Supplemental digital content is available in the text.
Key Words: TYPE I COLLAGEN, GENE VARIANT, BMD, MUSCLE STIFFNESS, SPORTS INJURY
ABSTRACT
Purpose
We aimed to investigate the hypothesis that type I collagen plays a role in increasing bone mineral density (BMD) and muscle stiffness, leading to low and high risks of fatigue fracture and muscle injury, respectively, in athletes. As a potential mechanism, we focused on the effect of the type I collagen alpha 1 chain gene (COL1A1) variant associated with transcriptional activity on bone and skeletal muscle properties.
Methods
The association between COL1A1 rs1107946 and fatigue fracture/muscle injury was evaluated in Japanese athletes. Effects of the polymorphism on tissue properties (BMD and muscle stiffness) and type I collagen α1/α2 chain ratios in muscles were examined in Japanese nonathletes.
Results
The C-allele carrier frequency was greater in female athletes with fatigue fracture than in those without (odds ratio = 2.44, 95% confidence interval [CI] = 1.17–5.77) and lower in female athletes with muscle injury than in those without (odds ratio = 0.46, 95% CI = 0.24–0.91). Prospective validation analysis confirmed that in female athletes, muscle injury was less frequent in C-allele carriers than in AA genotype carriers (multivariable-adjusted hazard ratio = 0.27, 95% CI = 0.08–0.96). Among female nonathletes, the C-allele of rs1107946 was associated with lower BMD and lower muscle stiffness. Muscle biopsy revealed that C-allele carriers tended to have a larger type I collagen α1/α2 chain ratio than AA genotype carriers (2.24 vs 2.05, P = 0.056), suggesting a higher proportion of type I collagen α1 homotrimers.
Conclusion
The COL1A1 rs1107946 polymorphism exerts antagonistic effects on fatigue fracture and muscle injury among female athletes by altering the properties of these tissues, potentially owing to increased levels of type I collagen α1 chain homotrimers.
Despite preventive efforts, many athletes face sports-related injuries. For example, 8% of the athletes in the Rio de Janeiro 2016 Olympic Games incurred at least one injury, with skeletal muscle, ligament, and bone injuries constituting the highest number of severe injuries (1). Although these injuries have been well described at the clinical level, an understanding of the biological mechanisms underlying susceptibilities to these injuries will contribute to the development of models to effectively identify injury risk and personalized prevention programs. Particularly, tissue properties can be associated with the risk of sports-related injuries, such as low bone mineral density (BMD) in fatigue fracture (2,3) and high skeletal muscle stiffness in muscle injury (4,5). Such tissue properties (4,6,7) and the incidence of sport-related injuries (8,9) are reportedly influenced by genetic factors. Although a hypothesis that genetic factors influence the incidence of sports-related injuries by altering tissue properties has been proposed (10), it has not been experimentally demonstrated.
Collagens are the most abundant proteins in mammals, constituting up to 30% of the total protein mass (11). Among the 28 types within the human collagen superfamily, type I is the most abundant protein constituent of the bone, with genes encoding type I collagen being considered as candidates associated with BMD (12). In comparison, muscle stiffness is influenced by intramuscular connective tissues such as perimysium and endomysium (13), which contain type I collagen as the major component (14). Collectively, genetic variants in the type I collagen genes represent likely candidates for affecting the susceptibility to fatigue fracture and muscle injury. Frequently studied polymorphisms in the type I collagen alpha 1 gene (COL1A1) include −1997A/C (rs1107946), −1663IndelA (rs11327935), and +1245C/A (rs1800012). These polymorphisms are located in the promoter and intron 1 regions of COL1A1 and are associated with the COL1A1 transcriptional activity by altering DNA–transcription factor interactions (15,16). Specifically, the COL1A1 C-del-A (rs1107946; rs11327935; rs1800012) haplotype showed increased transcriptional activity compared with the other haplotypes (16). These polymorphisms are associated with ligament and tendon injuries that are often sports-related (17,18). Considering type I collagen functions in the bone (19), increase in the production of type I collagen would result in an increase in bone strength and BMD, which in turn is associated with reduced risk of fatigue fracture (2,3). On the contrary, increased type I collagen in intramuscular connective tissues, such as perimysium and endomysium, would induce greater stiffness of the skeletal muscle (13) and is therefore considered to be associated with a greater risk of muscle injury (4,5). Accordingly, we hypothesized that the COL1A1 polymorphism-related transcriptional activity has opposite genotypic effects on risks of fatigue fracture and muscle injury. However, because rs11327935 and rs1800012 are nonpolymorphic in the Japanese population according to the Japanese Multi Omics Reference Panel (https://jmorp.megabank.tohoku.ac.jp/202001/variants), in the present study, we focused on the rs1107946 polymorphism within the COL1A1 promoter region.
To test the hypothesis, we determined the association of the COL1A1 rs1107946 A/C polymorphism with susceptibilities to fatigue fracture and muscle injury in athletes. We also assessed the effects of this polymorphism on the properties of bone and muscle and the potential contribution of type I collagen formation. Together, this information may lead to a reduction of sport-related injuries by identifying injury risk and developing effective injury prevention programs with particular focus on tissue properties.
METHODS
Study design
Associations of the COL1A1 rs1107946 polymorphism with fatigue fracture and muscle injury were examined in 1667 Japanese athletes from the Japanese Human Athlome Project (J-HAP) (stage 1 analyses) (20). To confirm our results, validation analyses were performed in 508 Japanese athletes from the Juntendo Fitness Plus (J-Fit+) study (stage 2 analyses). The effects of the rs1107946 polymorphism on tissue properties (BMD, n = 905; muscle stiffness, n = 250), collagen metabolism marker in serum (n = 133), type I collagen α1/α2 chain ratio, and COL1A1/COL1A2 mRNA expression in skeletal muscle (n = 23) were also examined in Japanese nonathlete populations.
Stage 1 analyses of sports-related injuries in J-HAP
Subjects for stage 1 analyses comprised 1667 Japanese athletes majoring in various sports from the J-HAP. J-HAP was part of the “Athlome Project Consortium” (20). These athletes were recruited from March 2015 to November 2017. The detailed selection process of subjects of stage 1 analyses is shown in a flow diagram (see Figure, Supplemental Digital Content 1, http://links.lww.com/MSS/C291). The final number of participants in the case–control association analyses for fatigue fracture and muscle injury was (i) 216 athletes with fatigue fracture and 1420 athletes without fatigue fracture and (ii) 191 athletes with muscle injury and 1373 athletes without muscle injury, respectively. In J-HAP, the history of up to three sports-related injuries in the descending order of severity was assessed using a questionnaire as described previously (4,21). A questionnaire was designed and administered on the basis of the consensus statement made available by the Fédération Internationale de Football Association (FIFA) (22). In the questionnaire, we asked the following details pertaining to each injury: injured body part, type of injury, cause of injury, when the injury occurred, time loss due to the injury, number of injuries of the same type at the same site, and whether a medical practitioner had diagnosed the injury or not. Information pertaining to the main sport, competitive level, and playing years was also obtained using the questionnaire. Only female athletes were asked about their menstrual status, with amenorrhea and oligomenorrhea being regarded as “irregular menstruation.” Case–control association analyses for fatigue fracture and muscle injury were performed within the J-HAP cohort. In the analyses, the case group included only athletes with noncontact injuries diagnosed by medical practitioners (i.e., athletes with contact injury or injury not diagnosed by medical practitioners were excluded). Characteristics of athletes, including case–control analyses of fatigue fracture and muscle injury, are shown in Supplemental Digital Contents 2 and 3, respectively, http://links.lww.com/MSS/C292 and http://links.lww.com/MSS/C293. Briefly, the fatigue fracture group showed a greater proportion of female and track and field athletes than the group with no-fatigue fracture (see Table, Supplemental Digital Content 2, http://links.lww.com/MSS/C292). Moreover, the muscle injury group exhibited a greater proportion of track and field athletes and longer playing years in main sports than the group with no muscle injury (see Table, Supplemental Digital Content 3, http://links.lww.com/MSS/C293). Written consent was obtained from each participant. The procedure was approved by the Ethics Committees of Juntendo University, Nippon Sport Science University, and Tenri University and performed in accordance with the Declaration of Helsinki.
Stage 2 analysis of sports-related injuries in the J-Fit+ study
In 508 Japanese college athletes (379 males, 129 females) from various sports, fatigue fracture and muscle injury occurrences in 2 yr (November 2017 to October 2019) were investigated using a questionnaire. The cause of injury, month of occurrence, and whether the injury was diagnosed by a doctor were also assessed. Furthermore, in the questionnaire, main sports, training exposure hours per day, and training frequencies per week were evaluated. The investigations were conducted once annually at the end of October (i.e., October 2018 and October 2019). Based on the information regarding injury month, training exposure hours per day, and training frequencies per week, we calculated training exposure hours to injury occurrence. As in stage 1 analyses, athletes with contact injury or injury not diagnosed by medical practitioners were excluded. The number of subjects at each stage is shown in a flow diagram (see Figure, Supplemental Digital Content 4, http://links.lww.com/MSS/C294). This study was part of the J-Fit+ study (https://www.juntendo.ac.jp/jfit/en/). Written consent was obtained from each participant. The study was approved by the Ethics Committee of Juntendo University and performed in accordance with the Declaration of Helsinki.
Analysis of BMD
The association of the COL1A1 polymorphism with BMD was examined in 905 Japanese individuals (610 males, 295 females) from Waseda Alumni’s Sports, Exercise, Daily Activity, Sedentariness, and Health (WASEDA’S Health) Study, the design of which was described previously (23). Whole-body BMD in all subjects and lumbar spine BMD in female subjects were measured using dual-energy x-ray absorptiometry (Delphi A, Hologic, Bedford, MA, or Horizon A, Hologic, Marlborough, MA). Written consent was obtained from each participant. The study was approved by the Ethics Committees of Waseda University and Juntendo University and performed in accordance with the Declaration of Helsinki.
Meta-analysis of BMD in Asian postmenopausal female subjects
Because a recent meta-analysis (12) on the effect of the COL1A1 rs1107946 polymorphism on BMD reported unclear results, we conducted a meta-analysis using data from homogeneous populations (i.e., Asian postmenopausal females). Data of lumbar spine BMD in each genotype of the rs1107946 polymorphism were extracted from four previous studies (24–27) and the present study. Meta-analyses were performed using Review Manager 5.3.5 (http://tech.cochrane.org/revman). The inverse variance method and the random effects model were used to estimate the pooled mean differences of BMD between the genotypes. Heterogeneity among study results was assessed using the I2 statistic.
Analyses of muscle stiffness
In 250 Japanese individuals (153 males, 97 females), muscle stiffness of the biceps femoris long head, semitendinosus, and semimembranosus of both the legs was measured using an ultrasound shear wave elastography scanner (Aixplorer, Supersonic Image, Aix-en-Provence, France) as described previously (4,28). Written consent was obtained from each participant. The study was approved by the Ethics Committees of the Juntendo University and performed in accordance with the Declaration of Helsinki.
Analysis of serum procollagen I N-terminal propeptide (collagen metabolism marker)
The association of the COL1A1 polymorphism with serum procollagen I N-terminal propeptide (PINP; a collagen metabolism marker) was examined in 133 postmenopausal female subjects from WASEDA’S Health Study. Blood samples were collected between 8:30 and 11:00 am after at least 10 h overnight fast and then centrifuged at 1690g for 15 min at 4°C. Serum samples were stored at −80°C until use. PINP concentrations were determined using a commercially available enzyme-linked immunosorbent assay kit for PINP (CEA957Hu; Cloud-Clone Corp., Katy, TX), according to the manufacturer’s protocol. Optical density at 450 nm was measured using a microplate reader (SpectraMax™ iD5, Molecular Devices, Sunnyvale, CA).
Analyses of COL1A1/COL1A2 mRNA expression and type I collagen α1/α2 chain ratio in skeletal muscle
To examine the effects of the COL1A1 rs1107946 polymorphism on COL1A1/COL1A2 mRNA expression and collagen composition in skeletal muscle, muscle biopsy samples from 23 healthy young adults (13 males, 10 females; age = 23 ± 3 yr, height = 166.9 ± 6.7 cm, body mass = 60.5 ± 6.1 kg; rs1107946 genotype CC, n = 8; AC, n = 8; AA, n = 7) were used. These muscle samples were obtained from the vastus lateralis muscle approximately 15 cm above the patella, as described previously (29). The obtained muscle samples were frozen immediately in liquid nitrogen and stored at −80°C until analysis. The frozen muscle samples were crushed with 5.0 mm zirconia beads using a Micro Smash MS-100R (Tomy Seiko, Japan) at 3000 rpm twice for 15 s at 2°C. Half of the powdered muscle samples was used for quantitative reverse transcription–polymerase chain reaction (RT-qPCR) and the remaining half for quantification of type I collagen α1 and α2 chains. All the subjects provided written informed consent before their inclusion in this study. The study was approved by the Ethics Committees of the Juntendo University and performed in accordance with the Declaration of Helsinki.
Total RNA was extracted from muscle samples using TRIzol® Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. RNA concentration and purity were checked using a NanoDrop 8000 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Total RNA was reverse-transcribed using SuperScript™ IV VILO™ Master Mix with the ezDNase enzyme (Thermo Fisher Scientific). The mRNA levels of COL1A1 and COL1A2 were analyzed using the TaqMan® gene expression assays (Assay ID: Hs00164004_m1 [COL1A1] and Hs01028956_m1 [COL1A2]) and the StepOne™ Real-Time PCR system (Thermo Fisher Scientific). Actin beta (ACTB) was used as an internal expression control (Assay ID: Hs01060665_g1). PCR was performed in a 20-μL reaction mixture containing 10 μL of TaqMan® universal master mix II, 1 μL of TaqMan® gene expression assay mix, and 9 μL of cDNA. The expression levels of COL1A1 and COL1A2 were normalized to the expression levels of ACTB using the comparative Ct-method (30) applying the formula 2−(Ct of COL1A1―Ct of ACTB). Each expression value was log2-transformed.
The ratio of α1/α2 chains of type I collagen and the estimated proportion of α1 homotrimer were determined by liquid chromatography–mass spectrometry (LC-MS) as reported previously (31,32). In brief, freeze-dried muscle biopsy samples were heated at 60°C for 30 min in 100 mM Tris–HCl/1 mM CaCl2 (pH 7.6) after adding stable isotope-labeled collagen (SI collagen) (33) as an internal standard. The samples were digested with sequencing grade modified trypsin (Promega, Madison, WI) in 100 mM Tris–HCl/1 mM CaCl2 (pH 7.6) at 37°C for 16 h. Subsequently, trypsin digestion was again performed at 37°C for 24 h after heating at 60°C for 30 min. After centrifugation, the supernatant was subjected to LC-MS analysis on a 3200 QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer (AB Sciex, Foster City, CA) coupled to an Agilent 1200 Series HPLC system (Agilent Technologies, Palo Alto, CA) using a BIOshell A160 Peptide C18 HPLC column (5 μm particle size, L × I.D. 150 × 2.1 mm; Supelco, Bellefonte, PA). Previously established specific marker peptides (two peptides for each chain) (33) were detected by multiple reaction monitoring mode. The molar concentrations of α1 and α2 chains were determined based on the ratio of the marker peptides to stable isotopically heavy peptides derived from SI collagen. We assumed the amount of α112 heterotrimer is equal to that of α2; the amount of α111 homotrimer was calculated as follows: (α1 − α2 × 2) × 1/3.
Genotyping analysis
Total DNA was isolated from the saliva (J-HAP, J-Fit+ study, and muscle stiffness study) or venous blood (WASEDA’S Health Study and muscle biopsy study) using the Oragene® DNA Collection Kit (DNA Genotek, ON, Canada) or QIAamp DNA Blood Mini or Midi Kit (Qiagen, Hilden, Germany), respectively. The samples were analyzed for the rs1107946 polymorphism in COL1A1 using a TaqMan® SNP Genotyping Assay (Assay ID: C___7477171_10) and LightCycler® 480 System (Roche Molecular Systems, Mannheim, Germany) or QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific). PCR was performed in a 5-μL genotyping mixture containing 2.5 μL of TaqMan™ GTXpress™ Master Mix (2×), 0.0625 μL of TaqMan® SNP Genotyping Assay mix (40×), 1.4375 μL of sterilized water, and 1 μL of genomic DNA (10 ng·μL−1). Two to four negative controls were included on each plate. Genotypes were called based on TaqMan® assay results using LightCycler® 480 SW (version 1.5, Roche Molecular Systems) or QuantStudio Design and Analysis Software (v1.2, Thermo Fisher Scientific). A total of 95 randomly selected samples were genotyped in duplicate for the rs1107946 polymorphism, from which we confirmed that the genotyping results entirely agreed between duplicates.
Statistical analysis
Data are expressed as the mean ± SD. Statistical significance was set at P < 0.05. Statistical analyses were performed using JMP Pro version 12 (SAS Institute, Cary, NC) or IBM SPSS Statistics version 26. The Hardy–Weinberg equilibrium of the rs1107946 polymorphism was assessed using a χ2 test.
In stage 1 analyses, logistic regression analysis was applied to investigate the associations of the rs1107946 polymorphism with fatigue fracture and muscle injury. Main sport (track and field or other), playing years, and competitive level were adjusted. Odds ratio (OR) and 95% confidence interval (CI) were calculated under dominant, recessive, and additive genetic models. Akaike information criterion was calculated for each model to determine the best fitting genetic model. In stage 2 analyses, the associations of the rs1107946 polymorphism with the incidence of fatigue fracture and muscle injury were assessed using Cox proportional hazards models. The unadjusted and multivariable-adjusted hazard ratios were computed. In the multivariable-adjusted model, the main sport (track and field or other) and the competitive level were adjusted. The incidence rates of injuries, according to the rs1107946 genotypes, were compared using a Fisher’s exact test.
To examine whether the genotypes are associated with the phenotype variables (BMD and muscle stiffness) independently of confounding factors, ANCOVA and multiple linear regression analysis were used for the dominant, recessive, and additive models, respectively. The association between histories of fatigue fracture and muscle injury was examined by logistic regression analysis in athletes with at least one history of sport-related injury from the J-HAP cohort. Sex, main sport (track and field or other), playing years, and competitive level were adjusted.
In statistical analysis other than that mentioned above, continuous values were compared between genotypes using an unpaired t-test for dominant and recessive models and a Spearman correlation test for the additive model. The categorical variables were compared between groups using Pearson’s χ2 test.
Using the data from our previous study, we calculated the necessary sample size to detect the association between the rs1107946 genotype and the history of muscle injury with an OR of 2.0 (α = 0.05, power = 0.8, probability of CC genotype carriers = 0.36, and the ratio of control to case subjects = 8.9). The critical sample size was estimated to be 723 (73 cases and 650 controls).
RESULTS
C-allele of the COL1A1 rs1107946 polymorphism is oppositely associated with fatigue fracture and muscle injury
In stage 1 analysis, the COL1A1 rs1107946 A/C polymorphism was significantly associated with fatigue fracture and skeletal muscle injury in female, but not male, athletes (Tables 1 and 2). The fatigue fracture group showed significantly higher frequency of the CC + AC genotype than the no-fatigue fracture group (CC + AC vs AA, OR = 2.44, 95% CI = 1.17–5.77, P = 0.016; Table 1). The muscle injury group showed significantly lower frequency of the CC + AC genotype than the no-muscle injury group (CC + AC vs AA, OR = 0.46, 95% CI = 0.24–0.91, P = 0.026; Table 2). When the analysis was limited to participants with irregular menstruation (36 cases and 97 controls for fatigue fracture; 18 cases and 108 controls for muscle injury), associations of the rs1107946 polymorphism with fatigue fracture (CC + AC vs AA, OR = 5.11, 95% CI = 1.29–34.50, P = 0.018) and muscle injury (CC + AC vs AA, OR = 0.18, 95% CI = 0.05–0.63, P = 0.008) were prominent.
TABLE 1.
Association between COL1A1 rs1107946 polymorphism and fatigue fracture in J-HAP (n = 1636).
| Genotype | n (%) | Dominant | Recessive | Additive | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fatigue Fracture | No-Fatigue Fracture | OR (95% CI) AIC | P* | OR (95% CI) AIC | P* | OR (95% CI) AIC | P* | ||
| rs1107946 | CC+ AC vs AA | CC vs AC + AA | CC vs AC vs AA | ||||||
| All | AA | 27 (12.5) | 238 (16.8) | 1.41 (0.93–2.21) | 0.108 | 1.11 (0.82–1.49) | 0.514 | 1.15 (0.93–1.42) | 0.198 |
| AC | 108 (50.0) | 678 (47.8) | 1254.0 | 1256.2 | 1254.9 | ||||
| CC | 81 (37.5) | 504 (35.5) | |||||||
| Male | AA | 19 (14.4) | 157 (15.9) | 1.10 (0.67–1.90) | 0.709 | 1.06 (0.72–1.54) | 0.774 | 1.06 (0.81–1.38) | 0.691 |
| AC | 65 (49.2) | 484 (48.9) | 814.7 | 814.8 | 814.7 | ||||
| CC | 48 (36.4) | 348 (35.2) | |||||||
| Female | AA | 8 (9.5) | 81 (18.8) | 2.44 (1.17–5.77) | 0.016 | 1.26 (0.76–2.07) | 0.362 | 1.39 (0.98–2.00) | 0.063 |
| AC | 43 (51.2) | 194 (45.0) | 437.5 | 442.4 | 439.8 | ||||
| CC | 33 (39.3) | 156 (36.2) | |||||||
Adjusted by main sport (athletics), playing years, and competitive level. AIC, Akaike information criterion. Values in bold indicate P < 0.05.
*P value by logistic regression analysis.
TABLE 2.
Association of COL1A1 rs1107946 polymorphism with muscle injury in J-HAP (n = 1564).
| Genotype | n (%) | Dominant | Recessive | Additive | |||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle Injury | No Muscle Injury | OR (95% CI) AIC | P* | OR (95% CI) AIC | P* | OR (95% CI) AIC | P* | ||
| rs1107946 | CC+ AC vs AA | CC vs AC + AA | CC vs AC vs AA | ||||||
| All | AA | 37 (19.4) | 223 (16.2) | 0.79 (0.54–1.19) | 0.261 | 0.92 (0.67–1.27) | 0.634 | 0.90 (0.73–1.12) | 0.352 |
| AC | 87 (45.6) | 652 (47.5) | 1130.4 | 1131.5 | 1130.8 | ||||
| CC | 67 (35.1) | 498 (36.3) | |||||||
| Male | AA | 21 (15.7) | 153 (16.4) | 1.04 (0.64–1.76) | 0.881 | 1.12 (0.77–1.63) | 0.551 | 1.07 (0.82–1.40) | 0.622 |
| AC | 61 (45.5) | 450 (48.3) | 795.5 | 795.1 | 795.3 | ||||
| CC | 52 (38.8) | 328 (35.2) | |||||||
| Female | AA | 16 (28.1) | 70 (15.8) | 0.46 (0.24–0.91) | 0.026 | 0.56 (0.29–1.04) | 0.067 | 0.61 (0.41–0.91) | 0.014 |
| AC | 26 (45.6) | 202 (45.7) | 341.9 | 343.5 | 340.8 | ||||
| CC | 15 (26.3) | 170 (38.5) | |||||||
Adjusted by main sport (athletics), playing years, and competitive level. AIC, Akaike information criterion. Values in bold indicate P < 0.05.
*P value by logistic regression analysis.
In validation analyses, no association was observed between the rs1107946 polymorphism and the fatigue fracture incidence (Fig. 1A and B, and Supplemental Digital Contents 5 and 6, http://links.lww.com/MSS/C295 and http://links.lww.com/MSS/C296). Figure 1C, D and Supplemental Digital Contents 7 and 8 (http://links.lww.com/MSS/C297 and http://links.lww.com/MSS/C298) show the relationship between the rs1107946 genotype and the muscle injury risk as estimated by the Cox proportional hazards model. In female athletes, CC + AC genotype carriers exhibited a low incidence of muscle injury than AA genotype carriers (hazard ratio = 0.27, 95% CI = 0.08–0.96, P = 0.043 adjusted for main sport; Fig. 1D and Supplemental Digital Content 8, http://links.lww.com/MSS/C298) similar to stage 1 analysis results.
FIGURE 1.
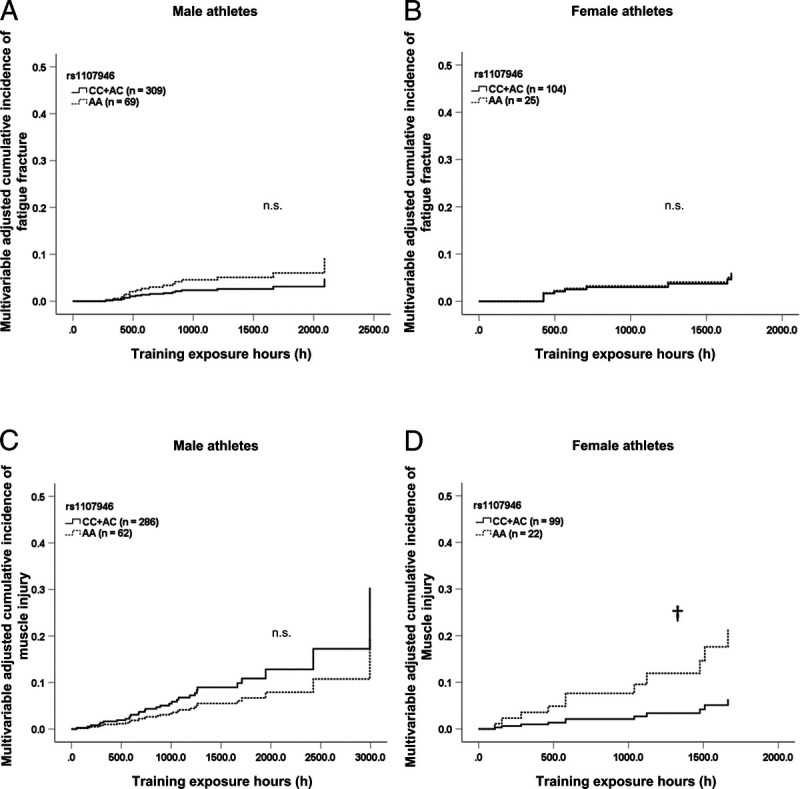
Cumulative incidence curve for fatigue fracture and muscle injury according to the COL1A1 rs1107946 genotypes. Multivariable-adjusted cumulative incidence curves of fatigue fracture in male athletes (A), fatigue fracture in female athletes (B), muscle injury in male athletes (C), and muscle injury in female athletes (D). n.s., no significance detected. †C-dominant model, P = 0.043 by the Cox proportional hazards model.
Association of the rs1107946 polymorphism with BMD
In male and female subjects, no significant association was observed between the rs1107946 A/C polymorphism and the whole-body BMD (see Figures, Supplemental Digital Content 9, http://links.lww.com/MSS/C299). However, in the subanalysis of postmenopausal female subjects, the rs1107946 A/C polymorphism tended to be associated with lumbar spine BMD, wherein the C-allele was associated with lower BMD (AA, 0.89 ± 0.12, vs AC, 0.88 ± 0.13, vs CC, 0.83 ± 0.12 g·cm−2, P = 0.057, under the C-additive model after adjustment for age and BMI, β for C-allele: −0.03; see Figure, Supplemental Digital Content 9, http://links.lww.com/MSS/C299).
Meta-analysis performed by using data from previous studies in Asian populations and the present study revealed that postmenopausal female subjects with the CC genotype presented a significantly lower lumbar spine BMD than those with the AA genotype (mean difference = −0.02, 95% CI = −0.04–0.00, I2 = 0%, P = 0.02; see Figure, Supplemental Digital Content 10, http://links.lww.com/MSS/C300).
Association of the rs1107946 polymorphism with skeletal muscle stiffness
In male subjects, no significant association was observed between the rs1107946 A/C polymorphism and the hamstring muscle stiffness (Fig. 2A). In female subjects, stiffness of the semitendinosus (AA, 25.5 ± 6.8; AC, 22.0 ± 4.9; CC, 22.3 ± 4.8 kPa) and semimembranosus (AA, 41.6 ± 17.7; AC, 37.6 ± 16.4; CC, 33.0 ± 11.0 kPa) significantly differed among different the rs1107946 genotype carriers (semitendinosus: P = 0.013 under the C-dominant model after adjustment for regular stretch, semimembranosus: P = 0.040 under the C-additive model after adjustment for regular stretch, β for C-allele: −4.5) (Fig. 2B).
FIGURE 2.
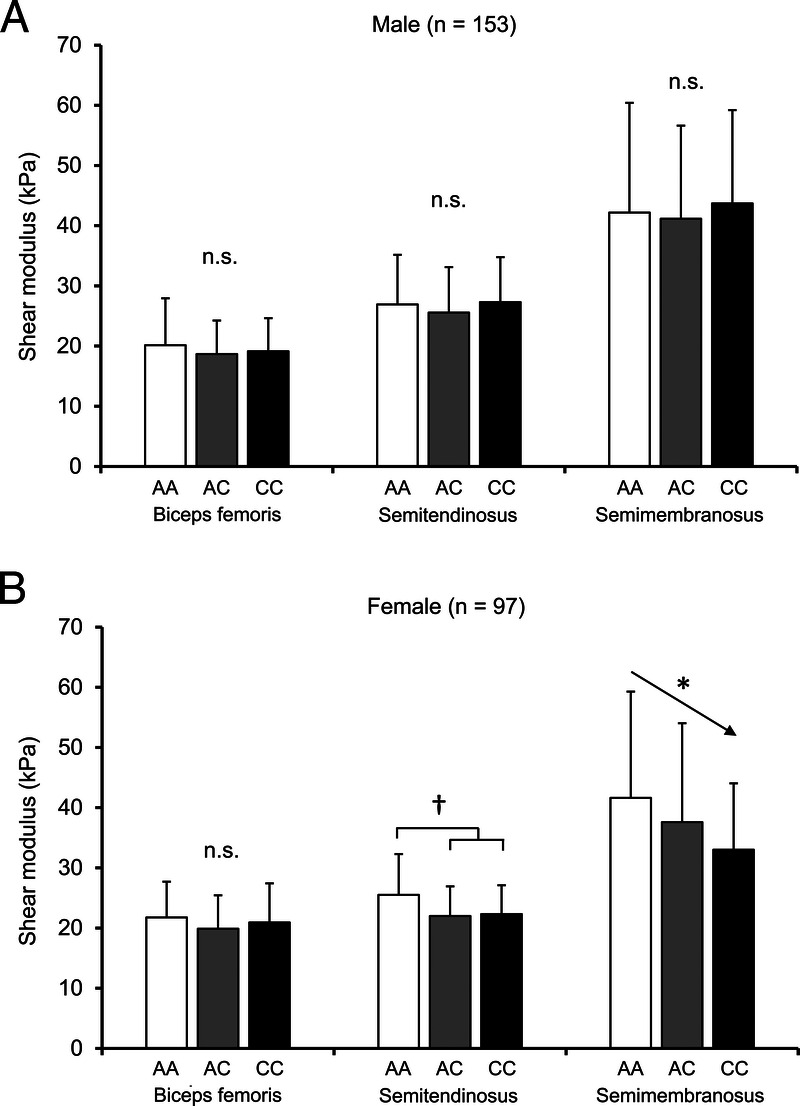
Association between the COL1A1 rs1107946 polymorphism and the muscle stiffness in male (A) and female (B) subjects. †C-dominant model, P = 0.013 by ANCOVA after adjustment for a regular stretch. *Additive model, P = 0.040 by multiple regression analysis after adjustment for regular stretch. The error bars show SD.
The rs1107946 polymorphism is not associated with serum PINP (collagen synthesis marker)
Serum samples of 137 Japanese females from WASEDA’S Health Study were analyzed for determining the levels of PINP, which reflect type I collagen synthesis in the body (mainly in bones). The serum PINP level did not significantly differ among genotypes of the rs1107946 A/C polymorphism (AA, 139.3 ± 40.3; AC, 149.4 ± 44.9; CC, 150.0 ± 49.9 ng·mL−1; P = 0.335 for the C-dominant model, P = 0.693 for the C-recessive model by the unpaired t-test, and P = 0.593 for the C-additive model by the Spearman correlation test; Fig. 3A).
FIGURE 3.
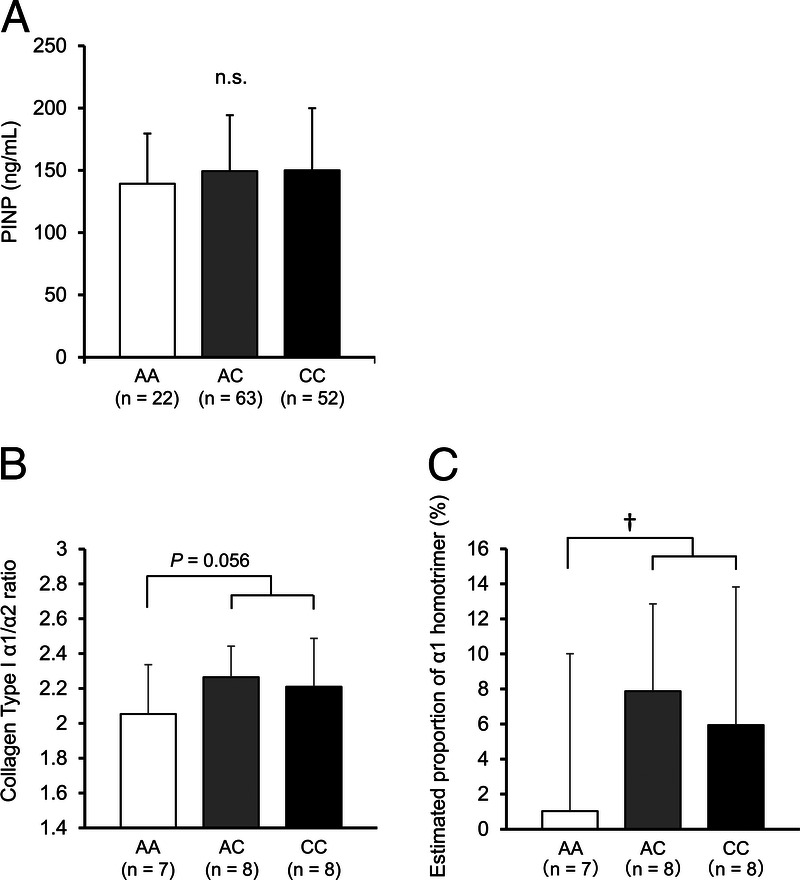
Association between the COL1A1 rs1107946 polymorphism and the PINP concentration in serum (A), collagen type I α1/α2 chain ratio (B), and estimated proportion of α1 homotrimers (C) in skeletal muscle. †C-dominant model, P = 0.044 by the unpaired t-test. The error bars show SD.
Association of the rs1107946 polymorphism with type I collagen α1/α2 chain ratio and COL1A1/COL1A2 mRNA expression in human skeletal muscle
Muscle samples of CC + AC genotype carriers tended to have a higher ratio of α1/α2 chains than muscle samples of AA genotype carriers (CC + AC; 2.24 ± 0.23, vs AA; 2.05 ± 0.28, P = 0.056 by unpaired t-test; Fig. 3B). The estimated proportion of α1 homotrimers was significantly higher in muscle samples of CC + AC genotype carriers than in AA genotype carriers (P = 0.044 by unpaired t-test; Fig. 3C). RT-qPCR analysis indicated that the COL1A1 mRNA expression level in skeletal muscle was higher in CC than in AC + AA genotype carriers (P = 0.034 by unpaired t-test), and the COL1A2 mRNA expression level was also significantly higher in CC than AC + AA genotype carriers (P = 0.026 by unpaired t-test; see Figures, Supplemental Digital Content 11, http://links.lww.com/MSS/C301). The ratio of COL1A1/COL1A2 mRNA expression tended to be higher in the skeletal muscle of CC than AC + AA genotype carriers (P = 0.074 by unpaired t-test; see Figure, Supplemental Digital Content 11, http://links.lww.com/MSS/C301).
Opposite association between fatigue fracture and muscle injury
Guided by the opposite effects of the rs1107946 polymorphism with regard to fatigue fracture and muscle injury, we examined the relationship between histories of fatigue fracture and muscle injury regardless of the rs1107946 polymorphism in 1185 athletes from the J-HAP cohort. Logistic regression analysis showed that athletes with a history of fatigue fracture exhibited significantly lower frequency of history of muscle injury than those in the no-fatigue fracture group (OR = 0.59, 95% CI = 0.36–0.92, P = 0.019 after adjustment for sex, main sport, playing years, and competitive level). This trend was observed even when examining each sex and main sport individually (see Table, Supplemental Digital Content 12, http://links.lww.com/MSS/C302).
DISCUSSION
We identified that the COL1A1 functional promoter region A/C polymorphism (rs1107946) is oppositely associated with the risks of fatigue fracture and muscle injury, respectively, in female athletes, where the C-allele was associated with higher risk of fatigue fracture (OR, 2.44) and lower risk of muscle injury (OR, 0.46). These associations were supported by the relationships between the polymorphism and the tissue properties that constitute potential risk factors of these injuries, namely, the C-allele was associated with lower BMD and lower muscle stiffness. Moreover, in vastus lateralis, C-allele carriers exhibited a higher ratio of type I collagen α1 to α2 chain than AA genotype carriers (α1/α2, 2.24 vs 2.05), suggesting a slight increase in α1 chain homotrimers (an increase of about 6%). These results imply that an increased COL1A1/COL1A2 mRNA expression ratio of the C-allele of the rs1107946 polymorphism might induce the production of α1 chain homotrimers of type I collagen in tissues, which in turn may result in susceptibility to fatigue fracture and resistance against muscle injury by decreasing the BMD and muscle stiffness.
Although the effects of the COL1A1 rs1107946 A/C polymorphism on fracture risk and BMD in the nonathletic population have been examined previously, conflicting and inconclusive results were reported (12,34). In the present study, we found that the C-allele of rs1107946 polymorphism was associated with higher risk of fatigue fracture in female athletes (OR, 2.44) in stage 1 analyses. Moreover, the association was prominent in female athletes with irregular menstruation who exhibited elevated fatigue fracture risk (OR, 5.11) (35). Furthermore, a meta-analysis performed using data from the present and previous studies confirmed that CC genotype carriers presented lower lumbar spine BMD than AA genotype carriers among Asian postmenopausal female subjects.
Conversely, the association between the rs1107946 polymorphism C-allele and the high fatigue fracture risk was not validated in stage 2 analyses. Because we could not assess the menstruation status in these analyses, the results could be accordingly affected. Therefore, further prospective studies considering menstruation status are necessary to confirm the association between the rs1107946 polymorphism and the fatigue fracture. Nevertheless, taken together, our findings suggest that although the COL1A1 rs1107946 polymorphism appears to play very little or no role in fatigue fracture and BMD in normal situations, under high-risk conditions (such as irregular menstruation in female athletes and postmenopausal women), the influence of the rs1107946 polymorphism on fatigue fracture and BMD markedly increases.
Notably, although the C-allele of the COL1A1 rs1107946 A/C polymorphism was associated with susceptibility to fatigue fracture in our study, an opposite association was observed on muscle injury, where the C-allele conferred resistance against muscle injury in female athletes in stage 1 analyses. This association was also confirmed during the stage 2 analyses. The rs1107946 polymorphism C-allele was also associated with lower stiffness of the semitendinosus and semimembranosus, but not of the biceps femoris muscles, in female subjects. Muscle stiffness is influenced by intramuscular collagenous connective tissues (13). However, the contribution of intramuscular connective tissues to muscle stiffness depends on how much the muscle is stretched (36); the contribution of collagenous tissues is high when the muscle is tensioned and stretched. Based on previous findings (37), the semitendinosus and semimembranosus would be more stretched than the biceps femoris in the posture in which muscle stiffness was measured in the present study (i.e., hip flexed at 70° and knee fully extended). Therefore, it is not surprising that the association of the COL1A1 polymorphism with stiffness was found in the semitendinosus and semimembranosus, but not in the biceps femoris. Collectively, these results suggest that the COL1A1 rs1107946 A/C polymorphism affects the risk of muscle injury in female athletes by altering muscle stiffness.
The specific mechanism by which the rs1107946 polymorphism influences the observed muscle phenotypes in females remains unclear. However, sex differences have been reported in joint flexibility and muscle stiffness (38,39), wherein females exhibit greater joint flexibility and lower muscle stiffness than males. These differences may be related to sex hormones such as estrogen. Notably, estrogen suppresses type I collagen synthesis (40); moreover, women exhibit reduced collagen content in tissues compared with men (41,42). The collagen fibril content in tissue is also highly correlated with tissue stiffness (42). In addition, interactions of collagen molecules and/or collagen fibrils with other extracellular matrix (ECM) components may also be involved in tissue stiffness. A previous proteomics analysis showed that there are sex differences in the expression of several ECM-related proteins other than collagen in human ligament and tendon tissues (43). Therefore, the effect of the COL1A1 polymorphism was possibly prominent in females because of reduced collagen content and/or altered expression of other ECM-related proteins in their muscle tissue.
Based on the Genotype-Tissue Expression database (https://www.gtexportal.org/home/), the rs1107946 polymorphism, located in the COL1A1 promoter region, is associated with COL1A1 mRNA expression in human tissues such that the C-allele is associated with higher expression than the A allele. The same association was observed in our RT-qPCR analysis of human skeletal muscle biopsy samples in which COL1A1 and COL1A2 mRNA expressions were higher in skeletal muscle samples of CC genotype carriers than in samples from A allele carriers. These results led to the conjecture that the CC genotype may be associated with higher protein expression of type I collagen in tissues. However, this would not be consistent with the observed association of the C-allele with lower BMD and lower muscle stiffness. We failed to find an association between the rs1107946 polymorphism and the serum PINP concentration, which reflects collagen synthesis. This result suggests that the rs1107946 polymorphism does not affect the protein expression level of type I collagen. Accordingly, we next examined the association between the rs1107946 polymorphism and the type I collagen α1/α2 chain ratio in tissue. We found that human skeletal muscle samples of C-allele carriers showed a greater ratio of type I collagen α1/α2 chains than those of AA genotype carriers, suggesting the existence of a higher proportion of α1 homotrimers. In addition, the ratio of COL1A1/COL1A2 mRNA expression was higher in skeletal muscle of CC genotype carriers in our study. These results suggest that an increased ratio of COL1A1/COL1A2 mRNA expression in CC genotype carriers may induce increased production of α1 chain homotrimers of type I collagen in tissues. However, it is important to note that collagen biosynthesis is one of the most complex processes among all protein production and involves many steps, from the transcription of COL1A1 and COL1A2 to the tissue deposition of a triple-helical molecule (44).
It was previously postulated that the altered mechanical properties induced by an increase in the homotrimeric type I collagen molecule act as a risk factor for bone fracture and as a protective factor against injuries to tendon/ligament tissues (45). In bone tissue, an increased ratio of α1/α2 chains (suggesting a higher proportion of type I collagen α1 homotrimers) was suggested to be associated with reduced bone strength and BMD, and therefore with the risk of fracture (46). On the contrary, the effect of the increased type I collagen α1 homotrimers on the mechanical properties of skeletal muscle is not well known. The osteogenesis imperfecta (OI) murine (oim) mouse model carries a spontaneous nucleotide deletion that causes a frameshift in Col1a2 resulting in the absence of functional α2 chains of type I collagen (47). Therefore, oim/oim mice exclusively produce homotrimeric (three α1 chains) type I collagen rather than heterotrimeric (two α1 chains and one α2 chain) type I collagen. Skeletal muscle in the oim/oim mouse exhibited lower fibrillary collagen content and decreased tetanic force compared with that in wild-type mice, whereas skeletal muscle of the +/oim mouse presented mild weakness of tetanic force (48). In our study, carriers of the C-allele of the rs1107946 polymorphism, associated with a higher proportion of α1 homotrimers, exhibited decreased muscle stiffness. Although the effect of the rs1107946 polymorphism is much smaller than the +/oim genotype effect, increased homotrimers may alter the overall structure of ECM and thus decrease muscle stiffness. Because low stiffness of skeletal muscle is related to a low risk of muscle injury (4,5), our results suggest that altered mechanical properties induced by an increase in homotrimeric type I collagen molecule oppositely affect the risk of injuries in bone and skeletal muscle.
We also found that athletes with fatigue fracture history showed a significantly lower frequency of muscle injury history than those without fatigue fracture history. The incidence of these injuries differs among sports discipline/events in addition to between sexes (49,50). Therefore, these factors may affect the observed association. However, we also confirmed the significant association between fatigue fracture and muscle injury after adjustment for main sport and sex. Furthermore, even when examining each event and sex individually, the same tendencies were observed (see Table, Supplemental Digital Content 12, http://links.lww.com/MSS/C302). These results suggest that susceptibilities to fatigue fracture and muscle injury are inversely related and that this phenomenon might be attributed to the material properties of these tissues. Although we focused only on the COL1A1 rs1107946 polymorphism, future elucidation of the polygenic profile that determines the material properties of these tissues will contribute to the development of personalized injury prevention programs.
A strength of the present study is the study design, which includes two-stage association analyses for injuries (stage 1, relatively large-scale cohort; stage 2, prospective design). Furthermore, we confirmed the effects of the polymorphism not only on tissue properties such as BMD and muscle stiffness but also on the collagen type I α1/α2 chain ratio using human tissue samples. These multistage investigations may reduce the possibility of false-positive results. By contrast, we are aware that the multiple statistical tests have the potential for false-positive results. Therefore, further investigations with a larger sample size are required to confirm our claim.
Our study had other limitations. First, assessments of injury history and incidence were conducted by questionnaire. Although we focused only on injuries to which the subjects had received a diagnosis by medical doctors/practitioners, the reliability of our data is lower than that obtained using medical records. Second, the association between the rs1107946 polymorphism C-allele and the high fatigue fracture risk was not validated in stage 2 analyses. Moreover, the lack of consideration of menstruation status and short follow-up period (2 yr) during stage 2 analyses may affect the results. Further prospective studies with longer periods and consideration of menstruation status will be required to confirm the causal association between the polymorphism evaluated herein and the fatigue fracture.
The present findings suggest that the C-allele of the COL1A1 rs1107946 polymorphism is associated with a higher risk of fatigue fracture and lower risk of muscle injury in female athletes through alteration of tissue properties, which is possibly induced by increased homotrimerization of the α1 chain of type I collagen. Furthermore, susceptibilities to fatigue fracture and muscle injury are inversely related, with this phenomenon being attributable to the material properties of bone and muscle. Collectively, our findings may facilitate the identification of injury risk in athletes and allow researchers and clinicians to develop injury prevention programs specifically targeting tissue properties.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the Japan Society for Promotion of Science (JSPS) KAKENHI Scientific Research (B) (18H03155 to N. F. and 16H03233 to N. M.), Young Scientists (A) (17H04752 to E. M.), Young Scientists (18 K17863 to H. K.), MEXT-Supported Program for the Private University Research Branding Projects (to Juntendo University) and for the Strategic Research Foundation at Private Universities (S1511017 to Waseda University), and a Tenri University Research Grant (N. Kamiya.). H. K. and K. H. were recipients of a Grant-in-Aid for JSPS Fellows from the JSPS. The authors thank Editage (www.editage.jp) for English language editing.
All authors declare that they have no conflict of interest. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The present study does not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
ERI MIYAMOTO-MIKAMI, Email: eri-miyamoto@juntendo.ac.jp.
HIROSHI KUMAGAI, Email: kumazin7@gmail.com.
KUMPEI TANISAWA, Email: tanisawa@waseda.jp.
YUKI TAGA, Email: y-taga@nippi-inc.co.jp.
KOSUKE HIRATA, Email: hirata.kosuke.q9@shibaura-it.ac.jp.
NAOKI KIKUCHI, Email: n.kikuchi@nittai.ac.jp.
NOBUHIRO KAMIYA, Email: nkamiya1@sta.tenri-u.ac.jp.
RYOKO KAWAKAMI, Email: r.kawakami@aoni.waseda.jp.
TAISHI MIDORIKAWA, Email: taishi@obirin.ac.jp.
TAKUJI KAWAMURA, Email: tkawamura@aoni.waseda.jp.
RYO KAKIGI, Email: rkakigi@jiu.ac.jp.
TOSHIHARU NATSUME, Email: natsumetoshiharu@gmail.com.
HIROFUMI ZEMPO, Email: zempo.hirofumi@gmail.com.
KOYA SUZUKI, Email: ko-suzuki@juntendo.ac.jp.
YOSHIMITSU KOHMURA, Email: ykoumura@juntendo.ac.jp.
KAZUNORI MIZUNO, Email: k-mizuno@nippi-inc.co.jp.
SUGURU TORII, Email: shunto@waseda.jp.
SHIZUO SAKAMOTO, Email: s.sakamoto@waseda.jp.
KOICHIRO OKA, Email: koka@waseda.jp.
MITSURU HIGUCHI, Email: mhiguchi@waseda.jp.
HISASHI NAITO, Email: hnaitou@juntendo.ac.jp.
NAOKAZU MIYAMOTO, Email: n-miyamoto@juntendo.ac.jp.
REFERENCES
- 1.Soligard T Steffen K Palmer D, et al. Sports injury and illness incidence in the Rio de Janeiro 2016 Olympic summer games: a prospective study of 11274 athletes from 207 countries. Br J Sports Med. 2017;51(17):1265–71. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman KE, Cano Sokoloff N, Nardo Maffazioli GD, Clarke HM, Lee H, Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015;47(8):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrack MT Gibbs JC De Souza MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42(4):949–58. [DOI] [PubMed] [Google Scholar]
- 4.Kumagai H Miyamoto-Mikami E Hirata K, et al. ESR1 rs2234693 polymorphism is associated with muscle injury and muscle stiffness. Med Sci Sports Exerc. 2019;51(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witvrouw E, Danneels L, Asselman P, D’Have T, Cambier D. Muscle flexibility as a risk factor for developing muscle injuries in male professional soccer players. A prospective study. Am J Sports Med. 2003;31(1):41–6. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto N, Miyamoto-Mikami E, Hirata K, Kimura N, Fuku N. Association analysis of the ACTN3 R577X polymorphism with passive muscle stiffness and muscle strain injury. Scand J Med Sci Sports. 2018;28(3):1209–14. [DOI] [PubMed] [Google Scholar]
- 7.Morris JA Kemp JP Youlten SE, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larruskain J Celorrio D Barrio I, et al. Genetic variants and hamstring injury in soccer: an association and validation study. Med Sci Sports Exerc. 2018;50(2):361–8. [DOI] [PubMed] [Google Scholar]
- 9.Varley I Hughes DC Greeves JP, et al. The association of novel polymorphisms with stress fracture injury in elite athletes: further insights from the SFEA cohort. J Sci Med Sport. 2018;21(6):564–8. [DOI] [PubMed] [Google Scholar]
- 10.Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev. 2011;39(4):191–8. [DOI] [PubMed] [Google Scholar]
- 11.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie P Liu B Zhang L, et al. Association of COL1A1 polymorphisms with osteoporosis: a meta-analysis of clinical studies. Int J Clin Exp Med. 2015;8(9):14764–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon). 2001;16(2):87–101. [DOI] [PubMed] [Google Scholar]
- 14.Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 1984;219(3):1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Giralt N Enjuanes A Bustamante M, et al. In vitro functional assay of alleles and haplotypes of two COL1A1-promoter SNPs. Bone. 2005;36(5):902–8. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, van’t Hof RJ, Albagha OM, Ralston SH. Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet. 2009;18(15):2729–38. [DOI] [PubMed] [Google Scholar]
- 17.Collins M, September AV, Posthumus M. Biological variation in musculoskeletal injuries: current knowledge, future research and practical implications. Br J Sports Med. 2015;49(23):1497–503. [DOI] [PubMed] [Google Scholar]
- 18.Ficek K Cieszczyk P Kaczmarczyk M, et al. Gene variants within the COL1A1 gene are associated with reduced anterior cruciate ligament injury in professional soccer players. J Sci Med Sport. 2013;16(5):396–400. [DOI] [PubMed] [Google Scholar]
- 19.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319–36. [DOI] [PubMed] [Google Scholar]
- 20.Pitsiladis YP Tanaka M Eynon N, et al. Athlome project consortium: a concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol Genomics. 2016;48(3):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massidda M Miyamoto-Mikami E Kumagai H, et al. Association between the ACE I/D polymorphism and muscle injuries in Italian and Japanese elite football players. J Sports Sci. 2020;38(21):2423–9. [DOI] [PubMed] [Google Scholar]
- 22.Fuller CW Ekstrand J Junge A, et al. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Br J Sports Med. 2006;40(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito T Kawakami R Tanisawa K, et al. Dietary patterns and abdominal obesity in middle-aged and elderly Japanese adults: Waseda Alumni’s sports, exercise, daily activity, sedentariness and health study (WASEDA’S health study). Nutrition. 2019;58:149–55. [DOI] [PubMed] [Google Scholar]
- 24.Lau HH, Ng MY, Ho AY, Luk KD, Kung AW. Genetic and environmental determinants of bone mineral density in Chinese women. Bone. 2005;36(4):700–9. [DOI] [PubMed] [Google Scholar]
- 25.Singh M, Singh P, Singh S, Juneja PK, Kaur T. A haplotype derived from the common variants at the -1997G/T and Sp1 binding site of the COL1A1 gene influences risk of postmenopausal osteoporosis in India. Rheumatol Int. 2013;33(2):501–6. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Ando F, Niino N, Shimokata H. Association of a -1997G—>T polymorphism of the collagen Ialpha1 gene with bone mineral density in postmenopausal Japanese women. Hum Biol. 2005;77(1):27–36. [DOI] [PubMed] [Google Scholar]
- 27.Zhang LQ, Liu H, Huang XF. Relation of JAGGED 1 and collagen type 1 alpha 1 polymorphisms with bone mineral density in Chinese postmenopausal women. Int J Clin Exp Pathol. 2014;7(10):7142–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto N, Hirata K, Kimura N, Miyamoto-Mikami E. Contributions of hamstring stiffness to straight-leg-raise and sit-and-reach test scores. Int J Sports Med. 2018;39(2):110–4. [DOI] [PubMed] [Google Scholar]
- 29.Natsume T, Ozaki H, Kakigi R, Kobayashi H, Naito H. Effects of training intensity in electromyostimulation on human skeletal muscle. Eur J Appl Physiol. 2018;118(7):1339–47. [DOI] [PubMed] [Google Scholar]
- 30.Norman B, Esbjornsson M, Rundqvist H, Osterlund T, von Walden F, Tesch PA. Strength, power, fiber types, and mRNA expression in trained men and women with different ACTN3 R577X genotypes. J Appl Physiol (1985). 2009;106(3):959–65. [DOI] [PubMed] [Google Scholar]
- 31.Terajima M Taga Y Sricholpech M, et al. Role of Glycosyltransferase 25 domain 1 in type I collagen glycosylation and molecular phenotypes. Biochemistry. 2019;58(50):5040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii KK Taga Y Sakai T, et al. Lowering the culture temperature corrects collagen abnormalities caused by HSP47 gene knockout. Sci Rep. 2019;9(1):17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taga Y, Kusubata M, Ogawa-Goto K, Hattori S. Stable isotope-labeled collagen: a novel and versatile tool for quantitative collagen analyses using mass spectrometry. J Proteome Res. 2014;13(8):3671–8. [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Evangelou E, Ioannidis JP, Ralston SH. Polymorphisms in the 5′ flank of COL1A1 gene and osteoporosis: meta-analysis of published studies. Osteoporos Int. 2011;22(3):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine Position Stand: the female athlete triad. Med Sci Sports Exerc. 1997;29(5):i–ix. [DOI] [PubMed] [Google Scholar]
- 36.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutts A. The range of sarcomere lengths in the muscles of the human lower limb. J Anat. 1988;160:79–88. [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto N, Hirata K, Miyamoto-Mikami E, Yasuda O, Kanehisa H. Associations of passive muscle stiffness, muscle stretch tolerance, and muscle slack angle with range of motion: individual and sex differences. Sci Rep. 2018;8(1):8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamoto-Mikami E Miyamoto N Kumagai H, et al. COL5A1 rs12722 polymorphism is not associated with passive muscle stiffness and sports-related muscle injury in Japanese athletes. BMC Med Genet. 2019;20(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwan G Neugarten J Sherman M, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50(4):1173–9. [DOI] [PubMed] [Google Scholar]
- 41.Lemoine JK, Lee JD, Trappe TA. Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashemi J, Chandrashekar N, Mansouri H, Slauterbeck JR, Hardy DM. The human anterior cruciate ligament: sex differences in ultrastructure and correlation with biomechanical properties. J Orthop Res. 2008;26(7):945–50. [DOI] [PubMed] [Google Scholar]
- 43.Little D, Thompson JW, Dubois LG, Ruch DS, Moseley MA, Guilak F. Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS One. 2014;9(5):e96526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelse K, Poschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–46. [DOI] [PubMed] [Google Scholar]
- 45.Gibbon A, Raleigh SM, Ribbans WJ, Posthumus M, Collins M, September AV. Functional COL1A1 variants are associated with the risk of acute musculoskeletal soft tissue injuries. J Orthop Res. 2020;38(10):2290–8. [DOI] [PubMed] [Google Scholar]
- 46.Mann V Hobson EE Li B, et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107(7):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chipman SD Sweet HO McBride DJ Jr, et al. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90(5):1701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010;29(7):638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edouard P, Feddermann-Demont N, Alonso JM, Branco P, Junge A. Sex differences in injury during top-level international athletics championships: surveillance data from 14 championships between 2007 and 2014. Br J Sports Med. 2015;49(7):472–7. [DOI] [PubMed] [Google Scholar]
- 50.Edouard P, Branco P, Alonso JM. Muscle injury is the principal injury type and hamstring muscle injury is the first injury diagnosis during top-level international athletics championships between 2007 and 2015. Br J Sports Med. 2016;50(10):619–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


