Abstract
Introduction:
Secondary amyloidosis is a rare complication of rheumatoid arthritis (RA) that is histologically characterized by the deposition of amyloid fibrils in target organs, such as the kidneys and gastrointestinal tract. Controlling the inflammatory response is essential to prevent organ dysfunction in amyloid A (AA) amyloidosis secondary to RA, and no clear treatment strategy exists.
Patient Concerns and Diagnosis:
A 66-year-old woman with RA, who had been treated with disease-modifying anti-rheumatic drugs for 1 year, presented with recurrent abdominal pain and prolonged diarrhea. Endoscopy showed chronic inflammation, and colon tissue histology confirmed AA amyloidosis.
Interventions and Outcomes:
After tocilizumab therapy was begun, her diarrhea and abdominal pain subsided, and articular symptoms improved. Biologic drugs for RA have been used in patients with secondary AA amyloidosis, including tumor necrosis factor and Janus kinase inhibitors, interleukin 6 blockers, and a T cell modulator. Here, we systematically review existing case reports and compare the outcomes of RA-related AA amyloidosis after treatment with various drugs.
Conclusion:
The data indicate that biologic drugs like tocilizumab might be treatments of choice for AA amyloidosis secondary to RA.
Keywords: amyloidosis, arthritis, biological products, interleukin-6, rheumatoid
1. Introduction
Secondary amyloid A (AA) amyloidosis is a rare and fatal complication of rheumatic diseases, including rheumatoid arthritis (RA).[1,2] Serum AA (SAA) protein is an acute-phase reactant synthetized by hepatocytes in the presence of abundant proinflammatory cytokines and activated immune cells. Fibrils derived from SAA accumulate in the extracellular matrix in target organs. The kidneys are most frequently affected, resulting in proteinuria with or without renal impairment. Occasionally, the gastrointestinal (GI) tract and heart are also involved, with unexpected manifestations including uncontrolled diarrhea or generalized edema. Diagnosis requires histologic confirmation of deposited amyloid fibrils in tissue, which display a characteristic apple-green birefringence when stained with Congo red. There are no useful biomarkers for the development of secondary AA amyloidosis in RA, and its severity is not proportional to RA disease activity. In addition, there is no effective treatment strategy to prevent resultant organ dysfunction, including renal failure. Reducing the SAA level may also be necessary to prevent irreversible organ damage.
Recently, several promising biologic agents have been developed, and some have been identified as effective drugs for active RA with acceptable safety profiles.[3] They include tumor necrosis factor (TNF) inhibitors (etanercept, infliximab, adalimumab, and golimumab), a recombinant fusion protein modulating a co-stimulatory signal for T cell activation (abatacept), an interleukin (IL)6 receptor antagonist (tocilizumab), and a monoclonal antibody against CD20 (rituximab). The use of advanced strategies for RA treatment, including these biologic agents, might decrease the frequency of secondary AA amyloidosis.[4] To reduce the inflammatory response in RA, most biologic agents can be considered when secondary AA amyloidosis develops, and their outcomes have been reported.
Here, we report that anti-IL6 therapy abrogated uncontrolled symptoms of secondary AA amyloidosis in the GI tract of a patient with RA. We also review the use of biologic agents in AA amyloidosis secondary to RA to identify the most effective therapies.
2. Case report
A 66-year-old woman with a history of hypertension and RA was referred to the emergency room for fever for 1 week, and for watery diarrhea and abdominal discomfort for 1 month. She had been diagnosed with RA 2 years prior, after assessment for pain and swelling on her left wrist and morning stiffness that continued all day. At the time of RA diagnosis, her rheumatoid factor (RF) titer was 21 IU/mL (normal range, ≤ 14 IU/mL), anti-cyclic citrullinated peptide titer was 0.4 U/mL (normal range, ≤ 5 U/mL), C-reactive protein (CRP) level was 0.85 mg/dL (normal range, ≤ 0.5 mg/dL), and erythrocyte sedimentation rate (ESR) was 56 mm/h (normal range, ≤ 25 mm/h). Methotrexate and hydroxychloroquine with low-dose prednisone were started after intra-articular injection of triamcinolone into her left wrist. Her disease activity score 28 (DAS28) improved to 2.5 from 4.6, and she tolerated the RA well with the medication for 6 months.
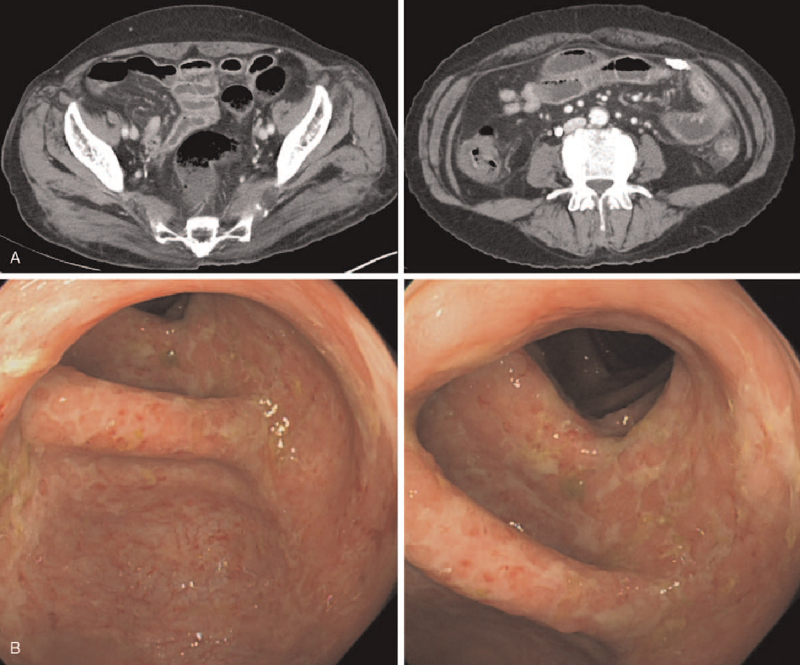
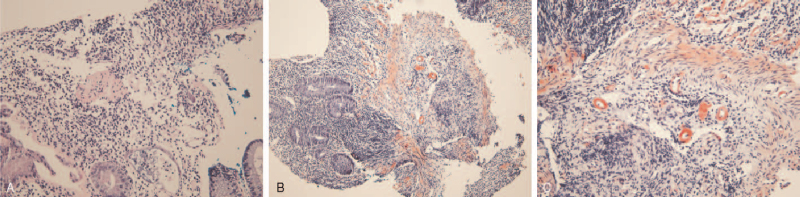
However, she suffered recurrent diarrhea with intermittent abdominal pain for 4 months and was admitted to the emergency room with a week-long fever and prolonged diarrhea with abdominal discomfort for the past month. Her temperature was 38.9°C and blood pressure was 110/83 mmHg. Physical examination revealed no direct or indirect tenderness on her abdomen, and joint tenderness and swelling were not observed. Laboratory results were: white blood cell count, 6,700 cells/μL; ESR, 64 mm/h; CRP, 10.55 mg/dL; RF, 255.4 U/mL; creatinine, 0.66 mg/dL; and albumin, 2.9 g/dL. There was no proteinuria or hematuria in urinalysis. The results of stool culture, parasite test, and clostridium difficile toxin A were negative. Abdominal computed tomography showed segmental wall thickening in the ascending to descending colon and distal jejunum (Fig. 1A). Endoscopy revealed chronic colitis with mucosal atrophy and erosion (Fig. 1B). Histopathology of her colon tissue confirmed chronic colitis with distorted crypt architecture, heavy infiltration of lymphoplasma cells, and amyloid deposition in the stroma and vessels, shown as a pink amorphous material positive for Congo red staining (Fig. 2).
Figure 1.
Abdominal computed tomography (A) and colonoscopy (B) results from the patient.
Figure 2.
Deposition of amyloid A in colon tissue (A). Hematoxylin and eosin staining (100 ×), B, C. Congo red staining (100 × (B), 200 × (C)).
After 2 weeks of prophylactic therapy for latent tuberculosis, she started 8 mg/kg intravenous tocilizumab and the prolonged diarrhea subsided, with decreased frequency within 1 week. Additionally, her inflammatory markers decreased (ESR 18 mm/h, CRP 0.98 mg/dL) after 1 month. Monthly tocilizumab therapy remains efficacious 2 years later.
3. Methods
The systematic review was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.[5] For the comprehensive literature search, all related journals were hand-searched, and the reference lists of included studies were scanned to obtain potentially related data on PubMed/MEDLINE and Scopus databases. Studies were selected based on the following Patients/Intervention/Comparisons/Outcomes/Study design criteria: P (RA with secondary AA amyloidosis), I (biologic agents, including TNF inhibitors, abatacept, rituximab, and tocilizumab, or tofacitinib), C (comparisons between biologics and previous medications in the resolution of AA amyloidosis), O (survival and drug safety), and S (original data with sufficient quantitative details). The literature search strategy is shown in Figure 3. The protocol was approved by the Institutional Review Board of our hospital (AJIRB-MED-MDB-20-574).
Figure 3.
PRISMA flow chart of study selection.
3.1. Systemic AA amyloidosis secondary to RA
Acute inflammatory stimuli alter the hepatic biosynthesis of acute-phase proteins, which can persist in chronic inflammatory diseases such as RA and juvenile idiopathic arthritis.[6] Upon initiation of the inflammatory cascade, activated monocytes and tissue macrophages release primary inflammatory mediators, which are members of the IL1 and TNF cytokine families. Then, secondary cytokines and chemokines, such as IL6, IL8, and monocyte chemoattractant proteins, are released, resulting in the recruitment of immune effector cells. Proinflammatory cytokines, including IL6, TNF, and IL1, cause and maintain elevations in SAA during chronic inflammation that induces AA amyloid fibrillogenesis. Amyloidogenic peptide processing produces amyloid fibril fragments, which accumulate in the lysosomes of mononuclear phagocytes.[7] Matrix metalloproteinases degrade SAA and AA, which aggregate into protofilaments.[8] Glycosaminoglycans and apolipoprotein E facilitate the deposition of AA amyloid fibrils in target tissues during amyloidosis.[9] Additionally, differences in the SAA genes also contribute to the occurrence of AA amyloidosis, along with abnormal fibril processing and deposition. An association between AA amyloidosis and SAA gene polymorphisms was found in patients with RA.[10] Aging was revealed as a risk factor for AA amyloidosis secondary to RA through animal model studies, as aging mice have elevated SAA levels. The analysis of 388 patients with RA and AA amyloidosis showed that the age at RA onset is independently associated with AA amyloidosis diagnosis.[11,12]
As circulating SAA causes the deposition of AA amyloid fibrils, its reduction may improve the prognosis of AA amyloidosis. In a study with 80 patients with systemic AA amyloidosis, maintaining a low SAA concentration improved target organ outcomes.[13] Controlling inflammation caused by the underlying disease is the most important strategy in secondary AA amyloidosis treatment. Dimethyl sulfoxide, which dissolves deposited amyloid proteins, has been used to treat systemic AA amyloidosis; however, its efficacy has not been conclusively demonstrated. Eprodisate was designed to inhibit the association between amyloidogenic proteins and glycosaminoglycans and reduce fibril deposition in tissues.[14] The pathogenesis of systemic AA amyloidosis suggests that SAA production must be reduced to inhibit deposition and prevent organ dysfunction, and controlling inflammation is critical to control SAA production. Therefore, biologic agents used to lower RA activity have been used to treat AA amyloidosis. Here, we review these cases.
3.2. Reports of AA amyloidosis secondary to RA treated by biologic agents
3.2.1. Tocilizumab
Tocilizumab, a recombinant monoclonal antibody, inhibits the biological activity of IL6.[15] IL6 is an essential proinflammatory cytokine in RA, as it promotes inflammatory cell infiltration of the synovial tissue, angiogenesis, metalloproteinase production, and osteoclast activation, leading to cartilage destruction. IL6 enhances the expression of SAA mRNA, and these effects are enhanced by TNFα or IL1β in hepatocytes.[16] Tocilizumab is regarded as more effective than disease-modifying anti-rheumatic drugs (DMARDs) for amyloidosis secondary to rheumatic diseases including RA and juvenile idiopathic arthritis.[17–20] As blocking the IL6 pathway results in decreased synthesis of inflammatory proteins, including SAA, IL6 antagonists are useful in AA amyloidosis treatment.[21] Successful outcomes for tocilizumab have been reported in several cases of GI AA amyloidosis secondary to RA. Most of these patients were female, and AA amyloid deposition was confirmed in biopsy specimens obtained by endoscopy.[17,18,22–24] One patient started tocilizumab therapy after two different TNF inhibitors failed to control the manifestations of AA amyloidosis, and another patient suffered a small intestine perforation with extensive amyloid deposition after one administration of tocilizumab. A 77-year-old female with massive ascites and bloody stool was diagnosed with AA amyloidosis after AA depositions were detected in her rectal mucosa, and her ascites disappeared after 5 months of tocilizumab therapy.[20] Tocilizumab is also effective in patients with renal AA amyloidosis, which manifests as proteinuria, hematuria, and renal failure.[19,24–31] Miyagawa et al reported four patients with RA and secondary AA amyloidosis who were treated with tocilizumab, and three had GI tract involvement with proteinuria.[28] Three patients had decreased proteinuria after 2 months, no progression to other organs, and normalized inflammatory markers, except for one patient who had low SAA levels before tocilizumab therapy. Two patients with decreased estimated glomerular filtration rates (9 and 19 mL·min−1·1.73 m−2) and proteinuria due to renal AA amyloidosis were treated with tocilizumab, and their renal function remained stable for 8 years.[32] In another report, six patients with RA and secondary AA amyloidosis were treated with tocilizumab after DMARDs and TNF inhibitors. Three showed decreased proteinuria, and two experienced stabilization of their renal manifestations with well-controlled RA.[29] In a case report, a patient with cardiac AA amyloidosis manifested as diffuse left ventricular hypertrophy with wall thickness improved after starting tocilizumab therapy. Her articular symptoms and increased inflammatory markers subsided, and her ventricular and interventricular septal thickness and left ventricle mass decreased.[33] These data, along with our case, suggest that tocilizumab is effective against AA amyloidosis in the GI tract.
3.2.2. TNF inhibitors
In 2004, two cases of RA-related renal AA amyloidosis treated with TNF inhibitors were reported.[34,35] Both patients were female and had long-term RA (17 and 27 years). Proteinuria gradually subsided, with decreased acute-phase reactants detected for > 30 months. Several case reports have demonstrated the efficacy of infliximab in the treatment of AA amyloidosis secondary to RA.[36,37] Kuroda et al prospectively studied the outcomes of TNF inhibitors in secondary GI amyloidosis with RA, and reported decreased proportions of amyloid-positive area in gastric biopsy specimens after therapy.[38] Nakamura et al reported that etanercept was more effective than cyclophosphamide in treating AA amyloidosis secondary to RA.[39] Survival, renal function, and serum CRP and albumin levels were improved in 24 patients treated with etanercept. When two patients with long-standing RA and renal AA amyloidosis were treated with adalimumab, one 57-year-old female improved, but a 78-year-old female with chronic kidney disease expired from cardiogenic shock.[40]
3.2.3. Other biologics
Abatacept, which contains the extracellular domain of human cytotoxic T-lymphocyte associated antigen 4, was used to treat two female patients with RA and AA amyloidosis.[41] They experienced clinical remission with stable renal function, their serum IL6 and TNF levels decreased, and AA amyloid fibrils that were visible in serial upper GI tract biopsies regressed completely. A patient with RA presented with refractory diarrhea despite etanercept, adalimumab, and certolizumab therapies, and was treated with tocilizumab.[42] When their RA disease activity nor their intractable diarrhea had subsided after 3 months, abatacept was started. The diarrhea subsided, and serum inflammatory markers normalized.
Four patients with secondary renal AA amyloidosis who were refractory to DMARDs and TNF inhibitors were treated with rituximab.[43] All had high disease activity (DAS28: 6.94 ± 0.9), three had kidney and GI tract involvement, and two had cardiac involvement. After therapy, the disease activities of all patients were controlled; however, renal manifestations such as serum creatinine and proteinuria were not significantly changed. A 61-year-old female with AA amyloidosis secondary to RA failed to respond to two TNF inhibitors, but rituximab therapy achieved symptom improvement and a reduction in SAA.[44] Three patients with AA amyloidosis secondary to RA were enrolled in a clinical trial, two with both renal and GI tract involvement, and one with renal involvement only.[45] All were refractory to TNF inhibitors (etanercept, adalimumab, or infliximab). After 2–7 cycles of rituximab therapy, their disease activity and articular symptoms improved, while there were no significant changes in renal function or proteinuria.
A patient with RA who had been treated with TNF inhibitors, tocilizumab, and abatacept presented with worsening renal function and proteinuria.[46] After the diagnosis of renal AA amyloidosis secondary to RA, tofacitinib, a small-molecule Janus kinase (JAK) inhibitor, was started. Renal impairment and proteinuria were ameliorated, and articular symptoms subsided, suggesting that JAK inhibition is a promising therapeutic strategy for AA amyloidosis secondary to RA.
3.2.4. Treatment outcomes of biologics
Kuroda et al compared the treatment outcomes of biologics including tocilizumab, TNF inhibitors, and non-biologic agents in patients with renal AA amyloidosis secondary to RA.[47] Among 53 patients who were treated with biologic agents, 22 received tocilizumab (12 first-line, 5 second-line, and 1 third-line). Survival and hemodialysis-free survival were significantly higher in the biologic therapy group than in the non-biologic therapy group. Changing to tocilizumab from TNF inhibitors or using tocilizumab as first-line therapy improved the prognosis of AA amyloidosis secondary to RA. However, no outcome comparisons were performed between TNF inhibitors and tocilizumab.
Patients with organ failure due to AA amyloidosis may not recover despite biologic therapy. In a report of biologic therapy use for secondary renal AA amyloidosis in RA and ankylosing spondylitis, 29 patients with RA and proteinuria and one patient with RA and end-stage renal disease (ESRD) were treated with biologic agents (TNF inhibitors in 23 patients, rituximab in 10, abatacept in 5, tocilizumab in 4, and anakinra in 1).[48] Proteinuria regressed by 25% in 13 patients and creatinine decreased by 25% in 2/23 patients; however, seven patients developed ESRD after biologic therapy. When the response of secondary amyloidosis to biologic therapy was evaluated using a composite response index based on disease activity, proteinuria, and renal functions, 52.2% (12/23) of patients with RA had good responses. Additionally, patients with positive RF values and higher DAS28 scores at the time of amyloidosis had good responses to biologics. These findings suggest that controlling inflammation prevents tissue damage during amyloidosis due to an active inflammatory reaction; however, when inflammation is minimal, the dysfunction cannot be reversed. Another study evaluated 15 patients with GI AA amyloidosis and the SAA1.3 allele, which is a risk factor for AA amyloidosis, who were treated with etanercept, tocilizumab, and abatacept.[4] Proteinuria was worse in the tocilizumab group than in the other biologic groups. Five patients died of end-stage renal AA amyloidosis, and two underwent peritoneal dialysis. Moreover, comparisons of the treatment response against secondary amyloidosis between tocilizumab and TNF inhibitor therapies revealed a higher treatment retention rate and decreased SAA levels in the tocilizumab group.[49]
4. Discussion
Our case showed that prolonged diarrhea due to GI AA amyloidosis was well-controlled by an IL6 receptor antibody in a patient with RA. Although her RA disease activity was mild after 1 year of DMARD therapy, diarrhea and abdominal pain developed, along with increases in her RF titer and inflammatory markers. Various etiologies, including inflammatory bowel disease and infectious colitis, were suspected prior to histologic confirmation of AA amyloidosis secondary to RA. Endoscopic and radiological findings are nonspecific in GI AA amyloidosis, and there is no pathological association between RA disease activity or disease-specific antibodies and the development of secondary AA amyloidosis.[50] It is important to perform an endoscopy with tissue biopsy for patients with RA who develop chronic symptoms such as diarrhea and abdominal pain or discomfort, even though they may have mild or well-controlled articular symptoms through DMARD use. Chronic diarrhea with malabsorption has been reported as a manifestation of secondary AA amyloidosis associated with inflammatory diseases.[17,18] Additionally, renal AA amyloidosis can lead to ESRD, proteinuria, or unexplained renal impairment, underscoring the need for detailed examinations.
The review of biologic therapy use for AA amyloidosis secondary to RA shows that TNF inhibitors, tocilizumab, abatacept, rituximab, and a JAK inhibitor can effectively prevent organ dysfunction and increase patient survival (Table 1). Many patients achieved remission or improvement of AA amyloidosis after the administration of several different biologics. For example, seven patients, who were treated with etanercept, adalimumab, or rituximab, improved and had SAA suppression after tocilizumab therapy, and one patient, who was treated with a TNF inhibitor and tocilizumab, achieved remission with abatacept.[30,42] This suggests that biologic agents need to be replaced to suppress the progression of AA amyloidosis secondary to RA, depending on the patient.
Table 1.
Previous reports of successful treatment of rheumatoid arthritis-associated amyloidosis with biologic agents.
| Author, year [Ref.] | Study design | Gender and age | Amyloidosis related symptoms | Involved organ | Disease duration | Used biologic agents or targeted synthetic DMARDs | Doses, routes of drug administration | Prior therapy | Outcome |
| Sawamura et al. 2020 sh[42] | Case report | F/72 | Diarrhea | Large intestine | 50 yr | ABA | 750 mg, IV, monthly | Anti-TNF agents, TCZ | Improved |
| Kovács et al. 2020 [31] | Case report | F/52 | Microscopic hematuria | kidney | 30 yr | TCZ | IV, monthly | Gold salt, MTX, LEF, ETA | Improved |
| Fukuda et al. 2021 [32] | Case reports (n = 2) | F/59M/71 | Deteriorating renal function | Kidney and duodenum | TCZ | 8mg/kg, monthly | Patient 1; Gold salt, BCA, ETAPatient 2; BCA, ETA, ABA | Improved | |
| Nakamura et al. 2019 [4] | Retrospective study (n = 15) | Upper gastrointestinal | ETA, TCZ, ABA | - | - | TCZ: Worse5: Expired | |||
| Shimagami et al. 2019 [20] | Case report | F/77 | Massive ascites | Rectum | 40 yr | TCZ | 320 mg IV twice and then 162 mg SQ | SZP | Improved |
| Kilic et al. 2018 [45] | Retrospective study (n = 4) | F = 4, mean age 55.5 | Kidney | 18.8 years | Rituximab (n = 4) | Two endogenous IV infusions of Ig per treatment cycle separated by a two-week interval | MTX, SSZ, HCQ, LEF, CS, ETA, ADM | 3: Switch to TCZ1: Improved | |
| Galmiche et al. 2018 [23] | Case report | F/78 | Diarrhea and leg edema | Duodenum | 0 | TCZ | 8mg/kg, monthly | MTX | Improved |
| Watanabe et al. 2018 [46] | Case report | F/76 | Pitting edema | kidney | 16 yr | Tofacitinib | Anti-TNF agents, TCZ, ABA | Improved | |
| Yamagata et al. 2017 [24] | Case report | F/67 | Diarrhea and pedal edema | Colon and kidney | TCZ | 8mg/kg, monthly | SZP | Improved | |
| Pamuk et al. 2016 [48] | Retrospective study (n = 30) | M = 11/F = 19, Mean age 51.7 | Proteinuria (29), ESRD (1) | Kidney | 14.2 yr | Anti-TNF agents (n = 23), RTM (n = 10), ABA (n = 5), TCZ (n = 4), ANA (n = 1) | - | MTX, SZP, LEF | 12: Improved |
| Courties et al. 2015 [29] | Retrospective study (n = 8) | M = 2/F = 6Mean age 69.9 | Renal failure | Kidney, liver, duodenum | 17.1 yr | TCZ | 8mg/kg, IV, every 4 wk | MTX, HCQ, SSZ, ETA, ADA, ABA | 6: Improved |
| Lane et al. 2015 [30] | Case series (n = 7) | M = 3/F = 4 | Renal impairment | Kidney | TCZ | 8mg/kg, IV, every 4 wk | MTX, SSZ, ETA, RTX, LFM | 1: complete response6: partial response | |
| Yamada et al. 2014 [25] | Case report | F/71 | Nephrotic syndrome | Kidney | 15 yr | TCZ | 8mg/kg, IV, every 4 wk | BCA | Improved |
| Matsui et al. 2014 [26] | Case report | F/60s | Heart failure and renal dysfunction | Kidney, stomach | 10 yr | TCZ | 8mg/kg, IV, every 4 wk | MTX, BCA | Improved |
| Miyagawa et al. 2014 [28] | Case series (n = 5) | All female, Mean age 59.2 | Renal involvement | Kidney, GI | 20.2 yr | TCZ | 8mg/kg, IV, every 4 wk | MTX, AZ, BCA, ETA | Improved |
| Nakamura et al. 2014 [41] | Case series (n = 2) | F/70, F/65 | Refractory diarrhea, weight loss, proteinuria | KidneyGI | ABA | 500 mg IV monthly | MTX, ETA, TCZ | Improved | |
| Vinicki et al. 2013 [19] | Case report | F/48 | Hematuria | Kidney | 10 yr | TCZ | 8mg/kg, IV, every 4 wk | MTX, SZP | Improved |
| Burkart et al. 2013 [44] | Case report | F/61 | N.A. | N.A | 34 yr | RTX | ADA | Improved | |
| Fikri-Benbrahim et al. 2013 [40] | Two case reports | F/57F/78 | ProteinuriaDilated cardiomyopathy and CKD | Kidney | 8 yr | ADA | MTX | 1: Improved1: expired | |
| Hakala M et al. 2013[27] | Case series (RA = 3) | M/53M/60F/64 | proteinuria | Kidney | TCZ | 8mg/kg, monthly | DMARDs, ETA, ADA | 2: Improved1: Maintained | |
| Nakamura et al. 2012 [39] | Retrospective study (n = 24) | M = 4/F = 20 | Proteinuria, thyroid dysfunction, weight loss, repeated constipation and diarrhea | Kidney, GI | 16.2 yr | ETA | MTX (62.5%) | ETN: Improved in survival and mean GFR | |
| Kuroda et al. 2012 [47] | Retrospective study (n = 53) | M = 7, F = 46, Mean age 63.2 | Kidney involvement | Kidney | 16.8 yr | ETA, IFX, TCZ | 7: expired9: HD | ||
| Hattori et al. 2012 [33] | Case report | F/58 | Cardiac and kidney involvement | Cardiac involvement | 10 yr | TCZ | 8mg/kg, IV, every 4 wk | Gold, BCA, ETA | Improved |
| Narvaez J et al. 2011[43] | Case series (RA = 4) | All F, 46 - 75 | Kidney, gastrointestinaltract, cardiac | 14 – 40 yr | RTX | 1g IV, 2 wk interval | MTX, IFX, ETA, ADM, AZ | 3: Improved1: Maintained | |
| Lee et al. 2011 [37] | Case report | F/62 | Diarrhea and abdominal pain | GI involvement | Long-standing | IFX | 5mg/kg at weeks 0, 2, and 6 | MTX, LEF | Improved |
| Inoue et al. 2010 [18] | Case report | F/64 | Persistent vomiting and diarrhea | GI involvement | 7 yr | TCZ | 8mg/kg, IV, every 4 wk | SSZ, BCM, MTX | Improved |
| Kuroda et al. 2009 [38] | Prospective study (n = 14) | M = 2, F = 12, Mean age 57.6 | N.A. | stomach | 15 yr | ETA (n = 10), IFX (n = 4) | ETA 25 mg SQ twice a wk, IFX 3 mg/kg at weeks 0, 2, and 6, and then every 8 wk. | Improved | |
| Sato H et al. 2009 [17] | Case report | F/53 | Diarrhea, Hypovolemic shock | GI involvement | 10 yr | TCZ | 8mg/kg, IV, every 4 wk | MTX, SZP | Improved |
| Nishida S et al. 2009 [22] | Case report | F/50 | Diarrhea, weight loss | GI involvement | 12 yr | TCZ | 8mg/kg, IV, every 4 wk | DMARD, ETA, IFX | Improved |
| Kuroda et al 2008 [36] | Case report | F/55 | Nephrotic syndrome | Kidney | 27 yr | IFX | MTX | Improved | |
| Ravindran et al. 2004 [34] | Case report | F/74 | Proteinuria | Kidney | 27 yr | ETA | 25 mg twice weekly SC | HCQ, gold, MTX | Regression of AA amyloid, no change in Felty's SD |
| Smith et al. 2004 [35] | Case report | F/56 | Proteinuria | Kidney | 17 yr | ETA | 25 mg twice weekly SC | HCQ, D-penicillamine, AZP, MTX | Improved |
The limitations of this review are that it may contain publication bias, and consists only of case reports, as large, well-controlled studies comparing the effectiveness of each biologic are currently lacking. These will be necessary to determine the optimal treatment strategy for AA amyloidosis secondary to RA. However, treatment results to date indicate that biologics are a good option to reduce morbidity and mortality due to AA amyloidosis.
Author contributions
Conceptualization: Ju-Yang Jung, Hyoun-Ah Kim.
Data curation: Ju-Yang Jung, Young-Bae Kim, Ji-won Kim, Chang-Hee Suh, Hyoun-Ah Kim.
Formal analysis: Hyoun-Ah Kim.
Funding acquisition: Hyoun-Ah Kim.
Investigation: Young-Bae Kim, Hyoun-Ah Kim.
Methodology: Hyoun-Ah Kim.
Resources: Young-Bae Kim.
Writing – original draft: Ju-Yang Jung.
Writing – review & editing: Ji-won Kim, Chang-Hee Suh, Hyoun-Ah Kim.
Footnotes
Abbreviations: AA = Amyloid A, CRP = C-reactive protein, DAS 28 = disease activity score 28, DMARDs = disease modifying anti-rheumatic drugs, ESR = erythrocyte sedimentation rate, ESRD = end-stage renal disease, GI = gastrointestinal, IL = interleukin, RA = rheumatoid arthritis, RF = rheumatoid factor, SAA = serum amyloid A, TNF = tumor necrosis factor.
How to cite this article: Jung JY, Kim YB, Kim Jw, Suh CH, Kim HA. Biologic therapy for amyloid A amyloidosis secondary to rheumatoid arthritis treated with interleukin 6 therapy: case report and review of literature. Medicine. 2021;100:32(e26843).
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0992).
Availability of data and material was not applicable.
Code availability was not applicable.
The protocol was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-MDB-20-574).
Consent to participate: Informed consent was provided by the patient.
Consent for publication: Informed consent was provided by the patient.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
ABA = abatacept, ADA = adalimumab, ANA = anakinra, AZP = azathioprine, BCA = Bucillamine, CKD = chronic kidney disease, DMARDs = disease-modifying antirheumatic drugs, ESRD = end-stage renal disease, ETA = etanercept, GI = gastrointestinal, GOL = golimumab, HCQ = hydroxychloroquine, IFX = infliximab, IV = intravenous, LEF = leflunomide, MTX = methotrexate, RA = rheumatoid arthritis, RTM = rituximab, SC = subcutaneous, SD = syndrome, SSZ = sulfasalazine, TAC = tacrolimus, TCZ = tocilizumab.
References
- [1].Papa R, Lachmann HJ. Secondary, AA, amyloidosis. Rheum Dis Clin North Am 2018;44:585–603. [DOI] [PubMed] [Google Scholar]
- [2].Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic aa amyloidosis. N Engl J Med 2007;356:2361–71. [DOI] [PubMed] [Google Scholar]
- [3].Law ST, Taylor PC. Role of biological agents in treatment of rheumatoid arthritis. Pharmacol Res 2019;150:104497. [DOI] [PubMed] [Google Scholar]
- [4].Nakamura T, Shiraishi N, Morikami Y, Fujii H, Kuratsu J. Systemic AA amyloidosis secondary to rheumatoid arthritis may be treatable but is still difficult to manage in daily clinical practice. Amyloid 2019;26:123–4. [DOI] [PubMed] [Google Scholar]
- [5].Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev 2021;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- [7].Kluve-Beckerman B, Manaloor J, Liepnieks JJ. Binding, trafficking and accumulation of serum amyloid a in peritoneal macrophages. Scand J Immunol 2001;53:393–400. [DOI] [PubMed] [Google Scholar]
- [8].an der Hilst JC, Yamada T, Op den Camp HJ, van der Meer JW, Drenth JP, Simon A. Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: potential explanation for higher risk of type AA amyloidosis. Rheumatology (Oxford) 2008;47:1651–4. [DOI] [PubMed] [Google Scholar]
- [9].Calamai M, Kumita JR, Mifsud J, et al. Nature and significance of the interactions between amyloid fibrils and biological polyelectrolytes. Biochemistry 2006;45:12806–15. [DOI] [PubMed] [Google Scholar]
- [10].Nakamura T, Higashi S, Tomoda K, Tsukano M, Baba S, Shono M. Significance of SAA1.3 allele genotype in Japanese patients with amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford) 2006;45:43–9. [DOI] [PubMed] [Google Scholar]
- [11].Hsu HC, Zhou T, Yang PA, Herrera GA, Mountz JD. Increased acute-phase response and renal amyloidosis in aged cd2-fas-transgenic mice. J Immunol 1997;158:5988–96. [PubMed] [Google Scholar]
- [12].Okuda Y, Yamada T, Matsuura M, Takasugi K, Goto M. Ageing: a risk factor for amyloid A amyloidosis in rheumatoid arthritis. Amyloid 2011;18:108–11. [DOI] [PubMed] [Google Scholar]
- [13].Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 2001;358:24–9. [DOI] [PubMed] [Google Scholar]
- [14].Dember LM, Hawkins PN, Hazenberg BP, et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med 2007;356:2349–60. [DOI] [PubMed] [Google Scholar]
- [15].Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biologics 2008;2:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun 2004;314:363–9. [DOI] [PubMed] [Google Scholar]
- [17].Sato H, Sakai T, Sugaya T, et al. Tocilizumab dramatically ameliorated life-threatening diarrhea due to secondary amyloidosis associated with rheumatoid arthritis. Clin Rheumatol 2009;28:1113–6. [DOI] [PubMed] [Google Scholar]
- [18].Inoue D, Arima H, Kawanami C, et al. Excellent therapeutic effect of tocilizumab on intestinal amyloid a deposition secondary to active rheumatoid arthritis. Clin Rheumatol 2010;29:1195–7. [DOI] [PubMed] [Google Scholar]
- [19].Vinicki JP, De Rosa G, Laborde HA. Renal amyloidosis secondary to rheumatoid arthritis: Remission of proteinuria and renal function improvement with tocilizumab. J Clin Rheumatol 2013;19:211–3. [DOI] [PubMed] [Google Scholar]
- [20].Shimagami H, Katada Y. Successful treatment with tocilizumab for massive ascites due to secondary amyloidosis complicating rheumatoid arthritis: a case report. Scand J Rheumatol 2019;48:511–2. [DOI] [PubMed] [Google Scholar]
- [21].Okuda Y. AA amyloidosis - benefits and prospects of IL-6 inhibitors. Mod Rheumatol 2019;29:268–74. [DOI] [PubMed] [Google Scholar]
- [22].Nishida S, Hagihara K, Shima Y, et al. Rapid improvement of aa amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis 2009;68:1235–6. [DOI] [PubMed] [Google Scholar]
- [23].Galmiche S, Buob D, Fellahi S, Bastard JP, Grateau G, Georgin-Lavialle S. Rheumatoid arthritis revealed by polyadenopathy, diarrhea and digestive aa amyloidosis. Joint Bone Spine 2019;86:397–8. [DOI] [PubMed] [Google Scholar]
- [24].Yamagata A, Uchida T, Yamada Y, et al. Rapid clinical improvement of amyloid a amyloidosis following treatment with tocilizumab despite persisting amyloid deposition: a case report. BMC Nephrol 2017;18:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yamada S, Tsuchimoto A, Kaizu Y, et al. Tocilizumab-induced remission of nephrotic syndrome accompanied by secondary amyloidosis and glomerulonephritis in a patient with rheumatoid arthritis. CEN Case Rep 2014;3:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Matsui M, Okayama S, Tsushima H, et al. Therapeutic benefits of tocilizumab vary in different organs of a patient with AA amyloidosis. Case Rep Nephrol 2014;2014:823093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hakala M, Immonen K, Korpela M, Vasala M, Kauppi MJ. Good medium-term efficacy of tocilizumab in DMARD and anti-TNF-(therapy resistant reactive amyloidosis. Ann Rheum Dis 2013;72:464–5. [DOI] [PubMed] [Google Scholar]
- [28].Miyagawa I, Nakayamada S, Saito K, et al. Study on the safety and efficacy of tocilizumab, an anti-IL-6 receptor antibody, in patients with rheumatoid arthritis complicated with AA amyloidosis. Mod Rheumatol 2014;24:405–9. [DOI] [PubMed] [Google Scholar]
- [29].Courties A, Grateau G, Philippe P, et al. AA amyloidosis treated with tocilizumab: case series and updated literature review. Amyloid 2015;22:84–92. [DOI] [PubMed] [Google Scholar]
- [30].Lane T, Gillmore JD, Wechalekar AD, Hawkins PN, Lachmann HJ. Therapeutic blockade of interleukin-6 by tocilizumab in the management of AA amyloidosis and chronic inflammatory disorders: a case series and review of the literature. Clin Exp Rheumatol 2015;33:S46–53. [PubMed] [Google Scholar]
- [31].Kovács A, Cserenyecz A, Baksay B, Kemény É, Szekanecz Z. Successful treatment of rheumatoid arthritis-associated renal aa amyloidosis with tocilizumab. Isr Med Assoc J 2020;22:455–7. [PubMed] [Google Scholar]
- [32].Fukuda M, Sawa N, Hoshino J, Ohashi K, Motoaki M, Ubara Y. Tocilizumab preserves renal function in rheumatoid arthritis with aa amyloidosis and end-stage kidney disease: two case reports. Clin Nephrol 2021;95:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hattori Y, Ubara Y, Sumida K, et al. Tocilizumab improves cardiac disease in a hemodialysis patient with AA amyloidosis secondary to rheumatoid arthritis. Amyloid 2012;19:37–40. [DOI] [PubMed] [Google Scholar]
- [34].Ravindran J, Shenker N, Bhalla AK, Lachmann H, Hawkins P. Case report: response in proteinuria due to AA amyloidosis but not felty's syndrome in a patient with rheumatoid arthritis treated with TNF-alpha blockade. Rheumatology (Oxford) 2004;43:669–72. [DOI] [PubMed] [Google Scholar]
- [35].Smith GR, Tymms KE, Falk M. Etanercept treatment of renal amyloidosis complicating rheumatoid arthritis. Intern Med J 2004;34:570–2. [DOI] [PubMed] [Google Scholar]
- [36].Kuroda T, Otaki Y, Sato H, et al. A case of AA amyloidosis associated with rheumatoid arthritis effectively treated with infliximab. Rheumatol Int 2008;28:1155–9. [DOI] [PubMed] [Google Scholar]
- [37].Lee CK, Park JY, Shim JJ, Jang JY. Successful treatment with anti-tumor necrosis factor alpha for reactive small-bowel amyloidosis. Endoscopy 2011;43: Suppl 2 UCTN: E326–7. [DOI] [PubMed] [Google Scholar]
- [38].Kuroda T, Wada Y, Kobayashi D, et al. Effective anti-TNF-alpha therapy can induce rapid resolution and sustained decrease of gastroduodenal mucosal amyloid deposits in reactive amyloidosis associated with rheumatoid arthritis. J Rheumatol 2009;36:2409–15. [DOI] [PubMed] [Google Scholar]
- [39].Nakamura T, Higashi S, Tomoda K, Tsukano M, Shono M. Effectiveness of etanercept vs cyclophosphamide as treatment for patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford) 2012;51:2064–9. [DOI] [PubMed] [Google Scholar]
- [40].Fikri-Benbrahim O, Rivera-Hernández F, Martínez-Calero A, Cazalla-Cadenas F, García-Agudo R, Mancha-Ramos J. Treatment with adalimumab in amyloidosis secondary to rheumatoid arthritis: two case reports. Nefrologia 2013;33:404–9. [DOI] [PubMed] [Google Scholar]
- [41].Nakamura T, Kumon Y, Hirata S, Takaoka H. Abatacept may be effective and safe in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Exp Rheumatol 2014;32:501–8. [PubMed] [Google Scholar]
- [42].Sawamura M, Sawa N, Fujiwara H, et al. Abatacept improves intractable protein-losing enteropathy secondary to AA amyloidosis in a patient with rheumatoid arthritis. Mayo Clin Proc Innov Qual Outcomes 2020;4:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Narváez J, Hernández MV, Ruiz JM, Vaquero CG, Juanola X, Nollaa JM. Rituximab therapy for AA-amyloidosis secondary to rheumatoid arthritis. Joint Bone Spine 2011;78:101–3. [DOI] [PubMed] [Google Scholar]
- [44].Burkart J, Benson DM, Jr. When first line therapy for AA-amyloidosis secondary to rheumatoid arthritis fails: a correspondence. Joint Bone Spine 2013;80:229–30. [DOI] [PubMed] [Google Scholar]
- [45].Kilic L, Erden A, Sener YZ, et al. Rituximab therapy in renal amyloidosis secondary to rheumatoid arthritis. Biomolecules 2018;8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Watanabe T, Hattori T, Ogawa Y, Jodo S. Successful treatment with tofacitinib for renal disorder due to amyloid A amyloidosis and immunoglobulin A nephropathy in a patient with rheumatoid arthritis. Clin Exp Rheumatol 2018;36:683–4. [PubMed] [Google Scholar]
- [47].Kuroda T, Tanabe N, Kobayashi D, et al. Treatment with biologic agents improves the prognosis of patients with rheumatoid arthritis and amyloidosis. J Rheumatol 2012;39:1348–54. [DOI] [PubMed] [Google Scholar]
- [48].Pamuk ÖN, Kalyoncu U, Aksu K, et al. A multicenter report of biologic agents for the treatment of secondary amyloidosis in turkish rheumatoid arthritis and ankylosing spondylitis patients. Rheumatol Int 2016;36:945–53. [DOI] [PubMed] [Google Scholar]
- [49].Okuda Y, Ohnishi M, Matoba K, et al. Comparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseases. Mod Rheumatol 2014;24:137–43. [DOI] [PubMed] [Google Scholar]
- [50].Sattianayagam PT, Hawkins PN, Gillmore JD. Systemic amyloidosis and the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2009;6:608–17. [DOI] [PubMed] [Google Scholar]