Abstract
Background
Heterologous vaccine regimens have been widely discussed as a way to mitigate intermittent supply shortages and to improve immunogenicity and safety of COVID-19 vaccines. We aimed to assess the reactogenicity and immunogenicity of heterologous immunisations with ChAdOx1 nCov-19 (AstraZeneca, Cambridge, UK) and BNT162b2 (Pfizer-BioNtech, Mainz, Germany) compared with homologous BNT162b2 and ChAdOx1 nCov-19 immunisation.
Methods
This is an interim analysis of a prospective observational cohort study enrolling health-care workers in Berlin (Germany) who received either homologous ChAdOx1 nCov-19 or heterologous ChAdOx1 nCov-19–BNT162b2 vaccination with a 10–12-week vaccine interval or homologous BNT162b2 vaccination with a 3-week vaccine interval. We assessed reactogenicity after the first and second vaccination by use of electronic questionnaires on days 1, 3, 5, and 7. Immunogenicity was measured by the presence of SARS-CoV-2-specific antibodies (full spike-IgG, S1-IgG, and RBD-IgG), by an RBD–ACE2 binding inhibition assay (surrogate SARS-CoV-2 virus neutralisation test), a pseudovirus neutralisation assay against two variants of concerns (alpha [B.1.1.7] and beta [B.1.351]), and anti-S1-IgG avidity. T-cell reactivity was measured by IFN-γ release assay.
Findings
Between Dec 27, 2020, and June 14, 2021, 380 participants were enrolled in the study, with 174 receiving homologous BNT162b2 vaccination, 38 receiving homologous ChAdOx1 nCov-19 vaccination, and 104 receiving ChAdOx1 nCov-19–BNT162b2 vaccination. Systemic symptoms were reported by 103 (65%, 95% CI 57·1–71·8) of 159 recipients of homologous BNT162b2, 14 (39%, 24·8–55·1) of 36 recipients of homologous ChAdOx1 nCov-19, and 51 (49%, 39·6–58·5) of 104 recipients of ChAdOx1 nCov-19–BNT162b2 after the booster immunisation. Median anti-RBD IgG levels 3 weeks after boost immunisation were 5·4 signal to cutoff ratio (S/co; IQR 4·8–5·9) in recipients of homologous BNT162b2, 4·9 S/co (4·3–5·6) in recipients of homologous ChAdOx1 nCov-19, and 5·6 S/co (5·1–6·1) in recipients of ChAdOx1 nCov-19– BNT162b2. Geometric mean of 50% inhibitory dose against alpha and beta variants were highest in recipients of ChAdOx1 nCov-19–BNT162b2 (956·6, 95% CI 835·6–1095, against alpha and 417·1, 349·3–498·2, against beta) compared with those in recipients of homologous ChAdOx1 nCov-19 (212·5, 131·2–344·4, against alpha and 48·5, 28·4–82·8, against beta; both p<0·0001) or homologous BNT162b2 (369·2, 310·7–438·6, against alpha and 72·4, 60·5–86·5, against beta; both p<0·0001). SARS-CoV-2 S1 T-cell reactivity 3 weeks after boost immunisation was highest in recipients of ChAdOx1 nCov-19–BNT162b2 (median IFN-γ concentration 4762 mIU/mL, IQR 2723–8403) compared with that in recipients of homologous ChAdOx1 nCov-19 (1061 mIU/mL, 599–2274, p<0·0001) and homologous BNT162b2 (2026 mIU/mL, 1459–4621, p=0·0008) vaccination.
Interpretation
The heterologous ChAdOx1 nCov-19–BNT162b2 immunisation with 10–12-week interval, recommended in Germany, is well tolerated and improves immunogenicity compared with homologous ChAdOx1 nCov-19 vaccination with 10–12-week interval and BNT162b2 vaccination with 3-week interval. Heterologous prime-boost immunisation strategies for COVID-19 might be generally applicable.
Funding
Forschungsnetzwerk der Universitätsmedizin zu COVID-19, the German Ministry of Education and Research, Zalando SE.
Introduction
Because of intermittent supply shortages of individual COVID-19 vaccines and evidence of rare, but severe adverse events after vaccination with vector-based vaccines such as the ChAdOx1 nCov-19 vaccine (AstraZeneca, Cambridge, UK),1, 2, 3, 4 heterologous prime-boost regimens for COVID-19 vaccines have gained substantial interest.5 Heterologous booster vaccination with an mRNA vaccine after initial immunisation with ChAdOx1 nCov-19 has been recommended in several countries, including Germany,6 despite scarce data on reactogenicity, safety, and immunogenicity of this prime-boost regimen in humans.
Research in context.
Evidence before this study
We searched PubMed from inception to June 15, 2021, with no language restrictions, using the search terms “heterologous” AND “vaccination” AND “COVID-19” NOT “BCG”. The search returned 44 articles. We found no studies on heterologous prime-boost immunisation for COVID-19 using ChAdOx1 nCov-19 and BNT162b2 published before the start of this study in December, 2020. A correspondence published on May 12, 2021, described the initial reactogenicity and safety data of the Com-Cov trial, which randomly assigned participants to receive either homologous ChAdOx1 nCov-19, heterologous ChAdOx1 nCov-19–BNT162b2, homologous BNT162b2, or heterologous BNT162b2–ChAdOx1 nCov-19 vaccination given 28 days apart. The authors reported an increase in systemic reactogenicity of the heterologous ChAdOx1 nCov-19–BNT162b2 boost in comparison with homologous boost vaccination. We did not find other peer-reviewed studies reporting reactogenicity data of heterologous COVID-19 vaccination. Immunogenicity data of the Com-CoV trial is being published presently. A case report published on May 31, 2021, described antibody responses in two participants who received a heterologous immunisation with ChAdOx1 nCov-19 (Covishield) followed by BNT162b2 33 days later. The authors reported increased neutralising antibodies after the heterologous boost. Besides the small sample size (n=2) and variable sampling intervals (13 days and 25 days), no comparison with homologous vaccination was done, limiting the interpretation of these results. Two preclinical studies in mouse models of heterologous vaccination combining adenoviral-vectored and mRNA-based vaccines reported increased immunogenicity of heterologous regimens compared with that of homologous regimens. So far, we found no published peer-reviewed study reporting reactogenicity, safety, and immunogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 prime-boost vaccination. The CombivacS trial also assessed the immunogenicity and safety of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination, yet it did not include a homologous vaccination group as comparison.
Added value of this study
To our knowledge, our prospective observational cohort study provides first real-world data on the reactogenicity and immunogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 prime-boost vaccination with a 10–12-week vaccination interval, compared with homologous ChAdOx1 nCov-19 vaccination with a 10–12-week vaccine interval or homologous BNT162b2 vaccination with a 3-week vaccine interval. We compared the reactogenicity and safety of regimens by self-reported local and systemic symptoms for 7 days after prime and boost immunisations in a cohort of 380 health-care workers. We have also assessed immunogenicity of the vaccine regimens 3–4 weeks after prime and boost immunisation. We found similar humoral immune responses, with evidence of increased neutralisation capacity and increased T-cell responses after heterologous ChAdOx1 nCov-19–BNT162b2 immunisation compared with homologous ChAdOx1 nCov-19 and BNT162b2 vaccination. In light of increased reactogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination reported by Robert H Shaw and colleagues when the vaccines were given 28 days apart, our data suggest that extended vaccine intervals might improve the tolerability of heterologous prime-boost vaccination.
Implications of all the available evidence
Heterologous vaccination has been widely discussed to mitigate intermittent vaccine supply shortages and to improve immunogenicity and efficacy of existing COVID-19 vaccines. Our study offers real-world evidence supporting the safety and immunogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination, which is currently recommended in several countries. Combined with preclinical studies reporting improved immunogenicity of heterologous vaccination regimens, our data support ongoing efforts to investigate heterologous vaccination regimens for COVID-19.
On Jan 29, 2021, the German standing committee on vaccination recommended that ChAdOx1 nCov-19 be administered only to individuals aged 18–64 years. Consequently, mainly younger people, including health-care workers, received ChAdOx1 nCov-19, while mRNA vaccines (BNT162b2 [Pfizer-BioNtech, Mainz, Germany] and mRNA-1273 [Moderna, Cambridge, MA, USA]) were prioritised for use in older individuals. In response to reports about rare blood clotting events, including cerebral venous sinus thrombosis, associated with ChAdOx1 nCov-19 vaccination especially in younger women,2, 3, 4 several European countries restricted their recommendations for ChAdOx1 nCov-19 vaccination to individuals older than a certain age limit (eg, older than 60 years in Germany and older than 55 years in France).7 Heterologous boost immunisation with an mRNA vaccine (BNT162b2 or mRNA-1273) was consequently recommended for individuals who had already received a first immunisation with ChAdOx1 nCov-19, but who were younger than the revised age limit for that vaccine.7 In phase 2/3 trials, both BNT162b2 and ChAdOx1 nCov-19 showed significant reactogenicity—most commonly pain at the injection site, fatigue, headache, chills, and fever—with only a minor proportion of study participants reporting severe reactions.8, 9 An interim analysis of reactogenicity data in the Com-COV trial, investigating various heterologous prime-boost regimens of licensed COVID-19 vaccines, reported no serious side-effects but a clearly increased reactogenicity after heterologous boost with BNT162b2 28 days after initial vaccination with ChAdOx1 nCov-19.10 In this interim analysis, up to 80% of individuals receiving a heterologous prime-boost with ChAdOx1 nCov-19 and BNT162b2 reported fatigue and other systemic reactions, an up to 40 times increase compared with the respective homologous boost vaccinations.10 Both BNT162b2 and ChAdOx1 nCov-19 have been shown to elicit robust immune responses, with a significant increase after homologous boost vaccination in clinical trials and real-world studies.8, 9, 11, 12, 13 Heterologous prime-boost immunisation has been shown to elicit increased immunogenicity for other vaccines,5, 14, 15 and early animal experiments suggested increased immunogenicity of boost vaccination with an mRNA vaccine after initial immunisation with adenovector-based COVID-19 vaccines.16 Another study reported increased immune responses in participants receiving a heterologous booster of BNT162b2 8 weeks after an initial dose of ChAdOx1 nCov-19, compared with participants who only received the initial vaccination with ChAdOx1 nCov-19.17 However, data comparing immunogenicity of heterologous ChAdOx1 nCov-19 and BNT162b2 prime-boost vaccination to homologous regimens are still needed.
Heterologous ChAdOx1 nCov-19 and mRNA vaccination has already commenced in several countries, despite insufficient robust immunogenicity and safety data for this regimen. In this study, we aimed to assess the reactogenicity and immunogenicity of homologous BNT162b2 vaccination with a 3-week interval, homologous ChAdOx1 nCov-19 vaccination with a 10–12-week interval, and heterologous ChAdOx1 nCov-19–BNT162b2 prime-boost immunisation with a 10–12-week interval in a prospective observational cohort study.
Methods
Study design
Health-care workers receiving routine COVID-19 vaccination were enrolled in the EICOV and COVIM prospective observational cohort studies done at Charité—Universitätsmedizin Berlin (Berlin, Germany), after written informed consent was obtained. EICOV was approved by the ethics committee of Charité—Universitätsmedizin Berlin (EA4/245/20), and COVIM (EudraCT-2021–001512–28) was approved by the Federal Institute for Vaccines and Biomedicines (Paul Ehrlich Institute) and by the Ethics committee of the state of Berlin. Both studies were done in accordance with the guidelines of Good Clinical Practice (ICH 1996) and the Declaration of Helsinki.
Health-care workers at Charité—Universitätsmedizin Berlin were offered either two doses of BNT162b2 3 weeks apart or an initial dose of ChAdOx1 nCov-19 followed by a heterologous boost with BNT162b2 10–12 weeks later. The vaccine regimen depended on availability and current official recommendations. Health-care workers who received an initial dose of ChAdOx1 nCov-19 were also free to choose a homologous booster with ChAdOx1 nCov-19 10–12 weeks later. The number of study participants was determined by feasibility of immunological analyses and enrolment. Baseline data on demographics were collected by questionnaire in an electronic case report form at enrolment. Blood samples for detection of SARS-CoV-2-specific antibodies and T-cell responses were collected immediately before the first vaccination, and 3–4 weeks after the first and second vaccination.
Assessment of reactogenicity and safety
Participants were asked to fill in electronic questionnaires on reactogenicity, adverse events, medication, and medical visits on days 1, 3, 5, and 7 after the first and second vaccination. Additionally, the use of antipyretic medication (nonsteroidal anti-inflammatory drugs or paracetamol) before and after vaccination was recorded. We assessed local and systemic reactions to the different vaccines by use of a modified US Food and Drug Administration toxicity scale18 of mild (does not interfere with daily activities), moderate (interferes with daily activities), and severe (daily activities no longer feasible). After the initial assessments, all participants were asked to self-report any systemic symptoms and intake of pain medication through an electronic questionnaire every 2 weeks. Here, we report on the results of questionnaires of the first 7 days after the first and second vaccination.
Assessment of immunogenicity
Participants with PCR-confirmed infection or detectable anti-nucleocapsid protein IgG at any timepoint during the study were excluded from the main immunogenicity analysis (appendix p 2). We selected a subset of study participants for immunogenicity analysis on the basis of multivariate matching for sex and age between vaccine groups. We assessed the presence of SARS-CoV-2-specific antibodies using a microarray-based immunoassay including spike protein (full spike, S1 subunit, and receptor-binding domain [RBD]) and nucleocapsid protein as antigens to discriminate between vaccine-induced antibody response and convalescent SARS-CoV-2 infection (SeraSpot Anti-SARS-CoV-2 IgG, Seramun Diagnostica, Heidesee, Germany).19 We investigated the functional neutralisation capacity using an RBD-ACE2 binding inhibition assay (surrogate SARS-CoV-2 virus neutralisation test [sVNT]; cPass, medac, Wedel, Germany), following the manufacturer's instructions.20 Additionally, we used a SARS-CoV-2 pseudovirus neutralisation assay (pNT) to test the neutralising capacity of the vaccine regimes by determining serum 50% inhibitory dilutions (ID50) 3 weeks after boost immunisation against the alpha (B.1.1.7) and beta (B.1.351) variants of concern. This assay has been described previously20 and further details are provided in the appendix (pp 5–6). Maturation of IgG avidity was characterised by a modified anti-SARS-CoV-2 S1 IgG ELISA (anti-SARS-CoV-2 S1 IgG ELISA Kit, Euroimmun Medizinische Labordiagnostika, Lübeck, Germany)19 in randomly selected samples from individuals who were seroreactive 3 weeks after prime vaccination, 30 each from the homologous and heterologous boost cohorts. Avidity indices between 40% and 60% were considered as borderline avidity and higher than 60% as high avidity. SARS-CoV-2 spike-specific T-cell responses were measured by an interferon-γ (IFN-γ) release assay (IGRA; Euroimmun Medizinische Labordiagnostika)19 of S1 peptide-stimulated T-cells in whole blood. IGRA was not done after prime immunisation with BNT162b2 due to technical and logistical reasons in the early weeks of the study. A subset of the antibody and T-cell data obtained from the BNT162b2 prime and homologous BNT162b2 prime-boost groups has been shown in a previous publication that compared immune responses of older people with younger people after vaccination.19
Statistical analysis
Data are presented as median (IQR), unless stated otherwise. For the statistical analysis, we used GraphPad PRISM, version 9.1.2, or JMP Pro, version 15.2.0. Group comparisons were done in a univariate analysis by use of Fisher's exact test or nonparametric Kruskal-Wallis test with Dunn's multiple comparisons test. All 95% CIs were calculated according to the Wilson and Brown method.21 Median and IQR are indicated for the results of ELISA-based immunological analyses. No imputation of missing data was done. For 50% inhibitory dilutions in pNT, geometric mean and 95% CIs are indicated. p<0·05 was considered significant. Detailed methods, including study design and immunogenicity analyses are described in detail in the appendix (pp 5–6).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
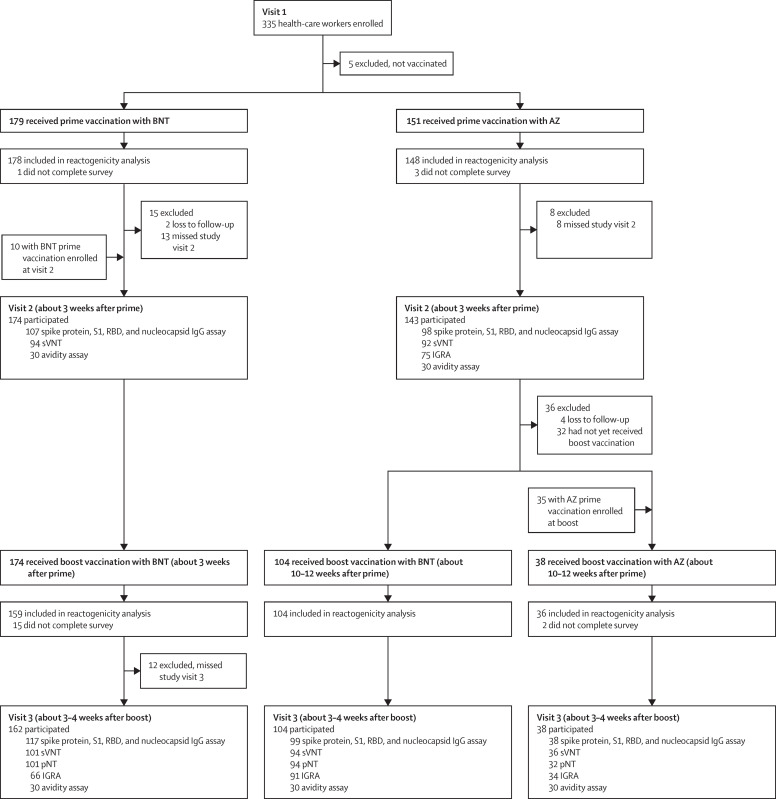
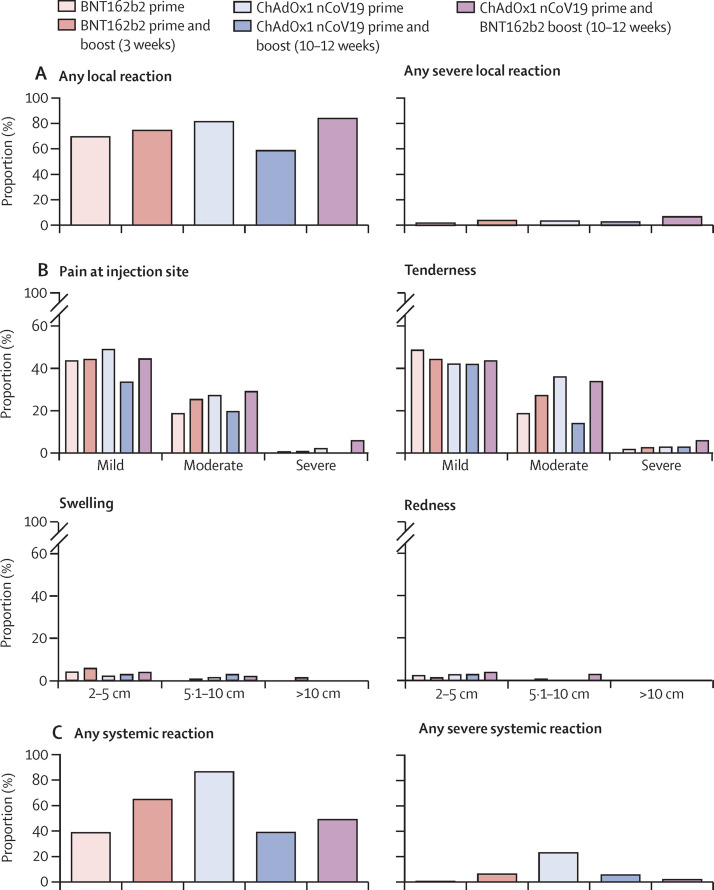
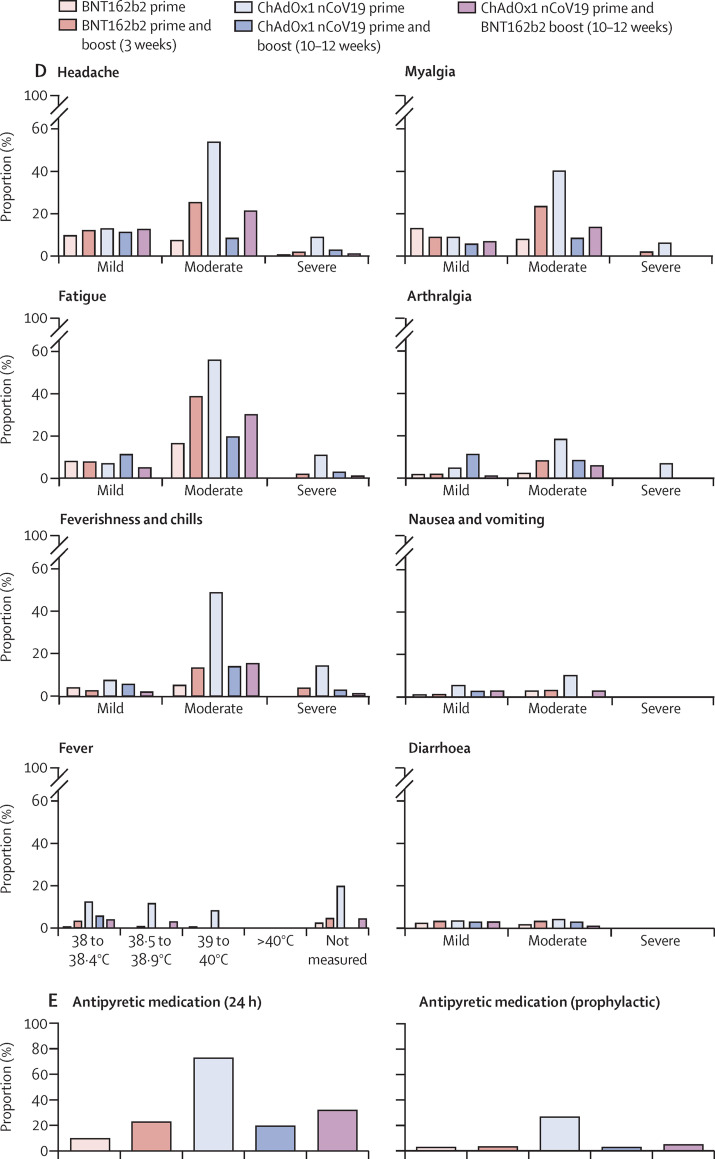
From Dec 27, 2020, to June 14, 2021, 380 health-care workers were enrolled in the study and received either BNT162b2 (n=179) or ChAdOx1 nCov-19 prime immunisation (n=151). For the boost vaccination, 174 participants received homologous BNT162b2 boost after 3 weeks, 104 received heterologous ChAdOx1 nCov-19–BNT162b2 after 10–12 weeks, and 38 received homologous ChAdOx1 nCov-19 after 10–12 weeks (figure 1 ). Baseline characteristics of the study population are provided in the table . All vaccination regimens were associated with a relatively high frequency of local reactions, most commonly pain at the injection site and tenderness. Local reactions were usually mild or moderate (figure 2A, B ; appendix p 4). No major differences were observed in the frequency or severity of local reactions after either of the prime or boost immunisations, with the exception of a slightly higher frequency of local reactions after heterologous ChAdOx1 nCov-19–BNT162b2 booster vaccination compared with that after homologous BNT162b2 booster vaccination and a lower frequency of local reactions after homologous ChAdOx1 nCov-19 booster vaccination compared with that after heterologous ChAdOx1 nCov-19–BNT162b2 and homologous BNT162b2 booster vaccinations (figure 2A, B; appendix p 4). Conversely, notable differences were reported for systemic reactions. These were most frequently reported after prime immunisation with ChAdOx1 nCov-19 (128 [86%] of 148, 95% CI 80·0–91·1) and after homologous BNT162b2 booster immunisation (103 [65%] of 159, 57·1–71·8). By contrast, systemic reactions were reported by 51 (49%, 39·6–58·5) of 104 participants after heterologous ChAdOx1 nCov-19–BNT162b2 booster vaccination, by 69 (39%, 31·9–46·1) of 178 participants after the first immunisation with BNT162b2, and by 14 (39%, 24·8–55·1) of 36 participants after homologous ChAdOx1 nCov-19 booster vaccination (figure 2C, appendix p 4). Severe systemic symptoms—including fatigue, myalgia, headache, feverishness or chills, and fever higher than 38°C—were most frequently reported after ChAdOx1 nCov-19 prime immunisation, were less frequent after homologous ChAdOx1 nCov-19 and homologous BNT162b2 booster immunisations, and were least common after heterologous ChAdOx1 nCov-19–BNT162b2 booster vaccination (figure 2D, appendix p 4).
Figure 1.
Study profile
AZ=ChAdOx1 nCov-19 COVID-19 vaccine. BNT=BNT162b2 mRNA COVID-19 vaccine. RBD=SARS-CoV-2 receptor-binding domain. S1= SARS-CoV-2 spike protein S1 domain. sVNT=surrogate virus neutralisation assay. pNT=pseudovirus neutralisation test. IGRA=interferon-γ release assay.
Table.
Baseline characteristics and vaccine schedule details of study participants
| BNT prime (n=179) | BNT prime and boost (n=174) | AZ prime (n=151) | AZ prime and BNT boost (n=104) | AZ prime and boost (n=38) | |||
|---|---|---|---|---|---|---|---|
| Prime to boost interval, days | NA | 21 (21–21) | NA | 71 (70–73) | 83 (71–84) | ||
| Participants with reactogenicity data | 178 (99%) | 159 (91%) | 148 (98%) | 104 (100%) | 36 (95%) | ||
| Age, years | 34 (29–44) | 34 (29–43) | 35 (28–47) | 37 (29–51) | 51 (33–59) | ||
| Sex | |||||||
| Female | 98 (55%) | 87 (55%) | 101 (68%) | 78 (75%) | 23 (64%) | ||
| Male | 80 (45%) | 72 (45%) | 47 (32%) | 26 (25%) | 13 (36%) | ||
| Participants with immunogenicity data | 94 (53%) | 101 (58%) | 92 (61%) | 94 (90%) | 36 (95%) | ||
| Vaccination to sampling interval, days | 21 (21–21) | 28 (27–31) | 23 (22–28) | 21 (20–21) | 24 (20–28) | ||
| Age, years | 35 (30–48) | 35 (30–47) | 37 (30–50) | 37 (29–48) | 51 (33–59) | ||
| Sex | |||||||
| Female | 66 (70%) | 73 (72%) | 73 (79%) | 71 (76%) | 23 (64%) | ||
| Male | 28 (30%) | 28 (28%) | 19 (21%) | 23 (24%) | 13 (36%) | ||
Data are n, n (%), or median (IQR). BNT=BNT162b2 mRNA COVID-19 vaccine. AZ=ChAdOx1 nCov-19 COVID-19 vaccine. NA=not applicable.
Figure 2.
Local and systemic reactogenicity of BNT162b2 or ChAdOx1 nCov-19 prime immunisations and homologous or heterologous boosting until day 7 after vaccination
Figure shows the proportion of participants reporting any local reaction (A) and indicated local reactions grouped by severity (B), proportion of participants reporting any systemic reaction (C) and indicated systemic symptoms grouped by severity (D), and proportion of participants reporting intake of antipyretic medication within 24 h after vaccination and prophylactic intake of antipyretic medication (E). Definition of severity according to modified US Food and Drug Administration criteria18 of mild (does not interfere with daily activities), moderate (interferes with daily activities), and severe (daily activities no longer feasible).
No potentially life-threatening reactions were reported after any of the vaccine regimens in this study. Intake of antipyretic medication within 24 h after vaccination was markedly higher after prime immunisation with ChAdOx1 nCov-19 (108 [73%] of 148, 65·3–79·5) compared with after heterologous ChAdOx1 nCov-19–BNT162b2 boost (33 [32%] of 104, 23·6–41·2), homologous BNT162b2 boost (36 [23%] of 159, 16·8–29·7), homologous ChAdOx1 nCov-19 boost (seven [19%] of 36, 9·8–35·0), and prime immunisation with BNT162b2 (17 [10%] of 178, 6·0–14·8; figure 2E). To assess potentially confounding effects of antipyretic medication, we compared the intake of prophylactic antipyretic medication in all groups. The proportion of participants who reported prophylactic antipyretic medication was highest in the ChAdOx1 nCov-19 prime immunisation group (40 [27%] of 148, 20·5–34·7) and distinctly lower in all other groups (five [3%] of 178, 1·2–6·4, for BNT162b2 prime; one [3%] of 36, 0·1–14·2, for homologous ChAdOx1 nCov-19 boost; five [5%] of 104, 2·1–10·8, for heterologous ChAdOx1 nCov-19–BNT162b2 boost; and five [3%] of 159, 1·4–7·1, for homologous BNT162b2 boost). Therefore, prophylactic intake of antipyretics did not account for lower adverse reactions with ChAdOx1 nCov-19–BNT162b2 boost vaccination compared with those with ChAdOx1 nCov-19 prime vaccination. Most vaccine reactions were reported on day 1 and 3 after vaccination and receded by day 7 (appendix p 1).
27 participants had a history of SARS-CoV-2 infection before enrolment, had a positive PCR result for SARS-CoV-2 during the study, or had a detectable anti-spike IgG antibody response at baseline or anti-nucleocapsid protein IgG antibody response at baseline or during follow-up, and were therefore analysed separately and excluded from the main analysis of immunogenicity (appendix p 2).
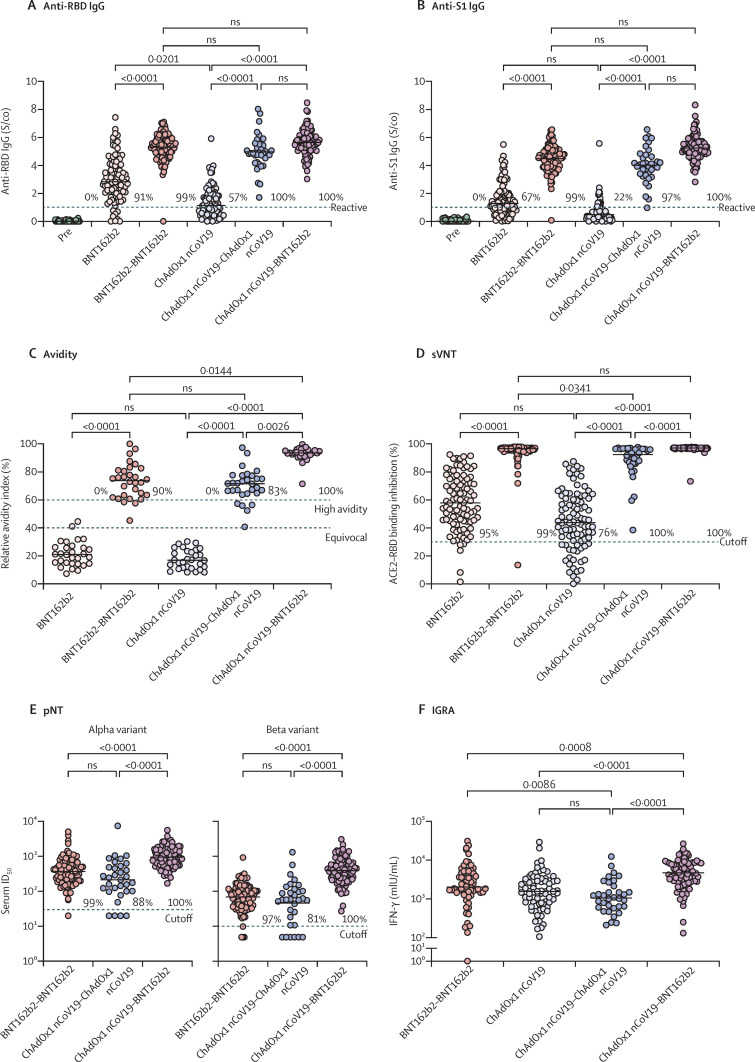
3 weeks after prime immunisation with BNT162b2, 86 (91%, 84·1–95·6) of 94 participants were reactive for anti-SARS-CoV-2-RBD IgG compared with 52 (57%, 46·3–66·2) of 92 participants after ChAdOx1 nCov-19 prime immunisation (p<0·0001, figure 3A ). The proportion of participants who were RBD-reactive increased to 100 (99%, 94·6–100) of 101 3 weeks after homologous BNT162b2 boost immunisation, to 36 (100%, 90·4–100) of 36 after homologous ChAdOx1 nCov-19 boost immunisation, and to 94 (100%, 96·1–100) of 94 after heterologous ChAdOx1 nCov-19–BNT162b2 boost immunisation (figure 3A). Compared with participants immunised with BNT162b2, those immunised with ChAdOx1 nCov-19 had significantly lower anti-RBD IgG levels (median 2·8 signal to cutoff ratio [S/co], IQR 2·0–4·1, vs 1·1 S/co, 0·5–1·9, p=0·020; figure 3A) 3 weeks after prime immunisation. Median levels of anti-S1 spike IgG (1·3 S/co, 0·8–2·0, for ChAdOx1 nCov-19 vs 0·5 S/Co, 0·2–0·8, for BNT162b2; p=0·18, figure 3B) and anti-full spike IgG (2·8 S/co, 1·5–3·0, for ChAdOx1 nCov-19 vs 1·1 S/co, 0·5–1·6, for BNT162b2; p=0·14, appendix p 3) were not significantly reduced when correcting for multiple testing after prime immunisation. 3 weeks after boost immunisation, SARS-CoV-2 spike-binding IgG responses in participants immunised with homologous BNT162b2 boost (anti-RBD IgG median 5·4 S/co, 4·8–5·9) were similar to those immunised with homologous ChAdOx1 nCov-19 boost (4·9 S/co, 4·3–5·6) and heterologous ChAdOx1 nCov-19–BNT162b2 boost (5·6 S/co, 5·1–6·1; figure 3A, B, appendix p 3).
Figure 3.
SARS-CoV-2-specific IgG and T-cell responses
Figure shows anti-RBD IgG (A) and anti-S1 IgG (B) assays, anti-S1 IgG avidity (C), and neutralising capacity measured by sVNT (D) in serum of participants who had received prime immunisation with BNT162b2 or ChAdOx1 nCov-19, and homologous BNT162b2 or ChAdOx1 nCov-19 or heterologous ChAdOx1 nCov-19–BNT162b2 boost; serum neutralisation activity against B.1.1.7 (alpha) and B.1.351 (beta) variants measured by pNT after boost immunisation (E); and T-cell reactivity in whole blood samples measured by IGRA (F). Samples were taken before first immunisation, 3 weeks after first vaccination, and 3–4 weeks after boost vaccination. Dotted lines indicate the manufacturer's pre-specified thresholds: higher than 1 S/co for anti-RBD IgG reactivity, 40–60% for borderline avidity, higher than 60% for high avidity, higher than 30% for sVNT cutoff, and 30-fold serum dilution for the alpha variant and 10-fold dilution for the beta variant for the limit of detection for pNT. Lines indicate the median, except for pNT, where the geometric mean is shown. p values are indicated. ACE2=angiotensin-converting enzyme 2. ID50=50% inhibition dilution. IFN-γ=interferon γ. ns=not significant. pNT=pseudovirus neutralisation test. pre=sample taken before first immunisation. RBD=SARS-CoV-2 receptor-binding domain. S1=SARS-CoV-2 spike protein S1 domain. S/co=signal-to-cutoff ratio. sVNT=surrogate virus neutralisation assay.
In addition to antibody levels, we measured serum antibody avidity. High avidity serum antibodies, defined as an antibody avidity index higher than 60%, were not detected after prime immunisation with either BNT162b2 or ChAdOx1 nCov-19 (figure 3C). 3 weeks after boost immunisation, 27 (90%, 95% CI 74·4–96·5) of 30 participants in the homologous BNT162b2 group, 25 (83%, 66·4–92·7) of 30 in the homologous ChAdOx1 nCov-19 group, and 30 (100%, 88·6–100) of 30 in the heterologous ChAdOx1 nCov-19–BNT162b2 immunised group had high anti-S1 IgG avidity indices (figure 3C). Hence, maturation of IgG avidity after boost vaccination was observed with all three regimens, but the median relative avidity index was higher after heterologous ChAdOx1 nCov-19–BNT162b2 boost (93·6%, IQR 91·9–95·5) compared with that of homologous ChAdOx1 nCov-19 boost (71·7%, 64·8–77·4, p=0·0026) and that of homologous BNT162b2 boost (73·9%, 63·0–81·6, p=0·014, figure 3C).
Neutralising antibodies were detected in 89 (95%, 95% CI 88·2–97·0) of 94 participants receiving BNT162b2 and in 70 (76%, 66·4–83·6) of 92 participants receiving ChAdOx1 nCov-19 prime vaccination (figure 3D). 3 weeks after boost immunisation, the neutralising antibody response rate had increased in all cohorts to 100 (99%, 94·6–99·9) of 101 after homologous BNT162b2 boost, 36 (100%, 90·3–100) of 36 after homologous ChAdOx1 nCov-19 boost, and 94 (100%, 96·1–100) of 94 after heterologous ChAdOx1 nCov-19–BNT162b2 boost (figure 3D). sVNT titers were similar after homologous BNT162b2 (median 96·6%, IQR 95·5–97·2) and heterologous ChAdOx1 nCov-19–BNT162b2 prime-boost immunisation (97·1%, 96·9–97·3; figure 3D). By contrast, neutralising capacity was significantly lower after homologous ChAdOx1 nCov-19 immunisation (92·4%, 86·4–96·4) compared with that after homologous BNT162b2 (p=0·034) and heterologous ChAdOx1 nCov-19–BNT162b2 immunisation (p<0·0001).
The observed effects on sVNT titers were supported with results of pseudovirus neutralisation assays against two SARS-CoV-2 variants of concern (figure 3E). 100 (99%, 95% CI 94·6–99·5) of 101 participants immunised with homologous BNT162b2 boost, 28 (88%, 71·9–95·0) of 32 immunised with homologous ChAdOx1 nCov-19 boost, and 94 (100%, 96·1–100) of 94 immunised with heterologous ChAdOx1 nCov-19–BNT162b2 boost showed neutralising capacity against alpha-variant pseudovirus particles. Similar proportions were observed against beta-variant pseudoviruses after homologous BNT162b2 and heterologous ChAdOx1 nCov-19–BNT162b2 boost immunisation. However, 26 (81%, 95% CI: 64·7–91·1) of 32 participants immunised with homologous ChAdOx1 nCov-19 boost showed neutralising capacity against beta-variant pseudoviruses (figure 3E). Accordingly, when tested against the alpha variant, participants immunised with heterologous ChAdOx1 nCov-19–BNT162b2 showed significantly higher serum neutralising activity (geometric mean ID50 956·6, 95% CI 835·6–1095·0) compared with those receiving homologous BNT162b2 (369·2, 310·7–438·6) and homologous ChAdOx1 nCov-19 (212·5, 131·2–344·4; p<0·0001; figure 3E). Although neutralising activity against the immune escape-associated beta variant was generally lower than that against the alpha variant, the difference between homologous and heterologous vaccine regimens was more pronounced. Compared with participants immunised with homologous BNT162b2 (geometric mean ID50 72·4, 95% CI 60·5–86·5) and homologous ChAdOx1 nCov-19 (48·5, 28·4–82·8), those immunised with heterologous ChAdOx1 nCov-19–BNT162b2 showed significantly higher serum neutralising activity against the beta variant (417·1, 349·3–498·2, p<0·0001; figure 3E).
Serological responses are most widely used to assess immunogenicity of vaccination, but T-cell responses are another important marker of anti-SARS-CoV-2 immunity. We used IGRA to measure the spike S1-specific T-cell response in 75 participants who received ChAdOx1 nCov-19 prime, 34 who received homologous ChAdOx1 nCov-19 boost, 91 who received heterologous ChAdOx1 nCov-19–BNT162b2 boost, and 66 who received homologous BNT162b2 boost. 3 weeks after ChAdOx1 nCov-19 prime immunisation, participants showed robust T-cell responses (figure 3F). Notably, T-cell reactivity was significantly higher after heterologous ChAdOx1 nCov-19–BNT162b2 boost immunisation compared with both homologous BNT162b2 (median IFN-γ concentration 4762 mIU/mL, IQR 2723–8403, vs 2026 mIU/mL, 1459–4621; p=0·0008) and homologous ChAdOx1 nCov-19 boosting (1061 mIU/mL, 599–2274; p<0·0001; figure 3F).
Discussion
Heterologous prime-boost vaccination against COVID-19 is being discussed as a means to improve immunogenicity and efficacy of existing vaccines and to mitigate intermittent supply shortages.5 After reports of rare thrombotic events associated with ChAdOx1 nCov-19, particularly in younger women,7 a heterologous boost with an mRNA vaccine (BNT162b2 or mRNA1273) with a dose interval of 12 weeks is currently recommended in Germany for individuals who have previously received one dose of ChAdOx1 nCov-19.6, 7 Several other countries also recommend heterologous vaccination after a first dose of ChAdOx1 nCov-19, despite a scarcity of robust safety and immunogenicity data for this vaccine regimen. In this observational cohort study, to our knowledge, we provide the first real-world data on reactogenicity and immunogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 prime-boost vaccination with a 10–12-week vaccine interval, compared with homologous ChAdOx1 nCoV19 vaccination with a similar interval, or homologous BNT162b2 vaccination with a 3-week vaccine interval.
Overall, all three regimens were well tolerated. We observed no major differences in reactogenicity between the prime-boost regimens. Local reactions were frequently observed for all vaccines. Systemic reactions, including severe reactions, were most frequent after prime immunisation with ChAdOx1 nCov-19, whereas reactogenicity of homologous BNT162b2, homologous ChAdOx1 nCoV19, and heterologous ChAdOx1 nCoV19–BNT162b2 were similar, with slightly decreased systemic reactions after heterologous ChAdOx1 nCov-19–BNT162b2 and homologous ChAdOx1 nCov-19. We speculated that the likelihood of intake of prophylactic medication would increase for the boost immunisation if participants had experienced strong symptoms after the prime vaccination. However, the data show that the reduced reactogenicity after the heterologous boost was not caused by a higher intake of prophylactic antipyretic medication. The observed similar reactogenicity of homologous BNT162b2 vaccination and good clinical tolerability of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination contrasts with interim results of the Com-COV trial, which reported increased systemic vaccine reactions after heterologous ChAdOx1 nCov-19–BNT162b2 vaccination compared with homologous ChAdOx1 nCov-19 and BNT162b2 regimens in a study of similar sample size.10 Several differences exist in study design (eg, randomised controlled trial vs observational study and differences in vaccine interval) and in study population demographics that might explain this discrepancy. The median age in Com-CoV was 57 years and 46% of participants were women, compared with 35 years and 62% of participants who were women in our study (table). The interval between first and second vaccination with either BNT162b2 or ChAdOx1 nCov-19 was 28 days in the Com-COV study, compared with 71 days for ChAdOx1 nCov-19–BNT162b2 and median 83 days for homologous ChAdOx1 nCov-19 reported here. Therefore, we hypothesise that extended vaccine intervals of 10–12 weeks might reduce the reactogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination.
We observed robust immunogenicity of both homologous and heterologous prime-boost regimens. Anti-S1-IgG avidity, S1-reactive T-cells, and neutralising capacity against two variants of concern were significantly increased 3 weeks after heterologous ChAdOx1 nCov-19–BNT162b2 boost compared with homologous BNT162b2 and ChAdOx1 nCov-19 boost vaccination. Phase 1/2 studies have previously reported robust immunogenicity of homologous BNT162b2 and ChAdOx1 nCov-19 immunisation.22, 23 By contrast, immunogenicity data of heterologous ChAdOx1 nCov-19–BNT162b2 immunisation in direct comparison with homologous immunisation have not been published so far. Despite a lower humoral response after prime immunisation with ChAdOx1 nCov-19 compared with that after BNT162b2, we observed no significant difference in anti-S1-IgG levels, anti-RBD-IgG levels, and neutralisation capacity determined by sVNT, and we observed an increase in anti-S1-IgG avidity 3 weeks after heterologous ChAdOx1 nCov-19–BNT162b2 boost compared with homologous BNT162b2 boost. SARS-CoV-2 pseudovirus neutralisation capacity against two variants of concern showed significantly increased ID50 titers after heterologous ChAdOx1 nCov-19–BNT162b2 vaccination compared with those of both homologous regimens. This finding is in line with previous studies reporting increased antibody responses in patients who recovered from COVID-19 after a single dose of BNT162b2 compared with seronegative individuals receiving two doses of BNT162b2.24 All three vaccine regimens induced robust T-cell responses, but T-cell reactivity was significantly increased after heterologous immunisation. Additionally, no significant increase of T-cell reactivity was achieved after homologous ChAdOx1 nCov-19 boost compared with ChAdOx1 nCov-19 prime vaccination. Taken together, heterologous ChAdOx1 nCov-19–BNT162b2 immunisation with a vaccine interval of 10–12 weeks is well tolerated and highly immunogenic and might offer significantly enhanced immunogenicity compared with homologous vaccination with ChAdOx1 nCov-19 and BNT162b2.
Our study has several limitations. First, our study was not masked and did not include a placebo group. Therefore, we cannot exclude that reporting of reactogenicity was influenced by the expectation of symptoms in some participants, for example, after vaccination with ChAdOx1 nCov-19. Additionally, the sample size is too small to address rare adverse events, such as thrombotic thrombocytopenia syndrome or myocarditis. Moreover, the allocation of different vaccination regimens was not randomised. Due to the current recommendations for heterologous ChAdOx1 nCov-19–mRNA vaccination in individuals younger than 60 years, most health-care workers enrolled in our study opted for the recommended heterologous booster, and the cohort of participants vaccinated with homologous ChAdOx1 nCov-19 boost was comparatively small, with participants who were older than those in the other groups. In addition to the different combinations of vaccines, the median interval between first and second dose was different for homologous BNT162b2 vaccination (21 days), homologous ChAdOx1 nCov-19 (83 days), and heterologous vaccination (71 days). Therefore, at this stage, it is unclear to which extent the observed differences in immunogenicity between homologous BNT162b2 and heterologous ChAdOx1 nCov-19–BNT162b2 or homologous ChAdOx1 nCov-19 might also be attributable to the extended vaccine interval in the latter two groups, and further research is needed to address these open questions. The observed increased anti-S1-IgG avidity in the ChAdOx1 nCov-19–BNT162b2 group compared with the homologous BNT162b2 group for instance, might be caused by the extended vaccination interval for ChAdOx1 nCov-19–BNT162b2 because antibody affinity maturation increases over time. However, vaccine intervals were similar for homologous ChAdOx1 nCov-19 and ChAdOx1 nCov-19–BNT162b2 vaccination, indicating that the heterologous combination itself might improve antibody maturation. A definitive immune correlate of protection has not been established for COVID-19 vaccines and our study does not provide data on effectiveness. Therefore, whether increased immunogenicity of heterologous vaccination translates into improved protection remains unclear, and this requires further studies. Given that both humoral and cellular immune responses were markedly improved by heterologous boosting, we speculate that heterologous ChAdOx1 nCov-19–BNT162b2 vaccination might provide superior protection, which is supported by the improved neutralisation capacity against a variant of concern with antibody escape mutations (beta). However, to make all vaccination regimens fully comparable, further research including homologous BNT162b2 vaccination with a 10–12-week interval is needed. Studies are also underway that investigate combinations of different mRNA vaccines (eg, BNT162b2 and mRNA-1273).25
In conclusion, our study provides important real-world evidence for the safety and immunogenicity of heterologous ChAdOx1 nCov-19–BNT162b2 vaccination. Besides the enhanced immunogenicity described for the regimen described in this study, heterologous vaccination schedules might also alleviate logistical challenges and mitigate intermittent supply shortages of individual vaccines. In light of increasing occurrence of new virus variants carrying immune escape mutations, it will be important to determine vaccine efficacy of heterologous vaccination regimens, particularly regarding protection against severe COVID-19. Our data support further studies into the applicability of heterologous prime-boost vaccination strategies for COVID-19.
Data sharing
Anonymised raw immunogenicity data for tested samples and anonymised reactogenicity data can be obtained upon request to the corresponding authors. Proposals will be reviewed and approved by the authors, collaborators, and the security department on the basis of scientific merit and absence of competing interests. If the proposal is approved and a data access agreement and confidentiality agreement has been signed, data will be shared through a secure online platform.
Declaration of interests
VMC is named together with Euroimmun on a patent application filed recently regarding the diagnosis of SARS-CoV-2 by antibody testing (application number EP20158626.0). HG and FKl are named on a patent application regarding neutralising antibodies against SARS-related coronaviruses (application number EP20177354). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We are grateful to all study participants at Charité—Universitätsmedizin Berlin for their participation. We also thank the entire staff of the Department for Occupational Medicine and the Charité Clinical Study Center at Charité—Universitätsmedizin Berlin and the Berlin Institute of Health for their support of the study. This study was supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19—COVIM [01KX2021] to CD, NS, FKu, FKl, VMC, and LES. Part of this work was funded by the German Ministry of Education and Research through projects VARIPath (01KI2021) to VMC and Deutsche Forschungsgemeinschaft (SFB-TR84 to NS and LES). The study was supported by a donation from Zalando SE to Charité Berlin.
Contributors
DH, TS, PT-L, PT, MLS, JR, HG, and KV did experiments and analysed data. DH, TS, PT-L, LES, VMC, and FKu have accessed and verified the data. DH, TS, PT-L, FKu, VMC, LES, FKl, HG, and KV wrote and revised the manuscript. FKu, VMC, and LES planned and supervised the study. HH, CT, SJ, AS, CvK, CD-H, PK, NS, CD, HB, JS, ETH, and LJL contributed data or samples. All authors contributed data to the study, revised manuscript, and had responsibility for the decision to submit for publication.
EICOV/COVIM Study Group
Claudia Conrad, Doris Frey, Anne-Sophie Sinnigen, Carolin Rubisch, Nadine Olk, Lisbeth Hasler, Angela Sanchez Rezza, Paolo Kroneberg, Alexandra Horn, Willi Koch, Paula Stubbemann, Julie-Anne Gabelich, Friederike Münn, Julia Tesch, Petra Mackeldanz, Leon Bergfeld, Tobias Bleicker, Jörn Ilmo Beheim-Schwarzbach, Anna Luisa Hiller, Sophia Brumhard, Lara Bardtke, Kai Pohl, Daniel Wendisch, Philipp Georg, Denise Treue, Dana Briesemeister, Jenny Schlesinger, Andreas Hetey, Luisa Kegel, Annelie Hermel, Ben Al-Rim, Birgit Maeß, Kerstin Behn, Michelle Lisy, Saskia Zvorc, Maria Rönnefarth, Sein Schmidt, Alexander Krannich, Isabelle Schellenberger, Georg Schwanitz, Viktoria Schenkel, Norma Bethke, Claudia Hülso, Sebastian Dieckmann, Christian Peiser.
Contributor Information
EICOV/COVIM Study Group:
Ben Al-Rim, Lara Bardtke, Jörn Ilmo Beheim-Schwarzbach, Kerstin Behn, Leon Bergfeld, Norma Bethke, Tobias Bleicker, Dana Briesemeister, Sophia Brumhard, Claudia Conrad, Sebastian Dieckmann, Doris Frey, Julie-Anne Gabelich, Philipp Georg, Ute Gläser, Lisbeth Hasler, Andreas Hetey, Anna Luisa Hiller, Alexandra Horn, Claudia Hülso, Luisa Kegel, Willi Koch, Alexander Krannich, Paolo Kroneberg, Michelle Lisy, Petra Mackeldanz, Birgit Maeß, Friederike Münn, Nadine Olk, Christian Peiser, Kai Pohl, Annelie Hermel, Maria Rönnefarth, Carolin Rubisch, Angela Sanchez Rezza, Isabelle Schellenberger, Viktoria Schenkel, Jenny Schlesinger, Sein Schmidt, Georg Schwanitz, Anne-Sophie Sinnigen, Paula Stubbemann, Julia Tesch, Denise Treue, Daniel Wendisch, and Saskia Zvorc
Supplementary Material
References
- 1.Committee EMAPRA. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 vaccine (ChAdOx1-S [recombinant])—COVID-19 vaccine AstraZeneca (other viral vaccines) EPITT. 2021;24 [Google Scholar]
- 2.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledford H. Could mixing COVID vaccines boost immune response? Nature. 2021;590:375–376. doi: 10.1038/d41586-021-00315-5. [DOI] [PubMed] [Google Scholar]
- 6.Robert Koch Institut Mitteilung der STIKO zur COVID-19-Impfung. Impfabstand und heterologes Impfschema nach Erstimpfung mit Vaxzevria (1.7.2021) 2021. https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/PM_2021-07-01.html
- 7.Robert Koch Institut Beschluss der STIKO zur 5. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. 2021. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/19/Art_03.html
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RH, Stuart A, Greenland M, Liu X, Van-Tam JSN, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahase E. COVID-19: one dose of vaccine cuts risk of passing on infection by as much as 50%, research shows. BMJ. 2021;373 doi: 10.1136/bmj.n1112. [DOI] [PubMed] [Google Scholar]
- 14.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard AJ, Launay O, Lelievre J-D, et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2021;21:493–506. doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]
- 16.Spencer AJ, McKay PF, Belij-Rammerstorfer S, et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Biologics Evaluation and Research Toxicity grading scale for volunteers in vaccine clinical trials. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical
- 19.Schwarz T, Tober-Lau P, Hillus D, et al. Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis. 2021;27:2174–2178. doi: 10.3201/eid2708.211145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanshylla K, Di Cristanziano V, Kleipass F, et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917. doi: 10.1016/j.chom.2021.04.015. 29.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 22.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.University of Oxford Comparing COVID-19 vaccine schedule combinations—Com-COV2. 2021. https://comcovstudy.org.uk/about-com-cov2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised raw immunogenicity data for tested samples and anonymised reactogenicity data can be obtained upon request to the corresponding authors. Proposals will be reviewed and approved by the authors, collaborators, and the security department on the basis of scientific merit and absence of competing interests. If the proposal is approved and a data access agreement and confidentiality agreement has been signed, data will be shared through a secure online platform.