Abstract
For normal cochlear function, outer hair cells (OHCs) require a precise control of intracellular Ca2+ levels. In the absence of regulatory elements such as proteinaceous buffers or extrusion pumps, OHCs degenerate, leading to profound hearing impairment. Influx of Ca2+ occurs both at the stereocilia tips and the basolateral membrane. In this latter compartment, two different origins for Ca2+ influx have been poorly explored: voltage-gated L-type Ca2+ channels (VGCCs) at synapses with Type II afferent neurons, and α9α10 cholinergic nicotinic receptors at synapses with medio-olivochlear complex (MOC) neurons. Using functional imaging in mouse OHCs, we dissected Ca2+ influx individually through each of these sources, either by applying step depolarizations to activate VGCC, or stimulating MOC axons. Ca2+ ions originated in MOC synapses, but not by VGCC activation, was confined by Ca2+-ATPases most likely present in nearby synaptic cisterns. Although Ca2+ currents in OHCs are small, VGCC Ca2+ signals were comparable in size to those elicited by α9α10 receptors, and were potentiated by ryanodine receptors (RyRs). In contrast, no evidence of potentiation by RyRs was found for MOC Ca2+ signals over a wide range of presynaptic stimulation strengths. Our study shows that despite the fact that these two Ca2+ entry sites are closely positioned, they differ in their regulation by intracellular cisterns and/or organelles, suggesting the existence of well-tuned mechanisms to separate the two different OHC synaptic functions.
SIGNIFICANCE STATEMENT Outer hair cells (OHCs) are sensory cells in the inner ear operating under very special constraints. Acoustic stimulation leads to fast changes both in membrane potential and in the intracellular concentration of metabolites such as Ca2+. Tight mechanisms for Ca2+ control in OHCs have been reported. Interestingly, Ca2+ is crucial for two important synaptic processes: inhibition by efferent cholinergic neurons, and glutamate release onto Type II afferent fibers. In the current study we functionally imaged Ca2+ at these two different synapses, showing close positioning within the basolateral compartment of OHCs. In addition, we show differential regulation of these two Ca2+ sources by synaptic cisterns and/or organelles, which could result crucial for functional segregation during normal hearing.
Keywords: α9α10, calcium, cistern, outer hair cell, synapses, voltage-gated Ca2+ channels
Introduction
Efficient compartmentalization of Ca2+ signaling is crucial for normal cell function and to enhance the computational power of individual cells (Bootman et al., 2001; Petersen, 2002; Augustine et al., 2003). However, if not tightly controlled spatially and temporally within cells, it could generate severe cell dysfunction and even cell death (Choi, 1995). Cochlear outer hair cells (OHCs) are a unique group of cells responsible for sound amplification within the inner ear that present a highly polarized structure, featuring a stereocilia bundle on their apical end and synaptic connections on the basolateral membrane (Fuchs, 1996; Dallos, 2008; Fettiplace, 2017). One important aspect of OHC physiology is the precise homeostasis and tight regulation of Ca2+ during normal activity. The main Ca2+ source are mechanotransducer channels located at the tip of stereocilia (Beurg et al., 2009; Fettiplace and Nam, 2019). Within OHCs stereocilia high concentrations of proteinaceous Ca2+ buffers coexist with large amounts of extrusion pumps, suggesting the need for quick clearing mechanisms (Sakaguchi et al., 1998; Yamoah et al., 1998; Dumont et al., 2001; Hackney et al., 2005; Chen et al., 2012). Deficits in these control pathways, because of mutations in Ca2+-ATPases or the absence of the Ca2+ buffer oncomodulin, lead to OHCs loss and profound auditory deficits (Street et al., 1998; Ficarella et al., 2007; Tong et al., 2016).
Two other important Ca2+ sources in OHCs have been less characterized: the voltage-gated L-type Ca2+ channels (VGCC; Knirsch et al., 2007), and the α9α10 cholinergic nicotinic receptors (Weisstaub et al., 2002; Gómez-Casati et al., 2005). Both VGCC and α9α10 receptors are located at the basolateral membrane of OHCs, at synapses with Type II afferent fibers in the case of the former (Saito, 1990), and on the postsynaptic side of synapses with medio-olivochlear complex (MOC) fibers in the latter (Elgoyhen et al., 1994, 2001). Compared with inner hair cells (IHCs), OHCs show smaller Ca2+ currents through VGCC (Knirsch et al., 2007; Beurg et al., 2008; Johnson and Marcotti, 2008; Wong et al., 2013), and present synaptic ribbons with irregular shapes and fewer vesicles in their vicinity (for review, see Fuchs and Glowatzki, 2015). These synapses are weak as they only release few vesicles on stimulation contrasting with the multivesicular exocytosis at IHCs afferent synapses (Glowatzki and Fuchs, 2002; Weisz et al., 2009; Fuchs and Glowatzki, 2015). It has been suggested that Type II fibers would only be activated in loud environments, and possibly be responsible for signaling pain in the inner ear (Flores et al., 2015; Liu et al., 2015; Weisz et al., 2021).
On the other hand, cholinergic MOC synapses onto OHCs are inhibitory and provide the means to modulate sound amplification (Guinan, 1996). Synaptic responses are mediated by the highly Ca2+-permeable α9α10 receptors, coupled to the activation of SK2 (Ca2+-activated K+) channels leading to OHCs hyperpolarization (Fuchs, 1996; Weisstaub et al., 2002; Gómez-Casati et al., 2005). Detailed electron micrographs have shown postsynaptic cisterns within OHCs, closely aligned with presynaptic efferent synaptic contacts (Engström, 1958; Smith and Sjöstrand, 1961; Saito, 1980; Fuchs et al., 2014). In analogy to the sarcoplasmic reticulum found in myocytes, synaptic cisterns have been proposed to serve as Ca2+ stores that modulates efferent synaptic responses through both Ca2+ ATPases (of the sarcoplasmic type, SERCA) responsible for pumping Ca2+ ions out of the cytoplasm and into the cistern; and ryanodine receptors (RyRs), which trigger the phenomenon of Ca2+-induced Ca2+ release (CICR; Evans et al., 2000; Grant et al., 2006; Lioudyno et al., 2004; Sridhar et al., 1997). The functional evidence to support these observations is indirect, but the presence of SERCA in synaptic cisterns, and RyR in both synaptic and lateral wall cisterns has been documented before (Lioudyno et al., 2004; Grant et al., 2006).
In the present work, we performed functional Ca2+ imaging studies to dissect OHCs Ca2+ dynamics at their basolateral compartment. Using an ex vivo preparation of the cochlea from posthearing onset mice, we found that Ca2+ signals from VGCC are much smaller than those observed in IHCs (Wong et al., 2013; Moglie et al., 2018) but comparable in amplitude to those through α9α10 receptors. Moreover, they can be potentiated by RyR. On the contrary, Ca2+ transients produced by α9α10 activation were not affected by RyR, and were efficiently confined by cisternal Ca2+-ATPases. Thus, the present results indicate that these two Ca2+ sources in OHCs present different regulation mechanisms, despite a very close physical proximity.
Materials and Methods
Electrophysiological recordings from OHCs
Euthanasia and tissue extraction were conducted according to approved animal protocols of INGEBI Institutional Animal Care and Use Committee. Excised apical turns of 12- to 14-d-old mouse cochleas (BALB/c, either sex) were placed into a chamber on the stage of an upright microscope (Olympus BX51WI) and used within 2 h. OHCs were visualized on a monitor via a water immersion objective (60×), difference interference contrast optics and a CCD camera (Andor iXon 885). All recordings were performed at room temperature (22–25°C). Because of the short viability of the cochlear preparation and OHCs at this age, only one cell could be recorded per animal.
The cochlear preparation was superfused continuously at 2–3 ml/min with extracellular saline solution of an ionic composition similar to that of the perilymph: 144 mm NaCl, 5.8 mm KCl, 1.3 mm CaCl2, 0.7 mm NaH2PO4, 5.6 mm D-glucose, 10 mm HEPES buffer, 2 mm pyruvate, and 3 mm myo-inositol, pH 7.4. Working solutions containing different drugs were made up in this same saline and delivered through the perfusion system. Recording pipettes were fabricated from 1 mm borosilicate glass (WPI), with tip resistances of 6–8 MΩ. Series resistance errors were not compensated for.
For all experiments, the basic pipette solution was made from a 1.25× stock to reach a final concentration of 95 mm KCl, 40 mm K-ascorbate, 5 mm HEPES, 2 mm pyruvate, 6 mm MgCl2, 5 mm Na2ATP, 10 mm phosphocreatine-Na2, 0.5 mm EGTA, and 0.4 mm Ca2+ indicator (Fluo-4), pH 7.2. To avoid variations in OHCs volume during experiments, pressure in the recording system was controlled with a digital manometer and kept within 5- to 9-cm H2O range.
Efferent synaptic currents were evoked by unipolar electrical stimulation of the MOC efferent axons as described previously (Goutman et al., 2005; Ballestero et al., 2011). Briefly, the electrical stimulus was delivered via a 20- to 80-µm-diameter glass pipette which position was adjusted until postsynaptic currents in OHCs were consistently activated. An electrically isolated constant current source (model DS3, Digitimer) was triggered via the data-acquisition computer to generate pulses of 40–220 µA, 1-ms width. Further increasing stimulation intensity, or duration, did not produce any rise in release probability (or reduction in failure rate). Solutions containing acetylcholine (ACh) were applied by a gravity-fed multichannel glass pipette (150-µm tip diameter).
Electrophysiological recordings were performed using a Multiclamp 700B amplifier (Molecular Devices), low-pass filtered at 6 kHz and digitized at 50 kHz via a National Instruments board. Data were acquired using WinWCP (J. Dempster, University of Strathclyde). To maximize Ca2+ driving force during imaging experiments, OHCs were voltage clamped at −100 mV, but only during a brief period of time when stimulation was applied and synaptic responses were recorded (650 ms in paired pulse experiments, and 2 s in trains). Otherwise, cells were held at −40 mV. Electric shocks to MOC fibers were separated by intervals of 5 s in paired pulse experiments, and 30 s for trains. Recordings were analyzed with custom-written routines in IgorPro 6.37 (Wavemetrics). IPSCs were evaluated as successfully activated when met a double criteria: (1) the amplitude of the current right after the stimulation artifact was >3× SD of the basal current; and (2) the integral of the IPSC was >0.3 pC, which is the average integral of a single synaptic event (Ballestero et al., 2011).
Chemicals
Stock solutions of dantrolene, thapsigargin, and ryanodine (both at 1 and 100 μm) were prepared in DMSO and added to the intracellular solution, such that the concentration of DMSO in the pipette solution was 0.5% v/v in every case. All salts and drugs were acquired from Sigma except for ryanodine, dantrolene and thapsigargin which were purchased from Tocris.
Ca2+ imaging experiments
Ca2+ indicators were included in the patch-pipettes (at the concentration indicated before) allowing the diffusion into the cells. The preparation was illuminated with a blue LED system (Tolket) through the epifluorescence port of the widefield microscope, and images were acquired using an Andor iXon 885 camera controlled through a Till Photonics interface system. The focal plane was set close to the basal pole of OHCs where synapses are found. The signal-to-noise ratio was improved with an on-chip binning of 4 × 4, giving a resolution of 0.533 µm/pixel with the 60× water immersion objective. The image size was set to 50 × 50 pixels which allowed an acquisition rate of 140 frames/s. Image acquisition started 5 min after whole-cell break in to ensure the proper dialysis of the cell content and lasted up to 45 min. Images were analyzed with custom-written routines in IgorPro 6.37 (Wavemetrics).
A time lapse consisting of 250 images were taken for each experiment. An averaged image in each time lapse was used to determine the edge of the cell by an automatic thresholding algorithm. Within the cell borders, a donut-shaped mask covering the cell's cytoplasm was defined comprising 40–90% of the maximal fluorescence signal. The mask was divided in 24 radial regions of interest (ROIs) with its center set at the maximal intensity pixel of the cell. Fluorescence intensity was measured in every ROI for each time frame as ΔF/F0, or ΔF in cases where experimental treatments produced an effect on basal fluorescence (F0). Normalization by F0 is less critical in our experimental conditions provided that dye concentration is homogeneous throughout the imaging area (OHCs' cytoplasm), and also across recordings (always set by intracellular solution, 400 μm; Bootman et al., 2013). Two criteria were used to determine that a successful synaptic Ca2+ event occurred at a particular ROI: (1) a fluorescence peak was identified right after the MOC stimulus, with 2.5× higher amplitude than the standard deviation of the baseline fluorescence; and (2) the area under the curve (fluorescence trace) was larger than 0.11 [arbitrary units (A.U.)/s]. Finally, those ROIs that exhibited a consistent pattern of activation were selected as hotspots and used for further analysis of the fluorescence signal. Photobleaching was corrected for long acquisition protocols by fitting a line between prestimulus baseline and final fluorescence.
To determine the spread of the fluorescence change across the OHC cytoplasm, the response image when the fluorescence signal peaked was normalized to prestimulus fluorescence. Then, it was thresholded by fitting a bimodal distribution to the image histogram and the area and center of mass of the resulting mask calculated. Signal spread area was divided by the cell total area for comparison. The center of mass, which takes into account not only the area of the signal but also the fluorescence intensity in each point, was used to determine the location of the Ca2+ entry sites (both MOC and VGCC), and thus the distance between them.
Statistical analysis
Data are presented as group mean ± SEM and were analyzed with Infostat (Universidad Nacional de Córdoba). Effect sizes were calculated using R Statistical Software (RRID:SCR_001905) and the statsExpressions package. The presence of outlier datapoints was evaluated with Grubb's test. The Mann–Whitney test was used to perform comparisons between two groups and Kruskal-Wallis one-way analysis of variance followed by Conover's test for comparisons between multiple groups (non-corrected). Wilcoxon signed-rank and Friedman's test were used for comparisons between two and multiple paired samples, respectively. Non-parametric statistics were preferred given our sample sizes and the impossibility of testing the assumptions of parametric procedures. r value was used to inform effect sizes for comparison between two groups and ϵ2 value for comparison between multiple groups. Kendall's W was used to inform effect sizes for comparison between multiple paired samples. F test was used to compare models fitting in Figure 5C. Differences between samples were considered significant when p < 0.05.
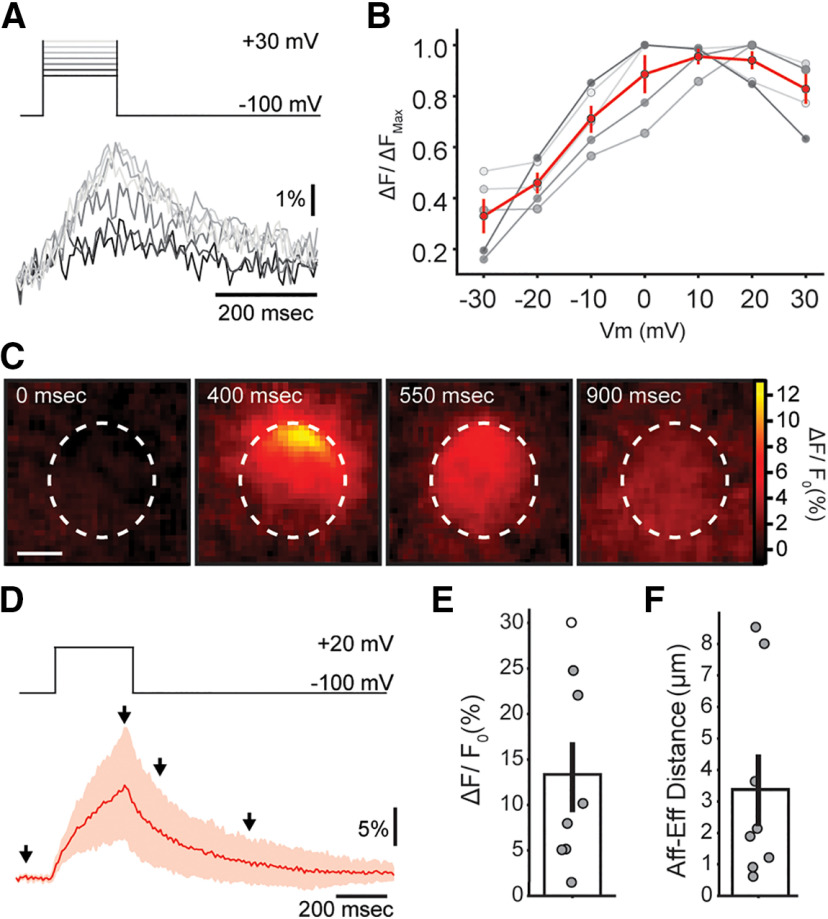
Figure 5.
Ca2+ transients during VGCC activation in OHCs. A, Representative traces of Ca2+ transients measured at the brightest ROI, for voltage steps between −30 and + 30 mV. B, Normalized maximum for Ca2+ transients in each cell (gray symbols) as a function of voltage. Red trace and marks represent mean ± SEM. C, Sequence of images showing the localized Ca2+ concentration increase in an OHC during 300-ms depolarization to +20 mV. Scale bar: 5 μm. D, Mean trace of the Ca2+ signal (in red, 95% confidence interval in light red) during a step pulse to +20 mV (top panel). E, Average ΔF/F0 for +20-mV steps in OHCs. F, Average distance between locations of afferent (VGCC) and efferent (MOC) Ca2+ signals within each OHC. Bar plots are mean ± SEM. Friedman's test; *p < 0.05.
Results
Local ACh application evokes a global Ca2+ rise in OHCs
To directly measure Ca2+ influx and spread by activation of α9α10 receptors, the Ca2+-sensitive indicator Fluo-4 was loaded into cells through the patch-clamp electrode (Fig. 1A). In a first approach, a local application pipette was used to puff ACh onto the organ of Corti preparation. Figure 1B presents a series of images taken at the OHCs base, before and after ACh application (Fig. 1A for representation of imaging focal plane). The first image in the sequence also shows an array of ROIs designed to measure fluorescence changes in the cytoplasm as a function of time (% ΔF/F0, unless otherwise indicated). The application of a nearly saturating ACh concentration (1 mm; Lioudyno et al., 2004; Gómez-Casati et al., 2005) produced a global and long lasting elevation of cytoplasmic Ca2+ (images in Fig. 1B; and traces of fluorescence intensity as a function of time in Fig. 1C). The mean peak of the ΔF/F0 signal was 311 ± 65%, with a corresponding electrophysiological response integral of 1.9 ± 0.3 nC (n = 6; Fig. 1C–E). These values were considered as an upper limit of efferent activation, since this very high concentration of an externally applied agonist activates receptors distributed throughout the surface of the cell and bypasses presynaptic synaptic vesicle availability.
Figure 1.
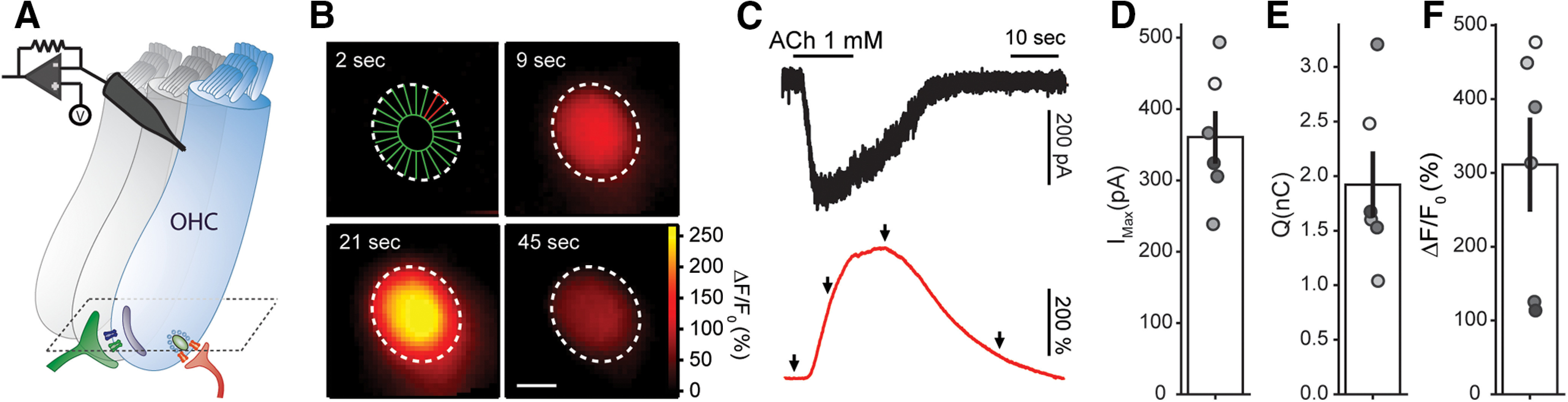
ACh evokes global Ca2+ transients in OHCs. A, Illustration of a recorded OHCs within the intact organ of Corti preparation, with synaptic connections (green MOC terminal, red Type II afferent), and a scheme of the imaging focal plane (dashed lines). B, Sequence of wide-field microscopy images of an OHC (loaded with Fluo-4) during ACh 1 mm perfusion. Dotted white lines represent the outer margin of the OHC's fluorescence signal. First image shows ROI design scheme in which the cell's cytoplasm was divided in 24 radial ROIs (see Materials and Methods). Scale bar: 5 μm. C, Black trace corresponds to the whole-cell current recorded during ACh perfusion (Vhold = –100 mV) and red trace to the ΔF/F0 signal measured at the ROI depicted in red on panel B. Arrows indicate the time points of images shown on panel B. D–F, Peak current (D), charge (E), and maximal ΔF/F0 (F) during ACh perfusion. Bar plots are mean ± SEM.
Cholinergic synaptic Ca2+ signals in OHCs
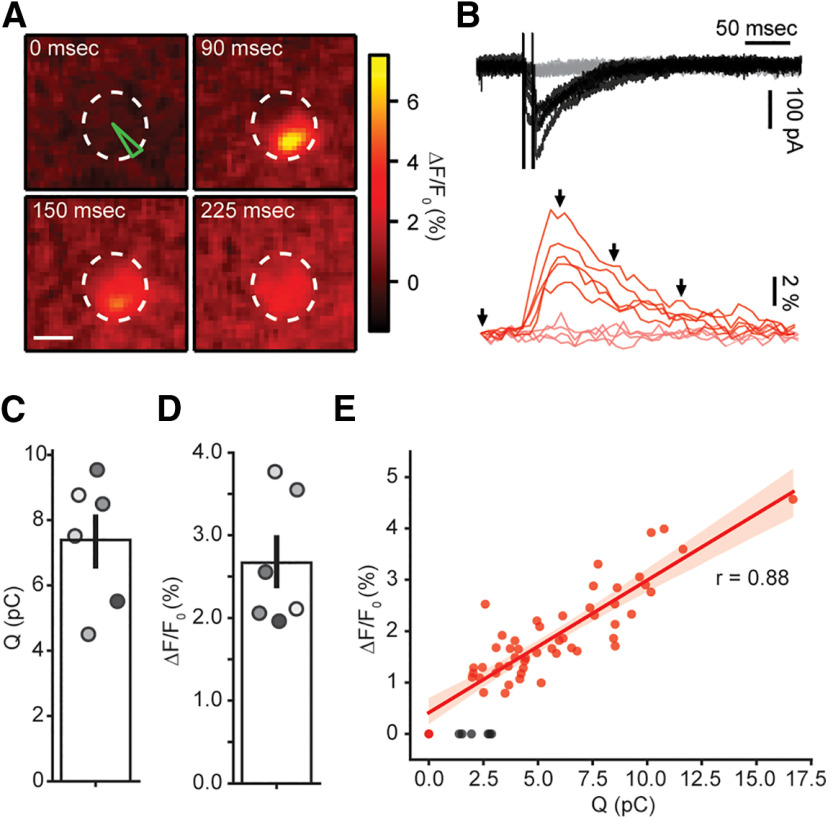
An alternative and more physiological approach to investigate efferent input to OHCs was undertaken by electrically stimulating MOC axons innervating these cells, to evoke ACh synaptic release. Figure 2A shows a series of images of an OHC during a typical protocol of MOC fibers stimulation. In contrast to that observed with ACh external applications, local and brief Ca2+ transients were seen in response to synaptic activation. Only one Ca2+ entry site was observed in each recorded OHC (n = 6 cells). Representative traces of fluorescence changes at the brightest ROI are shown in the bottom panel of Figure 2B, red traces, whereas the corresponding synaptic currents recorded in the same trials are included in the top panel (black traces). α9α10 receptors are highly Ca2+ permeable, but because of the much higher concentration of Na+ and K+ in the extracellular solution (150 mm of Na+ plus K+ vs 1.3 mm Ca2+; see Materials and Methods), the majority of the current allowed in physiological conditions is carried by monovalent cations (Weisstaub et al., 2002). Two technical measures contributed to improve the detection of the Ca2+ transients: the use of a high affinity indicator (Fluo-4) to resolve small changes in Ca2+ concentration; and the transient voltage-clamping at −100 mV (see Materials and Methods) to maximize Ca2+ driving force. Thus, under these experimental conditions efferent stimulation produced inward currents as the result of both α9α10 receptors and SK2 channels activation (K+ equilibrium potential: ∼−82 mV). In addition, paired pulses were used instead of single stimuli to increase the otherwise very low synaptic release probability (Ballestero et al., 2011; Vattino et al., 2020). Because of frame rate limitations, the imaging signal appears as the ensemble activation in response to both stimuli in a pair. An average of 100 ± 22 stimulation trials was performed per cell (range 60–200, n = 6 cells), with a synaptic success rate of 87 ± 3% (range: 73–98%, n = 6), judged by the presence of IPSCs (see Materials and Methods). In turn, Ca2+ signals were detected in 83 ± 5% (range: 64–99%, n = 6) of trials with successful synaptic currents. The average Ca2+ signal obtained with this protocol presented an amplitude of 2.7 ± 0.3% ΔF/F0 (n = 6 cells), whereas the integral of the synaptic currents was 7.4 ± 0.8 pC, excluding synaptic failures for the calculation of both averages (n = 6; Fig. 2C,D). Imaging and electrophysiological results were highly correlated, as shown in Figure 2E (r = 0.88 in this representative example, range: 0.65–0.95, n = 6). This strong correlation indicates that the imaging signal is a good proxy for α9α10 and SK2 synaptic activation, most likely reflecting Ca2+ influx through nicotinic receptors. Note that failures in detecting imaging events coincided with the smallest IPSCs (Fig. 2E, black symbols).
Figure 2.
Efferent fiber electrical stimulation evokes localized Ca2+ signals in OHCs. A, Sequence of images showing the localized Ca2+ increase in an OHC following efferent fiber stimulation. Scale bar: 5 μm. B, top, Representative whole-cell current traces during doble-pulse efferent electrical stimulation at 100 Hz (Vh = −100 mV). Black traces represent those trials where an eIPSC was detected after the stimulus artifact (failures, in gray). Bottom, Representative Ca2+ transients taken at the ROI indicated in green on panel A, for the same trials shown on the top panel. Red traces correspond to trials were an IPSC was detected and failures in pink. C, D, Mean charge (C) and ΔF/F0 (D) for successful efferent fiber stimulation trials. Bar plots are mean ± SEM. E, Size of Ca2+ transients as function of charge during efferent stimulation trials in a representative OHC. Black dots represent successful IPSC events with no detectable fluorescence signal.
Synaptic Ca2+ signals during trains of efferent stimuli
MOC neurons can be activated as a result of a feedback loop starting in the afferent pathway and producing steady firing of efferent neurons at rates of up to 200 s−1 (Liberman, 1988; Brown, 1989; Guinan, 2006). Repetitive activation of MOC axons results in presynaptic facilitation of neurotransmitter release (Ballestero et al., 2011). To explore how train stimulation affects synaptic Ca2+ transients, MOC fibers were stimulated at 20, 40, and 80 Hz (300-ms duration each; Fig. 3). Images included in Figure 3A were taken at the peak of the Ca2+ rise for each train, whereas Figure 3B shows average synaptic currents (top, in black) and the corresponding Ca2+ signals (bottom, in red) taken at the brightest ROI in each cell. Ca2+ levels varied with the frequency of the stimulation train, with average amplitudes of 5.1 ± 1.1% ΔF/F0, 9.5 ± 2.2% ΔF/F0 and 15.6 ± 2.5% ΔF/F0 at 20, 40, and 80 Hz, respectively (n = 8; Wkendall = 0.706, k = 3; p = 0.0001 Friedman's test; Fig. 3C). The integral of the ensemble synaptic response across the duration of the train was 23.6 ± 6.2 pC, 43.3 ± 11.0 pC and 64.1 ± 8.8 pC (n = 8; Wkendall = 0.937, k = 3; p < 0.0001 Friedman's test; Fig. 3D). A positive correlation was observed between the integral of synaptic currents elicited by trains at different frequencies and the amplitude of Ca2+ transients (r = 0.88; Fig. 3E). Moreover, whereas trains at 20 Hz elicited a localized Ca2+ rise with a measurable spread within the OHCs cytoplasm of 31 ± 5% (25.9 ± 4.8 µm2) of the total imaged area (74.0 ± 8.8 µm2), this value increased to 48 ± 6% (32.1 ± 4.4 µm2) and 63 ± 5% (43.8 ± 4.0 µm2) for 40 and 80 Hz, respectively (n = 8; Wkendall = 0.762, k = 3; p < 0.0001 Friedman's test; Fig. 3F).
Figure 3.
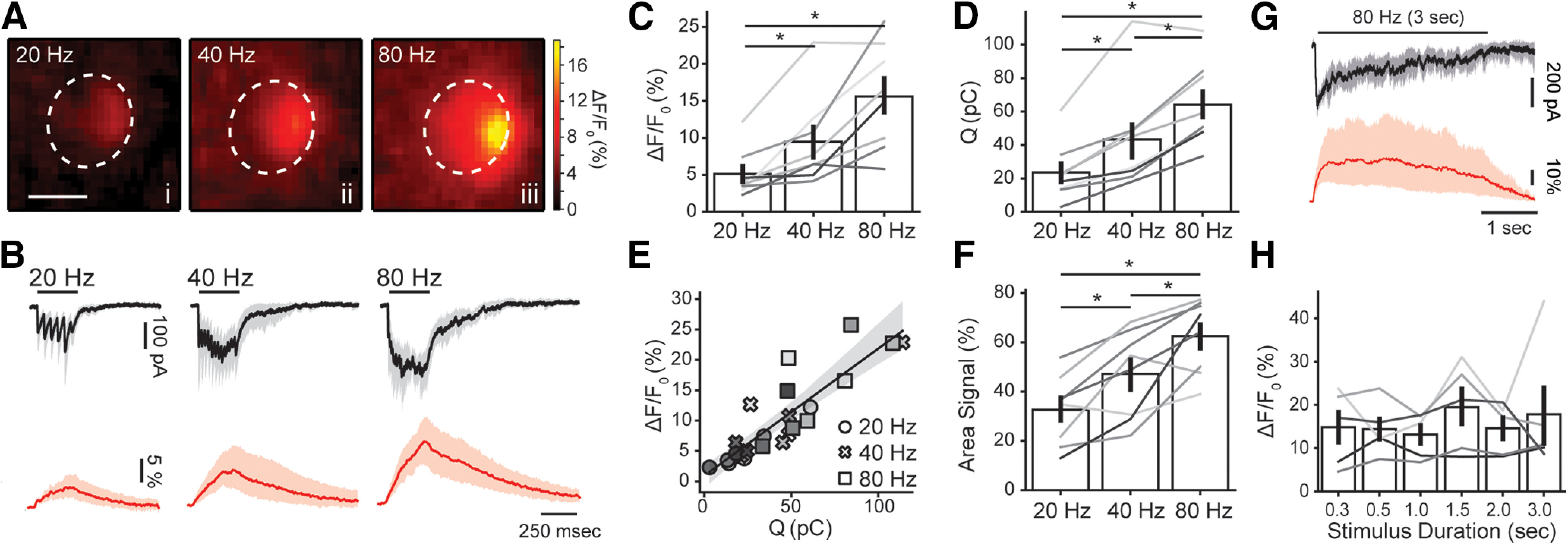
Amplitude and spread of efferent Ca2+ transients are dependent on stimulation frequency. A, Representative images of an OHC at the peak of the fluorescence signal during efferent fiber electrical stimulation at 20 (i), 40 (ii), and 80 (iii) Hz. B, Mean inhibitory current traces (black, 95% confidence intervals in gray) recorded during 300 ms efferent fiber electrical stimulation at 20, 40, and 80 Hz (Vh = −100 mV). Red traces show the mean ΔF/F0 (95% confidence intervals in light red) at the ROI with the highest fluorescence signal. Peak Ca2+ values (C) and charge (D) for 300-ms stimulation trains at tested frequencies. E, Amplitude of fluorescence signal as a function of charge. Each symbol represents a different stimulation frequency. F, Spread of the Ca2+ signal within each OHC cytoplasmic space (as percentage of the area in the imaged plane). Values were taken at the time point where the signal peaked. G, Mean whole-cell synaptic response (black, 95% confidence intervals in gray) and Ca2+ signals (red, 95% confidence intervals in light red) obtained during a 3-s electrical stimulation of efferent fibers at 80 Hz. H, Peak fluorescence amplitude for trains with duration between 0.3 and 3 s (at 80 Hz). Bar plots are mean ± SEM. Friedman's test; *p < 0.05.
Comparing Ca2+ signals in Figures 1–3, it becomes clear that synaptic activation of α9α10 receptors during trains is (at least) ∼20 times smaller than that obtained with locally applied ACh. This might result from the low release probability of MOC terminals (Ballestero et al., 2011; Vattino et al., 2020), in addition to the fact that the stimulation paradigm typically activates a single axon (see Materials and Methods). Moreover, activation by external ACh does not rely on vesicle availability and likely activates both synaptic and extrasynaptic receptors in OHCs. This drastic difference in measurable ΔF/F0 amplitude between exogenous ACh application (Fig. 1) and electrical stimulation (Figs. 2, 3) suggests that the Ca2+ indicator used in these experiments does not saturate even with high-frequency synaptic trains. The tight correlation between ΔF/F and charge in Figure 3E further supports this conclusion.
In order to obtain a better understanding of Ca2+ dynamics during sustained MOC activity, trains of stimuli at 80 Hz were applied for longer periods of time (0.3–3 s; Fig. 3G,H). A sustained Ca2+ load was observed in each of these train durations, but peak values did not differ (n = 5; Wkendall = 0.5, k = 6; p = 0.57 Friedman's test; Fig. 3H). This latter result suggests that OHCs have a tight mechanism for controlling Ca2+ overload and spread at high-frequency efferent stimulation. The role of the subsynaptic cistern in this phenomenon has been suggested in the past (Sridhar et al., 1997; Evans et al., 2000; Lioudyno et al., 2004) and was evaluated in the following section.
Modulation of efferent Ca2+ transients by subsynaptic cisterns
SERCA pumps, located in OHCs subsynaptic cisterns, have been suggested to modulate synaptic currents through α9α10 receptors (Lioudyno et al., 2004). To address a possible role of SERCA in shaping Ca2+ transients, the specific blocker thapsigargin (1 μm) was added to the bath during an OHC recording. In the absence of any stimulation, the basal fluorescence increased when perfusing thapsigargin from a control value of 1396 ± 551 to 1851 ± 502 A.U. (n = 6; r = 0.898; p = 0.024 Wilcoxon test; Fig. 4B, inset). This ∼30% increase in the basal cytoplasmic concentration of Ca2+ might represent the basal SERCA activity responsible for pumping ions out of the OHC cytoplasm at rest.
Figure 4.
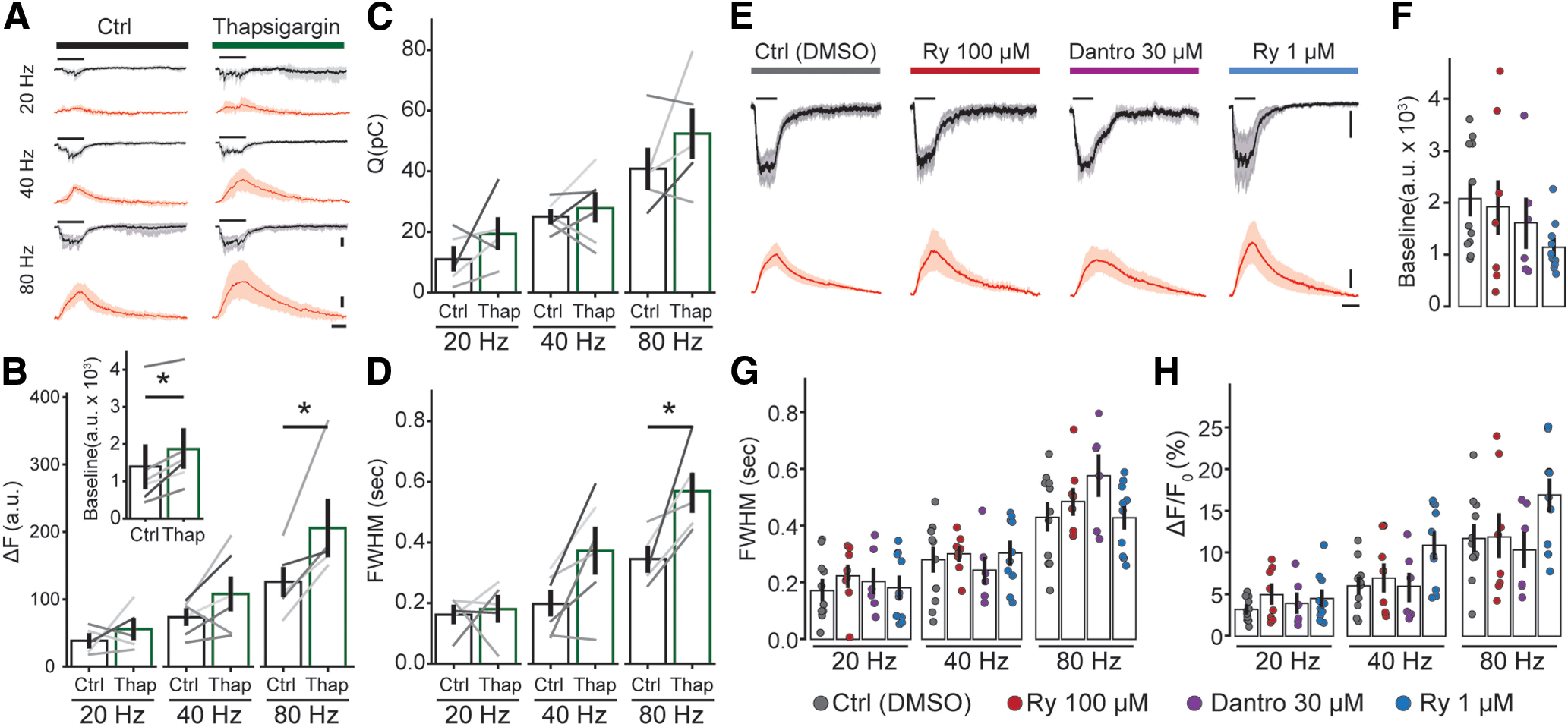
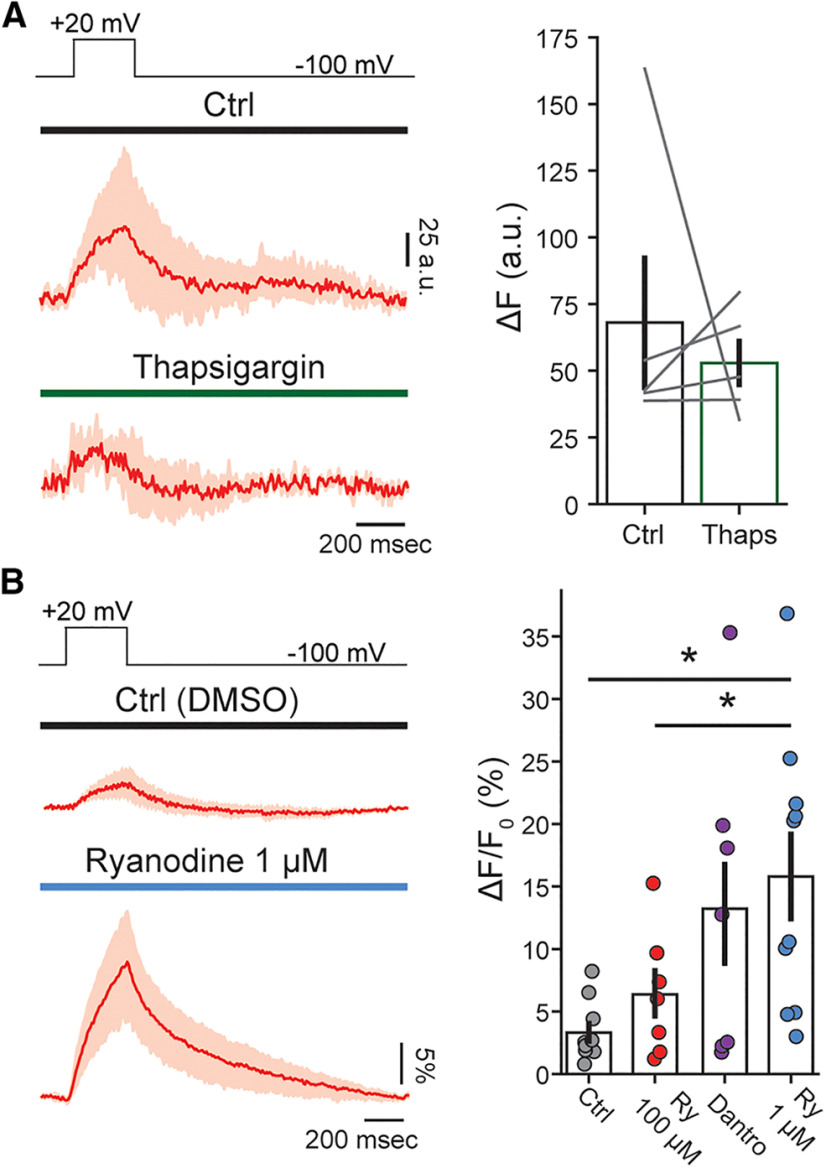
Efferent Ca2+ signals are affected by blocking cisternal ATPases, but not by modulators of RyRs. A, Mean synaptic responses (black, 95% confidence intervals in gray) and Ca2+ transients (red, 95% confidence intervals in light red) during a 300-ms electrical stimulation at 20, 40, and 80 Hz before and after perfusion of thapsigargin (x scale bar: 250 ms; y scale bar: 50 A.U. and 100 pA). B, Peak of Ca2+ transients (as ΔF). C, Charge of synaptic responses. D, Duration of Ca2+ transients (as FWHM). Inset, Baseline fluorescence signal before and after perfusion of thapsigargin. E, Synaptic currents (mean, black, confidence intervals in gray) and Ca2+ transients (red, confidence interval in light red) obtained during efferent fiber stimulation (300 ms, 80 Hz), using an intracellular solution containing vehicle (DMSO), RyR blockers (ryanodine 100 μm or dantrolene 30 μm), or a RyR agonist (ryanodine 1 μm; x scale bar: 250 ms; y scale bar: 50% and 100 pA). F, Baseline fluorescence for each condition. Duration (FWHM; G) and maximal fluorescence signal (as ΔF/F0; H) for each intracellular solution. Bar plots are mean ± SEM. Wilcoxon signed-rank test; *p < 0.05.
In a following set of experiments, trains of MOC stimuli were applied before and during the application of thapsigargin (Fig. 4A–D). Peak ΔF values were calculated instead of ΔF/F0 (Fig. 4B), to avoid an incorrect estimation of Ca2+ transients size that would result from dividing ΔF by a larger basal fluorescence in the presence of thapsigargin. A similar approach using ΔF has been applied in the past to avoid misleading calculations of Ca2+ transients size (Kennedy, 2002; Frank et al., 2009; Moglie et al., 2018; see Materials and Methods). Synaptic currents integral are shown in Figure 4C. As previously described for ΔF/F0 (Fig. 3), peak ΔF values increased with the frequency of stimulation in control conditions: 38.4 ± 8.6, 73.4 ± 10.5, and 125.9 ± 21.8 A.U. for 20-, 40-, and 80-Hz trains (n = 5, p = 0.0085 Friedman's test). In the presence of thapsigargin, ΔF values grew to 55.8 ± 14.1, 107.9 ± 24.5, and 205.7 ± 39.9 A.U. for 20, 40, and 80 Hz, respectively (n = 6), showing statistically significant differences for the 80-Hz train compared with control (r20 Hz = 0.301, r40 Hz = 0.641, r80 Hz = 0.903; p = 0.04, Wilcoxon signed-rank test).
The effect of thapsigargin on the duration of the synaptic Ca2+ transients was analyzed, by measuring the full width at half maximum (FWHM) of the signal. In control conditions, FWHM values varied with the stimulation frequency: 162 ± 27 ms at 20 Hz, 198 ± 42 ms at 40 Hz, and 345 ± 38 ms at 80 Hz (n = 5; p = 0.0097, Friedman's test). For the 20 Hz trains, the FWHM of the transient was largely shorter than the stimulating train duration (300 ms) because of sporadic synaptic activation. Thapsigargin prolonged Ca2+ transients with average values of 180 ± 42 ms at 20 Hz, 372 ± 75 ms at 40 Hz and 569 ± 62 ms at 80 Hz (n = 5; r20 Hz = 0.06, r40 Hz = 0.812, r80 Hz = 0.903; p = 0.048 Wilcoxon signed-ranked test for the 80-Hz train compared with control). Taken together, these results indicate that SERCA pumps, most likely present at subsynaptic cistern (Lioudyno et al., 2004), play a significant role in accelerating the removal of Ca2+ entering through α9α10 at efferent synapses and also in curtailing the peak of the transient.
Since the presence of RyR has been described morphologically, and indirectly through the modulation of MOC-OHC synaptic function by ryanodine (Evans et al., 2000; Lioudyno et al., 2004; Grant et al., 2006), in the following experiments we directly tested Ca2+ dynamics at efferent synapses in the presence of drugs that modulate RyR. To avoid indirect or presynaptic effects (Zhang et al., 2020), drugs were included in the patch pipette (control experiments were performed in the presence of vehicle, DMSO). Low (1 μm) and high (100 μm) concentrations of ryanodine were used to either activate or block RyR (McGrew et al., 1989; Lanner et al., 2010). Dantrolene, a specific inhibitor of these receptors, was also tested. Interestingly, none of these treatments showed any effect on either the amplitude of synaptic Ca2+ transients (ϵ220 Hz = 0.04/p20 Hz = 0.70, ϵ240 Hz = 0.19/p40 Hz = 0.08, ϵ280 Hz = 0.17/p80 Hz = 0.25; Kruskal–Wallis test; same for ΔF; Fig. 4E,H), duration (estimated as FWHM; ϵ220 Hz = 0.04/p20 Hz = 0.72, ϵ240 Hz = 0.04/p40 Hz = 0.71, ϵ280 Hz = 0.12/p80 Hz = 0.13; Kruskal–Wallis test; Fig. 4E,G), or basal Ca2+ (ϵ2 = 0.12; p = 0.24 Kruskal–Wallis test; Fig. 4F). The corresponding values for these experiments are shown in Table 1. These results preclude the participation of RyR in the modulation of OHCs efferent responses.
Table 1.
Values for integral of synaptic currents, amplitude and duration Ca2+ signals during step depolarization of OHCs or MOC stimulation at 20-, 40-, and 80-Hz trains, in different pharmacological conditions
| Q (pC) | ΔF/F0 (%) | FWHM (s) | ||
|---|---|---|---|---|
| Depolarization | Ctrl (DMSO) | — | 3.3 ± 0.8 (n = 10) | 0.22 ± 0.04 (n = 7) |
| Ryanodine 100 μm | — | 6.4 ± 1.9 (n = 7) | 0.21 ± 0.08 (n = 7) | |
| Dantrolene 30 μm | — | 13.2 ± 4.7 (n = 7) | 0.27 ± 0.07 (n = 7) | |
| Ryanodine 1 μm | — | 15.8 ± 3.5 (n = 10) | 0.35 ± 0.02 (n = 10) | |
| 20 Hz | Ctrl (DMSO) | 20.0 ± 4.0 (n = 11) | 3.2 ± 0.4 (n = 11) | 0.17 ± 0.03 (n = 11) |
| Ryanodine 100 μm | 20.6 ± 3.5 (n = 8) | 5.0 ± 1.1 (n = 8) | 0.22 ± 0.04 (n = 8) | |
| Dantrolene 30 μm | 12.8 ± 2.0 (n = 6) | 3.9 ± 1.2 (n = 6) | 0.20 ± 0.04 (n = 6) | |
| Ryanodine 1 μm | 17.1 ± 4.9 (n = 10) | 4.5 ± 0.9 (n = 10) | 0.18 ± 0.04 (n = 10) | |
| 40 Hz | Ctrl (DMSO) | 34.3 ± 5.0 (n = 11) | 6.0 ± 1.8 (n = 11) | 0.28 ± 0.04 (n = 11) |
| Ryanodine 100 μm | 31.6 ± 4.4 (n = 8) | 7.0 ± 1.6 (n = 8) | 0.30 ± 0.02 (n = 8) | |
| Dantrolene 30 μm | 25.6 ± 3.3 (n = 6) | 6.0 ± 1.7 (n = 6) | 0.24 ± 0.05 (n = 6) | |
| Ryanodine 1 μm | 37.1 ± 6.3 (n = 10) | 10.9 ± 1.5 (n = 10) | 0.30 ± 0.04 (n = 10) | |
| 80 Hz | Ctrl (DMSO) | 61.2 ± 6.3 (n = 11) | 11.7 ± 1.6 (n = 11) | 0.43 ± 0.05 (n = 11) |
| Ryanodine 100 μm | 47.7 ± 5.7 (n = 8) | 11.9 ± 2.6 (n = 8) | 0.49 ± 0.04 (n = 8) | |
| Dantrolene 30 μm | 42.4 ± 2.1 (n = 6) | 10.3 ± 2.2 (n = 6) | 0.58 ± 0.07 (n = 6) | |
| Ryanodine 1 μm | 60.3 ± 6.7 (n = 10) | 16.9 ± 1.9 (n = 10) | 0.43 ± 0.04 (n = 10) |
Ca2+ entry through L-type Ca2+ channels
Ca2+ influx through VGCC was investigated by applying step depolarizations to OHCs, from a holding potential of −100 mV, up to a range of potentials between −30 and +30 mV. Representative Ca2+ transients are shown in Figure 5A, with peak values that followed the expected bell-shaped dependence on membrane potential, and maxima at +10/+20 mV (Fig. 5B). Only one Ca2+ entry spot was observed per OHC in these experiments.
Step depolarizations to +20 mV for 300 ms (same duration as that of MOC train stimulation in Figs. 3, 4) evoked Ca2+ transients with an average peak of 13.3 ± 3.8% ΔF/F0., with highly variable values that ranged from 1.5% to 30.1% ΔF/F0. This average matched in size with transients obtained with 40 and 80 Hz MOC trains (n = 8; p20 HZ = 0.02, p40 Hz = 0.82, p80Hz = 0.37, Friedman's test; WKendall = 0.263, k = 4; Figs. 3C, 5C–E). Moreover, the spread of the fluorescence signal was also equivalent to that observed with the 40- and 80-Hz trains of efferent stimulation [57 ± 7% of OHC area (36.0 ± 3.2 µm2); p20 HZ < 0.001, p40 Hz = 0.37, p80Hz = 0.37, Friedman's test; WKendall = 0.692, k = 4].
Previous evidence indicates that Type II afferents on OHCs are closely positioned with efferent synapses and subsynaptic cisterns (Fuchs et al., 2014; Saito, 1980, 1990). A functional quantification of this proximity was evaluated by measuring the physical distance between the centers of mass of VGCC and MOC Ca2+ transients within a given cell (see Materials and Methods; Fig. 5C,F). An average value of 3.7 ± 1.1 µm (n = 8) was estimated, ranging from 0.6 to 8.5 µm, with five out of eight cells showing values ≤2 µm. Because of the proximity of VGCC Ca2+ signals to synaptic cisterns, and the large spread, the possible modulation by SERCA and RyR was further investigated.
No changes in the amplitude of the depolarization-evoked Ca2+ signals were observed in the presence of the SERCA blocker thapsigargin (control ΔF: 68 ± 24 A.U., thapsigargin ΔF: 53 ± 9, n = 5; r = 0.30; p = 0.58 Wilcoxon signed-rank test; Fig. 6A,B). In addition, neither 100 μm ryanodine nor dantrolene modified VGCC signals (6.4 ± 1.9% ΔF/F0; n = 7; p = 0.344 Kruskal–Wallis test, and 13.2 ± 4.7% ΔF/F0; n = 7; p = 0.073 Kruskal–Wallis test, respectively) indicating that CICR was not elicited during +20 mV voltage pulses in our recording conditions. In contrast, 1 μm ryanodine, a concentration known to increase RyR opening time (McGrew et al., 1989), elicited a strong potentiation of the Ca2+ transient, with average peak values of 15.8 ± 3.5% ΔF/F0, compared with 3.3 ± 0.8% ΔF/F0 in control experiments with vehicle (DMSO) in the pipette (n = 10 and n = 7, respectively; p = 0.001 Kruskal–Wallis test, and also p = 0.046 compared with 100 μm ryanodine; ϵ2 = 0.34; Fig. 6C,D), suggesting that RyR can be recruited during VGCC Ca2+ events. Taken together, these data suggest that VGCC Ca2+ transients are not controlled by SERCA pumps, but could be modulated by RyR present in synaptic and/or lateral wall cisterns (Lioudyno et al., 2004; Grant et al., 2006).
Figure 6.
Modulation of afferent Ca2+ influx through VGCC by thapsigargin and ryanodine. A, Mean traces of the Ca2+ transients obtained with 300-ms depolarization to +20 mV before (top) and during perfusion of thapsigargin (bottom). On the right, average peak Ca2+ level (ΔF) for step depolarizations. B, Mean traces of the Ca2+ transients during with steps to +20 mV, using intracellular solutions containing DMSO or ryanodine 1 μm. Average peak Ca2+ signal (ΔF/F0) are shown on the right for experimental conditions in low and high concentrations of ryanodine (Ry; 1 and 100 μm) and dantrolene (30 μm). Wilcoxon signed-rank test; *p < 0.05.
Discussion
The present results provide a direct analysis of synaptic Ca2+ dynamics in OHCs triggered by MOC (efferent) and VGCC (afferent) activation. It also shows evidence for cisternal modulation of amplitude, spread, and duration of Ca2+ transients. Significantly, only one efferent Ca2+ hotspot was found per OHC, suggesting that a single MOC fiber is stimulated at a time, although more MOC-OHC synaptic contacts are present (Liberman, 1990; Warr, 1992). The amplitude of Ca2+ transients shows a strong dependence on MOC stimulation at frequencies between 20 and 80 Hz (Fig. 3), which might explain the reported changes in MOC inhibitory strength as a function of stimulation rate (Galambos, 1956; Art et al., 1984; Gifford and Guinan, 1987). On the other hand, a single Ca2+ spot was also observed when activating VGCC by step depolarizations, although multiple contacts with Type II afferent coexist in one OHC within a close proximity (Fuchs and Glowatzki, 2015). Moreover, although Ca2+ signals produced by activation of VGCC were similar in amplitude to those through α9α10 receptors, they were significantly smaller to those reported for IHCs (Frank et al., 2009; Wong et al., 2013; Moglie et al., 2018). This most likely relates to the weak capacity of OHCs to release neurotransmitter and activate Type II afferents compared with the multivesicular release from IHC ribbon synapse, responsible for encoding timing, intensity and frequency in each Type I afferent fiber (Fuchs and Glowatzki, 2015).
Ca2+ homeostasis in OHCs and efferent regulation
Several studies have reported alternative mechanisms that operate in OHCs to handle Ca2+ influx, with special attention on Ca2+ entering through MET channels (Fettiplace and Nam, 2019). Thus, the integrity of OHCs and cochlear function relies on the normal operation of mechanisms such as buffering and extrusion by plasma membrane ATPases present in stereocilia (Kozel et al., 1998; Tong et al., 2016). In addition, mitochondria, abundantly found in a layer right below the cuticular plate in OHCs, play the important role of containing Ca2+ leak into the basolateral compartment (Furness and Hackney, 2006; Beurg et al., 2010).
In MOC synapses located at the basal pole of OHCs, Ca2+ influx through α9α10 receptors rapidly activates SK2 channels at the plasma membrane because of a tight functional coupling (Oliver et al., 2000). The large synaptic cisterns located at a close physical distance of OHCs plasma membrane determines a restricted diffusion space where Ca2+ ions entering through α9α10 accumulate (Fuchs et al., 2014). However, since efferent fibers operate best at prolonged and high-frequency MOC activation (Robertson and Gummer, 1985; Brown, 1989), the physical barrier provided by the synaptic cisterns might not be sufficient to prevent Ca2+ leak onto OHCs cytoplasm. The confinement of efferent Ca2+ might be crucial to avoid interaction with off targets, such as activation of exocytosis at Type II afferent synapses (see below), modulation of prestin-driven electromotility (Dallos et al., 1997; Frolenkov et al., 2000), or even excitotoxicity (Choi, 1995). The present results provide evidence indicating that at high-frequency efferent stimulation cisternal ATPases (SERCA) come into play to control excessive Ca2+ spread and prevent its spill over. This effect of cisternal ATPases is strongest at 80-Hz trains, a condition that leads to the largest Ca2+ load.
It should be noted that, because of the small domain determined by OHCs plasma membrane and synaptic cisterns, and also the spread of the Ca2+ transients (Fig. 3F), it is very likely that under our experimental conditions Ca2+ signals recorded at high-frequency MOC stimulation result, at least partially, from Ca2+ that escaped into the cytoplasm. Nevertheless, one should take into account that under normal physiological conditions the high concentration of intracellular buffers (Sakaguchi et al., 1998; Hackney et al., 2005) might restrain Ca2+ diffusion. Moreover, because of the fragility of the tissue, as well as the instability of OHCs recordings from older animals, experiments were performed within the postnatal day (P)12–P14 age range and room temperature. Therefore, some of our conclusions may differ in more mature OHCs and/or at body temperature.
Our results do not provide support for the participation of RyR during MOC activation within a wide range of stimulation strengths (20- to 80-Hz trains of 300 ms of duration). This result contrasts with previous observations in OHCs in which the role of RyR was evaluated indirectly by measuring efferent synaptic activation (Lioudyno et al., 2004). We cannot preclude that RyR might engage if preceded by hair cell excitation, which firstly raises cytoplasmic Ca2+ concentration, and as a consequence of this paves the way for cisternal load through SERCA activity. This mechanism has been proposed to occur in chick and immature hair cells from rats (Im et al., 2014; Zachary et al., 2018), but whether it is relevant in OHCs remains to be proven. The observation that MOC inhibition in vivo is potentiated by a preceding auditory stimulation might indicate that this phenomenon could be functional in hearing (Kujawa and Liberman, 1999).
Ca2+ influx through VGCC and Type II afferent activation
Ca2+ currents through VGCC in OHCs are several fold smaller than in IHCs and present a shift to the right in the current–voltage relation (Knirsch et al., 2007; Johnson and Marcotti, 2008; Wong et al., 2013). Accordingly, in the present work we show that Ca2+ signals peaked at +20 mV (−20 mV for IHCs), and their amplitudes (13.3% ΔF/F0, 300-ms step) were 3-fold smaller compared with brief depolarizations to submaximal potentials in IHCs (42% ΔF/F0, 20 ms to −30 mV; Moglie et al., 2018). This result adds to other features of afferent synapses in OHCs indicating that the release probability is small in these cells when compared with IHCs. Thus, ribbons in active zone areas with Type II afferents are small, irregular in shape, and can accommodate few vesicles (Fuchs and Glowatzki, 2015). However, Ca2+ transients activated by VGCC were further potentiated by a RyR agonist (Fig. 6), suggesting that CICR mechanisms can boost synaptic strength in contacts with Type II afferents. CICR has been shown to modulate vesicle release and recruitment on afferent synapses of hair cells from frogs and turtles (Lelli et al., 2003; Castellano-Muñoz et al., 2016). The fact that the specific RyR antagonists, dantrolene and ryanodine at high concentrations, failed to inhibit Ca2+ transients produced by VGCC activation (Fig. 6B) most likely indicates that in our stimulating conditions (300-ms step depolarization to +20 mV) intracellular Ca2+ does not reach the critical concentration necessary to trigger CICR processes. Taken together, it can be concluded that VGCC Ca2+ transients are susceptible to RyR modulation, but that stronger OHCs excitation is required to evoke CICR in the absence of pharmacological modulators.
Whether RyRs involved in VGCC-mediated Ca2+ boost are located in OHCs synaptic cisterns (Lioudyno et al., 2004; Grant et al., 2006), or lateral wall cisterns (Grant et al., 2006; Ranum et al., 2019), or even at other intracellular organelles is still unknown. If RyRs located in sublateral walls cisterns were responsible for VGCC potentiation (Grant et al., 2006; Ranum et al., 2019), an independent functioning of afferent and efferent synapses would be expected. Alternatively, if synaptic cisterns were involved in RyR potentiation of VGCC signals, a close interaction between afferent and efferent synapses could occur in OHCs. Thus, Ca2+ entering through α9α10 receptors would be taken up by SERCA pumps, filling the cisterns with Ca2+ and providing fuel for boosting transmitter release at Type II afferents through activation of RyRs.
Both functional (Fig. 6E) and structural (Saito, 1990; Fuchs et al., 2014) evidence indicates that the diffusion interval between afferent and efferent synaptic locations is very short. This observation together with the finding of a wide cytoplasmic Ca2+ spread obtained with high-frequency MOC stimulation (Fig. 3F) suggests that efferent to Type II afferent crosstalk in OHCs might occur during efferent activation. In contrast to what was observed in developing IHCs where afferent and efferent Ca2+ signals have opposing roles (Moglie et al., 2018), activation of MOC and Type II afferents synapses might both occur in loud environments (Robertson and Gummer, 1985; Brown, 1989; Fuchs and Glowatzki, 2015; Weisz et al., 2021). Therefore, coincident activation of the two synapses onto OHCs, whether causal or not, would not be rare.
In conclusion, in the present work, we show for the first time Ca2+ dynamics at the base of OHCs. Interestingly, intracellular cisterns and organelles play important and differential roles in Ca2+ transients elicited by the activation of afferent and efferent synaptic signaling.
Footnotes
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2016-2155 (to J.D.G.) and the National Institutes of Health Grant R01 DC001508 (to P.A.F. and A.B.E.). We thank Maryline Beurg for advice on the use of ascorbic acid in the intracellular solution and Joe Santos-Sacchi on OHCs recordings, J. Dempster for the use of WinWCP, and Paul A. Fuchs for comments on the manuscript.
The authors declare no competing financial interests.
References
- Art JJ, Fettiplace R, Fuchs PA (1984) Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol 356:525–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K (2003) Local calcium signaling in neurons. Neuron 40:331–346. 10.1016/s0896-6273(03)00639-1 [DOI] [PubMed] [Google Scholar]
- Ballestero J, Zorrilla de San Martín J, Goutman JD, Elgoyhen AB, Fuchs PA, Katz E (2011) Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells. J Neurosci 31:14763–14774. 10.1523/JNEUROSCI.6788-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D (2008) Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci 28:1798–1803. 10.1523/JNEUROSCI.4653-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam J, Ricci AJ (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12:553–558. 10.1038/nn.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Chen Q, Fettiplace R (2010) Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104:18–34. 10.1152/jn.00019.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Lipp P, Berridge MJ (2001) The organisation and functions of local Ca2+ signals. J Cell Sci 114:2213–2222. 10.1242/jcs.114.12.2213 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M (2013) Ca2+-sensitive fluorescent dyes and intracellular Ca2+ imaging. Cold Spring Harb Protoc 8:83–99. [DOI] [PubMed] [Google Scholar]
- Brown MC (1989) Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40:93–109. 10.1016/0378-5955(89)90103-2 [DOI] [PubMed] [Google Scholar]
- Castellano-Muñoz M, Schnee ME, Ricci AJ (2016) Calcium-induced calcium release supports recruitment of synaptic vesicles in auditory hair cells. J Neurophysiol 115:226–239. 10.1152/jn.00559.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Mahendrasingam S, Tickle JA, Hackney CM, Furness DN, Fettiplace R (2012) The development, distribution and density of the plasma membrane calcium ATPase 2 calcium pump in rat cochlear hair cells. Eur J Neurosci 36:2302–2310. 10.1111/j.1460-9568.2012.08159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW (1995) Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 18:58–60. [PubMed] [Google Scholar]
- Dallos P (2008) Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN (1997) Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci 17:2212–2226. 10.1523/JNEUROSCI.17-06-02212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG (2001) Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 21:5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann SF (1994) α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79:705–715. 10.1016/0092-8674(94)90555-X [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J (2001) alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98:3501–3506. 10.1073/pnas.051622798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström H (1958) On the double innervation of the sensory epithelia of the inner ear. Acta Otolaryngol 49:109–118. 10.3109/00016485809134734 [DOI] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P, Mammano F (2000) Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium 28:195–203. 10.1054/ceca.2000.0145 [DOI] [PubMed] [Google Scholar]
- Fettiplace R (2017) Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Compr Physiol 7:1197–1227. 10.1002/cphy.c160049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Nam JH (2019) Tonotopy in calcium homeostasis and vulnerability of cochlear hair cells. Hear Res 376:11–21. 10.1016/j.heares.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E (2007) A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA 104:1516–1521. 10.1073/pnas.0609775104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, García-Añoveros J (2015) A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 25:606–612. 10.1016/j.cub.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T (2009) Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci USA 106:4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B (2000) Two distinct Ca(2+)-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci 20:5940–5948. 10.1523/JNEUROSCI.20-16-05940.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA (1996) Synaptic transmission at vertebrate hair cells. Curr Opin Neurobiol 6:514–519. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E (2015) Synaptic studies inform the functional diversity of cochlear afferents. Hear Res 330:18–25. 10.1016/j.heares.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Lehar M, Hiel H (2014) Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol 522:717–729. 10.1002/cne.23478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Hackney CM (2006) The structure and composition of the stereociliary bundle of vertebrate hair cells. In: Springer handbook of auditory research. Vertebrate hair cells. New York: Springer US. [Google Scholar]
- Galambos R (1956) Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol 19:424–437. 10.1152/jn.1956.19.5.424 [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ (1987) Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res 29:179–194. 10.1016/0378-5955(87)90166-3 [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA (2002) Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5:147–154. 10.1038/nn796 [DOI] [PubMed] [Google Scholar]
- Gómez-Casati ME, Fuchs PA, Elgoyhen AB, Katz E (2005) Biophysical and pharmacological characterization of nicotinic cholinergic receptors in rat cochlear inner hair cells. J Physiol 566:103–118. 10.1113/jphysiol.2005.087155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E (2005) Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol 566:49–59. 10.1113/jphysiol.2005.087460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Slapnick S, Kennedy HJ, Hackney CM (2006) Ryanodine receptor localisation in the mammalian cochlea: an ultrastructural study. Hear Res 219:101–109. [DOI] [PubMed] [Google Scholar]
- Guinan JJ (1996) Physiology of olivocochlear efferents. In: The cochlea. (Dallos P, Popper AN, Fay RR, eds). New York: Springer New York, Inc. [Google Scholar]
- Guinan JJ (2006) Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27:589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R (2005) The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25:7867–7875. 10.1523/JNEUROSCI.1196-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im GJ, Moskowitz HS, Lehar M, Hiel H, Fuchs PA (2014) Synaptic calcium regulation in hair cells of the chicken basilar papilla. J Neurosci 34:16688–16697. 10.1523/JNEUROSCI.2615-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W (2008) Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells. J Physiol 586:1029–1042. 10.1113/jphysiol.2007.145219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ (2002) Intracellular calcium regulation in inner hair cells from neonatal mice. Cell Calcium 31:127–136. 10.1054/ceca.2001.0267 [DOI] [PubMed] [Google Scholar]
- Knirsch M, Brandt N, Braig C, Kuhn S, Hirt B, Münkner S, Knipper M, Engel J (2007) Persistence of Ca(v)1.3 Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J Neurosci 27:6442–6451. 10.1523/JNEUROSCI.5364-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE (1998) Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 273:18693–18696. 10.1074/jbc.273.30.18693 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (1999) Long-term sound conditioning enhances cochlear sensitivity. J Neurophysiol 82:863–873. 10.1152/jn.1999.82.2.863 [DOI] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2:a003996. 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Perin P, Martini M, Ciubotaru CD, Prigioni I, Valli P, Rossi ML, Mammano F (2003) Presynaptic calcium stores modulate afferent release in vestibular hair cells. J Neurosci 23:6894–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1988) Physiology of cochlear efferent and afferent neurons: direct comparisons in the same animal. Hear Res 34:179–191. 10.1016/0378-5955(88)90105-0 [DOI] [PubMed] [Google Scholar]
- Liberman MC (1990) Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear Res 49:209–223. 10.1016/0378-5955(90)90105-x [DOI] [PubMed] [Google Scholar]
- Lioudyno MI, Hiel H, Kong J, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA (2004) A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci 24:11160–11164. 10.1523/JNEUROSCI.3674-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Glowatzki E, Fuchs PA (2015) Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci USA 112:14723–14727. 10.1073/pnas.1515228112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew SG, Wolleben C, Siegl P, Inui M, Fleischer S (1989) Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry 28:1686–1691. 10.1021/bi00430a039 [DOI] [PubMed] [Google Scholar]
- Moglie MJ, Fuchs PA, Elgoyhen AB, Goutman JD (2018) Compartmentalization of antagonistic Ca2+ signals in developing cochlear hair cells. Proc Natl Acad Sci USA 115:E2095–E2104. 10.1073/pnas.1719077115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Klöcker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B (2000) Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26:595–601. 10.1016/S0896-6273(00)81197-6 [DOI] [PubMed] [Google Scholar]
- Petersen OH (2002) Calcium signal compartmentalization. Biol Res 35:177–182. 10.4067/s0716-97602002000200008 [DOI] [PubMed] [Google Scholar]
- Ranum PT, Goodwin AT, Yoshimura H, Kolbe DL, Walls WD, Koh JY, He DZZ, Smith RJH (2019) Insights into the biology of hearing and deafness revealed by single-cell RNA sequencing. Cell Rep 26:3160–3171.e3. 10.1016/j.celrep.2019.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Gummer M (1985) Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear Res 20:63–77. 10.1016/0378-5955(85)90059-0 [DOI] [PubMed] [Google Scholar]
- Saito K (1980) Fine structure of the sensory epithelium of the guinea pig organ of Corti: afferent and efferent synapses of hair cells. J Ultrasructure Res 71:222–232. 10.1016/S0022-5320(80)90108-2 [DOI] [PubMed] [Google Scholar]
- Saito K (1990) Freeze-fracture organization of hair cell synapses in the sensory epithelium of guinea pig organ of Corti. J Electron Microsc Tech 15:173–186. 10.1002/jemt.1060150209 [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA (1998) Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. J Histochem Cytochem 46:29–39. 10.1177/002215549804600105 [DOI] [PubMed] [Google Scholar]
- Smith CA, Sjöstrand FS (1961) Structure of the nerve endings on the external hair cells of the guinea pig cochlea as studied by serial sections. J Ultrasructure Res 5:523–556. 10.1016/S0022-5320(61)80025-7 [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC, Sewell WF (1997) Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci 17:428–437. 10.1523/JNEUROSCI.17-01-00428.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K (1998) Mutations in a plasma membrane Ca2+-atpase gene cause deafness in deafwaddler mice. Nat Genet 19:390–394. 10.1038/1284 [DOI] [PubMed] [Google Scholar]
- Tong B, Hornak AJ, Maison SF, Ohlemiller KK, Liberman MC, Simmons DD (2016) Oncomodulin, an EF-hand Ca2+ buffer, is critical for maintaining cochlear function in mice. J Neurosci 36:1631–1635. 10.1523/JNEUROSCI.3311-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattino LG, Wedemeyer C, Elgoyhen AB, Katz E (2020) Functional postnatal maturation of the medial olivocochlear efferent – outer hair cell synapse. J Neurosci 40:4842–4857. 10.1523/JNEUROSCI.2409-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB (1992) Organization of the olivocochlear efferent system in mammals. In: The mammalian auditory pathway: neuroanatomy. (Webster DB, Popper AN, Fay RR, eds). New York: Springer. [Google Scholar]
- Weisstaub N, Vetter DE, Elgoyhen AB, Katz E (2002) The α9α10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res 167:122–135. 10.1016/S0378-5955(02)00380-5 [DOI] [PubMed] [Google Scholar]
- Weisz CJC, Glowatzki E, Fuchs PA (2009) The postsynaptic function of type II cochlear afferents. Nature 461:1126–1129. 10.1038/nature08487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJC, Williams SS-P, Eckard CSC, Divito CBC, Ferreira DDW, Fantetti KNK, Dettwyler SSA, Cai HMH, Rubio ME, Kandler K, Seal RP (2021) Outer hair cell glutamate signaling through type II spiral ganglion afferents activates neurons in the cochlear nucleus in response to non-damaging sounds. J Neurosci 41:2930–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AB, Jing Z, Rutherford MA, Frank T, Strenzke N, Moser T, Rutherford MA, Frank T, Strenzke N, Moser T (2013) Concurrent maturation of inner hair cell synaptic Ca2+ influx and auditory nerve spontaneous activity around hearing onset in mice. J Neurosci 33:10661–10666. 10.1523/JNEUROSCI.1215-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah EN, Lumpkin EA, Dumont RA, Smith PJS, Hudspeth AJ, Gillespie PG (1998) Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci 18:610–624. 10.1523/JNEUROSCI.18-02-00610.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary S, Nowak N, Vyas P, Bonanni L, Fuchs PA (2018) Voltage-gated calcium influx modifies cholinergic inhibition of inner hair cells in the immature rat cochlea. J Neurosci 38:5677–5687. 10.1523/JNEUROSCI.0230-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Glowatzki E, Roux I, Fuchs PA (2020) Nicotine evoked efferent transmitter release onto immature cochlear inner hair cells. J Neurophysiol 124:1377–1387. 10.1152/jn.00097.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]