevant papers. Further, we identified and excluded 11 papers which included reviews, conference abstracts, case reports, meta-analyses, and papers lacking necessary parameters and data. Seven clinical studies were eventually selected and used for this meta-analysis[18–24] (Table 1).
Table 1.
Characteristics of included studies.
| Author | Year | Country | Case number |
| Lim et al | 1994 | Korea | 44 |
| Chen et al | 2009 | Taiwan | 24 |
| Cui et al | 2010 | China | 59 |
| Zheng et al | 2011 | China | 162 |
| Liu et al | 2015 | China | 288 |
| He et al | 2017 | China | 42 |
| Liu et al | 2015 | China | 687 |
3.2. Accuracy and sensitivity of TAUS in advanced and early GC diagnosis
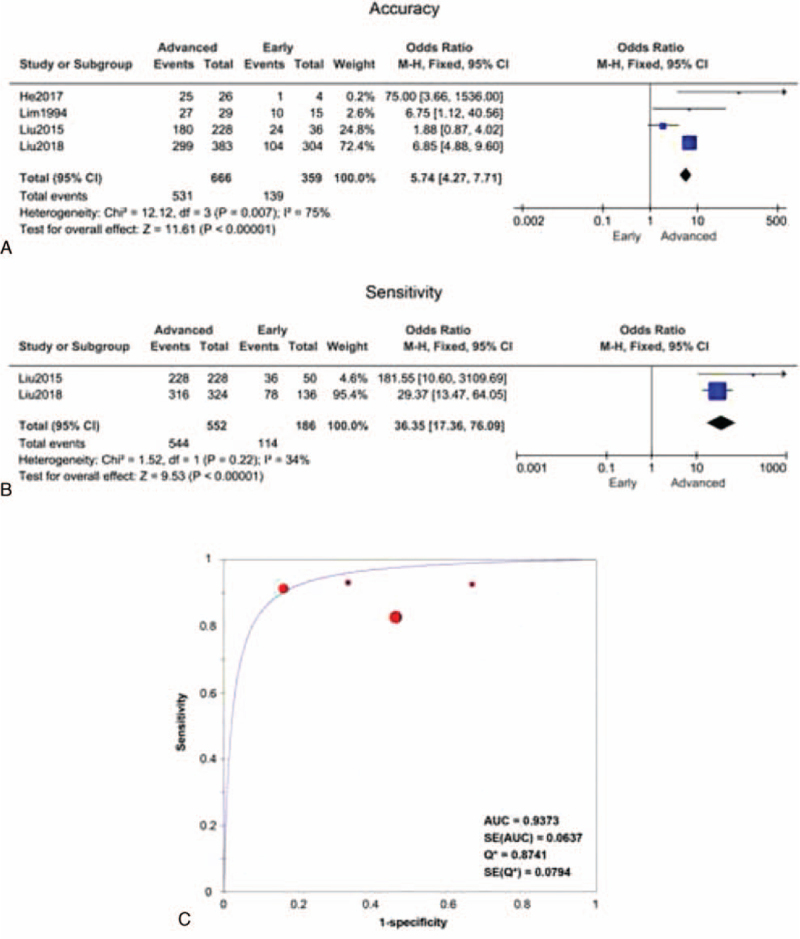
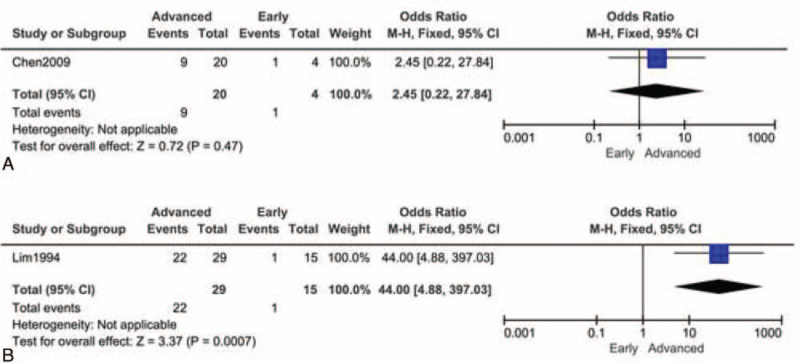
Since a limited number of studies were included, only 4 papers were used for accuracy comparison, and 2 papers were included for sensitivity comparison. We found TAUS had distinct diagnostic efficacies for early and advanced GC patients. The diagnostic accuracy odds ratio of advanced vs early was 5.74 (95% confidence interval: 4.27–7.17), and the overall pooled accuracy was 65.4%. The advanced group's pooled accuracy was 79.7%, and that of the early group was 38.7%. The accuracy of TAUS was much higher for the advanced cohort compared to the early ones (test for overall effect Z = 11.61, P < .00001) (Fig. 2A). There was significant heterogeneity across studies (Chi2 = 12.12, P = .007; I2 = 75%). Similarly, the sensitivity was also significantly higher in the advanced group (test for overall effect Z = 9.53, P < .00001). The overall sensitivity for advanced GC was 98.6% (544/552), and that for early GC was 61.2% (114/186) (Fig. 2B). No heterogeneity between 2 groups was observed in sensitivity comparison (Chi2 = 1.52, P = .22; I2 = 34%). The summary receiver operating characteristics curve, presented in Figure 2C, exhibited an AU = 0.937 in discrimination of early and advanced GC. Taken together, TAUS had higher practicability in advanced GC diagnosis when compared with early GC. For those patients suspected of advanced GC (with related symptoms), it is strongly recommendable to administer a timely TAUS examination. Next, we proposed some features of advanced GC based on limited papers. However, very few studies had focused on the distinguishable parameters between advanced and early patients; thus, only 2 studies were collected. In a study by Chen et al[18], the color Doppler vascularity index (CDVI) was claimed to be associated with lymph node metastasis of GC. But no significant difference was found in high-CDVI (≥11%) proportion between groups (Z = 0.72, P = .47) (Fig. 3A). Lim et al[21] found that the advanced group had a dramatically higher frequency of lesions larger than 1 cm (22/29 vs 1/15, Z = 3.37, P = .0007) (Fig. 3B). Collectively, TAUS was useful in recognizing large lesions, which might imply a higher stage.
Figure 2.
Cumulative meta-analysis (forest plots) of the TAUS accuracy and sensitivity between the advanced group and early group. (A) Difference in accuracy between the advanced and early groups. (B) Difference in sensitivity between the advanced and early groups. (C) SROC of 4 studies about TAUS specificity and sensitivity in discriminating advanced and early groups. SROC = summary receiver operating characteristics, TAUS = transabdominal ultrasound.
Figure 3.
Different features between advanced GC and early GC in TAUS examination (presented as forest plots). (A) High color Doppler vascularity index (>11%); (B) lesion larger than 1 cm. GC = gastric cancer, TAUS = transabdominal ultrasound.
3.3. Comparison of accuracy between TAUS and other techniques
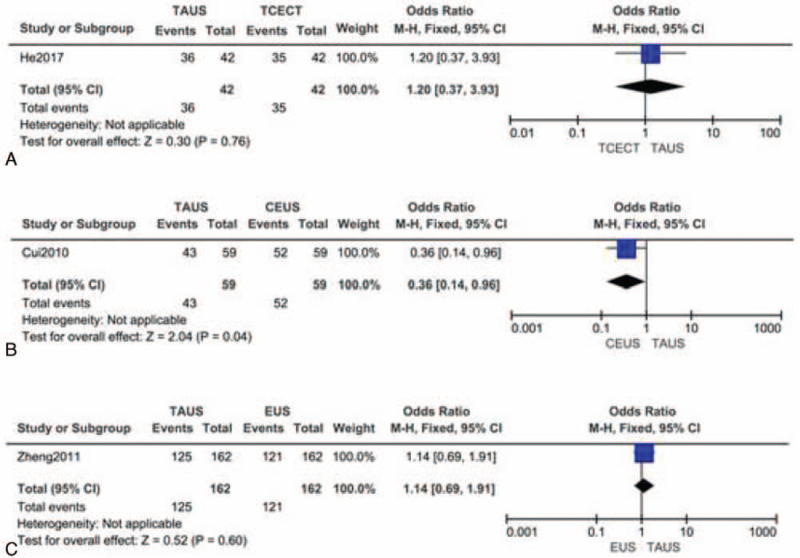
Another study in 2017 demonstrated no significant difference in the overall accuracy of TAUS (86%, 36/42) and transverse contrast-enhanced CT (83%, 35/42, P > .999) (Fig. 4A).[20] These findings suggested that TAUS could provide a satisfactory diagnosis with an accuracy similar to the CT method. However, in the work of Cui et al,[19] the accuracy of TAUS was 72.9%, and that of the contrast-enhanced ultrasonography was 88.1% (P = .04) (Fig. 4B). Their results indicated that contrast-enhanced ultrasonography was a more useful diagnostic tool for preoperative T-staging of GC; however, the TAUS accuracy was relatively lower when compared with other studies. Notably, Zheng et al[24] had compared the accuracies of TAUS and EUS and demonstrated a slight superiority of the TAUS method (77.2% vs 74.7%) (Fig. 4C). In T3 stage recognition, TAUS showed significantly higher accuracy than EUS. Moreover, lymph nodes were staged correctly with TAUS and EUS in 78.4% and 57.4% of subjects, respectively. In summary, TAUS showed an excellent performance in staging and GC discovery, which was comparable or even superior to EUS and contrast-enhanced CT methods.
Figure 4.
Comparison of accuracy between TAUS and other techniques (presented as forest plots). (A) TAUS vs transverse contrast-enhanced CT; (B) TAUS vs CEUS; (C) TAUS vs EUS. CEUS = contrast-enhanced ultrasonography, CT = computed tomography, EUS = endoscopic ultrasonography, TAUS = transabdominal ultrasound.
Finally, due to relatively limited existing studies, it was difficult to assess the publication bias. Therefore, the superiority of TAUS still needs to be interpreted cautiously. Further studies to explore the accuracy, sensitivity, and usefulness of TAUS in GC diagnosis are urgently needed.
4. Discussion
TAUS is a powerful tool in distinguishing advanced GC from early GC. The accurate detection and staging of GC are vital in selecting treatment strategies and significantly impact the prognosis of patients. Timely utilization of TAUS can identify patients suffering from advanced GC who might essentially benefit from non-invasive diagnostic procedures. So far, there are no published meta-analyses assessing TAUS performance for diagnosing or staging GC preoperatively. This paper analyzed 7 studies and revealed a significant evaluation value of TAUS for GC patients. According to our findings, TAUS is relatively more accurate and sensitive in diagnosing advanced GC than early GC, owing to the higher tumor burden in patients with advanced GC. These results are consistent with previous EUC and CT studies. In a study by Wakelin et al,[25] it had been reported that CT and EUS accurately identified advanced tumors (T3/T4) in 94% and 88% of subjects, respectively. EUS was able to visualize tumors in all the patients while correctly assessing 64% in T1/T2 stage and 92% in T3/T4 stage. However, there are still some controversies. A part of the surveys implied that EUS and CT had a poor performance in identifying T and N stages.[26] Theoretically, CDVI is very useful in evaluating stomach angiogenesis.[18,27] A high CDVI can be regarded as an excellent preoperative indicator of the short survival of GC patients. A study by Chen et al[28] (not included in the present meta-analysis due to insufficient data) revealed a linear correlation between CDVI and micro-vessel density. Therefore, patients with a high CDVI (11% or above) may have a higher severity and lower survival. CDVI could be regarded as an independent prognostic factor in patients with stage III GC. However, in our analysis, no significant difference was found in the high-CDVI proportion between groups, implying that CDVI is a more useful indicator for prognosis rather than the diagnosis of GC, which is consistent with previous studies. However, further studies are required to confirm this notion.
Our findings reveal that the accuracy of TAUS is comparable to CT. TAUS is a highly recommended investigation tool for it provides diagnosis with satisfactory accuracy but without radiative harm. Similar results have been obtained in other diseases. Some scholars evaluated the hemodynamics of gastric varices using color Doppler TAUS and reported that TAUS and CT findings were in complete agreement.[29] There are limited preliminary studies regarding the comparison of EUS and TAUS in preoperative staging of GC. Zheng et al (included in this analysis) showed a better performance of GC accuracy in the TAUS group. Another study reported similar results in N2 and N3 staging (68.29% vs 34.15%; 66.67% vs 12.50%). TAUS was obviously superior to EUS in the M1 stage (83.33% vs 16.67%). The accuracy of EUS increased from 64.18% to 79.10% when combined with TAUS.[30] In practice, EUS has poorer compliance than TAUS, which brings extra pain to the GC patients. In contrast, in our analysis of the published studies, TAUS, though a non-invasive technique, showed a superior diagnostic efficacy (at least not inferior to EUS). Although detections using TAUS may be influenced by air in the lungs and gastric varices, it is relatively easy to operate. Moreover, the development of color Doppler TAUS has made it possible to detect small amounts of blood flow and the portal venous system, providing additional references in tumor staging. Overall, TAUS exhibits a non-inferiority in comparison to other imaging techniques in the accuracy of GC diagnosis but is prominent in the non-invasive and non-radiative properties. A combined staging/identification approach and continuous optimization are recommended for accurate GC diagnosis in the future.
5. Conclusions
In summary, TAUS is more accurate and sensitive in diagnosing advanced GC compared to early GC. More features of advanced GC are required to improve its ability of recognition. TAUS has comparable or superior performance compared to other imaging modalities, but it is prominent in its non-invasive and non-radiative properties. At least, TAUS can be considered as a complementary imaging diagnostic tool to CT and EUS.
Acknowledgments
We thank everyone who has contributed to the study, in particular the journal editors and reviewers.
Author contributions
Conceptualization: Yuqin Zhang.
Formal analysis: Jianzhong Zhang.
Investigation: Liu Yang.
Methodology: Jianzhong Zhang, Liu Yang.
Resources: Songxiong Huang.
Supervision: Songxiong Huang.
Writing – original draft: Yuqin Zhang.
Writing – review & editing: Yuqin Zhang, Jianzhong Zhang.
Footnotes
Abbreviations: CDVI = color Doppler vascularity index, CT = computed tomography, EUS = endoscopic ultrasonography, GC = gastric cancer, TAUS = transabdominal ultrasound.
How to cite this article: Zhang Y, Zhang J, Yang L, Huang S. A meta-analysis of the utility of transabdominal ultrasound for evaluation of gastric cancer. Medicine. 2021;100:32(e26928).
YZ and JZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Wilke H, Stahl M. Therapy in gastric cancer. From an oncological perspective. Chirurg 2009;80:1023–7. [DOI] [PubMed] [Google Scholar]
- [2].von Rahden BH, Stein HJ. Staging and treatment of advanced esophageal cancer. Curr Opin Gastroenterol 2005;21:472–7. [PubMed] [Google Scholar]
- [3].Pollack BJ, Chak A, Sivak MV, Jr. Endoscopic ultrasonography. Semin Oncol 1996;23:336–46. [PubMed] [Google Scholar]
- [4].Mellinger JD, Ponsky JL. Endoscopy in gastric malignancy. Cancer Treat Res 1991;55:51–68. [DOI] [PubMed] [Google Scholar]
- [5].Cimavilla Roman M, de la Serna Higuera C, Loza Vargas LA, et al. Endoscopic ultrasound versus multidetector computed tomography in preoperative gastric cancer staging. Rev Esp Enferm Dig 2017;109:761–7. [DOI] [PubMed] [Google Scholar]
- [6].Lachter J, Bluen B, Waxman I, Bellan W. Establishing a quality indicator format for endoscopic ultrasound. World J Gastrointest Endosc 2013;5:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015;CD009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ly QP, Sasson AR. Modern surgical considerations for gastric cancer. J Natl Compr Canc Netw 2008;6:885–94. [DOI] [PubMed] [Google Scholar]
- [9].Kim SH, Choi YH, Kim JW, et al. Clinical significance of computed tomography-detected ascites in gastric cancer patients with peritoneal metastases. Medicine (Baltimore) 2018;97:e9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang H, Pan Z, Du L, et al. Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Korean J Radiol 2008;9:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giganti F, Orsenigo E, Arcidiacono PG, et al. Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging? Prospective comparison with EUS and multidetector computed tomography. Gastric Cancer 2016;19:216–25. [DOI] [PubMed] [Google Scholar]
- [12].Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 2010;25:512–8. [DOI] [PubMed] [Google Scholar]
- [13].Yan C, Bao X, Shentu W, et al. Preoperative gross classification of gastric adenocarcinoma: comparison of double contrast-enhanced ultrasound and multi-detector row CT. Ultrasound Med Biol 2016;42:1431–40. [DOI] [PubMed] [Google Scholar]
- [14].Liu Z, Guo J, Wang S, et al. Evaluation of transabdominal ultrasound with oral cellulose-based contrast agent in the detection and surveillance of gastric ulcer. Ultrasound Med Biol 2017;43:1364–71. [DOI] [PubMed] [Google Scholar]
- [15].Liu Z, Guo J, Li J, et al. Gastric lesions: demonstrated by transabdominal ultrasound after oral administration of an echoic cellulose-based gastric ultrasound contrast agent. Ultraschall Med 2016;37:405–11. [DOI] [PubMed] [Google Scholar]
- [16].Polkowski M, Palucki J, Butruk E. Transabdominal ultrasound for visualizing gastric submucosal tumors diagnosed by endosonography: can surveillance be simplified? Endoscopy 2002;34:979–83. [DOI] [PubMed] [Google Scholar]
- [17].Ishigami S, Yoshinaka H, Sakamoto F, et al. Preoperative assessment of the depth of early gastric cancer invasion by transabdominal ultrasound sonography (TUS): a comparison with endoscopic ultrasound sonography (EUS). Hepatogastroenterology 2004;51:1202–5. [PubMed] [Google Scholar]
- [18].Chen CN, Lin JJ, Lee H, et al. Association between color doppler vascularity index, angiogenesis-related molecules, and clinical outcomes in gastric cancer. J Surg Oncol 2009;99:402–8. [DOI] [PubMed] [Google Scholar]
- [19].Cui J, Yang YM, Ding LJ, et al. Diagnostic value of contrast-enhanced ultrasonography in preoperative T-staging of gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2010;13:141–4. [PubMed] [Google Scholar]
- [20].He X, Sun J, Huang X, et al. Comparison of oral contrast-enhanced transabdominal ultrasound imaging with transverse contrast-enhanced computed tomography in preoperative tumor staging of advanced gastric carcinoma. J Ultrasound Med 2017;36:2485–93. [DOI] [PubMed] [Google Scholar]
- [21].Lim JH, Ko YT, Lee DH. Transabdominal US staging of gastric cancer. Abdom Imaging 1994;19:527–31. [DOI] [PubMed] [Google Scholar]
- [22].Liu Z, Guo J, Wang S, et al. Evaluation of transabdominal ultrasound after oral administration of an echoic cellulose-based gastric ultrasound contrast agent for gastric cancer. BMC Cancer 2015;15: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Z, Ren W, Guo J, et al. Preliminary opinion on assessment categories of stomach ultrasound report and data system (Su-RADS). Gastric Cancer 2018;21:879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zheng Z, Yu Y, Lu M, et al. Double contrast-enhanced ultrasonography for the preoperative evaluation of gastric cancer: a comparison to endoscopic ultrasonography with respect to histopathology. Am J Surg 2011;202:605–11. [DOI] [PubMed] [Google Scholar]
- [25].Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol 2002;41:161–7. [DOI] [PubMed] [Google Scholar]
- [26].Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol 2015;111:1016–20. [DOI] [PubMed] [Google Scholar]
- [27].Chen JC, Chen Y, Lin JH, Wu JM, Tseng SH. Resveratrol suppresses angiogenesis in gliomas: evaluation by color Doppler ultrasound. Anticancer Res 2006;26(2A):1237–45. [PubMed] [Google Scholar]
- [28].Chen CN, Cheng YM, Lin MT, Hsieh FJ, Lee PH, Chang KJ. Association of color Doppler vascularity index and microvessel density with survival in patients with gastric cancer. Ann Surg 2002;235:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sato T, Yamazaki K, Akaike J. Diagnosis of gastric varices and evaluation of the effectiveness of treatment using transabdominal color Doppler ultrasonography. J Ultrasound Med 2009;28:1125–31. [DOI] [PubMed] [Google Scholar]
- [30].Hao-hui Z, Jian-jun Y, Ji-yun C, et al. Endoscopic ultrasonography and transabdominal ultrasonography in preoperative staging of gastric cancer. Chin J Clin Med Imaging 2012;23:841–4. [Google Scholar]