Abstract
Atrial fibrillation is considered to be the most common arrhythmia in the clinic, and it gradually increases with age. In recent years, there has been increasing evidence that atrial fibrillation may exacerbate the progression of cognitive dysfunction. The current guidelines recommend ablation for drug-refractory atrial fibrillation.
We aimed to prospectively analyze changes in cognitive function in patients with atrial fibrillation following treatment using different ablation methods.
A total of 139 patients, with non-valvular atrial fibrillation, were included in the study. The patients were divided into the drug therapy (n = 41) and catheter ablation (n = 98) groups, with the catheter ablation group further subdivided into radiofrequency ablation (n = 68) and cryoballoon (CY) ablation (n = 30). We evaluated cognitive function at baseline, 3- and 12-months follow-up using the Telephone Interview for Cognitive Status-modified (TICS-m) test, then analyzed differences in cognitive function between the drug therapy and catheter ablation groups, to reveal the effect of the different ablation methods.
We observed a significantly higher TICS-m score (39.56 ± 3.198) in the catheter ablation group at 12-month follow-up (P < .001), than the drug treatment group was. Additionally, we found no statistically significant differences in TICS-m scores between the radiofrequency ablation and CY groups at 3- and 12-month postoperatively (P > .05), although the two subgroups showed statistically significant cognitive function (P < .001).
Overall, these findings indicated that radiofrequency and CY ablation improve cognitive function in patients with atrial fibrillation.
Keywords: atrial fibrillation, cognitive function, cryoballoon ablation, radiofrequency ablation
1. Introduction
Atrial fibrillation (AF) is the most common form of arrhythmia in clinical practice. AF prevalence and incidence have been shown to range from 0.2% to 7.9% and 0.5–1.4/1000 patients per year, respectively.[1] The global burden of AF is currently on the rise, owing to aging of the society. Dementia, mainly manifested as cognitive dysfunction, is being more common in the elderly population in a similar fashion to AF. Dementia is a progressive cognitive impairment in the absence of any disturbances in consciousness, with a prevalence > 5% in people over 65 years. This prevalence reportedly doubles up every five years after the age of 65.[2] Previous studies have shown that AF is a risk factor for ischemic stroke and heart failure.[3] Functionally, various AF-related pathological mechanisms, including cerebral hypoperfusion, inflammation, brain atrophy, genetics factors, and shared risk factors have been shown to affect cognitive function.[4] Thrombus originating from the left atrial appendage has been proven to be one of the causes of embolic stroke in AF patients.[5] To date, systemic anticoagulation remains the cornerstone of stroke prevention treatment and has been shown to significantly reduce the risk of overall stroke and systemic embolism compared to warfarin, and non-vitamin K antagonist oral anticoagulants.[6] According to the current guidelines, catheter ablation represents the first-line therapy for treating symptomatic and drug-refractory AF. The most prevalent ways of ablation are radiofrequency ablation and cryoballoon (CY).[7] However, efficacy of catheter ablation on cognitive function is controversial, whereas the effect of radiofrequency ablation (RFCA) and CY on cognitive function is unclear. In this study, we sough to evaluate the effect of different treatment methods cognitive function in AF patients.
2. Methods
2.1. Study population
A total of 139 patients with nonvalvular atrial fibrillation (NVAF) were enrolled at the cardiology department and ward of Beijing Anzhen Hospital, Capital Medical University from August 2018 to August 2019. The study group, the average age of 60.90 ± 8.51 years old, comprised 97 and 42 males and females, respectively. 41 patients who received conventional antiarrhythmic treatment were placed in the drug treatment group, whereas 98 who underwent radiofrequency ablation (n = 68) and CY (n = 30) were in the catheter ablation group. We documented NVAF in all patients, whereas AF was recorded in at least one electrocardiogram (ECG) before hospitalization. AF was confirmed by surface ECG or 24-hour Holter. All patients were adults over 18 years old and volunteered to participate in the study.
Exclusion criteria were as follows: (1) patients with valvular heart disease, such as rheumatic heart disease; (2) presence of diseases affecting cognitive function, such as stroke, intracranial hemorrhage, brain tumor, severe carotid artery stenosis, severe intracranial artery stenosis, and mental illness, among other, (3) Various types of dementia, such as Alzheimer's disease and vascular dementia; (4) a history of prior catheter ablation or patient who underwent catheter ablation relapsed, then received another ablation treatment or a second ablation; (5) patients with intracardiac thrombosis, active inflammation, infectious diseases who could not undergo catheter ablation; (6) severe coagulopathy, hypertension (>180/110mm Hg and poor blood pressure control), liver insufficiency, renal insufficiency (creatinine clearance rate < 30 ml/min), and end-stage malignant tumors; (7) impaired hearing that could not allow follow-up.
We provided a detailed explanation of risks associated with ablation, all patients provided written informed consent before receiving treatment. The study was approved by the ethics committee of Beijing Anzhen Hospital affiliated with Capital Medical University (No.2018083X).
2.2. Treatment strategy
Treatment plans were provided according to patients’ condition, with patients allowed to select the final plans as per their wishes. Patients in the drug treatment group received traditional antiarrhythmic drugs, to lower ventricular rate below 110 beats/min and anticoagulant therapy according to the CHA2DS2-VASc score.[8] Non-vitamin K antagonist oral anticoagulants (rivaroxaban 20 mg QD or dabigatran 150 mg Bid) were selected according to the patient's own wishes.
Treatment procedures were performed under conscious sedation and analgesia, using appropriate doses of midazolam and fentanyl. For the catheter procedure, systemic anticoagulation was performed by infusion of low molecular weight heparin to maintain an activated clotting time of 300 to 500 seconds. Measurements were performed routinely, after every 30 min.
For RFCA, we used a transseptal approach to access the left atrium (LA) from the right femoral vein with an 8.5F sheath (Fast Cath, St. Jude Medical). Then, we sent a PentaRay catheter (Biosense Webster) into the LA and created a LA activation map. RFCA was performed using an irrigated contact force-sensing catheter (Navistar Smart Touch, Biosense Webster) at a temperature and power limits of 43°C and 35 W, respectively. This ablation aimed to isolate the antrum in front of pulmonary veins.
CY ablation was performed using a double-walled balloon (2AF283, Medtronic), a general single frozen time of 180 seconds (if the temperature was below -55°C, stop frozen). This was based on time to the isolation of pulmonary vein to decide whether to supplement ablation. Generally, the order of ablation entails ablation of left superior, left inferior, right superior, right inferior. During the ablation of the left pulmonary vein, the pacing electrode was kept in the right ventricle and the vagal reflex was prevented during rewarming. The pacing electrode was placed in the superior vena cava when ablating the right pulmonary vein, whereas the right phrenic nerve packed (2 V/1000ms) during ablation. Stimulation of the diaphragmatic muscle was then observed. Pulmonary vein isolation was confirmed with Achieve catheter (990063–020, Medtronic).
2.3. Assessment of cognitive function
Telephone Interview for Cognitive Status-modified (TICS-m) is an efficient and cost-effective screening instrument for detecting mild cognitive impairment. TICS-m scores range from 0 to 50 and are divided into 3 cognitive domains, namely memory (20 points), directivity (13 points), language, and attention (17 points). A cutoff score ≤of 27 indicates individuals with dementia, whereas scores ranging from 28 to 31 indicate individuals with mild cognitive impairment.[9] All patients underwent a prospective cognitive function assessment of using the TICS-m at baseline and 3- and 12-months follow-up. The procedure includes an education adjustment, where 5 points are added to the score for patients with less than 8 years of education, 2 points are added to the score for patients with 8 to 10 years of education, no points are added to the score for those with 11 to 15 years for education, whereas 2 points are subtracted from scores of patients with 16 or more years of education.
2.4. Follow up
All patients were asked to perform a 24-hour Holter ECG monitor during a 3-month and 12-months follow-up period, or ECGs when patients’ symptoms were suggestive of AF. Anticoagulation and antiarrhythmic therapy continued for 3 to 6 months after ablation, in the catheter group. We also evaluated TICS-m scores at 3- and 12-months of follow-up
2.5. Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 23.0 (SPSS Inc, Chicago, IL) and Microsoft Excel 2016 (Microsoft Office). Normally distributed continuous variables conforming to the normal distribution were expressed as the mean ± standard deviation, and continuous variables inconsistent with normal distribution were expressed as a median. Categorical variables were expressed as frequencies (percentage). Continuous variables were compared by Student t-tests or a Mann-Whitney U test, whereas categorical variables were compared using a χ2 test or Fisher exact test. Repeated measures analysis of variance was used to compare the serial changes in the cognitive function of the radiofrequency ablation groups and CY group at 3 months and 12 months after ablation. A multivariable linear regression analysis was used to explore the associated variables of a treatment impaired cognitive function. A value of P < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
A total of 139 patients with NVAF were included in this study, including 97 males and 42 females, with an average age of (60.90 ± 8.51) years. There were also no statistically significant differences between the two groups in terms of course of the disease, complications, LA size, ejection fraction, smoking, alcohol consumption, use of anticoagulant drugs, and cognitive function score before enrollment (P < .05), but there were statistically significant differences in gender, age, CHA2DS2-VASc score, HAS-BLED score, and type of AF (P < .05, Table 1).
Table 1.
Baseline characteristics of patients in the drug treatment and catheter ablation groups.
| drug treatment (n = 41) | RFCA (n = 68) | CY (n = 30) | P-values | |
| Male/n (%) | 22 (53.66%) | 56 (82.35%) | 19 (63.33%) | .005a,b |
| Age/yr | 64.51 ± 8.12 | 58.68 ± 8.46 | 61.00 ± 7.62 | .002a,b |
| BMI (kg/m2) | – | 26.33 ± 3.32 | 26.09 ± 2.85 | .730 |
| recurrence /n (%) | – | 18 (26.47%) | 5 (16.67%) | .291 |
| Persistent AF/n (%) | 22 (53.66%) | 16 (23.53%) | 6 (20.00%) | .001b |
| Hypertension/n (%) | 25 (60.98%) | 31 (45.59%) | 18 (60.00%) | .445 |
| Diabetes/n (%) | 14 (34.15%) | 13 (19.12%) | 5 (16.67%) | .127 |
| CHD/n (%) | 17 (41.46%) | 11 (16.18%) | 4 (13.33%) | .004b |
| Heart failure/n (%) | 10 (24.39%) | 10 (14.71%) | 6 (20.00%) | .209 |
| CHA2DS2-VASc score | 2 (0, 5) | 1 (0, 4) | 2 (0, 6) | .000a,b |
| HAS-BLED score | 2 (0, 4) | 1 (0, 4) | 1 (0, 3) | .008a,b |
| Time of operation /minutes | – | 150 (89, 239) | 100.5 (70, 245) | .000 |
| LA short axis/mm | 41 (30,68) | 39 (28, 53) | 40 (25, 48) | .232 |
| LVEF (%) | – | 63 (32, 75) | 62.5 (54, 73) | .997 |
| Heart rate | – | 70 (52, 120) | 70 (50, 95) | .957 |
| The course of disease/month | 12 (48) | 24 (40) | 36 (63) | .273 |
| Smoking/n (%) | 16 (39.02%) | 20 (29.41%) | 20 (29.41%) | .076 |
| Drinking/n (%) | 14 (34.15%) | 22 (32.35%) | 22 (32.35%) | .934 |
| Anticoagulant therapy/n (%) | 21 (51.22%) | 38 (55.88%) | 14 (46.67%) | .764 |
| Antithrombotic drugs /n (%) | 13 (31.71%) | 26 (38.24%) | 10 (33.33%) | .643 |
| Antihypertensive drugs/n (%) | 20 (48.78%) | 25 (36.76%) | 17 (56.67%) | .154 |
| Hypoglycemic agent /n (%) | 10 (24.39%) | 10 (14.71%) | 3 (10.00%) | .232 |
| Lipid-lowering drugs /n (%) | 12 (29.27%) | 22 (32.35%) | 9 (30.00%) | .937 |
| TICS-m score at baseline | 36.41 ± 3.03 | 36.90 ± 2.97 | 36.90 ± 2.97 | .647 |
We divided the catheter ablation group into two subgroups: RFCA and CY based on different ways of ablation, and observed a 100% postoperative success rate across these groups. We observed statistically significant differences (P < 0.05) between the two groups with regards to operation duration, but not in recurrence rates (P > 0.05, Table 1)
3.2. Changes in cognitive function during a 12-months follow-up period
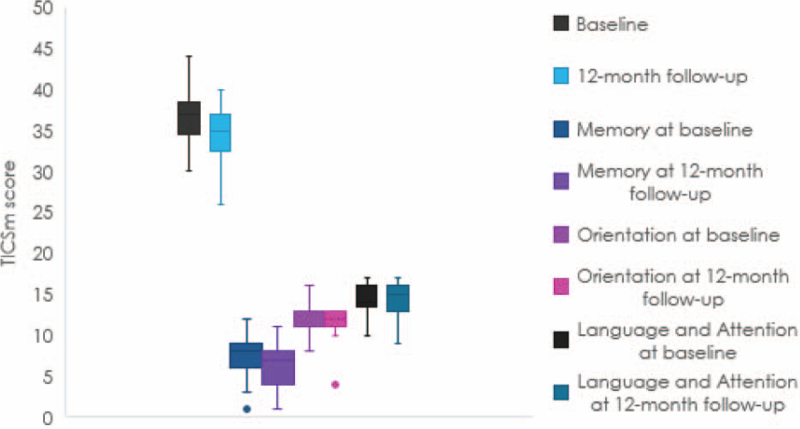
We compared changes in cognitive function scores for patients in the drug treatment and catheter ablation groups, at baseline and 12-months follow-up (Table 2). Results revealed no significant differences in total TICS-m scores between the groups at baseline. Conversely, TICS-m scores of patients in the catheter ablation group (39.56 ± 3.198) were significantly improved compared to those in the drug treatment group (34.44 ± 3.271) (t = 8.553, P = .000) at 12-month follow-up. The cognitive decline of patients in the drug treatment group was mainly reflected in memory (Fig. 1).
Table 2.
Cognitive function comparison between drug therapy group and catheter ablation group.
| Drug treatment | Catheter ablation | t-values | P-values | |
| Baseline | 36.41 ± 3.033 | 36.74 ± 3.097 | 0.577 | .565 |
| 12-mo | 34.44 ± 3.271 | 39.56 ± 3.198 | 8.553 | .000 |
Figure 1.
Changes in cognitive function in the drug treatment group.
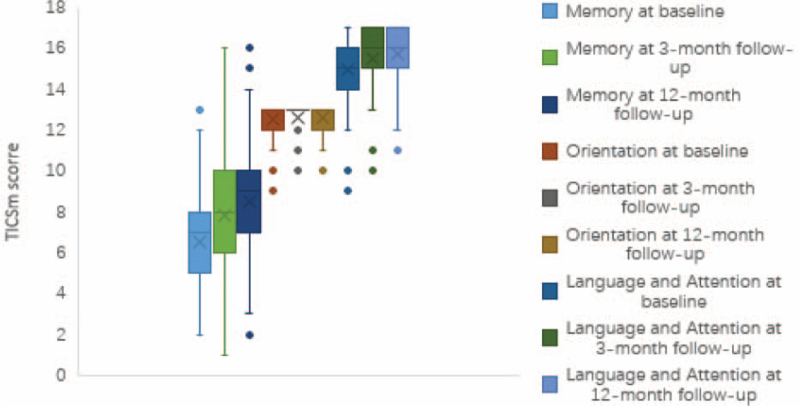
A comparison of cognitive function, between the radiofrequency ablation and CY subgroups, at baseline, 3- and 12-months after ablation revealed no statistically significant differences in TICS-m score (P > .05)(Table 3). However, we observed a significant improvement in postoperative cognitive function of patients in both subgroups, which was mainly reflected in memory, language, and attention (Table 4; Fig. 2).
Table 3.
Comparison of cognitive function between the radiofrequency ablation group and the cryoballoon group.
| Difference | 95%CI | F-values | P-values | |
| Groups | ||||
| Baseline | 0.497 | (−0.854, 1.848) | 0.533 | .467 |
| 3 mo | 0.475 | (−0.917, 1.868) | 0.460 | .499 |
| 12 mo | 0.136 | (−1.262, 1.535) | 0.037 | .847 |
| RFCA | ||||
| Time | 37.162 | P < .001 | ||
| Baseline vs 3 mo | −1.912 | (−2.727, −1.096) | P < .001 | |
| Baseline vs 12 mo | −2.706 | (−3.479, −1.933) | P < .001 | |
| 3 mo vs 12 mo | −0.794 | (−1.587, −0.001) | .049 | |
| CY | ||||
| Time | 20.547 | P < .001 | ||
| Baseline vs 3m | −1.933 | (−3.161, −0.705) | .001 | |
| Baseline vs 12 mo | −3.067 | (−4.230, −1.903) | P < .001 | |
| 3 mo vs 12 mo | −1.133 | (−2.327, 0.060) | .068 | |
| Group × Time | 0.247 | .782 | ||
Table 4.
Radiofrequency ablation and cryoballoon subgroup analysis of cognitive function.
| Total | Memory | Orientation | Language and Attention | |||||
| RFCA | CY | RFCA | CY | RFCA | CY | RFCA | CY | |
| Baseline | 36.897 ± 0.377 | 36.400 ± 0.467 | 6.765 ± 2.273 | 5.967 ± 0.656 | 12.485 ± 0.872 | 12.533 ± 0.900 | 14.882 ± 1.653 | 15.067 ± 1.964 |
| 3 mo | 38.809 ± 0.388 | 38.333 ± 0.584 | 8.059 ± 2.467 | 7.200 ± 2.976 | 12.602 ± 0.736 | 12.667 ± 0.844 | 15.485 ± 1.388 | 15.567 ± 1.591 |
| 12 mo | 39.603 ± 0.390 | 39.467 ± 0.587 | 8.647 ± 2.438 | 8.200 ± 2.987 | 12.618 ± 0.792 | 12.567 ± 0.774 | 15.632 ± 1.303 | 15.900 ± 1.398 |
| Overall analysis (F,P) | ||||||||
| Group | 0.372, 0.544 | 2.313, 0.132 | 0.019, 0.891 | 0.344, 0.559 | ||||
| Time | 49.552,0.000 | 32.157, 0.000 | 1.067, 0.346 | 22.584, 0.000 | ||||
| group∗time | 0.235, 0.790 | 0.369, 0.692 | 0.253, 0.771 | 0.298, 0.732 | ||||
| Groups | P-values | |||||||
| Baseline | .467 | .132 | .804 | .632 | ||||
| 3 mo | .499 | .140 | .707 | .799 | ||||
| 12 mo | .847 | .414 | .768 | .362 | ||||
| Time | P-values | |||||||
| 3 mo-baseline | .000 | .001 | .000 | .016 | .596 | .780 | .000 | .047 |
| 12 mo-baseline | .000 | .000 | .000 | .000 | .390 | .993 | .000 | .001 |
| 12 mo-3mo | .049 | .068 | .118 | .063 | .998 | .861 | .528 | .188 |
Figure 2.
Changes in cognitive function in catheter ablation group.
3.3. Factors affecting cognitive function
Baseline data and results from single-factor analysis allowed us to select group, gender, age, CHA2DS2-VASc score, HAS-BLED score, persistent atrial fibrillation, and coronary heart disease for inclusion in the multiple linear regression analysis (enter method). Results indicated that only the different groups had statistically significant effects on changes in cognitive function. Specifically, the catheter ablation group significantly improved cognitive function compared to the drug treatment group. The rest of the factors did not significantly change cognitive function in AF patients (Table 5).
Table 5.
Linear regression model of cognitive function changes after 12 months of drug treatment and catheter ablation.
| β (95%CI) | SE | P-values | |
| Group | 4.944 (3.595, 6.292) | 0.681 | .000 |
| Sex | −0.566 (−2.118, 0.987) | 0.785 | .472 |
| Age | −0.054 (−0.130, 0.023) | 0.039 | .168 |
| CHA2DS2-VASc | −0.083 (−0.827, 0.661) | 0.376 | .825 |
| HAS-BLED score | −0.258 (−0.935, 0.420) | 0.343 | .454 |
| Type of AF | −0.657 (−1.895, 0.582) | 0.626 | .286 |
| CHD | −0.090 (−1.777, 1.597) | 0.853 | .916 |
In two subgroups, the group and surgery time was included in the multiple linear regression analysis (Enter method). Results indicated that group had no significant effect on cognitive function scores, there was an influence of the procedure time at the cognitive function changes at 12-month follow-up. (Tables 6 and 7).
Table 6.
Linear regression model of cognitive function changes between radiofrequency ablation and cryoballoon at 3-month follow-up.
| β (95%CI) | SE | P-values | |
| Group | −0.993 (−2.625, 0.639) | 0.822 | .230 |
| Surgery time | −0.012 (−0.032, 0.008) | 0.010 | .233 |
Table 7.
Linear regression model of the cognitive function changes between radiofrequency ablation and cryoballoon at 12-month follow-up.
| β (95%CI) | SE | P-values | |
| Group | −1.057 (−2.670, 0.555) | 0.812 | .196 |
| Surgery time | −0.021 (−1.777, 1.597) | 0.010 | .033 |
4. Discussion
In this study, we successfully used the TICS-m test to prospectively evaluate changes in cognitive function in AF patients, following catheter ablation or drug therapy. Results revealed no major adverse events during the follow-up. However, a significantly higher cognitive function was recorded in the catheter ablation, relative to the drug treatment group, 12 months after the operation and this was mainly manifested in memory. Besides, we found no statistically significant difference in cognitive function between the RFCA and CY subgroups, 3- and 12 months after the operation, although the cognitive function of the two groups was significantly improved compared to preoperative cognitive function, especially in terms of memory, language and attention. These results indicate that the RFCA and CY treatment methods enhance cognitive function, and are not affected by other confounding factors.
Our study did not use other reported cognitive screening tests such as the Mini-Mental State Examination or the Montreal Cognitive Assessment. Because these screening tests require face-to-face interviews, which are difficult to conduct in a group of patients from all over the country. Using an easy-to-use cognitive assessment tool (TICS-m) by phone without face-to-face interactions. Many studies[10,11] have used this test to evaluate cognitive function and it has been shown to be well reproducible.
As for the comparison of cognitive function between the RFCA group and the CY group at 3 and 12 months after operation, there was no statistical difference between the two groups, which could be attributed to two factors. First, the sample size of the CY group was small; second, the baseline data of the two groups were basically matched, and there was no significant difference in postoperative recurrence, so there was no significant difference in cognitive function between the two groups.
Accumulating evidence indicates that AF may adversely affect cognitive function, and the incidence of cognitive as well as neurological dysfunction in AF patients is higher than that in the general population.[3] Consequently, this effect has been attributed to various mechanisms, including low cardiac output, cerebral hypoperfusion, endothelial dysfunction, vascular inflammation, thrombosis, genetic factors, as well as common risk factors, such as hypertension, sleep apnea, diabetes, and obesity, among others.[12] Catheter ablation, established therapy for patients with drug-refractory symptomatic AF, is extensively used to lower arrhythmia burden, minimize symptoms, alleviate patients’ clinical symptoms, and improve quality of life.[13,14] To date, however, the effect of RFCA and CY on cognitive function remains unclear.
Early data from some small observational studies showed that about 28 and 27% of patients with paroxysmal and persistent AF, respectively, developed acute cognitive dysfunction after RFCA. Besides, the overall cognitive function of 13–20% of these patients significantly declined at 3 months after ablation.[15]
During the ablation process, the presence of catheters stimulates the heart and blood vessels, thereby causing inflammation, and increasing the risk of hypercoagulability. Besides, catheters act as sources of iatrogenic embolization, such as air embolization and carbonization, which may increase the risk of thromboembolic events, and cause cognitive decline.[2] The prolongation of the operation time can increase the occurrence of the above-mentioned series of unfavorable factors, thereby affecting the patient's cognitive function. However, there is no significant difference in the long-term cognitive function changes between the radiofrequency ablation group and the cryo-balloon group in this study, which cannot be ruled out It is related to the relatively small number of cases in the CY group. (Supplemental Digital Content: ).
A recent prospective study, evaluating changes in cognitive function in 28 and 17 patients who received catheter ablation and drug therapy, respectively, found no significant differences concerning medical therapy or catheter ablation over time.[16] Another study also reported no association between AF burden and overall neurocognitive performance.[17] Contrary to these studies, systolic blood pressure and cardiac output were positively correlated with improvements in mental state and cerebral blood flow. A subsequent study showed that AF ablation can reduce the possibility of left atrial thrombosis caused by atrial asynchronism and hemodynamic changes by relieving clinical symptoms and maintaining sinus rhythm in patients, thus improving patients’ long-term cognitive function.[18]
Our results were consistent with some large-scale published studies. For example, a 9-year follow-up study found that catheter ablation reduced the risk of dementia and hospitalization in AF patients, relative to those without catheter ablation, especially in AF subjects aged above 65 years.[19] Similarly, Bunch et al[20] found that patients who underwent catheter ablation had significantly lower rates of incident dementia compared to those without ablation in an observational study with a 3-year follow-up. Also, REDUCE-TE studied the incidence of silent cerebral lesions (SCL) and changes in cognitive function after catheter ablation to isolate pulmonary veins, and found a 9.3% incidence of no SCL following pulmonary vein isolation, with no significant differences in neurocognitive tests between patients with and without SCL.[21] A MEDAFI trial, evaluating the incidence of acute brain injury after CY and radiofrequency ablation, found that both ablation techniques resulted in acute brain injury without neurological symptoms following pulmonary vein isolation, compared with patients without acute brain injury. Besides, the authors found no significant differences between the ablation techniques, despite patients with acute brain injury being much older.[22]
Results from our multi-factor analysis showed that operation time does not influence cognitive function, despite the observed differences between the two ablation techniques. This may be attributed to different energy forms from the two ablation techniques. For different energy forms of ablation, the time required for ablation, as well as the area and depth of the injury are different. However, it remains unclear whether the mechanism underlying cognitive function changes is related to the energy form. Further studies, using more centers, and larger sample sizes are expected to clarify this mechanism.
4.1. Limitations
There were several limitations in our study. First, this was a non-randomized single-center pilot study, although appropriate sample sizes of cognitive function in patients with AF have not yet been established, the small sample size in the CY group may have underpowered the analysis, therefore, our results may have certain biases and errors. Second, we did not perform a brain imaging study before or after the ablation procedure to exclude any potential silent cerebral emboli.
4.2. Future directions
Larger prospective studies and randomized trials are needed to validate our findings. We hope to further expand the sample size, conduct regular follow-ups, and combine objective evidence such as cranial magnetic resonance imaging, to improve the long-term effects of RFCA and CY on cognitive function.
5. Conclusion
A rhythm control strategy with catheter ablation has superior efficacy in maintaining or improving the cognitive function of AF patients, compared to medical therapy alone. Besides, RFCA and CY equally enhance cognitive function.
Author contributions
All authors participated in the conception, design, analysis, and interpretation of the data as well as writing, and revision of the manuscript. All authors approved the final version of the manuscript and agreed to act as guarantors of the work.
Conceptualization: Zefeng Wang, Yongquan Wu.
Data curation: Xinlu Wang, Xiaohan Yan, Manyun Huang.
Formal analysis: Xinlu Wang, Zefeng Wang, Xiaohan Yan.
Investigation: Xinlu Wang.
Methodology: Xinlu Wang, Xiaohan Yan, Manyun Huang.
Project administration: Xinlu Wang, Yongquan Wu.
Supervision: Zefeng Wang.
Visualization: Yongquan Wu.
Writing – original draft: Xinlu Wang.
Writing – review & editing: Zefeng Wang, Yongquan Wu.
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CY = cryoballoon, ECG = electrocardiogram, LA = left atrium, RFCA = radiofrequency ablation, SCL = silent cerebral lesion, TICS-m = telephone interview for cognitive status-modified.
How to cite this article: Wang X, Wang Z, Yan X, Huang M, Wu Y. Radiofrequency and cryoballoon ablation improve cognitive function in patients with atrial fibrillation. Medicine. 2021;100:32(e26914).
The study was supported by the Capital Health Research and Development of Special (Grant No. 2020-2-2062).
Informed consent was obtained from all patients for the publication of this article. All patients undergoing catheter ablation gave written informed consent before their enrolment into the study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
BMI = body mass indecx, CHD = coronary heart disease, CY = cryoballoon, LA = left atrial, LVEF = left ventricular ejection fraction, RFCA = radiofrequency ablation, TICS-m = Telephone Interview for Cognitive Status-modified.
Comparison between the drug treatment group and the cryoballoon group.
Comparison between the radiofrequency ablation group and the cryoballoon group.
12 mo = 12-month follow-up, 3 mo = 3-month follow-up, CI = confidence interval, CY = cryoballoon, RFCA = radiofrequency ablation.
12 mo = 12-month follow-up, 3 mo = 3-month follow-up, CY = cryoballoon, RFCA = radiofrequency ablation.
Interaction between group and time, F: Two-way Repeated Measures ANOVA statistics, P: Two-way Repeated Measures ANOVA P-values.
AF = Atrial fibrillation, CHD = coronary heart disease, CI = confidence interval, SE = standard error.
CI = confidence interval, SE = standard error.
CI = confidence interval, SE = standard error.
References
- [1].Guo Y, Tian Y, Wang H, et al. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest 2015;147:109–19. [DOI] [PubMed] [Google Scholar]
- [2].Chinta V, Askandar S, Nanda A, et al. Atrial fibrillation and deterioration in cognitive function. Curr Probl Cardiol 2018;44:100386. [DOI] [PubMed] [Google Scholar]
- [3].Madhavan M, Graff-Radford J, Piccini JP, et al. Cognitive dysfunction in atrial fibrillation. Nat Rev Cardiol 2018;15:744–56. [DOI] [PubMed] [Google Scholar]
- [4].Chopard R, Piazza G, Gale SA, et al. Dementia and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Am J Med 2018;131:1408–17. [DOI] [PubMed] [Google Scholar]
- [5].Mahajan R, Brooks AG, Sullivan T, et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart 2012;98:1120–6. [DOI] [PubMed] [Google Scholar]
- [6].Dagres N, Chao TF, Fenelon G, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? Europace 2018;20:1399–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [8].Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS)[J]. Eur Heart J 2020;00:01–126. [Google Scholar]
- [9].Lindgren N, Rinne JO, Palviainen T, et al. Prevalence and correlates of dementia and mild cognitive impairment classified with different versions of the modified Telephone Interview for Cognitive Status (TICS-m)[J]. Int J Geriatr Psychiatry 2019;34:1883–91. [DOI] [PubMed] [Google Scholar]
- [10].Ma N, Feng X, Wu Z, et al. Cognitive impairments and risk factors after ruptured anterior communicating artery aneurysm treatment in low-grade patients without severe complications: a multicenter retrospective study. Front Neurol 2021;12:613785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Garcia MA, Ortiz K, Arevalo SP, et al. Age of migration and cognitive function among older latinos in the United States[J]. J Alzheimers Dis 2020;76:1493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sepehri Shamloo A, Dagres N, Mussigbrodt A, et al. Atrial fibrillation and cognitive impairment: new insights and future directions. Heart Lung Circ 2019;29:69–85. [DOI] [PubMed] [Google Scholar]
- [13].Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- [14].Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Medi C, Evered L, Silbert B, et al. Subtle post-procedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol 2013;62:531–9. [DOI] [PubMed] [Google Scholar]
- [16].Tischer TS, Nitschke D, Krause I, et al. Prevalence and progression of cognitive impairment in atrial fibrillation patients after treatment with catheter ablation or drug therapy. Cardiol Res Pract 2019;2019:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Herm J, Schirdewan A, Koch L, et al. Impact of atrial fibrillation burden on cognitive function after left atrial ablation - Results of the MACPAF study. J Clin Neurosci 2020;73:168–72. [DOI] [PubMed] [Google Scholar]
- [18].Jin MN, Kim TH, Kang KW, et al. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol 2019;12:e007197. [DOI] [PubMed] [Google Scholar]
- [19].Yu-Cheng Hsieh, Yun-Yu Chen, Kuo-Liong Chien, et al. Catheter ablation of atrial fibrillation reduces the risk of dementia and hospitalization during a very long-term follow-up. Int J Cardiol 2019;304:75–81. [DOI] [PubMed] [Google Scholar]
- [20].Bunch TJ. Atrial fibrillation and dementia. Circulation 2020;142:618–20. [DOI] [PubMed] [Google Scholar]
- [21].Schmidt B, Szeplaki G, Merkely B, et al. Silent cerebral lesions and cognitive function after pulmonary vein isolation with an irrigated gold-tip catheter: REDUCE-TE Pilot study. J Cardiovasc Electrophysiol 2019;30:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neumann T, Kuniss M, Conradi G, et al. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace 2011;13:37–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.