Abstract
Background
Snakebite is a priority neglected tropical disease and causes a range of complications that vary depending on the snake species. Randomised clinical trials have used varied outcome measures that do not allow results to be compared or combined. In accordance with the Core Outcomes Measurements in Effectiveness Trials (COMET) initiative, this systematic review aims to support the development of a globally relevant core outcome set for snakebite.
Methods
All randomised controlled trials, secondary analyses of randomised controlled trials and study protocols investigating the efficacy of therapeutics for human snakebite envenoming were eligible for inclusion. Study screening and data extraction were conducted in duplicate by two independent reviewers. All primary and secondary outcome measures were extracted and compiled, as were adverse event outcome measures. Similar outcome measures were grouped into domains. The study was prospectively registered with PROSPERO: CRD42020196160.
Results
This systematic review included 43 randomised controlled trials, two secondary analyses and 13 study protocols. A total of 382 outcome measures were extracted and, after duplicates were merged, there were 153 unique outcomes. The most frequently used outcome domain (‘venom antigenaemia’) was included in less than one third of the studies. The unique outcomes were classified into 60 outcome domains. Patient-centred outcomes were used in only three of the studies.
Discussion
Significant heterogeneity in outcome measures exists in snakebite clinical trials. Consensus is needed to select outcome measures that are valid, reliable, patient-centred and feasible. The results of this systematic review strongly support the development of a core outcome set for use in snakebite clinical trials.
Author summary
Standardised outcome measures for snakebite randomised controlled trials are needed to enable results to be compared and combined between studies. This systematic review was conducted to understand the variations in outcome measure use in snakebite randomised controlled trials, and to create a comprehensive list of outcome measures from which to develop a core outcome set (COS). A total of 153 unique outcome measures were extracted from the 58 studies identified in this systematic review. Of these 153 unique outcome measures, 91 were used in only in a single study. Although a form of bedside whole blood clotting test was used in 30 of the 58 studies, 18 unique methods of measurement were identified. Only three studies, all conducted in the USA, included patient-centred outcomes. This systematic review demonstrates the strong need for a snakebite core outcome set, which will support the adoption of valid, reproducible, and patient-centred outcome measures, and enable downstream meta-analyses.
Introduction
Global estimates indicate that there are 1·8 million envenomings and 94,000 deaths each year due to snakebite, with the highest burden in sub-Saharan Africa and Asia [1].
There is significant within-species and between-species variability in the toxins found in snake venoms [2], which account for the broad range of clinical manifestations caused by envenoming [3]. Syndromes of systemic envenoming include neurotoxicity, haemorrhage, and coagulopathy. Local effects can range from swelling to tissue necrosis, and are an important cause of disability and limb amputation [4]. Other effects of envenoming include myotoxicity, hypotension, and renal injury.
There has been limited funding for snakebite research, with a global average of under 5 million USD invested per annum [5], and a resulting paucity of clinical trials [6]. Antivenom is the only specific therapy for treating the aetiological toxins injected during snakebite, yet their use is rarely supported by clinical efficacy data or a rigorous regulatory framework [7]. However, in 2017 the World Health Organization reinstated snakebite envenoming as a category A neglected tropical disease [8], and thus funding to support snakebite management is anticipated to increase. Appropriate outcome measures are vital for ensuring that findings are relevant to patients and can appropriately inform policy makers. They need to be valid and reliable, particularly when surrogate endpoints are relied upon.
The Core Outcome Measures in Effectiveness Trials (COMET) Initiative has advocated for and supported the development of core outcome sets (COS) in clinical research [9]. These are developed by collaborative groups of researchers, clinicians, and patients; to identify an agreed minimum set of outcome measures for a disease area. By using a core outcome set, it is easier to compare, contrast and combine results of clinical trials, which has rarely been possible in the field of snakebite [10,11]. The first step toward developing a COS is to undertake a systematic review of the existing literature to inform. This systematic review aims to describe the heterogeneity in outcome measures used across clinical trials and will provide a comprehensive resource of outcome measures that can be considered when developing a COS.
Methods
Search strategy and selection criteria
Databases were searched for randomised controlled trials and trial protocols wherein therapeutics that inhibit venom, or its downstream pathological effects, were studied. MEDLINE, Cochrane CENTRAL, Web of Science and Embase were searched from database inception until the 23rd of June 2020, with no language restriction, using the search terms ([“Snake bite” OR “snake envenomation” OR “snake venoms” OR “antivenoms” OR “antivenins] and [“randomised controlled trial” OR “randomised” OR “randomized” OR “randomly” OR “placebo” OR “double-blind” OR “single-blind” OR “clinical trial”]. Reference lists of included studies were searched. The following trial registries were searched: Australian trial register; International Standard Randomised Controlled Trial register; Clinical Trials Registry India; Chinese clinical trials registry; Clinical trial gov; Sri Lanka Clinical Trials Registry; Japan Primary Registries Network; WHO ICTRP. Full details of the search strategy were uploaded to PROSPERO (CRD42020196160).
Covidence systematic review software was used to compile, deduplicate and screen studies. Two reviewers (MA and DA) independently screened titles and abstracts, and subsequently the full-text articles. Full texts were translated to English language when necessary. Disagreements were resolved by consensus discussion with a third reviewer (HE). All reviewers (MA, DA and HE) are clinical academics with experience of interpreting clinical trials. Studies of adjunctive therapies that proposed to treat either antivenom hypersensitivity reactions or bite-site infection were excluded. Published secondary analyses of randomised controlled trials were included if they provided additional outcome measures.
Data extraction
Prespecified data (as reported in S1 Text) were independently extracted and standardised by two authors (MA and DA). All primary, secondary, and adverse event outcome measures were extracted verbatim from full-text articles and study protocols. Outcome measures were grouped into the following predefined categories: haemorrhage; coagulopathy; neurotoxicity; local tissue damage; renal injury; cardiotoxicity; myotoxicity; mortality; venom antigenaemia; additional antivenom requirement; functional status; scoring system; composite outcome; or other.
Data synthesis
After merging duplicate outcome measures, a data driven approach was used to classify them into domains. Each domain represented a grouping of outcomes that were deemed to be measuring a similar parameter. Consensus on domain allocations and domain names was reached by the primary authors. The characteristics of the studies and the outcome measures were summarised using descriptive statistics. The methodological quality of the primary and secondary outcome measures was assessed by the independent reviewers (MA and DA) using an established tool (as reported in S2 Text) [12]. The reviewers assessed whether each outcome measure was clearly stated; clearly defined; and patient-centred.
R version 4.0.3 was used for all analyses. The protocol was prospectively registered with PROSPERO (42020196160).
Results
Study screening
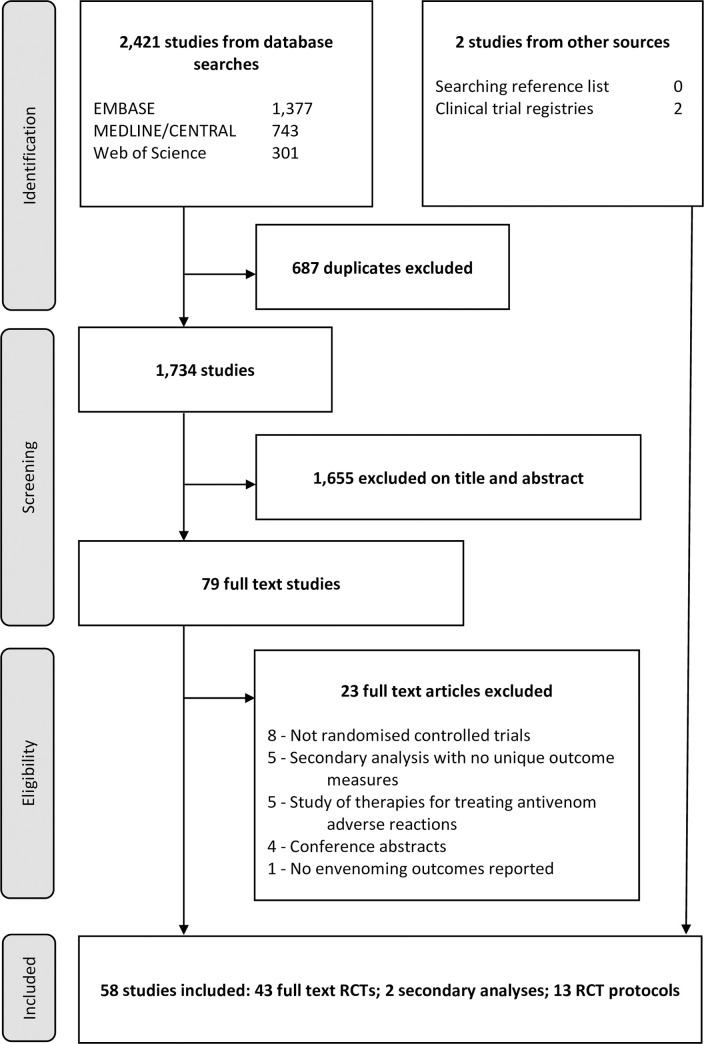
The database searches identified 2,421 studies, of which 687 were duplicates (Fig 1). Review of titles and abstracts identified 79 potentially eligible studies. All full texts were obtained and two required translation to English language. Searching of clinical trial registries identified two further protocols. Following full text review, 43 randomised controlled trials, 13 trial protocols and two published secondary analyses were included. Amongst the 13 included protocols, 6 had been terminated and 7 were ongoing. Two published secondary analyses [13,14] utilised data from the clinical trial published by Gerardo et al [15] and reported the additional outcome measures ‘opiate use’ and ‘the physical function domain of the SF-36 questionnaire’. Table 1 details the characteristics of all the included studies.
Fig 1. Study selection strategy.
RCT = randomised controlled trial.
Table 1. Characteristics of randomised controlled trials and trial protocols.
| Author (year)–protocol registration number | Country(s) | Blinding | N | Snake species | Syndrome of envenoming | Comparison | Products | Primary outcome measure* (verbatim)† | Primary outcome measure category* | Primary outcome clearly defined*‡ (yes/no)? | Primary outcome patient-centred* (yes/no)? | Adverse event outcome domains* (clearly defined‡–yes/no?) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||||||||
| Isbister et al. (2013) [24]—ACTRN12607000620426 | Australia | Open label | 65 | Pseudonaja textilis, Notechis scutatus, Tropidechis carinatus and Hoplocephalus spp. | Coagulopathy, haemorrhage and local tissue damage | FFP vs standard of care | Fresh frozen plasma | Proportion with an INR<2 at six hours post antivenom | Coagulopathy | Yes | No | Anaphylaxis (yes) |
| Mendonca-da-Silva et al. (2017) [62]—ISRCTN12845255 | Brazil | Open label | 116 | Bothrops Lachesis and Crotalus spp. | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Lyophilised Polyvalent Bothrops-Lachesis-Crotalus (Instituto Butantan) Liquid fill polyvalent antivenoms: Bothrops or Bothrops-Lachesis or Bothrops-Crotalus antivenom (Instituto Butantan/Instituto Vital Brazil/FUNED) |
NA | NA | NA | NA | Anaphylaxis (yes); serum sickness (no) |
| de Oliveira Pardal et al. (2004) [52] | Brazil | Open label | 74 | Bothrops atrox, Lachesis muta and Bothrops marajoensis | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Antibotropico-laquetico polyvalent Bothrops-Lachesis antivenom (Instituto Butantan) Antibotropico polyvalent Bothrops whole IgG antivenom (FUNED) |
NA | NA | NA | NA | Non-specific early reaction (no) |
| Jorge et al. (1995) [63] | Brazil | Double blind | 170 | Bothrops spp. | Coagulopathy, haemorrhage and local tissue damage | Different doses of antivenom | Antibotropico polyvalent Bothrops antivenom (Instituto Butantan) | NA | NA | NA | NA | NA |
| Cardoso et al. (1993) [54] | Brazil | Double blind | 121 | Bothrops spp. | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Antibotropico polyvalent Bothrops antivenom (Instituto Butantan) Antibotropico polyvalent Bothrops antivenom (Instituto Vital Brazil) Antibotropico polyvalent Bothrops whole IgG antivenom (FUNED) |
NA | NA | NA | NA | Serum sickness (no); non-specific early reaction (no) |
| Theakston et al. (1992) [84] | Brazil | Open label | 118 | Bothrops spp. | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Antibotropico polyvalent Bothrops antivenom (Instituto Butantan) Antibotropico polyvalent Bothrops antivenom (Instituto Vital Brazil) Antibotropico polyvalent Bothrops whole IgG antivenom (FUNED) |
NA | NA | NA | NA | NA |
| Miao et al. (2003) [28] | China | Open label | 46 | Not identified | Coagulopathy, haemorrhage and local tissue damage | Qingwen Baidu Decoction vs standard of care | Qingwen Baidu Decoction "anti-cobra serum" "anti-viper serum" "anti-acutus serum" |
NA | NA | NA | NA | NA |
| Otero-Patino et al. (2012) [53] | Colombia | Double blind | 72 | Bothrops asper | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Bothrops-Lachesis-Crotalus F(ab’)2 antivenom (ICP) [pepsin digestion caprylic acid fractionation] Polyvalent Bothrops-Lachesis-Crotalus whole IgG antivenom (ICP) [caprylic acid fractionation] |
NA | NA | NA | NA | Anaphylaxis (yes); early hypersensitivity reaction (yes); serum sickness (no) |
| Otero et al. (2006) [70] | Colombia | Double blind | 67 | Bothrops asper | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Bothrops-Lachesis-Crotalus whole IgG antivenom (ICP) [ammonium sulfate precipitation and pepsin digestion] Polyvalent Bothrops-Lachesis-Crotalus whole IgG antivenom (ICP) [caprylic acid fractionation] Monovalent Bothrops atrox whole IgG antivenom (Instituto Nacional de Salud) |
Cessation of local and systemic haemorrhage within the first 6 h of treatment and permanent recovery of blood coagulability no later than 6h after the onset of serotherapy were considered as the criteria of efficacy of the initial antivenom dose during phase II of the study. If these criteria were not fulfilled, an additional dose of three vials of the same antivenom was administered. | Composite outcome | No | No | Non-specific early reaction (no) |
| Otero et al. (1999) [65] | Colombia | Double blind | 53 | Bothrops spp. and Porthidium spp. | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Borthrops-Lachesis-Crotalus whole IgG antivenom (ICP) Monovalent Bothrops atrox whole IgG antivenom (ICP) |
NA | NA | NA | NA | Serum sickness (no); early hypersensitivity reaction (yes) |
| Otero-Patino et al. (1998) [64] | Colombia | Double blind | 79 | Bothrops spp. | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Bothrops F(ab’)2 antivenom (Instituto Butantan) Polyvalent Bothrops whole IgG antivenom (Instituto Butantan) Monovalent Bothrops atrox whole IgG antivenom (Instituto Nacional de Salud) |
The cessation of local and systemic bleeding and no progression of swelling between six and 12 hr after the initial dose of antivenom was considered an adequate therapeutic response | Composite outcome | No | No | Serum sickness (no); non-specific early reaction (no) |
| Otero et al. (1996) [67] | Colombia | Double blind | 39 | Bothrops atrox | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Borthrops-Lachesis-Crotalus antivenom (ICP) Monovalent Bothrops atrox whole IgG antivenom (ICP) |
NA | NA | NA | NA | Anaphylaxis (no); non-specific early reaction (no) |
| Smalligan et al. (2004) [69] | Ecuador | Double blind | 210 | Bothrops spp. and Lachesis muta | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent Bothrops antivenom whole IgG (Instituto Nacional de Higiene y Medicina Tropical “Leopoldo Izquieta Perez”) Polyvalent Bothrops antivenom (Instituto Butantan) Polyvalent Bothrops-Lachesis-Crotalus whole IgG antivenom (Instituto Nacional de Salud) |
Permanent restoration of blood coagulability after 6 hours and 24 hours (20WBCT) | Coagulopathy | Yes | No | Non-specific early reaction (no) |
| Sagar et al. (2020) [34]—CTRI/2015/05/005826 | India | Open label | 140 | Not identified | Coagulopathy, haemorrhage, local tissue damage and renal injury | Different doses of antivenom | Polyvalent F(ab’)2 antivenom (VINS) | Number developing AKI or mortality (AKI as per KDIGO criteria) | Composite outcome | Yes | No | Anaphylaxis (no); non-specific early reaction (no) |
| Sarin et al. (2017) [32]—CTRI/2015/06/005893 | India | Open label | 51 | Not identified | Neurotoxicity | Different doses of antivenom | Polyvalent F(ab’)2 antivenom (VINS) | Duration of mechanical ventilation (from time of intubation to time of extubation)—as per study protocol | Neurotoxicity | Yes | No | Non-specific early reaction |
| Paul et al. (2007) [19] | India | Open label | 80 | Not identified | Coagulopathy, haemorrhage, local tissue damage and renal injury | Heparin vs standard of care | Dalteparin Polyvalent Fab2 antivenom (Serum Institute of India) |
NA | NA | NA | NA | NA |
| Paul et al. (2004) [38] | India | Double blind | 100 | Not identified | Coagulopathy, neurotoxicity and renal injury | Different doses of antivenom | Polyvalent Fab2 antivenom (Serum Institute of India) | NA | NA | NA | NA | NA |
| Srimannarayana et al. (2004) [66] | India | Open label | 60 | Not identified | Coagulopathy and renal injury | Different doses of antivenom | Polyvalent Fab2 antivenom (Serum Institute of India) | NA | NA | NA | NA | Non-specific early reaction (no) |
| Paul et al. (2003) [22] | India | Open label | 122 | Not identified | Coagulopathy and renal injury | Heparin vs standard of care | Heparin infusion Polyvalent Fab2 antivenom (Serum Institute of India) |
NA | NA | NA | NA | NA |
| Tariang et al. (1999) [39] | India | Single blind | 60 | Not identified | Coagulopathy, local tissue damage, neurotoxicity and renal injury | Different doses of antivenom | Polyvalent Fab2 antivenom (Serum Institute of India) | Normalisation of coagulopathic (Clotting time more than 15 minutes) or neurotoxic (confusion, bilateral ptosis, dysarthria, dysphonia, respiratory distress, respiratory paralysis, muscle weakness, diplopia, bradycardia, hypotension) parameters or death | Composite outcome | No | No | NA |
| Thomas et al. (1985) [37] | India | Open label | 53 | Not identified | Coagulopathy local tissue damage and renal injury | Different doses of antivenom | Polyvalent Fab2 antivenom (Haffkine Biopharmaceutical Corporation) | NA | NA | NA | NA | NA |
| Reid et al. (1963)[16] | Malaysia | Double blind | 100 | Calloselasma rhodostoma | Coagulopathy, haemorrhage and local tissue damage | Antivenom vs placebo vs steroids | “Agkistrodon rhodostoma” antivenom (Queen Saovabha Institute) Prednisolone |
NA | NA | NA | NA | Anaphylaxis (no); serum sickness (no) |
| Tin et al. (1992) [20] | Myanmar | Open label | 20 | Daboia russelii | Coagulopathy and renal injury | Heparin vs placebo | Heparin infusion Monovalent Russell’s viper antivenom (Myanmar Pharmaceutical Industry) | NA | NA | NA | NA | NA |
| Myint-Lwin et al. (1989) [21] | Myanmar | Open label | 28 | Daboia russelii | Coagulopathy and renal injury | Heparin vs placebo | Heparin infusion Monovalent Russell’s viper antivenom (Burma Pharmaceutical Industry) |
NA | NA | NA | NA | NA |
| Alirol et al. (2017) [48]- NCT01284855 | Nepal | Double blind | 155 | Not identified | Neurotoxicity | Different doses of antivenom | Polyvalent F(ab’)2 antivenom (VINS) | In-hospital death OR the need for assisted ventilation (clinical indications for intubation and assisted ventilation were (1) absent gag reflex, (2) presence of paradoxical breathing, (3) respiratory distress or cyanosis, whichever was detected first, and/or (4) oxygen saturation <90% despite high flow oxygen supplementation) OR worsening or recurrence of neurotoxicity (defined as the appearance of 2 new neurotoxic signs OR the appearance of a severe neurotoxic sign) after the initial dose of antivenom | Composite outcome | Yes | No | Anaphylaxis (no); full adverse event reporting (yes) |
| Abubakar et al. (2010) [56]—ISRCTN01257358 | Nigeria | double blinded | 400 | Echis ocellatus | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | EchiTAb Plus-ICP polyvalent whole IgG antivenom (ICP) EchiTAb G monovalent E ocellatus whole IgG antivenom (MicroPharm) |
Permanent restoration of blood coagulability, judged by 20WBCT at 6 hours after initiation of antivenom treatment. Permanent implied restoration after which there was no (further) recurrence of blood incoagulability. This was assessed by repeating the 20WBCT 6, 12, 18, 24 and 48 hr after the initial dose of antivenom. | Coagulopathy | Yes | No | Anaphylaxis (yes); pyrogenic reaction (yes); serum sickness (no) |
| Meyer et al. (1997) [68] | Nigeria | Open label | 39 | Echis ocellatus | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Polyvalent IPSER Africa F(ab’)2 antivenom (Pasteur Mérieux Serum) EchiTab monovalent E. ocellatus Fab antivenom (Therapeutic Antibodies Ltd) |
NA | NA | NA | NA | Anaphylaxis (yes) |
| Warrell et al. (1980) [57] | Nigeria | Open label | 14 | Echis ocellatus | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | North and West African Bitis-Echis-Naja polyvalent antivenom (Behringwerke) Monovalent Echis ocellatus antivenom (Pasteur Paris) |
NA | NA | NA | NA | Non-specific early reaction (no) |
| Warrell et al. (1974) [58] | Nigeria | Open label | 46 | Echis ocellatus | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | North and West African Bitis-Echis-Naja polyvalent antivenom (Behringwerke) Monovalent Echis antivenom (SAIMR) |
NA | NA | NA | NA | Non-specific early reaction (no) |
| Qureshi et al. (2013) [71] | Pakistan | Double blind | 74 | Not identified | Coagulopathy and local tissue damage | Different antivenom products | Polyvalent Pakistani antivenom (National Institute of Health, Islamabad) Polyvalent F(ab’)2 antivenom (VINS) |
NA | NA | NA | NA | Early hypersensitivity reaction (yes) |
| Trevett et al. (1995) [26] | Papua New Guinea | Double blind | 50 | Oxyuranus scutellatus canni | Neurotoxicity | Edrophonium vs Amifampridine vs placebo (all groups received atropine) | Edrophonium Amifampridine Atropine |
NA | NA | NA | NA | Non-specific early reaction (no) |
| Watt et al. (1989) [46] | Philippines | Double blind | 8 | Naja philippinensis | Neurotoxicity | Different doses of antivenom | Monovalent Philippine cobra antivenom (Philippine MOH) All groups received edrophonium and atropine |
NA | NA | NA | NA | Non-specific early reaction (no); serum sickness (no) |
| Watt et al. (1986) [25] | Philippines | Double blind | 10 | Naja philippinensis | Neurotoxicity | Atropine and Edrophonium vs Placebo | Atropine and Edrophonium | Number of seconds that upper lid retraction could be maintained during upward gaze and the proportion of the iris uncovered during maximal effort to open the eyes | Neurotoxicity | Yes | No | NA |
| Isbister et al. (2017) [31]—SLCTR/2010/011 | Sri Lanka | Open label | 141 | Daboia russelii | Coagulopathy, haemorrhage and renal injury | Fresh frozen plasma vs standard of care | Fresh frozen plasma Polyvalent F(ab’)2 antivenom (VINS) |
The primary outcome was the proportion of patients with an INR of <2 at 6 h post-antivenom administration | Coagulopathy | Yes | No | Anaphylaxis (yes); Transfusion-related acute lung injury (yes) |
| Ariaratnam et al. (2001) [47] | Sri Lanka | Open label | 43 | Daboia russelii | Coagulopathy, local tissue damage, myotoxicity, neurotoxicity and renal injury | Different antivenom products | Monovalent Sri Lankan Daboia russelii Fab antivenom (Protherics) Polyvalent Fab2 antivenom (Haffkine Biopharmaceutical Corporation) |
NA | NA | NA | NA | Anaphylaxis (no); early hypersensitivity reaction (yes); non-specific early reaction (no); pyrogenic reaction (no) |
| Sellahewa et al. (1995) [17] | Sri Lanka | Single blind | 63 | Hypnale hypnale | Local tissue damage | Antivenom vs placebo | Polyvalent Fab2 antivenom (Haffkine Biopharmaceutical Corporation) | NA | NA | NA | NA | Early hypersensitivity reaction (no) |
| Sellahewa et al. (1994) [27] | Sri Lanka | Open label | 15 | Daboia russelii and Hypnale hypnale | Coagulopathy, local tissue damage and neurotoxicity | IVIG vs standard of care | Intravenous immunoglobulin (IVIG) (Sandoglobulin, Sandoz Pharmaceuticals) SII Polyvalent ASV IP Fab2 antivenom (Serum Institute of India) |
NA | NA | NA | NA | Anaphylaxis (no) |
| Rojnuckarin et al. (2006) [18] | Thailand | Double blind | 28 | Trimeresurus macrops, Trimeresurus albolabris and Trimeresurus macrops | Local tissue damage | Antivenom vs placebo | Polyvalent Green pit viper F(ab’)2 antivenom (QSMI, Thai Red Cross) | The degree of oedema is defined by the differences in circumferences of the affected and unaffected sides of limbs, measured (in cm) at the same distances from nearby joints. The points with the maximal differences were chosen and marked for the next measurements. The main outcome is the percentage reduction in limb circumference, calculated as the reduction in limb circumference on each day after intervention, divided by the initial limb circumference, and multiplied by 100. | Local tissue damage | Yes | No | NA |
| Warrell et al. (1986) [59] | Thailand | Open label | 46 | Calloselasma rhodostoma | Coagulopathy, haemorrhage and local tissue damage | Different antivenom products | Monovalent Malayan pit viper antivenom (QSMI, Thai Red Cross) Monovalent Malayan pit viper antivenom (Thai Government Pharmaceutical Organization) Monovalent Malayan pit viper antivenom (Twyford Pharmaceutical) |
NA | NA | NA | NA | Serum sickness (no); non-specific early reaction (no); pyrogenic reaction (yes) |
| Gerardo et al. (2017) [15]—NCT01864200 | USA | Double blind | 76 | Agkistrodon contortrix | Local tissue damage | Antivenom vs placebo | CroFab Polyvalent Crotalid Fab antivenom (Protherics) | Limb function 14 days after envenomation, measured by the Patient-Specific Functional Scale | Functional status | Yes | Yes | Full adverse event reporting (yes) |
| Gerardo et al. (2019) [13]–secondary analysis of NCT01864200 | ^ | ^ | ^ | ^ | ^ | ^ | ^ | NA | NA | NA | NA | NA |
| Freiermuth et al. (2018) [14]—secondary analysis of NCT01864200 | ^ | ^ | ^ | ^ | ^ | ^ | ^ | NA | NA | NA | NA | NA |
| Bush et al. (2015) [85]—NCT00636116 | USA | Double blind | 123 | Crotalinae | Coagulopathy | Different antivenom products | Anavip polyvalent Crotalid F(ab’)2 antivenom (Instituto Bioclon S.A.) CroFab Polyvalent Crotalid Fab antivenom (Protherics) |
Coagulopathy between the end of maintenance dosing and study day 8 (+/- 1 day). Coagulopathy was defined as platelet count less than 150,000/mm3, fibrinogen less than 150 mg/dL, or use of antivenom to treat a coagulation abnormality between the end of maintenance dosing and study day 5 | Composite outcome | Yes | No | Full adverse event reporting (yes); non-specific early reaction (no); serum sickness (no) |
| Boyer et al. (2013) [86]—NCT00868309 | USA | Open label | 12 | Crotalinae | Coagulopathy | Different antivenom products | CroFab Polyvalent Crotalid Fab antivenom (Protherics) Anavip polyvalent Crotalid F(ab’)2 antivenom (Instituto Bioclon S.A.) |
Detection of plasma venom levels during the post-acute treatment period | Venom antigenaemia | No | No | Anaphylaxis (no); full adverse event reporting (yes); serum sickness (no) |
| Dart et al. (2001) [42] | USA | Open label | 31 | Crotalinae (excluding Agkistrodon contortrix) | Coagulopathy and local tissue damage | Different doses of antivenom | CroFab Polyvalent Crotalid Fab antivenom (Protherics) | Snakebite severity score[41] | Scoring system | Yes | No | Serum sickness (yes); non-specific early reaction (no) |
| Protocols of active studies | ||||||||||||
| Isbister et al. (2011) [43]—ACTRN12611000588998 | Australia | Double blind | - | Pseudechis porphyriacus | Coagulopathy and myotoxicity | Antivenom vs placebo | Monovalent Tiger Snake F(ab’)2 antivenom (Commonwealth Serum Laboratories) Placebo |
The proportion of patients with myotoxicity defined as a peak creatine kinase greater than 1000U/L. | Myotoxicity | Yes | No | Early hypersensitivity reaction (yes); serum sickness (yes) |
| Ghorbani et al. (2013) [82]—IRCT2012122411873N1 | Iran | Double blind | - | Not reported | Local tissue damage | Steroid vs placebo | Dexamethasone infusion | Limb oedema (volume of limb according to shift of water in a dish scale in mL) | Local tissue damage | Yes | No | Full adverse event reporting (no) |
| Mousavi et al. (2020) [30]—IRCT20180515039672N2 | Iran | Double blind | - | Not identified | Coagulopathy, local tissue damage and neurotoxicity | Different antivenom products | SnaFab polyvalent antivenom (Padra Serum) Polyvalent Snake Antivenin (Razi Serum and Vaccine Research Institute) |
Percentage of victims with improving in snakebite symptoms (A) Stopping progression of swelling B) Normalized coagulation abnormalities C) Stopping the progression of neurotoxicity) | Other | No | No | NA |
| Lamb et al. (2020) [44]—NCT04210141 | Myanmar | Open label | - | Daboia siamensis | Coagulopathy and renal injury | Different doses of antivenom | Monospecific lyophilized F(ab)’2 viper antivenom (Burma Pharmaceutical Industry) | Blood coagulation at 6 hours as measured by the 20-minute WBCT (binary outcome) | Coagulopathy | Yes | No | Anaphylaxis (yes); full adverse event reporting (yes) |
| Jensen et al. (2012) [87]—ACTRN12612001062819 | Papua New Guinea | Double blind | - | Oxyuranus scutellatus | Coagulopathy and neurotoxicity | Different antivenom products | Monovalent Papuan taipan F(ab’)2 antivenom (ICP) Monovalent Taipan F(ab’)2 antivenom (Commonwealth Serum Laboratories) |
Prevention of airway obstruction or respiratory failure post-antivenom | Neurotoxicity | Yes | No | NA |
| Gawarammana et al. (2016) [29]—SLCTR/2016/012 | Sri Lanka | Double blind | - | Daboia russelii and Echis carnatus | Coagulopathy, local tissue damage, myotoxicity, neurotoxicity and renal injury | Different antivenom products | Polyvalent Sri Lankan antivenom (ICP) Polyvalent F(ab’)2 antivenom (VINS) |
1) Early anaphylactic-like reactions (up to 04 hrs) i. Mild: pruritus and/or urticarial only ii. Severe: Gastrointestinal symptoms (vomiting, diarrhoea, colicky abdominal pain) iii. Bronchospasm, or fall in systolic blood pressure below 90 mmHg 2) Early Pyrogenic reactions (up to 4 hours): increase oral temperature 38°C or above with or without rigors 3) Late serum sickness type antivenom reactions (2 weeks later): urticarial, pruritus, arthralgia, fever |
Other | Yes | No | NA |
| Kularatne et al. (2010) [33]—SLCTR/2010/006 | Sri Lanka | Open label | - | Bungarus Caeruleus | Neurotoxicity | Different doses of antivenom | Polyvalent F(ab’)2 antivenom (VINS) | The duration of mechanical ventilation (time in hours from the onset of intubation to extubation) | Neurotoxicity | Yes | No | NA |
| Protocols of terminated studies | ||||||||||||
| Grais et al. (2016) [88]—NCT02694952 | Central African Republic | Double blind | - | Not reported | Coagulopathy and haemorrhage | Different antivenom products | EchiTAb-Plus polyvalent whole IgG antivenom (ICP) FAV Afrique polyvalent F(ab’)2 antivenom (Sanofi Pasteur) |
Number of patients needing a third dose of antivenom, needing a blood transfusion, or dying [TimeFrame:28 days after enrolment] | Composite outcome | Yes | No | Full adverse event reporting (no) |
| Isbister et al. (2015) [36]—ACTRN12615000264583 | Australia | Open label | - | Notechis scutatus and Pseudonaja textilis | Coagulopathy, haemorrhage, local tissue damage, myotoxicity and renal injury | Early administration vs standard administration of antivenom | Monovalent Tiger Snake F(ab’)2 antivenom (Commonwealth Serum Laboratories) Brown Snake Antivenom (Commonwealth Serum Laboratories) |
Proportion of patients with significant envenomation defined as the development of one or more of the following effects (composite outcome): i) Myotoxicity defined as a peak CK greater than 1000U/L and local or systemic myalgia ii) Neurotoxicity: paralysis of 2 or more muscle groups (extra-ocular + bulbar) or respiratory paralysis iii) Major bleeding: defined by the International Society on Thrombosis and Haemostasis as fatal bleeding, symptomatic bleeding in a critical organ (e.g. intracranial haemorrhage) or bleeding resulting in a drop of haemoglobin >20g/L or requiring blood transfusion iv) Acute kidney failure injury: defined by the RIFLE criteria (creatinine increasing by 2x or more; <0·5ml/kg/hr urine output over12h) | Composite outcome | Yes | No | Anaphylaxis (yes) |
| Krishnan et al. (2016) [35]—CTRI/2016/10/007360 | India | Double blind | - | Not identified | Haemorrhage, local tissue damage, neurotoxicity and renal injury | N-Acetyl Cysteine vs placebo | N-Acetyl Cysteine Antivenom product not specified |
To compare the incidence and severity of acute kidney injury. | Renal injury | No | No | Non-specific early reaction (no) |
| Garcia et al. (2008) [89]—NCT00639951 | Mexico | Open label | - | Not identified | Coagulopathy, local tissue damage and myotoxicity | Different doses of antivenom | Antivipmyn Polyvalent Crotalinae F(ab’)2 antivenom (Instituto Bioclon) | Resolution of systemic signs and symptoms of snake bite envenomation expressed as percentage of patients requiring additional antivenom and percentage of patients that are stable. | Additional antivenom requirement | No | No | NA |
| Isbister et al. (2008) [23]—ACTRN12608000611325 | Sri Lanka | Open label | - | Daboia russelii | Coagulopathy and haemorrhage | FFP vs placebo | Fresh frozen plasma Polyvalent F(ab’)2 antivenom (VINS) Polyvalent Fab2 antivenom (Haffkine Biopharmaceutical Corporation) |
The proportion of patients with a significant return of coagulation function defined by an International Normalised Ratio (INR) < 2.0 (or prothrombin time (PT) < 24 seconds where INR was not performed) | Coagulopathy | Yes | No | Anaphylaxis (yes); Transfusion-related acute lung injury (yes) |
| Kerns et al. (2006) [40]—NCT00303303 | USA | Double blind | - | Agkistrodon contortrix | Coagulopathy and local tissue damage | Different doses of antivenom | CroFab Polyvalent Crotalid Fab antivenom (Protherics) | NA | NA | NA | NA | NA |
Studies are ordered alphabetically by country within each of the following groups: published randomised controlled trials; protocols of active, incomplete or terminated randomised controlled trials; and protocols of terminated randomised controlled trials.
Published secondary analyses are displayed below the corresponding primary randomised controlled trial.
Snake species is reported to species, genus or sub-family taxonomic rank, depending on the level of identification in each study.
Syndrome of envenoming is reported based on the study inclusion criteria and the descriptions of participant characteristics within each study.
20WBCT = 20-minute whole blood clotting test, AKI = acute kidney injury, CK = creatinine kinase, Fab = antigen-binding fragment, FFP = fresh frozen plasma, FUNED = Fundação Ezequiel Dias, ICP = Instituto Clodomiro Picado, Ig-immunoglobulin, INR = international normalised ratio, IVIG = intravenous immunoglobulin, KDIGO = Kidney Disease: Improving Global Outcomes, MOH = Ministry of Health, N = the number of participants randomised, PT = prothrombin time, RCT = randomised controlled trial, and SAIMR = South African Institute for Medical Research.
* For studies that did not clearly report a primary outcome measure, or adverse events, ‘NA’ has been listed in the table to highlight that this was not available.
† Where necessary, for the purposes of clarity, minor changes to the wording of outcome measures have been made. For example, in certain studies the outcome measure was partly defined in the methods section and partly defined in the results section, and thus required joining.
‡ ‘Clearly defined’ was defined as being reproducible by another researcher, including clear descriptions of time points, the person measuring the outcome, how the outcome was measured, and where the outcome was measured. ‘Patient-centred outcomes’ were defined as a measure of what the participant can do or how they feel, such as activities of daily living or ability to work.
Characteristics of included randomised controlled trials
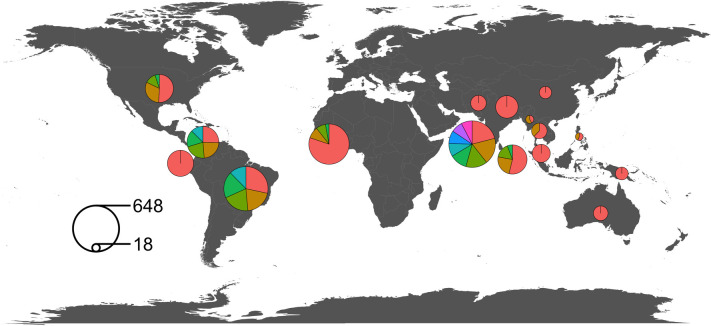
The proportion of randomised controlled trials conducted in each region were: Asia, 51·2% (n = 22 of 43); South America, 25·6% (n = 11); North America, 9·3% (n = 4); Africa, 9·3% (n = 4) (all in Nigeria); and Australasia, 4·7% (2) (Fig 2). The proportion published per decade were: 1960–69, 2·3% (n = 1 of 43); 1970–79, 2·3% (n = 1); 1980–89, 14·0% (n = 6); 1990–99, 27·9% (n = 12); 2000–09, 25·6% (n = 11); 2010–19, 25·6% (n = 11); and 2020, 2·3% (n = 1). Across the 43 trials, 3,418 participants were randomised. The mean sample size was 79 (IQR 41–100) and in 74·4% (n = 32 of 43) of studies the sample size was ≤100. The majority (55·8%; n = 24 of 43) were single centre studies, and the four trials with the greatest number of recruitment sites (range 7–28 sites) were conducted exclusively in Australia or the USA. Double blinding was adopted in 44·1% (n = 19 of 43); single blinding in 4·7% (n = 2); and 51·1% (n = 22) were open label. No pre-specified time-period of follow-up was defined in 55·8% (n = 24) trials. Among those where a follow-up period was reported (n = 19), 89.5% (n = 17) were for a period of 28 days or less; 5·3% (n = 1) were for 3-months; and 5·3% (n = 1) were for 6-months. Amongst trials published since January 2000 (N = 23), 43·5% (n = 10) reported a sample size calculation; 65·2% (n = 15) reported the numbers of participants screened for eligibility; and 43·5% (n = 10) were registered with a protocol available.
Fig 2. Total number of randomised controlled trial participants by country.
The area of each circle is proportionate to the total number of trial participants randomised per country in studies published between 1946 and 2020. The area of the segments of each circle are proportionate to the sample size of individual RCTs (e.g., there has been one large trial and three small trials conducted in Nigeria). Each circle overlies the country that it refers to. Where circles would overlap they have been moved, and the edge of the circle touches the corresponding country. The key demonstrates the samples size that corresponds to the surface area of two example circles. World map sourced from the Natural Earth project (1:50m resolution version) https://www.naturalearthdata.com.
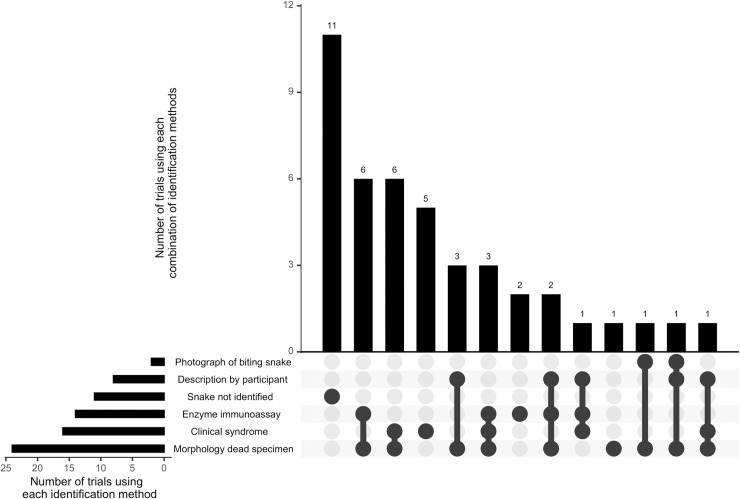
In 74·4% (n = 32 of 43) of randomised controlled trials, a method of identifying the biting snake, to species, genus or sub-family taxonomic rank, was used. The majority of trials combined two or more methods for identifying the snake. In 55·8% (n = 24) of clinical trials, the morphology of the dead snake was opportunistically assessed (when the specimen was brought into hospital), although other less specific methods of identification were often relied upon in these trials, such as an assessment of the clinical syndrome of envenoming. The clinical syndrome of envenoming (together with valid assumptions of locally prevalent snake species) was used to predict the biting species in 37·2% (n = 16) of clinical trials. Enzyme immunoassay, the participant’s description of the snake’s appearance, or a photograph of the biting snake (taken by the participant or a bystander) were assessed in 32·6% (n = 14), 18·6% (n = 8) and 4·7% (n = 2) of clinical trials, respectively. The UpSet plot (Fig 3) demonstrates the size of intersections between the different methods of snake identification used across the 43 included clinical trials. Amongst trials which identified the biting snake (n = 32), these were Viperidae in 87·5% (n = 28) of studies, and Elapidae in 12·5% (n = 4) of studies. The most commonly studied snake genera were Bothrops (34·4%; n = 11), Daboia (15·6%; n = 5) and Echis (12·1%; n = 4).
Fig 3. UpSet plot summarising methods of snake identification used in snakebite randomised controlled trials.
Upper bar chart, x axis: combinations of snake identification methods; y axis: number of randomised controlled trials using each combination of snake identification methods. Lower left bar chart, x axis: total numbers of randomised controlled trials using each individual method of snake identification; y axis: individual methods of snake identification.
Participants with coagulopathy or haemorrhagic envenoming were studied in 81·4% of trials (n = 35 of 43); local tissue damage in 69·8% (n = 30); renal injury in 25·6% (n = 11); neurotoxicity in 20·9% (n = 9); and myotoxicity in 2·3% (n = 1). Different antivenom products were compared in 44·2% (n = 19) of trials; different doses of the same antivenom product were compared in 23·3% (n = 10) of trials. Antivenom was compared to placebo in 9·3% (n = 4) of trials; in all of these, participants with severe envenoming were excluded [15,16] or the biting species was known to be associated with limited clinical manifestations [17,18]. Other therapies that were compared were: heparin, 9·3% (n = 4) [19–22]; fresh frozen plasma, 4·7% (n = 2) [23,24]; atropine and edrophonium, 2·3% (n = 1) [25]; edrophonium and amifampridine, 2·3% (n = 1) [26]; intravenous immunoglobulin, 2·3% (n = 1) [27]; and ‘Qingwen Baidu Decoction’ (a traditional Chinese medicine), 2·3% (n = 1) [28].
Quality of outcome measures
Amongst the trials and protocols (n = 56), 50·0% (n = 28) had a clearly stated primary outcome. Amongst studies published since January 2000 (n = 36), 69·4% (n = 25) had a clearly stated primary outcome. 80·0% (n = 20) of primary outcomes were clearly defined; 64% (n = 16) were clinical endpoints and 36% (n = 9) were laboratory markers; and 4·0% (n = 1) were patient centred. Across the secondary outcome measures from studies published since January 2000 (n = 226), 64·6% (n = 146) were clearly defined; 56.6% (n = 128) were clinical endpoints, 38.9% (n = 88) were laboratory markers, and 4.4% (n = 10) were exploratory; and 4·9% (n = 11) were patient centred.
Outcome measures
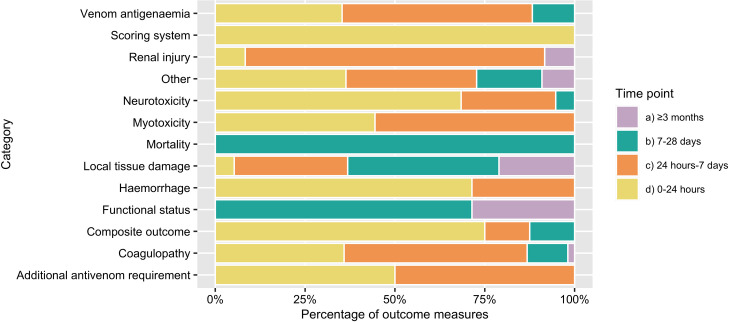
Across the 58 included studies, 382 outcome measures were extracted verbatim and, after duplicates were merged, 153 unique outcomes were identified. 59·5% of unique outcome measures were unique to a single study; 18·3% were used in two studies; 5·9%, in three studies; 4·6%, in four studies; and 11·8% were used in five or more studies. There was no single outcome that was used across all the studies, and the most frequently used outcome domain (‘venom antigenaemia’) was included in 32·8% of studies (n = 19 of 58). Venom antigenaemia was measured using various assays; the majority of which are not commercially available. 39·8% of the 382 extracted outcome measures did not report a specific timing of measurement. Amongst those with a time-point, 35·7% were measured for less than 24 hours; 42·6%, for less than a week; 16·5%, for less than a month; and 5·2% for up to 6-months. A summary of the durations of follow-up of outcome measures, grouped by category of envenoming, are presented in Fig 4. Full extracted outcome measure data is available in (S1 Data).
Fig 4. Duration of follow-up of outcome measures: grouped by category of envenoming.
Fig 4 depicts the time-period of follow-up of outcome measures within each category. For each outcome measure, the latest time point of follow-up was identified. Time points were grouped as: up to 24 hours; up to 7 days; up to 28 days; up to 3 months; and over 3 months. Within each category, the proportion of outcome measures with follow-up until each time-point is defined. For example, mortality outcome measures were always followed up for over 7 days but were never followed up for more than 28 days. No outcome measures were followed up until between 28 days and 3 months, and therefore this time point is not displayed in Fig 4.
The 28 primary outcome measures were categorised as follows: composite outcome, 8; coagulopathy, 6; neurotoxicity, 4; local tissue damage, 2; additional antivenom requirement, 1; functional status, 1; myotoxicity, 1; renal injury, 1; scoring system, 1; venom antigenaemia, 1; and other, 2. Table 1 summarises these primary outcomes, and includes the verbatim data extraction. The outcomes categorised as ‘other’ included one of antivenom hypersensitivity reaction [29] and one which was poorly defined [30]. The majority of primary outcome measures (82·1%) were unique to a single study. Amongst studies with shared primary outcome measures, three by Isbister et al adopted return of INR to less than two [23,24,31] and two studies measured duration of invasive ventilation [32,33].
For the remainder of the analysis herein, the 153 unique primary and secondary outcome measures will be considered together. Outcome measures were classified into 60 domains, and these are detailed in their corresponding categories in Table 2.
Table 2. Overview of outcome categories and domains.
| Category | Number of studies using an outcome measure within each category | Domain | Number of unique outcome measures within each domain | Number of studies using an outcome measure within each domain |
|---|---|---|---|---|
| Mortality | 18 | Mortality | 1 | 18 |
| Neurotoxicity | 14 | Ptosis | 3 | 6 |
| Requirement for invasive ventilation | 2 | 6 | ||
| Duration of invasive ventilation | 3 | 4 | ||
| Extraocular muscle palsy | 2 | 3 | ||
| Measure of other skeletal muscle weakness | 2 | 3 | ||
| Bulbar palsy | 3 | 2 | ||
| Electromyography | 1 | 2 | ||
| Spirometry | 3 | 1 | ||
| Neurotoxicity outcome poorly defined | 1 | 2 | ||
| Haemorrhage | 18 | Cessation of local or systemic bleeding | 4 | 9 |
| Anaemia | 3 | 4 | ||
| ISTH defined major bleeding | 1 | 4 | ||
| Blood transfusion requirement | 1 | 3 | ||
| Coagulopathy | 42 | Bedside clotting test | 18 | 30 |
| - 20-minute whole blood clotting test | (9) | (15) | ||
| - Lee White clotting time | (6) | (12) | ||
| - Bleeding time | (1) | (2) | ||
| - Other bedside clotting assay | (2) | (3) | ||
| Fibrinogen quantification | 5 | 17 | ||
| Clotting studies (INR, PT, APTT) | 9 | 14 | ||
| Platelet count | 3 | 9 | ||
| Clotting factor quantification | 6 | 7 | ||
| Fibrin and Fibrinogen-Degradation Products quantification | 5 | 6 | ||
| Clotting factor replacement | 1 | 2 | ||
| Myotoxicity | 7 | Creatinine kinase | 1 | 6 |
| Myoglobinuria | 2 | 2 | ||
| Myalgia | 1 | 1 | ||
| Renal injury | 14 | Acute kidney injury (non-specific criteria) | 4 | 6 |
| Requirement for renal replacement therapy | 1 | 6 | ||
| Serum creatinine | 2 | 5 | ||
| Serum urea | 1 | 3 | ||
| Acute kidney injury (RIFLE or KDIGO criteria) | 1 | 2 | ||
| Haematuria | 1 | 1 | ||
| Renal outcome poorly defined | 1 | 1 | ||
| Local tissue damage | 19 | Development of skin blistering or necrosis | 4 | 9 |
| Swelling measured by circumference of bitten limb | 3 | 9 | ||
| Pain—ordinal scale | 3 | 4 | ||
| Skin and soft tissue infection | 4 | 4 | ||
| Opioid requirement | 2 | 3 | ||
| Swelling measured as proximal extension | 1 | 3 | ||
| Swelling measured by limb volume | 1 | 2 | ||
| Need for amputation, skin grafting or debridement | 3 | 1 | ||
| Local tissue damage outcome poorly defined | 1 | 2 | ||
| Cardiotoxicity | 3 | Hypotension | 2 | 3 |
| Venom antigenaemia | 19 | Venom antigen quantification | 1 | 19 |
| Additional antivenom requirement | 10 | Total dose of antivenom required | 1 | 6 |
| Need for additional antivenom following initial dosing | 3 | 4 | ||
| Functional status | 12 | Measure of limb weakness | 2 | 3 |
| Multipoint scale of physical function | 8 | 3 | ||
| Number of therapy sessions | 1 | 1 | ||
| Return to work | 1 | 1 | ||
| Functional status outcome poorly defined | 1 | 1 | ||
| Scoring system | 1 | Snakebite severity score | 1 | 1 |
| Composite outcome | 14 | Composite outcome | 13 | 13 |
| Other | 19 | Duration of hospital admission | 1 | 16 |
| Allergic reaction | 1 | 1 | ||
| Anosmia | 1 | 1 | ||
| GI symptoms of envenoming | 2 | 1 | ||
| Hypoxic brain injury | 1 | 1 | ||
| Leucocyte count | 1 | 1 | ||
| Serum lactate dehydrogenase | 1 | 1 | ||
| Serum metalloproteinase | 1 | 1 | ||
| ‘Other’ outcome poorly defined | 1 | 7 |
Categories were predefined and each represent an established clinical syndrome of envenoming (e.g., neurotoxicity), or a broad categorisation of the outcome type (e.g., composite outcome). Two reviewers independently categorised outcome measures and consensus was reached on disagreements.
Domains were defined using a data driven approach. Each domain represents a grouping of outcomes that were deemed to be measuring a similar parameter. Consensus on domain allocations and domain names was reached by the primary authors.
APTT = activated partial thromboplastin time, GI = gastrointestinal, ISTH = International Society on Thrombosis and Haemostasis, INR = international normalised ratio, KDIGO = Kidney Disease: Improving Global Outcomes, and PT = prothrombin time.
Outcome measures in the category ‘coagulopathy’ were included in 72·4% of studies (n = 42 of 58). An outcome measure in the ‘bedside clotting test’ domain was used in 51·7% (n = 30) of studies and, within this domain, 18 unique methods of measurement were identified. These included various iterations of the ‘20-minute whole blood clotting test’, ‘Lee White clotting time’ and ‘bleeding time’, which were used in 15, 12 and 2 studies, respectively. The next most widely used coagulation domains were ‘fibrinogen quantification’ (29·3%; n = 17), and ‘clotting studies’ (24·1%; n = 14).
Outcome measures in the category ‘haemorrhage’ were adopted in 31·0% of studies (n = 18 of 58) and were grouped into the following domains: ‘cessation of local or systemic bleeding’; ‘anaemia’; ‘ISTH defined major bleeding’ and ‘blood transfusion requirement’. The most widely used was ‘cessation of local or systemic bleeding’ which was adopted in 15·5% of studies (n = 9) and was measured in four unique ways.
Neurotoxicity outcome measures were reported in 24·1% of studies. Amongst the 14 studies that used a neurotoxicity outcome measure, the most widely used were ‘ptosis’ (42·9% of studies); ‘requirement for invasive ventilation’ (42·9% of studies); and ‘duration of invasive ventilation’ (28·6% of studies). ‘Electromyography’ was used in 14·3% and ‘spirometry’ was used in 7·1% of studies with a neurotoxicity outcome measure.
Renal injury outcome measures were adopted in 24·1% of studies (n = 14 of 58) and were predominantly based on measurements of creatinine or urine output, with various cut-offs for defining abnormal. 5·2% (n = 3) of studies adopted the RIFLE or KDIGO criteria for defining acute kidney injury [34,35] including one study where it formed a component of a composite outcome [36]. The proportion of participants requiring renal replacement therapy were measured in 10·3% of studies (n = 6); all conducted in India [19,22,34,37–39].
Outcome measures that assessed local tissue damage were adopted in 32·8% of studies (n = 19 of 58). ‘Development of skin blistering or necrosis’ and ‘swelling measured by circumference of bitten limb’ were the most widely used, being included in 15.5% of studies (n = 9). The ‘need for amputation, skin grafting or debridement’ outcome domain was only adopted in one study.
The ‘multipoint scale of physical function’ domain was the most widely adopted measure of ‘functional status’ and included eight functional scales (as reported in S3 Text). All of these were patient-centred outcomes and were used in three studies; all conducted in the USA [13,15,40]. A scoring system, the ‘snakebite severity score’ [41], was used in a single study [42]. Composite outcomes were included in 13 studies, and each of these were unique and represented the primary outcome measures (Table 1).
Adverse event outcome measures
Amongst the trials and protocols (n = 56), there was a failure to record adverse event outcomes in 32·1% (n = 18) of studies. A total of 69 adverse event outcome measures were extracted verbatim, and were grouped as follows: ‘anaphylaxis’, 18; ‘early hypersensitivity reactions’, 6; ‘non-specific early reactions, 19; ‘pyrogenic reactions’, 3; full adverse event reporting (reporting of all serious adverse events), 8; ‘transfusion-related acute lung injury’, 2; and ‘serum sickness’, 13. Anaphylaxis was defined based on published criteria in five trials or protocols [23,24,31,43,44]. Serum sickness was defined based on reproducible clinical criteria in one published randomised controlled trial, and one trial protocol [42,43].
Discussion
Outcome measures used in clinical trials of snakebite envenoming vary considerably. Although varied outcome measures are needed to capture the diverse effects of envenoming by different species, variations within an outcome domain are undesirable. To achieve the WHO target of reducing snakebite deaths and disability by 50%, clinical trial outcome measures must include either direct measures of clinically relevant events or validated surrogate markers that are known to be associated with risk of disability or death.
This systematic review also demonstrates the troubling landscape of clinical trials in snakebite. Many recent trials did not use a sample size calculation, were single centre and were underpowered. Few clinical trials have been conducted in sub-Saharan Africa or the Middle East. Policy makers and clinicians are faced with a disturbing lack of data on which to evaluate antivenoms. Similar to our findings amongst randomised controlled trials of antivenoms, pre-clinical efficacy testing has used heterogenous methods that in a number of cases prevent comparisons between studies [45]. There is an urgent need for standardisation in the way that antivenoms are assessed, both pre-clinically and clinically.
Of further concern, many of the included clinical trials used unreliable methods for identifying the biting snake species. As the efficacy of antivenom is often snake species specific, knowing the biting species is important. Although the majority of trials utilised an assessment of the morphology of the dead specimen brought to the hospital, this was invariably opportunistic. For those participants who did not attend with the dead specimen, less specific methods were largely relied upon. Amongst eight of the 43 included clinical trials, participants were asked to recall and describe the appearance of the snake, and in a further 11 clinical trials no efforts were made to identify the biting species. The clinical syndrome of envenoming was used to predict the biting species in 16 clinical trials and, although this method can be reliable in settings where a single species is the predominant cause of coagulopathy, such as parts of West Africa, this is not reliable in various other settings. Unfortunately, reliable identification of the biting species remains challenging, particularly in LMIC settings, and further development of enzyme immunoassay and molecular based methods for snake identification are urgently needed.
There have been just nine randomised controlled trials that have included participants with neurotoxicity, with a combined sample size of 492 [25–27,32,38,39,46–48]. All except one study [26] took place in Asia. Many studies adopted measures of eyelid strength or requirement for mechanical ventilation. Outcomes used in other neuromuscular disorders may be useful. For example, the ‘myasthenic muscle score’ is a validated 100-point scale used to assess therapeutic efficacy in myasthenia gravis [49,50]. A scoring system has the advantage of capturing weakness of various muscle groups and providing a semi-quantitative measure that may more sensitively detect response to therapeutics. Spirometry, including measurement of forced vital capacity, offers a potentially sensitive and quantifiable measure of respiratory muscle strength and was used in one included study [25], although its validity in other neuromuscular disorders has been disappointing [51]. For phase III clinical trials, pragmatic endpoints with high clinical relevance will be important, such as the proportion of participants requiring intubation and ventilation.
Bleeding events were often poorly defined with insufficient detail to allow consistent replication in future studies [19,52–54]. Bleeding due to snake envenoming tends to involve small volume blood loss from the bite site, gums, or venepuncture sites. Although the International Society on Thrombosis and Haemostasis (ISTH) definition [55] of haemorrhage [23,24,31,36], or laboratory-based measures of anaemia [56–59], provide objective tools, bleeding events of this severity are rare in snakebite envenoming [60]. Furthermore, measures of haematocrit or packed cell volume [56–59] may under-estimate anaemia due to the concentration effect of venom-induced capillary leak syndrome [61].
A range of laboratory assays were used to assess for coagulopathy, and it is uncertain which is the most useful. The bedside clotting tests do not require any specialist equipment and can be conducted in remote rural settings where the majority of snakebites occur. The Lee White clotting time was used exclusively in studies from South America and Asia [19,37,38,62–67], whereas the 20-minute whole blood clotting test (20WBCT) has been used more widely [20,34,47,52–54,56,59,68–71]. Although the 20WBCT has been subject to more frequent validation than the Lee White clotting time, this has rarely been amongst participants that have received antivenom [24,72,73] and, therefore, bedside tests of whole blood clotting are inadequately validated for measuring response to treatment. A disadvantage of the 20WBCT is that it is binary rather than continuous. Sensitive continuous outcome measures are desirable for smaller studies such as phase II clinical trials.
Renal injury can result from envenoming by a range of snake species [74]. Outcome measures in snakebite clinical trials have focussed on acute renal injury; based on various thresholds of serum creatinine and oliguria. Internationally recognised criteria for the diagnosis of acute kidney injury are available [75–77], and these were adopted in three of the included studies [34,35], including within one composite outcome [36]. Although the need for renal replacement therapy (RRT) is an important measure, there is wide variation and no consensus on the optimal timing for initiating and stopping this in acute kidney injury [78]. Follow-up studies of adults and children with snake venom induced renal injury have demonstrated a 30% risk of progression to chronic kidney disease [79,80]. Chronic kidney disease is defined as an abnormality in the structure or functioning of the kidneys present for a minimum of 3 months [81]. No outcome measures fulfilled this definition and the need for longer follow-up of renal function in clinical trials should be considered.
Snakebite associated local tissue damage represents a varied spectrum of disease, ranging from swelling to necrosis, with complications including infection, contractures, and amputation. Although this range of disease was captured across the extracted outcomes, this was inconsistent between studies. Consensus on a list of outcomes, including details of how they should be measured, is needed. For example, limb swelling has been measured by circumference [18,27,35,47,63], distance of proximal extension [40,58,59], or limb volume [40,82].
Complications of local tissue damage can cause loss of physical function with a varying impact depending on an individual’s circumstances. Many people with snakebite are vulnerable and disability may significantly impact on their ability to work, subsistence farm or care for children. Patient-centred outcomes are key for capturing this, but such outcomes were only adopted in trials based in the USA [13,15,40]. The patient specific functional scale (PSFS) is simple (although does require numeracy) and allows patients to identify functions that are important to them. This tool has been validated for snakebite envenoming [13], although not in an LMIC setting.
Adverse event reporting varied significantly between the randomised controlled trials, and 32·1% of the included studies failed to report adverse events. As antivenom is an animal derived product, there is a significant risk of life-threatening anaphylaxis, yet only five of the 56 included studies used standardised published criteria for defining anaphylaxis. Given that the risk of anaphylaxis can vary substantially between antivenom products [7,83], it is essential that the rate of occurrence of these events can be reliably and consistently measured in clinical trials. Serum sickness was only reported as an outcome measure in 13 of the 58 included studies, and only two studies used clearly defined clinical criteria [42,43]. A standardised definition of anaphylaxis and serum sickness should be included in a core outcome set.
Limitations
When considering outcome measures for use in a core outcome set, it is important to ascertain whether they are valid, reliable, and feasible. Such an assessment was outside the scope of this study and will form the next stage of COS development. This systematic review did not restrict on the age or quality of the trials; however, this was important to ensure all outcome measures were captured. When describing the characteristics and quality of trials, data for studies published more recently were presented. At this stage there has not been any patient involvement and future work on COS development will strive to involve people who have directly experienced snakebite.
Conclusions
This study has identified significant heterogeneity of outcome measures in snakebite clinical trials. There is a strong need for a core outcome set, which will support the adoption of valid, reproducible, and patient-centred outcome measures, and enable downstream meta-analyses. Validated outcome measures are particularly important when assessing antivenom efficacy, as this expensive therapy is associated with a relatively high risk of adverse events. To provide global relevance that can span the diversity of snake species, outcomes that represent each of the syndromes of envenoming are needed. Through better outcome measures, together with increased global recognition of the importance of snakebite envenoming, high quality clinical trials in populations with the greatest burden of disease can be achieved.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(CSV)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author MA was supported by a Wellcome Trust clinical PhD fellowship (grant number: 203919/Z/16/Z; The Wellcome Trust, https://wellcome.org/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Medicine. 2008;5: e218. doi: 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casewell NR, Jackson TNW, Laustsen AH, Sunagar K. Causes and Consequences of Snake Venom Variation. Trends in Pharmacological Sciences. 2020;41: 570–581. doi: 10.1016/j.tips.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nature Reviews Disease Primers. 2017;3: 17063. doi: 10.1038/nrdp.2017.63 [DOI] [PubMed] [Google Scholar]
- 4.Halilu S, Iliyasu G, Hamza M, Chippaux J-P, Kuznik A, Habib AG. Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon. 2019;159: 1–4. doi: 10.1016/j.toxicon.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Global funding for snakebite envenoming research. [cited 6 Apr 2020]. Available: https://wellcome.ac.uk/sites/default/files/global-funding-for-snakebite-envenoming-research-2007-2018.pdf
- 6.Alirol E, Lechevalier P, Zamatto F, Chappuis F, Alcoba G, Potet J. Antivenoms for Snakebite Envenoming: What Is in the Research Pipeline? PLOS Neglected Tropical Diseases. 2015;9: e0003896. doi: 10.1371/journal.pntd.0003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potet J, Smith J, McIver L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Negl Trop Dis. 2019;13: e0007551–e0007551. doi: 10.1371/journal.pntd.0007551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancet editorial. Snake-bite envenoming: a priority neglected tropical disease. The Lancet. 2017;390: 2. doi: 10.1016/S0140-6736(17)31751-8 [DOI] [PubMed] [Google Scholar]
- 9.Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, et al. Core Outcome Set-STAndards for Development: The COS-STAD recommendations. PLOS Medicine. 2017;14: e1002447. doi: 10.1371/journal.pmed.1002447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habib AG, Warrell DA. Antivenom therapy of carpet viper (Echis ocellatus) envenoming: Effectiveness and strategies for delivery in West Africa. Toxicon. 2013;69: 82–89. doi: 10.1016/j.toxicon.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 11.Maduwage K, Buckley NA, de Silva HJ, Lalloo DG, Isbister GK. Snake antivenom for snake venom induced consumption coagulopathy. Cochrane Database Syst Rev. 2015; CD011428. doi: 10.1002/14651858.CD011428.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O’Brien K, et al. MOMENT–Management of Otitis Media with Effusion in Cleft Palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials. 2013;14: 70. doi: 10.1186/1745-6215-14-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerardo CJ, Vissoci JRN, de Oliveira LP, Anderson VE, Quackenbush E, Lewis B, et al. The validity, reliability and minimal clinically important difference of the patient specific functional scale in snake envenomation. PLoS One. 2019;14. doi: 10.1371/journal.pone.0213077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freiermuth C., Gerardo C.J., Lavonas E.J., Rapp-Olsson M., Kleinschmidt K.C., Sharma K., et al. Antivenom administration was associated with shorter duration of opioid use in copperhead envenomation patients. Academic Emergency Medicine. 2018;25: S89. doi: 10.1111/acem.13424 [DOI] [PubMed] [Google Scholar]
- 15.Gerardo CJ, Quackenbush E, Lewis B, Rose SR, Greene S, Toschlog EA, et al. The Efficacy of Crotalidae Polyvalent Immune Fab (Ovine) Antivenom Versus Placebo Plus Optional Rescue Therapy on Recovery From Copperhead Snake Envenomation: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Annals of Emergency Medicine. 2017;70: 233–244.e3. doi: 10.1016/j.annemergmed.2017.04.034 [DOI] [PubMed] [Google Scholar]
- 16.Reid H.A., Thean P.C., Martin W.J. Specific antivenene and prednisone in viper-bite poisoning: Controlled trial. British medical Journal. 1963;2: 1378–1380. doi: 10.1136/bmj.2.5369.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellahewa KH, Gunawardena G, Kumararatne MP. Efficacy of Antivenom in the Treatment of Severe Local Envenomation by the Hump-Nosed Viper (Hypnale hypnale). The American Journal of Tropical Medicine and Hygiene. 1995;53: 260–262. doi: 10.4269/ajtmh.1995.53.260 [DOI] [PubMed] [Google Scholar]
- 18.Rojnuckarin P, Chanthawibun W, Noiphrom J, Pakmanee N, Intragumtornchai T. A randomized, double-blind, placebo-controlled trial of antivenom for local effects of green pit viper bites. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100: 879–884. doi: 10.1016/j.trstmh.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 19.Paul V, Pudoor A, Earali J, John B, Anil Kumar CS, Anthony T. Trial of low molecular weight heparin in the treatment of viper bites. J Assoc Physicians India. 2007;55: 338–342. [PubMed] [Google Scholar]
- 20.Na Swe T, Lwin M, Ei Han K, Tun T, Tun P. Heparin therapy in Russell’s viper bite victims with disseminated intravascular coagulation: a controlled trial. Southeast Asian J Trop Med Public Health. 1992;23: 282–287. [PubMed] [Google Scholar]
- 21.Lwin M, Nu Swe T, Than T, Than T, Tun P. Heparin therapy in Russell’s viper bite victims with impending DIC (a controlled trial). Southeast Asian J Trop Med Public Health. 1989;20: 271–277. [PubMed] [Google Scholar]
- 22.Paul V, Prahlad KA, Earali J, Francis S, Lewis F. Trial of heparin in viper bites. J Assoc Physicians India. 2003;51: 163–166. [PubMed] [Google Scholar]
- 23.Isbister GK. Randomised controlled trial of fresh frozen plasma to speed the recovering of venom induced consumption coagulopathy in patients with Russell’s Viper envenoming in Sri Lanka. 2008 Dec. Report No.: ACTRN12608000611325. Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83196
- 24.Isbister GK, Buckley NA, Page CB, Scorgie FE, Lincz LF, Seldon M, et al. A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18). Journal of Thrombosis and Haemostasis. 2013;11: 1310–1318. doi: 10.1111/jth.12218 [DOI] [PubMed] [Google Scholar]
- 25.Watt G., Theakston R.D.G., Hayes C.G. Positive response to edrophonium in patients with neurotoxic envenoming by cobras Naja naja philippinensis. A placebo-controlled study. New England Journal of Medicine. 1986;315: 1444–1448. doi: 10.1056/NEJM198612043152303 [DOI] [PubMed] [Google Scholar]
- 26.Trevett AJ, Lalloo DG, Nwokolo NC, Naraqi S, Kevau IH, Theakston RDG, et al. Failure of 3,4-diaminopyridine and edrophonium to produce significant clinical benefit in neurotoxicity following the bite of Papuan taipan (Oxyuranus scutellatus canni). Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89: 444–446. doi: 10.1016/0035-9203(95)90051-9 [DOI] [PubMed] [Google Scholar]
- 27.Sellahewa KH, Kumararatne MP, Dassanayake PB, Wijesundera A. Intravenous immunoglobulin in the treatment of snake bite envenoming: a pilot study. Ceylon Med J. 1994;39: 173–175. [PubMed] [Google Scholar]
- 28.Miao Y, Chen M, Huang Z. Clinical observation on treatment of snake bite induced disseminated intravascular coagulation by qinwen baidu decoction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23: 590–592. [PubMed] [Google Scholar]
- 29.Gawarammana IB. A Randomized controlled trial on the safety of ICP-AVRI-UOP Sri Lankan polyspecific antivenom compared to Indian AVS in patients with snakebite. 2016 Jun. Report No.: SLCTR/2016/012. Available: https://slctr.lk/trials/slctr-2016-012
- 30.Mousavi SR. Phase 3, multi-center, randomized, two-arm, parallel, double blinded, active controlled for non-inferiority evaluation of efficacy and safety of snake anti-venom produced by Padra Serum Alborz in comparison with snake anti-venom produced by Razi Vaccine and Serum Research Institute in snakebite victims. 2020Feb. Available: https://www.irct.ir/trial/41983 [Google Scholar]
- 31.Isbister GK, Jayamanne S, Mohamed F, Dawson AH, Maduwage K, Gawarammana I, et al. A randomized controlled trial of fresh frozen plasma for coagulopathy in Russell’s viper (Daboia russelii) envenoming. J Thromb Haemost. 2017;15: 645–654. doi: 10.1111/jth.13628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarin K, Dutta TK, Vinod KV. Clinical profile & complications of neurotoxic snake bite & comparison of two regimens of polyvalent anti-snake venom in its treatment. Indian J Med Res. 2017;145: 58–62. doi: 10.4103/ijmr.IJMR_1319_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kularatne S. Low dose versus high dose of Indian polyvalent snake antivenom in reversing neurotoxic paralysis in common krait (Bungarus cearulus) bites: an open labeled randomised controlled clinical trial in Sri Lanka. 2010 Jul. Report No.: SLCTR/2010/006. Available: https://slctr.lk/trials/slctr-2010-006
- 34.Sagar P, Bammigatti C, Kadhiravan T, Harichandrakumar KT, Swaminathan RP, Reddy MM. Comparison of two Anti Snake Venom protocols in hemotoxic snake bite: A randomized trial. Journal of Forensic and Legal Medicine. 2020;73: 1–7. doi: 10.1016/j.jflm.2020.101996 [DOI] [PubMed] [Google Scholar]
- 35.Krishnan B. Clinical effects of N-acetylcysteine on acute kidney injury and other serious morbidities in childrenwith snake envenomation: A randomized double blind placebo controlled study. 2016 Oct. Report No.: CTRI/2016/10/007360. Available: http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=15943&EncHid=&modid=&compid=%27,%2715943det%27
- 36.Isbister GK. Randomised controlled trial investigating the effects of early snake antivenom administration. 2015 Mar. Report No.: ACTRN12615000264583. Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367828
- 37.Thomas P, Jacob J. Randomised trial of antivenom in snake envenomation with prolonged clotting time. British Medical Journal. 1985;291: 177–178. doi: 10.1136/bmj.291.6489.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul V, Pratibha S, Prahlad K, Earali J, Francis S, Lewis F. High-dose anti-snake venom versus low-dose anti-snake venom in the treatment of poisonous snake bites—a critical study. J Assoc Physicians India. 2004;52: 14–17. [PubMed] [Google Scholar]
- 39.Tariang D, Philip P, Alexander G, Macaden S, Jeyaseelan L, Peter J, et al. Randomized controlled trial on the effective dose of anti-snake venom in cases of snake bite with systemic envenomation. J Assoc Physicians India. 1999;47: 369–371. [PubMed] [Google Scholar]
- 40.Kerns W. The Efficacy of Crotaline Fab Antivenom for Copperhead Snake Envenomations. clinicaltrials.gov; 2006 Mar. Report No.: NCT00303303. Available: https://clinicaltrials.gov/ct2/show/NCT00303303
- 41.Dart RC, Hurlbut KM, Garcia R, Boren J. Validation of a Severity Score for the Assessment of Crotalid Snakebite. Annals of Emergency Medicine. 1996;27: 321–326. doi: 10.1016/s0196-0644(96)70267-6 [DOI] [PubMed] [Google Scholar]
- 42.Dart RC, Seifert SA, Boyer LV, Clark RF, Hall E, McKinney P, et al. A randomized multicenter trial of crotalinae polyvalent immune Fab (ovine) antivenom for the treatment for crotaline snakebite in the United States. Arch Intern Med. 2001;161: 2030–2036. doi: 10.1001/archinte.161.16.2030 [DOI] [PubMed] [Google Scholar]
- 43.Isbister GK. A multicentre double-blind randomised placebo-controlled trial of early antivenom versus placebo in the treatment of red bellied black snake envenoming. 2011 Jun. Report No.: ACTRN12611000588998. Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343001
- 44.Lamb T. An Adaptive Clinical Trial to Determine the Optimal Initial Dose of Lyophilized, Species Specific Monovalent Antivenom for the Management of Systemic Envenoming by Daboia Siamensis (Eastern Russell’s Viper) in Myanmar. clinicaltrials.gov; 2020 Sep. Report No.: NCT04210141. Available: https://clinicaltrials.gov/ct2/show/NCT04210141
- 45.Ainsworth S, Menzies SK, Casewell NR, Harrison RA. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLOS Neglected Tropical Diseases. 2020;14: e0008579. doi: 10.1371/journal.pntd.0008579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watt G, Meade BD, Theakston RD, Padre LP, Tuazon ML, Calubaquib C, et al. Comparison of Tensilon and antivenom for the treatment of cobra-bite paralysis. Trans R Soc Trop Med Hyg. 1989;83: 570–573. doi: 10.1016/0035-9203(89)90301-5 [DOI] [PubMed] [Google Scholar]
- 47.Ariaratnam C.A., Sjostrom L., Raziek Z., Abeyasinghe S., Kularatne M., Arachchi R.W.K.K., et al. An open, randomized comparative trial of two antivenoms for the treatment of envenoming by Sri Lankan Russell’s viper (Daboia russelii russelii). Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95: 74–80. doi: 10.1016/s0035-9203(01)90339-6 [DOI] [PubMed] [Google Scholar]
- 48.Alirol E, Sharma SK, Ghimire A, Poncet A, Combescure C, Thapa C, et al. Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: Evidence from a randomised controlled trial in Nepal. PLoS Negl Trop Dis. 2017;11: e0005612. doi: 10.1371/journal.pntd.0005612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnett C, Herbelin L, Dimachkie MM, Barohn RJ. Measuring Clinical Treatment Response in Myasthenia Gravis. Neurol Clin. 2018;36: 339–353. doi: 10.1016/j.ncl.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharshar T, Chevret S, Mazighi M, Chillet P, Huberfeld G, Berreotta C, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. J Neurol. 2000;247: 286–290. doi: 10.1007/s004150050585 [DOI] [PubMed] [Google Scholar]
- 51.Rieder P, Louis M, Jolliet P, Chevrolet J-C. The repeated measurement of vital capacity is a poor predictor of the need for mechanical ventilation in myasthenia gravis. Intensive Care Med. 1995;21: 663–668. doi: 10.1007/BF01711545 [DOI] [PubMed] [Google Scholar]
- 52.Pardal PP de O, Souza SM, Monteiro MR de C da C, Fan HW, Cardoso JLC, França FOS, et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans R Soc Trop Med Hyg. 2004;98: 28–42. doi: 10.1016/s0035-9203(03)00005-1 [DOI] [PubMed] [Google Scholar]
- 53.Otero-Patiño R, Segura A, Herrera M, Angulo Y, León G, Gutiérrez JM, et al. Comparative study of the efficacy and safety of two polyvalent, caprylic acid fractionated [IgG and F(ab’)2] antivenoms, in Bothrops asper bites in Colombia. Toxicon. 2012;59: 344–355. doi: 10.1016/j.toxicon.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 54.Cardoso JL, Fan HW, França FO, Jorge MT, Leite RP, Nishioka SA, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993;86: 315–325. [PubMed] [Google Scholar]
- 55.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3: 692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 56.Abubakar IS, Abubakar SB, Habib AG, Nasidi A, Durfa N, Yusuf PO, et al. Randomised controlled double-blind non-inferiority trial of two antivenoms for saw-scaled or carpet viper (Echis ocellatus) envenoming in Nigeria. PLoS Negl Trop Dis. 2010;4: e767. doi: 10.1371/journal.pntd.0000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warrell DA, Warrell MJ, Edgar W, Prentice CR, Mathison J, Mathison J. Comparison of Pasteur and Behringwerke antivenoms in envenoming by the carpet viper (Echis carinatus). Br Med J. 1980;280: 607–609. doi: 10.1136/bmj.280.6214.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warrell D, Davidson N, Omerod L, Pope H, Watkins B, Greenwood B, et al. Bites by the saw-scaled or carpet viper (Echis carinatus): trial of two specific antivenoms. Br Med J. 1974;4: 437–440. doi: 10.1136/bmj.4.5942.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warrell D.A., Looareesuwan S., Theakston R.D.G. Randomized comparative trial of three monospecific antivenoms for bites by the Malayan pit viper (Calloselasma rhodostoma) in Southern Thailand: Clinical and laboratory correlations. American Journal of Tropical Medicine and Hygiene. 1986;35: 1235–1247. doi: 10.4269/ajtmh.1986.35.1235 [DOI] [PubMed] [Google Scholar]
- 60.Lavonas EJ, Khatri V, Daugherty C, Bucher-Bartelson B, King T, Dart RC. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63: 71–78.e1. doi: 10.1016/j.annemergmed.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 61.Kendre PP, Jose MP, Varghese AM, Menon JC, Joseph JK. Capillary leak syndrome in Daboia russelii bite—a complication associated with poor outcome. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2018;112: 88–93. doi: 10.1093/trstmh/try026 [DOI] [PubMed] [Google Scholar]
- 62.Mendonça-da-Silva I, Tavares AM, Sachett J, Sardinha JF, Zaparolli L, Gomes Santos MF, et al. Safety and efficacy of a freeze-dried trivalent antivenom for snakebites in the Brazilian Amazon: An open randomized controlled phase IIb clinical trial. Calvete JJ, editor. PLOS Neglected Tropical Diseases. 2017;11: e0006068. doi: 10.1371/journal.pntd.0006068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorge MT, Cardoso JLC, Castro SCB, Ribeiro L, Franca F.OS, Sbrogio de Almeida ME, et al. A randomized “blinded” comparison of two doses of antivenom in the treatment of Bothrops envenoming in Sao Paulo, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89: 111–114. doi: 10.1016/0035-9203(95)90678-9 [DOI] [PubMed] [Google Scholar]
- 64.Otero-Patino R, Cardoso JLC, Higashi HG, Nunez V, Diaz A, Toro MF, et al. A randomized, blinded, comparative trial of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. American Journal of Tropical Medicine and Hygiene. 1998;58: 183–189. doi: 10.4269/ajtmh.1998.58.183 [DOI] [PubMed] [Google Scholar]
- 65.Otero R, Gutiérrez JM, Rojas G, Núñez V, Díaz A, Miranda E, et al. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon. 1999;37: 895–908. doi: 10.1016/s0041-0101(98)00220-7 [DOI] [PubMed] [Google Scholar]
- 66.Srimannarayana J, Dutta TK, Sahai A, Badrinath S. Rational use of anti-snake venom (ASV): trial of various regimens in hemotoxic snake envenomation. J Assoc Physicians India. 2004;52: 788–793. [PubMed] [Google Scholar]
- 67.Otero R, Gutiérrez JM, Núñez V, Robles A, Estrada R, Segura E, et al. A randomized double-blind clinical trial of two antivenoms in patients bitten by Bothrops atrox in Colombia. The Regional Group on Antivenom Therapy Research (REGATHER). Trans R Soc Trop Med Hyg. 1996;90: 696–700. doi: 10.1016/s0035-9203(96)90442-3 [DOI] [PubMed] [Google Scholar]
- 68.Meyer WP, Habib AG, Onayade AA, Yakubu A, Smith DC, Nasidi A, et al. First clinical experiences with a new ovine Fab Echis ocellatus snake bite antivenom in Nigeria: randomized comparative trial with Institute Pasteur Serum (Ipser) Africa antivenom. Am J Trop Med Hyg. 1997;56: 291–300. doi: 10.4269/ajtmh.1997.56.291 [DOI] [PubMed] [Google Scholar]
- 69.Smalligan R, Cole J, Brito N, Laing GD, Mertz BL, Manock S, et al. Crotaline snake bite in the Ecuadorian Amazon: randomised double blind comparative trial of three South American polyspecific antivenoms. BMJ. 2004;329: 1129. doi: 10.1136/bmj.329.7475.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otero R, León G, Gutiérrez JM, Rojas G, Toro MF, Barona J, et al. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans R Soc Trop Med Hyg. 2006;100: 1173–1182. doi: 10.1016/j.trstmh.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 71.Qureshi H, Alam SE, Mustufa MA, Nomani NK, Asnani JL, Sharif M. Comparative cost and efficacy trial of Pakistani versus Indian anti snake venom. J Pak Med Assoc. 2013;63: 1129–1132. [PubMed] [Google Scholar]
- 72.Thongtonyong N, Chinthammitr Y. Sensitivity and specificity of 20-minute whole blood clotting test, prothrombin time, activated partial thromboplastin time tests in diagnosis of defibrination following Malayan pit viper envenoming. Toxicon. 2020;185: 188–192. doi: 10.1016/j.toxicon.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 73.Ratnayake I, Shihana F, Dissanayake DM, Buckley NA, Maduwage K, Isbister GK. Performance of the 20-minute whole blood clotting test in detecting venom induced consumption coagulopathy from Russell’s viper (Daboia russelii) bites. Thromb Haemost. 2017;117: 500–507. doi: 10.1160/TH16-10-0769 [DOI] [PubMed] [Google Scholar]
- 74.Waiddyanatha S, Silva A, Siribaddana S, Isbister GK. Long-term Effects of Snake Envenoming. Toxins (Basel). 2019;11. doi: 10.3390/toxins11040193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter, Suppl. 2012;2: 1–138. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 76.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8: R204–212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11: R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palevsky PM, Metnitz PGH, Piccinni P, Vinsonneau C. Selection of endpoints for clinical trials of acute renal failure in critically ill patients. Current Opinion in Critical Care. 2002;8: 515–518. doi: 10.1097/00075198-200212000-00006 [DOI] [PubMed] [Google Scholar]
- 79.Sinha R, Nandi M, Tullus K, Marks SD, Taraphder A. Ten-year follow-up of children after acute renal failure from a developing country. Nephrol Dial Transplant. 2009;24: 829–833. doi: 10.1093/ndt/gfn539 [DOI] [PubMed] [Google Scholar]
- 80.Herath HMNJ, Wazil AWM, Abeysekara DTDJ, Jeewani NDC, Weerakoon KG a. D, Ratnatunga NVI, et al. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J. 2012;88: 138–142. doi: 10.1136/postgradmedj-2011-130225 [DOI] [PubMed] [Google Scholar]
- 81.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013. [cited 2 Oct 2020]. Available: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [Google Scholar]
- 82.Ghorbani A. Determination of injective dexamethasone efficacy associate with anti venum in decrease of limb edema in snake bite patients. 2013May. Available: https://en.irct.ir/trial/12056 [Google Scholar]
- 83.Moran NF, Newman WJ, Theakston RDG, Warrell DA, Wilkinson D. High incidence of early anaphylactoid reaction to SAIMR polyvalent snake antivenom. Transactions of The Royal Society of Tropical Medicine and Hygiene. 1998;92: 69–70. doi: 10.1016/s0035-9203(98)90959-2 [DOI] [PubMed] [Google Scholar]
- 84.Theakston RD, Fan HW, Warrell DA, Da Silva WD, Ward SA, Higashi HG. Use of enzyme immunoassays to compare the effect and assess the dosage regimens of three Brazilian Bothrops antivenoms. The Butantan Institute Antivenom Study Group (BIASG). The American journal of tropical medicine and hygiene. 1992;47: 593–604. doi: 10.4269/ajtmh.1992.47.593 [DOI] [PubMed] [Google Scholar]
- 85.Bush SP, Ruha A-M, Seifert SA, Morgan DL, Lewis BJ, Arnold TC, et al. Comparison of F(ab’)2 versus Fab antivenom for pit viper envenomation: a prospective, blinded, multicenter, randomized clinical trial. Clin Toxicol (Phila). 2015;53: 37–45. doi: 10.3109/15563650.2014.974263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyer LV, Chase PB, Degan JA, Figge G, Buelna-Romero A, Luchetti C, et al. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab’)2 antivenoms. Toxicon. 2013;74: 101–108. doi: 10.1016/j.toxicon.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 87.Jensen S. A randomized controlled trial (RCT) of a new monovalent antivenom (ICP Papuan taipan antivenom) for the treatment of Papuan taipan (Oxyuranus scutellatus) envenoming in Papua New Guinea to measure safety, minimum dose and effectiveness in the prevention of neurotoxic paralysis, arrest of coagulopathy and other effects of envenoming. 2012 Oct. Report No.: ACTRN12612001062819. Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=361999
- 88.Grais R. Randomized, Double-blind, Non-inferiority Trial of Two Antivenoms for the Treatment of Snakebite With Envenoming. clinicaltrials.gov; 2016 Mar. Report No.: NCT02694952. Available: https://clinicaltrials.gov/ct2/show/NCT02694952
- 89.Garcia W. Multicentric, Randomized,Controlled and Comparative Study to Evaluate the Efficacy of Two Treatment Schemes With Antivipmyn ® for the Treatment of Snake Bite Envenomation. clinicaltrials.gov; 2008 Mar. Report No.: NCT00639951. Available: https://clinicaltrials.gov/ct2/show/NCT00639951