Abstract
Cancer is driven by genomic mutations in ‘cancer driver’ genes, which have essential roles in tumor development. These mutations may be caused by exposure to mutagens in the environment or by endogenous DNA-replication errors in tissue stem cells. Recent observations of abundant mutations, including cancer driver mutations, in histologically normal human tissues suggest that mutations alone are not sufficient for tumor development, thus prompting the question of how single mutant cells give rise to neoplasia. In a concept supported by decades-old data from mouse tumor models, non-mutagenic tumor-promoting agents have been posited to activate the proliferation of dormant mutated cells, thus generating actively growing lesions, with the promotion stage as the rate-limiting step in tumor formation. Non-mutagenic promoting agents, either endogenous or environmental, may therefore have a more important role in human cancer etiology than previously thought.
Mutations that accumulate in DNA are generally accepted to be harmful for human health. The mutations that result primarily from damage and misrepair of DNA in most tissues, with the possible exception of the immune system, have been linked to the aging process as well as to a host of diseases, including neurodegeneration and cancer1,2. However, recent studies exploiting deep-sequencing approaches have uncovered abundant somatic mutations in several adult-human normal tissues including the skin, esophagus, colon, liver, lung and endometrium3-12. Remarkably, healthy cells in the esophageal epithelium can carry several hundred mutations per cell in younger individuals, and this number can rise to more than 2,000 mutations per cell by around 60 years of age4. Many mutations found in normal cells are not simply non-functional passengers but are additionally found in cancers from the same tissues. For example, between 30% and 80% of the cells in normal esophagus tissue in older individuals have mutations in NOTCH1—a known cancer driver gene in esophageal cancer4,5.
These data prompt several important questions regarding the mechanisms that allow mutations to persist over such long periods and prevent them from giving rise to malignant tumors in the same tissues. One proposed explanation for the persistence of these mutations is that comprehensive repair of lesions in DNA would be an energetically unfavorable investment for normal cells, and consequently mutations that are tolerated in normal aging tissue accumulate13. However, many mutations are selected in clones that can be extensive in their size and coverage of the epithelium, thus suggesting that these mutations may confer a growth advantage on cells. Certain mutations, for example those in the NOTCH1 gene, might potentially even have a positive role in epithelial tissues by helping to replace cells lost through tissue damage or apoptosis. Notably, NOTCH1 mutations are more prevalent in normal epithelia than in cancers arising from the same tissue4. The notion of functional selection of mutations that increase fitness in normal tissues but are not cancer associated is supported by analysis of the mutational landscape in cirrhosis of the human liver. Recurrent mutations have been demonstrated in several genes including PKD1, PKHD1 and PPARGC1B, which are not thought to be drivers of liver cancer, thus suggesting that they act by conferring survival ability under conditions of chronic liver damage12.
The relative rarity of malignant progression despite the presence of many mutations could be due to the total number of mutations, or combinations thereof, being below that required for full transformation. Indeed, the numbers of mutations in tumors have been found to exceed those in clones of cells from normal aging tissues4,7. Mutations might also accumulate in cell types that do not readily undergo transformation. Studies in mouse models have shown that the nature of the cell of origin, in which mutations can be induced genetically, can determine the probability of malignant growth14. In yet another scenario, poorly understood exogenous environmental risk factors may have critical roles in stimulating the conversion of mutated cells into actively growing lesions. The goal of this Perspective is to highlight some prior studies on cancer development in mouse models that exactly recapitulate this scenario, in which exposure to mutagens gives rise to highly mutated but nevertheless dormant ‘initiated’ cells, which can be activated and form tumors shortly after exposure to a non-mutagenic tumor-promoting agent. These early mouse models offer an alternative view of the relative cancer risk conferred by exogenous mutagenic agents versus non-mutagenic environmental factors, and may have implications for approaches to cancer prevention.
Animal models of skin cancer: mutations are essential but not sufficient
The first animal-model studies were published more than 100 years ago and established a causal relationship between exposure to exogenous carcinogens and cancer risk15. Subsequent studies led to the concept of two-stage skin carcinogenesis, in which an initiating phase induced by treatment of mouse skin with a low dose of a carcinogen was followed by a promotion phase involving repeated treatment with an irritant inflammatory but non-mutagenic agent (croton oil)16 (Fig. 1a). A more modern version of this experiment involves initiation by a single treatment with a specific mutagen, most commonly dimethylbenzanthacene (DMBA), which causes development of squamous carcinomas carrying activating mutations in the Hras oncogene17,18. The most frequently used promoter is 12-O-tetradecanoyl-phorbol-13-acetate (TPA), although other chemicals or wounding can also promote initiated cells19.
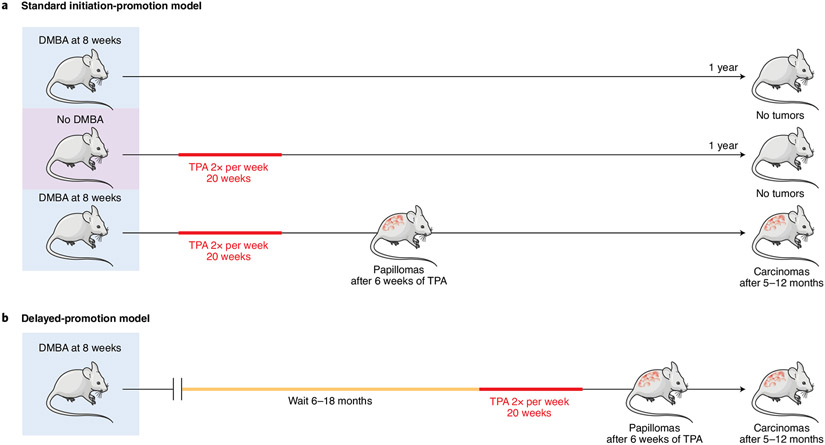
Fig. 1 ∣. The permanence of the initiated state.
a, Standard initiation-promotion model. Mice are treated once with a carcinogen (usually DMBA) at 8 weeks of age and 1 week later with twice-weekly treatment with a promoting agent (usually TPA). Treatment with either DMBA alone (top row) or TPA alone (second row) does not give rise to tumor development during a follow-up period of 1 year. However, DMBA followed by TPA induces benign tumors (papillomas) in 1–2 months, some of which progress to carcinomas after 5–12 months. b, Delayed-promotion model. Treatment with the carcinogen is followed a delay of 6–18 months before the start of promoter treatment. Benign tumors appear 1–2 months after starting TPA, and carcinomas develop after 5–12 months. The overall tumor yield decreases slightly after longer delay periods20-22.
Early studies by Berenblum and Shubik20 showed that, in the absence of any promotion phase, initiated cells do not disappear but remain dormant for most of the lifespan of the mice. When promotion is started 1 week after initiation, benign papillomas appear approximately 6–8 weeks thereafter. Even if promotion is delayed for more than 1 year after initiation, most of the mutated cells remain present and capable of giving rise to tumors, thus showing that the mutations induced by the initiating agent are persistent21 (Fig. 1b). These experiments were repeated by Loehrke et al.22, who also performed studies involving initiation in utero by systemic treatment of pregnant mice with low doses of carcinogens23,24. Although no skin tumors were observed in the offspring of carcinogen-exposed mice for up to 1 year after birth, repeated treatment with TPA starting at 12 weeks of age resulted in the appearance of multiple papillomas. The consequences of systemic initiation in utero followed by topical skin treatment with an inflammatory agent have implications beyond the well-known skin model, because increased tumor development was also seen in some other tissues, such as the liver and stomach24. We can conclude that the major risk factor for cancer development in initiated mice is not the persistence of strong oncogenic mutations but instead exposure to agents that act as promoters of preinitiated cells.
Although these studies were completed before anything was known about cellular oncogenes or cancer drivers, a single dose of carcinogen is now known to induce mutations in sufficient numbers, and in the correct combinations and in the appropriate target cells, for transformation. Tumor DNA-sequencing studies have shown that DMBA-initiated mouse skin cells, in addition to activating Hras mutations, contain thousands of mutations including additional known driver mutations (refs. 25,26 and Y. R. Li, K. Halliwill, R. Delrosario, Q. Tran, A.B. et al., unpublished data). The growth of cells carrying these mutations is stimulated by TPA almost immediately after the start of promoter treatment, and they produce visible precursor lesions after only 2–3 weeks of exposure27. Because this short time frame is not sufficient for the acquisition of additional mutations followed by clonal selection, we can conclude that all genetic changes required for benign skin tumor formation are present in initiated cells and that repeated exposure to the promoter is necessary for appearance of early-stage tumors.
Although these data suggest that thousands of mutations are insufficient for skin papilloma development, the same conclusion cannot yet be drawn for other mouse tumor models, in which the sensitivity to promoters may be variable, or for human samples. Although multiple rodent models have demonstrated the effects of promoters in different tissues, such as butylated hydroxytoluene for the lung28, dextran sodium sulfate for the colon29, phenobarbital for the liver30, cerulein for the pancreas31 and hot liquids for the esophagus32, the persistence of initiating mutations over time, as performed for the skin, has not yet been comprehensively investigated.
Promotion and human cancer risk
The possibility that a strong promotion phase, leading to the activation of mutant cells, may contribute to human cancer risk has not been adequately considered in a recent debate on the major human cancer risk factors33-35. Concluding that some, and possibly many, of the environmental factors that contribute to human cancer do so through non-mutational mechanisms related to promotion appears logical. Even well-known human cancer risk factors such as smoking and ultraviolet light, although best characterized as mutagens that directly damage DNA and have characteristic mutational signatures36, have been shown to induce pathways strongly linked to promotion. Nicotine in cigarette smoke stimulates cell growth, angiogenesis and metastasis in tumor model systems37, whereas ultraviolet light induces strong activation of the AP-1 transcription pathway, which is essential for TPA-induced promotion38. Obesity, now recognized as the major human cancer risk factor after smoking, is associated with a high-fat diet, which leads to stimulation of bile-acid production and alterations in lineage tracing of tissue stem cells in the colon and small intestine39, with possible major consequences for cancer risk. Other well-known and probably inter-related factors also have roles in promoting tumorigenesis, including chronic inflammation40 or wounding41. Although some of these mechanisms could be argued to also potentially lead to mutagenesis by stimulating proliferation and DNA-replication errors, or by the inflammation-associated generation of reactive oxygen species, evidence of a mutation-based route is lacking. In conclusion, whereas genomic mutations, whether induced by replication errors in stem cells or by environmental mutagens, are clearly essential for tumor development, many studies, particularly those based on mouse models, indicate that they are not sufficient. Exposure to a plethora of different and possibly tissue-specific, environmental or endogenous promoting agents may therefore be a critical rate-limiting step for tumor development in both mouse models and humans.
The good news: promotion is (potentially) preventable
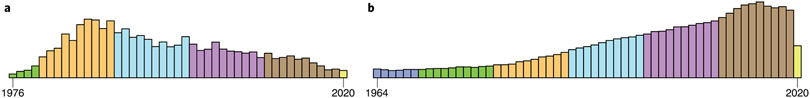
Incorporation of the promotion stage into risk modeling gives more room for some optimism regarding cancer prevention. Mutations induce a permanent ‘scar’ in tumor DNA and are essentially irreversible, whereas promotion may be a more manageable risk factor that is amenable to prevention strategies. Because long-term, continued treatment with promoters is required for optimal tumor yield, decreasing the overall dose or frequency of exposure, rather than complete elimination, may have a disproportionate effect in reducing cancer risk. A deeper understanding of the mechanisms through which promoters act, and the identification of potential environmental promoting agents, may help limit the effects of this important phase of carcinogenesis on human cancer risk. Unfortunately, no concerted efforts are underway to develop assays to screen for environmental agents with promoter activity. Indeed, the number of publications related to the prototypic tumor promoter TPA identified in a PubMed search for ‘TPA tumor promotion’ shows that research in this field, which peaked in the 1980s to 1990s, is drawing to a close, having been supplanted by excitement engendered by the discovery of mutations in oncogenes in the early 1980s42 (Fig. 2). Although publications that address the roles of other factors such as diet, obesity and inflammation in cancer risk have increased in emphasis over the same time frame, there is no consensus regarding the mechanisms through which these agents promote carcinogenesis or how they relate to the classical view of tumor promotion by chemicals such as TPA.
Fig. 2 ∣. Publications per year from 1974–2018.
These publications were identified through a search for ‘TPA tumor promotion’ (left) or ‘oncogene’ (right), showing the shift in emphasis.
Approaches to dissecting the roles of mutagens and promoters in human and mouse cancer development
Further analysis of the relationships among environmental exposures, somatic mutations and non-mutational promotion processes in human cancer is in progress as part of the Cancer Grand Challenges Mutographs Project funded by Cancer Research UK (https://www.mutographs.org/). A major goal of this project is to perform whole-genome sequencing of >5,000 human cancers in people from five different continents, with a primary focus on cancers of the esophagus, colon, kidney and pancreas. The rationale behind this project is that analysis of mutational signatures in tumor DNA, which have been associated with specific patterns of exposure to carcinogens36, may provide insights into the causes underlying the very large variation in the incidence of the same type of cancer in distinct geographical locations43. A unique feature of this project is the availability of extensive lifestyle and exposure information for each patient, including dietary habits, consumption of alcohol and hot drinks, body mass index and history of other chronic conditions that may affect cancer incidence. Sequencing of tumors in people from these diverse geographical areas may reveal features of the mutational burden, or specific types of novel mutational signatures, that could identify potential causative agents linked to variation in cancer incidence. However, the lack of any clear mutational differences between tumors from high-risk and low-risk areas may indicate important roles of lifestyle factors or non-mutagenic tumor-promoting agents that leave no genomic imprint on tumor DNA.
The Mutographs Project also includes a mouse-model component to investigate the causal relationships between specific exposures and the mutation burden or mutational signatures induced. These models offer the possibility of tailoring the route, dose and frequency of exposure to the agents being tested, which include suspected environmental chemical carcinogens; different types of radiation; or other major cancer risk factors, such as obesity, diet, chronic inflammation and wounding. The first results from these large-scale mouse tumor genome-sequencing projects are now becoming available44,45 and demonstrate the utility of mouse models in establishing definitive mechanistic links between DNA-damaging agents and mutational signatures. The US National Toxicology Program, over several decades, has performed assays in rodents to examine the in vivo carcinogenicity of hundreds of environmental chemicals classified as known or suspected human carcinogens by the International Agency for Research on Cancer (IARC). Whole-genome sequencing of tumors induced by long-term oral or inhalation exposure to one of 20 common environmental chemicals revealed that only a small proportion of these agents for which there was clear evidence of carcinogenicity actually induced a high mutation load and/or specific mutational signatures45. Most of the tested chemicals induced tumors that were indistinguishable from those arising spontaneously in the same mouse strain, with very low mutation burdens and no novel mutation patterns that could have indicated direct mutagenic activity. These agents may therefore act primarily through mechanisms that involve promotion of cells carrying spontaneously arising mutations, rather than by substantially contributing to the mutational burden.
Tumors from a range of additional mouse models designed to test the mutational effects of a variety of promoting factors, including long-term TPA treatment or wounding, a high-fat diet or genetically determined obesity, have also been sequenced. These data (Y. R. Li, K. Halliwill, R. Delrosario, Q. Tran, A.B. et al., unpublished data) provide strong support for the essential, rate-limiting roles of promoting factors in cancer causation, when the exposure is preceded by the induction of strong driver mutations. The analyses in progress will aid in determining whether some promoting or lifestyle factors contribute to overall mutation burden by stimulating endogenous mutation processes, or act epigenetically by changing chromatin landscapes or otherwise by altering gene expression and subsequently affecting tumor growth rate, stem cell fate changes or immune recognition.
In conclusion, efficient cancer prevention through minimizing the effects of environmental promoters would require better methods for their detection as well as for elucidating their mechanisms of action. Several new technologies are now available that could greatly increase the probability of detecting agents with promoter activity as well as those that act as direct mutagens. In vivo long-term bioassays are expensive and time consuming, but other approaches are now feasible for the detection of very rare mutant cells in normal tissues, as well as the quantification of their expansion and selection in response to treatment with candidate promoting agents46,47. Novel approaches to single-cell DNA sequencing may begin to address questions related to the numbers and combinations of mutations in single cells in normal tissues before frank tumor development48. Given their diversity, all chemical agents and other factors that can act as tumor promoters appear unlikely to act through the same mechanism, although they may ultimately converge on a common pathway related to conserved wound-healing responses to tissue damage. A deeper understanding of the mechanisms of action of non-genotoxic promoting agents may reveal the ‘holy grail’ of cancer-prevention research—a common pathway through which disparate agents cause tissue damage and consequently activate the latent mutant cells that lurk within normal tissues.
Acknowledgements
This work was supported by a Cancer Research UK Grand Challenge Award (C98/A24032), US National Cancer Institute (NCI) grants R35CA210018 and UO1CA176287, and the Barbara Bass Bakar Professorship of Cancer Genetics (to A.B.). The author thanks numerous colleagues for discussions.
Footnotes
Competing interests
A.B. is a member of the Scientific Advisory Board of Mission Bio, Inc. and has received funding support from Novartis and Bristol Myers Squibb.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hasty P, Campisi J, Hoeijmakers J, van Steeg H & Vijg J Aging and genome maintenance: lessons from the mouse? Science 299, 1355–1359 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Odegard VH & Schatz DG Targeting of somatic hypermutation. Nat. Rev. Immunol 6, 573–583 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Martincorena I et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martincorena I et al. Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama A et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565, 312–317 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Lee-Six H et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 574, 532–537 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Brunner SF et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature 574, 538–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore L et al. The mutational landscape of normal human endometrial epithelium. Nature 580, 640–646 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Suda K et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 24, 1777–1789 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK et al. Comprehensive analysis of genetic aberrations linked to tumorigenesis in regenerative nodules of liver cirrhosis. J. Gastroenterol 54, 628–640 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Zhu M et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic liver disease. Cell 177, 608–621.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nik-Zainal S & Hall BA Cellular survival over genomic perfection. Science 366, 802–803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown K, Strathdee D, Bryson S, Lambie W & Balmain A The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol 23, 516–24 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Yamagiwa K & Ichikawa K Über die künstliche Erzeugung von Papillom. Verh Jap Path Ges. 5, 142–148 (1915). [Google Scholar]
- 16.Berenblum I & Shubik P The role of croton oil applications, associated with a single painting of a carcinogen, in tumour induction of the mouse’s skin. Br. J. Cancer 1, 379–382 (1947). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balmain A & Pragnell IB Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature 303, 72–74 (1983). [DOI] [PubMed] [Google Scholar]
- 18.Quintanilla M, Brown K, Ramsden M & Balmain A Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 322, 78–80 (1986). [DOI] [PubMed] [Google Scholar]
- 19.McCreery MQ & Balmain A Chemical carcinogenesis models of cancer: back to the future. Annu. Rev. Cancer Biol 1, 295–312 (2017). [Google Scholar]
- 20.Berenblum I & Shubik P The persistence of latent tumour cells induced in the mouse’s skin by a single application of 9:10-dimethyl-1:2-benzanthracene. Br. J. Cancer 3, 384–386 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenbäck F, Peto R & Shubik P Initiation and promotion at different ages and doses in 2200 mice. Br. J. Cancer 44, 1–14 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loehrke H et al. On the persistence of tumor initiation in two-stage carcinogenesis on mouse skin. Carcinogenesis 4, 771–775 (1983). [DOI] [PubMed] [Google Scholar]
- 23.Goerttler K, Loehrke H, Schweizer J & Hesse B Two-stage skin carcinogenesis by systemic initiation of pregnant mice with 7,12-dimethylbenz(a)anthracene during gestation days 6-20 and postnatal promotion of the F 1-generation with the phorbol ester 12-tetradecanoylphorbol-13-acetate. J. Cancer Res. Clin. Oncol 98, 267–275 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goerttler K, Loehrke H, Hesse B, Milz A & Schweizer J Diaplacental initiation of NMRI mice with 7,12-dimethylbenz[a]anthracene during gestation days 6–20 and postnatal treatment of the F1-generation with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate: tumor incidence in organs other than the skin. Carcinogenesis 2, 1087–1094 (1981). [DOI] [PubMed] [Google Scholar]
- 25.McCreery MQ et al. Evolution of metastasis revealed by mutational landscapes of chemically induced skin cancers. Nat. Med 21, 1514–1520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassar D, Latil M, Boeckx B, Lambrechts D & Blanpain C Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat. Med 21, 946–954 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Binder RL et al. Squamous cell hyperplastic foci: precursors of cutaneous papillomas induced in SENCAR mice by a two-stage carcinogenesis regimen. Cancer Res. 58, 4314–4323 (1998). [PubMed] [Google Scholar]
- 28.Bauer AK, Dwyer-Nield LD, Keil K, Koski K & Malkinson AM Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp. Lung Res 27, 197–216 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Snider AJ et al. Murine model for colitis-associated cancer of the colon. Methods Mol. Biol 1438, 245–254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diwan BA, Rice JM & Ward JM Strain-dependent effects of phenobarbital on liver tumor promotion in inbred mice. Prog. Clin. Biol. Res 331, 69–83 (1990). [PubMed] [Google Scholar]
- 31.Guerra C et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Rapozo DCM et al. Recurrent acute thermal lesion induces esophageal hyperproliferative premalignant lesions in mice esophagus. Exp. Mol. Pathol 100, 325–331 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Tomasetti C & Vogelstein B Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Powers S, Zhu W & Hannun YA Substantial contribution of extrinsic risk factors to cancer development. Nature 529, 43–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wild C et al. Cancer risk: role of chance overstated. Science 347, 728 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaal C & Chellappan SP Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res 12, 14–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dérijard B et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76, 1025–1037 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Fu T et al. FXR regulates intestinal cancer stem cell proliferation. Cell 176, 1098–1112.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coussens LM & Werb Z Inflammation and cancer. Nature 420, 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolberg DS, Hollingsworth R, Hertle M & Bissell MJ Wounding and its role in RSV-mediated tumor formation. Science 230, 676–678 (1985). [DOI] [PubMed] [Google Scholar]
- 42.Weinberg RA Oncogenes and the molecular basis of cancer. Harvey Lect. 80, 129–136 (1984). -1985–1985. [PubMed] [Google Scholar]
- 43.Murphy G et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann. Oncol 28, 2086–2093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose, Li Y et al. Mutational signatures in tumours induced by high and low energy radiation in Trp53 deficient mice. Nat. Commun 11, 394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riva L et al. The mutational signature profile of known and suspected human carcinogens in mice. Nat. Genet 10.1038/s41588-020-0692-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salk JJ, Schmitt MW & Loeb LA Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat. Rev. Genet 19, 269–285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, MacAlpine DM & Counter CM Capturing the primordial Kras mutation initiating urethane carcinogenesis. Nat. Commun 11, 1800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon CM et al. Clonal selection with Ras pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 9, 1050–1063 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]