Abstract
Background:
Papillary thyroid carcinoma (PTC) incidence has been increasing worldwide. Obesity, that is, having a high body mass index, is associated with the incidence of several cancers including colon, breast, esophageal, and kidney cancer. However, the association between obesity and the clinical features of PTC is still unknown. This study aimed to determine the impact of obesity on the clinical features of PTC.
Method:
A database search was conducted for articles published up to 2020 on obesity and clinical features of PTC. Data were extracted from articles that met the meta-analysis inclusion criteria.
Results:
A total of 11 retrospective cohorts and 11,729 patients were included. Obesity was associated with the following variables in PTC patients: older age (difference in means = 1.95, 95% confidence interval [CI] 0.16–3.74, P = .03), male sex (odds ratio [OR] = 3.13, 95%CI 2.24–4.38, P < .00001), tumor size ≥1 cm (OR = 1.34, 95%CI 1.11–1.61, P < .002), multifocality (OR = 1.54, 95%CI 1.27–1.88, P < .0001), extrathyroidal extension (OR = 1.78, 95%CI 1.22–2.59, P = .003) and advanced tumor, node, metastasis stage (OR = 1.68, 95%CI 1.44–1.96, P < .00001). Preoperative serum thyroid-stimulating hormone level (difference in means = 0.09, 95%CI 0.35–0.52, P = .70), Vascular invasion (OR = 0.84, 95%CI 0.56–1.26, P = .41), lymph node metastasis (OR = 1.07, 95%CI 0.87–1.32, P = .50), distant metastasis (OR = 1.14, 95%CI 0.64–2.04, P = .66), and recurrence (OR = 1.45, 95%CI 0.97–2.15, P = .07) were not associated with obesity.
Conclusion:
Obesity was associated with several poor clinicopathologic prognostic features: older age, male gender, tumor size ≥1 cm, extrathyroidal extension, multifocality, and advanced tumor/node/metastasis stage. However, thyroid-stimulating hormone level, vascular invasion, lymph node metastasis, distant metastasis, and recurrence were not associated with obesity in PTC.
Keywords: body mass index, clinicopathologic progression, papillary thyroid carcinoma
1. Introduction
Over the past 20 years, the incidence of thyroid cancer, especially papillary thyroid carcinoma (PTC), has increased significantly worldwide. According to the Surveillance, Epidemiology, and End Results database, incidence of PTC in the USA increased from 4.56 per 100,000 person-years in 1974 to 1977 to 14.42 per 100,000 person-years in 2010 to 2013.[1] According to data from the China Cancer Center, thyroid cancer ranks seventh in cancer incidence in China and is the fourth most common malignancy in women.[2] The increase in the incidence of PTC is largely due to the application of high-resolution ultrasonography to detect thyroid nodules. Nevertheless, this does not explain why the incidence of thyroid cancer with a larger diameter is on the rise, with studies reporting increased incidence of PTCs, with larger size and at later stage.[3] One study based on data from the Surveillance Epidemiology and End Results database showed that environmental and other factors play an important role in the occurrence and development of thyroid cancer.[3]
Overweight and obesity, that is, having a high body mass index (BMI), are considered to be is associated with incidence of several cancers, including cancer of the colon, breast, esophagus, and kidney.[4] Furthermore, high BMI is related to advanced stage or aggressive cancers including prostate, colon and breast cancers.[5] However, whether BMI is associated with the incidence or prognosis of PTC is still controversial. Although the current (8th edition) American Joint Committee on Cancer staging system has stratified prognostic risk factors according to age, tumor size, lymph node metastasis (LNM), grossly extrathyroidal extension, and distant metastasis, the impact of these factors on PTC prognosis is still unknown.[6] Some studies have reported a correlation between obesity or elevated BMI with more advanced tumor/node/metastasis (TNM) stage and extrathyroidal extension in PTC.[7,8] In contrast, other studies have found no association between obesity and PTC progression.[9,10] In light of these conflicting reports, we aimed to conduct a meta-analysis to assess whether obesity was associated with the clinical features and progression of PTC.
2. Methods
We performed this meta-analysis according to the preferred reporting items for systematic reviews and meta-analyses statement.[11] A systematic search of the PubMed, Web of Science, and China National Knowledge Infrastructure databases was performed to find relevant articles published up to 8th February 2020. The following search terms were used: (“thyroid cancer” OR “thyroid neoplasm” OR “thyroid carcinoma”) AND (“obesity” OR “body mass index” OR “weight”). The study selection was done by two independent authors who screened the titles, abstracts, and full text. Relevant references and reviews were also identified. Discrepancies were resolved via discussion. Our study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University, Shenyang, China.
2.1. Selection criteria and exclusion criteria
BMI was calculated as weight (kilograms) divided by the square of height (meters). On the basis of the standardized guidelines set by the World Health Organization, for enrolled patients with PTC, normal weight was defined as 18.5 ≤ BMI < 25 kg/m2 and obesity was defined as BMI ≥30 kg/m2. Studies met the inclusion criteria if the following conditions were met:
-
(1)
Studies were required to be retrospective or prospective cohort studies;
-
(2)
included patients had PTC with thyroidectomy and central lymph node dissection or lateral lymph node dissection;
-
(3)
the relevant BMI records were complete to allow data extraction; and
-
(4)
data were presented as odds ratio (OR) or hazard ratio with 95% confidence interval (CI) reported.
We excluded studies that met at least one of the following criteria:
-
(1)
studies with patients who did not accept initial treatment;
-
(2)
articles that were comments, case reports, reviews, letters, conference abstracts, or expert opinions;
-
(3)
studies with patients with a family history of thyroid malignancy.
2.2. Data extraction and quality assessment
Data extraction was performed by two authors independently. The collected information was extracted and recorded as follows: first author, publication year, country, study design, and case number. Possible risk factors associated with elevated BMI were investigated and the corresponding numbers of patients were recorded. The identified risk factors were age, sex, tumor size, serum levels of thyroid-stimulating hormone (TSH) before operation, multifocality, extrathyroidal extension, vascular invasion, LNM, advanced TNM stage, distant metastasis, and recurrence. We assessed the quality of each included cohort study using the Newcastle-Ottawa Scale (NOS) which yielded a minimum score of 0 and a maximum score of 9. The quality assessments were performed by two independent reviewers.
2.3. Statistical analysis
Review Manager 5.3 (https://community.cochrane.org) was used for statistical analyses. The results are presented as ORs with 95% CIs. Unless otherwise specified, P-values < .05 were considered statistically significant. In addition, the I2 statistic and the Q test were used to quantify heterogeneity. When I2 was < 50% and P > .1, a fixed-effects model was used. To test for possible publication bias, Begg's funnel plots were used.
3. Results
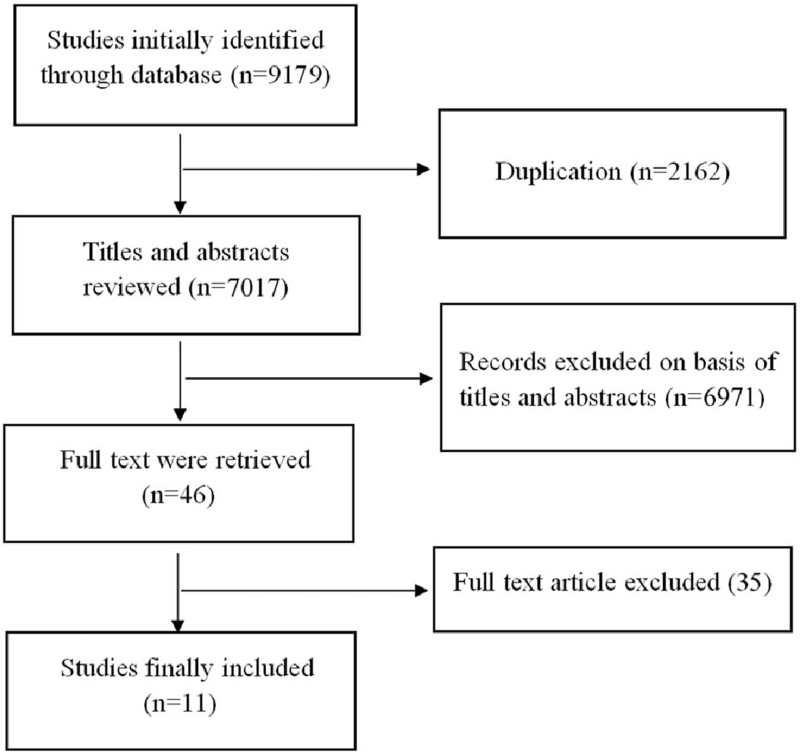
A total of 3,059 studies from PubMed, 6071 studies from Web of Science and 49 studies from China National Knowledge Infrastructure were initially found. After removing duplicate articles, 7,017 articles were included for screening. Following consideration of the inclusion and exclusion criteria for the review, a total of 46 full-text articles were selected after carefully checking the titles and abstracts. After the full-text review, 11 retrospective cohort studies met our selection criteria. A flow chart with details of the study selection process is displayed in Figure 1 and the study characteristics are shown in Table 1.
Figure 1.
Flow chart of the study selection process.
Table 1.
Basic characteristics of the included studies.
| Author | Year | Country | Study design | PTC/PTMC | Case number obese | Case number normal weight |
| Trésallet C[7] | 2014 | France | retrospective | PTC | 176 | 684 |
| Choi JS[8] | 2014 | Korea | retrospective | PTC | 32 | 451 |
| Choi JS[36] | 2015 | Korea | retrospective | PTMC | 13 | 426 |
| Feng JW[37] | 2019 | China | retrospective | PTC | 25 | 247 |
| Yu ST[38] | 2017 | China | retrospective | PTC | 304 | 826 |
| Kim SH[39] | 2015 | Korea | retrospective | PTC | 33 | 481 |
| Kim SK[40] | 2016 | Korea | retrospective | PTC | 165 | 2740 |
| Wu C[41] | 2017 | China | retrospective | PTC | 64 | 403 |
| Zhao S[42] | 2019 | China | retrospective | PTC | 426 | 1809 |
| Gąsior Perczak D[43] | 2018 | Poland | retrospective | PTC | 398 | 339 |
| Zhu X[44] | 2019 | China | retrospective | PTC | 208 | 1479 |
3.1. Age of patients
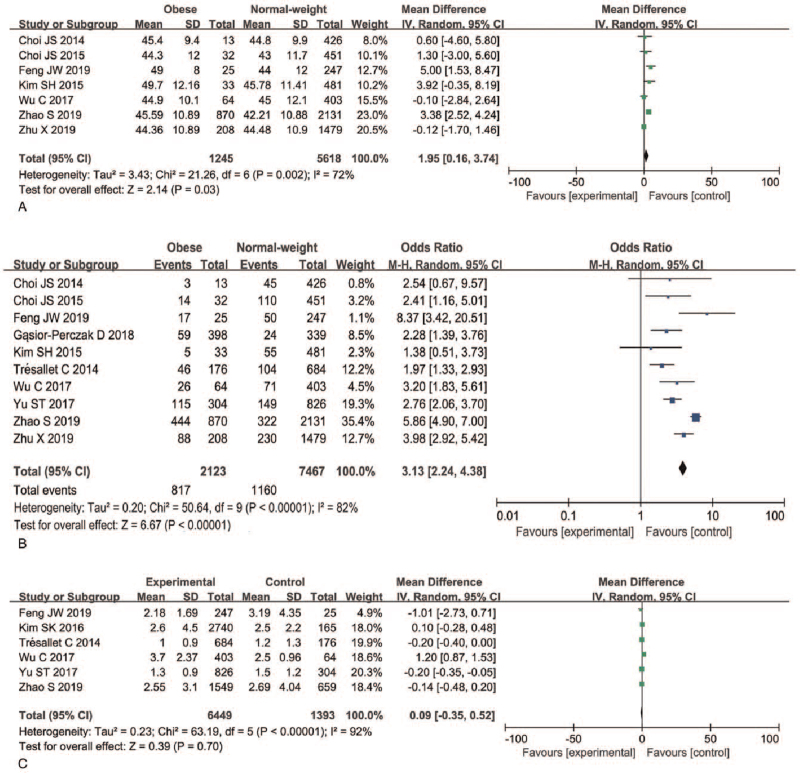
A total of seven articles were included in the analysis of whether obesity was associated with age in PTC patients. A random-effects model was applied to analyze this risk factor (P = .002, I2 = 72%). Older age was associated with obesity in PTC patients, with a significant difference in age found between the obese group and the normal weight group (difference in means [MD] = 1.95, 95%CI 0.16–3.74, P = .03) (Fig. 2A, Figure S1A, Supplemental Digital Content).
Figure 2.
Forest plots of the association between age, gender, preoperative serum TSH level, and obesity in PTC. (A) Age; (B) gender; (C) preoperative serum TSH level. PTC = papillary thyroid carcinoma, TSH = thyroid-stimulating hormone.
3.2. Gender
A total of 10 articles and 9,590 patients were included in the analysis of whether obesity was associated with sex in PTC patients. A random-effects model was applied to analyze this risk factor (P < .00001, I2 = 82%). Our results indicated that male PTC patients were more likely to be obese than female patients (38.48% vs 15.53%, OR = 3.13, 95%CI 2.24–4.38, P < .00001) (Fig. 2B, Figure S1B, Supplemental Digital Content).
3.3. Preoperative serum TSH level
A total of six articles were included in the analysis of whether obesity was associated with preoperative serum TSH level in PTC patients. A random-effects model was applied to analyze this risk factor (P < .00001, I2 = 92%) and our results indicated that preoperative serum TSH level was not associated with obesity in PTC patients. No difference in preoperative serum TSH level was found between the normal weight group and the obese group (MD = 0.09, 95%CI 0.35–0.52, P = .70) (Fig. 2C, Figure S1C, Supplemental Digital Content).
3.4. Primary tumor size
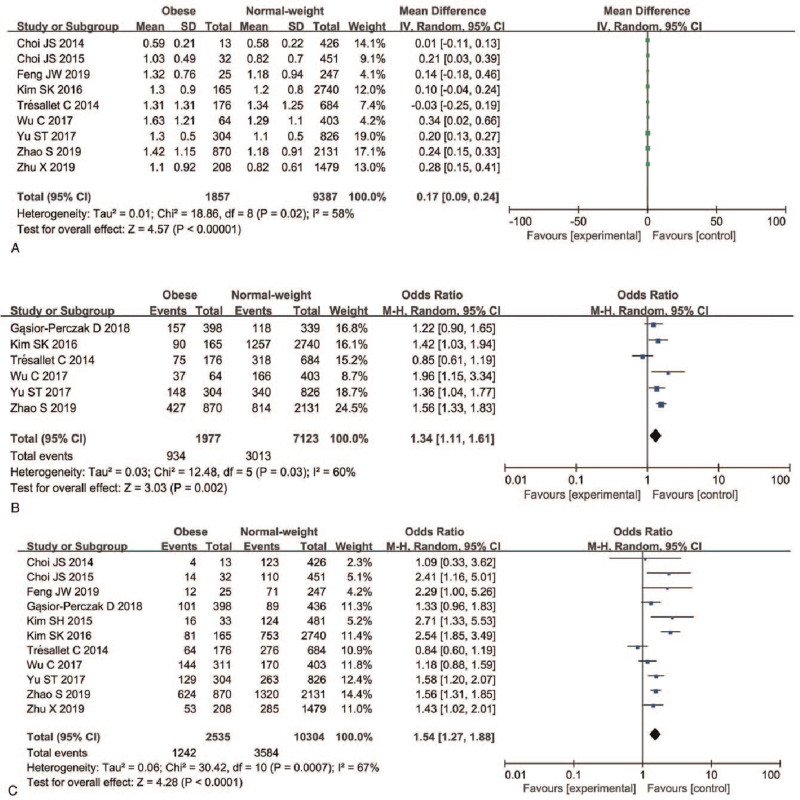
A total of nine studies with continuous variables and six articles with dichotomous variables were included in the analysis of whether obesity was associated with primary tumor size in PTC patients. For the studies with continuous variables, a random-effects model was used to detect this risk factor (P = .02, I2 = 58%); for dichotomous variables a random-effects models was used and 1 cm was used as a cutoff value (P = .03, I2 = 60%). Our meta-analysis indicated that obesity was associated with larger primary tumor size (MD = 0.17, 95%CI 0.09 − 0.24, P < .00001; OR = 1.34, 95%CI 1.11–1.61, P < .002) (Fig. 3A, Figure S2A, Supplemental Digital Content and Figure S3B, Supplemental Digital Content, Figure S2B, Supplemental Digital Content).
Figure 3.
Forest plots of the association between primary tumor size, multifocality, and obesity in PTC. (A) Primary tumor size (continuous variable); (B) primary tumor size (dichotomous variable); (C) multifocality. PTC = papillary thyroid carcinoma.
3.5. Multifocality
A random-effects model was applied to analyze whether obesity was associated with multifocality in PTC (P < .00001, I2 = 81%). A total of 11 articles with a total of 12,839 patients were included. Our meta-analysis indicated a higher rate of multifocality in the obese group than in the normal weight PTC patients (48.99% vs 34.78%). There was a significant difference in focality between the normal weight group and the obese group (OR = 1.54, 95%CI 1.27–1.88, P < .0001) (Fig. 3C, Figure S2C, Supplemental Digital Content).
3.6. Extrathyroidal extension
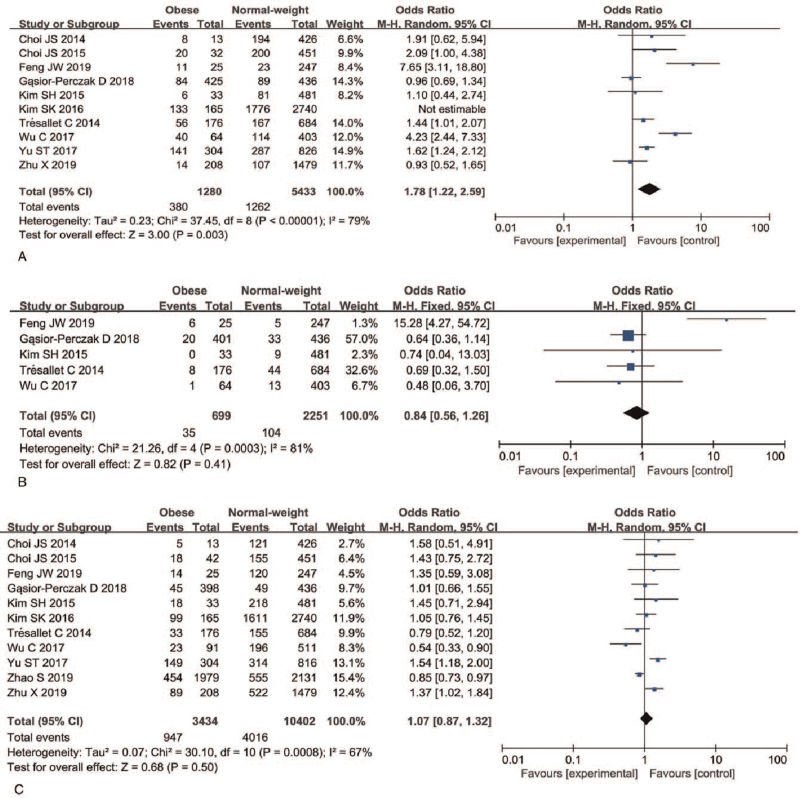
A total of 10 articles and 6713 patients were included in the analysis of whether obesity was associated with extrathyroidal extension in PTC patients. A random-effects model was applied to analyze this risk factor (P < .00001, I2 = 79%). Meta-analysis indicated that extrathyroidal extension-positive PTC patients were more likely to be obese than extrathyroidal extension-negative PTC patients (29.68% vs 23.23%; OR = 1.78, 95%CI 1.22–2.59, P = .003) (Fig. 4A, Figure S3A, Supplemental Digital Content).
Figure 4.
Forest plots of the association between extrathyroidal extension, vascular invasion, lymph node metastasis, and obesity in PTC. (A) Extrathyroidal extension; (B) vascular invasion; (C) lymph node metastasis. PTC = papillary thyroid carcinoma.
3.7. Vascular invasion
A total of five articles and 2950 patients were included in the analysis of whether obesity was associated with vascular invasion in PTC patients. A fixed-effects model was applied to analyze this risk factor (P < .0003, I2 = 81%) and the meta-analysis indicated that vascular invasion was not associated with obesity in PTC patients (OR = 0.84, 95%CI 0.56–1.26, P = .41) (Fig. 4B, Figure S3B, Supplemental Digital Content).
3.8. Lymph node metastasis
A total of 11 articles and 13,836 patients were included in the analysis of whether obesity was associated with LNM in PTC patients. A random-effects model was applied (P < .0008, I2 = 67%) and the meta-analysis indicated that being LNM positive was not associated with obesity in PTC patients (OR = 1.07, 95%CI 0.87–1.32, P = .50) (Fig. 4C, Figure S3C, Supplemental Digital Content).
3.9. Advanced TNM stage
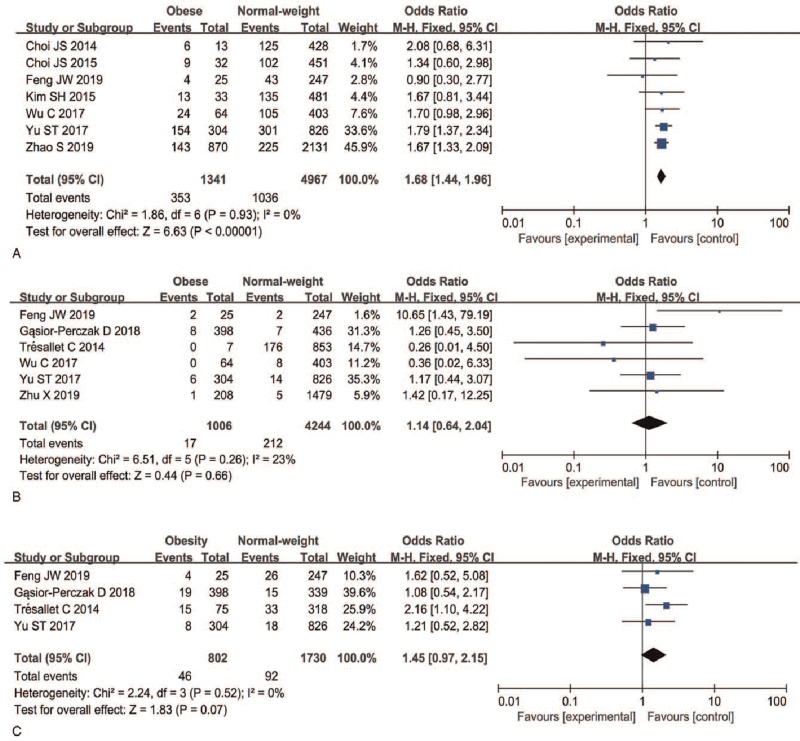
A fixed-effects model was applied to analyze advanced TNM stage as a risk factor (P = .93, I2 = 0%). The meta-analysis indicated that advanced TNM stage (III/IV) PTC was associated with obesity in PTC patients (26.32% vs 20.86%; OR = 1.68, 95%CI 1.44–1.96, P < .00001) (Fig. 5A, Figure S4A, Supplemental Digital Content).
Figure 5.
Forest plots of the association between advanced TNM stage, distant metastasis, recurrence, and obesity in PTC. (A) Advanced TNM stage; (B) distant metastasis; (C) recurrence. PTC = papillary thyroid carcinoma, TNM = tumor/node/metastasis.
3.10. Distant metastasis
A total of six articles and 5250 patients were included in the analysis of whether obesity was associated distant metastasis in PTC patients. A fixed-effects model was applied to the analysis of this risk factor (P < .26, I2 = 23%) and indicated that distant metastasis was not associated with obesity in PTC patients (OR = 1.14, 95%CI 0.64–2.04, P = .66) (Fig. 5B, Figure S4B, Supplemental Digital Content).
3.11. Recurrence
A total of four articles and 2532 patients were included in the analysis of whether obesity was associated with recurrence of disease in PTC patients. A fixed-effects model was applied (P < .52, I2 = 0%) and the meta-analysis indicated that recurrence was not associated with obesity in PTC patients (OR = 1.45, 95%CI 0.97–2.15, P = .07) (Fig. 5C, Figure S4C, Supplemental Digital Content).
4. Discussion
Thyroid cancer incidence has increased rapidly across the world in the last several decades. PTC is the most common type, representing approximately 85% to 90% of all cases.[12] In the USA, thyroid cancer incidence increased from 4.56 per 100,000 person-years in 1974 to 1977 to 14.42 per 100,000 person-years in 2010 to 2013, an average increase of 3.6% per year between 1974 and 2013.[1] In China, the age-standardized incidence was 3.21/105 in 2005, and had increased to 9.61/105 in 2015. This represents an average annual percentage change of 12.4% for the period 2005 to 2015.[13] However, the cause of the increasing incidence of thyroid cancer is still unclear.
The prevalence of obesity is also increasing worldwide and the impact of obesity on cancer risk has been extensively studied.[14] Epidemiologic studies of the relationship between excess body weight and cancer risk have shown that BMI is positively associated with incidence of and poor pathologic prognosis for multiple cancers, including colon, esophageal, and breast cancer, among others. However, the relationship between BMI and the clinical features of PTC remains unclear. Some studies have reported a positive association between BMI and PTC. Higher BMI has been associated with extrathyroidal extension, LNM, and advanced tumor stages.[15,16] However, the possible associations between BMI and clinicopathologic features of thyroid cancer have remained controversial, and some studies have found no association between obesity, stages of PTC, and clinical features of thyroid cancer.[9,10] In addition, Paes et al[17] reported that higher BMI was not associated with more aggressive tumor features, and that obesity may be correlated with less nodal metastases. Although many studies have focused on the relationship between high BMI and the clinicopathologic features of PTC, the results are inconsistent. Thus, we performed a meta-analysis to assess the correlation between obesity and clinicopathologic factors in patients with PTC.
Age and male sex were two prognostic factors in PTC. Age > 55 years has been found to be associated with extrathyroidal extension and the significant factors that affect overall survival in patients with PTC.[18–20] The incidence of thyroid cancer is nearly three times higher in women than in men.[21] However, the aggressive histologic subtypes of thyroid cancer are more common in men.[21] In this meta-analysis, we concluded that obesity was associated with older age and male sex in PTC patients.
Preoperative serum TSH has been reported to play a major role in carcinogenesis, and several studies have reported an association between TSH level and thyroid cancer. TSH cooperates with insulin and IGF-1 signaling to activate the downstream MAPK and PI3K pathways that are central in thyroid carcinogenesis.[22,23] TSH level has been regarded as a factor that promotes aggressiveness of PTC in obese patients.[24] However, we found that obesity was not associated with preoperative serum TSH level in PTC patients.
Tumor size is considered a critical factor according to AJCC staging, and larger tumors are prone to being more aggressive.[25] From our data, we observed that tumor size ≥1 cm was 1.4 times more frequent in obese patients than in normal-weight patients. Whether multifocality is related to central lymph node metastasis in clinical lymph node-negative patients remains controversial. Multifocal PTC may be more aggressive than unifocal PTC, and multifocality is an independent risk factor for PTC recurrence after total thyroidectomy.[26] In this study, we found that obesity was associated with larger tumor size and multifocality in PTC patients.
The progression of PTC is slow and the primary tumor may break through the thyroid capsule and invade the surrounding strap muscles, the recurrent laryngeal nerve, and the internal jugular vein. It is widely believed that macroscopic extrathyroid extension has an important impact on the poor prognosis of PTC.[27] Some studies have reported that serum leptin levels are elevated in patients with PTC, and that PTC with expression of leptin and/or its receptor is associated with tumor aggressiveness.[28] Adiponectin levels are inversely associated with obesity, and low adiponectin level in obesity has been found to have an association with risk of several malignancies, including thyroid cancer.[29] Our results showed that obese patients had a higher probability of extrathyroid extension. However, vascular invasion may not be associated with obesity in PTC.
LNM is frequent in PTC, seen in 20% to 90% of patients.[30] It occurs in the central or lateral compartments and is an important determinant of the locoregional recurrence of PTC. Although there is controversy, some recent studies have reported that LNM can affect survival, particularly in elderly PTC patients.[31] In this study, LNM was not detected more frequently in obese patients than in normal weight PTC patients.
TNM stage is the most commonly used staging system applied in PTC. Classification is elaborated on by the AJCC, and allows prediction of the risk of cancer-related death. Our study was limited by the included studies, all of which used TNM staging according to the AJCC 7th edition. At the III/IV TNM stage of the AJCC, the 5-year survival rate is 41.4% to 82% for PTC.[32] In our study we found that high BMI was associated with advanced TNM stage. Distant metastasis and local recurrence are two important factors that may lead to a poor prognosis for PTC. Local recurrence has an effect on the survival of patients, and the 10-year overall survival rate is 78%–97%.[33,34] The 5-year and 10-year specific survival rates of patients with distant recurrence are 71% and 50%, respectively.[35] In our meta-analysis, we found that obesity was not associated with local recurrence and distant metastasis.
In conclusion, this study demonstrated that obesity was associated with several clinicopathologic features for poor prognosis of PTC: older age, male gender, tumor size ≥1 cm, extrathyroidal extension, multifocality, and advanced TNM stage. However, Preoperative serum TSH level, vascular invasion, LNM, distant metastasis, and recurrence were not associated with obesity in PTC.
Author contributions
Data curation: Qian Sun.
Investigation: Ningning Cui, Qian Sun.
Methodology: Ningning Cui.
Resources: Li Chen.
Software: Ningning Cui.
Validation: Li Chen.
Writing – original draft: Ningning Cui.
Writing – review & editing: Ningning Cui.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, LNM = lymph node metastasis, MD = difference in means, OR = odds ratio, PTC = papillary thyroid carcinoma, TNM = tumor/node/metastasis, TSH = thyroid-stimulating hormone.
How to cite this article: Cui N, Sun Q, Chen L. A meta-analysis of the influence of body mass index on the clinicopathologic progression of papillary thyroid carcinoma. Medicine. 2021;100:32(e26882).
Ethical standards was not applicable.
The authors have no conflicts of interest to disclose.
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
PTC = papillary thyroid carcinoma; PTMC = papillary thyroid microcarcinoma.
References
- [1].Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [5].Hahn KM, Bondy ML, Selvan M, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol 2007;166:1035–44. [DOI] [PubMed] [Google Scholar]
- [6].Lamartina L, Grani G, Arvat E, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer 2018;25:L7–11. [DOI] [PubMed] [Google Scholar]
- [7].Tresallet C, Seman M, Tissier F, et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery 2014;156:1145–52. [DOI] [PubMed] [Google Scholar]
- [8].Choi JS, Kim EK, Moon HJ, et al. Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine 2015;48:264–71. [DOI] [PubMed] [Google Scholar]
- [9].Iribarren C, Haselkorn T, Tekawa IS, et al. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer 2001;93:745–50. [DOI] [PubMed] [Google Scholar]
- [10].Kim JY, Jung EJ, Jeong SH, et al. The indices of body size and aggressiveness of papillary thyroid carcinoma. J Korean Surg Soc 2011;80:241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [12].Zhang W, Sun W, Qin Y, et al. Knockdown of KDM1A suppresses tumour migration and invasion by epigenetically regulating the TIMP1/MMP9 pathway in papillary thyroid cancer. J Cell Mol Med 2019;23:4933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Yu F, Shang Y, et al. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine 2020;68:163–73. [DOI] [PubMed] [Google Scholar]
- [14].Abelson P, Kennedy D. The obesity epidemic. Science 2004;304:1413. [DOI] [PubMed] [Google Scholar]
- [15].Goodman MT, Kolonel LN, Wilkens LR. The association of body size, reproductive factors and thyroid cancer. Br J Cancer 1992;66:1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253–6. [DOI] [PubMed] [Google Scholar]
- [17].Paes JE, Hua K, Nagy R, et al. The relationship between body mass index and thyroid cancer pathology features and outcomes: a clinicopathological cohort study. J Clin Endocrinol Metab 2010;95:4244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun W, Lan X, Zhang H, et al. Risk factors for central lymph node metastasis in CN0 Papillary thyroid carcinoma: a systematic review and meta-analysis. Plos One 2015;10:e139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jonklaas J, Nogueras-Gonzalez G, Munsell M, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 2012;97:E878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf) 2005;63:87–93. [DOI] [PubMed] [Google Scholar]
- [21].Ortega J, Sala C, Flor B, et al. Efficacy and cost-effectiveness of the UltraCision harmonic scalpel in thyroid surgery: an analysis of 200 cases in a randomized trial. J Laparoendosc Adv Surg Tech A 2004;14:09–12. [DOI] [PubMed] [Google Scholar]
- [22].Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiao J, Zhou Y. Relationship between serum thyroxin-stimulating hormone and papillary thyroid micrcarcinoma in nodular thyroid disease. Zhonghua Yi Xue Za Zhi 2015;95:908–11. [PubMed] [Google Scholar]
- [24].Wasikowa R, Iwanicka Z, Zak T, et al. Nodular goiter and thyroid carcinoma in children and adolescents in a moderate endemic area (lower Silesia-Sudeten endemia) in the last twelve years. J Pediatr Endocrinol Metab 1999;12:645–52. [DOI] [PubMed] [Google Scholar]
- [25].Tran B, Roshan D, Abraham E, et al. The prognostic impact of tumor size in papillary thyroid carcinoma is modified by age. Thyroid 2018;28:991–6. [DOI] [PubMed] [Google Scholar]
- [26].Genpeng L, Jianyong L, Jiaying Y, et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine (Baltimore) 2018;97:e9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ito Y, Miyauchi A, Kihara M, Kobayashi K, Miya A. Prognostic values of clinical lymph node metastasis and macroscopic extrathyroid extension in papillary thyroid carcinoma. Endocr J 2014;61:745–50. [DOI] [PubMed] [Google Scholar]
- [28].Celano M, Maggisano V, Lepore SM, et al. Expression of leptin receptor and effects of leptin on papillary thyroid carcinoma cells. Int J Endocrinol 2019;2019:5031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mele C, Sama MT, Bisoffi AA, et al. Circulating adipokines and metabolic setting in differentiated thyroid cancer. Endocr Connect 2019;8:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg 2018;50:94–103. [DOI] [PubMed] [Google Scholar]
- [31].Patron V, Hitier M, Bedfert C, et al. Predictive factors for lateral occult lymph node metastasis in papillary thyroid carcinoma. Eur Arch Otorhinolaryngol 2013;270:2095–100. [DOI] [PubMed] [Google Scholar]
- [32].Nam SH, Bae MR, Roh JL, et al. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol 2018;87:158–64. [DOI] [PubMed] [Google Scholar]
- [33].Saito Y, Matsuzu K, Sugino K, et al. The impact of completion thyroidectomy followed by radioactive iodine ablation for patients with lymph node recurrence of papillary thyroid carcinoma. Surgery 2019;166:342–8. [DOI] [PubMed] [Google Scholar]
- [34].Chinn SB, Zafereo ME, Waguespack SG, et al. Long-term outcomes of lateral neck dissection in patients with recurrent or persistent well-differentiated thyroid cancer. Thyroid 2017;27:1291–9. [DOI] [PubMed] [Google Scholar]
- [35].Ito Y, Higashiyama T, Takamura Y, et al. Clinical outcomes of patients with papillary thyroid carcinoma after the detection of distant recurrence. World J Surg 2010;34:2333–7. [DOI] [PubMed] [Google Scholar]
- [36].Choi JS, Lee HS, Kim EK, et al. The influence of body mass index on the diagnostic performance of pre-operative staging ultrasound in papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2015;83:550–5. [DOI] [PubMed] [Google Scholar]
- [37].Feng JW, Yang XH, Wu BQ, et al. Influence of body mass index on the clinicopathologic features of papillary thyroid carcinoma. Ann Otol Rhinol Laryngol 2019;128:625–32. [DOI] [PubMed] [Google Scholar]
- [38].Yu ST, Chen W, Cai Q, et al. Pretreatment BMI is associated with aggressive clinicopathological features of papillary thyroid carcinoma: a multicenter study. Int J Endocrinol 2017;2017:5841942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim SH, Park HS, Kim KH, et al. Correlation between obesity and clinicopathological factors in patients with papillary thyroid cancer. Surg Today 2015;45:723–9. [DOI] [PubMed] [Google Scholar]
- [40].Kim SK, Woo JW, Park I, et al. Influence of body mass index and body surface area on the behavior of papillary thyroid carcinoma. THYROID 2016;26:657–66. [DOI] [PubMed] [Google Scholar]
- [41].Wu C, Wang L, Chen W, et al. Associations between body mass index and lymph node metastases of patients with papillary thyroid cancer: a retrospective study. Medicine (Baltimore) 2017;96:e6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao S, Jia X, Fan X, et al. Association of obesity with the clinicopathological features of thyroid cancer in a large, operative population: a retrospective case-control study. Medicine (Baltimore) 2019;98:e18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gasior-Perczak D, Palyga I, Szymonek M, et al. The impact of BMI on clinical progress, response to treatment, and disease course in patients with differentiated thyroid cancer. PLOS ONE 2018;13:e204668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu X, Jiang L, Chen S, et al. Correlation between Body Mass Index and Clinicopathological Factors of Papillary Thyroid Cancer. Journal of Cancer Control and Treatment 2019;32:1088–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.