Abstract
Overweight/obesity can influence bone mineral accretion, but the conclusions are not consistent. We aimed to examine the association between bone mineral density (BMD) levels and body mass index (BMI) in 12 to 15 years old adolescents.
We performed a cross-sectional study including 8365 adolescents. BMD was evaluated using a quantitative ultrasound device. Z scores for BMI were evaluated using World Health Organization references. Logistic regression models were performed to evaluate the association between BMD levels and BMI.
Totally 1866 (22.3%) adolescents had low /reduced BMD, and boys had a higher rate than girls (72.6% vs 27.4%, P < .001). The rates of thinness, normal weight, overweight, and obesity were 2.8%, 57.1%, 22.3%, and 17.8%, respectively. The multivariable-adjusted (age, sex, systolic blood pressure, and height Z score) ORs (95% CIs) of low/reduced BMD associated with BMI groups (thinness, normal [reference], overweight, and obesity) were 0.59 (0.39–0.89), 1.00, 1.61 (1.41–1.84), and 1.98 (1.69–2.30), respectively (Ptrend < .001). This positive association existed in boys and girls though the differences were not significant between normal weight and thin girls. The multivariable-adjusted ORs for each 1-unit increase in BMI Z score were 1.36 (1.24–1.49) for girls, and 1.23 (1.16–1.30) for boys, and 1.26 (1.20–1.32) for all participants.
We observed a positive association between BMI and low/reduced BMD in 12 to 15 years old adolescents. More attention should be paid on overweight and obese adolescents to reduce the risk of low BMD. Further studies are needed to explore the mechanisms of this association.
Keywords: adolescent, body mass index, bone mineral density, obesity
1. Introduction
Osteoporosis, which is characterized by compromised bone strength, is associated with bone fragility and increased risk of fractures.[1] In China, approximately 13% adults suffered from osteoporosis, and the prevalence varied by regions, sex, and bone sites.[2] Bone mineral density (BMD) is an important determinant of osteoporosis and low BMD could lead to increased fracture risk, associated with increased mortality and morbidity with advancing age.[3] It was suggested that >90% of the adult bone mass is formed by the end of adolescence, and bone mass increase after maturation is minimal.[4] Data showed that boys attained 86% of adult bone mineral content (BMC) and BMD by 17 years old, and 95% by 18 years old.[5] In women, 40% of peak bone mass was accumulated during the adolescence, and 92% of total BMC was attained by age 18 years.[6] In Chinese girls, the peak bone mineral accretion occurred at menarche, which is consistent with studies in western girls.[7] Therefore, maximizing bone mineral mass accretion during adolescent years may contribute to reducing fracture and osteoporosis risks in later life.[8]
A number of factors have influences on bone mineral accretion, including weight, height, physical activity (PA), body composition, dietary intake, vitamin D level, genetic predisposition, hormones (such as insulin like growth factors 1, leptin), and exposure to smoking and alcohol intake.[8–10] Furthermore, genetic polymorphisms, including leptin system and vitamin D receptor may permit investigation of bone mass acquisition and the risk of osteoporosis independent from biological level measurement.[11,12] However, the associations between overweight/obesity and bone mass acquirement are controversial, and the mechanisms are still lack of fully investigation. Some studies reported that obese adolescents had relatively high bone mineral density because of higher mechanical load[10,13]; whereas others demonstrated lower bone mass and density in obese adolescents compared with normal weight adolescents as a result of low vitamin D and PA levels.[14,15] In the past decades, there was a continuous increase in overweight and obesity rates among children and adolescents in China. From 1991 to 2015, the overweight rate increased from 5.0% to 11.7% with little fluctuation in 2006 to 2010, and the obesity rate increased from 1.7% to 6.8%.[16] Therefore, the relationships between overweight/obesity in adolescents and bone health are drawing more attention.
There are several methods for measuring bone status, including dual energy x-ray absorptiometry (DXA), quantitative computed tomography, and quantitative ultrasonometry (QUS). By measuring quantitative parameters, including ultrasound attenuation and ultrasound velocity (speed of sound, SOS), QUS can be used to evaluate not only bone mass but also elasticity and bone structure in multiple sites, such as calcaneus, phalanges, tibia, and patella.[17] Comparing to other methods, QUS is a non-evasive, radiation-free, low cost, transportable, and easy to use technique, and was suggested to be associated with BMD measured by DXA[18] therefore it is regarded as a suitable method using in children and adolescents.[19–21]
The aim of this study was to investigate the association between overweight/obesity and BMD levels measured by QUS among adolescents aged 12 to 15 years old from a sub-urban area of Tianjin, China.
2. Methods
2.1. Study population
This study is based on data from Youth Health Care Promotion Program from 2014 to 2016. The Youth Health Care Promotion Program is a general survey conducted annually among the first grade students of the 16 junior middle schools in Dongli District, a sub-urban area of Tianjin, China. This program includes questionnaire survey, health examinations, health education, and intervention. The study was approved by the Ethics Committee for Clinic Research of Tianjin Women and Children's Health Center, and informed written consents were obtained from students and their parents, and the Health Bureau of Doling District was responsible to organize the health examinations and developmental assessment. Combined with questionnaire survey, targeted health education and intervention would be provided.
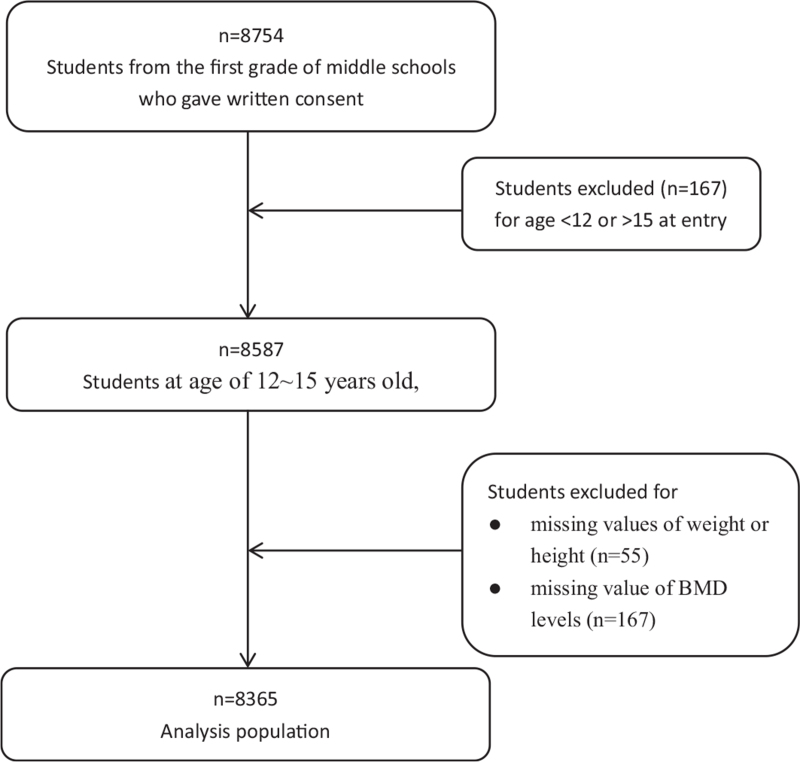
In total, 8754 students from the first grade of middle schools, aged from 9 to 19 years old, finished health examinations and questionnaire survey. Students at age of 12 to 15 years old, which accounted for 98% of all the students, were selected in this study. Students were excluded if they had missing values of weight or height (55 students), or BMD levels (167 students) (Fig. 1). The final sample consisted of 8365 students: 4432 boys (53%) and 3933 girls (47%).
Figure 1.
Inclusion and exclusion of participants. In total, 8754 students were recruited. After excluding students with incomplete information of weight, height, or bone mass density results, 8365 students aged 12 to 15 years old were included in the study.
2.2. Measurements
Adolescents’ weight and height were measured by specially-trained practitioners. Weight was measured after removing outer clothing, heavy pocket items, and shoes, and to the nearest 0.01 kg by using a digital scale (TCS-60, Tianjin Weighing Apparatus Co., China).
Standing height was measured without shoes, with the participant's head in the Frankfurt plane, and to the nearest 0.1 cm using a stadiometer (SZG-180, Shanghai Zhengdahengqi, China). Body mass index (BMI) was calculated as weight/height squared (kg/m2). BMI Z score and height Z score were derived by using the 2007 World Health Organization growth reference, and overweight is defined as >+1SD, obesity >+2SD, thinness: <–2SD.[22] Blood pressure was determined by single measurement using the standard mercury sphygmomanometer.
The BMD was measured by a portable quantitative ultrasound bone device (Sunlight Omnisense 7000), which measures the speed of ultrasonic waves propagating along the bone. Axially transmitted SOS is measured to indicate bone strength, such as mineral density, elasticity, cortical thickness, and microstructure, and the result is obtained after performing 3 consistent measurement cycles. BMD Z-scores were obtained by using an age- and sex-matched SOS value references for Asian children from the manufacturer. In this study, the SOS of mid-shaft tibia was measured. The measurement was performed at school in a separate classroom. Students were wearing loose clothes and keeping upright sitting position. They were asked to place their right legs on the test board with exposing their right shins, and the right mid-shafts was measured. Qualitative results were given as normal, reduced, or low BMD if the Z scores were >–1.0, ≤–1.0, >–2.0, and ≤–2.0, respectively, following the recommendation by the international society for clinical densitometry.[23,24] All ultrasound measurement was performed by experienced doctors.
2.3. Statistical analyses
The SPSS statistical software version 21.0 (IBM SPSS, Chicago, USA) was used to perform statistical analyses. Continuous variables were expressed as means ± SD, and t test was used to evaluate the differences between groups. Categorical variables were expressed as numbers and percentages, and Chi-squared test was used to compare the distribution. Logistic regression models were performed to evaluate the association between low BMD levels and BMI, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. In logistic regression, low BMD group and reduced BMD group were combined as low/reduced BMD group to compare with normal BMD group. The multivariate logistic regression analyses were adjusted for age, sex, systolic blood pressure (SBP), and height Z score. BMI was evaluated in the following 2 ways: as 4 categories (thin, normal, overweight, and obese), and normal BMI group was used as reference, and BMI Z score as a continuous variable. The different categories of BMI were included in the models as dummy variables, and the significance of the trend over different categories were tested in the same models with the median of each category as a continuous variable. P value < .05 was considered statistically significant.
3. Results
The 8365 adolescents in the study were aged 12 to 15 years old (12.83 ± 0.77 years), and 4432 (53%) were boys. The rates of thinness, normal weight, overweight, and obese were 2.8%, 57.1%, 22.3%, and 17.8%, respectively. In total, 6499 (77.7%) adolescents’ BMD were within normal range, 1421 (17.0%) had reduced BMD, and 445 (5.3%) had low BMD.
Participant characteristics according to BMD levels are shown in Table 1. In total low/reduced BMD group, boys accounted for 72.6% of adolescents, while in normal group, boys accounted for 47.3% (P < .001). Significant differences were also found in age, height, weight, BMI, height Z score, BMI Z score, SBP, and diastolic blood pressure in normal and reduced/low BMD groups. As shown in Table 1, adolescents having low BMD were older, taller, and had higher BMI Z score and blood pressures (P < .001).
Table 1.
General characteristics of participants according to BMD levels.
| Total | Low/reduced BMD | |||||
| Characteristics | (N = 8365) | Total (N = 1866) | Low BMD (N = 445) | Reduced BMD (N = 1421) | Normal BMD (N = 6499) | P ∗ |
| Gender (boys, %) | 4432 (53) | 1355 (72.6) | 370 (83.1) | 985 (69.3) | 3077 (47.3) | <.001 |
| Age, yrs | 12.83 (0.774) | 12.88 (0.818) | 12.93 (0.797) | 12.87 (0.825) | 12.82 (0.760) | .001 |
| Height, cm | 158.89 (7.523) | 160.36 (7.79) | 161.79 (7.75) | 159.91 (7.76) | 158.47 (7.39) | <.001 |
| Weight, kg | 53.12 (13.50) | 57.39 (14.30) | 61.42 (14.79) | 56.12 (13.92) | 51.89 (13.0) | <.001 |
| BMI, kg/m2 | 20.88 (4.31) | 22.16 (4.53) | 23.32 (4.72) | 21.79 (4.41) | 20.51 (4.17) | <.001 |
| Height-Z | 0.54 (1.15) | 0.70 (1.20) | 0.86 (1.22) | 0.64 (1.20) | 0.50 (1.12) | <.001 |
| BMI-Z | 0.60 (1.36) | 1.02 (1.34) | 1.33 (1.33) | 0.92 (1.33) | 0.48 (1.34) | <.001 |
| SBP | 107.34 (11.68) | 109.72 (77.83) | 111.83 (11.90) | 109.06 (11.74) | 106.66 (11.55) | <.001 |
| DBP | 67.99 (7.57) | 68.97 (7.45) | 70.06 (7.31) | 68.63 (7.50) | 67.71 (7.58) | <.001 |
| BMI groups∗∗ | <.001 | |||||
| Thinness (%) | 233 (2.8) | 27 (11.6) | 2 (0.9) | 25 (10.7) | 206 (88.4) | |
| Normal (%) | 4779 (57.1) | 830 (17.4) | 153 (3.2) | 677 (14.2) | 3949 (82.6) | |
| Overweight (%) | 1867 (22.3) | 493 (26.4) | 122 (6.5) | 371 (19.9) | 1374 (73.6) | |
| Obesity (%) | 1486 (17.8) | 516 (34.7) | 168 (11.3) | 348 (23.4) | 970 (65.3) | |
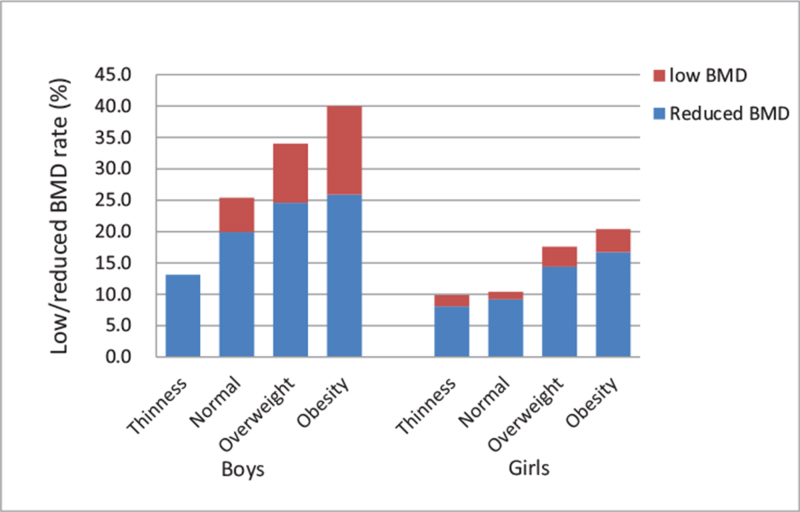
When reduced/low BMD rates were compared across different BMI groups, there was a positive association between BMI with the risk reduced/low BMD (Table 1). The rate of reduced/low BMD was the lowest in thinness adolescents, followed by normal and overweight adolescents, and the highest in obese adolescents. When stratified, this positive association existed in both boys and girls (Fig. 2).
Figure 2.
The rates of low/reduced BMD across different BMI and sex groups. The rates of low/reduced BMD were compared across different BMI groups in boys and girls. There was a positive association between low/reduced BMD rates and BMI groups in both boys and girls. BMD = bone mineral density, BMI = body mass index.
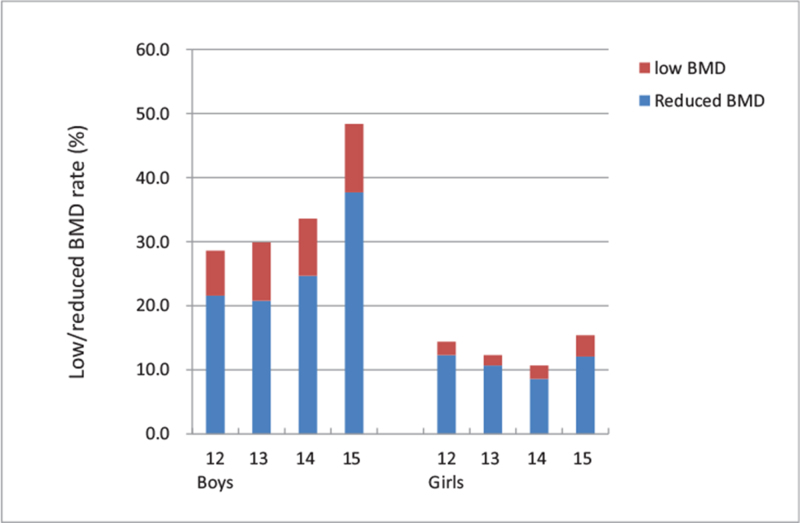
Low/reduced BMD rates were further calculated in different age and sex groups (Fig. 3). In linear × linear association tests, the rate of low/reduced BMD significantly increased with age in boys (Ptrend < .001). However, the rate decreased with age in girls across age groups of 12 to 14 years old (Ptrend = .018), but not significant after including 15 years old group into analysis (P = .072). Results indicated that the trend of low/reduced BMD with age might be different in boys and girls.
Figure 3.
The rates of low/reduced BMD across different age and sex groups. The rate of low/reduced BMD significantly increased with age in boys, while in girls, the rate decreased in girls across age groups of 12 to 14 years old. BMD = bone mineral density, BMI = body mass index.
Logistics regression models were conducted to investigate the association between low/reduced BMD and BMI groups. In total participants, the multivariable-adjusted (age, sex, SBP, and height Z score) ORs (95% CIs) of low/reduced BMD associated with BMI groups (thin, overweight, and obese) were 0.59 (0.39–0.89), 1.61 (1.41–1.84), and 1.98 (1.69–2.30), respectively comparing with normal BMI group (Ptrend < .001) (Table 2). After stratified by sex, this positive association existed in boys (Ptrend < .001). In girls, there was still a positive trend of low BMD associated with BMI groups (Ptrend < .001), though the difference was not significant between normal weight and thin girls.
Table 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for low/reduced BMD according to different BMI groups.
| Univariate analysis | Multivariate analysis∗ | |||
| BMI groups | OR | 95% CI | OR | 95% CI |
| Total | ||||
| Thin | 0.61 | 0.47–0.83 | 0.57 | 0.42–0.77 |
| Normal (reference) | 1.00 | - | 1.00 | - |
| Overweight | 1.58 | 1.42–1.76 | 1.54 | 1.38–1.73 |
| Obese | 2.196 | 1.95–2.47 | 1.88 | 1.64–2.15 |
| Ptrend | <.001 | <.001 | ||
| Boys | ||||
| Thin | 0.501 | 0.34–0.74 | 0.49 | 0.33–0.73 |
| Normal (reference) | 1.00 | - | 1.00 | - |
| Overweight | 1.41 | 1.21–1.64 | 1.40 | 1.20–1.63 |
| Obese | 1.80 | 1.56–2.09 | 1.77 | 1.50–2.10 |
| Ptrend | <.001 | <.001 | ||
| Girls | ||||
| Thin | 0.71 | 0.45–1.12 | 0.68 | 0.43–1.09 |
| Normal (reference) | 1.00 | - | 1.00 | - |
| Overweight | 1.67 | 1.42–1.97 | 1.74 | 1.47–2.06 |
| Obese | 1.87 | 1.51–2.32 | 2.02 | 1.59–2.57 |
| Ptrend | <.001 | <.001 | ||
Considering the sex and age differences in low/reduced BMD rates, the association between BMI Z score and low/reduced BMD was analyzed in boys and girls as well as different age groups separately (Table 3).
Table 3.
Association between BMI-Z score and low/reduced BMD in different sex and age groups∗.
| Total‡ | Boys | Girls | |||||||
| Age groups | N | OR (95% CI) | P | N | OR (95% CI) | P | N | OR (95% CI) | P |
| Without adjustment for height-Z | |||||||||
| 12∼ | 3117 | 1.39 (1.28–1.51) | <.001 | 1572 | 1.34 (1.21–1.48) | <.001 | 1545 | 1.50 (1.30–1.73) | <.001 |
| 13∼ | 3756 | 1.30 (1.21–1.39) | <.001 | 1981 | 1.31 (1.21–1.43) | <.001 | 1775 | 1.25 (1.09–1.43) | .001 |
| 14∼ | 1279 | 1.16 (1.03–1.29) | .011 | 757 | 1.22 (1.07–1.38) | .002 | 522 | 0.93 (0.72–1.20) | .571 |
| 15∼ | 213 | 0.97 (0.72–1.28) | .787 | 122 | 0.90 (0.65–1.25) | .546 | 91 | 1.14 (0.64–2.03) | .649 |
| 12∼15† | 8365 | 1.26 (1.20–1.32) | <.001 | 4432 | 1.24 (1.17–1.30) | <.001 | 3933 | 1.30 (1.20–1.42) | <.001 |
| With adjustment for height-Z | |||||||||
| 12∼ | 3117 | 1.38 (1.27–1.50) | <.001 | 1572 | 1.31 (1.19–1.45) | <.001 | 1545 | 1.54 (1.33–1.78) | <.001 |
| 13∼ | 3756 | 1.30 (1.21–1.39) | <.001 | 1981 | 1.12 (1.01–1.24) | <.001 | 1775 | 1.30 (1.13–1.49) | <.001 |
| 14∼ | 1279 | 1.15 (1.03–1.29) | .013 | 757 | 1.21 (1.07–1.38) | .003 | 522 | 0.96 (0.74–1.23) | .736 |
| 15∼ | 213 | 0.96 (0.73–1.28) | .803 | 122 | 0.90 (0.65–1.25) | .539 | 91 | 1.13 (0.63–2.02) | .684 |
| 12–15† | 8365 | 1.26 (1.20–1.32) | <.001 | 4432 | 1.23 (1.61–1.30) | <.001 | 3933 | 1.36 (1.24–1.49) | <.001 |
In boys of age 12 to 15 years old, BMI Z score was positively associated with low/reduced BMD (OR = 1.24, 95% CI = 1.17–1.30, P < .001). The association was also found significant in sub-age groups of 12, 13, and 14 years old, but not significant in the group of 15 years old.
In girls, BMI Z score was also positively associated with low/reduced BMD (OR = 1.30, 95% CI = 1.20–1.42, P < .001). The association existed in sub-age groups of 12 and 13 years old, but not found in groups of 14 and 15 years old.
In total, BMI Z score was positively associated with low/reduced BMD (OR = 1.26, 95% CI = 1.20–1.32, P < .001), with 1 unit increase in BMI Z score increasing the risk of low/reduced BMD by 26%. The positive associations in sub age groups of 12 and 13 years old were found more significant (P < .001) than 14-year-old group (P = .013).
4. Discussion
The present study showed that overweight or obese adolescents had higher risks of having low BMD in both sexes. With the BMI groups moving from thinness to obesity, the rate of low BMD would increase significantly. The results also suggested that sex might also be risk factors influencing low BMD incidence, as adolescents with low BMD were more likely to found in boys.
The amount of bone mass accrued during the growing years is a major determinant of the risk of fractures in later life.[9] The effects of overweight and obesity on bone mass and density are controversial. Some studies found positive correlations between bone mass/density and overweight/obesity,[10,13,25] whereas others showed the opposite conclusions.[26–28] In most of those studies, bone mineral density was measured at multiple sites by DXA which may better reflect adolescents’ bone mineral density. Moreover, fat mass and fat free mass , instead of total body mass, were assessed to evaluate the relationship between overweight/obese and BMD. However, these studies have various limitations respectively, including limited sample size, missing puberty stage, measurement method, and not involving calcium intake and physical activities into analysis. Therefore, the conclusion on overweight/obesity and BMD was not consistent. Data from this study indicated that the risk of low BMD would increase with the elevated BMI, comparing with adolescents with normal BMI, the risk of low BMD was lower in lean adolescents, while highest in obese adolescents. The discrepancies between different results may be explained by bone health measurement methods, measurement sites, skeleton maturation, and different standards and cutoffs to assess and define the weight groups. The mechanism of overweight/obese children having greater bone strength has been explained by the biomechanical load of body weight in weighting-bear bone[28] that high body mass, especially increased lean muscle forces, may have contributed to bone mass,[13] stimulating bone formation by suberiosteal expansion.[29] The positive association between leptin and BMD was also identified. It has been found that obese girls showed both higher serum leptin concentration and higher BMD.[10] On the other hand, overweight/obese children have several risk factors impairing bone health, including vitamin D deficiency, inadequate calcium intake, and sedentary behavior.[10,13] The association that increased PA level could predict better bone strength gain[30] may also explain the result of thin students having lower risk of low BMD in that thin students had a better PA status than overweight/obese students.[31] There was evidence suggested that obese children and adolescents were more likely to be identified with hypovitaminosis D,[32] and low vitamin D concentration could result in accelerated bone loss.[33] Meanwhile, the mechanisms of adiposity and bone mineralization are still incomplete. It was indicated that increased secretion of factors, such as cytokins, adiponectins, and resistin, resulting from excess adiposity, could mediate positively or negatively effects on bone status.[34] Moreover, the effects of body weight on bone mineral accrual were influenced by various factors, such as measurement site, sex, age, anabolic hormone status, puberty stage,[10,13,26,28] and may change over the life span. Therefore, further well designed studies that include more variables are suggested before any conclusion can be drawn.
Our data also suggests that sex was correlated with BMD level. In this study, male adolescents were more likely to have low BMD than women. Studies using quantitative ultrasound measurements or DXA to evaluate bone tissue both showed that girls had greater bone mass/BMD than boys until late adolescence.[26,35,36] A 7-year longitudinal study showed that, on average, girls were 1.5 years earlier than boys in terms of peak BMC accrual, and coincided in girls with the timing of menarche.[36] Ribeiro et al[37] showed that girls experienced early pubertal had more bone mass than boys at same age, indicating that sex hormones, especially estrogen, may have effects on bone mass accrual.[38,39]
The present study has several limitations. First, this study is cross-sectional designed, which was not allowed for a causal analysis between BMD and overweight/obese. Second, one-site qualitative measurement of BMD was used to evaluate bone status, which impeded detailed analysis of the linear correlation between bone health and relevant factors. Third, biological markers, including biomarkers of bone remodeling and hormonal changes during adolescence, were not available, precluding the investigation of overweight/obesity on bone health from the respects of internal nutrition status and endocrine level. Besides, lifestyle information, including PA, dairy intake, eating habits, etc which may have obvious effects on BMD, was not available in the study. Moreover, the subjects were all from one sub-urban district, thus not being able to represent the adolescents from the whole city. Nevertheless, this is a large-scale investigation of adolescents from middle-schools. The large sample size enabled to provide a general understanding of wide existing health problems among adolescents, which could provide directions for further research.
In conclusion, this study shows overweight/obese adolescents were more likely to low BMD, and the risk of having low BMD was higher in male adolescents. Since adolescence is a critical period of bone mineral accrual, effective interventions are needed to increase bone strength. Sex disparity was found in overweight/obese and BMD problems, and men were at higher risk, which may suggest sex-specific health promotion programs. Further well-designed studies, taking into account sex, quantitative bone mass measurement, multiple measurement sites, body composition, puberty stages, and biomarkers levels, are needed to fully investigate the correlation between overweight/obese and bone mass in adolescents.
Author contributions
Conceptualization: Leishen Wang, Zhongxian Xu, Yanmei Deng.
Data curation: Nan Li, Shuo Wang, Weiqin Li.
Formal analysis: Leishen Wang, Nan Li, Shuo Wang, Junhong Leng.
Investigation: Zhongxian Xu, Xuemei Meng, Chengshu Yu, Ming Zhao.
Methodology: Shuo Wang.
Project administration: Xuemei Meng.
Resources: Chengshu Yu, Ming Zhao.
Supervision: Zhongxian Xu, Chengshu Yu, Junhong Leng, Weiqin Li, Yanmei Deng.
Validation: Zhongxian Xu, Junhong Leng.
Writing – original draft: Leishen Wang.
Writing – review & editing: Leishen Wang, Weiqin Li.
Footnotes
Abbreviations: BMC = bone mineral content, BMD = bone mineral density, BMI = body mass index, CIs = confidence intervals, DXA = dual energy x-ray absorptiometry, ORs = odds ratios, PA = physical activity, QUS = quantitative ultrasonometry, SBP = systolic blood pressure, SOS = speed of sound.
How to cite this article: Wang L, Xu Z, Li N, Meng X, Wang S, Yu C, Leng J, Zhao M, Li W, Deng Y. The association between overweight and obesity on bone mineral density in 12 to 15 years old adolescents in China. Medicine. 2021;100:32(e26872).
LW and ZX and WL and YD contributed equally to this work.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data were shown as mean (SD) or number (percentage).
BMD = bone mineral density, BMI = body mass index, DBP = diastolic blood pressure, SBP = systolic blood pressure.
P values < .05 were shown in bold.
P values were calculated between adolescents with low/reduced BMD and adolescents with normal BMD.
P value was calculated to compare the distribution of normal, reduced, and low BMD through different BMI groups.
Adjusted for age, height Z score and SBP, and also for sex in the total analysis.
BMD = bone mineral density.
P values < .05 were shown in bold.
SBP was adjusted for all groups.
Age was adjusted for 12–15 years old adolescents.
Sex was adjusted for total groups.
References
- [1].Nelson DA, Barondess DA, Hendrix SL, et al. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res 2000;15:1992–7. [DOI] [PubMed] [Google Scholar]
- [2].Wang Y, Tao Y, Hyman ME, et al. Osteoporosis in china. Osteoporos Int 2009;20:1651–62. [DOI] [PubMed] [Google Scholar]
- [3].Miller LE, Pierson LM, Pierson ME, et al. Age influences anthropometric and fitness-related predictors of bone mineral in men. Aging Male 2009;12:47–53. [DOI] [PubMed] [Google Scholar]
- [4].Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med 1997;18: suppl: S191–4. [DOI] [PubMed] [Google Scholar]
- [5].Liberato SC, Maple-Brown L, Bressan J. Association between bone mineralization, body composition, and cardiorespiratory fitness level in young Australian men. J Clin Densitom 2015;18:187–91. [DOI] [PubMed] [Google Scholar]
- [6].Harel Z, Gold M, Cromer B, et al. Bone mineral density in postmenarchal adolescent girls in the United States: associated biopsychosocial variables and bone turnover markers. J Adolesc Health 2007;40:44–53. [DOI] [PubMed] [Google Scholar]
- [7].Zhu K, Greenfield H, Zhang Q, et al. Growth and bone mineral accretion during puberty in Chinese girls: a five-year longitudinal study. J Bone Miner Res 2008;23:167–72. [DOI] [PubMed] [Google Scholar]
- [8].Rizzoli R, Bianchi ML, Garabedian M, et al. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010;46:294–305. [DOI] [PubMed] [Google Scholar]
- [9].Bailey DA, McKay HA, Mirwald RL, et al. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res 1999;14:1672–9. [DOI] [PubMed] [Google Scholar]
- [10].Maggio AB, Belli DC, Puigdefabregas JW, et al. High bone density in adolescents with obesity is related to fat mass and serum leptin concentrations. J Pediatr Gastroenterol Nutr 2014;58:723–8. [DOI] [PubMed] [Google Scholar]
- [11].Ahmad I, Jafar T, Mahdi F, et al. Association of Vitamin D receptor (FokI and BsmI) gene polymorphism with bone mineral density and their effect on 25-hydroxyvitamin D Level in North Indian postmenopausal women with osteoporosis. Indian J Clin Biochem 2018;33:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Richert L, Chevalley T, Manen D, et al. Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J Clin Endocrinol Metab 2007;92:4380–6. [DOI] [PubMed] [Google Scholar]
- [13].Kim HY, Jung HW, Hong H, et al. The role of overweight and obesity on bone health in Korean adolescents with a focus on lean and fat mass. J Korean Med Sci 2017;32:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee YA, Kim HY, Hong H, et al. Risk factors for low vitamin D status in Korean adolescents: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2009. Public Health Nutr 2014;17:764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].do Prado WL, de Piano A, Lazaretti-Castro M, et al. Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab 2009;27:613–9. [DOI] [PubMed] [Google Scholar]
- [16].Guo Y, Yin X, Wu H, et al. Trends in overweight and obesity among children and adolescents in China from 1991 to 2015: a meta-analysis. Int J Environ Res Public Health 2019;16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wuster C, Heilmann P, Pereira-Lima J, et al. Quantitative ultrasonometry (QUS) for the evaluation of osteoporosis risk: reference data for various measurement sites, limitations and application possibilities. Exp Clin Endocrinol Diabetes 1998;106:277–88. [DOI] [PubMed] [Google Scholar]
- [18].Alwis G, Rosengren B, Nilsson JA, et al. Normative calcaneal quantitative ultrasound data as an estimation of skeletal development in Swedish children and adolescents. Calcif Tissue Int 2010;87:493–506. [DOI] [PubMed] [Google Scholar]
- [19].Halaba ZP, Pluskiewicz W. Quantitative ultrasound in the assessment of skeletal status in children and adolescents. Ultrasound Med Biol 2004;30:239–43. [DOI] [PubMed] [Google Scholar]
- [20].Baroncelli GI, Federico G, Bertelloni S, et al. Bone quality assessment by quantitative ultrasound of proximal phalanxes of the hand in healthy subjects aged 3--21 years. Pediatr Res 2001;49:713–8. [DOI] [PubMed] [Google Scholar]
- [21].Leung TF, Wang SS, Kwok FY, et al. Assessment of dietary food and nutrient intake and bone density in children with eczema. Hong Kong Med J 2017;23:470–9. [DOI] [PubMed] [Google Scholar]
- [22].WHO. The WHO Growth reference 5-19 years; 2018. Available at: http://www.who.int/growthref/en/Accessed June 7. [Google Scholar]
- [23].Rauch F, Plotkin H, DiMeglio L, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2007 Pediatric Official Positions. J Clin Densitom 2008;11:22–8. [DOI] [PubMed] [Google Scholar]
- [24].Shaiykova A, Pasquet A, Goujard C, et al. Reduced bone mineral density among HIV-infected, virologically controlled young men: prevalence and associated factors. AIDS 2018;32:2689–96. [DOI] [PubMed] [Google Scholar]
- [25].Gracia-Marco L, Ortega FB, Jimenez-Pavon D, et al. Adiposity and bone health in Spanish adolescents. The HELENA study. Osteoporos Int 2012;23:937–47. [DOI] [PubMed] [Google Scholar]
- [26].de Moraes AM, Goncalves EM, Barbeta VJ, et al. Cross-sectional study of the association of body composition and physical fitness with bone status in children and adolescents from 11 to 16 years old. BMC Pediatr 2013;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goulding A, Taylor RW, Jones IE, et al. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord 2000;24:627–32. [DOI] [PubMed] [Google Scholar]
- [28].Ivuskans A, Latt E, Maestu J, et al. Bone mineral density in 11-13-year-old boys: relative importance of the weight status and body composition factors. Rheumatol Int 2013;33:1681–7. [DOI] [PubMed] [Google Scholar]
- [29].Leonard MB, Shults J, Wilson BA, et al. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr 2004;80:514–23. [DOI] [PubMed] [Google Scholar]
- [30].Kehrig AM, Bjorkman K, Muhajarine N, et al. Moderate to vigorous physical activity and impact loading independently predict variance in bone strength at the tibia but not at the radius in children. Appl Physiol Nutr Metab 2019;44:326–31. [DOI] [PubMed] [Google Scholar]
- [31].Ying-Xiu Z, Jin-Shan Z, Jing-Yang Z, et al. Comparison on physical activity among adolescents with different weight status in Shandong, China. J Trop Pediatr 2013;59:226–30. [DOI] [PubMed] [Google Scholar]
- [32].Alemzadeh R, Kichler J, Babar G, et al. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008;57:183–91. [DOI] [PubMed] [Google Scholar]
- [33].Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014;383:146–55. [DOI] [PubMed] [Google Scholar]
- [34].Farr JN, Amin S, LeBrasseur NK, et al. Body composition during childhood and adolescence: relations to bone strength and microstructure. J Clin Endocrinol Metab 2014;99:4641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carvalho WR, Goncalves EM, Ribeiro RR, et al. Influence of body composition on bone mass in children and adolescents. Rev Assoc Med Bras (1992) 2011;57:662–7. [DOI] [PubMed] [Google Scholar]
- [36].Baxter-Jones AD, Mirwald RL, McKay HA, et al. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8-19-year-old boys and girls. Ann Hum Biol 2003;30:160–75. [DOI] [PubMed] [Google Scholar]
- [37].Ribeiro RR, Guerra-Junior G, de Azevedo Barros-Filho A. Bone mass in schoolchildren in Brazil: the effect of racial miscegenation, pubertal stage, and socioeconomic differences. J Bone Miner Metab 2009;27:494–501. [DOI] [PubMed] [Google Scholar]
- [38].Arabi A, Nabulsi M, Maalouf J, et al. Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone 2004;35:1169–79. [DOI] [PubMed] [Google Scholar]
- [39].Yilmaz D, Ersoy B, Bilgin E, et al. Bone mineral density in girls and boys at different pubertal stages: relation with gonadal steroids, bone formation markers, and growth parameters. J Bone Miner Metab 2005;23:476–82. [DOI] [PubMed] [Google Scholar]