Abbreviations
- 4‐NQO
4‐Nitroquinoline 1‐oxide
- AMPK
adenosine monophosphate‐activated protein kinase
- ESCC
esophageal squamous cell carcinoma
- FoxP3
forkhead box P3
- IFN‐γ
interferon‐gamma
- IL
interleukin
- LKB1
liver kinase B1
- PD‐L1
programmed death‐ligand 1
- SIRP
signal regulatory protein
- STAT3
signal transducer and activator of transcription 3
- TIME
tumor immune microenvironment
- TNF
tumor necrosis factor
Metformin, a biguanide derivate, has been in use as an anti‐diabetic agent for over 60 years and has been widely accepted as first‐line therapy for the management of type II diabetes. Interestingly, the treatment of type II diabetes with metformin is associated with reduced risk and improved prognosis for many types of cancers [1]. This observation has led to an explosion of interest in the potential application of metformin for cancer chemopreventive and direct antitumor effects, but also more recently for potential immunomodulatory activity.
Cancer immunotherapy has a major impact on the treatment outcomes of various solid tumors, with long‐term remission and even cure in certain patients. Nevertheless, the heterogeneity of the tumor microenvironment, such as those found in esophageal cancer, can influence the response to immunotherapy [2]. The use of drugs such as metformin may help to alter the tumor immune microenvironment (TIME) and could improve the efficiency of immunotherapy. The antitumor immune activity of metformin has only become a focus in the past 2‐3 years in preclinical studies, including enhancing the quality and quantity of T cells [3], switching the phenotype of tumor‐promoting macrophages to tumor‐suppressive [3], altering cytokine expression and downregulating programmed death‐ligand 1 (PD‐L1) [4, 5].
We recently reported on a phase II clinical trial of low‐dose metformin treatment in patients with esophageal squamous cell carcinoma (ESCC) that provided clear evidence of the immunomodulatory properties of metformin in the TIME of cancer patients [6]. In this double‐blinded study, patients underwent endoscopic biopsy of esophageal tumors. Upon pathological diagnosis with ESCC, patients were randomized to receive tablets of 250 mg metformin or placebo of identical appearance for 7‐14 days before surgery (Figure 1). Interestingly, although no impact on cell proliferation or apoptosis levels was detected in ESCC tissues, metformin treatment markedly altered the immune repertoire in ESCC tissues. In pre‐treatment biopsies, there was no significant difference between placebo‐ and metformin‐treated groups in any of the markers assayed for innate or adaptive immunity. However, in post‐treatment biopsies of metformin‐treated patients, CD8+ T cell infiltration was significantly increased, whereas CD4+ T cell infiltration was significantly decreased. For innate immune cells, metformin treatment significantly increased the percentage of CD11c+ tumor‐suppressive macrophages and decreased the percentage of CD163+ tumor‐promoting macrophages. In multivariate regression analysis, metformin treatment remained an independent factor for changes in CD8+ T cells, CD11c+ myeloid cells, CD163+ myeloid cells, and CD20+ B cells. Notably, although PD‐L1 is a crucial checkpoint protein associated with T cell exhaustion and non‐inflamed TIME [7], PD‐L1 expression did not change in either study arm.
FIGURE 1.
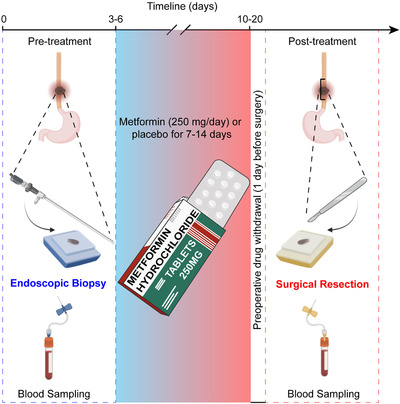
Patient treatment and sample collection scheme for the clinical trial of low‐dose metformin treatment in patients with esophageal squamous cell carcinoma
These clinical observations were closely recapitulated in an ESCC mouse model induced by carcinogen 4‐nitroquinoline 1‐oxide (4‐NQO), where low‐dose and short‐term metformin treatment yielded almost identical changes in TIME as detected in ESCC patients. Upon extended treatment with metformin, the antitumoral TIME changes of the mice became more pronounced. Specifically, the percentage of CD11c+ tumor‐suppressive macrophages was further increased, whereas CD206+ tumor‐promoting macrophages were strongly suppressed. In line with this, treatment of ESCC cells with low‐dose metformin in vitro significantly potentiated phagocytic uptake of ESCC cells via macrophages. Further, the percentage of forkhead box P3 (FoxP3)+ regulatory T cells was reduced. In contrast to short‐term treatment, the expression of PD‐L1 was significantly decreased following long‐term metformin treatment compared to placebo treatment. Moreover, long‐term metformin treatment decreased ESCC cell proliferation as well as the number of tumors per mouse. Thus, the mouse model mirrored the alterations of metformin‐induced TIME reprogramming in ESCC patients and provided a window to capture the impact of extended treatment with low‐dose metformin in ESCC.
One of the main mechanisms of action for the antitumor activity of metformin is thought to be the regulation of liver kinase B1 (LKB1)/adenosine monophosphate‐activated protein kinase (AMPK) pathways. In addition, dephosphorylation of signal transducer and activator of transcription 3 (STAT3) has been recently identified to be triggered by metformin [8]. Both AMPK and STAT3 are known to participate in the modulation of the metabolic reprogramming and immunoregulation in immune cells (i.e., CD8+ T cells and CD11c+ tumor‐suppressive macrophages) [9, 10]. Indeed, clear activation of AMPK and inactivation of STAT3 were detected in intra‐tumoral immune cells, associating with significant increases in tumor necrosis factor‐α(TNF‐α)+CD8+ T cells, interferon‐γ(IFN‐γ)+CD8+ T cells, TNF‐α+CD11c+ macrophages, and IFN‐γ+CD11c+ macrophages. At the same time, a significant decrease in interleukin (IL)‐10+CD11c+ macrophages was detected. Thus, these data provide evidence that low‐dose metformin mediates metabolic‐immune signaling pathways, most likely via AMPK‐STAT3 signaling, leading to an increase in pro‐inflammatory cytokine expression in key immune effector cell types in the tumor microenvironment.
From our data, it is clear that metformin alters the TIME in patients and in a closely‐matched mouse model, possibly via metabolic reprogramming. However, the potential implementation of metformin as a bona fide immuno‐metabolic adjuvant in clinics still warrants further evaluation. Its effect may also be influenced by particular body conditions including high blood glucose, obesity, and other metabolic disturbances which may change the metabolism locally or systemically [4]. In addition, it has been reported that the metabolic program is different between resting immune cells and proliferative immune cells, as well as between normal cells and cancer cells [11, 12]. Further, the TIME contains a host of diverse cell types with distinct and flexible metabolic states. Thus, the exact outcome of metformin treatment may partly depend on the composition of the TIME before treatment. Future efforts on the immuno‐metabolic mechanism contributing to the antitumor actions of metformin are warranted, and the strategy targeting metabolic reprogramming may have the potential to be optimized to selectively target cancer cells or the TIME.
Of note, metformin increased the number of tumor‐suppressive macrophages and decreased that of tumor‐promoting macrophages. In addition, the phagocytic activity of metformin‐treated macrophages toward ESCC cells was enhanced, indicating that metformin plays an important role in (re)activating macrophage‐mediated immunity. Our findings, thus, suggest that the potential combination of metformin with innate immune‐targeting strategies, such as combination with CD47‐blocking or CD24‐blocking antibodies that remove CD47/signal regulatory protein‐alpha‐ or CD24/Siglec‐10‐mediated “don't eat me” signaling, might be worth further (pre)clinical investigation [13, 14].
Low‐dose metformin also clearly increased the number of B lymphocytes. Currently, increasing evidence supports a pivotal role of B lymphocytes in tumor immunology but the function of tumor‐infiltrating B cells remains controversial [15, 16], suggesting that the specific subpopulation and function of tumor‐infiltrating B cells need to be carefully assessed. Therefore, the potential impact of metformin‐induced functional alterations on B lymphocytes will need to be delineated in further studies.
Classification of the TIME into subtypes has been attempted previously to give a readily interpretable and broad‐stroke overview of the tumor microenvironment status. Such subtype classification of the TIME is a potentially useful tool for evaluating the immunological status and predicting immunotherapy response and prognosis [17]. Using histochemistry and immunofluorescence, we similarly developed a generalized model for the microenvironment in our study, with a suppressive (S‐TIME), equilibrated (E‐TIME), or activated (A‐TIME) status, based on the relative presence of immune cell subtypes. Metformin treatment clearly and positively impacted the TIME status, with significant shifts of TIME in ESCC patients from S‐TIME or E‐TIME towards A‐TIME status. It will be interesting to implement additional technologies to get a further detailed understanding and optimize the TIME definitions for ESCC. Hereto, alternative approaches, such as flow cytometry or single‐cell sequencing can be exploited, with the landscape of immune cells in ESCC microenvironment in a mouse model and patients recently being reported [2, 18]. Therefore, the conclusions of metformin treatment for the TIME remodeling in ESCC may be further refined and fine‐tuned to provide better predictive value.
Given the pivotal role of TIME status during immunotherapies, it would be of great worth to develop a “gold standard” method for defining the TIME status that may aid in defining the impact of drugs and combinatorial treatment strategies. With the development of quantitative multiplex immunohistochemistry, single‐cell sequencing, and spatial transcriptomics, the classification and definition of TIME status could be further improved, and the relative impact of individual cell type changes can be better weighed. Ultimately, TIME classification may be implemented in clinical care for diagnosis and patient stratification.
Due to the asymptomatic feature of patients with early‐stage ESCC, most ESCC cases present in late stages [19, 20], with ∼48% of patients having stage III ESCC in our study. Thus, the potential impact of metformin in early developmental stages of ESCC (i.e., its influence on inhibiting the formation of tumor metastasis) remains to be determined. It remains to be elucidated whether the antitumor immune response mediated by metformin is related to the treatment duration or dosage. Importantly, since the mouse model closely corresponds to the data obtained from patients, this mouse model may serve as a tool to more rapidly investigate different timing and treatment strategies in order to steer the design of next clinical trials with metformin in ESCC.
There are increasing clinical trials evaluating metformin for cancer prevention and treatment, with our study providing compelling evidence for the immunomodulatory role of metformin in the tumor microenvironment. For future clinical studies, one should bear in mind that the antitumor benefits of metformin include not only direct tumor‐killing effects but also TIME activation with diverse mechanisms (Figure 2). A clinical study with a detailed physical examination, a meticulous assessment of tumor status, and a thoughtful plan for TIME evaluation would help to provide meaningful information and lead to successful outcomes. Before metformin can be properly implemented as an effective adjuvant for immune boosting in clinical practice, carefully designed prospective investigations are required to confirm the impact of metformin on the TIME.
FIGURE 2.
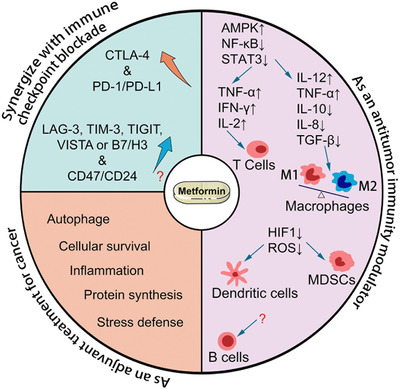
Model of metformin action in cancer cells and the tumor immune microenvironment. Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte antigen 4; PD‐1, programmed cell death 1; PD‐L1, programmed death‐ligand 1; LAG‐3, lymphocyte activating 3; TIM‐3, T cell immunoglobulin, and mucin domain‐containing protein 3; TIGIT, T cell immunoreceptor with Ig and ITIM domains; VISTA, V‐domain Ig suppressor of T cell activation; B7/H3, B7 homolog 3; AMPK, adenosine monophosphate‐activated protein kinase; NF‐κB, nuclear factor kappa B; STAT3, signal transducer and activator of transcription 3; TNF, tumor necrosis factor; IFN‐γ, interferon‐gamma; IL, interleukin; TGF‐β, transforming growth factor‐beta; HIF‐1, hypoxia‐inducible factor 1; ROS, reactive oxygen species; MDSCs, myeloid‐derived suppressor cells
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
All authors consent to publish the paper.
AUTHORS’ CONTRIBUTIONS
YL, SW, EB, and HZ wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by the following funding agencies: National Natural Science Foundation of China (82072683, 81773087, 81071736, 81572876 and 30973508 to H.Z.); Clinical Research Enhancement Initiative of Shantou University Medical College (201421); Natural Science Foundation of Guangdong Province of China (2021A1515012522 and 9151018004000000 to H.Z.); Science and Technology Planning Project of Guangdong Province of China (2019A030317024 to H.Z.); Special Project on the Integration of Industry, Education and Research of Guangdong Province (2011A090100024 to H.Z.).
Contributor Information
Edwin Bremer, Email: e.bremer@umcg.nl.
Hao Zhang, Email: haolabcancercenter@163.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1.Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta‐analysis. Ann Oncol. 2016;27(12):2184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, Chen Z, Han Y, Han L, Zou X, Zhou B, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun. 2020;11(1):6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, et al. Metformin modulates innate immune‐mediated inflammation and early progression of NAFLD‐associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70(4):710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen GG, Woo PYM, Ng SCP, Wong GKC, Chan DTM, van Hasselt CA, et al. Impact of metformin on immunological markers: Implication in its anti‐tumor mechanism. Pharmacol Ther. 2020;213:107585. [DOI] [PubMed] [Google Scholar]
- 5.Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X, et al. Metformin Liposome‐Mediated PD‐L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl Mater Interfaces. 2021;13(7):8026–41. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, et al. Low‐Dose Metformin Reprograms the Tumor Immune Microenvironment in Human Esophageal Cancer: Results of a Phase II Clinical Trial. Clin Cancer Res. 2020;26(18):4921–32. [DOI] [PubMed] [Google Scholar]
- 7.Sacher AG, Gandhi L. Biomarkers for the Clinical Use of PD‐1/PD‐L1 Inhibitors in Non‐Small‐Cell Lung Cancer: A Review. JAMA Oncol. 2016;2(9):1217–22. [DOI] [PubMed] [Google Scholar]
- 8.Kurelac I, Umesh Ganesh N, Iorio M, Porcelli AM, Gasparre G. The multifaceted effects of metformin on tumor microenvironment. Semin Cell Dev Biol. 2020;98:90–7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. STAT3 Activation‐Induced Fatty Acid Oxidation in CD8(+) T Effector Cells Is Critical for Obesity‐Promoted Breast Tumor Growth. Cell Metab. 2020;31(1):148–61 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walton K, Fernandez MR, Sagatys EM, Reff J, Kim J, Lee MC, et al. Metabolic reprogramming augments potency of human pSTAT3‐inhibited iTregs to suppress alloreactivity. JCI Insight. 2020;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell. 2021;39(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. [DOI] [PubMed] [Google Scholar]
- 14.Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, et al. Interaction between tumour‐infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, et al. Tumor‐educated B cells selectively promote breast cancer lymph node metastasis by HSPA4‐targeting IgG. Nat Med. 2019;25(2):312–22. [DOI] [PubMed] [Google Scholar]
- 17.Duan J, Xie Y, Qu L, Wang L, Zhou S, Wang Y, et al. A nomogram‐based immunoprofile predicts overall survival for previously untreated patients with esophageal squamous cell carcinoma after esophagectomy. J Immunother Cancer. 2018;6(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao J, Cui Q, Fan W, Ma Y, Chen Y, Liu T, et al. Single‐cell transcriptomic analysis in a mouse model deciphers cell transition states in the multistep development of esophageal cancer. Nat Commun. 2020;11(1):3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y, Dong H, Deng W, Lin W, Li K, Xiong X, et al. Evaluation of Salivary Exosomal Chimeric GOLM1‐NAA35 RNA as a Potential Biomarker in Esophageal Carcinoma. Clin Cancer Res. 2019;25(10):3035–45. [DOI] [PubMed] [Google Scholar]
- 20.Xiong X, Ke X, Wang L, Yao Z, Guo Y, Zhang X, et al. Splice variant of growth hormone‐releasing hormone receptor drives esophageal squamous cell carcinoma conferring a therapeutic target. Proc Natl Acad Sci U S A. 2020;117(12):6726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


