Abstract
Rationale:
Despite the development of human papillomavirus vaccines and significant improvement in cervical cancer screening over the past few years, cervical cancer remains the fourth most common cancer in women of childbearing age after breast cancer, melanoma, and thyroid cancer.
Patient concerns:
In this case report, the patients are all cervical cancer with stage IB2 and IB3 during pregnancy, the management constitutes a major medical challenge related to the impact of treatment on both maternal and fetal outcomes. Neoadjuvant chemotherapy (NACT) is an innovative option for cervical cancer patients with stage IB2 and IB3 before cesarean delivery and radical hysterectomy, and many chemotherapeutic agents are available, cisplatin plus paclitaxel yielded good maternal and fetal outcomes to the authors’ knowledge.
Diagnoses:
Masses were discovered in the cervix of 4 pregnant women with a history of vaginal bleeding. Biopsy examination of the masses revealed cervical carcinoma, which was staged in accordance with the International Federation of Gynecology and Obstetrics (i.e., FIGO) system.
Interventions:
The patients were treated with paclitaxel plus cisplatin, followed by cesarean delivery and radical hysterectomy.
Outcomes:
The 4 patients were treated successfully, with no recurrence during follow-up periods of 14 to 56 months, and all of the children were doing well with no anomalies.
Lessons:
Although further data are required, in pregnant women with invasive cervical cancer, NACT with cisplatin plus paclitaxel followed by cesarean delivery and radical hysterectomy was a practical treatment option.
Keywords: case report, locally advanced cervical cancer, neoadjuvant chemotherapy, pregnancy
1. Introduction
Cervical cancer is the leading cause of cancer-related death among women worldwide, with approximately 300,000 deaths annually (National Institute of Health. Cervical Cancer 2020). However, invasive cervical cancer during pregnancy is an extremely rare event, with an incidence between 0.05% and 0.1%.[1] The birth rate for women >30 years of age has steadily increased over the past few decades, coupled with the fact that the peak age at most tumor occurrences is quite different than the peak age at pregnancy; as such, gestational tumors are relatively rare. When cervical cancer is diagnosed during pregnancy, successful treatment is possible if management is implemented by the collaboration of a multidisciplinary team of healthcare providers, which enables further optimization of maternal treatment while considering fetal development and providing psychological support and long-term follow-up to the infants.
The standard treatment for early stage cervical cancer is conization or simple hysterectomy, and that for locally invasive cervical cancer is concurrent chemoradiotherapy or neoadjuvant chemotherapy (NACT) followed by surgery.[2–4] During pregnancy, radiotherapy and surgery can lead to spontaneous abortion, congenital malformations, and pediatric malignancies. Therefore, in pregnant women with cervical cancer, surgery must be delayed, and NACT may help prevent disease progression until the fetal lungs mature.[5] In these cases, NACT is considered with subsequent cesarean delivery (CD) and radical hysterectomy (RH).[6] NACT with cisplatin or a combination of cisplatin, vincristine, and bleomycin has been widely used. In non-pregnant cervical cancer patients, the response rates to NACT with cisplatin plus paclitaxel reportedly range from 40% to 50%.[7] In one study involving 15 pregnant women with cervical cancer, NACT with cisplatin plus paclitaxel, followed by CD and RH[8–14] yielded good maternal and fetal outcomes (Table 1).
Table 1.
NACT with cisplatin plus paclitaxel in pregnant patients with cervical cancer.
| Author | FIGO stage | NACT regimen | Type of surgery | Adjuvant therapy | Follow-up (mo) | Maternal outcome | Fetal outcome |
| Chun et al[8] | IB1 | P + T | CD + RH + PLND + PALND | None | 49 | DOD | NL |
| IB2 | P + T | CD + RH + PLND + PALND | CT | 60 | NED | NL | |
| Palaia et al[15] | IIB | P + T | CD + RH + PLND | None | 10 | NED | NL |
| Yousefi et al[16] | IB2 | P + T | CD + RH + PLND + PALND | RT | 6 | NED | NL |
| Li et al[17] | IB2 | P + T | CD + RH + PLND | CT + RT | 21 | NED | NL |
| IB2 | P + T | CD + RH + PLND | None | 13 | NED | NL | |
| Fruscio et al[18] | IB2 | P + T | CD + RH + PLND | None | 113 | NED | NL |
| IB2 | P + T | CD + RH + PLND | None | 115 | NED | NL | |
| Kong et al[19] | IB1 | P + T | CD + RH + PLND + PALND | None | 104 | NED | NL |
| IB2 | P + T | CD + RH + PLND + PALND | CT | 35 | NED | NL | |
| IB1 | P + T | CD + RH + PLND + PALND | CT | 24 | NED | NL | |
| Ricci et al[20] | IIA | P + T | CD + RH + PLND | CT + RT | 63 | DOD | NL |
| IIA | P + T | CD + RH + PLND | RT | 31 | NED | NL | |
| IB2 | P + T | CD + RH + PLND | None | 27 | NED | NL | |
| IB2 | P + T | CD + RH + PLND | CT + RT | 18 | NED | NL |
Herein, we report a case series of 4 pregnant women with invasive cervical cancer who were treated with NACT with cisplatin plus paclitaxel, followed by CD and RH. A review of the relevant literature is also presented and discussed.
2. Case presentations
The outcomes of 4 pregnant women with cervical cancer, who were treated with NACT in the authors’ hospital between January 2013 and January 2020, are reported (Table 2). These cases were staged according to the International Federation of Gynecology and Obstetrics (i.e., “FIGO”) system. The patients were carefully supervised and provided informed written consent to treatment. Fetal evaluation was managed through serial ultrasound assessments throughout the pregnancies (Table 2). The babies’ health status was assessed according to Apgar scores at 1, 5, and 10 minutes after birth. Follow-up data regarding the babies’ health and patient survival statuses were collected every 6 months. All 4 of the patients had stage IB disease and were treated with NACT with cisplatin plus paclitaxel, in accordance with current guidelines.[9] All women underwent CD after completion of NACT. RH with bilateral salpingectomy and extensive lymph node resection was performed at the time of delivery.
Table 2.
Clinical characteristics of the patients in our case series.
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Age (yr) | 37 | 26 | 31 | 37 |
| Obstetric history | G8P3 + 4 | G4P1 + 2 | G3P1 + 1 | G2P0 |
| Stage | IB1 | IB1 | IB2 | IB2 |
| Pathological diagnosis | SC | SC | SC | SC |
| GA at diagnosis (wk) | 20 | 30 + 6 | 29 + 4 | 16 + 1 |
| GA at start of NACT (wk) | 21 + 3 | 31 + 4 | 32 + 5 | 20 + 1 |
| NACT (mg/m2, courses) | P (50, 3), T (125, 3) | P (50, 1), T (125, 1) | P (50, 1), T (135, 1) | T (135, 3), C (AUC 4) |
| GA at delivery (wk) | 35 + 3 | 34 + 5 | 36 + 4 | 36 + 3 |
| Type of surgery | CD + RH + BS + PLND + PALND | CD + RH + BS + PLND + PALND | CD + RH + BS + PLND + PALND | CD + RH + BS + PLND + PALND |
| Response to NACT | LVSI + , N + | LVSI + , N- | LVSI + , N + | LVSI + , N- |
| Postoperative treatment | RT | RT | RT | RT |
| Neonatal weight (g) | 2470 | 2330 | 2600 | 3300 |
| Neonatal sex | Male | Female | Female | Female |
| Apgar scores | 10–10-10 | 9–10-10 | 10–10-10 | 10–10-10 |
| Follow-up (mo) | 18 | 56 | 14 | 10 |
| Maternal outcome | NED | NED | NED | NED |
| Fetal outcome | NL | NL | NL | NL |
2.1. Case 1
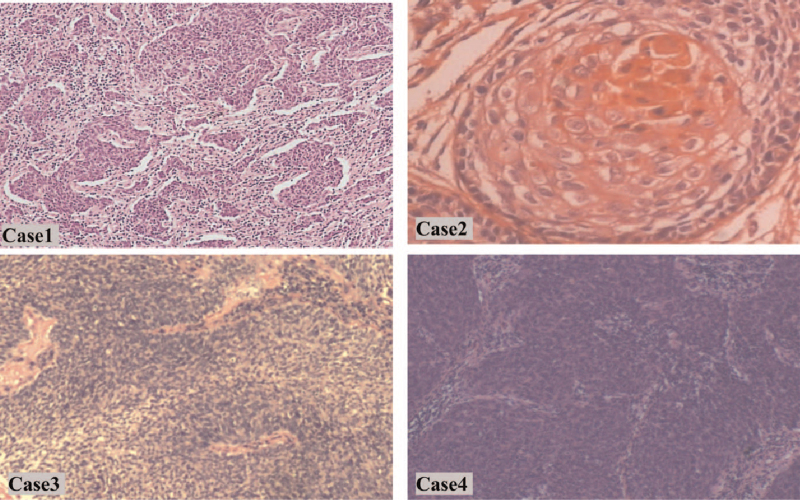
A 37-year-old woman was admitted to the authors’ hospital at 20 weeks’ gestation due to a one-month history of vaginal bleeding (Table 2). A mass measuring 2 cm in width was found in the cervix. Biopsy examination of the mass revealed cervical squamous cell carcinoma (Fig. 1). Invasive cervical cancer was diagnosed at stage IB1.
Figure 1.
Histological examination of cervical tissue biopsy samples from Cases 1 (× 100), 3 (× 100), and 4 (× 100) revealed squamous cell carcinoma. Histological examination of cervical tissue biopsy samples from Case 2 (× 400) revealed mucinous adenocarcinoma cell carcinoma.
The patient underwent NACT with cisplatin (50 mg/m2) and paclitaxel (125 mg/m2) every 3 weeks starting at 21 + 3 weeks’ gestation, and 2 more cycles were initiated at 26 + 5 weeks and 30 weeks. She experienced no major adverse reaction, after chemotherapy, the local lesions of the cervix was partial response (RECIST 1.1) and subsequently underwent RH at the time of CD at 35 + 3 weeks. Neonatal birth weight was 2470 g, and Apgar scores at 1, 5, and 10 min were 10, 10, and 10, respectively. The baby exhibited no overt malformations.
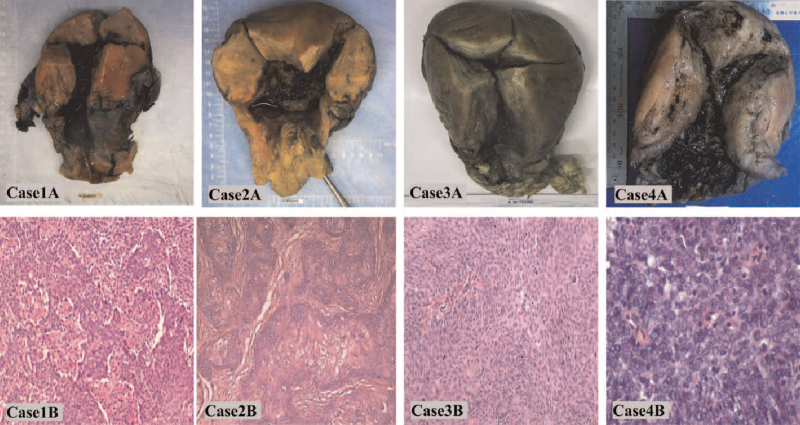
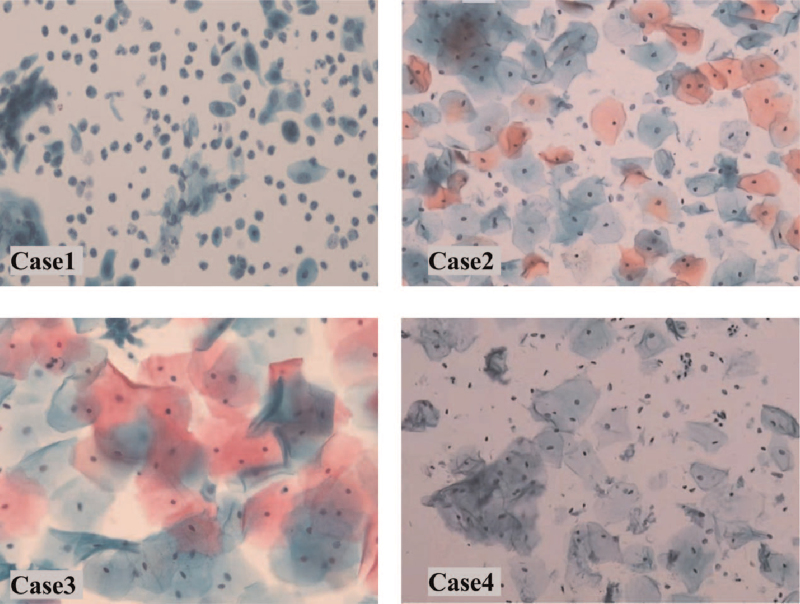
Postoperative pathological examination of the resected tumor confirmed lymphovascular space involvement (LVSI) with positive lymph nodes (Fig. 3). Accordingly, radiotherapy was administered. No recurrence or metastasis was observed in this patient during 18 months of follow-up (Fig. 4) and her child was doing well.
Figure 3.
Case 1A-4A: Surgical specimen from the uterus and parametria after caesarean section and radical hysterectomy. Case 1B (× 100), 3B (× 100), 4B (× 400). Pathological images of hematoxylin and eosin stained samples revealing squamous cell carcinoma. Case 2B (× 100). Pathological images of hematoxylin and eosin stained samples revealing mucinous adenocarcinoma.
Figure 4.
Cases 1-4. Images of liquid-based cytology testing of the vaginal cuff when followed-up recently.
2.2. Case 2
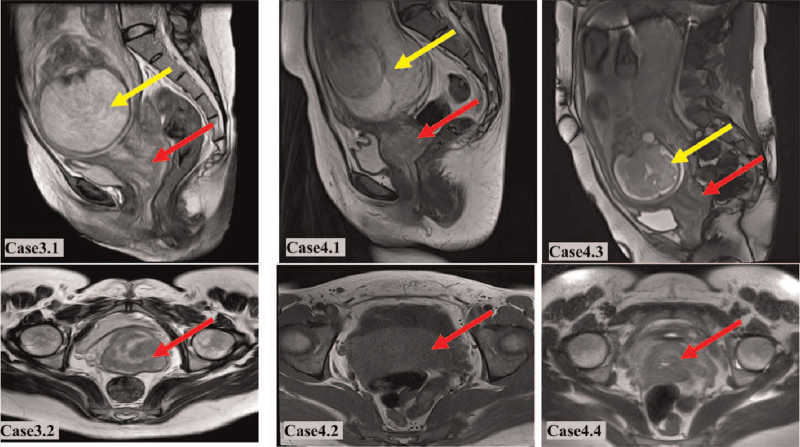
A 26-year-old woman was admitted to the authors’ hospital with vaginal bleeding at 30 + 6 weeks’ gestation (Table 2). The amount of vaginal blood loss was greater than that of normal menstrual blood loss. A cervical mass, measuring 3 cm in width, was detected on pelvic examination, and biopsy examination confirmed the presence of cervical mucinous adenocarcinoma (Fig. 1). T2 sagittal and T2 coronal pelvic magnetic resonance imaging (MRI) revealed the fetus and tumor at 29 + 4 weeks’ pregnancy (Fig. 2). Invasive cervical cancer was diagnosed at stage IB1.
Figure 2.
Case 3: T2 sagittal (3.1) and T2 coronal (3.2) pelvic magnetic resonance imaging (MRI) at 29 + 4 weeks of pregnancy reveals the fetus (yellow arrows) and tumor (red arrows). Case 4: T2 sagittal (4.1) and T2 coronal (4.2) pelvic MRI at 16 weeks and 29 weeks (4.3 and 4.4) of pregnancy reveals the fetus (yellow arrows) and tumor (red arrows).
The patient underwent treatment with NACT with cisplatin (50 mg/m2) and paclitaxel (125 mg/m2) at 31 + 4 weeks’ gestation. Because she was in the third trimester at the time cervical cancer was diagnosed, she completed only 1 cycle of NACT before surgery, and the local lesions of the cervix was SD (RECIST 1.1). The patient experienced no serious adverse reactions and then underwent RH at the time of CD at 34 + 5 weeks. Neonatal birth weight was 2330 g, and Apgar scores of the newborn at 1, 5, and 10 minutes were 9, 10, and 10, respectively. No anomalies were observed in the baby.
Postoperative pathological examination of the surgical specimen confirmed LVSI with no lymph node involvement (Fig. 3); therefore, radiotherapy was performed. No recurrence or metastasis occurred in this patient during the 56 months of follow-up (Fig. 4) and her child was healthy, with no anomalies.
2.3. Case 3
A 31-year-old woman presented with vaginal bleeding at 29 + 4 weeks’ gestation (Table 2). Pelvic examination revealed a mass, measuring > 4 cm in diameter, in the cervix. Biopsy examination of the mass confirmed cervical squamous cell carcinoma (Fig. 1). Invasive cervical cancer was diagnosed at stage IB2.
This patient was treated with NACT with cisplatin (50 mg/m2) and paclitaxel (135 mg/m2) at 32 + 5 weeks’ gestation. Because the cancer was diagnosed in the third trimester, the patient completed a single cycle of NACT before undergoing surgery, and the local lesions of the cervix was SD (RECIST 1.1). She did not experience adverse reactions and underwent RH at the time of CD at 36 + 4 weeks’ gestation. Neonatal birth weight was 2600 g, and 1, 5, and 10 min Apgar scores for the newborn were 10, 10, and 10, respectively. No anomalies were observed in the baby.
Postoperative pathological examination confirmed LVSI with positive lymph nodes (Fig. 3) and radiotherapy was performed. No recurrence or metastasis was detected in this patient during 14 months of follow-up (Fig. 4), and her baby exhibited normal growth and development.
2.4. Case 4
A 37-year-old woman, with no history of vaginal bleeding, underwent liquid-based cytology testing that revealed atypical squamous cells of undetermined significance (i.e., “ASCUS”) at 16 weeks’ gestation (Table 2). Pelvic examination revealed a mass in the cervix measuring > 2 cm in diameter. Biopsy examination of the mass confirmed cervical squamous cell carcinoma (Fig. 1). Invasive cervical cancer was diagnosed at stage IB2. This patient was treated with 3 cycles of NACT with cisplatin (50 mg/m2) and paclitaxel (135 mg/m2) every 3 weeks, which began at 20 weeks’ gestation and finished at 29 weeks. She experienced no adverse reactions and underwent RH at the time of CD at 36 + 3 weeks’ gestation. T2 sagittal and T2 coronal pelvic MRI revealed the fetus and tumor at 16 weeks and 29 weeks of pregnancy, respectively (Fig. 2), the cervical mass was partial response (RECIST 1.1) after 3 cycles of NACT. Neonatal birth weight was 3300 g, and 1, 5, and 10 minutes Apgar scores for the newborn were 10, 10, and 10, respectively. No anomalies were observed in the baby.
Postoperative pathological examination of the surgical specimen confirmed LVSI with no lymph node involvement (Fig. 3). Therefore, radiotherapy was administered. No recurrence or metastasis occurred in this patient during months of follow-up (Fig. 4) and her child was healthy, with no anomalies.
3. Discussion and conclusion
Ideally, treatment for cervical cancer during pregnancy should yield good outcomes for both mother and child. However, selecting a treatment strategy is challenging for both the patient and physician. The treatment of cervical cancer during pregnancy depends mainly on gestational age (first, second, and third trimester) at diagnosis, the extent of local invasion (stage and size of tumor), lymph node metastasis, tumor histological type, and the patient's wishes. The management of pregnancy complicated by cervical cancer follows the principle of individualization, which is based mainly on the 5 aspects of gestational age, tumor stage, grade, pathological type, and the patient's fertility desires at the time of diagnosis. Members of the International Network on Cancer, Infertility, and Pregnancy, in collaboration with other international experts, provided guidelines for gynecological cancers in pregnancy.[21] According to these guidelines, simple cervical resection or wide conization is feasible for patients with stage IA2–IB1 disease, a tumor diameter <2 cm, and negative lymph nodes. Radical trachelectomy is not recommended during pregnancy.[22] For pregnancy-preserving locally advanced cervical cancer, NACT or termination of pregnancy is recommended; NACT is the only option for patients who wish to continue the pregnancy until fetal maturity. Considering current clinical practices in China and the lack of adequate technology and experience, we recommend adopting a prudent attitude toward laparoscopic lymphadenectomy and cervical resection during pregnancy.
Chemotherapy should be administered before 35 weeks’ gestation to minimize the risk for transient neonatal myelosuppression, and to avoid maternal and fetal sepsis and hemorrhage. To reduce the risk for complications, delivery should be planned within 3 weeks after the last chemotherapy cycle.[10] Drugs such as cisplatin, vincristine, and bleomycin have been used to treat cervical cancer during pregnancy, with cisplatin one of the most widely used. In 2 studies involving pregnant women with cervical cancer, cisplatin concentrations were significantly lower in the umbilical cord blood and amniotic fluid than in the maternal blood, and all newborns were healthy.[14,23] This suggests that NACT with cisplatin can be safely used during pregnancy. Data regarding the safety of paclitaxel for the fetus are limited. A baboon model demonstrated that paclitaxel concentration in fetal blood was <2% of its maternal blood concentration.[24] In addition, a retrospective study demonstrated the relative safety of paclitaxel during the second and third trimesters of pregnancy.[17] However, the authors of that study emphasized that longer-term oncological and pediatric follow-up was necessary to confirm their findings.
In pregnant patients with early stage cervical cancer, 1 study reported that the risk for disease progression was relatively low. Most patients with stage IB disease experience satisfactory outcomes after planned treatment delay.[25] Each case is unique, and the postponement of treatment should be carefully individualized. If fetal viability is achievable, pregnant women with cervical cancer who wish to continue their pregnancies and postpone treatment must be closely monitored and followed for a prolonged period. In such women, NACT may be a feasible method for managing cervical cancer during pregnancy.
To our knowledge, 21 cases of cervical cancer during pregnancy treated using NACT with platinum-based drugs have been reported in the literature; however, no specific/standardized regimen has been established.[23] To date, when added to our 4 cases, 19 case reports have described pregnant women with cervical cancer undergoing NACT with cisplatin combined with paclitaxel before CD and RH (Table 1).[8,15–20] Significant tumor regression was observed in these women. During delivery, RH was scheduled to occur between 33 and 36 weeks’ gestation when the fetal lung exhibited sufficient maturity. Among these 19 patients, 2 died. One patient with small cell neuroendocrine carcinoma who did not undergo postoperative adjuvant therapy died of widespread metastases 2 years after the diagnosis. Another patient with squamous cell carcinoma died of recurrence 1 year after the last cycle of platinum-based chemotherapy. In the remaining 16 patients who underwent NACT with cisplatin plus paclitaxel, the tumor did not recur.
We also reviewed the relevant literature over the past 20 years. We found that 31 pregnant women with cervical cancer were treated with NACT, cisplatin alone, cisplatin plus vincristine, bleomycin, or adriamycin. Follow-up data indicated that 3 patients were lost to follow-up, 7 died of tumor recurrence, and one was alive with disease.[18,25–40]
In conclusion, pregnant patients with cervical cancer treated with NACT with cisplatin plus paclitaxel, followed by CD and RH, experienced relatively good maternal and fetal outcomes. While primary surgery and radiotherapy can adversely affect fetal outcomes, the regimen we recommend for pregnant women with cervical cancer is an efficacious and feasible alternative. However, to date, few patients have received this treatment, and further studies with longer-term follow-up are required to confirm its safety and effectiveness during pregnancy.
Author contributions
Conceptualization: Yi Quan, Ping Liu.
Data curation: Huiqiong Huang, Yi Quan.
Formal analysis: Huiqiong Huang, Yi Quan, Ping Liu.
Investigation: Huiqiong Huang, Ping Liu.
Methodology: Huiqiong Huang, Xiaorong Qi, Ping Liu.
Project administration: Huiqiong Huang, Xiaorong Qi.
Resources: Huiqiong Huang.
Supervision: Xiaorong Qi, Ping Liu.
Writing – original draft: Huiqiong Huang, Yi Quan.
Writing – review & editing: Xiaorong Qi, Ping Liu.
Footnotes
Abbreviations: CD = cesarean delivery, LVSI = lymphovascular space involvement, NACT = neoadjuvant chemotherapy, RH = radical hysterectomy .
How to cite this article: Huang H, Quan Y, Qi X, Liu P. Neoadjuvant chemotherapy with paclitaxel plus cisplatin before radical surgery for locally advanced cervical cancer during pregnancy: a case series and literature review. Medicine. 2021;100:32(e26845).
This study was approved by the ethics committee of west China Second University Hospital, Sichuan University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The patients were carefully advised and signed informed consent forms before treatment.
Written informed consent was obtained from the patients/guardians of the patient for publication of this Case Report. A copy of the written consent is available for review by the Editor of this journal.
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
ADCA = adenocarcinoma, AWD = alive with disease, CCRT = concurrent chemoradiation therapy, CD = cesarean delivery, CR = complete response, CT = chemotherapy, DOD = died of disease, FIGO = International Federation of Gynecology and Obstetrics, GADD = gestational age at diagnosis and delivery, NA = not available, NACT = neoadjuvant chemotherapy, NED = no evidence of disease, NL = normal, P = cisplatin, PALND = para-aortic lymph node dissection, PLND = pelvic lymph node dissection, PR = partial response, RH = radical hysterectomy, RT = radiotherapy, SC = squamous cell carcinoma, SCENC = small cell neuroendocrine carcinoma, T = paclitaxel.
BS = bilateral salpingectomy, GA = gestational age, LVSI = lymphovascular space involvement, N = lymph node.
References
- [1].La Russa M, Jeyarajah AR. Invasive cervical cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol 2016;33:44–57. [DOI] [PubMed] [Google Scholar]
- [2].De Vincenzo R, Amadio G, Ricci C, et al. Treatment of cervical cancer in Italy: strategies and their impact on the women. Vaccine 2009;27:A39–45. [DOI] [PubMed] [Google Scholar]
- [3].Fanfani F, Fagotti A, Ferrandina G, et al. Neoadjuvant chemoradiation followed by radical hysterectomy in FIGO Stage IIIB cervical cancer: feasibility, complications, and clinical outcome. Int J Gynecol Cancer 2009;19:1119–24. [DOI] [PubMed] [Google Scholar]
- [4].Ferrandina G, Ercoli A, Fagotti A, et al. Completion surgery after concomitant chemoradiation in locally advanced cervical cancer: a comprehensive analysis of pattern of postoperative complications. Ann Surg Oncol 2014;21:1692–9. [DOI] [PubMed] [Google Scholar]
- [5].Ricci C, Scambia G, Vincenzo RD. Locally advanced cervical cancer in pregnancy: overcoming the challenge: a case series and review of the literature. Int J Gynecol Cancer 2016;26:1490–6. [DOI] [PubMed] [Google Scholar]
- [6].Hunter MI, Tewari K, Monk BJ. Cervical neoplasia in pregnancy. Part 2: current treatment of invasive disease. Am J Obstet Gynecol 2008;199:10–8. [DOI] [PubMed] [Google Scholar]
- [7].Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 1999;17:2676–80. [DOI] [PubMed] [Google Scholar]
- [8].Chun KC, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical surgery in early cervical cancer during pregnancy: three case reports. Jpn J Clin Oncol 2010;40:694–8. [DOI] [PubMed] [Google Scholar]
- [9].Peccatori FA, Azim HA, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi160–70. [DOI] [PubMed] [Google Scholar]
- [10].Brewer M, Kueck A, Runowicz CD. Chemotherapy in pregnancy. Clin Obstet Gynecol 2011;54:602–18. [DOI] [PubMed] [Google Scholar]
- [11].Amant F, Van Calsteren K, Halaska MJ, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol 2012;13:256–64. [DOI] [PubMed] [Google Scholar]
- [12].Amant F, Van Calsteren K, Halaska MJ, et al. Gynecologic cancers in pregnancy: guidelines of an international consensus meeting. Int J Gynecol Cancer 2009;19:S1–2. [DOI] [PubMed] [Google Scholar]
- [13].Amant F, Halaska MJ, Fumagalli M, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer 2014;24:394–403. [DOI] [PubMed] [Google Scholar]
- [14].Marnitz S, Kohler C, Oppelt P, et al. Cisplatin application in pregnancy: first in vivo analysis of 7 patients. Oncology 2010;79:72–7. [DOI] [PubMed] [Google Scholar]
- [15].Palaia I, Pernice M, Graziano M, Bellati F, Panici PB. Neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer during pregnancy: a case report. Am J Obstet Gynecol 2007;197:e5–6. [DOI] [PubMed] [Google Scholar]
- [16].Yousefi Z, Hoshyar AH, Kadkhodayan S, Hasanzade M, Kalantari MR, Mottaghi M. Neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer during pregnancy: case report and review of literature. Oman Med J 2013;28:60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li J, Wang LJ, Zhang BZ, Peng YP, Lin ZQ. Neoadjuvant chemotherapy with paclitaxel plus platinum for invasive cervical cancer in pregnancy: two case report and literature review. Arch Gynecol Obstet 2011;284:779–83. [DOI] [PubMed] [Google Scholar]
- [18].Fruscio R, Villa A, Chiari S, et al. Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review. Gynecol Oncol 2012;126:192–7. [DOI] [PubMed] [Google Scholar]
- [19].Kong TW, Lee EJ, Lee Y, Chang SJ, Son JH, Ryu HS. Neoadjuvant and postoperative chemotherapy with paclitaxel plus cisplatin for the treatment of FIGO stage IB cervical cancer in pregnancy. Obstet Gynecol Sci 2014;57:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ricci C, Scambia G, De Vincenzo R. Locally Advanced Cervical Cancer in Pregnancy: Overcoming the Challenge. A Case Series and Review of the Literature. Int J Gynecol Cancer 2016;26:1490–6. [DOI] [PubMed] [Google Scholar]
- [21].Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol 2019;30:1601–12. [DOI] [PubMed] [Google Scholar]
- [22].Ilancheran A. Neoadjuvant chemotherapy in cervical cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol 2016;33:102–7. [DOI] [PubMed] [Google Scholar]
- [23].Kohler C, Oppelt P, Favero G, et al. How much platinum passes the placental barrier? Analysis of platinum applications in 21 patients with cervical cancer during pregnancy. Am J Obstet Gynecol 2015;213:206.e1-5. [DOI] [PubMed] [Google Scholar]
- [24].Calsteren KV, Verbesselt R, Devlieger R, et al. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int J Gynecol Cancer 2010;20:1456–64. [DOI] [PubMed] [Google Scholar]
- [25].Karam A, Feldman N, Holschneider CH. Neoadjuvant cisplatin and radical cesarean hysterectomy for cervical cancer in pregnancy. Nat Clin Pract Oncol 2007;4:375–80. [DOI] [PubMed] [Google Scholar]
- [26].Hecking T, Abramian A, Domrose C, et al. Individual management of cervical cancer in pregnancy. Arch Gynecol Obstet 2016;293:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giacalone PL, Laffargue F, Benos P, Rousseau O, Hedon B. Cis-platinum neoadjuvant chemotherapy in a pregnant woman with invasive carcinoma of the uterine cervix. Br J Obstet Gynaecol 1996;103:932–4. [DOI] [PubMed] [Google Scholar]
- [28].Lai CH, Hsueh S, Chang TC, et al. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol 1997;64:456–62. [DOI] [PubMed] [Google Scholar]
- [29].Tewari K, Cappuccini F, Gambino A, Kohler MF, Pecorelli S, DiSaia PJ. Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer 1998;82:1529–34. [DOI] [PubMed] [Google Scholar]
- [30].Marana HR, de Andrade JM, da Silva Mathes AC, Duarte G, da Cunha SP, Bighetti S. Chemotherapy in the treatment of locally advanced cervical cancer and pregnancy. Gynecol Oncol 2001;80:272–4. [DOI] [PubMed] [Google Scholar]
- [31].Caluwaerts S, K. VANC, Mertens L, et al. Neoadjuvant chemotherapy followed by radical hysterectomy for invasive cervical cancer diagnosed during pregnancy: report of a case and review of the literature. Int J Gynecol Cancer 2006;16:905–8. [DOI] [PubMed] [Google Scholar]
- [32].Bader AA, Petru E, Winter R. Long-term follow-up after neoadjuvant chemotherapy for high-risk cervical cancer during pregnancy. Gynecol Oncol 2007;105:269–72. [DOI] [PubMed] [Google Scholar]
- [33].Benhaim Y, Pautier P, Bensaid C, Lhomme C, Haie-Meder C, Morice P. Neoadjuvant chemotherapy for advanced stage cervical cancer in a pregnant patient: report of one case with rapid tumor progression. Eur J Obstet Gynecol Reprod Biol 2008;136:267–8. [DOI] [PubMed] [Google Scholar]
- [34].Boyd A, Cowie V, Gourley C. The use of cisplatin to treat advanced-stage cervical cancer during pregnancy allows fetal development and prevents cancer progression: report of a case and review of the literature. Int J Gynecol Cancer 2009;19:273–6. [DOI] [PubMed] [Google Scholar]
- [35].Seamon LG, Downey GO, Harrison CR, Doss B, Carlson JW. Neoadjuvant chemotherapy followed by post-partum chemoradiotherapy and chemoconsolidation for stage IIIB glassy cell cervical carcinoma during pregnancy. Gynecol Oncol 2009;114:540–1. [DOI] [PubMed] [Google Scholar]
- [36].Smyth EC, Korpanty G, McCaffrey JA, Mulligan N, Carney DN. Small-cell carcinoma of the cervix at 23 weeks gestation. J Clin Oncol 2010;28:e295–7. [DOI] [PubMed] [Google Scholar]
- [37].Rabaiotti E, Sigismondi C, Montoli S, Mangili G, Candiani M, Vigano R. Management of locally advanced cervical cancer in pregnancy: a case report. Tumori 2010;96:623–6. [DOI] [PubMed] [Google Scholar]
- [38].Favero G, Chiantera V, Oleszczuk A, et al. Invasive cervical cancer during pregnancy: laparoscopic nodal evaluation before oncologic treatment delay. Gynecol Oncol 2010;118:123–7. [DOI] [PubMed] [Google Scholar]
- [39].de Lima CA, Barcelos AC, Paschoini Mde C, et al. Conservative treatment of uterine cervical adenocarcinoma in pregnancy. Case Rep Obstet Gynecol 2013;2013:692017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Peculis LD, Ius Y, Campion M, Friedlander M, Hacker N. Stage IB2 adenosquamous cervical cancer diagnosed at 19-weeks’ gestation. Aust N Z J Obstet Gynaecol 2015;55:94–7. [DOI] [PubMed] [Google Scholar]