Abstract
Background:
Adjuvants to local anesthetics, such as nalbuphine and dexmedetomidine, can be used to improve the quality and duration of peripheral nerve block effects. Dexmedetomidine has been successfully used as an adjuvant of erector spinae plane block (ESPB) with ropivacaine in video-assisted thoracoscopic lobectomy surgeries (VATLS). This study aimed to compare the effects of nalbuphine and dexmedetomidine used as adjuvants to ropivacaine for ESPB in VATLS.
Methods:
A total of 102 patients undergoing VATLS with ESPB were enrolled and randomized into 3 groups, each of which received a different adjuvant to ropivacaine. The visual analogue scale score, onset and duration of sensory block, use of patient-controlled analgesia (PCA), rate of rescue analgesia, duration of postoperative hospitalization, incidence of postoperative nausea and vomiting, and chronic pain were measured and observed.
Results:
The visual analogue scale score, total PCA use, rate of rescue analgesia, and postoperative chronic pain in the ropivacaine with dexmedetomidine (RD), and ropivacaine with nalbuphine (RN) groups were lower than those in the ropivacaine (RC) group (P < .05). The duration of sensory block was longer and the first use of PCA occurred later in the RD and RN groups than they did in the RC group (P < .05).
Conclusions:
As an adjuvant to ropivacaine in ESPB, nalbuphine and dexmedetomidine are comparable in terms of the associated analgesia, sensory block duration, need for rescue analgesia, and incidence of chronic pain in patients after VATLS.
Keywords: adjuvant, analgesia, dexmedetomidine, erector spinae plane block, nalbuphine
1. Introduction
Video-assisted thoracoscopic lobectomy surgery (VATLS) is increasingly common in the treatment of early stage non-small cell lung cancer,[1,2] as it involves a minimally invasive approach to advanced resections.[3] VALTS does not require the ribs to be spread and the incisions involved are relatively small, which may account for the decreased pain experienced by patients.[4] However, postoperative pain management, particularly early postoperative pain, remains a matter of concern for anesthesiologists and thoracic surgeons.[5] Erector spinae plane block (ESPB) is a novel posterior thoracic wall block that the local anesthetics is injected locally in the deep erector spinae muscle surface, as a part of multimodal analgesia. Given that erector spinae muscles anatomically situate along the thoracolumbar spine, ESPB promotes an extensive craniocaudal spread.[6,7] Previous studies have shown that ESPB can provide effective analgesia in breast, chest, and abdominal surgery, and in thoracotomy.[8–10] Ultrasound is a non-invasive visualization technology that helps capture the anatomical structure of target tissues; it can help guide the direction and depth of anesthesia puncture needles, thus reducing the risk of complications.[11,12] Adjuvants to local anesthetics, such as nalbuphine[10,13] and dexmedetomidine,[10,14] may improve the quality and duration of peripheral nerve block effects. Dexmedetomidine is a potent α2 agonist, emerging as an adjuvant to regional anesthesia and analgesia, as it may prolong and enhance the analgesic effect of the epidural,[15] caudal,[16] subarachnoid,[17] brachial plexus,[18] and paravertebral block.[14,19] Recently, Gao et al[5] have shown that dexmedetomidine may be used as an adjuvant to ESPB with ropivacaine, achieving prolonged sensory block duration, providing effective acute pain control after surgery, and reducing the need for rescue analgesia in VATLS.
Nalbuphine is an opioid agonist-antagonist of the phenanthrene series, associated with analgesia without side effects such as respiratory depression, which may occur with the use of pure agonists; in addition, its analgesic and some anti-pruritic effects are mediated by the μ and κ receptors.[20] Moreover, it has been used safely and successfully as epidural,[21] intrathecal,[22] and brachial plexus block.[23] Whether nalbuphine can be successfully used as an adjuvant to ESPB with ropivacaine and improve analgesia in a manner similar to that associated with dexmedetomidine remains unclear. This study aimed to compare the effects of nalbuphine and dexmedetomidine as adjuvants to ropivacaine for ESPB in VATLS.
2. Methods
This study was registered at the Chinese Clinical Trial Registry on July 13, 2019 (ChiCTR1900024498) and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Anhui, China) on December 10, 2019. This study adhered to the CONSORT guidelines and other relevant institutional guidelines and governmental regulations. The authors take full responsibility for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This randomized, controlled, double-blind study enrolled patients scheduled for VATLS at the First Affiliated Hospital of Anhui Medical University (Hefei, China); all patients provided written informed consent. Patients were eligible for inclusion in the present study if they met the following criteria: American Society of Anesthesiologists physical status I or II, age 29 to 75 years, body mass index of ≤35 kg/m2, and scheduled for VATLS. Patients were excluded from the present study if they met the following criteria: chronic pain drug use, alcohol or drug abuse, any contraindications for ESPB, refused consent, infection at the site of injection, and diagnosis of psychiatric disorders, bradycardia, cardiac conduction block, significant cardiopulmonary disease, or uncontrolled diabetes mellitus, or body mass index of >35 kg/m2.
A total of 102 patients undergoing surgery were divided into 3 groups (n = 34 per group), using a computer-generated random numbers table. A staff anesthesiologist, who was not involved in the study, prepared the injectate, based on group assignment. Study investigators were blinded to group assignment. Patients were trained to use a 10-point visual analog pain scale (VAS) (0 points, no pain; 10 points, maximum pain imaginable) to assess pain intensity,[24] and to use a patient-controlled analgesia (PCA) device.[25] Anesthesia technique was the same for all participating patients.
2.1. Anesthesia and perioperative treatment
When patients were transferred into the operating room, peripheral intravenous (IV), right internal jugular vein, and radial artery catheters were placed. Electrocardiogram, invasive blood pressure, central venous pressure, heart rate, pulse oximetry, and the bispectral index (BIS) (Vista; Aspect Medical Systems Inc., Norwood, MA) were monitored throughout surgery.[26] ESPB was performed in the standard lateral position before general anesthesia was administered. Ultrasound imaging was performed, using SonoSite M-Turbo (Bothell, WA). All blocks were performed with a linear transducer (3–13 MHz, SL1543). Transducers were enclosed in a sterile plastic cover. All injections were administered with a 22 G, 120-mm needle (stimuplex D; B. Braun Melsungen AG, Melsungen, Germany), inserted by an in-plane technique until the tip reached the interfacial plane deep within the erector spinae muscle, after standard skin disinfection (Figs. 1 and 2). In all patients, 30 mL of the drug was injected into the corresponding surgical posterior hemithorax at the level of T5 for ESPB.[6] The RC group (n = 34) received 0.5% ropivacaine at a dose of 30 mL; the RD group (n = 34) received dexmedetomidine (1 μg/kg) and 0.5% ropivacaine at a dose of 30 mL; the RN group (n = 34) received 20 mg nalbuphine and 0.5% ropivacaine at a dose of 30 mL. The sensory block of the 5th intercostal space in the midaxillary line was assessed by testing bilateral cold perception for 30 minutes after applying the nerve block. Patients were excluded from the study if the sensory blockade was unsuccessful. The time of sensory block onset was the time from the end of block drug injection to the onset of sensory block.
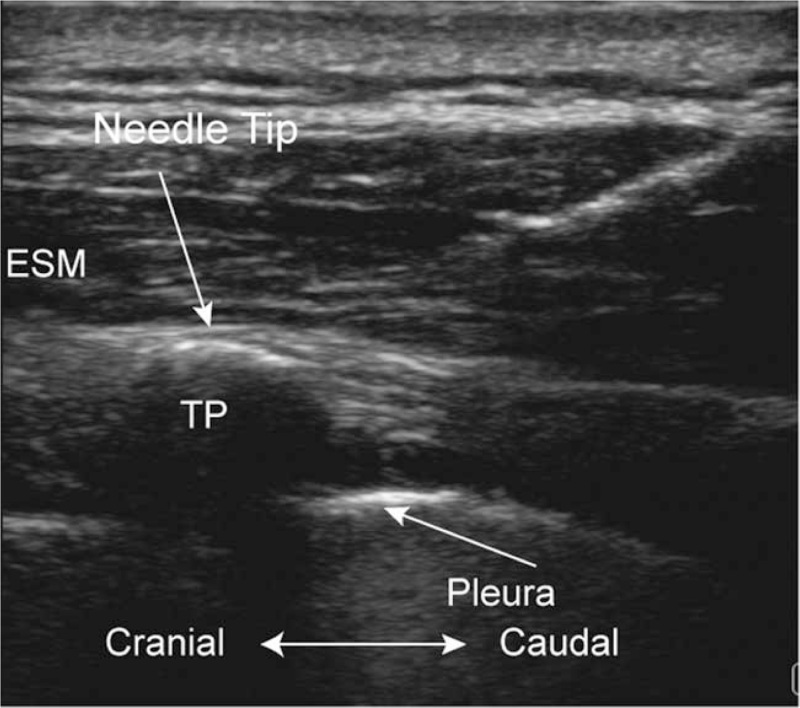
Figure 1.
Ultrasound image taken before the erector spinae plane block (ESPB). ESM = erector spinae muscle, TP = transverse process.
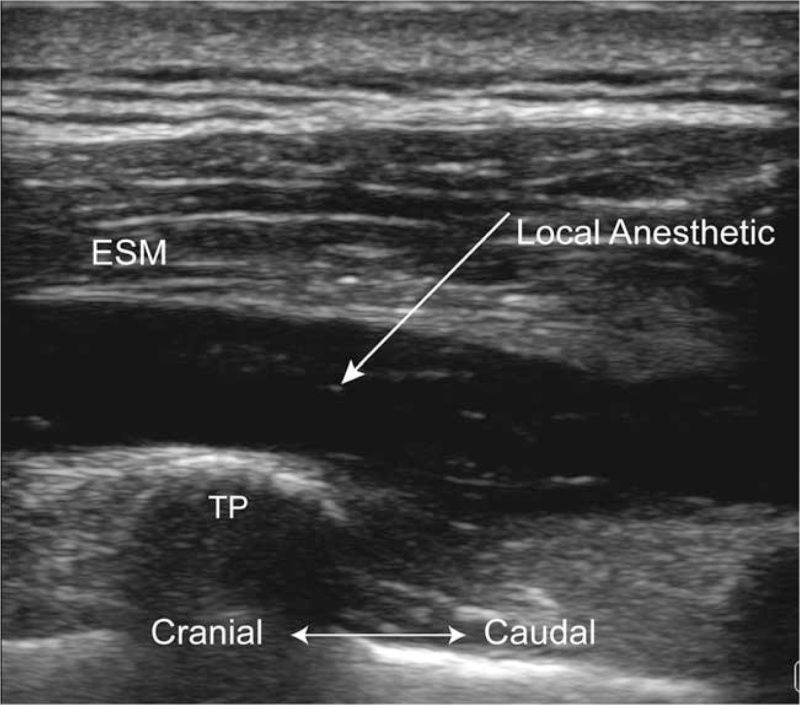
Figure 2.
Ultrasound image taken after the erector spinae plane block (ESPB). ESM = erector spinae muscle, TP = transverse process.
All patients underwent a standard general anesthesia protocol: propofol (Diprivan; AstraZeneca plc, London, UK) was injected with a target-controlled infusion (Graseby 3500; Smiths Medical, Wat -ford, UK) during anesthetic induction. With an initial target concentration of 1.0 μg/mL, the concentration progressively increased by 0.3 μg/mL until the BIS value reached 40 to 60, when 0.03 mg/kg midazolam and 0.5 μg/kg sufentanil were injected (IV). Rocuronium bromide (0.9 mg/kg) was used to facilitate double-lumen endobronchial intubation. After tracheal intubation, patients were ventilated with 100% oxygen, and a volume-controlled ventilator was used; tidal volume was set to 8 mL/kg of the ideal body weight, an inspiratory-to-expiratory ratio was set to 1:2, and respiratory frequency was set to 10 breaths/min. Propofol and remifentanil were continuously infused to maintain anesthesia; sufentanil and cisatracurium were injected, as required. BIS values were maintained in the range of 40 to 60 throughout surgery by changing the effect-site concentration of propofol. The ventilation mode was switched to one-lung ventilation at the time of skin incision, and maintained until lobectomy was completed; ventilator parameters were adjusted to maintain satisfactory pulse oximetry and end-tidal carbon dioxide values throughout the procedure.
Propofol and remifentanil administration was discontinued, when the last skin suture was completed. Neostigmine (20 μg/kg) and atropine (5–10 μg/kg) were administered, as required, according to tidal volume and frequency, to reverse residual muscle relaxation at the end of surgery. Patients were admitted to the post-anesthesia care unit (PACU) after spontaneous breathing was recovered. Patients were extubated in the PACU according to a standard extubation protocol and subjects were moved to the ward when a Steward recovery score exceeded 4 points. At the end of surgery, sufentanil (0.1–0.2 μg/kg) was administered before the PCA pump was set up. The PCA capacity was 150 mL and contained 4.5 μg/kg sufentanil and 150 mg flurbiprofen. The infusion rate was maintained at 2 mL/h, and the patient-controlled bolus was 2 mL with a lockout interval of 15 minutes. Patients could press for an additional bolus, when their VAS pain scores were ≥3 points; the first-time request for pressing PCA was recorded. When the VAS score remained ≥4 points after PCA delivery, patients received intramuscular injection of tramadol 100 mg as rescue analgesia. Cold perception test in comparison with the contra-lateral intercostal area was performed. The duration of sensory block was the time from the end of block drug injection to the point in time where any differences in cold perception between both sides disappeared.
Throughout anesthesia, the mean arterial pressure (MAP) was maintained between –20% and +20% of the baseline value. Hypotension was defined as a 20% decrease below the baseline the MAP or MAP of <60 mm Hg, lasting >30 seconds. Phenylephrine (40 μg, IV) was given when fluid therapy was not appropriate. Atropine (0.3 mg, IV) was given for bradycardia, which was defined as heart rate of <60 bpm. Ephedrine (3–6 mg, IV) was given to treat bradycardia and hypotension. The primary endpoint was PCA use during the first 72 hours postoperatively. Secondary outcomes included: timing of the first-time request for PCA use; timing of sensory block onset; duration of sensory block; VAS pain scores at various time points (awakening in the PACU and 2, 4, 6, 8, 12, 24, 48, 72 hours after surgery); consumption of sufentanil, remifentanil, and propofol during anesthesia; incidence of postoperative nausea and vomiting (PONV) and rescue analgesia use in the ward and the hospital stay after surgery; incidence of chronic pain 3 months postoperatively, assessed during a telephone follow-up interview.[5]
2.2. Statistical analysis
Data analysis was performed in SPSS, version 17.0 (SPSS Inc., Chicago, IL). Sample size calculations were performed with an online sample size calculator, using values derived from our previous pilot study. This previous study has shown a decreased mean effective PCA pressing frequency among patients under general anesthesia combined with ESPB, using ropivacaine with dexmedetomidine and ropivacaine with nalbuphine (2.8 ± 2.9 and 2.2 ± 1.5, respectively), compared with that of patients undergoing general anesthesia combined with ESPB using ropivacaine (8.8 ± 5.1) at 72 hours after surgery. To detect differences in PCA use 72 hours postoperatively with an SD of 4.5, the sample size required was 26 patients per group at a power of 80% and a two-tailed α-error of 5%. We enrolled 102 patients in total (N = 34/group) to account for potential dropouts. The Kolmogorov–Smirnov test was used to determine the normality of data distribution. Continuous variables were reported as mean ± standard deviation, and median (25th–75th percentiles), and categorical variables were reported as counts (percentages). The homogeneity of variance test was performed for variables that showed evidence of normal distribution; one-way ANOVA was used for variance analysis, and the least significant difference test was used for subsequent inter-group comparisons. The Kruskal–Wallis test was used to examine continuous variables that did not follow a normal distribution. Categorical variables were compared, using the chi-squared or Fisher exact tests P-values of <.05 were considered indicative of a statistically significant finding.
3. Results
The study flow is depicted in Fig. 3. The patients’ characteristics are presented in Table 1. There was no significant difference in patient or intraoperative characteristics among the groups, including sex, age, body mass index, surgery duration, or the consumption of sufentanil, remifentanil, or propofol.
Figure 3.
Flowchart of the study. RC, 0.5% ropivacaine; RD, 0.5% ropivacaine, and 1 μg/kg dexmedetomidine; RN, 0.5% ropivacaine and 20 mg nabluphine.
Table 1.
Patient characteristics and intraoperative data.
| Variables | Group RC (n = 32) | Group RD (n = 33) | Group RN (n = 30) | P value |
| Gender | .992 | |||
| Male | 15 (46.9%) | 15 (45.5%) | 14 (46.7%) | |
| Female | 17 (53.1%) | 18 (54.5%) | 16 (53.3%) | |
| Age, yr | 56.2 (9.8) | 56.0 (9.6) | 54.8 (9.9) | .838 |
| BMI, kg/m2 | 23.8 (2.8) | 23.5 (2.9) | 22.6 (3.0) | .277 |
| Duration of surgery, min | 177.5 (57.1) | 173.8 (50.5) | 164.8 (51.5) | .638 |
| Consumption of sufentanil, μg | 44.7 (9.4) | 46.3 (7.6) | 48.6 (8.7) | .226 |
| Consumption of remifentanil, mg | 1.7 (0.6) | 1.5 (0.5) | 1.6 (0.5) | .462 |
| Consumption of propofol, mg | 771.1 (306.6) | 788.0 (213.5) | 761.5 (285.2) | .927 |
Sensory block onset in the RD group occurred sooner than it did in the RN or RC groups (Table 2). In addition, sensory block duration was prolonged and first-time PCA use was delayed in both RD and RN groups, compared with those in the RC group. Total PCA use and the requirement for rescue analgesia in the RD and RN groups were reduced, compared with those in the RC group. There was no significant difference in the duration of postoperative hospitalization or the incidence of PONV among the groups. The incidence of postoperative chronic pain was lower in the RN and RD groups than in the RC group (3 months post-VATLS).
Table 2.
Postoperative analgesia and postoperative hospital stays.
| Variables | Postoperative time | Group RC (n = 32) | Group RD (n = 33) | Group RN (n = 30) | P value |
| Onset time of sensory block, min | 10.9 (3.0) | 6.2 (1.9)∗,∗∗ | 8.9 (3.6) | <.001 | |
| Duration of sensory block, h | 8.9 (5.9) | 16.0 (4.0)∗,∗∗ | 14.1 (4.5)∗ | <.001 | |
| First time request for PCA use, h | 14.5 (9–20) | 23 (14–33)∗ | 21 (15–42)∗ | .001 | |
| Sum of effective pressing numbers | 72 h | 7 (5–10) | 2 (1–3)∗ | 2 (1–4)∗ | <.001 |
| Postoperative stay in hospital, d | 6 (4–7.5) | 5 (4–6) | 5 (4–6) | .116 | |
| Rescue analgesia | 10 (31.3%) | 2 (6.1%)∗ | 2 (6.7%)∗ | .005 | |
| PONV | 5 (15.6%) | 5 (15.2%) | 7 (23.3%) | .642 | |
| Chronic pain | 8 (25.0%) | 1 (3.0%)∗ | 1 (3.3%)∗ | .005 |
The VAS scores were significantly lower in the RD and RN groups than in the RC group at postoperative 4, 6, 8, 12, 24, 48, 72 hours at rest and at 2, 4, 6, 8, 12, 48 hours during coughing. In addition, the VAS scores were significantly lower in the RD group at postoperative 24 and 72 hours during coughing than they were in the RN and RC groups (Table 3).
Table 3.
The visual analogue scale (VAS) score at varied points in resting and cough.
| Variables | Postoperative time | Group RC (n = 32) | Group RD (n = 33) | Group RN (n = 30) | P value |
| VAS in resting | Wake up | 0 (0–0) | 0 (0–0) | 0 (0–0) | .067 |
| 2 h | 0 (0–0) | 0 (0–0) | 0 (0–0) | .047 | |
| 4 h | 0 (0–0.75) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 6 h | 0 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 8 h | 0 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 12 h | 1 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 24 h | 1 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 48 h | 1 (0–1.75) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 72 h | 1 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| VAS in cough | Wake up | 0 (0–0) | 0 (0–0) | 0 (0–0) | .067 |
| 2 h | 0 (0–0) | 0 (0–0)∗ | 0 (0–0)∗ | .001 | |
| 4 h | 0 (0–1) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 6 h | 0 (0–1.75) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 8 h | 0 (0–2) | 0 (0–0)∗ | 0 (0–0)∗ | <.001 | |
| 12 h | 1 (0–1) | 0 (0–0)∗ | 0 (0–2)∗ | .002 | |
| 24 h | 2 (1–4) | 2 (1–2)∗,∗∗ | 2 (2–2.25) | .014 | |
| 48 h | 2 (0–4) | 1 (1–2)∗ | 2 (1–2)∗ | .029 | |
| 72 h | 2 (1–3) | 1 (0–1)∗,∗∗ | 2 (0.75–2) | <.001 |
4. Discussion
VATLS was introduced >20 years ago, providing a less traumatic alternative to thoracotomy, reducing postoperative pain, perioperative bleeding, and duration of hospitalization, facilitating the recovery of normal activities.[3] However, postoperative pain management, in particular, early postoperative pain, remains a concern for anesthesiologists and thoracic surgeons.[5] ESPB is a fascial plane block, which can be injected into the deep erector spinae muscle surface as an anesthetic, effectively relieving the pain associated with chest and abdomen surgery,[7,27] including VATLS. However, admitting patients with peripheral catheters is neither feasible nor desirable; thus, there remains a need to extend the analgesic effect of the single-shot nerve block into the postoperative period. Previous clinical studies have shown that the use of adjuvants with local anesthetics, for example, dexamethasone, dexmedetomidine, and nalbuphine can improve the quality and duration of the sensory block, and help prevent toxicity. Dexmedetomidine has been used successfully as an adjuvant to ESPB with ropivacaine, prolonging sensory block, providing effective acute pain control after surgery, and reducing the need for rescue analgesia in VATLS. The present study is first to examine whether nalbuphine can be successfully used as an adjuvant to ESPB with ropivacaine for improving analgesia.
Our study has shown that the addition of 20 mg nalbuphine to 0.5% ropivacaine in ESPB can provide effects comparable to those of 1 μg/kg dexmedetomidine, concurrently improving analgesia, postoperative pain levels, sensory block duration, and delaying and reducing the need for PCA use, relative to those observed with the use of 0.5% ropivacaine alone in patients undergoing VATLS.
Dexmedetomidine is a highly selective α2 receptor agonist. Previous studies have proposed candidate mechanisms of the improved blockade efficacy associated with dexmedetomidine use. First, dexmedetomidine may interact with local anesthetics. Dexmedetomidine may cause vasoconstriction around the site of injection, which delays the absorption of the local anesthetic and prolongs its effect. Second, perineural dexmedetomidine may directly affect peripheral nerve activity and attenuate acute local anesthetic-induced perineural inflammation without causing nerve damage, while blocking the hyperpolarization-activated cation current. Finally, dexmedetomidine itself has analgesic effects and analgesic-sparing properties; the involvement of peripheral α2A-ARs receptors may account for the effect of dexmedetomidine as a peripheral nerve block. Presynaptic α2 adrenoceptor activation inhibits the release of a transmitter from the primary afferent fibers. Postsynaptic α2 adrenoceptor stimulation at the level of the spinal cord increases acetylcholine concentrations in the superficial dorsal horn and inhibits nociceptive neurotransmission by reducing the release of neurotransmitters such as substance P and glutamate.[28]
Nalbuphine is chemically related to both the agonist analgesic oxymorphone and the antagonist naloxone, and acts as an antagonist of the μ receptor and as an agonist of the κ receptor, resulting in analgesia and sedation with minimal effects on the cardiovascular and respiratory systems.[29] Previous studies examined the mechanisms of nalbuphine that may improve blockade efficacy. First, nalbuphine may act synergistically with ropivacaine. Second, given the characteristics of nalbuphine, which acts on opioid receptors, the inhibition of neuronal serotonin uptake may be involved, leading to the augmentation of the spinal inhibitory pathways for pain. However, other mechanisms may be involved and should be examined in future studies.
Some studies on nalbuphine as an adjuvant to ropivacaine have reported improved quality and duration of analgesia without serious side effects. Borah et al[30] found that nalbuphine as an adjuvant to ropivacaine in elective lower limb surgery provides prolonged analgesia and a reliable sensory block with satisfactory efficacy and fewer side effects. Meanwhile, Yadav et al[31] found that nalbuphine significantly extends the duration of analgesia of the brachial plexus block under supraclavicular approach, when used with 0.75% ropivacaine; no adverse effects were observed. Mavaliya et al. found that nalbuphine prolongs the duration of the sensory block and postoperative analgesia, compared to those observed with the use of fentanyl as an intrathecal adjuvant to 0.75% isobaric ropivacaine for subarachnoid block.[32] In the present study, intraoperative analgesic effects were similar across protocols; however, postoperative analgesic effects differed among groups. There was no difference in the use of intraoperative analgesics among groups. This finding may be due to the double-blind design of this study, and the fact that the dose of intraoperative analgesics was determined, based on body weight. Meanwhile, the amount of postoperative analgesics required in the adjuvant groups was reduced, suggesting that the use of adjuvants may prolong the duration of analgesia and relieve hyperalgesia.[33]
Our research also has some limitations. The primary outcome is the effective pressing number of PCA pump which is not as good as postoperative PCA consumption. Because postoperative PCA consumption is more intuitive. The pain level and sensory blockade assessments were performed, using patient-reported measures; no objective measures were used to assess these outcomes. The present study was a small randomized double-blind trial, designed to inform clinical practice. However, few previous studies have investigated the mechanisms associated with peripheral dexmedetomidine and nalbuphine use in ESPB. Further studies are required to elucidate the mechanism and optimal doses of dexmedetomidine and nalbuphine for use as adjuvants in clinical practice.
5. Conclusion
The present findings suggest that nalbuphine and dexmedetomidine, used as local anesthetic adjuvants to ESPB, provide comparable acute pain control, prolong sensory block, reduce the need for rescue analgesia, and decrease the incidence of chronic pain in patients recovering from VATLS.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Yiqiao Wang, Yuanhai Li.
Data curation: Jin Rao.
Formal analysis: Jin Rao, Zhixin Gao, Qing Wang, Yiqiao Wang.
Funding acquisition: Weiwei Zhong, Yiqiao Wang.
Investigation: Jin Rao, Qing Wang.
Methodology: Jin Rao, Qing Wang.
Project administration: Jin Rao, Pei Gao, Qing Wang.
Resources: Jin Rao, Pei Gao.
Software: Jin Rao, Pei Gao.
Supervision: Jin Rao, Pei Gao.
Validation: Jin Rao, Pei Gao.
Visualization: Jin Rao, Pei Gao.
Writing – original draft: Jin Rao, Gaolin Qiu.
Writing – review & editing: Jin Rao, Zhixin Gao, Gaolin Qiu, Weiwei Zhong, Yiqiao Wang, Yuanhai Li.
Footnotes
Abbreviations: BIS = bispectral index, ESPB = erector spinae plane block, MAP = mean arterial pressure, PACU = post-anesthesia care unit, PCA = patient-controlled analgesia, RC = ropivacaine, RD = ropivacaine with dexmedetomidine, RN = ropivacaine with nalbuphine, VAS = visual analog scale, VATLS = video-assisted thoracoscopic lobectomy surgeries.
How to cite this article: Rao J, Gao Z, Qiu G, Gao P, Wang Q, Zhong W, Wang Y, Li Y. Nalbuphine and dexmedetomidine as adjuvants to ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Medicine. 2021;100:32(e26962).
JR and ZG have contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (Beijing, China; grant Nos. 82001185 to Weiwei Zhong); Natural Science Foundation of Anhui Provincial Department of Education (Anhui, China; grant Nos. kj2019A1111 to Yiqiao Wang); Research Fund of Anhui Medical University (Anhui, China; grant Nos. 2019xkj246 to Yiqiao Wang).
Trial registration: This trial was registered at the Chinese Clinical Trial Registry (data of registration, 13/07/2019, ChiCTR1900024498).
Availability of data and materials: The datasets generated and analyzed to support the findings of this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate: The study was registered at the Chinese Clinical Trial Registry on 13/07/2019(ChiCTR1900024498) and was approved by Institutional Ethics Committee (The First Affiliated Hospital of Anhui Medical University Ethics Committee, kuai 10/12/2019, Anhui, China) and written informed consents have been obtained from all patients.
Consent for publication: Not applicable.
The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The datasets generated during and/or analyzed during the current study are publicly available.
Data represent mean (SD) or number (%). P < .05 is considered as a statistically significant difference. RC, 0.5% ropivacaine; RD, 0.5% ropivacaine, and 1 μg/kg dexmedetomidine; RN, 0.5% ropivacaine and 20 mg nalbuphine. BMI = body mass index.
Data are presented as mean (SD), median (interquartile range) or number (%). PONV = postoperative nausea and vomiting.
P < .05 compared with the Group RC.
P < .05 compared with the Group RN, RC, 0.5% ropivacaine; RD, 0.5% ropivacaine and 1 μg/kg dexmedetomidine; RN, 0.5% ropivacaine and 20 mg nalbuphine.
Data are presented as median (interquartile range). P < .05 is considered as a statistically significant difference.
P < .05 compared with the Group RC.
P < .05 compared with the Group RN. RC, 0.5% ropivacaine; RD, 0.5% ropivacaine and 1 μg/kg dexmedetomidine; RN, 0.5% ropivacaine and 20 mg nalbuphine.
References
- [1].Yun JK, Park I, Kim HR, et al. Long-term outcomes of video-assisted thoracoscopic lobectomy for clinical N1 non-small cell lung cancer: a propensity score-weighted comparison with open thoracotomy. Lung Cancer 2020;150:201–8. [DOI] [PubMed] [Google Scholar]
- [2].Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705–9. [DOI] [PubMed] [Google Scholar]
- [3].Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- [4].Berfield KS, Farjah F, Mulligan MS. Video-assisted thoracoscopic lobectomy for lung cancer. Ann Thorac Surg 2019;107:603–9. [DOI] [PubMed] [Google Scholar]
- [5].Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med 2019;7:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 2016;41:621–7. [DOI] [PubMed] [Google Scholar]
- [7].El-Boghdadly K, Pawa A. The erector spinae plane block: plane and simple. Anaesthesia 2017;72:434–8. [DOI] [PubMed] [Google Scholar]
- [8].Hamed MA, Goda AS, Basiony MM, Fargaly OS, Abdelhady MA. Erector spinae plane block for postoperative analgesia in patients undergoing total abdominal hysterectomy: a randomized controlled study original study. J Pain Res 2019;12:1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med 2019;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hamilton D, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth 2017;118:474–5. [DOI] [PubMed] [Google Scholar]
- [11].Chiu YH, Chang KV, Chen IJ, Wu WT, Özçakar L. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis. Eur Radiol 2020;30:6663–72. [DOI] [PubMed] [Google Scholar]
- [12].Chang PH, Chen YJ, Chang KV, Wu WT, Özçakar L. Ultrasound measurements of superficial and deep masticatory muscles in various postures: reliability and influencers. Sci Rep 2020;10:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jyothi B, Gowda S, Shaikh SI. A comparison of analgesic effect of different doses of intrathecal nalbuphine hydrochloride with bupivacaine and bupivacaine alone for lower abdominal and orthopedic surgeries. Ind J Pain 2014;28:18. [Google Scholar]
- [14].Mohamed SA, Fares KM, Mohamed AA, Alieldin NH. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician 2014;17:E589–98. [PubMed] [Google Scholar]
- [15].Bajwa SJS, Arora V, Kaur J, Singh A. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth 2011;5:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009;103:268–74. [DOI] [PubMed] [Google Scholar]
- [17].Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol 2011;27:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 2014;39:37–47. [DOI] [PubMed] [Google Scholar]
- [19].Sinha S, Mukherjee M, Chatterjee S, et al. Comparative study of analgesic efficacy of ropivacaine with ropivacaine plus dexmedetomidine for paravertebral block in unilateral renal surgery. Anaesth Pain Intensive Care 2012;16:38–42. [Google Scholar]
- [20].Zacny JP, Conley K, Marks S. Comparing the subjective, psychomotor and physiological effects of intravenous nalbuphine and morphine in healthy volunteers. J Pharmacol Exp Ther 1997;280:1159–69. [PubMed] [Google Scholar]
- [21].Chatrath V, Attri JP, Bala A, Khetarpal R, Ahuja D, Kaur S. Epidural nalbuphine for postoperative analgesia in orthopedic surgery. Anesth Essays Res 2015;9:326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shakooh S, Bhosle P. Intrathecal nalbuphine: an effective adjuvant for post operative analgesia. Innov J Med Health Sci 2014;40:46–90. [Google Scholar]
- [23].Abdelhaq MM, Elramely MA. Effect of nalbuphine as adjuvant to bupivacaine for ultrasound-guided supraclavicular brachial plexus block. Open J Anesthesiol 2016;6:20–6. [Google Scholar]
- [24].Escalona-Marfil C, Coda A, Ruiz-Moreno J, Riu-Gispert LM, Gironès X. Validation of an electronic visual analog scale mHealth tool for acute pain assessment: prospective cross-sectional study. J Med Internet Res 2020;22:e13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hausken J, Fretland ÅA, Edwin B, et al. Intravenous patient-controlled analgesia versus thoracic epidural analgesia after open liver surgery: a prospective, randomized, controlled, noninferiority trial. Ann Surg 2019;270:193–9. [DOI] [PubMed] [Google Scholar]
- [26].Suzuki M, Larkum ME. General anesthesia decouples cortical pyramidal neurons. Cell 2020;180:666.e13–76.e13. [DOI] [PubMed] [Google Scholar]
- [27].Kendall MC, Alves L, Traill LL, De Oliveira GS. The effect of ultrasound-guided erector spinae plane block on postsurgical pain: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2020;20:01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abdelhamid BM, Omar H. Nalbuphine as an adjuvant to 0.25% levobupivacaine in ultrasound-guided supraclavicular block provided prolonged sensory block and similar motor block durations (RCT). J Anesth 2018;32:551–7. [DOI] [PubMed] [Google Scholar]
- [29].Gupta K, Jain M, Gupta PK, et al. Nalbuphine as an adjuvant to 0.5% bupivacaine for ultrasound-guided supraclavicular brachial plexus blockade. Ind J Pain 2016;30:176. [Google Scholar]
- [30].Borah TJ, Dey S, Yunus M, Dev P, Karim HMR, Bhattacharyya P. Effect of different doses of intrathecal nalbuphine as adjuvant to ropivacaine in elective lower limb surgeries: a dose finding study. Indian J Anaesth 2018;62:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yadav VK, Choudhary AK, Prasad MK, et al. Role of nalbuphine as an adjuvant to ropivacaine in supraclavicular block-a randomized control study. Anaesth Pain Intensive Care 2019;23:186–91. [Google Scholar]
- [32].Mavaliya V, Tak M, Singh B, et al. Comparison of nalbuphine versus fentanyl as an adjuvant to 0.75% isobaric ropivacaine in subarachnoid block for orthopedic surgery of lower limbs: a randomized, double-blind study. Bali J Anesthesiol 2020;4:161. [Google Scholar]
- [33].An K, Elkassabany NM, Liu J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One 2015;10:e0123459. [DOI] [PMC free article] [PubMed] [Google Scholar]