PURPOSE:
Value-based programs, such as the Oncology Care Model (OCM), seek to improve care for patients undergoing chemotherapy, while reducing total costs. The purpose of this study is to quantify the impact of adopting biosimilar granulocyte colony-stimulating factors (G-CSFs) for febrile neutropenia (FN) primary prophylaxis (PP) from a US practice perspective.
METHODS:
A 1-year economic analysis on real-world direct drug costs and health care resource utilization was conducted in a hypothetical cohort of 500 patients with nonmyeloid cancer receiving chemotherapy. The first model simulated total cost savings of biosimilar versus reference G-CSFs over six cycles of chemotherapy. The second model evaluated cost and outcome implications of expanding the use of biosimilar G-CSFs to an additional 10% of patients at intermediate FN risk.
RESULTS:
Based on real-world evidence over 1 year, a total of 121 of 500 patients received G-CSF prophylaxis resulting in cost savings that ranged from $0.54M US dollars (USD) (short-acting, eg, filgrastim) to $1.68M USD (long-acting, eg, pegfilgrastim) when switching from reference to biosimilar G-CSFs. Expanding the use of biosimilar G-CSFs allowed an additional 24 patients to receive prophylaxis of FN, leading to cost savings of $0.03M USD or $1.19M USD, with a reduction of $0.08M USD in FN-related resource utilization cost. The per-patient per-year cost saving for long-acting G-CSFs was about $3,000 USD.
CONCLUSION:
The implementation of biosimilar versus reference G-CSFs to OCM-participating practices results in a reduction of costs and facilitates achieving OCM metrics by improving patients' outcomes while expanding biosimilar G-CSFs access to patients at intermediate risk of chemotherapy-induced FN.
INTRODUCTION
Granulocyte colony-stimulating factors (G-CSFs) are used to reduce febrile neutropenia (FN) incidence associated with systemic chemotherapy.1 Based on published evidence from clinical and economic studies, routine prophylaxis using G-CSFs has been recommended by both the National Comprehensive Cancer Network (NCCN) and the ASCO practice guidelines in patients receiving high-risk chemotherapy (ie, those with a > 20% risk of developing chemotherapy-induced FN without G-CSF prophylaxis).2-4 Recommendations for using G-CSF prophylaxis in patients at intermediate risk (ie, those with a 10%-20% risk of developing chemotherapy-induced FN) are less clear, and guidelines state that independent clinical judgment should be exercised based on the individual patient's situation.4 However, emerging evidence suggests that it is cost effective to provide routine, primary prophylaxis of FN using G-CSFs within the intermediate-risk population, which may lead to reductions in health care utilization and costs.5-7 The provision of cost-effective care is one of the drivers of various alternative payment models, such as the Oncology Care Model (OCM).
Owing to the high cost of managing patients with cancer, the OCM was developed with the ultimate goal of reducing costs and improving the quality of care that beneficiaries receive by fostering coordinated, high-quality, and cost-effective cancer care.8,9 Under the OCM, practices enter into arrangements in the form of retrospective performance-based payments if they meet the cost and quality goals. Specifically, these practices must demonstrate a reduction in drug-related costs and in hospitalizations following the use of chemotherapy.10 The TrACER study is evaluating one way to potentially reduce hospitalizations through instituting outcome improvement initiatives aimed at appropriate G-CSF utilization for FN prophylaxis.11 However, despite the known benefits in clinical practice, primary prophylaxis (ie, prophylaxis with the first cycle of chemotherapy) for FN may still be suboptimal.12 The NCCN has provided short-term guidance stating that more expanded primary prophylaxis in patients at intermediate risk of FN following chemotherapy may be considered.13
A reason for the low rate of G-CSF prophylaxis is the historically high acquisition cost of G-CSFs (ie, when only reference G-CSFs were available), causing practices and health systems to be mindful about the potential impact on their overall and drug-related costs.14 However, with the introduction of biosimilar G-CSFs, significant cost savings with the same quality of care may be realized. It is estimated that in the United States, biosimilars have the potential to generate $44.2 billion US dollars (USD) in cost savings from 2014 to 2024.15 Moreover, these cost savings can be reinvested by expanding access for additional patients to receive G-CSF prophylaxis. This would likely lower expenses for the practice, patients, and the health care system, while also offering opportunities for practices to meet their OCM goals through improved outcomes.5,16 Nevertheless, there is a lack of economic analysis modeling using real-world data to estimate the cost differences that include both direct drug costs and health care resource utilization (HCRU) from an OCM-participating practice's perspective; the budget impact models previously published take into consideration the payer's perspective.17 There is also lag of acceptance of biosimilar products, although clinical trials have demonstrated the equivalence in efficacy and safety of biosimilar to reference G-CSFs.18-21 Accordingly, an economic analysis was performed to quantify the potential effect of conversion to biosimilar G-CSFs on OCM metrics. The first objective is to quantify, from a US practice perspective, the potential financial impact of conversion from reference to biosimilar G-CSFs on drug-related costs. The second objective is to assess the economic implications of expanded access to biosimilar G-CSFs in patients receiving myelosuppressive chemotherapy at intermediate risk of FN.
METHODS
Model
For this economic analysis, a sequential set of two simulation models were developed in Microsoft Excel22 to simulate the economic impact of adopting biosimilar G-CSFs in the OCM. Following the conversion from reference to biosimilar G-CSFs, outcomes measured within the models were (1) G-CSF administration and drug-related costs and (2) FN-related HCRU including emergency visits, outpatient visits, and hospitalizations, as well as costs associated with HCRU.
A hypothetical panel included 500 oncology patients with a heterogenous mix of nonmyeloid malignancies receiving six cycles of chemotherapy in a 1-year time horizon (Fig 1). Patients were stratified into three categories based on the risk of developing FN when treated with chemotherapy as defined by the NCCN Guidelines: high risk (> 20% risk), intermediate risk (10%-20% risk), and low risk (< 10% risk).4,23 Based on real-world evidence, the model assumed that a certain proportion of patients received prophylaxis with reference G-CSFs in each chemotherapy risk category.23
FIG 1.
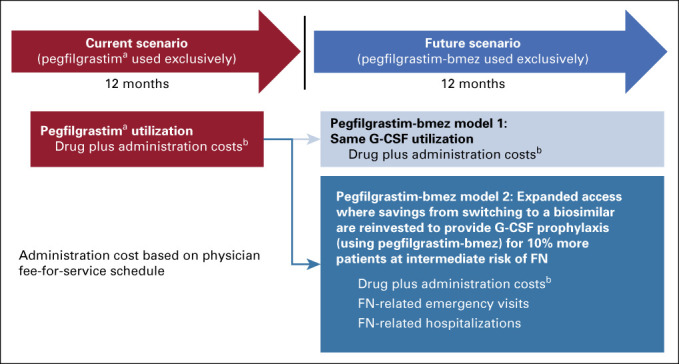
Practice switches from reference pegfilgrastim to biosimilar pegfilgrastim-bmez. aPegfilgrastim dosage forms include both the PFS and the OBI. bCosts based on current WAC; administration cost based on physician fee-for-service schedule. FN, febrile neutropenia; G-CSF, granulocyte colony-stimulating factor; OBI, on-body injector; PFS, prefilled syringe; WAC, wholesale acquisition cost.
The first model (model 1) estimated cost savings when patients received prophylactic use of biosimilar G-CSFs for FN instead of reference G-CSFs (ie, switch from reference pegfilgrastim prefilled syringe [PFS] to biosimilar pegfilgrastim-bmez, from reference pegfilgrastim on-body injector [OBI] to biosimilar pegfilgrastim-bmez or from reference filgrastim to biosimilar filgrastim-sndz) as well as differences in FN-related HCRU and costs. The second model (model 2) estimated cost savings when more patients received prophylactic use of biosimilar G-CSFs instead of reference G-CSFs. Specifically, model 2 increased the number of patients in the intermediate-risk category who received prophylactic G-CSFs for prevention of FN by 10%, while keeping the proportion of patients receiving prophylactic G-CSF in both high- and low-risk categories constant. In all, model 2 simulated a situation where oncology practices reinvested cost savings accrued from model 1 back to patients with cancer, hence expanded access with biosimilar G-CSF prophylaxis. The robustness of the results was tested via one-way sensitivity analyses: reference pegfilgrastim PFS cost, biosimilar pegfilgrastim-bmez cost, percentage of patients on chemotherapy at intermediate risk of FN, and percentage of intermediate risk patients receiving G-CSFs were varied by ±50%.
Cost Inputs
In both models, total costs (including direct drug costs, drug administration costs, nursing labor costs, and FN-related HCRU costs) were compared between reference and biosimilar G-CSF prophylaxis and cost savings per patient per year (PPPY) were estimated. Direct drug costs were based on current wholesale acquisition costs for reference pegfilgrastim PFS ($6,231.06 USD), reference pegfilgrastim OBI ($6,231.06 USD), reference filgrastim ($531.43 USD), biosimilar pegfilgrastim-bmez ($3,925.53 USD), and biosimilar filgrastim-sndz ($438.98 USD).24 Drug administration costs were based on physician fee schedules for administration costs of G-SCFs, published in April 2020, by the Centers for Medicare and Medicaid Services.25 Nursing labor costs were calculated by multiplying the number of observed nursing hours needed to administer each drug26 with published national average nurse wages in 2020. Finally, HCRU costs associated with FN were estimated based on average costs reported in the literature.26,27 The proportion of patients who received prophylactic use of G-CSF, but still experienced FN events, was estimated. Costs of such FN events that would result in (1) emergency department (ED) visits with hospitalization, (2) ED visit without hospitalization, (3) outpatient visit with hospitalization, and (4) outpatient visit without hospitalization were estimated.28-30
Clinical Inputs
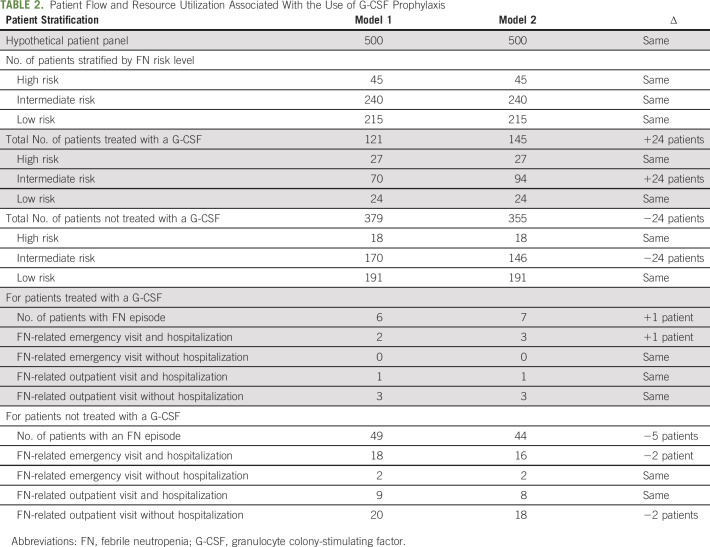
Stratified prevalence to estimate the number of patients receiving prophylactic G-CSF based on the risk of FN after chemotherapy was derived from published, real-world data. Baig et al23 estimated the prevalence of high-, intermediate-, and low-FN risk regimens among patients with heterogeneous mix of nonmyeloid cancers and the percentage of those patients who received G-CSF prophylaxis within each risk category (see Appendix Table A1, online only). These percentages were used to calculate the number of patients receiving high-, intermediate-, and low-risk regimens among the hypothetical 500-patient panel (Table 1) and to subsequently calculate the number of patients receiving G-CSF prophylaxis within each risk category.23 In model 1, there were 59% of patients in the high-risk category, 29% of patients in the intermediate-risk category, and 11% of patients in the low-risk category who received prophylactic G-CSF (Table 2).23 In model 2, the proportion of patients at intermediate risk of FN receiving prophylactic G-CSFs increased from 29% to 39%. Other assumed input values are shown in Table 1.
TABLE 1.
Modeling Inputs and Assumptions
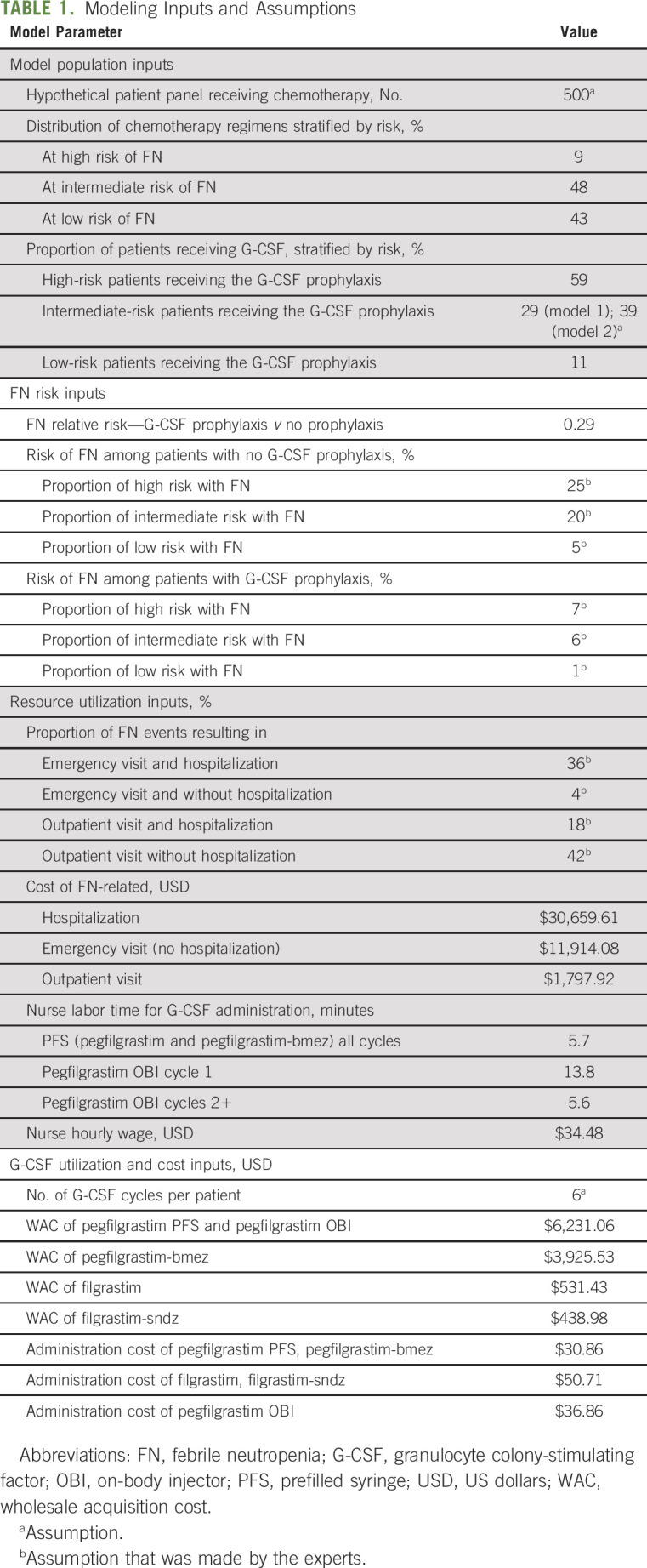
TABLE 2.
Patient Flow and Resource Utilization Associated With the Use of G-CSF Prophylaxis
RESULTS
For a hypothetical panel of 500 patients with nonmyeloid cancer being treated with chemotherapy during a year, it was estimated that 45 patients (9%) were at high risk of FN, 240 patients (48%) were at intermediate risk of FN, and 215 patients (43%) were at low risk of FN (Table 1).
Model 1: Reference Versus Biosimilar G-CSFs
Of all patients treated with chemotherapy, 27 patients (59%) at high risk, 70 patients (29%) at intermediate risk, and 24 patients (11%) at low risk would receive prophylactic use of G-CSF. Thus, in total, there would be 121 patients receiving G-CSF for prophylaxis of FN. Among these, six patients would experience an FN event, resulting in two ED visits with subsequent hospitalization, one outpatient visit with subsequent hospitalization, and three outpatient visits only (Table 2). The results in cost savings simulations are shown in Table 3.
TABLE 3.
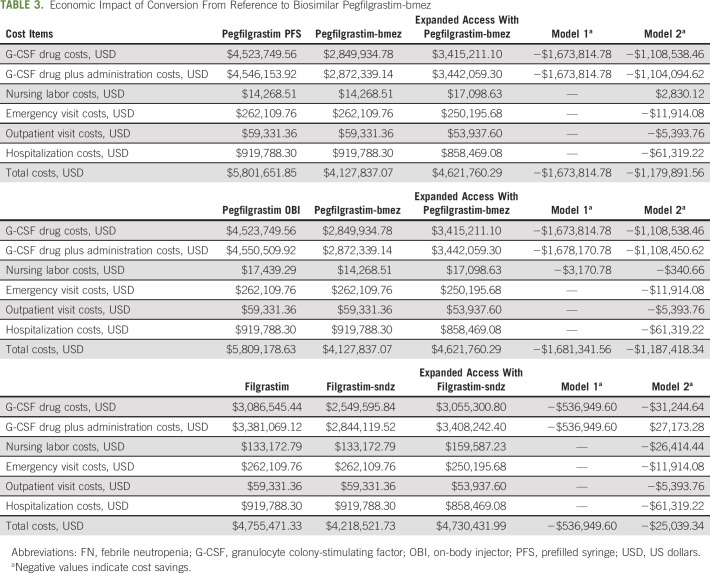
Economic Impact of Conversion From Reference to Biosimilar Pegfilgrastim-bmez
Conversion from reference pegfilgrastim PFS to biosimilar pegfilgrastim-bmez.
The cost of reference pegfilgrastim PFS over six cycles was estimated to be $4.53M USD. The administration and nursing labor costs added up to $36,673 USD. HCRU costs were estimated to be $1.24M USD. In total, it would cost $5.80M USD to manage 121 patients with cancer for prophylaxis of FN with reference pegfilgrastim PFS. In contrast, the cost of biosimilar pegfilgrastim-bmez was estimated to be $2.85M USD, which was $1.67M USD less than reference pegfilgrastim PFS. Since G-CSF prophylaxis patterns did not change, FN-related HCRU and costs remained the same after conversion (because of the same efficacy). Therefore, it would cost $4.13M USD to manage the same patients with biosimilar pegfilgrastim-bmez, resulting in total cost savings of $1.67M USD or $3,347.63 USD PPPY.
Conversion from reference pegfilgrastim OBI to biosimilar pegfilgrastim-bmez.
The direct drug cost of pegfilgrastim OBI would be the same as that of pegfilgrastim PFS; however, it would cost $4,356 USD more to administer pegfilgrastim OBI, because of higher administration costs, to 121 patients over six cycles with a total cost of $5.81M USD. Although the cost of biosimilar pegfilgrastim-bmez and FN-related health care costs would remain the same, the cost savings from converting reference pegfilgrastim OBI to biosimilar pegfilgrastim-bmez would be $1.68M USD or $3,362.68 USD PPPY.
Conversion from reference filgrastim to biosimilar filgrastim-sndz.
Both reference filgrastim and biosimilar filgrastim-sndz were assumed to be administered to 121 patients for 8 days per cycle over six cycles. The cost of the drug and administration would be $3.38M USD for reference filgrastim. Because patients typically return to the practice to receive daily short-acting G-CSF injections, administration and nursing labor costs increased to approximately $0.43M USD. The total costs of managing patients with reference filgrastim were $4.76M USD. After converting to biosimilar filgrastim-sndz, the total costs would decrease to $4.22M USD for a cost savings of $0.54M USD or $1,073.90 USD PPPY.
Model 2: Expanded Access to Biosimilar G-CSFs for Patients at Intermediate Risk of FN
For this expanded access model, we reinvested cost savings to provide G-CSF prophylaxis for incrementally 10% more patients at intermediate risk of FN, so that another 24 patients could receive prophylactic G-CSF, leading to 145 patients being managed for FN. Among these patients, seven would experience FN events leading to three ED visits with subsequent hospitalization, one outpatient visit with subsequent hospitalization, and three outpatient visits without hospitalization (Table 2).
Conversion from reference pegfilgrastim PFS to expanded access with biosimilar pegfilgrastim-bmez.
With a higher number of patients treated in the intermediate-risk category (from 70 to 94 patients), the cost of biosimilar pegfilgrastim-bmez would rise to $3.42M USD. Nonetheless, there would still be a $1.11M USD savings compared with the cost of reference pegfilgrastim PFS in model 1. The increase in nursing labor and administration costs of $7,274 USD was offset by reduction in ED visit costs ($11,914 USD reduction), in outpatient visit costs ($5,394 USD reduction), and in hospitalization costs ($61,319 USD reduction). Upon conversion to biosimilar pegfilgrastim-bmez, increasing the number of patients at intermediate risk of FN and receiving biosimilar G-SCF would lead to a $1.18M USD cost savings or $2,359.78 USD PPPY.
Conversion from reference pegfilgrastim OBI to expanded access with biosimilar pegfilgrastim-bmez.
The savings in direct drug costs would be $1.11M USD when converting from reference pegfilgrastim OBI to biosimilar pegfilgrastim-bmez in the expanded access scenario, with a total health care savings of $1.19M USD or $2,374.84 USD PPPY.
Conversion from reference filgrastim to expanded access with biosimilar filgrastim-sndz.
Direct drug costs were decreased by $31,244 USD when converting from reference filgrastim to biosimilar filgrastim-sndz in the expanded access scenario. The administration and nursing labor costs increased by $84,832 USD, because of an incremental number of patients with cancer receiving G-CSF prophylaxis. With savings in reduction of FN events, converting from reference filgrastim to expanded access with biosimilar filgrastim-sndz would lead to cost savings of $25,039 USD or $50.08 USD PPPY.
Sensitivity Analysis
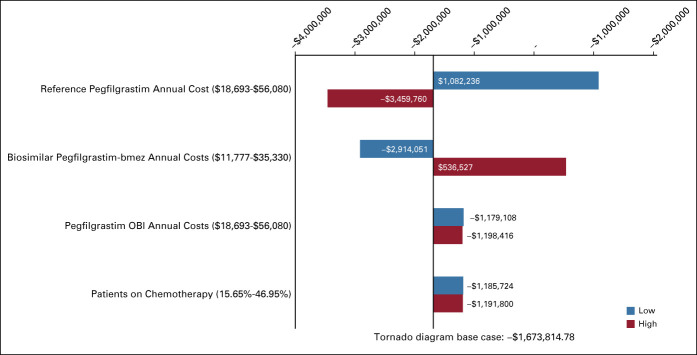
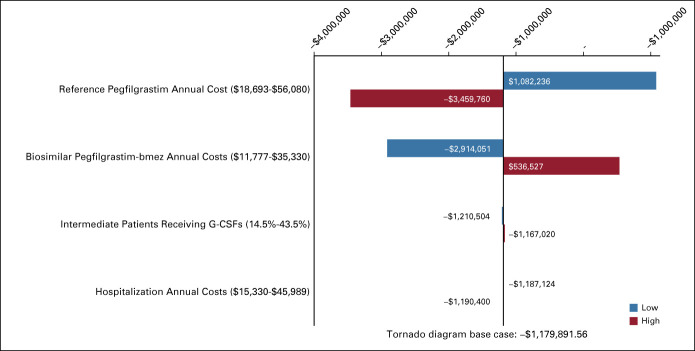
In Appendix Figures A1 and A2 (online only), tornado diagrams show one-way sensitivity analysis results for models 1 and 2 (respectively), when switching from reference pegfilgrastim to biosimilar pegfilgrastim-bmez. This analysis demonstrated that the budget impact was the most sensitive to change in reference pegfilgrastim cost, followed by change in biosimilar pegfilgrastim-bmez cost.
DISCUSSION
This study is unique as it focuses on the OCM and how expanding primary prophylaxis with biosimilar G-CSFs can lead to substantial cost savings compared with reference G-CSFs. The goal of the OCM is to use appropriately aligned financial incentives to enable improved care coordination, appropriateness of care, and access to care for beneficiaries undergoing chemotherapy.10 Conversion to biosimilar G-CSFs would allow more patients at intermediate risk of FN to be treated with prophylactic biosimilar G-CSF, with fewer patients experiencing FN events and still generating savings to the practices and health care system. It fits the OCM intention to improve health outcomes and produce higher quality care at the same or lower cost to Medicare. In fact, evaluation of OCM practices has demonstrated a straightforward strategy for therapeutic substitution of a biosimilar for the reference G-CSF.31
The potential for biosimilar medicines to yield cost savings in the OCM framework is remarkable and will free up resources in participating practices, allowing them to implement practice redesign activities to improve the quality of care being delivered and ultimately patient outcomes.31-35 Biosimilar G-CSF offers the same benefit as its reference at a lower cost, which is an opportunity to reduce lower-value care.36 Furthermore, reinvesting some of these savings to treat additional patients may lead to improved overall population outcomes, meeting key criteria of the OCM key metrics. In fact, when reviewing data from 2014 to 2018, CMS has found more than a 50% increase in biosimilar filgrastim utilization in OCM-participating practices.31 The findings of this study reinforce the need to expanding value-based use of G-CSFs to fit for the goals of OCM.
In November 2019, CMS initiated discussions about the next phase of the OCM, namely, the Oncology Care First (OCF) Model. Specifically, the OCF model encompasses two primary payment models: (1) Monthly Population Payment: in which practices would be paid monthly in lump sums for Evaluation and Management services, including enhanced services and drug administration costs, and (2) Performance-Based Payment: intended to hold practices accountable for total cost of care, including drug costs, and to encourage practices to reduce their cost of care for 6-month episodes of care.37 Hence, the use of biosimilar medicines could be key in the OCF model for enhanced affordability, reduced costs, and increased access to biologic treatments. Biosimilar G-CSFs in oncology practices could offer an effective strategy for reducing costs while also ensuring that patients receive medications with the same benefits. This could provide significant support to achieve episode target cost thresholds in the future OCF model.
Current clinical practice guidelines are less clear about the need for primary prophylaxis for intermediate-risk patients. This is historically due to concerns about the cost of G-CSFs. The availability of biosimilar medicines presents an opportunity for oncology practices to reassess the cost effectiveness of expanding primary FN prophylaxis to more patients, potentially generating additional real-world evidence to update treatment recommendations for a selected group of patients. In light of COVID-19, both ASCO and NCCN have issued recommendations to expand the use of G-CSF prophylaxis among patients at intermediate and high level of expected risk (eg, > 10% risk) for prevention of neutropenic fevers.38,39 The primary goal is to minimize the risk of hospitalization, reduce patient exposure to SARS-CoV-2, and free up hospital resources to battle the ongoing pandemic. Although the results of this budget impact model further highlight the importance of biosimilar uptake in clinical practice to control or reduce total costs, it cannot answer questions related to cost effectiveness or the optimal FN risk threshold by which primary prophylaxis should be considered. Given that FN treatment patterns and the costs related to FN-related hospitalizations and G-CSFs have changed significantly since the FN risk threshold was established, future economic models using a decision analysis and real-world data should be constructed to evaluate whether the 20% FN risk threshold is still applicable Additionally, cost-effectiveness studies evaluating prophylaxis strategies using biosimilar G-CSFs can help clinicians and payers gain a better understanding of the benefit of various prophylaxis strategies in the current climate of value-based care.
Some of the limitations of this study are associated with the economic model itself. A model represents a simplification of the complex utilization patterns of G-CSF prophylaxis within a hypothetical oncology practice population. Hence, the generalization of the model results is limited to the patient scenarios and the chemotherapy risk assumptions examined. The model also assumes the exclusive use of one long-acting G-CSF within a practice and does not consider a scenario where multiple products are used. Publicly available data were used to estimate the cost of pegfilgrastim and the biosimilar pegfilgrastim-bmez; data on discounts and rebates are confidential and could not be integrated into the model. In addition, clinician input was used to estimate the distribution of patients with an emergency or outpatient visit when experiencing an FN event, which might not necessarily be generalizable to other practices.
In conclusion, our study demonstrates benefits of conversion from reference to biosimilar G-CSFs for prophylaxis of FN among patients with cancer who are undergoing myelosuppressive chemotherapy and further clinical and economic benefits of expanded access with biosimilar G-CSFs in the intermediate-risk category. The cost savings are substantial and can provide significant benefits to oncology practices that participate in the OCM by delivering on the mission to improve population-based outcomes at a lower cost.
Appendix
FIG A1.
Impact on total costs: model 1. Low and high are referring to the low and high values varied by ±50% for the average of each variable.
FIG A2.
Impact on total costs: model 2. Low and high are referring to the low and high values varied by ±50% for the average of each variable. G-CSF, granulocyte colony-stimulating factor.
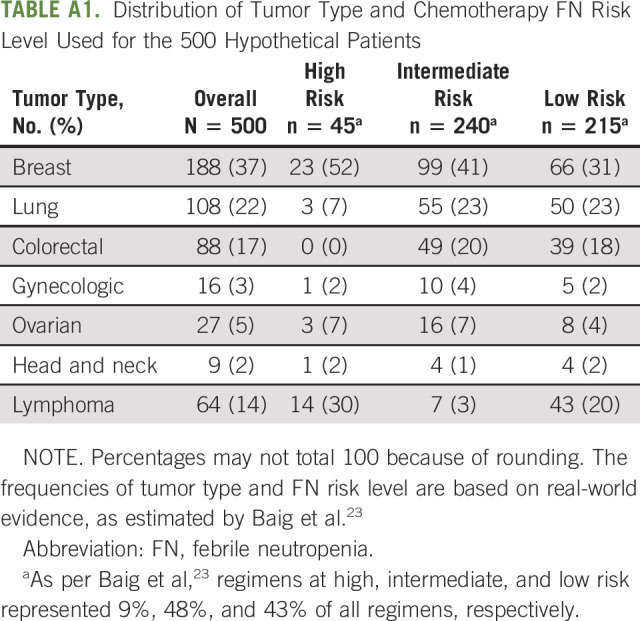
TABLE A1.
Distribution of Tumor Type and Chemotherapy FN Risk Level Used for the 500 Hypothetical Patients
Weijia Wang
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Sandoz
Edward Li
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Kim Campbell
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Novartis
Ali McBride
Consulting or Advisory Role: Pfizer, Sandoz, EMD Serono
Speakers' Bureau: Coherus Biosciences, Incyte, Bristol Myers Squibb
Steve D'Amato
Honoraria: ION Pharma, G1 Therapeutics
Consulting or Advisory Role: Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: ION Pharma
No other potential conflicts of interest were reported.
DISCLAIMER
The sponsor was involved in the study design, analysis, and publication. All authors vouch for the accurate representation of the data within this manuscript.
SUPPORT
Supported by Sandoz Inc, Princeton, NJ.
AUTHOR CONTRIBUTIONS
Conception and design: Weijia Wang, Edward Li, Kim Campbell, Ali McBride
Collection and assembly of data: Weijia Wang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Economic Analysis on Adoption of Biosimilar Granulocyte Colony-Stimulating Factors in Patients With Nonmyeloid Cancer at Risk of Febrile Neutropenia Within the Oncology Care Model Framework
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Weijia Wang
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Sandoz
Edward Li
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Kim Campbell
Employment: Sandoz
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Novartis
Ali McBride
Consulting or Advisory Role: Pfizer, Sandoz, EMD Serono
Speakers' Bureau: Coherus Biosciences, Incyte, Bristol Myers Squibb
Steve D'Amato
Honoraria: ION Pharma, G1 Therapeutics
Consulting or Advisory Role: Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: ION Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kuderer NM Dale DC Crawford J, et al. : Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol 25:3158-3167, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Freifeld AG Bow EJ Sepkowitz KA, et al. : Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56-e93, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Smith TJ Bohlke K Lyman GH, et al. : Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199-3212, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Becker PS Griffiths EA Alwan LM, et al. : NCCN guidelines insights: Hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw 18:12-22, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Lyman GH Mezzio D Li EC, et al. : A cost-effectiveness analysis of primary prophylaxis (PP) versus secondary prophylaxis (SP) with biosimilar myeloid growth factors (MGFs) for preventing chemotherapy-induced febrile neutropenia (FN) in non-Hodgkin lymphoma (NHL) patients at intermediate risk. J Clin Oncol 37, 2019. (suppl; abstr 107) [Google Scholar]
- 6.Li E Mezzio D Spargo A, et al. : Cost-effectiveness of filgrastim-sndz as primary prophylaxis (PP) versus secondary prophylaxis (SP) to prevent chemotherapy-induced febrile neutropenia (FN) in non-small cell lung cancer (NSCLC) patients at intermediate risk. J Clin Oncol 38, 2020. (suppl; abstr e19401) [Google Scholar]
- 7.Li E Mezzio D Spargo A, et al. : Cost-effectiveness of filgrastim-sndz as primary prophylaxis (PP) versus secondary prophylaxis (SP) to prevent chemotherapy-induced febrile neutropenia (FN) in breast cancer patients at intermediate risk. J Clin Oncol 38, 2020. (suppl; abstr 73) [Google Scholar]
- 8.DeMartino JK, Larsen JK: Equity in cancer care: Pathways, protocols, and guidelines. J Natl Compr Canc Netw 10:S1-S9, 2012. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 9.Page RD Newcomer LN Sprandio JD, et al. : The patient-centered medical home in oncology: From concept to reality. Am Soc Clin Oncol Ed Book:e82-89, 2015 [DOI] [PubMed] [Google Scholar]
- 10.CMS.gov : Oncology Care Model. https://innovation.cms.gov/initiatives/Oncology-Care/, 2020 [Google Scholar]
- 11.Bansal A Sullivan SD Hershman DL, et al. : A stakeholder-informed randomized, controlled comparative effectiveness study of an order prescribing intervention to improve colony stimulating factor use for cancer patients receiving myelosuppressive chemotherapy: The TrACER study. J Comp Eff Res 6:461-470, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitolo U Angrili F DeCosta L, et al. : G-CSF use in patients receiving first-line chemotherapy for non-Hodgkin's lymphoma (NHL) and granulocyte-colony stimulating factors (G-CSF) as observed in clinical practice in Italy. Med Oncol 33:139, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Griffiths EA Alwan LM Bachiashvili K, et al. : Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic. J Natl Compr Canc Netw doi: 10.6004/jnccn.2020.7610 [epub ahead of print on September 1, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotta F Mayer F Mecozzi A, et al. : Impact of guidance on the prescription patterns of G-CSFs for the prevention of febrile neutropenia following anticancer chemotherapy: A population-based utilization study in the Lazio region. BioDrugs 31:117-124, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulcahy AW, Predmore Z, Mattke S: The Cost Savings Potential of Biosimilar Drugs in the United States. https://www.rand.org/pubs/perspectives/PE127.readonline.html, 2014 [Google Scholar]
- 16.Patel K Patel M Gor A, et al. : Oncology practice transformation helps deliver patient-centered cancer care in a community oncology practice. Am J Manag Care 24:SP147-SP148, 2018 [PubMed] [Google Scholar]
- 17.Wang W Ribes D Campbel K, et al. : A Budget Impact Analysis on the Prophylactic Use of Biosimilar Pegfilgrastim in Non-Myeloid Cancer Patients at Risk of Chemotherapy-Induced Febrile Neutropenia and Its Expanded Use to Intermediate-Risk Patients. Abstract presented at Academy of Managed Care Pharmacy (AMCP) Annual Meeting, April, 2020; AMCP eLearning
- 18.Harbeck N Lipatov O Frolova M, et al. : Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12:1359-1367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackwell K Donskih R Jones CM, et al. : A comparison of proposed biosimilar LA-EP2006 and reference pegfilgrastim for the prevention of neutropenia in patients with early-stage breast cancer receiving myelosuppressive adjuvant or neoadjuvant chemotherapy: Pegfilgrastim randomized oncology (supportive care) trial to evaluate comparative treatment (PROTECT-2), a phase III, randomized, double-blind trial. Oncologist 21:789-794, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon K Braithwaite J Berry B, et al. : Biosimilar perceptions among healthcare professionals and commercial medical benefit policy analysis in the United States. BioDrugs 35:103-112, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Leonard E Wascovich M Oskouei S, et al. : Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. J Manag Care Spec Pharm 25:102-112, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Microsoft Excel. Redmond, WA, Microsoft Press, Division of Microsoft Corp, 2016 [Google Scholar]
- 23.Baig H Somlo B Eisen M, et al. : Appropriateness of granulocyte colony-stimulating factor use in patients receiving chemotherapy by febrile neutropenia risk level. J Oncol Pharm Pract 25:1576-1585, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micromedex Solutions : Pegfilgrastim, filgrastim, pegfilgrastim-bmez and filgrastim-sndz. Grand Rapids, MI, Truven Health Analytics IAA. http://www.micromedexsolutions.com [Google Scholar]
- 25.CMS.gov : CY 2020 Physician Fee Schedule Final Rule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched, 2020 [Google Scholar]
- 26.Hatfield MD Reitan S Moser L, et al. : A time-motion observational study of clinic staff: Administration of pegfilgrastim primary prophylaxis via next-day manual injection and on-body injector (OBI). Value in Health 21:S137-S138, 2018 [Google Scholar]
- 27.US Bureau of Labor Statistics : Occupational Outlook Handbook: Registered Nurses. https://www.bls.gov/ooh/healthcare/registered-nurses.htm, 2020 [Google Scholar]
- 28.Elting LS Lu C Escalante CP, et al. : Outcomes and cost of outpatient or inpatient management of 712 patients with febrile neutropenia. J Clin Oncol 26:606-611, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Schwartzberg LS Lal LS Balu S, et al. : Clinical outcomes of treatment with filgrastim versus a filgrastim biosimilar and febrile neutropenia-associated costs among patients with nonmyeloid cancer undergoing chemotherapy. J Manag Care Spec Pharm 24:976-984, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai E Guy GP Dunbar A, et al. : Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 13:e552-e561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CMS.gov : Oncology Care Model—Third Annual Evaluation Report: Performance Periods 1-3. https://innovation.cms.gov/data-and-reports/2020/ocm-evaluation-annual-report-2, 2020 [Google Scholar]
- 32.Aapro M, Cornes P, Abraham I: Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract 18:171-179, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Sun D Andayani TM Altyar A, et al. : Potential cost savings from chemotherapy-induced febrile neutropenia with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European union G5 countries: A simulation study. Clin Ther 37:842-857, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Aapro M Cornes P Sun D, et al. : Comparative cost efficiency across the European G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in patients with cancer. Ther Adv Med Oncol 4:95-105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulacsi L Brodszky V Baji P, et al. : The rituximab biosimilar CT-P10 in rheumatology and cancer: A budget impact analysis in 28 European countries. Adv Ther 34:1128-1144, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker B Frytak J Hayes J, et al. : Evaluation of practice patterns among oncologists participating in the Oncology Care Model. JAMA Netw Open 3:e205165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CMS.gov : Oncology Care First Model: Informal Request for Information. https://innovation.cms.gov/files/x/ocf-informalrfi.pdf, 2020 [Google Scholar]
- 38.American Society of Clinical Oncology : COVID-19 Patient Care Information. https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19, 2020 [Google Scholar]
- 39.NCCN Hematopoietic Growth Factors . Short-term recommendations specific to issues with COVID-19 (SARS-CoV-2). https://www.nccn.org/covid-19/pdf/HGF_COVID-19.pdf, 2020