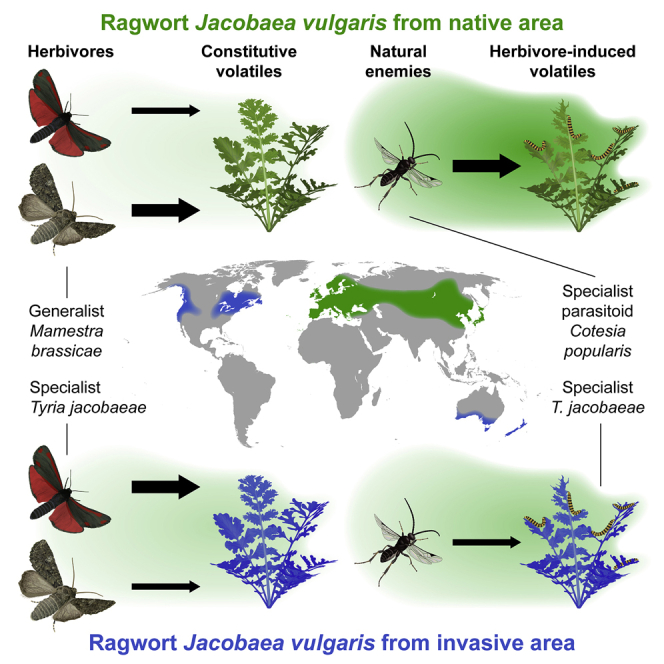
Summary
It is increasingly evident that plants interact with their outside world through the production of volatile organic compounds,1, 2, 3, 4, 5 but whether the volatiles have evolved to serve in plant defense is still a topic of considerable debate.3,6, 7, 8 Unharmed leaves constitutively release small amounts of volatiles, but when the leaves are damaged by herbivorous arthropods, they emit substantially more volatiles. These herbivore-induced plant volatiles (HIPVs) attract parasitoids and predators that kill insect herbivores,9, 10, 11, 12 and this can benefit the plants.13,14 As yet, however, there is no tangible evolutionary evidence that this tritrophic interplay contributes to the selection forces that have shaped the volatile emissions of plants.2,3,5, 6, 7, 8,15 With this in mind, we investigated the evolutionary changes in volatile emissions in invasive common ragwort and the respective defensive roles of its constitutive and inducible volatiles. This Eurasian plant has invaded other continents, where it evolved for many generations in the absence of specialized herbivores and their natural enemies. We found that, compared to native ragworts, invasive plants release higher levels of constitutive volatiles but considerably lower levels of herbivore-induced volatiles. As a consequence, invasive ragwort is more attractive to a specialist moth but avoided by an unadapted generalist moth. Importantly, conforming to the indirect defense hypothesis, a specialist parasitoid was much more attracted to caterpillar-damaged native ragwort, which was reflected in higher parasitism rates in a field trial. The evolution of foliar volatile emissions appears to be indeed driven by their direct and indirect roles in defenses against insects.
Keywords: ragwort, Jacobaea vulgaris, cinnabar moth, Tyria jacobaeae, Cotesia popularis, constitutive plant volatiles, herbivore-induced plant volatiles, Mamestra brassicae, shifting defense hypothesis, invasive species evolution
Graphical abstract
Highlights
-
•
The evolution of invasive plants can provide insight into the function of volatiles
-
•
Invasive ragwort releases more constitutive but fewer inducible volatiles
-
•
The invader is more attractive to a specialist herbivore but less to its parasitoid
-
•
The evolution of volatiles from invasive ragwort supports their defensive function
Lin et al. compare constitutive and herbivore-induced volatiles emitted by ragwort plants from native and invasive areas. The observed differences and the corresponding behavioral responses of a specialist and a generalist herbivore as well as of a parasitic wasp support the hypothesis that volatiles evolved as plant defenses.
Results and discussion
Plant invasions can be considered large-scale evolutionary experiments, with major shifts in herbivore and natural enemy pressures.16 Invasive plants often escape from the coevolved herbivores that occur in their native range and encounter novel, unadapted herbivores in the invaded ranges.17,18 Such shifts in insect associations exert altered selection pressures, which can lead to evolutionary changes in allocation patterns to defense and growth.19 Invasive plants are therefore also highly suited to test the importance of insects for the evolutionary trajectory of plant volatiles. We did this for Jacobaea vulgaris (common ragwort), which is native to Eurasia and has been introduced more than a century ago independently in New Zealand, Australia, and North America.20 In its native range, J. vulgaris is attacked by a number of specialist herbivores, but most damage is caused by the caterpillars of the cinnabar moth Tyria jacobaeae, which can have a strong impact on the plant’s fitness.21 The caterpillars are frequently parasitized by the specialist parasitoid Cotesia popularis, with parasitism rates that can reach up to 37% in the field.22 No specialist herbivores occur in the invasive ranges.17 If herbivores and their natural enemies play a role in the evolution of volatile emissions, we would therefore expect differences in the emissions between native and invasive J. vulgaris populations. Based on this expectation, we formulated the hypotheses that (1), in its native range, J. vulgaris has been under selective pressure to minimize the release of constitutive plant volatiles (CPVs) to avoid detection by its specialist herbivores but to produce high levels of herbivore-induced plant volatiles (HIPVs) to reliably attract natural enemies and (2), in its invasive range, in the absence of its main specialist herbivore and the natural enemies of this herbivore, J. vulgaris has been freed from the selective pressure to produce HIPVs, and the emissions of CPVs may have been boosted to better deter generalist herbivores. We test these hypotheses with a series of manipulative experiments.
Moth preferences and constitutive volatiles
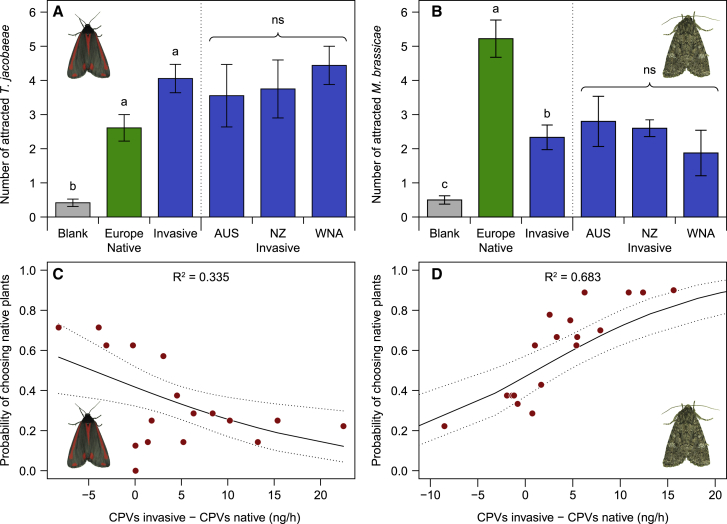
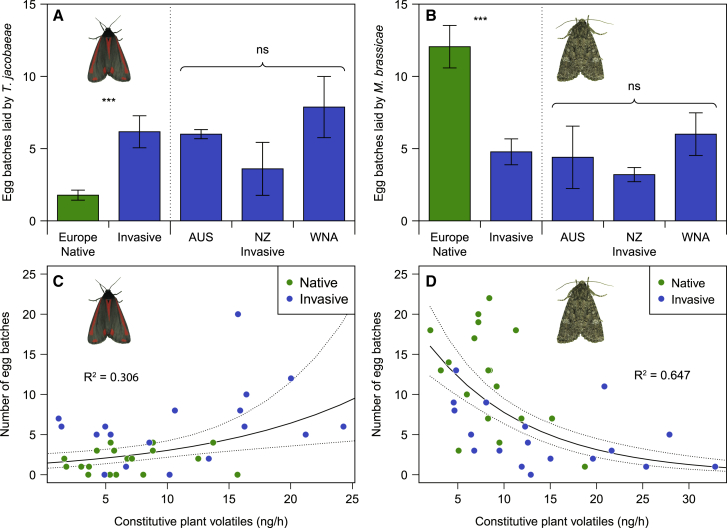
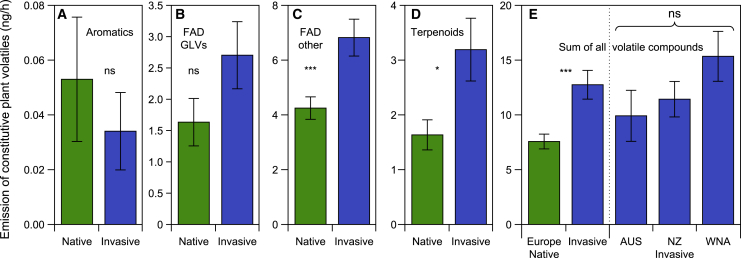
In a first experiment, we collected CPVs from undamaged native and invasive J. vulgaris genotypes and conducted olfactometer experiments to test the responses of two moth species, one whose larvae are adapted to feed on J. vulgaris and one whose larvae rarely survive on it. The specialist T. jacobaeae preferred (by a marginal difference; p = 0.0767) the odor of invasive genotypes over the odor of native plants, whereas the generalist Mamestra brassicae strongly preferred the odor of native genotypes over invasive ones (Figures 1A and 1B). For both moths, the percentage of females choosing a plant in each invasive-native pair was correlated with the difference in CPV production between the two plants but in opposite directions: T. jacobaeae preferred the high-emitting plant within each pair, whereas M. brassicae preferred the low-emitting plant (Figures 1C and 1D). In the oviposition assays, T. jacobaeae females laid three times as many egg batches on invasive genotypes than on native genotypes (Figure 2A). In contrast, M. brassicae females laid more than double the number of egg batches on native genotypes compared to invasive genotypes (Figure 2B). For both moths, there were no significant differences among plants from the three invasive ranges in the number of egg batches they laid (Figures 2A and 2B). The number of egg batches laid by T. jacobaeae increased with increasing total CVP emissions, although it decreased for M. brassicae (Figures 2C and 2D), further corroborating the importance of these volatiles for the moths’ oviposition decisions. Figures 3A–3D present the amounts of emitted volatiles, classified on the basis of the common metabolic pathways through which they are produced.23 On average, invasive J. vulgaris genotypes used in this experiment produced 74% more CPVs than native genotypes (Figure 3E; invasive = 192.6 ± 20.6 ng/12 h versus native = 110.8 ± 10.6 ng/12 h). The difference in CPV emissions between native and invasive plants was due to the higher emission by invasive plants of fatty acid derivatives and terpenoids (Figures 3A–3D).
Figure 1.
Host plant preferences of a specialist and generalist moth species
(A and B) Attraction of female T. jacobaeae moths (A) and female M. brassicae moths (B) to the odors of undamaged native and invasive Jacobaea vulgaris genotypes and two controls (blank) in a four-arm olfactometer (n = 5 for Australia [AUS], n = 5 for New Zealand [NZ], n = 8 for Western North America [WNA], and n = 18 for Europe). Values are means of number of moths choosing an olfactometer arm ± SE of 18 replicates for both moth species. 75% and 86% of the T. jacobaeae and M. brassicae, respectively, made a choice. Generalized linear mixed models (GLMMs) with a Poisson error distribution: (A) origin: χ2(2) = 64.813, p < 0.001; range within origin: χ2(2) = 0.724, p = 0.70; (B) origin: χ2(2) = 88.348, p < 0.001; range within origin: χ2(2) = 1.324, p = 0.52. Different letters indicate significant differences among treatments at p < 0.05 with a Tukey post hoc test, whereas ns above the three invasive ranges represents no significant difference among the three invasive ranges.
(C and D) Proportion of T. jacobaeae (C) and M. brassicae (D) females that chose an undamaged native J. vulgaris over an undamaged invasive J. vulgaris in the olfactometer assay plotted against the differences in the amounts of constitutive plant volatiles (CPVs) released between the invasive and native J. vulgaris of the same pair. Logistic regressions (binomial GLM): (C) χ2(1) = 7.635, p = 0.006; (D) χ2(1) = 28.147, p < 0.001. The solid line indicates the predictions from the model and the dotted lines the confidence intervals of the predictions.
See also Figures S1, S3, and S4A and Table S2.
Figure 2.
Oviposition preferences of a specialist and generalist moth species
(A and B) Mean number of egg batches laid by (A) T. jacobaeae and (B) M. brassicae on native and invasive J. vulgaris genotypes originating from different ranges (n = 5 for AUS, n = 5 for NZ, n = 8 for WNA, and n = 18 for Europe). Values are means ± SE. GLMMs with a Poisson error distribution: (A) origin: χ2(1) = 20.169, p < 0.001; cage: χ2(1) = 2.704, p = 0.10; range within origin: χ2(2) = 5.968, p = 0.05; (B) origin: χ2(1) = 21.488, p < 0.001; cage: χ2(1) = 3.923, p = 0.048; range within origin: χ2(2) = 1.188, p = 0.55; origin × cage: χ2(1) = 0.108, p = 0.74; range within origin × cage: χ2(2) = 9.976, p = 0.007. Different letters indicate significant differences between plant origin at p < 0.05 with a Tukey post hoc test. ns above the three invasive ranges represents no significant difference among the three invasive ranges.
(C and D) Log-linear regressions (Poisson GLMM) between CPV emissions of a given plant and the number of egg batches it received from Tyria jacobaeae (C) and Mamestra brassicae (D). (C) CPVs: χ2(1) = 9.673, p = 0.002; cage: χ2(1) = 1.456, p = 0.23; CPVs × cage: χ2(1) = 3.581, p = 0.058; (D) CPVs: χ2(1) = 44.257, p < 0.001; cage: χ2(1) = 6.287, p = 0.012; CPVs × cage: χ2(1) = 1.600, p = 0.21. The solid line indicates the predictions from the model and the dotted lines the confidence intervals of the predictions.
Figure 3.
Constitutive plant volatiles (CPVs) emission by native and invasive Jacobaea vulgaris genotypes
(A–D) Total emission of CPVs from different metabolic pathways: aromatic compounds (A); fatty-acid-derived (FAD) green leave volatiles (GLVs) (B); other FADs (C); and terpenoids (D). This comparison of VOC groups emitted by invasive and native Jacobaea vulgaris genotypes is based on the volatiles collected during the moth olfactometer test. Values are means ± SE. Linear mixed models (LMMs): (A) χ2(1) = 0.531, p = 0.47; (B) χ2(1) = 2.654, p = 0.10; (C) χ2(1) = 14.752, p < 0.001; (D) χ2(1) = 6.001, p = 0.014. This classification of VOCs is based on the most common metabolic pathways used by plants to produce volatile compounds. Asterisks indicate significant differences between origin; ∗p < 0.05; ∗∗∗p < 0.001; ns, not significant.
(E) Total leaf volatile emission of invasive and native J. vulgaris genotypes originating from different ranges used in the moth olfactometer experiment. Values are means ± SE (n = 10 for AUS, n = 10 for NZ, n = 16 for WNA, and n = 36 for Europe). LMM, origin: χ2(1) = 13.257, p < 0.001; range within origin: χ2(2) = 5.409, p = 0.067. ∗∗∗p < 0.001, ns above the three invasive ranges represents no significant difference among the three invasive ranges.
Contrary to HIPVs, the importance of CPVs in multitrophic interactions has been largely ignored. Relatively few studies have looked at how CPVs may repel or attract herbivores,24,25 and it has not been considered that these effects may differ for specialist and generalist herbivores. Clearly, CPVs of invasive J. vulgaris plants are far less attractive to the generalist M. brassicae, and this is reflected in reduced oviposition on invasive plants (Figures 2B and 2D). In a previous study,26 female moths of another generalist (Spodoptera exigua) showed the same oviposition preference for native plants. In the native range, where generalists and specialists are both present, the production of CPVs therefore presents an ecological dilemma for the plant because, unlike generalists, the specialist T. jacobaeae is attracted by CPVs, as is evident from its preference for invasive J. vulgaris genotypes (Figures 2A and 2C). This implies that the change in herbivore selection pressures is driving the evolution of plant CPVs, as supported by significant differences in all of the measured variables (egg batches, attractiveness, and CPVs) between invasive and native genotypes. These changes go in the same direction for all three invasive ranges (Figures 1, 2, and 3E), which have distinct climatic conditions (Figure S2), and therefore, differences in abiotic factors do not explain the observed changes. The recent introduction of T. jacobaeae in some invasive populations27 offers a great opportunity to study what happens if the selection pressure on CPV is reversed.
Herbivore-induced volatiles and parasitoid attraction
In a second experiment, we collected volatiles from native and invasive J. vulgaris genotypes before and after infestation by caterpillars of the specialist moth and compared the volatile composition and quantities of these HIPVs. In an olfactometer experiment, we tested the odor preferences of females of the parasitic wasp C. popularis when offered choices between the HIPVs of plants from different origins.
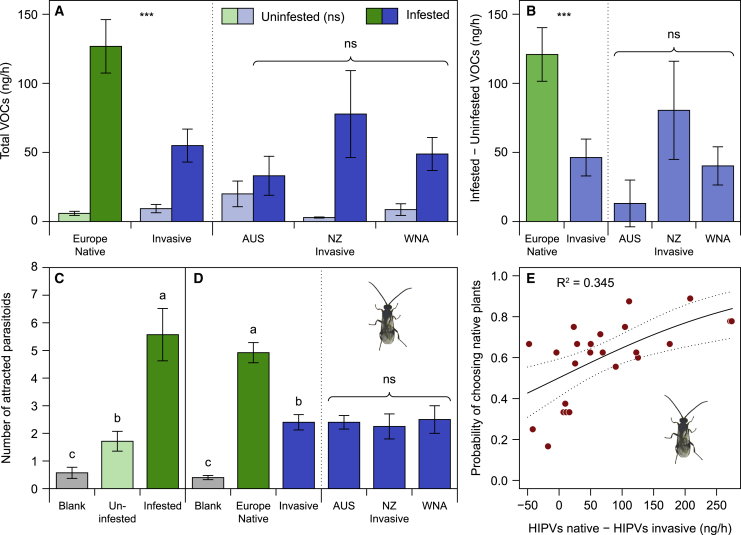
After infestation by T. jacobaeae larvae, native genotypes emitted on average 27.3 times more volatiles, whereas the invasive genotypes showed only a 4.9-fold increase in the total amount of volatiles after infestation (Figure 4A). Moreover, the difference in total emissions before and after T. jacobaeae infestation was considerably greater in plants from native than from invasive ranges (Figure 4B). These differences go in the same direction for all three invasive ranges (Figures 4A and 4B), which have distinct climatic conditions (Figure S2). In the parasitoid olfactometer bioassays, the odors of native J. vulgaris genotypes infested with T. jacobaeae larvae were three times as attractive to C. popularis females than the odors of the non-infested native J. vulgaris genotypes (Figure 4C). More importantly, native J. vulgaris plants that were damaged by T. jacobaeae were more than twice as attractive as similarly damaged invasive plants (Figure 4D). The wasps’ behavioral responses matched the measured differences in HIPV emissions between invasive and native J. vulgaris plants (Figure 4E). The preference of female C. popularis wasps for native J. vulgaris genotypes infested with T. jacobaeae in each invasive-native plant pair tested (10 parasitoids used per pair) was positively associated with the difference in HIPVs emission between the pairs. It should be noted that, in cases where the total amount of HIPVs did not differ between native and invasive genotypes, the parasitoids did not distinguish between native or invasive plant odors (Figure 4E). The differences in HIPVs emission between invasive and native plants were only quantitative, as the composition of the VOC blends emitted by native genotypes after infestation was not different from that of invasive genotypes (Figure S3).
Figure 4.
Differences in herbivore-induced volatile (HIPV) emission between native and invasive plants and its effect on parasitoid attraction
(A) Total emission of HIPVs of invasive and native J. vulgaris genotypes originating from different ranges used in the parasitoid olfactometer experiment. One paired sample from NZ was lost. Values are means ± SE (n = 5 for AUS, n = 7 for NZ, n = 12 for WNA, and n = 25 for Europe). LMM, infestation: χ2(1) = 205.147, p < 0.001; origin: χ2(1) = 3.49, p = 0.062; range within origin: χ2(2) = 0.396, p = 0.82; infestation × origin: χ2(1) = 12.235, p < 0.001; range within origin × infestation: χ2(2) = 8.238, p = 0.013. Asterisks indicate significant differences between origin in infested plants; ∗∗∗p < 0.001; ns above the three invasive ranges represents no significant difference among the three invasive ranges. There were no differences between origin or among ranges in uninfested plants.
(B) Differences in total VOC emissions (after − before) between native and invasive regions. LMM, origin: χ2(1) = 18.936, p < 0.001; range within origin: χ2(2) = 1.801, p = 0.41. Asterisks indicate significant differences between origin; ∗∗∗p < 0.001; ns above the three invasive ranges represents no significant difference among the three invasive ranges.
(C and D) Parasitoid attraction.
(C) Number of females of C. popularis attracted to the odors of native J. vulgaris genotypes infested with 15-s instar T. jacobaeae versus uninfested native J. vulgaris genotypes.
(D) Number of females of C. popularis attracted to odors of native J. vulgaris infested with 15-s instar T. jacobaeae versus invasive J. vulgaris infested with 15-s instar T. jacobaeae from three ranges. Overall, 84% and 81% of the C. popularis parasitoids made a choice, respectively, in (C) and (D). GLMM with a Poisson error distribution: (C) origin: χ2(2) = 40.411, p < 0.001; (D) origin: χ2(2) = 114.003, p < 0.001; range within origin: χ2(2) = 0.125, p = 0.94. Different letters indicate significant differences among treatments at p < 0.05 with a Tukey post hoc test. ns above the three invasive ranges represents no significant difference among ranges.
(E) Proportion of females of the parasitoid C. popularis that chose the odor of native plants over invasive plants of J. vulgaris, both infested with 15-s instar of T. jacobaeae, plotted against the differences in the amounts of HIPVs produced between the native and invasive genotype of the same plant pair. Logistic regression (binomial GLM): χ2(1) = 11.449; p < 0.001. The solid line indicates the predictions from the model and the dotted lines the confidence intervals of the predictions.
See also Figures S1, S3, and S4 and Table S2.
Native J. vulgaris released a more than 2-fold higher amount of HIPVs than invasive genotypes after infestation with T. jacobaeae, which was reflected in reduced attraction of C. popularis females to HIPVs from invasive plants. This supports our hypothesis that invasive J. vulgaris genotypes have evolved reduced levels of HIPVs because, in the absence of T. jacobaeae, they have no longer a need to attract parasitoids. This is in general agreement with the notion that HIPVs serve as an indirect defense function by attracting natural enemies.6,10,12
Parasitism rates in the field
In a final experiment, we tested, under realistic field conditions, whether the observed odor preferences of the parasitoid result in differences in parasitism rates. Lab-reared T. jacobaeae caterpillars were distributed equally over native and invasive J. vulgaris plants that had been placed in a native J. vulgaris population with naturally occurring T. jacobaeae and parasitoids (Figure S4B). Twelve days after placing caterpillars on the plants, they were recollected and brought to the laboratory. We retrieved circa 18% of the released T. jacobaeae larvae, with fewer larvae (on average 5.7 larvae) retrieved from pairs of native than from pairs of invasive plants (on average 8.53 larvae). In total, 614 larvae were found back on invasive plant pairs versus 422 on native plant pairs (generalized linear mixed model [GLMM] with a Poisson error distribution: origin, χ2(1) = 8.119, p = 0.004; block: χ2(2) = 21.3, p < 0.001; Table S1). The parasitism rate of T. jacobaeae larvae retrieved from native genotypes was significantly higher (14.3%) than for larvae from invasive genotypes (8.6%; GLMM with a binomial error distribution: origin, χ2(1) = 6.2652, p = 0.012; block: χ2(2) = 28.473, p < 0.001), and this difference remained significant after removing the two pairs of invasive plants from the analysis that carried additional T. jacobaeae larvae from a natural laid egg batch (GLMM with a binomial error distribution: origin, χ2(1) = 4.445, p = 0.035; block: χ2(2) = 17.811, p < 0.001; Table S1). Hence, T. jacobaeae caterpillars had a higher probability of being parasitized by naturally occurring C. popularis wasps on native J. vulgaris genotypes than on invasive J. vulgaris genotypes. Interestingly, the number of plant pairs with parasitized larvae did not differ between native and invasive plant pairs (GLMM with a binomial error distribution: origin, χ2(1) = 1.305, p = 0.253; block: χ2(2) = 7.231, p = 0.027). Yet the fact that a higher proportion of larvae was parasitized on native than on invasive plants suggests that parasitoids forage longer on plants with a high HIPV production, supporting the notion that natural selection for HIPVs is favored by their attractiveness to natural enemies.
It is noteworthy that we retrieved fewer larvae from native than from invasive plants. This may be because of a higher predation level on the native plants, again well in line with the observed higher volatile production upon damage, leading to higher attractiveness to predators. Moreover, when perceiving C. popularis wasps, T. jacobaeae larvae may let themselves drop from the plant to escape parasitism.28 The lower recapture rate on native plants is therefore indicative of higher levels of attacks by C. popularis and other natural enemies. Disappearance of larvae due to dispersal is unlikely, as larvae of the T. jacobaeae do not leave their food plant before the third instar.29 The considerable difference in the number of retrieved larvae has important consequences for the feeding damage inflicted on the plants, as just a few larvae can completely defoliate an average-sized ragwort plant.30
Overall, the results fully support the hypothesis that the selection for HIPV production has been relaxed for invasive J. vulgaris because of the absence of T. jacobaeae and its coevolved parasitoid C. popularis. The assumed function of HIPVs as an indirect defense to attract natural enemies is further substantiated by the preferences of the parasitoid C. popularis and parasitism rates in the field. The rapid evolution of decreased emissions of HIPVs in invasive J. vulgaris genotypes suggests that the production of these volatiles is costly and that HIPVs are only adaptive in the native range of J. vulgaris. In conclusion, this study provides critical evidence that the exclusion from a specialized key herbivore and its main parasitoid has led to repeated and convergent changes in both CPV and HIPV emissions in J. vulgaris. Plants have to cope simultaneously with the pressure to hide from their specialist herbivores, signal their toxicity to generalist herbivores, and recruit parasitoids and other natural enemies to fend off attackers. Our results confirm that the balance between these different selective forces contributes to the evolution of foliar volatile emissions.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Jacobaea vulgaris | Seeds were collected from Europe, Australia, North America west coast and North America east coast | N/A |
| Tyria jacobaeae | Pupae were collected from the dunes of Meijendel, the Netherlands | N/A |
| Mamestra brassicae | Eggs were collected from a lab culture of Wageningen University31 | N/A |
| Cotesia popularis | Cocoons of C. popularis were obtained from T. jacobaeae larvae collected in the dunes of Meijendel, the Netherlands | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| E-2-hexenal | Sigma-aldrich | CAS: 6728-26-3 |
| Z-3-hexen-1-ol | Sigma-aldrich | CAS: 928-96-1 |
| E-2-hexen-1-ol | Sigma-aldrich | CAS: 928-95-0 |
| Z-3-hexenyl acetate | Sigma-aldrich | CAS: 3681-71-8 |
| n-octane | Sigma-aldrich | CAS: 111-65-9 |
| nonanal | Sigma-aldrich | CAS: 124-19-6 |
| Pentadecane | Sigma-aldrich | CAS: 629-62-9 |
| β-ocimene and trans-ocimene | Sigma-aldrich | CAS: 13877-91-3 |
| E-β-caryophyllene | Sigma-aldrich | CAS: 87-44-5 |
| E-β-farnesene | Sigma-aldrich | CAS: 18794-84-8 |
| α-humulene | Sigma-aldrich | CAS: 6753-98-6 |
| benzaldehyde | Sigma-aldrich | CAS: 100-52-7 |
| 1-nonene | Sigma-aldrich | CAS: 124-11-8 |
| decanal | Sigma-aldrich | CAS: 112-31-2 |
| 1-pentadecene | Sigma-aldrich | CAS: 13360-61-7 |
| Methylene chloride | Honeywell | CAS: 75-09-2 |
| Nonyl acetate | Sigma-aldrich | CAS: 143-13-5 |
| Deposited data | ||
| Datasets and statistical analyses | This paper | https://doi.org/10.5061/dryad.fxpnvx0rk |
| Software and algorithms | ||
| R v4.03 | R development core team, 2021 | https://www.r-project.org |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ted C.J. Turlings (ted.turlings@unine.ch)
Materials availability
This study did not generate new unique reagents.
Data and code availability
The datasets and code generated during this study are available at Dryad https://doi.org/10.5061/dryad.fxpnvx0rk
Experimental model and subject details
We used Jacobaea vulgaris (common ragwort, formerly Senecio jacobaea, family Asteraceae) and associated insects as our study system. J. vulgaris is a monocarpic perennial plant that is native to Eurasia and was introduced into Australia,32 New Zealand,33 Eastern North America and Western North America.33 The populations of J. vulgaris from West and Eastern North America are geographically isolated. Doorduin et al.20 found that the amount of genetic variation, using molecular markers, of native J. vulgaris populations does not differ from the different invasive ranges, suggesting that introductions from multiple source populations have occurred.
In the USA, an introduced range, J. vulgaris has been observed to be fed upon by more than 40 species of generalist arthropods but no specialist herbivores.17 On the other hand, in the native range, J. vulgaris is attacked by more than 70 herbivores, but most herbivory is due to two highly specialized herbivores: the cinnabar moth, Tyria jacobaeae and a flea beetle, Longitarsus jacobaeae.26,32 The foliar-feeding larvae of T. jacobaeae can remove all the aboveground parts of J. vulgaris plants within a short time period.22,34,35 In the native range, up to 37% of the larvae of T. jacobaeae can be parasitized by the specialist braconid parasitoid Cotesia popularis.22 In the past decades, Tyria jacobaeae and L. jacobaeae were introduced as biological control agents into to the three invasive ranges.27,36, 37, 38 But evidences collected so far showed no evolutionary adaptation of invasive J. vulgaris populations after this renewed exposure to T. jacobaeae.26,39
The invasion of J. vulgaris took place 100-160 years ago, depending on range, and until a few decades ago no specialist herbivores were present in invasive populations. Enough time has passed without specialized herbivores for selection to lead to evolutionary changes in defense chemistry in this species with a lifespan of two years. Abiotic factors such as climatic condition in the introduced areas could also potentially affect post-introduction evolution.40, 41, 42 For this reason, we selected populations from a variety of distinct invasive ranges, covering a wide spectrum of climatic conditions, to compare them with the native range. Previous studies showed that, within less than 70 generations, natural selection in response to the release from specialist herbivores changed resource allocation patterns of invasive J. vulgaris populations in favor of better growth and competitive ability at the expense of costly defenses.26,43,44 Therefore J. vulgaris is a highly suitable model plant species to study evolutionary changes after invasion.
As a specialist herbivore we used the univoltine cinnabar moth T. jacobaeae, which is native to Europe and western central Asia. In spring, adults emerge from pupae between the end of April and June, and lay eggs in clusters on the underside of the lower leaves of J. vulgaris. The number of eggs per batch can range from 1 to more than 80. Eggs hatch within 2 weeks after oviposition. Larval development takes over one month and consists of five larval stages. Fully grown larvae leave the plant and pupate in the upper layers of the soil. In Northwestern Europe, dune populations of J. vulgaris are particularly susceptible to T. jacobaeae larvae and suffer complete defoliation once every 2 or 3 years in mid-June. More than 1000 pupae of T. jacobaeae were collected from the dunes of Meijendel, the Netherlands, at the end of May, 2015. Pupae were kept in a cold room and in April 2016 they were transferred to a climate chamber (20°C, 50 to 70% RH, 16:8 h L/D). Adult moths that emerged from the pupae were used for both the olfactometer and oviposition experiments. Eggs were collected and larvae were reared on J. vulgaris plants until they reached the second instar for later infestation. The moth Mamestra brassicae (cabbage moth, Noctuidae), which has a distribution range from Europe to eastern Asia, was used as a generalist herbivore. The larvae feed on the leaves of a wide range of plants and adults can be found at any time from May to October due to the two or three overlapping generations.45 Eggs were collected from a lab culture of Wageningen University31 and larvae were reared on Chinese cabbage until pupation. Then adult moths were maintained in a climate chamber (20°C, 50 to 70% RH, 16:8 h L/D) for later use.
The gregarious braconid parasitoid Cotesia popularis (Apanteles popularis) is a specialist on T. jacobaeae. The adults emerge at the beginning of May and only parasitize the first and second instar larvae of their host. The parasitoid larvae do not kill their host immediately, but develop inside the living host, which dies shortly before the parasitoids pupate. Parasitoids overwinter in a woolly, white cocoon and emerge as adults the following spring.46 More than 1000 cocoons of C. popularis were obtained from T. jacobaeae larvae collected in the dunes of Meijendel, the Netherlands, at the end of May, 2015. Cocoons were kept in a cold room until April 2016. They were then transferred to a climate chamber (20°C, 50 to 70% RH, 16:8 h L/D), where the adults emerged and were kept with honey and water until they were used in the experiments. The insects had no experience with HIPVs prior to the experiments.
Method details
Plant growth conditions
Seeds of J. vulgaris were collected from Europe, Australia, North America west coast and North America east coast (Table S2). A redundancy analysis using 19 climatic variables was performed to compare the climatic differences among the geographic ranges (see Quantification and statistical analysis section). The analysis shows that the sample-collecting sites of the four ranges have actually different climatic conditions (Figure S2). Seeds originating from the same plant were germinated in a Petri dish lined with moistened filter papers, and seedlings were transferred into cylindrical plastic pots (4 × 10 cm) with commercial soil (Ricoter Aussaaterde, Aarberg, Switzerland). Plants were grown in a controlled growth chamber under 16:8 L:D light regime at 25°C (GroBanks, Model BB-XXL3+, CLF plant climatics, Emersacker, Germany; photosynthetic photon flux density (PPFD): 100 μmol m-2 s-1).
Moth olfactometer and oviposition assays
Moth olfactometer bioassays and oviposition bioassays were conducted for both T. jacobaeae and M. brassicae using 8-week old vegetative J. vulgaris plants with a rosette diameter of circa 10 cm and 8-12 leaves. For bioassays with each moth species, 18 native J. vulgaris genotypes from Europe were randomly paired with 18 invasive J. vulgaris genotypes from Australia (5 populations), New Zealand (5 populations), North America east coast (6 populations) and North America west coast (2 populations), resulting in 18 pairs (Table S2).
The preferences of female moths for different odors were measured using a four-arm olfactometer47 (Figure S4A), which had different dimensions from the one used for parasitoids (see below), notably wider arms allowing the moths to pass. Mated female moths were given the choice between four air flows from four different glass bottles. Two bottles were empty (blanks), one contained a native and one an invasive J. vulgaris plant, with the latter two positioned opposite to each other. The air flows converged in a central glass piece,48 where 2 moths were released. After 30 min, the moths were recollected and the treatment they chose was recorded. If a moth had walked more than 8 cm into one of the olfactometer arms (12 cm long in total), it was considered to have made a choice for that odor source. Moths that did not make a choice were recorded as “no choice.” An olfactometer test ( = 1 pair of plants tested) consisted of five consecutive releases of 2 moths and moths were used only once. Due to the limited space, moths were not able to fly in the olfactometer, but they were capable to walk into each arm. The position of each treatment was carefully assigned before each test to make sure the positions would be homogeneously distributed among treatments at the end of the experiment. The glassware was cleaned between tests and the cleaning process of the glassware consisted of rinsing the glassware sequentially with three solvents: water, acetone, and pentane, and putting the glassware in an oven at 250°C for a minimum of 3 h. The blends of volatiles emitted by each plant were collected after the olfactometer test with a volatile collection setup for 12h.49 VOCs were collected using a trapping filter containing 25 mg of 80–100 mesh SuperQ adsorbent. Before use, trapping filters were cleaned with 300 μL of methylene chloride. After each collection, the volatiles were extracted from the filters with 150 μL of methylene chloride. The samples were stored at −80°C before analysis.
After each olfactometer test and volatile collection, plants were transferred and randomly placed into two net cages (1 × 2 m) for oviposition bioassays. For each moth species the tested 18 pairs of plants were split into two cages, with first 9 pairs in one cage and the remaining 9 pairs in another cage. For each cage, 35 female moths and 15 male moths were released in the center of the cage. The position of each plant was randomly changed every day. After one week all moths were removed and the number of egg batches were counted and total leaf area of each plant was measured by a scanner and calculated using Adobe Photoshop CS6 software (Adobe Systems, San Jose, CA, USA). There was no significant difference in the total leaf area between native and invasive plant genotypes (ANOVA, F1,70 = 2.917, p = 0.092).
Parasitoid olfactometer assay
After 5 weeks of plant growth (4-5 leaves), 25 native J. vulgaris genotypes from Europe were paired with 25 invasive J. vulgaris genotypes from Australia (5 populations), New Zealand (8 populations), North America east coast (8 populations) and North America west coast (4 populations) (Table S2), resulting in 25 pairs. Individual plants from each pair received 15 s instar larvae of T. jacobaeae, resembling the herbivore pressure resulting from a small egg batch. After 24 hours of feeding, the preferences of C. popularis females for different odors were measured using a four-arm olfactometer setting.47 For each pair of plants, mated female C. popularis were given the choice between 4 air flows from four different glass bottles. Two bottles were empty (blanks), one contained an infested native and one an infested invasive J. vulgaris plant, with the latter two positioned opposite to each other. The air flows converged in a central glass piece, where two female C. popularis wasps were released.48 After 30 min, we recorded the choice of the wasps by counting the number that were trapped in a glass bulb of each arm (Figure S4A). Wasps that did not make a choice were recorded as “no choice.” The wasps were then removed and a new pair was introduced. An olfactometer test ( = 1 pair of plants) consisted of five consecutive releases of 2 wasps, and wasps were only used once. Plant positions were changed and glassware was cleaned between plant pairs as described above. The blends of volatiles emitted by each plant were collected for 3h just before infesting them with T. jacobaeae larvae and again after the olfactometer test had ended, using a volatile collection setup. Larvae were not removed from the plants during the volatile collection. Volatiles were collected using a trapping filter containing 25 mg of 80–100 mesh SuperQ adsorbent. Before use, trapping filters were cleaned with 300 μL of methylene chloride. After each collection, volatile compounds were extracted from the filters with 150 μL of methylene chloride. After the volatile collection, total leaf area of each plant was recorded by a scanner and the damaged leaf area was calculated the same way as stated above. Native and invasive genotypes did not differ in the total leaf area (ANOVA, F1,48 = 1.455, p = 0.234) and damaged leaf area (ANOVA, F1,48 = 0.537, p = 0.467).
In order to test whether the parasitoids were specifically attracted to HIPVs produced by infested J. vulgaris rather than CPVs, additional olfactometer tests were conducted with 2 sibs, seeds of the same flower head, of 7 genotypes from different native populations. For this additional test, wasps were given the choice between a sib infested with 15 s instar larvae of T. jacobaeae, an uninfested sib, and two empty odor sources (blanks). An olfactometer test ( = 1 pair of plants) consisted of five consecutive releases of 2 wasps, and wasps were only used once (n = 7).
Volatile analyses
Volatile compounds were analyzed and identified as described in D’Alessandro and Turlings.48 An internal standard (nonyl acetate, each 200 ng in 10 mL methylene chloride) was added to each sample. Volatile compounds were analyzed with an Agilent 6850 gas chromatograph coupled to a 5973 Network mass selective detector (transfer line 230°C, source 230°C, ionization potential 70eV). A 2 μL aliquot of each sample was injected in the pulsed split less mode onto a non-polar column (HP-1 ms, 30 m, 0.25 mmID, 0.25 μm film thickness, Agilent J&W Scientific, USA). Helium at constant flow (1.9 mL/min) was used as carrier gas. Compounds were identified by comparing the spectra obtained from the samples with those from a reference database (NIST mass spectral library). Additionally, the identity of compounds of particular interest to our study was verified by comparing the spectra and retention times found in our samples to those of pure compounds (Sigma-Aldrich). The compounds of verified identity were: benzaldehyde, E-2-hexenal, Z-3-hexen-1-ol, Z-2-hexen-1-ol, Z-3-hexenyl acetate, 1-nonene, nonanal, decanal, 1-pentadecene, trans-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene, β-caryophyllene, β-bergamotene and (E)-β-farnesene, (Table S4). The quantities of the major components of the blends were estimated based on the total ion count of the compounds compared to the total ion count of the internal standard and corrected for an appropriate response factor.50 The response factor relative to nonyl acetate was calculated separately for several standards: benzaldehyde, E-2-hexenal, Z-3-hexen-1-ol, E-2-hexen-1-ol, Z-3-hexenyl acetate, n-octane, nonanal, pentadecane, β-ocimene, E-β-caryophyllene, E-β-farnesene and α-humulene. For compounds not present in the standard list, we used the response factor of the closest standard in retention time of the same class. In case a given compound had a different molecular mass compared to that of its corresponding standard, the response factor was normalized based on the molecular mass.
Field experiment
We conducted a field experiment in the native range of J. vulgaris to measure the parasitism rates of T. jacobaeae larvae by naturally occurring C. popularis wasps on native and invasive J. vulgaris genotypes. The experiment was conducted in Meijendel, a sand dune area in the west of the Netherlands close to The Hague (52°13’N, 4°34’E), where native J. vulgaris, T. jacobaeae and C. popularis are naturally present. In Meijendel, parasitism rates of T. jacobaeae larvae by C. popularis varied from 0 to 37% over a 17-year period (1988-2004) with an average parasitism rate of circa 10%.51
In October 2016, seeds from 19 native populations and 16 invasive populations (Table S3) were germinated resulting in 294 native seedlings and 272 invasive seedlings. Seedlings were potted in small pots on 21 November 2016 and were grown in a climate room (Reftech) at a constant temperature of 20°C, 70% RH with 16 hours light with a photosynthetic photon flux density (PPFD) of 113 μmol m−2 s−1. On January 17, 2017, 197 native and 197 invasive plants were re-potted in 13 cm pots with a 50% soil/dune sand mixture and transferred on the same day to a frost-free greenhouse without supplemental light. Plants were watered when needed. An infestation with the aphid Myzus persicae was observed on April 3, 2017 and the biological control agents Chrysoperla carnea and Aphelinus abominalis (Koppert) were released on the plants. The biocontrol agents were able to control the aphid infestation. The plants were placed outside the greenhouse on 6 April 2017. Plants grew well outside and all received a 2ml volume of Osmocote, a slow nutrient releaser. Five plants were vernalized and started to flower and were discarded. On May 3, 2017, 148 plants from 19 native populations and 144 plants from 16 invasive populations were brought to the field to let them acclimatize to the local conditions. On May 8, 2017, the field experiment was initiated by placing the plants in three blocks in the dunes of Meijendel (Figure S4B). Blocks were circa 50 m apart and in all blocks a background population of J. vulgaris plants was present. Plants were grouped in pairs. A pair of plants consisted of two plants from the same origin (native or invasive). Experimental plants were put in pairs in the field to generate a larger odor plume. Pairs of plants were randomly put in a grid in such a way that a pair of native genotypes was always neighboring 4 pairs of invasive genotypes, and vice versa (Table S1; Figure S4B). Plants within a pair were placed about 15 cm apart so that leaves were just not touching. Pairs were placed 5 m apart. The three blocks consisted of 45, 49 and 52 pairs of plants respectively (Table S1; Figure S4B). Plants were placed on trays (18 cm diam.) and watered when needed.
For the field experiment larvae of the cinnabar moths T. jacobaeae were collected in 2016 from the dunes close to The Hague where also the field experiment was conducted in 2017. Larvae were grown until pupation and kept in a cold room at 4°C. In April 2017, pupae were taken from the cold room and within 2-3 weeks moths emerged. Within a week after emergence the moths laid eggs. After hatching, first instar larvae were used for infesting the plants in the field trial.
Just before artificially infesting the plants with T. jacobaeae caterpillars, we counted and removed the egg batches naturally deposited by local T. jacobaeae of 18 plants by cutting off the infested leaf with a sharp razorblade. Naturally deposited egg batches were equally distributed over native and invasive plants (Chi square test, χ2 = 0.83, p = 0.361). For two pairs an egg batch escaped our attention and these pairs therefore contained additional T. jacobaeae larvae, which could not be distinguished from experimental larvae.
Only first and second instar larvae of T. jacobaeae are parasitized by C. popularis.28 The infestation20protocol of plants in the field went as follows: every plant was infested with twenty freshly hatched larvae. We chose to add 20 larvae because in the oviposition experiment the cinnabar moth egg batches varied from 10 to 44 eggs with an average of 23 eggs, and in the HIPV experiment feeding by 15 freshly hatched larvae led to a strong increased HIPVs. Twenty freshly hatched larvae were transferred into an Eppendorf tube, and then the tubes were immediately brought to the field. Each tube was opened and placed in the middle of the rosette of a single plant. Larvae left the tubes by themselves. The next day plants were checked to verify that larvae had left the tubes and those that had not were counted. Infestation started on 16 May 2017 and for the next 10 days each day a different section of the pairs of plants were infested. During the first five consecutive days each day about 8 pairs of native and 8 pairs of invasive plants (circa 5 pairs per block) were infested. The 6th day no larvae were placed on plants and from the 7th day infestations continued for another four consecutive days until all plants had received neonate larvae. In total, we infested 72 pairs of invasive and 74 pairs of native J. vulgaris plants in the course of 10 days (Table S1). Of the 5840 cinnabar moth larvae transferred in tubes to the plants 124 larvae were still in the tubes the next day and were excluded from the experiment. In the end, each pair of plants received on average 39.2 first instar larvae (19.6 larvae per plant).
Exactly twelve days after infestation, the larvae were removed from the plants, counted and taken to the laboratory, where they were reared until pupation in individual boxes per pair of plants. Harvesting of the larvae, just as the infestation, took 10 days, and they were harvested from the plants in the same order as they were placed on the plants. Therefore, all larvae were exposed to natural enemies in the field for exactly twelve days. After harvesting the larvae, plant shoots were harvested, dried for at least three days at 60°C and weighed. The shoot weights of plants from the invasive range were larger than the shoot weights of genotypes from the native range (7.49 g versus 4.97 g, ANOVA, F1,290 = 70.2, p < 0.001). In the laboratory experiment, we did not find a significant correlation between shoot weight and plant VOC emissions (Pearson correlation, r = 0.187, n = 50, p > 0.05) suggesting that plant size has little effect on volatile production in common ragwort.
In the laboratory, caterpillars were fed with leaves from native J. vulgaris collected from natural populations from Zoeterwoude and Leiden. When the cinnabar moth larvae reached the fifth instar, they were placed in separate tubes. Parasitoid larvae emerge just before pupation. The number of parasitized larvae and the number of (unparasitized) T. jacobaeae pupae were recorded.
Quantification and statistical analysis
For the olfactometer experiments, the effects of plant volatiles on the preference of female moths and parasitoids were analyzed using generalized linear mixed models (GLMM) with a Poisson error distribution (both moth species: n = 18, wasps: n = 25, where n represents number of olfactometer test). The number of moths/wasps that chose any olfactometer arm was the response variable, with plant origin (blank, native and invasive) and range nested within plant origin as fixed explanatory factors. An extra analysis was performed for wasp preference only with native plants that were either uninfested or infested by Tyria jacobaeae caterpillars. In all models the olfactometer assay was used as a random factor. Moths/wasps that did not make a choice were excluded from the analyses but are mentioned in the results section.
We also tested if the variation in plant volatiles explains the variation in the olfactometer preference of moths/wasp using logistic regressions (binomial GLM). For the moth preference, we tested if the difference in constitutive volatile emissions between the invasive and the native plant for a given assay explains the proportion of moths that chose native plants over invasive plants. For the wasp preference, we tested if the difference in herbivore-induce volatiles emissions between the native and the invasive plant for a given assay explains the proportion of wasps that chose native plants over invasive plants. The difference between invasive and native plants in total leaf area from the moth bioassays and in total leaf area and damaged leaf area from the parasitoid bioassays were evaluated with an ANOVA.
For the cage experiments, the effects of plant origin on the number of egg batches laid by moths were analyzed with a GLMM with a Poisson error distribution (for both moth species: n = 36, where n represent number of individual plants). Here the number of egg batches was the response variable, while plant origin (native or invasive), range nested within plant origin, cage and the interaction of cage by plant origin were taken as fixed factors. When the interaction was not significant it was removed from the model. We also tested how the total emissions of constitutive volatiles explain the number of egg batches per plant using a log-linear regression (Poisson GLMM). We used cage ID as a covariate to control for differences between cages and the plant pair was included as a random factor.
The differences between plant origin (native versus invasive) in constitutive VOCs were analyzed with linear mixed models (LMM; n = 72, where n represents number of plants). To analyze the variation in HIPVs we also used LMMs (n = 99, where n represents number of plants). Here the response variable was the total volatile emission (log transformed), the fixed factors were the infestation state (infested or uninfested), the plant origin (native versus invasive), their interaction and the range nested within plant origin and plant identity as a random factor. In another model we used the difference between infested and uninfested HIPVs as response factor and plant origin and range nested within plant origin as fixed factors. As these plants were used in pairs in the olfactometers assays, we included a random factor for the plant pair ID to account for a possible influence of the experimental procedure.
For the field experiment we analyzed whether the plant origin can explain the number of caterpillars retrieved from plants using a GLMM with a Poisson error distribution (n = 146, where n represents number of plant pairs). The effects of plant origin on parasitism rate and parasitism presence were analyzed using a GLMM with a binomial error distribution. In addition, we repeated the model for parasitism rate after removing a couple of observations which contained caterpillars from natural origin. In these models the field was used as a covariate.
To analyze whether the plant origin and infestation state explain the volatile composition we performed a redundancy discriminant analyses (RDA, R package vegan). The response compound matrix was normalized and the explanatory variables were the plant origin (native versus invasive), the plant state (uninfested versus infested) and their interaction (n = 99, where n represents number of plants). We also, performed an RDA on climatic variables across the sampling sites to explore climatic differences among ranges (Europe, Australia, New Zealand and Western North America). For this, 19 climatic variables (1950-2000) were downloaded from the World Clim dataset (https://www.worldclim.org/current) in 5 arc-minutes resolution for each sampled population (n = 49, where n represents number of locations).
For all the linear models, a type 2 Wald chi square test was applied to test overall significance of explanatory variables. We used Tukey post hoc tests to analyze the differences between levels of a given factor. Statistical significance was defined with by α = 0.05. Whenever a GLM(M) was overdispersed (dispersion value ≥ 2), we included an observation-level factor as random factor to correct for overdispersion. All the analyses were performed in R 4.03 (R core developing team, 2020), except the analyses of leaf area and damage that were performed in SPSS 18.0 (SPSS: An IBM Company, Chicago, USA). The details for each statistical test can be found in the results section and in the figure legends.
Acknowledgments
We are very thankful to J. Shepherd, R. Holloway, J. Müller, H. Auge, S. Brunzel, N. Schidlo, L. de Witte, A. Eycott, J. van Alphen, J. Bale, K. Wolff, W. Spoor, M. Crawley, S. Derridj, R. Abott, S. Luijten, J. Ireson, T. Morley, M. Jonsson, C. Griffin, L. Bertola, S. Decombel, L. Doorduin, J. Blythel, P. Pelser, U. Schaffner, V. Vanparys, N. Agerbirk, S. Luijten, D. Ober, A. Balogh, M. Bartelheimer, L. Joosten, N. Sletvold-Hommelvik, P. Olejniczak, and S. Andersson for collecting seeds. We thank Eddy van der Meijden for commenting on the manuscript and N. Raes and Y. Chen for help with processing the climatic data and principal-component analysis (PCA). We thank K. van der Veen-van Wijk and H. Nell for their technical assistance in plant growth and caterpillar collecting. We thank Florian Schiestl for providing some of the pure compounds used for volatile identification. The study was jointly sponsored by the National Natural Science Foundation of China (grant no. 31520103912) and the earmarked fund for China Agricultural Research System (CARS-01-40; to Y.L.). T.L. thanks the National Natural Science Foundation of China (grant no. 32001204) for financial support. D.L., T.C.J.T., T.D., C.B.-S., and G.A.D. were supported by the Swiss National Science Foundation (grant no. 31003A_166632 and no. 315230_185319) and by European Research Council advanced grant 788949.
Author contributions
T.L., P.G.L.K., K.V., Y.L., and T.C.J.T. conceived the ideas and designed methodology; T.L., D.L., L.B., and G.A.D. collected the data; T.L., K.V., C.B.-S., T.D., and G.A.D. analyzed the data; and T.L. and G.A.D. initiated the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Declaration of interests
The authors declare no competing interests.
Published: June 18, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2021.05.055.
Contributor Information
Klaas Vrieling, Email: k.vrieling@biology.leidenuniv.nl.
Yonggen Lou, Email: yglou@zju.edu.cn.
Ted C.J. Turlings, Email: ted.turlings@unine.ch.
Supplemental information
References
- 1.Unsicker S.B., Kunert G., Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009;12:479–485. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Dicke M., Baldwin I.T. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Heil M. Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol. 2014;204:297–306. [Google Scholar]
- 4.Mescher M.C., Pearse I.S. Communicative interactions involving plants: information, evolution, and ecology. Curr. Opin. Plant Biol. 2016;32:69–76. doi: 10.1016/j.pbi.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Turlings T.C.J., Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018;63:433–452. doi: 10.1146/annurev-ento-020117-043507. [DOI] [PubMed] [Google Scholar]
- 6.Hare J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2011;56:161–180. doi: 10.1146/annurev-ento-120709-144753. [DOI] [PubMed] [Google Scholar]
- 7.Kessler A., Heil M. The multiple faces of indirect defences and their agents of natural selection. Funct. Ecol. 2011;25:348–357. [Google Scholar]
- 8.Kaplan I. Trophic complexity and the adaptive value of damage-induced plant volatiles. PLoS Biol. 2012;10:e1001437. doi: 10.1371/journal.pbio.1001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicke M., Sabelis M.W. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 1987;38:148–165. [Google Scholar]
- 10.Turlings T.C.J., Tumlinson J.H., Lewis W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 11.De Moraes C.M., Lewis W.J., Paré P.W., Alborn H.T., Tumlinson J.H. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 12.Kessler A., Baldwin I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 13.Schuman M.C., Barthel K., Baldwin I.T. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife. 2012;1:e00007. doi: 10.7554/eLife.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange E.S., Farnier K., Degen T., Gaudillat B., Aguilar-Romero R., Bahena-Juárez F., Oyama K., Turlings T.C.J. Parasitic wasps can reduce mortality of teosinte plants infested with fall armyworm: support for a defensive function of herbivore-induced plant volatiles. Front. Ecol. Evol. 2018;6:55. [Google Scholar]
- 15.Kergunteuil A., Röder G., Rasmann S. Environmental gradients and the evolution of tri-trophic interactions. Ecol. Lett. 2019;22:292–301. doi: 10.1111/ele.13190. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell C.E., Agrawal A.A., Bever J.D., Gilbert G.S., Hufbauer R.A., Klironomos J.N., Maron J.L., Morris W.F., Parker I.M., Power A.G. Biotic interactions and plant invasions. Ecol. Lett. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 17.Frick K.E. Third list of insects that feed upon tansy ragwort, Senecio jacobaea, in the western United States. Ann. Entomol. Soc. Am. 1972;65:629–631. [Google Scholar]
- 18.Castells E., Morante M., Blanco-Moreno J.M., Sans F.X., Vilatersana R., Blasco-Moreno A. Reduced seed predation after invasion supports enemy release in a broad biogeographical survey. Oecologia. 2013;173:1397–1409. doi: 10.1007/s00442-013-2718-4. [DOI] [PubMed] [Google Scholar]
- 19.Blossey B., Nötzold R. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecol. 1995;83:887–889. [Google Scholar]
- 20.Doorduin L.J., van den Hof K., Vrieling K., Joshi J. The lack of genetic bottleneck in invasive Tansy ragwort populations suggests multiple source populations. Basic Appl. Ecol. 2010;11:244–250. [Google Scholar]
- 21.Coombs E.M., Clark J.K., Piper G.L., Cofrancesco A.F. First Edition. Oregon State University; 2004. Biological Control of Invasive Plants in the United States. [Google Scholar]
- 22.van der Meijden E. Can hosts escape from their parasitoids? Neth. J. Zool. 1979;30:382–391. [Google Scholar]
- 23.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. [Google Scholar]
- 24.Brilli F., Ciccioli P., Frattoni M., Prestininzi M., Spanedda A.F., Loreto F. Constitutive and herbivore-induced monoterpenes emitted by Populus x euroamericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ. 2009;32:542–552. doi: 10.1111/j.1365-3040.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- 25.Lehrman A., Boddum T., Stenberg J.A., Orians C.M., Björkman C. Constitutive and herbivore-induced systemic volatiles differentially attract an omnivorous biocontrol agent to contrasting Salix clones. AoB Plants. 2013;5:plt005. doi: 10.1093/aobpla/plt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi J., Vrieling K. The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005;8:704–714. [Google Scholar]
- 27.McEvoy P., Cox C., Coombs E. Successful biological control of ragwort, Senecio Jacobaea, by introduced insects in Oregon. Ecol. Appl. 1991;1:430–442. doi: 10.2307/1941900. [DOI] [PubMed] [Google Scholar]
- 28.van der Meijden E., van der Veen-van Wijk C.A.M., van Ginneken V.J.T. Cotesia (Apanteles) popularis L. parasitoids do not always kill their host. Entomol. Mon. Mag. 2000;136:117–120. [Google Scholar]
- 29.van der Meijden E. Changes in the distribution pattern of Tyria jacobaeae during the larval period. Neth. J. Zool. 1975;26:136–161. [Google Scholar]
- 30.van der Meijden E., Nisbet R.M., Crawley M.J. The dynamics of a herbivore-plant interaction, the cinnabar moth and ragwort. In: Dempster J.P., McLean I.F.G., editors. Insect Populations - in Theory and in Practice. Kluwer Academic Publishers; 1998. pp. 291–308. [Google Scholar]
- 31.Gols R., Bukovinszky T., van Dam N.M., Dicke M., Bullock J.M., Harvey J.A. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 2008;34:132–143. doi: 10.1007/s10886-008-9429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harper J.L., Wood W. Senecio Jacobaea L. J. Ecol. 1957;45:617–637. [Google Scholar]
- 33.Poole A.L., Cairns D. Botanical aspects of ragwort (Senecio jacobaea L.) control. Bull. NZ Depart. Sci. Ind. Res. 1940;82:1–66. [Google Scholar]
- 34.Dempster J.P. The population ecology of the cinnabar moth, Tyria jacobaeae L.(Lepidoptera, Arctiidae) Oecologia. 1971;7:26–67. doi: 10.1007/BF00346293. [DOI] [PubMed] [Google Scholar]
- 35.Crawley M.J., Gillman M.P. Population dynamics of cinnabar moth and ragwort in grassland. J. Anim. Ecol. 1989;58:1035–1050. [Google Scholar]
- 36.Syrett P. Biological control of ragwort in New Zealand: a review. Australian Weeds. 1983;2:96–101. [Google Scholar]
- 37.Bain J.F. The biology of Canadian weeds. 96. Senecio jacobaea L. Can. J. Plant Sci. 1991;71:127–140. [Google Scholar]
- 38.McLaren D.A., Ireson J.A., Kwong R.M. Biological control of ragwort in Australia. In: Spencer N.R., editor. Proceedings of the Xth International Symposium on Biological Control of Weeds. Montana State University; 2000. pp. 67–79. [Google Scholar]
- 39.Rapo C., Müller-Schärer H., Vrieling K., Schaffner U. Is there rapid evolutionary response in introduced populations of tansy ragwort, Jacobaea vulgaris, when exposed to biological control? Evol. Ecol. 2010;24:1081–1099. [Google Scholar]
- 40.Colautti R.I., Ricciardi A., Grigorovich I.A., MacIsaac H.J. Is invasion success explained by the enemy release hypothesis? Ecol. Lett. 2004;7:721–733. [Google Scholar]
- 41.Liu H., Stiling P. Testing the enemy release hypothesis: a review and meta-analysis. Biol. Invasions. 2006;8:1535–1545. [Google Scholar]
- 42.Bradley B.A., Oppenheimer M., Wilcove D.S. Climate change and plant invasions: restoration opportunities ahead? Glob. Change Biol. 2009;15:1511–1521. [Google Scholar]
- 43.Lin T., Klinkhamer P.G.L., Vrieling K. Parallel evolution in an invasive plant: effect of herbivores on competitive ability and regrowth of Jacobaea vulgaris. Ecol. Lett. 2015;18:668–676. doi: 10.1111/ele.12445. [DOI] [PubMed] [Google Scholar]
- 44.Lin T., Klinkhamer P.G.L., Pons T.L., Mulder P.P.J., Vrieling K. Evolution of increased photosynthetic capacity and its underlying traits in invasive Jacobaea vulgaris. Front. Plant Sci. 2019;10:1016. doi: 10.3389/fpls.2019.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chougule N.P., Doyle E., Fitches E., Gatehouse J.A. Biochemical characterization of midgut digestive proteases from Mamestra brassicae (cabbage moth; Lepidoptera: Noctuidae) and effect of soybean Kunitz inhibitor (SKTI) in feeding assays. J. Insect Physiol. 2008;54:563–572. doi: 10.1016/j.jinsphys.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Esch S., Klinkhamer P.G., van der Meijden E. Do distances among host patches and host density affect the distribution of a specialist parasitoid? Oecologia. 2005;146:218–226. doi: 10.1007/s00442-005-0214-1. [DOI] [PubMed] [Google Scholar]
- 47.Turlings T.C.J., Davison A.C., Tamò C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2004;29:45–55. [Google Scholar]
- 48.D’Alessandro M., Turlings T.C.J. In situ modification of herbivore-induced plant odors: a novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses. 2005;30:739–753. doi: 10.1093/chemse/bji066. [DOI] [PubMed] [Google Scholar]
- 49.Ton J., D’Alessandro M., Jourdie V., Jakab G., Karlen D., Held M., Mauch-Mani B., Turlings T.C. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 50.Kreuzwieser J., Scheerer U., Kruse J., Burzlaff T., Honsel A., Alfarraj S., Georgiev P., Schnitzler J.-P., Ghirardo A., Kreuzer I. The Venus flytrap attracts insects by the release of volatile organic compounds. J. Exp. Bot. 2014;65:755–766. doi: 10.1093/jxb/ert455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Meijden E., van der Veen-van Wijk C.A. Tritrophic metapopulation dynamics: a case study of ragwort, the cinnabar moth, and the parasitoid Cotesia popularis. In: Hanski I., Gilpin M., editors. Metapopulation Biology. Elsevier; 1997. pp. 387–405. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and code generated during this study are available at Dryad https://doi.org/10.5061/dryad.fxpnvx0rk