Prevention of hospital-acquired infections is a critical aspect of clinical management of COVID-19 as hospital-acquired infections have been a common feature of previous novel coronavirus outbreaks.1 The number of COVID-19 patients in UK hospitals reached high levels during the first pandemic wave of 2020, and higher levels still in the subsequent winter wave. We assessed the magnitude of nosocomial COVID-19 in acute and long-term National Health Service (NHS) hospital facilities in the UK during the first pandmic wave.
We examined records of COVID-19 patients in UK hospitals enrolled in the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study, with symptom onset before Aug 1, 2020.2, 3 We identified patients as having hospital-acquired infections using a combination of their admission date and symptom onset date, and estimates of their infection date based on the known incubation period distribution of SARS-CoV-2.4 To incorporate uncertainty in individual patient's incubation periods, we imputed infection dates for patients and identified those admitted before infection as having hospital-acquired infections. Multiple imputation was used to generate estimates and CIs (appendix). We estimate that 11·3% (95% CI 11·1–11·6) of patients with COVID-19 in 314 UK hospitals became infected after hospital admission. This proportion increased to at least 15·8% (17·6%; 15·8–19·6) of patients with COVID-19 by the middle of May, 2020, long after the peak of admissions. Using an extremely conservative threshold of symptom onset at least 14 days after admission to identify hospital-acquired infections, we estimate that 6·8% (95% CI 6·7–7·0) of all patients with COVID-19 had nosocomial infections, with a peak of 8·2% (7·0–9·6) of patients having nosocomial infections in mid-May.
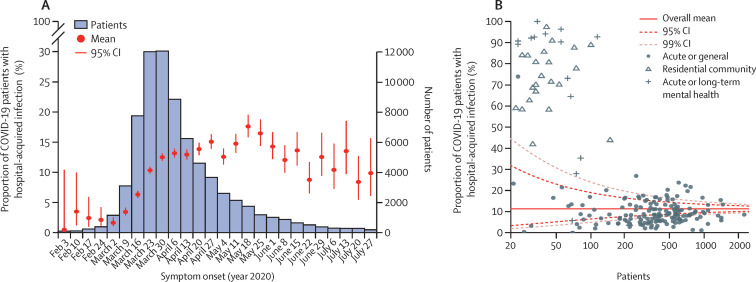
There was marked heterogeneity in hospital-acquired infection proportion between hospital trusts and by the nature of care they provide (figure ). Hospitals providing acute and general care had lower hospital-acquired infection proportions (9·7%; 95% CI 9·4–9·9) than residential community care hospitals (61·9%; 56·4–68·0) and mental health hospitals (67·5%; 60·1–75·8), reflecting outbreaks seen in care homes. The reasons for the variation between hospitals providing the same type of care require urgent investigation to identify and promote best infection control practice for future treatment of COVID-19 patients.
Figure.
Estimated proportion of COVID-19 patients with hospital-acquired infection based on 72 157 patients with complete admission and symptom onset dates (A) and funnel plot of trust-level proportion of hospital-acquired infections, stratified by care type (B)
As ISARIC WHO CCP-UK data are a subset of all admissions (approximately two-thirds sample in the period of observation), we estimated the number of hospital-acquired infections by hospital trust, accounting for the participation rate of each hospital trust in comparison to NHS Digital Secondary Uses Service data. We estimated that of 82 624 patients admitted before Aug 1, 2020, 5699–11 862 patients were infected during their hospital stay. Underestimation is probable since ISARIC4C data cannot identify patients infected during admission but discharged before manifesting symptoms, or patients infected during another health-care visit before admission.
Limited access to testing early in the outbreak, false negative results for nasopharyngeal swabs in early stages of disease, and presentation with gastrointestinal symptoms may have led to some patients with COVID-19 being misclassified and placed in non-COVID-19 areas with different infection prevention control processes.3 Enteric features, and the ability of SARS-CoV-2 to persist on surfaces, raise the possibility of faecal-oral transmission in care settings under severe pressure, although the role of this transmission route is uncertain.5
As SARS-CoV-2 is likely to persist as an endemic or seasonal virus in coming years, it is critical to use the lessons learned so far in the pandemic to minimise the burden of hospital-acquired infections, and to consider new approaches to reduce the burden further. Surveillance afforded by this study has helped to rapidly identify changes in hospital-acquired infection incidence in different health-care settings. Unlike at the beginning of the pandemic, there are opportunities to pre-empt hospital-acquired infections and break chains of transmission through regular patient, resident, and staff testing including point-of-care diagnostics, as recently introduced for NHS staff, coupled with robust hospital infection prevention and control policies that include staff vaccination, environmental disinfection, and appropriate isolation, all supported by sentinel monitoring systems.
Acknowledgments
JMR reports grants from the Engineering and Physical Sciences Research Council, the Medical Research Council (MRC), and Wellcome; and personal fees from University of Oxford, Centra Technology, Al Jazeera, University of Warwick, and London School of Hygiene & Tropical Medicine, unrelated to this Correspondence. Lancaster University received payment from the Ministry of Health of Saudi Arabia for a training course delivered by JMR in September, 2019. SF reports grants from Wellcome, unrelated to this Correspondence. LT reports grants from the MRC, Wellcome, Innovate UK, National Institute for Health Research (NIHR), and EU Horizon 2020, unrelated to this Correspondence. JSN-V-T is seconded to the Department of Health and Social Care, England (DHSC). The views expressed in this Correspondence are those of the authors and not necessarily those of the DHSC. PJMO reports personal fees for consultancy from Janssen and from the European Respiratory Society; grants from the MRC and Wellcome; funding from the EU and the European Federation of Pharmaceutical Industries and Associations for the respiratory syncytial virus consortium in Europe; and funding from the NIHR, the MRC, and GSK to the EMINENT Network. PJMO participated in a Nestle Discussion Forum in November, 2020, was a member of Pfizer's antivirals advisory board in December, 2020, and was president of the British Society for Immunology from 2013 to 2018. MGS reports grants from the NIHR, the MRC, and the Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool. MGS is minority owner of Integrum Scientific. All other authors declare no competing interests.
Supplementary Material
References
- 1.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunning JW, Merson L, Rohde GG, et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14:8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Tao W, Flavell RA, et al. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18:269–283. doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.