Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignant disease with a unique tumor microenvironment surrounded by an interlaced network of cancer and noncancerous cells. Recent works have revealed that the dynamic interaction between cancer cells and neuronal cells leads to perineural invasion (PNI), a clinical pathological feature of PDAC. The formation and function of PNI are dually regulated by molecular (e.g., involving neurotrophins, cytokines, chemokines, and neurotransmitters), metabolic (e.g., serine metabolism), and cellular mechanisms (e.g., involving Schwann cells, stromal cells, T cells, and macrophages). Such integrated mechanisms of PNI not only support tumor development, growth, invasion, and metastasis but also mediate the formation of pain, all of which are closely related to poor disease prognosis in PDAC. This review details the modulation, signaling pathways, detection, and clinical relevance of PNI and highlights the opportunities for further exploration that may benefit PDAC patients.
Keywords: neurotrophins, pancreatic ductal adenocarcinoma, perineural invasion, schwann cells, tumor microenvironment
This review details the modulation, function, and detection of perineural invasion, and highlights the opportunities for further exploration that may benefit pancreatic cancer patients.
Abbreviations
- ACh
acetylcholine
- ADRB2
adrenoceptor beta 2
- ARTN
artemin
- CAFs
cancer‐associated fibroblasts
- CCL2
C‐C motif chemokine ligand 2
- CCR2
C‐C motif chemokine receptor 2
- CX3CL1
C‐X3‐C motif chemokine ligand 1
- CX3CR1
C‐X3‐C motif chemokine receptor 1
- CXCL12
C‐X‐C motif chemokine ligand 12
- CXCR4
C‐X‐C motif chemokine receptor 4
- DFS
disease‐free survival
- DRG
dorsal root ganglia
- GDNF
glial cell‐derived neurotrophic factor
- GFRA
GDNF family receptor alpha
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HDAC1
histone deacetylase 1
- HIF1A
hypoxia‐inducible factor 1 subunit alpha
- HPGD
15‐hydroxyprostaglandin dehydrogenase
- IFNG/IFNγ
interferon‐gamma
- IL‐6
interleukin‐6
- KLRB1
killer cell lectin‐like receptor B1
- KRAS
KRAS proto‐oncogene, GTPase
- L1CAM
L1 cell adhesion molecule
- MAP1LC3A/B
microtubule‐associated protein 1 light chain 3 alpha/beta
- MAPK
mitogen‐activated protein kinase
- MDK
midkine
- MHC‐II
major histocompatibility complex class II
- MMP
matrix metallopeptidase
- NCAM1
neural cell adhesion molecule
- NECTIN1
nectin cell adhesion molecule 1
- NGF
nerve growth factor family
- NGFR
nerve growth factor receptor
- NTRK1
neurotrophic receptor tyrosine kinase 1
- OS
overall survival
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- PLXND1
plexin D1
- PNI
perineural invasion
- PSCs
pancreatic stellate cells
- PTN
pleiotrophin
- RET
Ret proto‐oncogene
- ROBO1
roundabout guidance receptor 1
- SDC3
syndecan 3
- SEMA3D
semaphorin 3D
- SLIT2
slit‐guiding ligand 2
- SNCG
synuclein gamma
- SP
substance P
- STAT3
signal transducer and activator of transcription 3
- TAMs
tumor‐associated macrophages
- TGFB
transforming growth factor‐beta
- TRPV1
transient receptor potential cation channel subfamily V member 1
- VGF
VGF nerve growth factor inducible
1. BACKGROUND
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and the fourth‐leading cause of cancer death in the United States, with a 5‐year overall survival (OS) rate of 10% [1]. Despite advances in medicine, the incidence of PDAC continues to rise, with 60,430 new cases and 48,220 deaths reported in 2020 [1]. It is one of the most aggressive solid malignant tumors, and its poor prognosis is due to many reasons, including but not limited to late diagnosis, early metastasis, and therapy resistance [2]. These challenging clinical features of PDAC are determined by its tumor microenvironment, which has hypoxia and immune escape characteristics and is formed by various cellular and noncellular components [3]. Understanding the composition of the tumor microenvironment and its role in tumor progression is the cornerstone of the development of new anti‐PDAC therapies.
The nerves are fibers that receive and send information between tissues, and they are usually parallel to blood vessels throughout the body. As early as the 19th century, researchers have observed the phenomenon of perineural invasion (PNI) of cancer [4]. PNI is usually defined as the appearance of tumor cells along the nerves and/or within the epineural, perineural, and endoneurial places of the neuronal sheath, with cancer cells surrounding at least 33% of the nerves [4, 5]. Recently, there has been an increasing interest in understanding the phenotypes and molecular basis of the interaction between the nerves and cancer cells that drive pancreatic tumorigenesis and treatment resistance. Histopathological studies have found that PNI can occur in over 80% of PDAC tumor tissues and is an early event of tumorigenesis in preclinical and clinical models [6, 7, 8], which leads to significant tumor characteristics. In particular, PNI is associated with tumor progression, increased local recurrence, strong pain, and poor prognosis in PDAC patients [4]. Therefore, targeting PNI may provide an alternative strategy to improve the prognosis of PDAC patients.
The nerves and tumor cells can influence each other to provide a suitable microenvironment for tumor survival and growth. On one hand, the infiltrated nerves promote the proliferation of tumor cells and the formation of pancreatic intraepithelial neoplasia in KRASG12D‐driven PDAC mouse models by neuronal inflammation [9]. Ablation of the sensory neurons in the pancreas protects the nerves from inflammatory damage, thereby delaying tumor formation in mouse models [9]. These findings establish the potential role of the nerves in shaping an inflammatory tumor microenvironment. On the other hand, cancer cells invading around or inside the nerves may ultimately lead to neurological dysfunction, increased plasticity, or structural destruction [10, 11, 12, 13]. Such dynamic communication between the nerves and PDAC cells further facilitates immune escape, tumor growth, and metastasis.
Complementing previous reviews [14, 15, 16], in this Review, we aim to provide new insights into the relationship between the nerves and pancreatic cancer. We will not only delineate the key mediators in controlling the interplay between the nerves and cancer cells in the tumor microenvironment but also highlight the detection, function, and significance of PNI in PDAC.
2. MOLECULAR MEDIATORS OF PNI
2.1. Neurotrophins
Neurotrophins are a family of proteins that induce the survival, development, and function of neurons. They can also be expressed in PDAC cells and have a direct effect on the interaction between cancer cells and the nerves in the tumor microenvironment through corresponding receptors. Below, we review recent findings that different neurotrophin subfamilies exert various effects in regulating PNI and pancreatic tumor metastasis (Figure 1).
FIGURE 1.
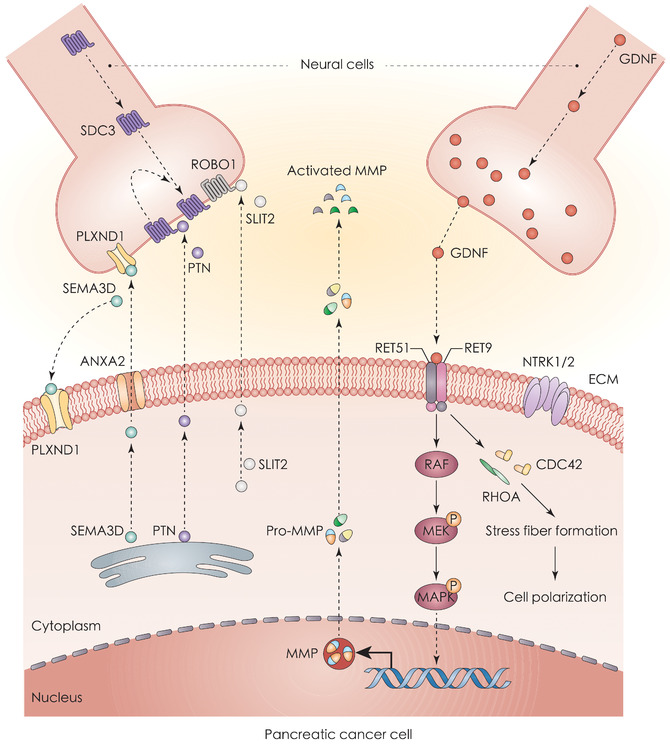
Neurotrophins in PNI and PDAC. Neurotrophins are associated with PNI in pancreatic tumorigenesis. A series of interactions between multiple neurotrophin ligands and receptors, such as SEMA3D‐PLXND1, ARTN‐GFRA3, and PTN‐SDC3, promote the movement of cancer cells toward neural cells. The binding of GDNF to RET receptors activates downstream KRAS signaling and upregulates the expression of MMPs. It also accelerates the formation of stress fibers, thus resulting in cellular polarization and metastasis during PNI. Abbreviations: ANXA2, annexin A2; CDC42, cell division cycle 42; ECM, extracellular matrix; GDNF, glial cell‐derived neurotrophic factor; MAPK, mitogen‐activated protein kinase; MAP2K, mitogen‐activated protein kinase kinase; MMP, matrix metallopeptidase; NTRK1, neurotrophic receptor tyrosine kinase 1; NTRK2, neurotrophic receptor tyrosine kinase 2; PDAC, pancreatic ductal adenocarcinoma; PLXND1, plexin D1; PNI, perineural invasion; PTN, pleiotrophin; RAF, Raf‐1 proto‐oncogene, serine/threonine kinase; RET, ret proto‐oncogene; RHOA, Ras homolog family member A; ROBO1, roundabout guidance receptor 1; SDC3, syndecan 3; SEMA3D, semaphorin 3D; SLIT2, slit guidance ligand 2
2.1.1. The nerve growth factor family (NGF)
Neuronal cells and cancer cells can release neurotrophins to promote cell growth, survival, and maintenance [17]. NGF and other known members of the neurotrophin family, such as brain‐derived neurotrophic factor, neurotrophin 3, and neurotrophin 4, as well as their sharing receptors, neurotrophic receptor tyrosine kinase 1 (NTRK1), neurotrophic receptor tyrosine kinase 2, and nerve growth factor receptor (NGFR), are expressed in different PDAC cell lines, indicating that PDAC cells and neural cells may influence each other through these neurotrophins. Apart from the research conducted using cell lines, the mRNA levels of genes encoding the neurotrophin family members and NTRK1 in the tumor tissues from PDAC patients have been found to be upregulated, mainly in neuronal cells [18]. Interestingly, NTRK1 and NGFR are used as opposite prognostic biomarkers for PDAC patients. For example, a retrospective study of 56 PDAC patients showed that a high expression of NTRK1 was associated with poor prognosis while the overexpression of NGFR was linked to a relatively long survival [19], suggesting that different neurotrophin signals play a selective role in predicting patient survival.
Pancreatic cancer cells require more nutrients or energy to support their growth and metastasis by altering their metabolism. The reprogramming of energy metabolism is a dynamic process that can be achieved through multiple mechanisms involving amino acid metabolism, glucose utilization, and autophagic degradation. For example, PDAC cells rely on exogenous serine (a conditionally essential amino acid) in varying degrees for tumor proliferation [20]. Regarding the nervous system, axons and dorsal root ganglia (DRG) can release serine into the pancreatic tumor microenvironment to provide additional energy support [20]. After serine deprivation, PDAC cells express and release more NGFs by upregulating their mRNA translation [20], thereby enhancing the movement of axons toward the tumor nest. These findings represent the interaction between cancer and axons during nutrition starvation which promotes PNI formation. Targeting serine supply or NGF secretion may be an attractive strategy to inhibit tumor growth in the pancreatic tumor microenvironment. Anti‐NGF treatment significantly reduced the ratio of neurogenic inflammation and PNI in an 8‐week KRASG12D‐driven PDAC mouse model [21], indicating that NGF is also a driving factor of nerve‐related inflammation within the tumor microenvironment.
Not all neurotrophins are highly expressed in PDAC cells. For example, slit‐guiding ligand 2 (SLIT2) is a neurotrophic protein related to cell navigation, and its mRNA level was low in PDAC cell lines and patient tumor tissues [22]. Functional studies have shown that the overexpression of SLIT2 in human PDAC cell lines (MiaPACA2 and PANC1) suppressed cell migration and invasion. Conversely, blocking the interaction between SLIT2 and its receptor, roundabout guidance receptor 1 (ROBO1), enhanced the motility and invasiveness of PDAC cells, further supporting the negative role of SLIT2 in cancer cell migration and invasion [22]. In the co‐culture system with Schwann cells or a Matrigel/DRG culture system, SLIT2 restricted bidirectional movement between PDAC cells and neural cells [22]. However, another group reported that cancer‐associated fibroblasts (CAFs) from PDAC patients expressed higher levels of SLIT2 than those from healthy controls, thereby promoting neurite outgrowth and Schwann cell migration by activating the cadherin 2 pathway [23]. Moreover, the inhibition of SLIT2‐ROBO1 signaling hampered this neural remodeling effect [23]. Altogether, these data indicate the possibility of inhibiting PNI by targeting different NGF family members expressed in PDAC cells and other cells of the tumor microenvironment.
2.1.2. Glial cell‐derived neurotrophic factor family ligands and receptors
Glial cell‐derived neurotrophic factor (GDNF) family ligands are a series of proteins including GDNF, neurturin, artemin (ARTN), and persephin, which can bind to specific receptors to perform distinct functions [24]. GDNF is an essential protein for the maintenance of neuron growth and is highly expressed in the peripheral and central nervous systems [25]. Compared to patients without PNI (24/40, 60.0%), PDAC patients with PNI had a higher frequency of GDNF expression (16/18, 88.9%), indicating that GDNF is involved in the neural invasion process [26]. During the growth process of neurons, certain neural cells (e.g., Schwann cells and motor neurons) could secrete GDNF into extracellular space [27]. Ret proto‐oncogene (RET) is the receptor of GDNF and is selectively expressed in some PDAC cell lines (e.g., MiaPACA2, ASPC1, SW1990, and CAPAN2) [28]. Compared with RET‐negative PDAC cells or GDNF‐nonsecreting neuronal cells (such as IMR32 cells), RET+ PDAC cells (such as MiaPACA2 and ASPC1) co‐culture with GDNF‐secreting neuronal cells (such as T98G cells) promoted the migration of tumor cells to neuronal cells [28]. Cell migration mediated by the GDNF‐RET axis depends on the activation of KRAS proto‐oncogene, GTPase (KRAS), and the phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic signaling pathway [29], indicating that classical oncogene signals are involved in promoting the neurotrophin pathway.
Structural studies have documented that RET has two isoforms, namely RET9 and RET51, which are completely different in terms of protein interactions and oncogenic potentials [30]. RET51 plays a leading role in the GDNF‐RET‐mediated PDAC invasion process [31]. After RET was activated, it not only induced the polarization and invadopodia of cancer cells but also facilitated matrix degradation by producing more matrix metallopeptidase 2 (MMP2), MMP9, and MMP14 [31], thereby accelerating cell invasion. Similarly, the knockdown of GDNF family receptor alpha 1 (GFRA1), the co‐receptor of RET, limited the migration of MiaPACA2 cells. Accordingly, the combination of exogenous GFRA1 and GDNF stimulated the RET mitogen‐activated protein kinase cascade and induced the invasion of cancer cells [32]. These findings establish a model for neural signals to promote PDAC metastasis, namely the GDNF‐GFRA1‐RET axis.
ARTN is another member of the GDNF family. PDAC cell lines (ASPC1, BxPC3, CAPAN1, MiaPACA2, PANC1, SU86.86, T3M4, and COLO357) and tissues showed a high expression of ARTN and its receptor GDNF family receptor alpha 3 (GFRA3) [33, 34]. ARTN had the ability to trigger GFRA3‐dependent invasion in PDAC cells [33, 34]. Further research is needed to explore the structural basis of the ARTN‐GFRA3 pathway in promoting cell invasion. It is also unclear whether neurons are the main source for extracellular ARTN to drive tumor metastasis.
2.1.3. The midkine (MDK) family
The MDK family includes two members, namely MDK and pleiotrophin (PTN). They share the same receptor, syndecan 3 (SDC3), to promote the outgrowth of neural cells [35]. The level of MDK in pancreatic cancer specimens was higher than that in normal pancreatic tissues [36, 37], which was associated with PNI and poor prognosis [38]. The neurotrophic factor PTN was also highly expressed in PDAC cells and can function as a damage‐associated molecular pattern in the tumor microenvironment [39]. Once released by necrotic tumor cells, extracellular PTN was recognized by the receptor SDC3 on the pancreatic nerves, which leads to nerve proliferation [40]. However, the upregulation of SDC3 in neurons and Schwann cells might cause an accumulation of PTN+ PDAC cells around neuronal cells, thereby aggravating nerve damage [40]. These findings indicate that PTN‐SDC3 signaling plays a dual role in regulating neuroplasticity during PNI.
2.1.4. The axon guidance gene family
Members of the axon guidance gene family, such as semaphorin 3D (SEMA3D), play a role in the formation of neuronal networks. The knockdown of SEMA3D or blockade of its receptor plexin D1 (PLXND1) attenuated the invasion of tumor cells (primary murine KPC cells) towards the nerves in vitro [41]. Reducing SEMA3D or PLXND1 expression also decreased the nerve density in tumor tissues in vivo [41]. Overall, these preclinical and clinical studies indicate that high levels of SEMA3D and PLXND1 may be implicated in PNI, and lay the foundation for further study on the changes of different neurotrophins in the pancreatic tumor microenvironment.
2.2. Chemokines
The C‐X‐C motif chemokine ligand and C‐X‐C motif chemokine receptor chemokine family consists of a series of peptides, which primarily act as a chemoattractant for immune cells and neural cells. In this family of chemokines, the overproduction of some of them is associated with the PNI process (Figure 2). For example, C‐X3‐C motif chemokine receptor 1 (CX3CR1) was detected in some PDAC cell lines (MiaPACA2, CFPAC, PACA44, T3M4, PANC1, ASPC1, and A8184) and surgical specimens of patients but it was undetectable in normal pancreatic tissues [42]. C‐X3‐C motif chemokine ligand 1 (CX3CL1) is a specific ligand of CX3CR1 which is expressed by neurons and the nerves. From the histological evaluation, increased CX3CR1 expression was closely associated with tumor PNI in PDAC patients (P = 0.026), implying a role of CX3CR1 signaling in the pancreatic tumor microenvironment [42]. Mechanistically, the migration of CX3CR1+ PDAC tumor cells towards recombinant CX3CL1 protein or CX3CL1+ neural cells depended on the activation of integrins and G protein‐coupled receptors in vitro and in vivo [42].
FIGURE 2.
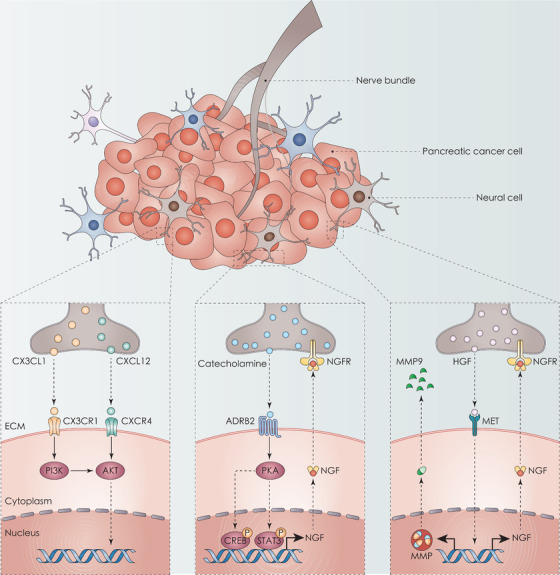
Chemokines and neurotransmitters in PNI and PDAC. Neural cells release C‐X‐C motif chemokine ligands (CX3CL and CXCL12), which can bind to receptors CX3CR1 and CXCR4, respectively, and activate the PI3K‐AKT pathway. In addition, neural cells secrete catecholamine and then induce tumor invasion to the nerves through the ADRB2‐PKA‐STAT3 signaling pathway. Similarly, HGF secreted by DRG binds to MET receptors on PDAC cells in a paracrine manner. The activation of MET leads to the upregulation of NGF and MMP9, which may enhance PNI. Abbreviations: ADRB2, adrenoceptor beta 2; CREB, CREB/ATF BZIP transcription factor; CX3CL1, C‐X3‐C motif chemokine ligand 1; CX3CR1, C‐X3‐C motif chemokine receptor 1; CXCL12, C‐X‐C motif chemokine ligand 12; CXCR4, C‐X‐C motif chemokine receptor 4; DRG, dorsal root ganglia; ECM, extracellular matrix; HGF, hepatocyte growth factor; MET, met proto‐oncogene, receptor tyrosine kinase; MMP9, matrix metallopeptidase 9; NGF, nerve growth factor; NGFR, nerve growth factor receptor; PDAC, pancreatic ductal adenocarcinoma; PKA, protein kinase CAMP‐activated catalytic; PNI, perineural invasion; STAT3, signal transducer and activator of transcription 3
The expression of C‐X‐C motif chemokine receptor 4 (CXCR4) in PDAC human specimens was also significantly related to PNI (P = 0.0001) [43]. C‐X‐C motif chemokine ligand 12 (CXCL12) is a main ligand of CXCR4. Subsequent co‐culture experiments showed that DRG releases CXCL12 in a paracrine fashion, thereby attracting PDAC cells to DRG [43]. In vivo evidence demonstrated that the blockade of CXCL12‐CXCR4 signaling could remarkably reduce tumor size, nerve injury degree, and PNI level in tumor tissues [43]. Although the exact mechanism of activation of these pathways needs to be further studied, neutralizing antibodies or inhibitors that block CX3CR1 or CXCR4 may be a way to inhibit PDAC by reducing PNI.
Alternatively, hepatocyte growth factor, a multifunctional chemokine expressed by DRG, interacts with MET proto‐oncogene, receptor tyrosine protein‐coding kinase, which leads to the upregulation of NGF and MMP9 of PDAC cells, thereby promoting PDAC cell migration and invasion through a paracrine manner [44]. The co‐culture model showed that hepatocyte growth factor enhances the invasion of tumor cells toward DRG, leading to a subsequent outgrowth of DRG [44]. Since these signaling axes play a vital role in normal health and function, researchers cannot readily assess the balance between physical and pathological functions. In addition to cancer cells and the nerves, chemokines could directly affect the infiltration of various immune cells in the tumor microenvironment [45], highlighting a complex influence of these chemokines in promoting the development and progress of PDAC.
2.3. Neurotransmitters
Catecholamines are neurotransmitters containing epinephrine, norepinephrine, and dopamine. They play an important role in maintaining different physiological functions and stress responses. In preclinical PDAC models, norepinephrine induced tumor invasion to the nerves by activating the signaling pathway involving adrenoceptor beta 2 (ADRB2), protein kinase CAMP‐activated catalytic, and signal transducer and activator of transcription 3 (STAT3) [46]. The STAT3 transcription factor is an important regulator of pancreatic tumorigenesis, and for promoting tumor stem cell proliferation and maintaining an inflammatory tumor microenvironment [47]. However, clinical studies have not confirmed the role of STAT3 in PNI. A study based on the histological results of 79 PDAC patients showed that the phosphorylation of STAT3 has no relationship with PNI [48]. Regardless, the interaction of catecholamines with ADRB2 would promote the PNI of PDAC by stimulating NGF secretion and increasing the nerve density in PDAC tissues [49]. These studies provide new insights into the relationship between neuropsychological stress or sympathetic nerve stress and the progression of PDAC [49]. Despite the need to evaluate potential adverse effects, the inhibition of the catecholamine pathway may be a valuable treatment for advanced PDAC.
3. CELLULAR MEDIATORS OF PNI
As mentioned earlier, the pancreatic tumor microenvironment is composed of various cellular components that form a dynamic network, which is conducive to immune tolerance, metabolic reprogramming, cancer metastasis, and PNI. In this section, we summarize the role of various types of cells in regulating PNI within the tumor microenvironment (Figure 3).
FIGURE 3.
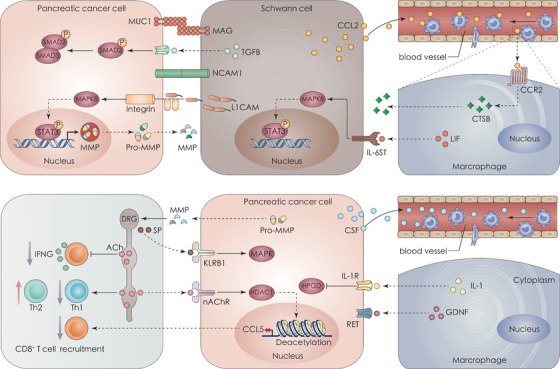
Cellular crosstalk in PNI and PDAC. The pancreatic tumor microenvironment is infiltrated by various cells, such as immune cells, neural cells, and CAFs. The crosstalk between these cells promotes tumor proliferation, invasion, and immunosuppression. Cancer cells interact with neural cells through membrane proteins (MAG‐MUC1 and NCAM1) or secretory proteins (L1CAM), and then promote the movement of tumor cells toward neural cells. The combination of IL‐6 released by cancer cells and IL‐6R on Schwann cells is related to less pain in PDAC patients. Both neuronal cells and cancer cells release cytokines, such as CCL2 or CSF1, which attract macrophages from the circulation. Activated macrophages release CTSB to degrade collagen IV (a component of the nerves’ perineurium) and IL‐1 to downregulate the expression of HPGD. The expression of HPGD is positively correlated with PNI. ACh in DRG can directly inhibit the recruitment, activation, and function of CD8+ T cells, leading to immunosuppression. Abbreviations: ACh, acetylcholine; CAF, cancer‐associated fibroblast; CCL2, C‐C motif chemokine ligand 2; CCR2, C‐C motif chemokine receptor 2; CSF, colony‐stimulating factor; CTSB, cathepsin B; DRG, dorsal root ganglia; GNDF, glial cell‐derived neurotrophic factor; HDAC1, histone deacetylase 1; HPGD, 15‐hydroxyprostaglandin dehydrogenase; IFNG, interferon gamma; IL‐1, interleukin 1; IL‐1R, interleukin 1 receptor; IL‐6, interleukin 6; IL‐6R, interleukin 6 receptor; IL‐6ST, interleukin 6 signal transducer; KLRB1, killer cell lectin‐like receptor B1; LICAM, L1 cell adhesion molecule; LIF, LIF interleukin 6 family cytokine; MAG, myelin‐associated glycoprotein; MAPK, mitogen‐activated protein kinase; MAPK8, mitogen‐activated protein kinase 8; MMP, matrix metallopeptidase; MUC1, mucin 1, cell surface‐associated; nAChR, nicotinic acetylcholine receptors; NCAM1, neural cell adhesion molecule 1; PDAC, pancreatic ductal adenocarcinoma; RET, ret proto‐oncogene; SMAD2, SMAD family member 2; SMAD3, SMAD family member 3; SP, substance P; STAT3, signal transducer and activator of transcription 3; T cell, T lymphocyte; TGFB, transforming growth factor beta; Th1, T helper type 1; Th2, T helper type 2
3.1. Schwann cells
Schwann cells are one of the common cell types in peripheral nerves and play a role in nerve repair and regeneration. The direct interaction between Schwann cells and tumor cells can be fine‐tuned at multiple levels. First, Schwann cells directly interacted with tumor cells through plasma membrane proteins, such as myelin‐associated glycoprotein and neural cell adhesion molecule 1 (NCAM1) [50, 51, 52]. Second, Mucin 1 further promoted the adhesion of PDAC cells to Schwann cells in vitro and the invasion of PDAC cells into sciatic nerves in vivo [50, 51]. Third, Schwann cells elicited structural changes in PDAC cells, showing the formation of protrusions to Schwann cells and dispersion among cancer cells [52]. These changes depend on NCAM1 on the Schwann cell membranes, thereby gradually promoting PNI in vitro and in vivo [52].
Schwann cells can also communicate with tumor cells through secretory proteins, including L1 cell adhesion molecule (L1CAM) and transforming growth factor‐beta (TGFB) [53, 54]. Schwann cells express and secrete L1CAM, which displays a powerful chemotaxis to PDAC cells [53]. After tumor cells encounter Schwann cells, L1CAM activated the downstream STAT3 pathway and upregulates the expression of MMP2 and MMP9, ultimately enhancing PNI [53]. In a transgenic mouse model, the administration of anti‐L1CAM antibody significantly alleviated PNI [53], confirming that L1CAM is a mediator of PNI. Additionally, TGFB, a multifunctional cytokine belonging to the transforming growth factor superfamily, was produced and released by Schwann cells [54]. The released TGFB augmented the aggressive capacity of PDAC cells, which contributes to PNI progression in preclinical models [54]. The expression and release of TGFB were also increased in PDAC patients [55, 56]. The multiple roles of TGFB in the process of malignant progression may provide abundant opportunities for therapeutic intervention in PDAC.
Apart from the crosstalk between Schwann cells and tumor cells, Schwann cells build a connection with tumor‐associated macrophages through secretory proteins [57]. Molecularly, Schwann cells secreted the chemokine C‐C motif chemokine ligand 2 (CCL2), which recruits inflammatory macrophages from the circulatory system to the site of PNI [57]. The recruited macrophages were further differentiated into specific macrophages with highly expressed cathepsin B through the CCL2‐C‐C motif chemokine receptor 2 (CCR2) pathway [57]. Consequently, cathepsin B degraded collagen IV (one of the components in nerve perineurium), thereby aggravating nerve injury and tumor cell invasion [57]. Interleukin‐6 (IL‐6) family cytokine was produced and released by fibroblasts and mast cells and maintained high levels in the serum or tissues of PDAC patients [58]. After binding to its receptor on the Schwann cells, the IL‐6 family cytokine activated the STAT3 pathway to trigger the migration and outgrowth of Schwann cells, thereby driving neuronal plasticity [58].
In particular, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), a monomeric glycoprotein secreted by immune cells and fibroblasts, acted as a key intercellular cytokine in a pancreatic hypoxic tumor microenvironment during the formation of PNI [59]. The expression of GM‐CSF was upregulated by the oxygen‐dependent transcriptional activator hypoxia‐inducible factor 1 subunit alpha (HIF1A) [60]. Consequently, the activation of the HIF1A‐GM‐CSF pathway mediated PNI by promoting the migration and accumulation of Schwann cells in tumor tissues, resulting in a poor prognosis [60]. In addition, the upregulation of GM‐CSF promoted the immune escape of PDAC, while the suppression of GM‐CSF enabled the immune system to clear tumor cells [61]. These findings could help us design effective strategies against PDAC by targeting neuroimmunity.
Altogether, Schwann cells play a crucial role in the process of PNI through extensive communications with various cells in the pancreatic tumor microenvironment. Therefore, a comprehensive understanding of the function of Schwann cells in PNI may open a window for early therapeutic intervention before PNI occurrence.
3.2. Stromal cells and fibroblasts
Pancreatic stellate cells (PSCs) are the most prominent cell type in the PDAC stroma, accounting for about 50% of PDAC stromal cells [62]. During the co‐culture process, PSCs upregulated the production and release of MMP proteins in PDAC (CFPAC1) cells, thereby enhancing tumor invasion ability [63]. The release of the extracellular matrix glycoprotein tenascin C by PSCs also enhanced the interaction between cancer cells and axonal DRG, which may be positively related to PNI, tumor stage, and tumor recurrence [64]. In addition, PDAC cells could release sonic hedgehog signaling molecules to activate hedgehog signaling pathways in PSCs, leading to cancer invasion and nerve dysfunction [65]. These findings indicate that abnormal hedgehog signals are involved in the communication between cancer cells, PSCs, and the nerves in the tumor microenvironment.
CAFs are highly differentiated stromal cells that promote tumor growth, angiogenesis, and matrix remodeling. CAFs express various unique proteins, which are related to the prognosis of PDAC. For example, nectin cell adhesion molecule 1 (NECTIN1)+ CAFs were an independent prognostic factor in PDAC [66]. The level of NECTIN1 in the CAFs of PDAC patients was positively correlated with advanced tumor stage (P = 0.016), PNI (P = 0.022), and short OS (P = 0.003) [66]. Fibroblast activation protein alpha was a broad marker of CAFs in various cancers and was closely associated with PNI (P = 0.009), tumor size (P < 0.001), and poor prognosis (P = 0.0085) in PDAC patients [67]. Although inhibiting CAFs is a challenge in clinical practice, stromal response still may be a potential target for PDAC treatment.
3.3. Macrophages
Tumor‐associated macrophages (TAMs) produce an immunosuppressive tumor microenvironment in PDAC and participate in every stage of tumor formation and development [68]. By co‐staining with macrophage markers (CD68 and CD163) and nervous system marker S100 calcium‐binding protein, an immunohistochemical analysis of 59 PDAC patients found that the number and density of TAMs in a PNI‐positive group were higher than those in the negative group [69]. Of note, the PNI‐negative group may be the case where PNI is present but not detected. Increased TAM invasion in PNI was associated with poor prognosis in PDAC patients [69]. Subsequent mechanistic studies confirmed that IL‐1 secreted by CD163+ macrophages inhibited the expression of 15‐hydroxyprostaglandin dehydrogenase (HPGD) in PDAC cells [70]. Although the specific mechanism was not yet clear, low levels of HPGD were related to PNI and short survival [70]. The colony‐stimulating factor 1 secreted by PDAC cells could recruit and activate macrophages [71]. Activated macrophages produced GDNF, thereby increasing PNI [71]. These findings provide a good example of the molecular communication between cancer cells and macrophages that drives PNI.
Other pathways for TAM‐related PNI include CCR2 signaling. In animal models, the invasion of F4/80+ wild‐type macrophages in the nerves was much more severe than that of CCR2‐deficient macrophages [71]. Activated macrophages stimulated human PDAC cells (PANC1 and MiaPACA2) to secrete MMP1 [72]. Soluble MMP1 further induced DRG to release substance P (SP), which activated killer cell lectin‐like receptor B1 (KLRB1) and then induced PNI through the pathway involving SP, KLRB1, and mitogen‐activated protein kinase (MAPK) in vitro and in vivo [72]. To illustrate the complex nature of this process, it is necessary to further identify the feedback mechanism of TAM‐associated signal transduction.
3.4. T cells
T cells are one of the main components of the adaptive immune system and are used to kill infected host cells or cancer cells. The vagus nervous system plays an immunomodulatory role in the pancreatic tumor microenvironment, partly by controlling the infiltration or activation of T cells [73]. The level of acetylcholine (ACh), a neurotransmitter produced by the vagus nerve, was elevated in the PNI‐detected samples from PDAC patients [73]. Elevated Ach can impair T cell recruitment and subsequent T cell‐mediated anti‐tumor immunity [73]. Mechanically, once captured by PDAC cells, ACh suppressed the expression of CCL5 in PDAC via histone deacetylase 1 (HDAC1)‐mediated histone deacetylation [73]. Conversely, low levels of CCL5 failed to recruit CD8+ T cells to the tumor site, leading to an immunosuppressive tumor microenvironment [73]. ACh also inhibited interferon‐gamma (IFNG/IFNγ) produced by CD8+ T cells, thereby favoring differentiation in T helper type 2 cells over T helper type 1 cells [73]. Therefore, in the orthotopic PDAC mouse model, it was not surprising that disrupting the vagus PNI process through vagotomy could inhibit tumor growth and prolong survival [73]. However, these findings were challenged by another study using a spontaneous model which showed that subdiaphragmatic vagotomy could accelerate KRASG12D‐driven PDAC tumorigenesis in mice [74]. In contrast, complementary treatment with muscarinic agonists was shown to reverse this tumor‐promoting phenotype (such as increased cancer stem cells and tumor metastasis) [74]. These different animal model studies together suggest a dual role of cholinergic nervous system invasion in PDAC, coupling the modulation of T cell recruitment and activation.
4. AUTOPHAGY AND PNI
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved degradation mechanism in which cytosol components are engulfed by autophagosome and then delivered to the lysosome for degradation [75]. Dysregulated autophagy plays an essential role across all stages of PDAC, including the formation of PNI [76]. The expression of the autophagosome marker microtubule‐associated protein 1 light chain 3 alpha/beta (MAP1LC3A/B) was positively correlated with PNI (P < 0.05), and high MAP1LC3A/B expression was an independent risk factor for PNI and poor prognosis of PDAC (P < 0.05) [77]. Although the mechanism by which autophagy in PDAC cells contributes to PNI is still poorly understood, ubiquitin C (one of the sources of ubiquitin) has been shown to act as a bridge between PNI and autophagy [78]. Another area worth exploring in the future is whether PNI can be used as an evaluation index for the clinical effect of the antimalarial agent hydroxychloroquine, a potent inhibitor of autophagy used in clinical trials in PDAC patients [79, 80, 81, 82]. It is also interesting to determine whether blocking PNI formation will impair the autophagic process.
5. BIOMARKERS OF PNI
5.1. Protein expression and secretion
Several studies have identified upregulation of a panel of proteins in the tumor samples from PDAC patients with PNI (Table 1). These proteins include CD74 (a regulator of antigen presentation by mediating the assembly and subcellular trafficking of the major histocompatibility complex class II [MHC‐II] complex) [83, 84, 85], cadherin 1, and other membrane proteins [86], which were closely related to the survival rate of PDAC patients. However, an increase in cyclin D1, a member of the G1 cyclin family, was associated with PNI, but not prognosis [87], suggesting that PNI regulators are not always parallel to the course of PDAC. Compared with the PNI‐undetected group, a lower expression of proteins, such as kinesin family member 14 and Fas cell surface death receptor [88, 89], were also detected in the PNI detected group. However, the function of these proteins and their effect on prognosis are unclear. Apart from protein expression, the serum levels of collagen type VI alpha 3 chain and baculoviral IAP repeat‐containing 5 were positively correlated with PNI [90, 91], providing a convenient way to monitor the occurrence of nerve damage in PDAC.
TABLE 1.
Protein expression and secretion associated with PNI in PDAC
| Protein | Sample sources | Testing method(s) | Expression | Association with PNI | P value | Reference(s) |
|---|---|---|---|---|---|---|
| BIRC5 | Serum from 80 PDAC patients and 80 healthy controls | ELISA | Upregulated | Positive | < 0.01 | [91] |
| CADM4 | Tissues from 258 patients | IHC | Downregulated | Negative | 0.001 | [126] |
| CCND1 | Tissues from 59 patients | IHC | Upregulated | Positive | NA | [87] |
| CDH1 | Tissues from 46 patients | IHC | Upregulated | Positive | NA | [86] |
| CD74 | Tissues from 67 patients | IHC | Upregulated | Positive | NA | [83] |
| CD74 | Tissues from 68 patients | IHC | Upregulated | Positive | 0.006 | [84, 85] |
| COL6A3 | Serum from 44 PDAC patients, 46 benign lesion patients, and 30 age‐matched healthy volunteers | ELISA; RT‐PCR | Upregulated | Positive | 0.0337 | [90] |
| CTHRC1 | Tissues from 40 patients | IHC; qRT‐PCR | Upregulated | Positive | 0.025 | [127] |
| CXCR4 | Tissues from 51 patients | IHC | Upregulated | Positive | 0.042 | [128] |
| FAS | Tissues from 162 patients | IHC | Upregulated | Negative | < 0.05 | [89] |
| HPGD | Tissues from 127 patients | IHC; RT‐PCR; Western blotting | Downregulated | Negative | 0.013 | [70] |
| IL‐13RA2 | Tissues from 236 patients | IHC; In situ hybridization | Upregulated | Positive | < 0.001 | [129] |
| LRP1 | Tissues from 478 patients | IHC; RT‐PCR; Western blotting | Upregulated | Positive | 0.001 | [130] |
| L1CAM | Tissue microarray containing 94 cases | IHC | Upregulated | Positive | 0.001 | [131] |
| MAP1LC3A/B | Tissues from 109 patients | IHC | Upregulated | Positive | < 0.05 | [77] |
| MDK | Tissues from 42 patients | IHC | Upregulated | Positive | 0.018 | [38] |
| MDK | Tissues from 114 patients | IHC; Western blotting | Upregulated | Positive | 0.03 | [132] |
| MYBL2 | Tissues from 93 patients | IHC | Upregulated | Positive | 0.013 | [133] |
| MYC | Tissues from 162 patients | IHC | Upregulated | Positive | < 0.0001 | [89] |
| PODXL | Tissues from 168 patients | IHC | Upregulated | Positive | 0.005 | [134] |
| POP1 | Tissues from 67 patients | IHC | Upregulated | Negative | < 0.05 | [135] |
| PTN | Tissues from 38 patients | IHC | Upregulated | Positive | 0.016 | [39] |
| PTN | Orthotopic tumor mouse model | IHC | Upregulated | Positive | 0.019 | [136] |
| SDC2 | Tissues from 42 patients; SUB8B8, T3M4, and PANC1 cells | Western blotting; qRT‐PCR | Upregulated | Positive | NA | [137] |
| SDC3 | Orthotopic tumor mouse model | IHC | Upregulated | Positive | 0.032 | [136] |
| SNCG | Tissues from 62 patients; PNI and orthotopic tumor mouse model | Proteomics; IHC | Upregulated | Positive | 0.009 | [92] |
| TIMP2 | Tissues from 51 patients | IHC | Upregulated | Positive | 0.042 | [128] |
| VGF | Five matched PNI and non‐PNI human cancer samples | Proteomics; IHC; Western blotting | Upregulated | Positive | 0.0086 | [12] |
| WASL | Tissues from 86 patients | IHC | Upregulated | Positive | 0.038 | [138] |
Abbreviations: BIRC5, baculoviral IAP repeat containing 5; CADM4, cell adhesion molecule 4; CCND1, cyclin D1; CDH1, cadherin 1; COL6A3, collagen type VI alpha 3 chain; CTHRC1, collagen triple helix repeat containing 1; CXCR4, C‐X‐C motif chemokine receptor 4; ELISA, enzyme‐linked immunosorbent assay; FAS, FAS cell surface death receptor; HPGD, 15‐hydroxyprostaglandin dehydrogenase; IHC, immunohistochemistry; IL‐13RA2, interleukin 13 receptor subunit alpha 2; LRP1, LDL receptor related protein 1; L1CAM, L1 cell adhesion molecule; MAP1LC3A/B, microtubule associated protein 1 light chain 3 alpha/beta; MDK, midkine; MYBL2, MYB proto‐oncogene like 2; MYC, MYC proto‐oncogene, BHLH transcription factor; PODXL, podocalyxin like; POP1, homolog, ribonuclease P/MRP subunit; PTN, pleiotrophin; QRT‐PCR, real‐time quantitative reverse transcription polymerase chain reaction; RT‐PCR, reverse transcription polymerase chain reaction; SDC2, syndecan 2; SDC3, syndecan 3; SNCG, synuclein gamma; TIMP2, TIMP metallopeptidase inhibitor 2; VGF, VGF, nerve growth factor inducible; WASL, WASP like action nucleation promoting factor; NA, not available.
In addition to researches involving traditional immunohistochemical staining, high‐throughput analysis has been applied to identify novel regulators of PNI. Through proteomic analysis of the nerves within PDAC tissues, compared with normal nerves, investigators found that invading the nerves had an increased VGF nerve growth factor inducible (VGF) level [12]. VGF is a secreted protein and neuropeptide precursor, which may play a role in maintaining energy homeostasis and neurite expansion [12]. By using a combination of proteomics and transcriptomics analysis, researchers showed that synuclein gamma (SNCG) had higher expression levels in a PNI group than that in a non‐PNI group [92]. Importantly, the overexpression of SNCG was an independent predictor of the OS of PDAC patients, whereas suppressing SNCG expression leaded to a significant decrease in the formation of PNI in preclinical animal models [92]. Since SNCG was overexpressed in various invasive and metastatic cancers [93, 94], it will be interesting to further evaluate its role in tumor metastasis caused by PNI.
5.2. Gene and noncoding RNA abnormalities
Gene alternations in human PDAC samples are also linked to PNI (Table 2). For example, Ras homolog family member C was abundantly expressed in PNI tissues and was related to poor disease prognosis [95]. The number of noncoding RNAs (such as microRNAs, piRNAs, and lncRNAs) within the human genome is unknown; however, recent transcriptomic studies suggest that some of them were significantly downregulated or upregulated during PNI in PDAC. For instance, the downregulation of MIR429 in tumor tissues inhibited neurotrophin 3 expression, thereby relieving PNI [96]. LncRNA, AFAP1 antisense RNA 1 was abundantly expressed in PDAC tissues and is positively associated with PNI [97]. However, the effect and mechanism of lncRNAs in PNI and PDAC have not been extensively characterized. An open question is how many transposable elements are reused within lncRNAs for PNI.
TABLE 2.
Gene and noncoding RNA abnormalities associated with PNI in PDAC
| Gene | Sample sources | Testing method(s) | Expression | Association with PNI | P value | Reference(s) |
|---|---|---|---|---|---|---|
| AFAP1‐AS1 | Tissues from 90 patients | RT‐PCR | Upregulated | Positive | 0.006 | [97] |
| ARHGDIB | PANC1, COLO357, and T3M4 cells | 51K human cDNA chips | Upregulated | Positive | NA | [88] |
| KIF14 | PANC1, COLO357, and T3M4 cells | 51K human cDNA chips | Downregulated | Negative | 0.05 | [88] |
| MIR429 | Tissues from 22 PDAC patients, 11 healthy controls | RT‐PCR | Downregulated | Negative | NA | [139] |
| RHOC | Tissues from 33 patients | RT‐PCR | Upregulated | Positive | NA | [95] |
| TMEM238L | Tissues from 22 PDAC patients, 11 healthy controls | RT‐PCR | Upregulated | Positive | 0.003 | [139] |
AFAP1‐AS1, AFAP1 antisense RNA 1; ARHGDIB, Rho GDP dissociation inhibitor beta; KIF14, kinesin family member 14; MIR429, microRNA 429; RHOC, Ras homolog family member C; RT‐PCR, reverse transcription polymerase chain reaction; TMEM238L, transmembrane protein 238 like; NA, not available.
6. CLINICAL SIGNIFICANCE OF PNI
6.1. Overall survival prediction
A number of retrospective studies have shown that the postoperative survival rate of PDAC patients was negatively correlated with nerve infiltration [10, 98‐101] (Table 3). One recent multicenter retrospective study enrolling 778 PDAC patients who received surgical treatment between 2009 and 2014 showed that the rate of PNI was 87% among these patients (T0/1, 61.6%; T2, 86.7%; T3/4, 90.1%; N0, 72.5%; and N1, 93.0%) [102]. PNI was an independent risk factor for disease‐free survival (DFS) and/or OS (P < 0.0001) [103], and could serve as a prognostic indicator [99, 104, 105]. Being PNI‐free was independently protective for long‐term survival (longer than 10 years) in PDAC patients [106]. Hence, the existence of PNI is one of the critical references to guide personalized antineoplastic therapeutic protocols [107].
TABLE 3.
Retrospective database analysis for PNI in PDAC
| Year | No. of patients | Analysis model(s) | Association with PNI | Prognosis significance | P value/OR | Reference(s) |
|---|---|---|---|---|---|---|
| 1996 | 113 (69.9%) |
Kaplan‐Meier analysis; Cox proportional hazards analysis |
Negative | Not an independent risk factor | 0.01 | [98] |
| 2002 | 24 (70.8%) |
Kaplan‐Meier analysis; Cox proportional hazards analysis |
Negative | NA | NA | [99] |
| 2006 | 56 (NA) |
Kaplan‐Meier analysis; Cox proportional hazards analysis |
Negative | Prognostic parameters | < 0.01 | [19] |
| 2007 | 33 (57.6%) | Kaplan‐Meier analysis | Negative | NA | 0.023 | [100] |
| 2007 | 75 (65.3%) |
Kaplan‐Meier analysis; Cox proportional hazards analysis |
Negative | Prognostic parameters | 0.034 | [10] |
| 2010 | 96 (53.1%) | Log‐rank test; Cox multi‐regression analysis | Negative | Prognostic parameters | 0.0001 | [101] |
| 2011 | 95 (88.0%) | Kaplan‐Meier analysis; univariate and multivariate Cox regression analysis | Negative | NA | 0.02 | [140] |
| 2012 | 212 (58%) |
Kaplan‐Meier analysis; Cox regression analysis; Cox proportional hazards models |
Negative | NA | 0.03 | [108] |
| 2015 | 209 (94%) |
Kaplan‐Meier analysis; Cox proportional hazards analysis |
Negative | Prognostic parameters | 0.0001 | [104] |
| 2016 | 173 (60.7%) | Multivariate Logistic Regression | Negative | Absence of PNI is independently associated with increased odds of long‐term survival | 0.036 | [106] |
| 2016 | 59 (50.8%) | Kaplan‐Meier analysis; multivariate Cox regression analysis | Negative | Prognostic parameters | 0.0009 | [109] |
| 2017 | 3538 (76.2‐97.8%) | Meta‐analysis | Negative | Prognostic parameters | < 0.00001 | [141] |
| 2019 | 17,313 (NA) | Meta‐analysis | Negative | Risk factors for recurrence | OR: 5·19, 2·79 to 9·64 | [110] |
| 2020 | 778 (87%) | Kaplan‐Meier analysis; multivariable Cox proportional hazards models | Negative | Prognostic parameters | 0.01 | [102] |
| 2020 | 400 (87.7%) | Kaplan‐Meier analysis; univariate and multivariate Cox regression analysis | Negative | Prognostic parameters | 0.05 | [103] |
The depth of nerve infiltration has also been shown to influence patients’ survival time. Patients with PNI had the shortest survival period (P = 0.034) [10]. For patients with tumor cells infiltrated beyond the axon of the nerves, DFS and OS were 13.4 months and 28.1 months, respectively [108]. In patients whose disease did not reach the axon, the DFS and OS were 32.9 months and 45.7 months, correspondingly [108]. Interestingly, a retrospective study of 59 PDAC patients showed that there was no parasympathetic nerve in normal pancreatic tissues, but parasympathetic nerves were abundant in tumor samples (50.8%) [109]. These changes in parasympathetic nerves acted as independent risk factors for poor prognosis [109]. Likewise, meta‐analysis data showed that PNI was obviously associated with DFS reduction (hazard ratio = 2.53; P = 0.0001) [110]. Not only DFS and OS but also the rate of peritoneal metastases (a sign of recurrence) have been shown to highly correlate with PNI [110]. These clinical studies support the oncogenic effects of PNI in the initiation and development of PDAC. Whether PNI can be used as an indicator of early diagnosis may depend on the development of more sensitive image detection technology.
6.2. Pain occurrence
In the late stage of disease, PDAC patients usually suffer severe pain due to tumor progression [16]. Considered as a negative prognostic factor for survival [111], severe pain not only greatly influences the quality of life for such patients but also may increase their risk of drug abuse. The invasion of tumor cells destroys the normal structure of the neural sheath and produces various molecules, contributing to neuropathic and inflammatory pain [6]. As mentioned earlier, GDNF contributed to the PNI process in PDAC patients [26]. Surprisingly, a rich expression of GDNF was closely related to the degree of back pain before surgery (P = 0.045) or 12 months after PDAC resection (P = 0.028) [26]. The accumulation of mast cells around the intrapancreatic nerves was also correlated with neuropathic pain [112]. Mechanically, NGF released by cancer cells stimulated the sensitive sensory nerves by interacting with transient receptor potential cation channel subfamily V member 1 (TRPV1), resulting in severe pain in PDAC patients [113, 114]. ARTN and GDNF secreted by tumor cells might upregulate TRPV1 expression, leading to more pain sensitivity [115].
In addition, hypoxic cancer cells secreted IL‐6 into the tumor microenvironment, leading to the growth and activation of Schwann cells [116]. The activated Schwann cells suppressed the signal transmission of spinal astroglia and microglia, which may be associated with the relief of pain during tumor initiation in KRASG12D‐driven PDAC mouse models [116]. Subsequent studies addressed the specific regulatory role of PNI in cancer‐associated pain through several animal models. For example, a PDAC orthotopic K8484 mouse model showed the main characteristics of human PDAC, including the structural remodeling of histopathology and nerve fibers [117]. In vivo methods to quantify pain or hypersensitivity are important to understanding the molecular mechanism of pain and its impact on the process of PDAC [117]. Of note, PHA‐848125, a dual inhibitor of cyclin‐dependent kinase and NTRK1, has been tested in preclinical trials to suppress PNI and alleviated pain generation in human PDAC cancer cells (BXPC3, MiaPACA2, and CAPAN1) in a xenograft mouse model [118].
6.3. Hyperglycemia management
Diabetes or hyperglycemia can lead to nerve damage. Several studies have investigated the clinical significance of poor blood glucose control and PNI in PDAC [119, 120]. First, through multivariate analysis, diabetes was proved to be an independent predictor of survival after resection in PDAC patients [121]. Second, pathological analysis confirmed the correlation between diabetes and PNI (P = 0.026) [121]. By comparing the tumor specimens of 61 PDAC patients with healthy controls, the frequency and severity of PNI in a hyperglycemia group were higher and heavier [119]. Third, both in vivo and in vitro experiments have shown that hyperglycemia can enhance the proliferation, NGF expression, neurotropism, and invasiveness of pancreatic cancer cells, thereby destroying the structure of the nerves [19, 120, 122]. These impaired nerves provide an avenue for tumor metastasis. Therefore, controlling blood glucose levels is very important for PDAC patients.
6.4. Treatment relevance
Traditionally, for PDAC patients who received neoadjuvant therapy, a PNI rate of 58% was reported, which was lower than that in those who did not receive neoadjuvant therapy (80%) (P = 0.002) [108]. Radiotherapy might also contribute to limiting PNI, because 4 Gy irradiation could remarkably reduce GDNF release in DRG, thereby inhibiting the invasion capacity of MiaPACA2 cells [123]. In vivo, 8 Gy radiotherapy was associated with a decrease in the secretion of GDNF from the sciatic nerves, which inhibited the frequency of PNI and protected sciatic nerve functions from destruction by cancer cells [123]. Sustained low‐dose irradiation from iodine‐125 seeds inhibited tumor growth and PNI formation in preclinical models [124]. Iodine‐125 seeds planted in the local sites of tumors even relieved pain in PDAC patients [124]. It has also been reported that telomerase‐specific oncolytic adenoviruses might have therapeutic potential for PDAC patients [125]. Moreover, these viruses repressed the migration and invasion of pancreatic cell lines in a DRG co‐culture system [125], indicating their potential role in preventing PNI. Overall, comprehensive multidisciplinary clinical trials are urgently needed to optimize the treatment of PDAC patients.
7. CONCLUSION AND PERSPECTIVES
In the past decade, we have witnessed progress in understanding the components and functions of the pancreatic tumor microenvironment, including the nerves. The nerves are involved in various tumor biological processes associated with the progression and recurrence of PDAC. In particular, PNI plays a complicated and unfavorable role in the prognosis of human PDAC, coupling various molecular, metabolic, and cellular components of the tumor microenvironment. However, preclinical animal studies have some contradictory or uncertain results on the effects of neurotransmitters or the vagus nerve on PDAC, which has led to concerns about the plasticity of PNI mechanisms and strategies to block the nervous system.
Some issues deserve further study. First, neurotransmitters are essential for many human physiological functions. Targeting neurotransmitters by neutralizing antibodies or compounds may cause undesirable or even toxic side effects. Second, the pancreatic tumor microenvironment is complex, composed of different systems and cell types. Although many studies have focused on the communication between tumors and the nervous system, research on the connection between the nervous system and other systems (e.g., immunity and microorganism) or conditions (e.g., hypoxia and oxidative stress) needs to be expanded. Third, PDAC relies on autophagy for the survival of cancer cells. How autophagy regulates the PNI process by degrading specific proteins remains an open question. Fourth, the neuronal and molecular machinery underlying pain transitions deserve further comprehensive study, as this may help early diagnosis or improve the quality of life of patients with advanced cancer. Collectively, identifying specific molecular targets or exploring new ways to inhibit the progression of PNI and other neurological abnormalities may have a potential impact on PDAC treatment.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All authors wrote, revised, and approved the manuscript.
ACKNOWLEDGMENTS
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript.
Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 2021;41:642–660. 10.1002/cac2.12188
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 2.Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70(5):375‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 4.Demir IE, Ceyhan GO, Liebl F, D'Haese JG, Maak M, Friess H. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010;2(3):1513‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379‐91. [DOI] [PubMed] [Google Scholar]
- 6.Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12(11):649‐59. [DOI] [PubMed] [Google Scholar]
- 7.Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demir IE, Schafer KH, Tieftrunk E, Friess H, Ceyhan GO. Neural plasticity in the gastrointestinal tract: chronic inflammation, neurotrophic signals, and hypersensitivity. Acta Neuropathol. 2013;125(4):491‐509. [DOI] [PubMed] [Google Scholar]
- 9.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113(11):3078‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31(11):1636‐44. [DOI] [PubMed] [Google Scholar]
- 11.Bockman DE, Buchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107(1):219‐30. [DOI] [PubMed] [Google Scholar]
- 12.Alrawashdeh W, Jones R, Dumartin L, Radon TP, Cutillas PR, Feakins RM, et al. Perineural invasion in pancreatic cancer: proteomic analysis and in vitro modelling. Mol Oncol. 2019;13(5):1075‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demir IE, Ceyhan GO, Rauch U, Altintas B, Klotz M, Muller MW, et al. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil. 2010;22(4):480‐90, e112‐3. [DOI] [PubMed] [Google Scholar]
- 14.Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, Doglioni C, et al. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers (Basel). 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan X, Sivakumar S, Bednarsch J, Wiltberger G, Kather JN, Niehues J, et al. Nerve fibers in the tumor microenvironment in neurotropic cancer‐pancreatic cancer and cholangiocarcinoma. Oncogene. 2021;40(5):899‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695‐707. [DOI] [PubMed] [Google Scholar]
- 17.Miknyoczki SJ, Lang D, Huang L, Klein‐Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81(3):417‐27. [DOI] [PubMed] [Google Scholar]
- 18.Ketterer K, Rao S, Friess H, Weiss J, Buchler MW, Korc M. Reverse transcription‐PCR analysis of laser‐captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9(14):5127‐36. [PubMed] [Google Scholar]
- 19.Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006;21(5):850‐8. [DOI] [PubMed] [Google Scholar]
- 20.Banh RS, Biancur DE, Yamamoto K, Sohn ASW, Walters B, Kuljanin M, et al. Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer. Cell. 2020;183(5):1202‐18 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saloman JL, Singhi AD, Hartman DJ, Normolle DP, Albers KM, Davis BM. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas. 2018;47(7):856‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gohrig A, Detjen KM, Hilfenhaus G, Korner JL, Welzel M, Arsenic R, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74(5):1529‐40. [DOI] [PubMed] [Google Scholar]
- 23.Secq V, Leca J, Bressy C, Guillaumond F, Skrobuk P, Nigri J, et al. Stromal SLIT2 impacts on pancreatic cancer‐associated neural remodeling. Cell Death Dis. 2015;6:e1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383‐94. [DOI] [PubMed] [Google Scholar]
- 25.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line‐derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130‐2. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, et al. The relationship between overexpression of glial cell‐derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36(4):656‐64. [DOI] [PubMed] [Google Scholar]
- 27.Cintron‐Colon AF, Almeida‐Alves G, Boynton AM, Spitsbergen JM. GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020;382(1):47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada Y, Takeyama H, Sato M, Morikawa M, Sobue K, Asai K, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic‐cancer cells bearing c‐ret proto‐oncogene with reference to glial‐cell‐line‐derived neurotrophic factor (GDNF). Int J Cancer. 1999;81(1):67‐73. [DOI] [PubMed] [Google Scholar]
- 29.Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, et al. Activation of phosphatidylinositol 3‐kinase and extracellular signal‐regulated kinase is required for glial cell line‐derived neurotrophic factor‐induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64(15):5291‐300. [DOI] [PubMed] [Google Scholar]
- 30.Tahira T, Ishizaka Y, Itoh F, Sugimura T, Nagao M. Characterization of ret proto‐oncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene. 1990;5(1):97‐102. [PubMed] [Google Scholar]
- 31.Lian EY, Hyndman BD, Moodley S, Maritan SM, Mulligan LM. RET isoforms contribute differentially to invasive processes in pancreatic ductal adenocarcinoma. Oncogene. 2020;39(41):6493‐510. [DOI] [PubMed] [Google Scholar]
- 32.He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, et al. GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF‐RET signaling. Proc Natl Acad Sci U S A. 2014;111(19):E2008‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244(2):274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao L, Bo H, Wang Y, Zhang J, Zhu M. Neurotrophic Factor Artemin Promotes Invasiveness and Neurotrophic Function of Pancreatic Adenocarcinoma In Vivo and In Vitro. Pancreas. 2015;44(1):134‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204(2):127‐43. [DOI] [PubMed] [Google Scholar]
- 36.Ohhashi S, Ohuchida K, Mizumoto K, Egami T, Yu J, Cui L, et al. Midkine mRNA is overexpressed in pancreatic cancer. Dig Dis Sci. 2009;54(4):811‐5. [DOI] [PubMed] [Google Scholar]
- 37.Maeda S, Shinchi H, Kurahara H, Mataki Y, Noma H, Maemura K, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97(3):405‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao J, Li WY, Li SG, Feng XS, Gao SG. Midkine promotes perineural invasion in human pancreatic cancer. World J Gastroenterol. 2014;20(11):3018‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao J, Ma Q, Wang L, Zhang M. Pleiotrophin expression in human pancreatic cancer and its correlation with clinicopathological features, perineural invasion, and prognosis. Dig Dis Sci. 2009;54(4):895‐901. [DOI] [PubMed] [Google Scholar]
- 40.Yao J, Hu XF, Feng XS, Gao SG. Pleiotrophin promotes perineural invasion in pancreatic cancer. World J Gastroenterol. 2013;19(39):6555‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurcak NR, Rucki AA, Muth S, Thompson E, Sharma R, Ding D, et al. Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice. Gastroenterology. 2019;157(3):838‐50 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68(21):9060‐9. [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Wang Z, Chen X, Duan W, Lei J, Zong L, et al. Stromal‐derived factor‐1alpha/CXCL12‐CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer. Oncotarget. 2015;6(7):4717‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nan L, Qin T, Xiao Y, Qian W, Li J, Wang Z, et al. Pancreatic Stellate Cells Facilitate Perineural Invasion of Pancreatic Cancer via HGF/c‐Met Pathway. Cell Transplant. 2019;28(9‐10):1289‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo K, Ma Q, Li J, Wang Z, Shan T, Li W, et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12(3):264‐73. [DOI] [PubMed] [Google Scholar]
- 47.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS‐induced pancreatic tumorigenesis. Cancer Res. 2011;71(14):5020‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koperek O, Aumayr K, Schindl M, Werba G, Soleiman A, Schoppmann S, et al. Phosphorylation of STAT3 correlates with HER2 status, but not with survival in pancreatic ductal adenocarcinoma. APMIS. 2014;122(6):476‐81. [DOI] [PubMed] [Google Scholar]
- 49.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. beta2 Adrenergic‐Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell. 2018;33(1):75‐90 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter‐receptor for myelin‐associated glycoprotein (Siglec‐4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67(21):10222‐9. [DOI] [PubMed] [Google Scholar]
- 51.Schwann Cells Promote Cancer Cell Invasion. Cancer Discov. 2016;6(5):473. [DOI] [PubMed] [Google Scholar]
- 52.Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126(4):1538‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Na'ara S, Amit M, Gil Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene. 2019;38(4):596‐608. [DOI] [PubMed] [Google Scholar]
- 54.Roger E, Martel S, Bertrand‐Chapel A, Depollier A, Chuvin N, Pommier RM, et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFbeta signaling. Cell Death Dis. 2019;10(12):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed S, Bradshaw AD, Gera S, Dewan MZ, Xu R. The TGF‐beta/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J Clin Med. 2017;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javle M, Li Y, Tan D, Dong X, Chang P, Kar S, et al. Biomarkers of TGF‐beta signaling pathway and prognosis of pancreatic cancer. PLoS One. 2014;9(1):e85942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakst RL, Xiong H, Chen CH, Deborde S, Lyubchik A, Zhou Y, et al. Inflammatory Monocytes Promote Perineural Invasion via CCL2‐Mediated Recruitment and Cathepsin B Expression. Cancer Res. 2017;77(22):6400‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bressy C, Lac S, Nigri J, Leca J, Roques J, Lavaut MN, et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res. 2018;78(4):909‐21. [DOI] [PubMed] [Google Scholar]
- 59.Sperb N, Tsesmelis M, Wirth T. Crosstalk between Tumor and Stromal Cells in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2020;21(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Jia R, Zhao T, Li X, Lang M, Lan C, et al. HIF‐1alpha mediates tumor‐nerve interactions through the up‐regulation of GM‐CSF in pancreatic ductal adenocarcinoma. Cancer Lett. 2019;453:10‐20. [DOI] [PubMed] [Google Scholar]
- 61.Pylayeva‐Gupta Y, Lee KE, Hajdu CH, Miller G, Bar‐Sagi D. Oncogenic Kras‐induced GM‐CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21(6):836‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y, Zhang C, Jiang K, Werner J, Bazhin AV, D'Haese JG. The Role of Stellate Cells in Pancreatic Ductal Adenocarcinoma: Targeting Perspectives. Front Oncol. 2020;10:621937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang D, Zhang J, Yuan Z, Gao J, Wang S, Ye N, et al. Pancreatic satellite cells derived galectin‐1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS One. 2014;9(3):e90476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuhashi S, Sakaguchi T, Murakami T, Fukushima M, Morita Y, Ikegami K, et al. Tenascin C in the Tumor‐Nerve Microenvironment Enhances Perineural Invasion and Correlates With Locoregional Recurrence in Pancreatic Ductal Adenocarcinoma. Pancreas. 2020;49(3):442‐54. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res. 2014;20(16):4326‐38. [DOI] [PubMed] [Google Scholar]
- 66.Yamada M, Hirabayashi K, Kawanishi A, Hadano A, Takanashi Y, Izumi H, et al. Nectin‐1 expression in cancer‐associated fibroblasts is a predictor of poor prognosis for pancreatic ductal adenocarcinoma. Surg Today. 2018;48(5):510‐6. [DOI] [PubMed] [Google Scholar]
- 67.Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18(8):840‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng L, Guo Y, Liang J, Chen S, Peng P, Zhang Q, et al. Perineural Invasion and TAMs in Pancreatic Ductal Adenocarcinomas: Review of the Original Pathology Reports Using Immunohistochemical Enhancement and Relationships with Clinicopathological Features. J Cancer. 2014;5(9):754‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arima K, Komohara Y, Bu L, Tsukamoto M, Itoyama R, Miyake K, et al. Downregulation of 15‐hydroxyprostaglandin dehydrogenase by interleukin‐1beta from activated macrophages leads to poor prognosis in pancreatic cancer. Cancer Sci. 2018;109(2):462‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavel O, Shomron O, Shabtay A, Vital J, Trejo‐Leider L, Weizman N, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72(22):5733‐43. [DOI] [PubMed] [Google Scholar]
- 72.Huang C, Li Y, Guo Y, Zhang Z, Lian G, Chen Y, et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics. 2018;8(11):3074‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang MW, Tao LY, Jiang YS, Yang JY, Huo YM, Liu DJ, et al. Perineural Invasion Reprograms the Immune Microenvironment through Cholinergic Signaling in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020;80(10):1991‐2003. [DOI] [PubMed] [Google Scholar]
- 74.Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018;8(11):1458‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Chen X, Kang R, Zeh H, Klionsky DJ, Tang D. Regulation and function of autophagy in pancreatic cancer. Autophagy. 2020:1‐22. 10.1080/15548627.2020.1847462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang YH, Liu JB, Gui Y, Lei LL, Zhang SJ. Relationship between autophagy and perineural invasion, clinicopathological features, and prognosis in pancreatic cancer. World J Gastroenterol. 2017;23(40):7232‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang YH, Zhang YX, Gui Y, Liu JB, Sun JJ, Fan H. Analysis of the autophagy gene expression profile of pancreatic cancer based on autophagy‐related protein microtubule‐associated protein 1A/1B‐light chain 3. World J Gastroenterol. 2019;25(17):2086‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boone BA, Bahary N, Zureikat AH, Moser AJ, Normolle DP, Wu WC, et al. Safety and Biologic Response of Pre‐operative Autophagy Inhibition in Combination with Gemcitabine in Patients with Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22(13):4402‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeh H, Bahary N, Boone BA, Singhi AD, Miller‐Ocuin JL, Normolle DP, et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition With High‐Dose Hydroxychloroquine and Gemcitabine/Nab‐Paclitaxel in Pancreatic Cancer Patients. Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karasic TB, O'Hara MH, Loaiza‐Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, et al. Effect of Gemcitabine and nab‐Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(7):993‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist. 2014;19(6):637‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12(8):2419‐26. [DOI] [PubMed] [Google Scholar]
- 84.Zhang JF, Hua R, Liu DJ, Liu W, Huo YM, Sun YW. Effect of CD74 on the prognosis of patients with resectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2014;13(1):81‐6. [DOI] [PubMed] [Google Scholar]
- 85.Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, et al. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol. 2009;16(9):2531‐8. [DOI] [PubMed] [Google Scholar]
- 86.Torer N, Kayaselcuk F, Nursal TZ, Yildirim S, Tarim A, Noyan T, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol. 2007;96(5):419‐23. [DOI] [PubMed] [Google Scholar]
- 87.Lebe B, Sagol O, Ulukus C, Coker A, Karademir S, Astarcioglu H, et al. The importance of cyclin D1 and Ki67 expression on the biological behavior of pancreatic adenocarcinomas. Pathol Res Pract. 2004;200(5):389‐96. [DOI] [PubMed] [Google Scholar]
- 88.Abiatari I, DeOliveira T, Kerkadze V, Schwager C, Esposito I, Giese NA, et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther. 2009;8(6):1494‐504. [DOI] [PubMed] [Google Scholar]
- 89.He C, Jiang H, Geng S, Sheng H, Shen X, Zhang X, et al. Expression of c‐Myc and Fas correlates with perineural invasion of pancreatic cancer. Int J Clin Exp Pathol. 2012;5(4):339‐46. [PMC free article] [PubMed] [Google Scholar]
- 90.Kang CY, Wang J, Axell‐House D, Soni P, Chu ML, Chipitsyna G, et al. Clinical significance of serum COL6A3 in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2014;18(1):7‐15. [DOI] [PubMed] [Google Scholar]
- 91.Ren YQ, Zhang HY, Su T, Wang XH, Zhang L. Clinical significance of serum survivin in patients with pancreatic ductal adenocarcinoma. Eur Rev Med Pharmacol Sci. 2014;18(20):3063‐8. [PubMed] [Google Scholar]
- 92.Hibi T, Mori T, Fukuma M, Yamazaki K, Hashiguchi A, Yamada T, et al. Synuclein‐gamma is closely involved in perineural invasion and distant metastasis in mouse models and is a novel prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(8):2864‐71. [DOI] [PubMed] [Google Scholar]
- 93.Inaba S, Li C, Shi YE, Song DQ, Jiang JD, Liu J. Synuclein gamma inhibits the mitotic checkpoint function and promotes chromosomal instability of breast cancer cells. Breast Cancer Res Treat. 2005;94(1):25‐35. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J, Liu XH, Li C, Wu XX, Chen YL, Li WW, et al. SNCG promotes the progression and metastasis of high‐grade serous ovarian cancer via targeting the PI3K/AKT signaling pathway. J Exp Clin Cancer Res. 2020;39(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77(1):147‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu D, Song L, Dai Z, Guan H, Kang H, Zhang Y, et al. MiR‐429 suppresses neurotrophin‐3 to alleviate perineural invasion of pancreatic cancer. Biochem Biophys Res Commun. 2018;505(4):1077‐83. [DOI] [PubMed] [Google Scholar]
- 97.Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang Y, et al. High expression of AFAP1‐AS1 is associated with poor survival and short‐term recurrence in pancreatic ductal adenocarcinoma. J Transl Med. 2015;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83(5):625‐31. [DOI] [PubMed] [Google Scholar]
- 99.Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, et al. Perineural invasion in pancreatic cancer. Pancreas. 2002;24(1):15‐25. [DOI] [PubMed] [Google Scholar]
- 100.Garcea G, Dennison AR, Ong SL, Pattenden CJ, Neal CP, Sutton CD, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33(7):892‐7. [DOI] [PubMed] [Google Scholar]
- 101.Chen JW, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke‐Smith M, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford). 2010;12(2):101‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, Di Salvo F, et al. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 103.Li B, Wang Y, Jiang H, Li B, Shi X, Gao S, et al. Pros and Cons: High Proportion of Stromal Component Indicates Better Prognosis in Patients With Pancreatic Ductal Adenocarcinoma‐A Research Based on the Evaluation of Whole‐Mount Histological Slides. Front Oncol. 2020;10:1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kondo N, Murakami Y, Uemura K, Hashimoto Y, Nakagawa N, Sasaki H, et al. An Increased Number of Perineural Invasions Is Independently Associated With Poor Survival of Patients With Resectable Pancreatic Ductal Adenocarcinoma. Pancreas. 2015;44(8):1345‐51. [DOI] [PubMed] [Google Scholar]
- 105.Zhou W, Jin W, Wang D, Lu C, Xu X, Zhang R, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a propensity score matching analysis. Cancer Commun (Lond). 2019;39(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stark AP, Sacks GD, Rochefort MM, Donahue TR, Reber HA, Tomlinson JS, et al. Long‐term survival in patients with pancreatic ductal adenocarcinoma. Surgery. 2016;159(6):1520‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kadera BE, Sunjaya DB, Isacoff WH, Li L, Hines OJ, Tomlinson JS, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph‐node negativity and overall survival. JAMA Surg. 2014;149(2):145‐53. [DOI] [PubMed] [Google Scholar]
- 108.Chatterjee D, Katz MH, Rashid A, Wang H, Iuga AC, Varadhachary GR, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36(3):409‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L, Guo L, Tao M, Fu W, Xiu D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin J Cancer Res. 2016;28(2):180‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanaka M, Mihaljevic AL, Probst P, Heckler M, Klaiber U, Heger U, et al. Meta‐analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106(12):1590‐601. [DOI] [PubMed] [Google Scholar]
- 111.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, et al. Pancreatic neuropathy and neuropathic pain–a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(1):177‐86 e1. [DOI] [PubMed] [Google Scholar]
- 112.Demir IE, Schorn S, Schremmer‐Danninger E, Wang K, Kehl T, Giese NA, et al. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One. 2013;8(3):e60529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li C, et al. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology. 2011;141(1):370‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, et al. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55(4):519‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line‐derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S, et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016;65(6):1001‐14. [DOI] [PubMed] [Google Scholar]
- 117.Selvaraj D, Hirth M, Gandla J, Kuner R. A mouse model for pain and neuroplastic changes associated with pancreatic ductal adenocarcinoma. Pain. 2017;158(8):1609‐21. [DOI] [PubMed] [Google Scholar]
- 118.Albanese C, Alzani R, Amboldi N, Avanzi N, Ballinari D, Brasca MG, et al. Dual targeting of CDK and tropomyosin receptor kinase families by the oral inhibitor PHA‐848125, an agent with broad‐spectrum antitumor efficacy. Mol Cancer Ther. 2010;9(8):2243‐54. [DOI] [PubMed] [Google Scholar]
- 119.Li J, Ma Q, Liu H, Guo K, Li F, Li W, et al. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6(2):e17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Ma J, Han L, Xu Q, Lei J, Duan W, et al. Hyperglycemic tumor microenvironment induces perineural invasion in pancreatic cancer. Cancer Biol Ther. 2015;16(6):912‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hank T, Sandini M, Qadan M, Weniger M, Ciprani D, Li A, et al. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long‐term survival in resected pancreatic cancer. Pancreatology. 2020;20(1):125‐31. [DOI] [PubMed] [Google Scholar]
- 122.Larrieta ME, Vital P, Mendoza‐Rodriguez A, Cerbon M, Hiriart M. Nerve growth factor increases in pancreatic beta cells after streptozotocin‐induced damage in rats. Exp Biol Med (Maywood). 2006;231(4):396‐402. [DOI] [PubMed] [Google Scholar]
- 123.Bakst RL, Lee N, He S, Chernichenko N, Chen CH, Linkov G, et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS One. 2012;7(6):e39925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu Z, Dong TH, Si PR, Shen W, Bi YL, Min M, et al. Continuous Low‐dose‐rate Irradiation of Iodine‐125 Seeds Inhibiting Perineural Invasion in Pancreatic Cancer. Chin Med J (Engl). 2016;129(20):2460‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koujima T, Tazawa H, Ieda T, Araki H, Fushimi T, Shoji R, et al. Oncolytic Virus‐Mediated Targeting of the ERK Signaling Pathway Inhibits Invasive Propensity in Human Pancreatic Cancer. Mol Ther Oncolytics. 2020;17:107‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawanishi A, Hirabayashi K, Yamada M, Takanashi Y, Hadano A, Kawaguchi Y, et al. Clinicopathological significance of Necl‐4 expression in pancreatic ductal adenocarcinoma. J Clin Pathol. 2017;70(7):619‐24. [DOI] [PubMed] [Google Scholar]
- 127.Liu W, Fu XL, Yang JY, Yang MW, Tao LY, Liu DJ, et al. Elevated expression of CTHRC1 predicts unfavorable prognosis in patients with pancreatic ductal adenocarcinoma. Am J Cancer Res. 2016;6(8):1820‐7. [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang YM, Li G, Sun BC, Zhao XL, Zhou ZK. Study on the relationship between CXCR4 expression and perineural invasion in pancreatic cancer. Asian Pac J Cancer Prev. 2014;15(12):4893‐6. [DOI] [PubMed] [Google Scholar]
- 129.Fujisawa T, Shimamura T, Goto K, Nakagawa R, Muroyama R, Ino Y, et al. A Novel Role of Interleukin 13 Receptor alpha2 in Perineural Invasion and its Association with Poor Prognosis of Patients with Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2020;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gheysarzadeh A, Ansari A, Emami MH, Razavi AE, Mofid MR. Over‐expression of low‐density lipoprotein receptor‐related Protein‐1 is associated with poor prognosis and invasion in pancreatic ductal adenocarcinoma. Pancreatology. 2019;19(3):429‐35. [DOI] [PubMed] [Google Scholar]
- 131.Ben QW, Wang JC, Liu J, Zhu Y, Yuan F, Yao WY, et al. Positive expression of L1‐CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2010;17(8):2213‐21. [DOI] [PubMed] [Google Scholar]
- 132.Petrusel L, Rusu I, Leucuta DC, Seicean R, Suharoschi R, Zamfir P, et al. Relationship between cachexia and perineural invasion in pancreatic adenocarcinoma. World J Gastrointest Oncol. 2019;11(12):1126‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu R, Li C, Lin X, Chen Q, Li J, Song L, et al. Clinicopathologic features and prognostic implications of MYBL2 protein expression in pancreatic ductal adenocarcinoma. Pathol Res Pract. 2017;213(8):964‐8. [DOI] [PubMed] [Google Scholar]
- 134.Saukkonen K, Hagstrom J, Mustonen H, Juuti A, Nordling S, Fermer C, et al. Podocalyxin Is a Marker of Poor Prognosis in Pancreatic Ductal Adenocarcinoma. PLoS One. 2015;10(6):e0129012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee SH, Kim H, Hwang JH, Lee HS, Cho JY, Yoon YS, et al. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathol Int. 2012;62(3):167‐75. [DOI] [PubMed] [Google Scholar]
- 136.Yao J, Zhang LL, Huang XM, Li WY, Gao SG. Pleiotrophin and N‐syndecan promote perineural invasion and tumor progression in an orthotopic mouse model of pancreatic cancer. World J Gastroenterol. 2017;23(21):3907‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Oliveira T, Abiatari I, Raulefs S, Sauliunaite D, Erkan M, Kong B, et al. Syndecan‐2 promotes perineural invasion and cooperates with K‐ras to induce an invasive pancreatic cancer cell phenotype. Mol Cancer. 2012;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo JC, Li J, Zhao YP, Zhou L, Cui QC, Zhou WX, et al. N‐wasp in pancreatic ductal adenocarcinoma: associations with perineural invasion and poor prognosis. World J Surg. 2014;38(8):2126‐31. [DOI] [PubMed] [Google Scholar]
- 139.Li DD, Fu ZQ, Lin Q, Zhou Y, Zhou QB, Li ZH, et al. Linc00675 is a novel marker of short survival and recurrence in patients with pancreatic ductal adenocarcinoma. World J Gastroenterol. 2015;21(31):9348‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maithel SK, Coban I, Kneuertz PJ, Kooby DA, El‐Rayes BF, Kauh JS, et al. Differential expression of ERCC1 in pancreas adenocarcinoma: high tumor expression is associated with earlier recurrence and shortened survival after resection. Ann Surg Oncol. 2011;18(9):2699‐705. [DOI] [PubMed] [Google Scholar]
- 141.Schorn S, Demir IE, Haller B, Scheufele F, Reyes CM, Tieftrunk E, et al. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma ‐ A systematic review and meta‐analysis. Surg Oncol. 2017;26(1):105‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


