Abstract
Radix Isatidis (Banlangen) is a well-known traditional Chinese medicine for the treatment of different diseases and prevention of many body disorders. Besides, it also plays a pivotal role in novel coronavirus pneumonia, coronavirus disease 2019 (COVID-19). However, few researchers know its active ingredients and mechanism of action for COVID-19. To find whether Banlangen has a pharmacological effect on COVID-19. In this research, we systematically analyze Banlangen and COVID-19 through network pharmacology technology. A total of 33 active ingredients in Banlangen, 92 targets of the active ingredients, and 259 appropriate targets of COVID-19 were obtained, with 11 common targets. The analysis of the biological process of gene ontology and the enrichment of Kyoto Encyclopedia of Genes and Genomes signaling pathway suggests that Banlangen participated in the biological processes of protein phosphatase binding, tetrapyrrole binding, the apoptotic process involving cysteine-type endopeptidase activity, etc. The COVID-19 may be treated by regulating advanced glycation end products/a receptor for advanced glycation end products signaling pathway, interleukin-17 signaling pathway, tumor necrosis factor signaling pathway, sphingolipid signaling pathway, and p53 signaling pathway. Banlangen has a potential pharmacological effect on COVID-19, which has the value of further exploration in the following experiment and clinical application.
Keywords: Banlangen, COVID-19, network pharmacology
1. Introduction
The novel coronavirus pneumonia broke out in Wuhan, Hubei Province, China, in December 2019 and spread rapidly throughout the world. On January 12, 2020, the pandemic was named coronavirus disease 2019 (COVID-19) by the World Health Organization.[1] In China, a total of 4,882,733 confirmed cases, 7,960,577 cured cases, and 577,375 deaths from COVID-19 were reported by 23:00 on July 15, and this data came from the website of National Health Commission of the People's Republic of China. The United States, Brazil, and Russia have emerged as the countries with the highest number of confirmed COVID-19 cases. In particular, the United States is the first country with 3,545,077 confirmed cases, 1,600,195 cured cases, and 139,143 deaths from COVID-19. The data came from the report of China Daily and Xinhua News Agency, and the data source was Johns Hopkins University in the United States. According to epidemiological investigations, the coronavirus affects males more than females. Moreover, the elderly and individuals with underlying diseases like diabetes, cancer, asthma, and heart diseases account for most of the confirmed cases, and most of them are seriously ill; that is, males with pre-existing medical conditions are more susceptible to COVID-19.[2,3]
At present, there is no specific drug for COVID-19, and the application of a brand-new drug to patients generally requires 12 to 15 years on average, so the development of new drugs from scratch will not be of much help to the current epidemic. Hence, “conventional drug in new use” is currently the most crucial treatment approach to resist COVID-19.[4] The COVID-19 Diagnosis and Treatment Program (7th trial version) recommends the integrated use of traditional Chinese medicine (TCM) and Western medicine (WM) to treat patients diagnosed with coronavirus.[5] The recommended WM include α-interferon, lopinavir/ritonavir, and ribavirin. The TCM is subdivided depending on the disease condition; that is, different prescriptions are recommended for different stages and degrees of illnesses.[5] As compared to the WM that focuses on symptomatic treatment, TCM emphasizes holism and the treatment based on syndrome differentiation.[6] There have been several large epidemic outbreaks in China's long history, and hence TCM has a rich anti-epidemic experience. According to the Chinese Epidemiological History, 321 large outbreaks were recorded since the Western Han Dynasty until the late Qing Dynasty, wherein TCM has saved innumerable lives.[7] TCM played an immense role in the battle against severe acute respiratory syndrome (SARS) in 2003.[8–10] In response to COVID-19, the National Health Commission of China has officially included TCM treatment in the third trial version of the Diagnosis and Treatment Program, and significantly expanded the content of TCM treatment to the 7th trial version.[5]
Several TCMs exhibit anti-epidemic functions, for example, Radix Isatidis (Banlangen), which could resist influenza and prevent plague.[11,12] The dry root of the cruciferous plant Isatis tinctoria, Banlangen, is a heat-clearing and detoxifying agent that can be used to treat exogenous fever, warm diseases at an initial stage, sore throat, spots induced by epidemic heat syndrome, mumps, erysipelas, and carbuncle sores. Besides strong resistance against viruses, endotoxin, bacteria, and cancer, Banlangen functions in enhancing immunity and detoxification.[13] Banlangen and its different preparations are commonly used for the clinical treatment of respiratory viral infections, viral hepatitis, and viral skin diseases.[14] Banlangen has been used in the prevention and control program for COVID-19, as Puji Disinfection Drink, Fanggan Decoction (2nd edition of the Hainan Preventive Herbal Prescription), Yinqiao Decoction (3rd edition of the Hainan Preventive Herbal Prescription), and Fanggan Decoction (Shenzhen Traditional Chinese Medicine Hospital).[15,16] Modern experimental studies have shown that water extracts, alcohol extracts, and alkaloids of Banlangen, such as epigoitrin, and alkaloid extract, exhibit strong anti-influenza effects.[17]
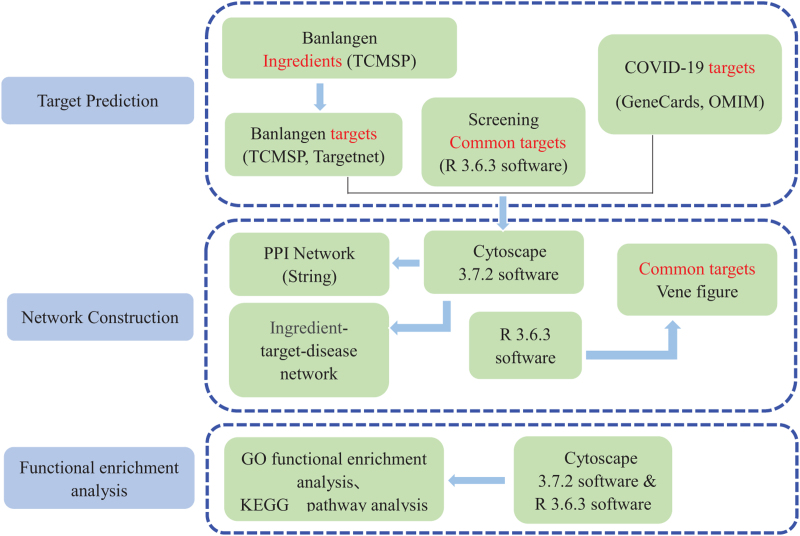
The advent of network pharmacology has enabled the development process of diseases to be viewed as the intricate mechanism of biological network balance in system biology. It allows the relationship between the body and the drug to be considered from the holistic perspective of the improvement or repair of biological network balance, which helps guide the research and development of new drugs based on multi-target therapy.[18] To date, no reports on the mechanism of action between Banlangen and COVID-19, based on using network pharmacology, have been published. Therefore, the authors conducted research to provide a more comprehensive and detailed analysis between Banlangen and COVID-19 by network pharmacology and investigated whether Banlangen has a pharmacological effect on COVID-19. The flow chart is shown in Figure 1.
Figure 1.
Flow chart of this study.
2. Material and methods
2.1. Screening of active ingredients in Banlangen and their targets
To screen the active ingredients meeting the requirements, the keyword “Banlangen” was entered into the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (http://tcmspw.com/tcmsp.php), and the condition was set to oral bioavailability >30% and drug-likeness >0.18.[19,20] The related targets in the TCMSP database were then employed to obtain corresponding targets of the active ingredients.
2.2. Screening of COVID-19 targets
The Gene Cards platform (https://www.genecards.org/) and the Online Mendelian Inheritance in Man platform (https://www.omim.org/) were used to retrieve the targets of COVID-19 using “novel coronavirus pneumonia” as the keyword. Targets of the active ingredients of Banlangen against COVID-19 were then acquired by overlapping targets of the active ingredients with the relevant targets of COVID-19 via the R 3.6.3 software.
2.3. Construction of protein–protein interaction network and active ingredient-disease-target network
The related targets of Banlangen against COVID-19 were uploaded to the STRING (https://string-db.org/) platform to explore the interaction between target proteins; the species column was set to “Homo sapiens” without combined score screening to build the protein–protein interaction (PPI) network. Next, Cytoscape 3.7.2 software was applied to construct the active ingredient of the Banlangen-disease-target network.
2.4. Gene ontology function analysis and Kyoto Encyclopedia of Genes and Genomes enrichment pathway analysis
The relevant bubble charts and bar graphs were plotted by conducting the gene ontology (GO) function analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment pathway analysis of the targets of Banlangen using Cytoscape 3.7.2 software and R 3.6.3 software with P < .05. Further, the mechanism of action of Banlangen in COVID-19 was revealed.
3. Results
3.1. Screening results of the active ingredients and targets of Banlangen
Through the TCMSP platform, 169 active ingredients of Banlangen were retrieved. After screening, based on the condition of oral bioavailability >30% and DL >0.18, 39 active ingredients were obtained, of which 33 contained available targets (92 in total). The results have been shown in Table 1.
Table 1.
Basic information on Banlangen composition.
| No. | Mol ID | Mol name | Related targets | OB% | DL |
| 1 | MOL001689 | Acacetin | NOS2, PTGS1, AR, PTGS2, PRSS1, NCOA2, NCOA1, CHEK1, ADRB2, RELA, BCL2, CDKN1A, BAX, CASP3, TP63, CASP8, FASN, FASLG | 34.97 | 0.24 |
| 2 | MOL002322 | Isovitexin | PTGS2, AR, RELA, IKBKB, TNFSF15 | 31.29 | 0.72 |
| 3 | MOL001721 | Isaindigodione | PTGS1, PTGS2, CHRM4, OPRD1, ADRA1B | 60.12 | 0.41 |
| 4 | MOL001722 | 2-O-beta-d-glucopyranosyl-2H-1,4-benzoxazin-3(4H)-one | PTGS2 | 43.62 | 0.31 |
| 5 | MOL001733 | EUPATORIN | NOS2, PTGS1, SCN5A, PTGS2, F7, PRSS1, NCOA2, NCOA1 | 30.23 | 0.37 |
| 6 | MOL001734 | 3-[[(2R,3R,5R,6S)-3,5-dihydroxy-6-(1H-indol-3-yloxy)-4-oxooxan-2-yl]methoxy]-3-oxopropanoic acid | PTGS2 | 85.87 | 0.47 |
| 7 | MOL001735 | Dinatin | NOS2, PTGS1, PTGS2, PRSS1, NCOA2, NCOA1, ACHE, RHO | 30.97 | 0.27 |
| 8 | MOL001736 | (−)-taxifolin | PTGS1, PTGS2 | 60.51 | 0.27 |
| 9 | MOL001749 | ZINC03860434 | SCN5A, CHRM3, ADRB2, CHRM1 | 43.59 | 0.35 |
| 10 | MOL001755 | 24-Ethylcholest-4-en-3-one | PGR, NR3C2 | 36.08 | 0.76 |
| 11 | MOL002311 | Quindoline | PTGS1, PTGS2, MAOB, NCOA2, PKIA | 33.17 | 0.22 |
| 12 | MOL001767 | Hydroxyindirubin | NOS2, PTGS1, ESR1, AR, PPARG, PTGS2, MAPK14, GSK3B, CCNA2 | 63.37 | 0.30 |
| 13 | MOL001771 | Poriferast-5-en-3beta-ol | PGR, NCOA2 | 36.91 | 0.75 |
| 14 | MOL001774 | Ineketone | NR3C2 | 37.14 | 0.30 |
| 15 | MOL001779 | Sinoacutine | NOS2, PTGS1, CHRM3, CHRM1, ESR1, AR, SCN5A, CHRM5, PTGS2, RXRA, OPRD1, ACHE, ADRA1B, OPRM1, ESR2 | 49.11 | 0.46 |
| 16 | MOL001781 | Indigo | PTGS1, PTGS2, RXRA, CCNA2 | 38.20 | 0.26 |
| 17 | MOL001782 | (2Z)-2-(2-oxoindolin-3-ylidene)indolin-3-one | NOS2, PTGS1, ESR1, AR, PTGS2, RXRA, GABRA1, MAPK14, GSK3B, CHEK1, CCNA2 | 48.40 | 0.26 |
| 18 | MOL001783 | 2-(9-((3-Methyl-2-oxopent-3-en-1-yl)oxy)-2-oxo-1,2,8,9-tetrahydrofuro[2,3-h]quinolin-8-yl)propan-2-yl acetate | KCNH2, PTGS2, PRSS1, NCOA2 | 64.00 | 0.57 |
| 19 | MOL001792 | DFV | PTGS1, ESR1, PTGS2, RXRA, ADRB2, MAOB, SLC6A4, PKIA | 32.76 | 0.18 |
| 20 | MOL001793 | (E)-2-[(3-indole)cyanomethylene-]-3-indolinone | NOS2, PTGS1, ESR1, AR, PTGS2, RXRA, MAPK14, GSK3B, CHEK1, CCNA2 | 54.59 | 0.32 |
| 21 | MOL001798 | neohesperidin_qt | PTGS1, SCN5A, PTGS2 | 71.17 | 0.27 |
| 22 | MOL001800 | Rosasterol | PGR | 35.87 | 0.75 |
| 23 | MOL001803 | Sinensetin | NOS2, KCNH2, AR, SCN5A, PTGS2, F7, ACHE, ADRB2, ESR2, CHEK1, PRSS1, NCOA2, NCOA1, PTGS1, ADRA1B | 50.56 | 0.45 |
| 24 | MOL001804 | Stigmasta-5,22-diene-3beta,7alpha-diol | PGR, NCOA2 | 43.04 | 0.82 |
| 25 | MOL001810 | 6-(3-Oxoindolin-2-ylidene)indolo[2,1-b]quinazolin-12-one | PTGS1, ESR1, PTGS2, KDR, PRSS1 | 45.28 | 0.89 |
| 26 | MOL001814 | (E)-3-(3,5-dimethoxy-4-hydroxy-benzylidene)-2-indolinone | PTGS1, SCN5A, PTGS, RXRA, GABRA1 | 57.18 | 0.25 |
| 27 | MOL001820 | (E)-3-(3,5-dimethoxy-4-hydroxyb-enzylidene)-2-indolinone | PTGS1, CHRM1, SCN5A, PTGS2, RXRA, ADRB2, GABRA1 | 65.17 | 0.25 |
| 28 | MOL001828 | 3-[(3,5-Dimethoxy-4-oxo-1-cyclohexa-2,5-dienylidene)methyl]-2,4-dihydro-1H-pyrrolo[2,1-b]quinazolin-9-one | PTGS1, KCNH2, SCN5A, PTGS2, F7, PRSS1 | 51.84 | 0.56 |
| 29 | MOL001833 | Glucobrassicin-1-Sulfonate_qt | ESR1, AR, PTGS2, CCNA2 | 42.52 | 0.24 |
| 30 | MOL000358 | Beta-sitosterol | PGR, NCOA2, PTGS1, PTGS2, KCNH2, CHRM3, CHRM1, SCN5A, CHRM4, ADRA1A, CHRM2, ADRA1B, ADRB2, CHRNA2, SLC6A4, OPRM1, GABRA1, BCL2, BAX, CASP9, JUN, CASP3, CASP8, PRKCA, PON1, MAP2 | 36.91 | 0.75 |
| 31 | MOL000359 | Sitosterol | PGR, NCOA2, NR3C2 | 36.91 | 0.75 |
| 32 | MOL000449 | Stigmasterol | PGR, NR3C2, NCOA2, ADH1C, RXRA, NCOA1, PTGS1, PTGS2, ADRA2A, SLC6A2, SLC6A3, ADRB2, AKR1B1, PLAU, LTA4H, MAOB, MAOA, CTRB1, CHRM3, CHRM1, ADRB1, SCN5A, ADRA1A, CHRM2, ADRA1B, GABRA1 | 43.83 | 0.76 |
| 33 | MOL000953 | CLR | PGR, NR3C2, NCOA2 | 37.87 | 0.68 |
3.2. Screening results of COVID-19 targets
The treatment targets of COVID-19 were retrieved through the Gene Cards platform, and the Online Mendelian Inheritance in Man platform and 259 related targets were obtained after deduplication. R 3.6.3 software was then utilized to obtain the confluence of the targets of active ingredients of Banlangen and the relevant targets of COVID-19. A total of 11 targets of the active ingredients of Banlangen against COVID-19 were obtained. The Venn diagram of the associated targets has been depicted in Figure 2.
Figure 2.
The Venn diagram of the associated targets of Banlangen against COVID-19. COVID-19 = coronavirus disease 2019.
3.3. Construction results of PPI network
The relevant targets of Banlangen against COVID-19 were uploaded to the STRING (https://string-db.org/) platform, and the species column was set to “Homo sapiens” with no combined score screening. A PPI network (Fig. 3) was then constructed, with 11 protein nodes, 32 interaction lines, and a PPI enrichment P value of 4.44e–16 (P < .05). Cytoscape 3.7.2 software was used to construct the active ingredient-disease-target network, and the results have been presented in Figure 4. The blue in the figure represents Banlangen, red signifies COVID-19, the yellow illustrates the active ingredients of Banlangen, while green portrays the shared genes among the two. Banlangen has a potential effect on COVID-19 through many active ingredients and multiple targets.
Figure 3.
The PPI networks of targets of the active ingredients of Banlangen. PPI = protein–protein interaction.
Figure 4.
Drug-target-disease network between Banlangen and COVID-19. COVID-19 = coronavirus disease 2019.
3.4. GO function enrichment analysis results
The GO function enrichment analysis of Banlangen was performed using R 3.6.3 software, and a total of 71 related biological processes, molecular functions, and cellular components were obtained with P < .05. The GO bar graphs and bubble charts of the first 20 entries are illustrated in Figures 5 and 6. The results show that the common targets are mainly concentrated in protein phosphatase binding, tetrapyrrole binding, the apoptotic process involving cysteine-type endopeptidase activity, death receptor binding, phosphatase binding, actin-binding, peroxidase activity, ubiquitin-protein ligase binding, ubiquitin-like protein ligase binding, transcription inhibitor binding, transcription activator binding, and antioxidant activity, to name some.
Figure 5.
Results of the GO function enrichment analysis of the targets of active ingredients of Banlangen. GO = gene ontology.
Figure 6.
Bubble diagrams of the GO function enrichment analysis of the targets of the active ingredients of Banlangen. GO = gene ontology.
3.5. KEGG pathway enrichment analysis results
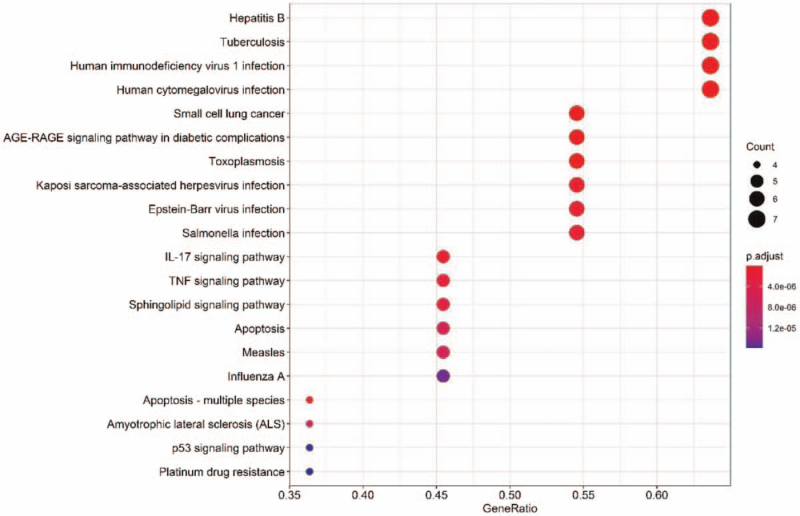
The R 3.6.3 software was used to perform the KEGG pathway enrichment analysis of Banlangen, and a total of 91 related signal pathways were obtained with P < .05. The KEGG bar charts and bubble charts of the first 20 entries are described in Figures 7 and 8. The results revealed that the common targets are mainly concentrated in hepatitis B, tuberculosis, small cell lung cancer, human immunodeficiency virus 1 infection, human cytomegalovirus infection, Epstein-Barr virus infection, salmonella infection, measles, Influenza A, and other diseases, as well as in the advanced glycation end products/a receptor for advanced glycation end products (RAGE) signaling pathway, interleukin-17 (IL-17) signaling pathway, tumor necrosis factor (TNF) signaling pathway, sphingolipid signaling pathway, and p53 signaling pathway, to name a few.
Figure 7.
KEGG pathway enrichment analysis results of the targets of active ingredients of Banlangen. KEGG = Kyoto Encyclopedia of Genes and Genomes.
Figure 8.
Bubble diagrams of the KEGG pathway enrichment analysis of the targets of active. KEGG = Kyoto Encyclopedia of Genes and Genomes.
4. Discussion
At present, COVID-19 is still in a widespread epidemic stage. No specific medication has been established for its treatment yet; therefore, the correct selection of COVID-19 drugs is crucial. TCM characteristically comprises several active substances, multiple targets, and low toxicity; it can effectively inhibit the production of viruses to cure diseases.[21] TCM has played a significant role in the prevention, control, diagnosis, and treatment of the SARS virus in 2003, Influenza A virus subtype H1N1 in 2009, and the Influenza B virus in 2012.[22–24] The COVID-19 Diagnosis and Treatment Program (7th trial version), jointly issued by the General Office of the National Health Commission of China and the General Office of the State Administration of Traditional Chinese Medicine, clearly stated that TCM has a noticeable therapeutic effect on the disease.[5] Therefore, the complete utilization of TCM's excellent resources to improve the therapeutic effects is extremely important to overcome this epidemic.
Banlangen is a classic example of a TCM drug that clears heat and removes toxicity from the body. During the outbreak of the SARS virus and Influenza A virus subtype H1N1, Banlangen played a vital role in the prevention, control, and treatment of these epidemics.[10,25] Besides, it is also a well-known drug found in the common man's home.[26] This study isolated 33 active substances and 92 therapeutic targets of Banlangen for the treatment of COVID-19. The top 3 active substances include acacetin, hydroxyindirubin, and β-sitosterol. Wu et al[27] found that acacetin possesses a significant anti-influenza virus effect and has low toxicity. Fan et al[28] proposed that acacetin could effectively protect the nerve damage against PC12-syn cells induced by MMP, and poses selective cytotoxicity to the human cancer cell line A459. Jie et al[29] also found hydroxyindirubin in Banlangen, and claimed that hydroxyindirubin could enhance the production of interferon-β by promoting the mitochondrial-mediated anti-viral signaling pathway, thus indicating that hydroxyindirubin may be a candidate drug for treating influenza virus. Kwok et al[30] discovered that hydroxyindirubin also possessed anti-inflammatory effects, inhibiting H9N2-induced cytokine production through MAPK and STAT3 signaling pathways. Additionally, Le et al[31] found that β-sitosterol plays an immunomodulatory role and could significantly suppress the proliferation of T helper cells and cytotoxic T cells. Weiliang et al[32] confirmed anti-viral, anti-inflammatory, anti-tumor, anti-immune, and other pharmacological activities of β-sitosterol.
A total of 11 targets, including PTGS1, PTGS2, PPARG, NOS2, RELA, PRKCA, CASP3, CASP8, BCL2, BAX, and MAPK14, were obtained in the PPI network. According to the interaction between the visible targets in the drug-disease-target network, it was observed that the connection node was directly proportional to the possibility of Banlangen in treating COVID-19 through this target. Among the targets, PTGS1, PTGS2, and NOS2 ranked the highest. PTGS is prostaglandin-endoperoxide synthase, also known as cyclooxygenase, and is an essential enzyme in the biosynthesis of prostaglandin. It acts as both dioxygenase and peroxidase. PTGS has 2 isozymes: constitutive PTGS1 and inducible PTGS2, which differ in the regulation of expression and tissue distribution.[33] PTGS1 regulates angiogenesis in endothelial cells and is inhibited by non-steroidal anti-inflammatory drugs (such as aspirin). PTGS1 is known to maintain tissue homeostasis by participating in cell signal transduction.[34] PTGS2 is involved in prostaglandin biosynthesis, related to inflammation and mitosis. Wang et al[35] found that PTGS1 can also regulate the osteogenesis of human adipose stem cells by regulating the NF-κB signaling pathway. Also, inhibition of PTGS1 and PTGS2 activity can effectively alleviate lung infections.[36] Studies have found that NOS2, an inducible nitric oxide synthase, is highly expressed during viral infections and inhibits arginine activity in cells that are persistently infected by the respiratory syncytial virus through Nω-hydroxy-nor-arginine. In conditioned media, Nω-hydroxy-nor-arginine increases the utilization of l-arginine, thereby down-regulating NOS2 and increasing the content of nitrite, thereby effectively reducing the viral genome replication.[37]
The GO bio-enrichment of Banlangen in the treatment of COVID-19 mainly involves protein phosphatase binding, tetrapyrrole binding, apoptotic processes comprising cysteine-type endopeptidase activity, death receptor binding, phosphatase binding, actin-binding, peroxidase activity, ubiquitin-protein ligase binding, ubiquitin-like protein ligase binding, transcription inhibitor binding, transcription activator binding, and antioxidant activity. KEGG functional enrichment analysis showed that targets of the first active substances of Banlangen are mainly involved in hepatitis B, tuberculosis, small cell lung cancer, human immunodeficiency virus 1 infection, human cytomegalovirus infection, Epstein-Barr virus infection, Salmonella infection, measles, Influenza A, and other diseases, as well as the receptor for advanced glycation end products in the complications of diabetes, IL-17, TNF, sphingolipid, p53, and other signaling pathways. Among them, 4 diseases with more than 6 targets of Banlangen, namely hepatitis B, tuberculosis, human immunodeficiency virus 1, and human cytomegalovirus infection, are involved. Signaling pathways with multiple targets of Banlangen include the RAGE signaling pathway, IL-17 signaling pathway, and TNF signal pathways, suggesting the likely role of Banlangen via the pathways mentioned above. The RAGE signaling pathway has a substantial regulatory effect on diabetes,[38] while the IL-17 and TNF signaling pathways mainly mediate inflammation and immune response.[39] Banlangen has been speculated to treat COVID-19 by inhibiting these pathways, consistent with the research outcomes of Xu et al[40] and Huang et al[41] on the treatment mechanisms of COVID-19.
It should be noted that the network pharmacology research data is based on a public database, which has limited data information and needs to be continuously improved. Moreover, this study also ignored the influence of the content and concentration of Banlangen on the disease, because only a certain amount of the drug can reach the target site to be effective. Therefore, this study needs further verification, but it is still valuable.
5. Conclusion
The present study explored the efficient active substances and related targets in Banlangen through network pharmacological methods and technologies. In summary, Banlangen regulates RAGE, IL-17, TNF, and other signaling pathways to inhibit inflammation and immune response by acting on PTGS1, PTGS2, NOS2, and other targets, thereby efficiently resisting COVID-19. Hence, Banlangen has a potential pharmacological effect on COVID-19, which has the value of further exploration in the following experiment and clinical application.
Author contributions
Bin Yu and Hong Ning conceived and designed the studies. Baodong Ling participated in this work. All authors participated in the drafting of the manuscript and revising it before final submission.
Conceptualization: Hong Ning.
Data curation: Bin Yu, Fei Lin, Hong Ning.
Funding acquisition: Baodong Ling.
Methodology: Fei Lin, Baodong Ling.
Project administration: Bin Yu, Baodong Ling.
Resources: Baodong Ling.
Software: Fei Lin.
Supervision: Fei Lin, Baodong Ling.
Validation: Hong Ning.
Visualization: Hong Ning.
Writing – original draft: Bin Yu, Hong Ning.
Writing – review & editing: Hong Ning.
Footnotes
Abbreviations: COVID-19 = coronavirus disease 2019, DL = drug-likeness, GO = gene ontology, KEGG = Kyoto Encyclopedia of Genes and Genomes, OB = oral bioavailability, OMIM = online mendelian inheritance in man, PPI = protein–protein interaction, SARS = severe acute respiratory syndrome, TCM = traditional Chinese medicine, TCMSP = Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, WM = Western medicine.
How to cite this article: Yu B, Lin F, Ning H, Ling B. Network pharmacology study on the mechanism of the Chinese medicine Radix Isatidis (Banlangen) for COVID-19. Medicine. 2021;100:32(e26881).
This work was supported by the National Natural Science Foundation of China (grant no. 81373454).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
DL = drug-likeness, OB = oral bioavailability.
References
- [1].Sifuentes-Rodríguez E, Palacios-Reyes D. COVID-19: the outbreak caused by a new coronavirus. Bol Med Hosp Infant Mex 2020;77:47–53. [DOI] [PubMed] [Google Scholar]
- [2].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hughes JP, Rees S, Kalindjian SB, et al. Principles of early drug discovery. Br J Pharmacol 2011;162:1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].General Office of National Health Commission of the People's Republic of China, Office of National Administration of Traditional Chinese Medicine. New coronavirus pneumonia diagnosis and treatment promram (trial version 7). China Med 2020;15:801–5. [Google Scholar]
- [6].Liu M, Gao Y, Yuan Y, et al. Efficacy and safety of integrated traditional chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res 2020;158:104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Qin Y, Zhao MJ, Tan YY, et al. History of influenza pandemics in China during the past century. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:1028–31. [DOI] [PubMed] [Google Scholar]
- [8].Chen Z, Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res 2004;18:592–4. [DOI] [PubMed] [Google Scholar]
- [9].Liu J, Manheimer E, Shi Y, Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Altern Complement Med 2004;10:1041–51. [DOI] [PubMed] [Google Scholar]
- [10].Xiao PG, Wang YY, Chen HS. Some research clues on Chinese herbal medicine for SARS prevention and treatment. Zhongguo Zhong Yao Za Zhi 2003;28:481–3. [PubMed] [Google Scholar]
- [11].Xiao P, Ye W, Chen J, Li X. Antiviral activities against influenza virus (FM1) of bioactive fractions and representative compounds extracted from Banlangen (Radix Isatidis). J Tradit Chin Med 2016;36:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang ZJ, Morris-Natschke SL, Cheng YY, Lee K-H, Li R-T. Development of anti-influenza agents from natural products. Med Res Rev 2020;40:2290–338. [DOI] [PubMed] [Google Scholar]
- [13].Zhou W, Zhang X-Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen). Am J Chin Med 2013;41:743–64. [DOI] [PubMed] [Google Scholar]
- [14].Zhao W-L, Jin L, Wang H-Z, et al. Production regionalization study of Isatidis Radix. Zhongguo Zhong Yao Za Zhi 2017;42:4414–8. [DOI] [PubMed] [Google Scholar]
- [15].Tong H. Commonness and individuality: a comprehensive inventory of Chinese medicine programs for the prevention and treatment of new coronary pneumonia in 31 provinces, autonomous regions and municipalities across the country. Fam Chin Med 2020;27:30–2. [Google Scholar]
- [16].Jiao Y-H, Yan Z-Q, He X-P, et al. Analysis of Fanggan Decoction in the prevention and treatment of new coronavirus pneumonia in Lingnan area. Henan J Tradit Chin Med 2020;40:660–2. [Google Scholar]
- [17].Luo Z, Liu L-F, Wang X-H, et al. Epigoitrin, an alkaloid from Isatis indigotica, reduces H1N1 infection in stress-induced susceptible model in vivo and in vitro. Front Pharmacol 2019;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu C-W, Lu L, Liang S-W, Chen C, Wang SM. Application of drug-target prediction technology in network pharmacology of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2016;41:377–82. [DOI] [PubMed] [Google Scholar]
- [19].Zheng W, Wu J, Gu J, et al. Modular characteristics and mechanism of action of herbs for endometriosistreatment in Chinese medicine: a data mining and network pharmacology-based identification. Front Pharmacol 2020;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Z, Yi P, Yang J, et al. Integrated network pharmacology analysis and serum metabolomics to reveal the cognitive improvement effect of Bushen Tiansui formula on Alzheimer's disease. J Ethnopharmacol 2020;249:112371. [DOI] [PubMed] [Google Scholar]
- [21].Lan S, Duan J, Zeng N, et al. Network pharmacology-based screening of the active ingredients and mechanisms of Huangqi against aging. Medicine (Baltimore) 2021;100:e25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen LL, Lin CJ, Chang ML, Lin JD. SARS prevention and nursing in traditional Chinese medicine. Hu Li Za Zhi 2004;51:32–8. [PubMed] [Google Scholar]
- [23].Li JH, Wang RQ, Guo WJ, Li JS. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): a meta-analysis. J Chin Med Assoc 2016;79:281–91. [DOI] [PubMed] [Google Scholar]
- [24].Wang J. Observation of the therapeutic effect and prognosis analysis of Yiqijiebiao prescription for the treatment of children with influenza B. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:1310–4. [DOI] [PubMed] [Google Scholar]
- [25].Li Z, Li L, Zhou H, et al. Radix isatidis polysaccharides inhibit influenza A virus and influenza A virus-induced inflammation via suppression of host TLR3 signaling in vitro. Molecules 2017;22:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu H, Li TN, Ran Q, Huang QW, Wang J. Strobilanthes cusia (Nees) Kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: a comprehensive review. J Ethnopharmacol 2021;265:113325. [DOI] [PubMed] [Google Scholar]
- [27].Wu Q, Yu C, Yan Y, Chen J, Zhang C, Wen X. Antiviral flavonoids from Mosla scabra. Fitoterapia 2010;81:429–33. [DOI] [PubMed] [Google Scholar]
- [28].Fan X, Lin S, Zhu C, et al. Aromatic constituents of Heteroplexis micocephal and their bioactivities. Zhongguo Zhong Yao Za Zhi 2011;36:48–56. [PubMed] [Google Scholar]
- [29].Jie C, Luo Z, Chen H, et al. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget 2017;8:105615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kwok H-H, Poon P-Y, Fok S-P, et al. Anti-inflammatory effects of indirubin derivatives on influenza A virus-infected human pulmonary microvascular endothelial cells. Sci Rep 2016;6:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Le C-F, Kailaivasan TH, Chow S-C, Abdullah Z, Ling SK, Fang CM. Phytosterols isolated from Clinacanthus nutans induce immunosuppressive activity in murine cells. Int Immunopharmacol 2017;44:203–10. [DOI] [PubMed] [Google Scholar]
- [32].Weiliang L, Yu J, Aixiang H. Research and development progress of β-sitosterol. Farm Prod Process 2019;1:77–9. [Google Scholar]
- [33].Agundez JA, Gonzalez-Alvarez DL, Vega-Rodriguez MA, Botello E, Garcia-Martin E. Gene variants and haplotypes modifying transcription factor binding sites in the human cyclooxygenase 1 and 2 (PTGS1 and PTGS2) genes. Curr Drug Metab 2014;15:182–95. [DOI] [PubMed] [Google Scholar]
- [34].Chan MV, Hayman MA, Sivapalaratnam S, et al. Identification of a homozygous recessive variant in PTGS1 resulting in a congenital aspirin-like defect in platelet function. Haematologica 2021;106:1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Y, Liu Y, Zhang M, et al. Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling. Stem Cell Res Ther 2019;10:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Soni KK, Meshram D, Lawal TO, Patel U, Mahady GB. Fractions of Boswellia serrata suppress LTA4, LTC4, cyclooxygenase-2 activities and mRNA in HL-60 cells and reduce lung inflammation in BALB/c mice. Curr Drug Discov Technol 2021;18:95–104. [DOI] [PubMed] [Google Scholar]
- [37].Santiago-Olivares C, Rivera-Toledo E, Gómez B. Nitric oxide production is downregulated during respiratory syncytial virus persistence by constitutive expression of arginase 1. Arch Virol 2019;164:2231–41. [DOI] [PubMed] [Google Scholar]
- [38].Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol 2017;39:365–83. [DOI] [PubMed] [Google Scholar]
- [39].Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 2011;1243:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu D, Xu Y, Wang Z, et al. Mechanism of Qingfei paidu decoction on COVID-19 based on network pharmacology. Pharmacol Clin Chin Materia Medica 2020;36:26–32. [Google Scholar]
- [41].Huang L, Wang J, Xu R, et al. Study on mechanism of Huanglian Jiedu decoction in treating novel coronavirus pneumonia based on network pharmacology. J Chin Med Mater 2020;3:779–85. [Google Scholar]