Abstract
Toddalia asiatica (L.) Lam., belonging to Toddalia genus of Rutaceae family, is a folk medicine in China used for hundreds of years. The whole plant can be used as medicine, especially the root that used to be applied in the folk. In recent decades, with the in-depth research from domestic and foreign researchers, it has gradually been discovered that the chemical components in T. asiatica are mainly coumarins and alkaloids. Its pharmacological effects are manifested in anti-inflammatory and analgesic, hemostatic coagulation, anti-tumor, treatment of cardiovascular diseases, etc. It has a wide range of clinical applications and significant effects on rheumatism, pain, wound bleeding, and bruises. Due to its important research value, in this article, the chemical compositions and pharmacological effects of T. asiatica are comprehensively expounded in recent years in order to provide a reference for the related research and application of this medicinal material, which were carried out through a bibliometric search using the Science Citation Index- Expanded (SCIE) database, web of science, Google scholar and Chinese National Knowledge Infrastructure (CNKI) and all that.
Keywords: Toddalia asiatica, Folk medicine, Chemical composition, Pharmacological effect
1. Introduction
Toddalia asiatica Lam., a plant of the genus Toddalia of the family Rutaceae, earliestly recorded in “Plant name and real picture ”, is a woody vine, and also the only plant in the genus (Xia and Liu, 2007). There are many aliases for T. asiatica, such as Jian xue fei, San bai bang, Fei long zhuang xue, Da jiu jia and so forth (Shi et al., 2011). The plant generally grows in secondary forests of roadsides, mountain forests, thickets and small trees, mainly distributed in tropical Africa and southeastern Asia, especially in the south of Wuling Mountainous areas in China (Tsai et al., 1998, Watanabe et al., 2014, Zhang et al., 2017). The old stem is brown with longitudinally lobed and protruding yellow-gray lenticels owning a thick cork layer, however, the young shoots have small round lenticels. In addition, there are many downward curved sharp thorns on the stem branches and leaf axes. The leaflets have no near stalk, while dense transparent oil spots can be seen in the light. After kneading, the aroma is similar to that of citrus leaves. The male inflorescences are panicles and umbellate but the female inflorescences are thyrses, which can blossom all the year round (Xia and Liu, 2007). Its fruit is scarlet or orange-red, 8–10 mm in diameter or slightly larger, with multiple longitudinal shallow grooves which becomes more pronounced after being dehydrated. Seeds are about 5–6 mm long on the brownish black seed coat with tiny pits (Xia and Liu, 2007).
T. asiatica has played a very important role in traditional medicine, and the whole plant can be used as medicine, especially its root often used in the folk (Yang et al., 2013, Li et al., 2018). The studies of chemical composition showed that the main chemical components were alkaloids and coumarins in T. asiatica (Wang et al., 2009, Hu et al., 2014, Sukieum et al., 2018, Lin and Chen, 2020). Otherwise, there were also some triterpenes (Huang et al., 2005), flavonoids (Shi et al., 2014), phenolic acids (Phatchana and Yenjai, 2014), lignans (Tsai et al., 1998) and what not. Modern pharmacological studies have suggested that T. asiatica shows anti-inflammatory and analgesic (Hao et al., 2004, Yang et al., 2013, Lu et al., 2015), antioxidant (Tian et al., 2011, Stephen Irudayaraj et al., 2012, Chen and Long, 2013, Tian et al., 2013), antibacterial (Ding et al., 2007, Hu et al., 2014), cardiovascular protection (Ren et al., 1993, He and Ren, 1998, Ren and He, 1998, He and Ren, 1999), anti-tumor (Iwasaki et al., 2006, Iwasaki et al., 2010, Li et al., 2018) and other pharmacological effects. According to our literature researches, this paper would review the studies on the chemical constituents and pharmacological effects of T. asiatica systematically in the following sections.
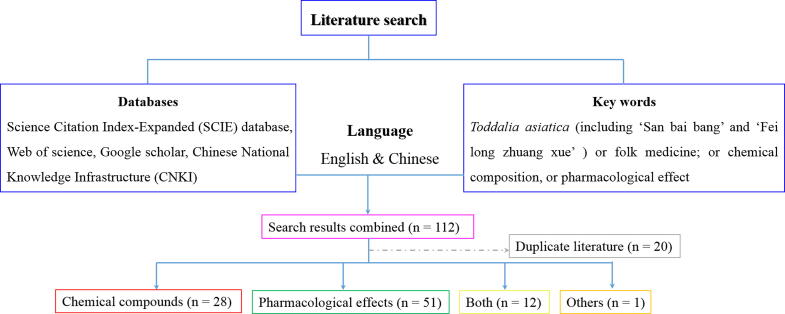
In this study, we employed the database such as Science Citation Index- Expanded (SCIE) database, web of science, Google scholar and Chinese National Knowledge Infrastructure (CNKI) et al., searching for researches that have been reported by scholars both at home and abroad during 1976–2020, in which ‘T. asiatica’ including ‘San bai bang’ and ‘Fei long zhuang xue’ was used as the main key words. Otherwise, folk medicine, chemical composition and pharmacological effect were also used for assisting us in looking for more valuable information in all directions, the aim of which is to make the review more detailed and systematic to help researchers for their studies. The detail research procedure was shown in Fig. 1.
Fig. 1.
Flow diagram of study selection.
2. Chemical composition
So far, a total of more than 165 compounds have been reported from T. asiatica, including 69 coumarins, 69 alkaloids, 8 terpenoids, 5 flavonoids, and 14 other compositions such as lipids, alcohols, phenolic acids, lignans, steroids and fatty acids. Among them, the most characteristic compounds for T. asiatica are coumarins and alkaloids.
2.1. Coumarins and its glycosides
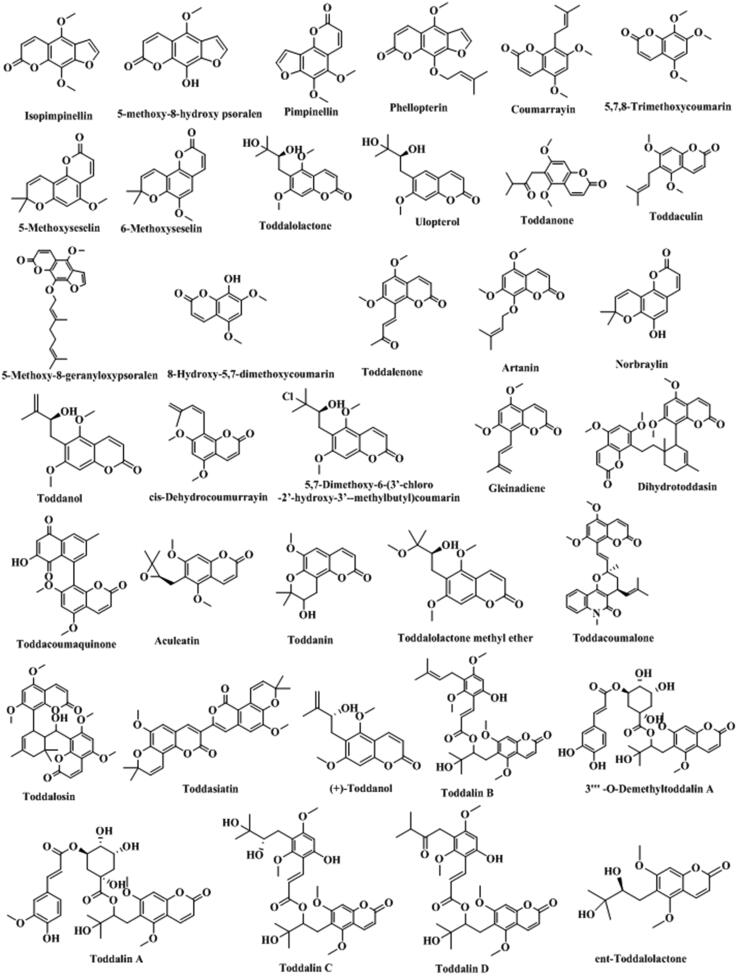
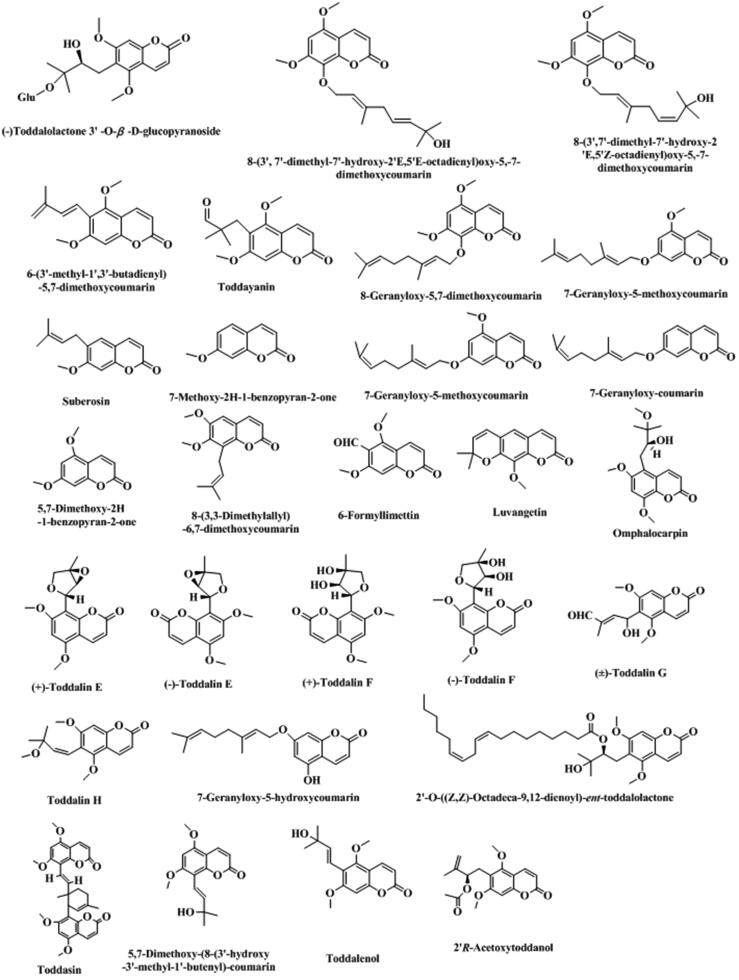
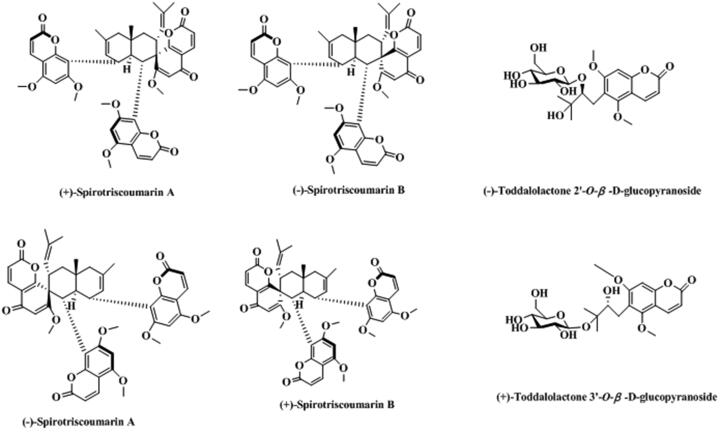
T. asiatica is rich in coumarins, including some simple coumarins, furanocoumarins, pyrancoumarins, and dicoumarin, summarized in Table 1. The structure of each corresponding chemical component were shown in Fig. 2. Coumarins are one of the important active ingredients in T. asiatica.
Table 1.
Coumarins reported from T. asiatica.
| Num. | Compound name | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | Toddasin | C32C32O5 | 544.210 | (Sharma et al., 1980) |
| 2 | Dihydrotoddasin | C32H32O8 | 544.601 | (Sharma et al., 1980) |
| 3 | 5,7-Dimethoxy-6-(3′-chloro-2′-hydroxy-3′-methylbutyl) coumarin | C16H19ClO5 | 326.777 | (Sharma et al., 1981a, Sharma et al., 1981b) |
| 4 | Toddaculin | C16H18O4 | 274.317 | (Ishii et al., 1983) |
| 5 | Toddalenone | C15H14O5 | 274.273 | (Ishii et al., 1983) |
| 6 | 8-(3,3-Dimethylallyl)-6,7-dimethoxycoumarin | C16H20O4 | 276.328 | (Ishii et al., 1983) |
| 7 | 6-Formyllimettin | C12H10O5 | 234.205 | (Ishii et al., 1983) |
| 8 | Toddacoumalone | C31H31NO6 | 513.590 | (Ishii et al., 1991a, Ishii et al., 1991b) |
| 9 | Toddanone | C16H18O5 | 290.316 | (Chen et al., 1993) |
| 10 | Toddanol | C16H18O5 | 290.316 | (Chen et al., 1993) |
| 11 | Toddalolactone methyl ether | C17H22O6 | 322.358 | (Chen et al., 1993) |
| 12 | Toddasiatin | C30H26O8 | 514.532 | (Tsai et al., 1997) |
| 13 | (+)-Toddanol | C16H18O5 | 290.316 | (Tsai et al., 1998) |
| 14 | 5,7,8-Trimethoxycoumarin | C12H12O5 | 236.224 | (Tsai et al., 1998) |
| 15 | 6-Methoxyseselin | C15H14O4 | 258.274 | (Tsai et al., 1998) |
| 16 | Toddalolactone | C16H20O6 | 308.331 | (Tsai et al., 1998) |
| 17 | Ulopterol | C15H18O5 | 278.305 | (Tsai et al., 1998) |
| 18 | Toddanin | C15H16O5 | 276.289 | (Tsai et al., 1998) |
| 19 | Phellopterin | C17H16O5 | 300.311 | (Tsai et al., 1998, Zhang et al., 2017) |
| 20 | 5,7-Dimethoxy-(8-(3′-hydroxy-3′-methyl-1′-butenyl)-coumarin | C16H18O5 | 290.30 | (Oketch-Rabah et al., 2000) |
| 21 | 8-(Geranyloxy)-5,7-dimethyloxycoumarin | C21H26O5 | 358.434 | (Wang et al., 2009) |
| 22 | 7-Geranyloxy-5-methoxycoumarin | C20H26O | 330.418 | (Wang et al., 2009) |
| 23 | Norbraylin | C14H12O4 | 244.247 | (Shi et al., 2013) |
| 24 | Suberosin | C15H16O3 | 244.286 | (Nyahanga et al., 2013) |
| 25 | Toddalenol | C16H18O5 | 290.31 | (Nyahanga et al., 2013) |
| 26 | Isopimpinellin | C13H10O5 | 246.219 | (Liu et al., 2014) |
| 27 | Coumarrayin | C16H18O4 | 274.317 | (Lin et al., 2014) |
| 28 | 5-Methoxyseselin | C15H14O4 | 258.274 | (Lin et al., 2014) |
| 29 | cis-Dehydrocoumurrayin | C16H16O4 | 272.301 | (Lin et al., 2014) |
| 30 | Gleinadiene | C16H16O4 | 272.301 | (Lin et al., 2014) |
| 31 | Toddacoumaquinone | C23H18O7 | 406.392 | (Lin et al., 2014) |
| 32 | Toddalin B | C32H38O10 | 582.648 | (Lin et al., 2014) |
| 33 | 3′’’ -O-Demethyltoddalin A | C32H36O14 | 644.629 | (Lin et al., 2014) |
| 34 | Toddalin A | C33H38O14 | 658.656 | (Lin et al., 2014) |
| 35 | Toddalin C | C32H40O12 | 616.662 | (Lin et al., 2014) |
| 36 | Toddalin D | C32H38O11 | 598.647 | (Lin et al., 2014) |
| 37 | ent-Toddalolactone | C16H20O6 | 308.331 | (Lin et al., 2014) |
| 38 | (−)-Toddalolactone 3′-O-β-D-glucopyranoside | C22H30O11 | 470.474 | (Lin et al., 2014) |
| 39 | 5-Methoxy-8-hydroxy psoralen | C12H8O5 | 232.193 | (Phatchana and Yenjai, 2014) |
| 40 | 5-Methoxy-8-geranyloxypsoralen | C22H24O5 | 368.430 | (Phatchana and Yenjai, 2014) |
| 41 | 8-Hydroxy-5,7-dimethoxycoumari | C11H10O5 | 222.197 | (Phatchana and Yenjai, 2014) |
| 42 | Artanin | C16H18O5 | 290.316 | (Phatchana and Yenjai, 2014) |
| 43 | Toddalosin | C32H34O9 | 562.617 | (Phatchana and Yenjai, 2014) |
| 44 | 8-(3′,7′-dimethyl-7′-hydroxy-2′E,5′E-octadienyl)oxy-5,7-dimethoxycoumarin | C21H26O6 | 374.434 | (Phatchana and Yenjai, 2014) |
| 45 | 8-(3′,7′-dimethyl-7′-hydroxy-2′E,5′Z-octadienyl)oxy-5,7-dimethoxycoumarin | C21H26O6 | 374.434 | (Phatchana and Yenjai, 2014) |
| 46 | 6-(3-Methyl-1,3-butadienyl)-5,7-dimethoxycoumarin | C16H16O4 | 272.301 | (Phatchana and Yenjai, 2014) |
| 47 | Omphalocarpin | C17H22O6 | 332.350 | (Tong et al., 2014) |
| 48 | Aculeatin | C16H18O5 | 290.316 | (Watanabe et al., 2014) |
| 49 | Toddayanin | C16H18O5 | 290.316 | (Hirunwong et al., 2016) |
| 50 | 7-Methoxy-2H-1-benzopyran-2-one | C10H10O3 | 170.185 | (Hirunwong et al., 2016) |
| 51 | 5,7-Dimethoxy-2H-1-benzopyran-2-one | C13H16O4 | 236.264 | (Hirunwong et al., 2016) |
| 52 | 7-Geranyloxy-coumarin | C10H10O3 | 170.185 | (Hirunwong et al., 2016) |
| 53 | Luvangetin | C15H14O4 | 258.269 | (Tang et al., 2016) |
| 54 | (+)-Spirotriscoumarin A | C47H46O12 | 802.306 | (Tang et al., 2016) |
| 55 | (−)-Spirotriscoumarin A | C47H46O12 | 802.306 | (Tang et al., 2016) |
| 56 | (−)-Spirotriscoumarin B | C47H46O12 | 802.307 | (Tang et al., 2016) |
| 57 | (+)-Spirotriscoumarin B | C47H46O12 | 802.307 | (Tang et al., 2016) |
| 58 | (+)-Toddalin E | C16H16O6 | 304.086 | (Li et al., 2017) |
| 59 | (−)-Toddalin E | C16H16O6 | 304.086 | (Li et al., 2017) |
| 60 | (+)-Toddalin F | C16H18O7 | 311.11 | (Li et al., 2017) |
| 61 | (−)-Toddalin F | C16H18O7 | 311.11 | (Li et al., 2017) |
| 62 | (±)-Toddalin G | C16H16O6 | 304.085 | (Li et al., 2017) |
| 63 | Toddalin H | C17H20O5 | 304.13 | (Li et al., 2017) |
| 64 | 7-Geranyloxy-5-hydroxycoumarin | C19H22O4 | 314.144 | (Li et al., 2017) |
| 65 | 2′O-((Z,Z)-Octadeca-9,12-dienoyl)-ent-toddalolactone | C34H50O7 | 570.36 | (Li et al., 2017) |
| 66 | Pimpinellin | C13H10O5 | 246.219 | (Zhang et al., 2017) |
| 67 | 2′R-acetoxytoddanol | C18H20O6 | 332.14 | (Sukieum et al., 2018) |
| 68 | (−)-Toddalolactone 2′-O-β-D-glucopyranoside | C22H30O11 | 470.47 | (Li et al., 2020) |
| 69 | (+)-Toddalolactone 3′-O-β-D-glucopyranoside | C22H30O11 | 470.47 | (Li et al., 2020) |
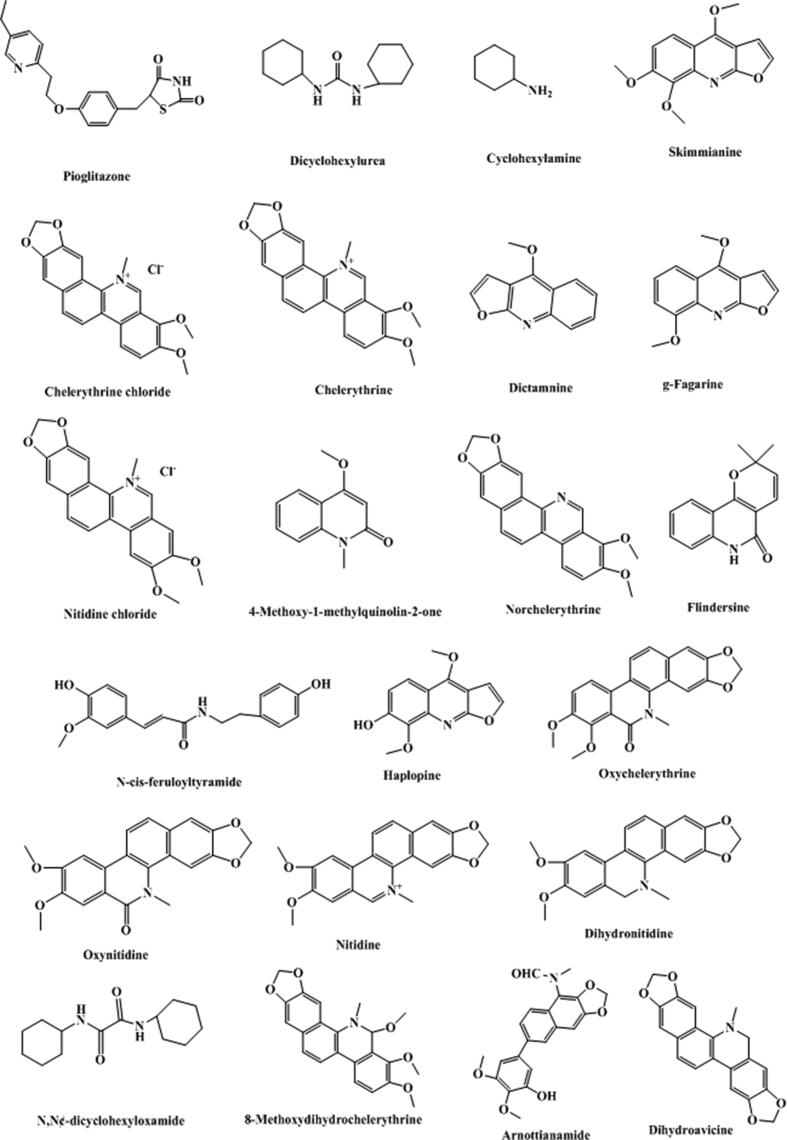
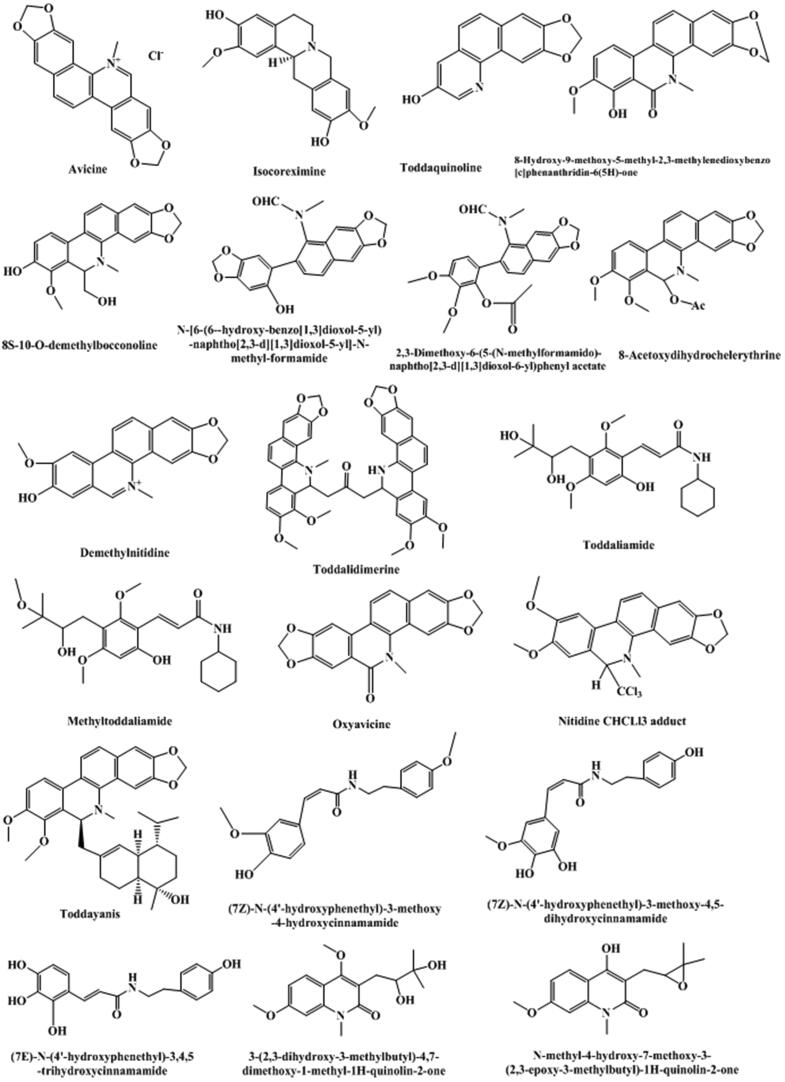
Fig. 2.
Structure and corresponding chemical name of coumarins and its derivants from Toddalia asiatica.
Japanese scholar Ishii et al., (Ishii et al., 1991a, Ishii et al., 1991b) isolated 12 known simple coumarins from methanol extracts of T. asiatica root collected from Taiwan, China, which are toddaculin, coumurrayin, toddalenol, 6-(2-hydroxy-3-methoxy-3-methylbutyl)-5, 7-dimethoxycoumarin, toddalolactone, 5,7,8-trimethoxycoumarin, 5-methoxysuberenon, 8-(3,3-Dimethylallyl)-6, 7-dimethoxycoumarin, 8-formyllimettin), 6-(3-chloro-2-hydroxy-3-methylbutyl)7-dimethoxycoumarin, 6-formyllimettin, toddalenone. Oketch-Rabah et al., (Oketch-Rabah et al., 2000) isolated a new coumarin compound (5,7-dimethoxy-(8-(3′-hydroxy-3′-methyl-1′-butenyl)-coumarin) from the ethyl acetate extract of T. asiatica root using a separation method guided by biological activity. Wang et al., (Wang et al., 2009) isolated two simple coumarin compounds from the 95% ethanol extract of T. asiatica grown in Yunnan province, China, which are 8-geranyloxy-5,7-dimethoxycoumarin and 7-geranyloxy-5-methoxycoumarin. Qiu et al., (Qiu et al., 2012) using microwave-assisted extraction isolated three furanocoumarins that were pimpinellin, isopimpinellin, and phellopterin, from T. asiatica root purchased from the Chinese herbal wholesale market in Hebei province, China. Phatchana and Chavi (Phatchana and Yenjai, 2014) identified three new coumarins that were 8-(3′,7′-dimethyl-7′-hydroxy-2′E,5′E-octadienyl)oxy-5,7-dimethoxycoumarin, 8-(3′,7′-dimethyl-7′-hydroxy-2′E,5′Z-octadienyl)oxy-5,7-dimethoxycoumarin, and 6-(3-Methyl-1,3-butadienyl)-5,7-dimethoxycoumarin, and 13 known compounds. Tsai et al., (Tsai et al., 1998) isolated 30 compounds from the wood of Formosan T. asiatica, including a new coumarin identified as toddanin. By bioassay-guided fractionation of the ethanol extract of T. asiatica roots, Lin et al., (Lin et al., 2014) isolated 21 coumarins, including seven new prenylated coumarins, that were toddalin A, 3‴-O-demethyltoddalin A, toddalin B, toddalin C, toddalin D, ent-toddalolactone, and (−)-toddalolactone 3′-O-β-D-glucopyranoside. Ishii et al., (Ishii et al., 1983) isolated a new coumarin toddalenone, and ten known coumarins that were toddalolactone, isopimpinellin, coumarrayin, toddanone, toddaculin, toddanol, 5,7,8-trimethoxycoumarin, toddasin, 5,7-Dimethoxy-6-(3′-chloro-2′-hydroxy-3′-methylbutyl) coumarin, and 8-(3,3-Dimethylallyl)-6,7-dimethoxycoumarin. Nyahanga et al., (Nyahanga et al., 2013) obtained 8 compounds including 6 coumarins characterized as aculeatin, toddaculin, isopimpinellin, suberosin, toddalenol, and toddalolactone from chromatographic fractionation of n-hexane, ethyl acetate and methanol extracts of T. asiatica root bark. Moreover, two coumarin glycosides, (−)-toddalolactone 2′-O-β-D-glucopyranoside and (+) –toddalolactone-3′-O-β-D-glucopyranoside, were firstly separated using two-dimensional HPLC (Li et al., 2020). There were also some other coumarins reported in the literature, quoted in Table 1, and would not be described in detail.
2.2. Alkaloids
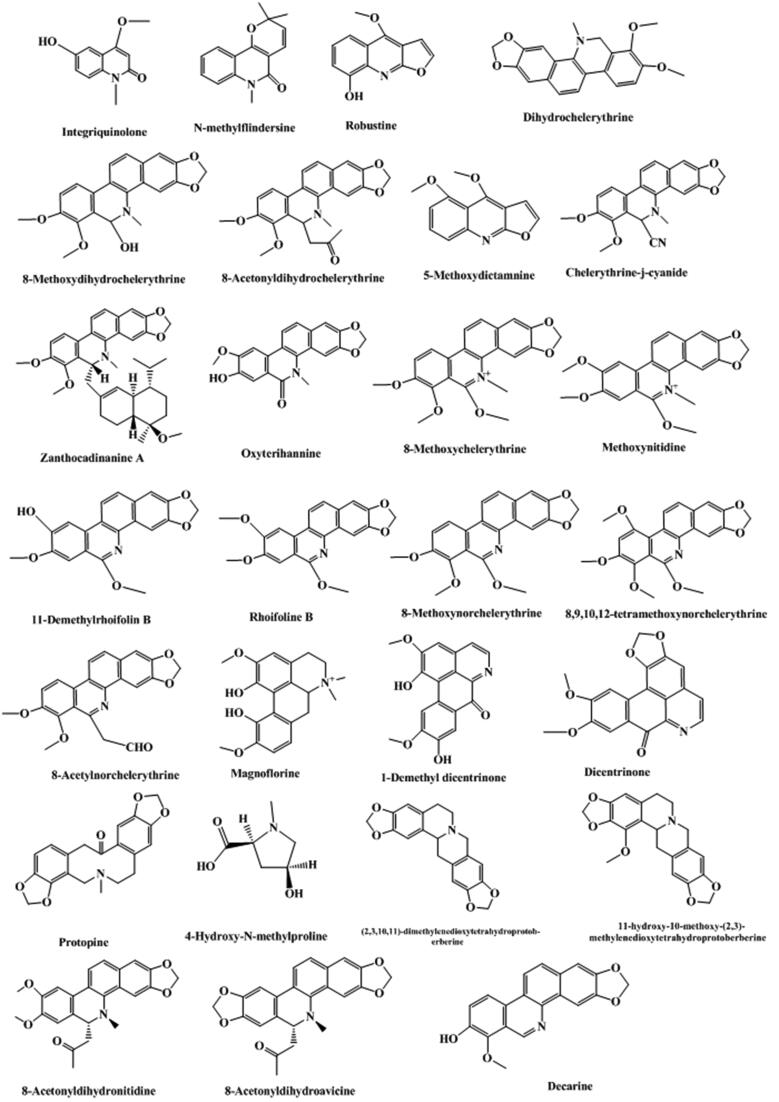
Alkaloids, second only to coumarins, are another mainly kind components in T. asiatica, such as quinoline alkaloids, phenanthroline alkaloids, and other alkaloids (summarized in Table 2 and Fig. 3). In recent years, with the continuous researches reported at home and abroad, scholars have found that alkaloids in T. asiatica played an important role in anti-inflammatory, analgesic, and anti-tumor effects (Hao et al., 2004, Hu et al., 2014). A rapid method using ultrafiltration liquid chromatography combined with stepwise flow rate counter-current chromatography was developed to screen and purify alkaloid that could be used as neuraminidase inhibitors (Cao et al., 2019). Quinoline alkaloids in the plant are primarily N-methylflindersine, toddacoumalone, skimmianine, integriquinolone, dictamnine, hannine, and oxyterihannine, etc. (Tsai et al., 1997, Tsai et al., 1998). Benzophenanthridine alkaloids in the plant are principally oxychelerythrine, des-N-methylchelerythrine, oxyavicine, oxyavicine, avicine, chelerythrine, chelerythrine-φ-cyanide, dihydroavicine, dihydrochelerythrine, 8-acetonyldihydrochelerythrin, 8-methoxydihydrochelerythrine, dihydronitidine, nitidine, oxynitidine, 8-hydroxydihydrochelerythrine, toddalidimerine and 6-methylnitidine etc. (Sharma et al., 1981a, Sharma et al., 1981b, Ishii et al., 1991a, Ishii et al., 1991b, Tsai et al., 1997, Tsai et al., 1998). The other ones are chiefly γ-fagarine, toddaquinoline, arnottianaide, methyltoddaliamide, isocoreximine, protopine, toddaliamide, N,N'-dicyclohexylurea, N, N'-dicyclohexyloxamide, methyltoddaliamide, toddaliamide, flindersine, etc. (Tsai et al., 1997, Tsai et al., 1998, Duraipandiyan and Ignacimuthu, 2009). In addition, three known alkaloids, 8-acetonyldihydronitidine, 8-acetonyldihydroavicine and decarine, were firstly discovered from the genus Toddalia and 8-acetonyldihydronitidine has showed strong inhibitory effect against phosphodiesterase-4 with an IC50 value of 5.14 µM (Lin and Chen, 2020).
Table 2.
Alkaloids reported from T. asiatica.
| Num. | Compound name | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | Robustine | C12H9NO3 | 215.06 | (Deshmukh et al., 1976) |
| 2 | 8-Acetonyldihydrochelerythrine | C24H23NO5 | 405.44 | (Sharma et al., 1981a, Sharma et al., 1981b) |
| 3 | Toddalidimerine | C44H38N2O9 | 738.79 | (Sharma et al., 1981a, Sharma et al., 1981b) |
| 4 | 8-Methoxydihydrochelerythrine | C22H21NO5 | 379.41 | (Sharma et al., 1982) |
| 5 | 2,3-Dimethoxy-6-(5-(N-methylformamido)naphtho[2,3-d][1,3]dioxol-6-yl)phenyl acetate | C23H21NO7 | 423.42 | (Sharma et al., 1982) |
| 6 | 8-Acetoxydihydrochelerythrine | C23H21NO6 | 407.42 | (Sharma et al., 1982) |
| 7 | Chelerythrine-φ-cyanide | C22H18N2O4 | 374.39 | (Ishii et al., 1983) |
| 8 | Chelerythrine | C21H18NO4 | 348.38 | (Ishii et al., 1983) |
| 9 | 4-Methoxy-1-methylquinolin-2-one | C11H11NO2 | 189.21 | (Ishii et al., 1983) |
| 10 | Oxychelerythrine | C21H17NO5 | 363.37 | (Ishii et al., 1983) |
| 11 | Integriquinolone | C11H13NO3 | 207.27 | (Ishii et al., 1983) |
| 12 | N-methylflindersine | C15H15NO2 | 241.11 | (Ishii et al., 1983) |
| 13 | Oxyavicine | C20H13NO5 | 347.32 | (Ishii et al., 1991a, Ishii et al., 1991b) |
| 14 | Avicine | C20H14NO4Cl | 367.79 | (Chen et al., 1993) |
| 15 | Toddaquinoline | C14H9NO3 | 239.23 | (Chen et al., 1993) |
| 16 | Dicyclohexylurea | C13H24N2O | 224.35 | (Tsai et al., 1997) |
| 17 | Norchelerythrine | C20H15NO | 333.34 | (Tsai et al., 1997) |
| 18 | N,N’ -dicyclohexyloxamide | C14H24N2O2 | 252.36 | (Tsai et al., 1997) |
| 19 | Toddaliamide | C22H33NO6 | 407.51 | (Tsai et al., 1997) |
| 20 | Methyltoddaliamide | C23H35NO6 | 421.53 | (Tsai et al., 1997) |
| 21 | Toddayanis | C36H43NO5 | 569.74 | (Tsai et al., 1997) |
| 22 | Cyclohexylamine | C6H13N | 99.18 | (Tsai et al., 1998) |
| 23 | Haplopine | C13H11NO4 | 245.24 | (Tsai et al., 1998) |
| 24 | Isocoreximine | C19H21NO4 | 327.38 | (Tsai et al., 1998) |
| 25 | 8-Hydroxy-9-methoxy-5-methyl-2,3-methylenedioxybenzo[c]phenanthridin-6(5H)-one | C20H15NO5 | 349.34 | (Tsai et al., 1998) |
| 26 | N-[6-(6-hydroxy-benzo[1,3]dioxol-5-y l)-naphtho[2,3-d][1,3]dioxol-5-yl]-N-methyl-formamide | C20H15NO6 | 365.34 | (Tsai et al., 1998) |
| 27 | Nitidine CHCLl3 adduct | C22H18Cl3NO4 | 466.75 | (Tsai et al., 1998) |
| 28 | Oxyterihannine | C20H15NO5 | 349.34 | (Tsai et al., 1998) |
| 29 | Chelerythrine chloride | C21H18ClNO4 | 383.83 | (Jain et al., 2006) |
| 30 | Nitidine chloride | C21H18NO4Cl | 383.83 | (Jain et al., 2006) |
| 31 | 3-(2,3-Dihydroxy-3-methylbutyl)-4,7-dimethoxy-1-methyl-1H-quinolin-2-one | C17H23NO5 | 321.37 | (Jain et al., 2006) |
| 32 | N-methyl-4-hydroxy-7-methoxy-3-(2,3-epoxy-3-methylbutyl)-1H-quinolin-2-one | C16H19NO4 | 289.33 | (Jain et al., 2006) |
| 33 | Flindersine | C14H13NO2 | 227.26 | (Duraipandiyan and Ignacimuthu, 2009) |
| 34 | Demethylnitidine | C20H16NO4 | 334.35 | (Iwasaki et al., 2010) |
| 35 | Dihydroavicine | C20H15NO4 | 333.34 | (Shi et al., 2011) |
| 36 | Skimmianine | C14H13NO4 | 259.26 | (Shi et al., 2013) |
| 37 | γ-Fagarine | C13H11NO3 | 229.24 | (Shi et al., 2013) |
| 38 | Dihydrochelerythrine | C21H19NO4 | 349.38 | (Shi et al., 2013) |
| 39 | Dihydronitidine | C21H19NO4 | 349.39 | (Nyahanga et al., 2013) |
| 40 | Zanthocadinanine A | C37H45NO5 | 583.76 | (Liu et al., 2014) |
| 41 | Protopine | C20H19NO5 | 353.37 | (Liu et al., 2014) |
| 42 | Oxynitidine | C21H17NO5 | 363.37 | (Hu et al., 2014) |
| 43 | Arnottianamide | C21H19NO6 | 381.39 | (Hu et al., 2014) |
| 44 | 5-Methoxydictamnine | C13H11NO3 | 229.23 | (Hu et al., 2014) |
| 45 | 8-Methoxychelerythrine | C22H20NO5 | 378.40 | (Hu et al., 2014) |
| 46 | Methoxynitidine | C22H20NO5 | 378.40 | (Hu et al., 2014) |
| 47 | 11-Demethylrhoifolin B | C20H15NO5 | 349.34 | (Hu et al., 2014) |
| 48 | Rhoifoline B | C21H17NO5 | 363.36 | (Hu et al., 2014) |
| 49 | 8-Methoxynorchelerythrine | C21H17NO5 | 363.36 | (Hu et al., 2014) |
| 50 | 8,9,10,12-tetramethoxynorchelerythrine | C22H19NO6 | 393.39 | (Hu et al., 2014) |
| 51 | 8-Acetylnorchelerythrine | C22H17NO5 | 375.37 | (Hu et al., 2014) |
| 52 | 1-Demethyl dicentrinone | C18H13NO5 | 323.30 | (Hu et al., 2014) |
| 53 | Dicentrinone | C18H11NO5 | 321.28 | (Hu et al., 2014) |
| 54 | (2,3,10,11)-dimethylenedioxytetrahydroprotob-erberine | C19H17NO4 | 323.34 | (Hu et al., 2014) |
| 55 | 11-hydroxy-10-methoxy-(2,3)-methylenedioxytetrahydroprotoberberine | C20H19NO5 | 353.13 | (Hu et al., 2014) |
| 56 | 4-Hydroxy-N-methylproline | C6H11NO3 | 145.16 | (Shi et al., 2014) |
| 57 | Dictamnine | C12H9NO2 | 199.21 | (Shi et al., 2014) |
| 58 | 8-hydroxy-dihydrochelerythrine | C21H19NO5 | 365.38 | (Shi et al., 2014) |
| 59 | Nitidine | C21H18NO4 | 348.38 | (Rashid et al., 2014) |
| 60 | Magnoflorine | C20H24NO4 | 342.41 | (Rashid et al., 2014) |
| 61 | Pioglitazone | C19H20N2O3S | 356.45 | (Watanabe et al., 2014) |
| 62 | N-cis-feruloyltyramide | C18H19NO4 | 313.35 | (Hu et al., 2015) |
| 63 | (7E)-N-(4′ -hydroxyphenethyl)-3,4,5-trihydroxycinnamamide | C17H17NO5 | 315.33 | (Hu et al., 2015) |
| 64 | (7Z)-N-(4′ -hydroxyphenethyl)-3-methoxy-4,5-dihydroxycinnamamide | C18H19NO5 | 329.35 | (Hu et al., 2015) |
| 65 | (7Z)-N-(4′ -methoxyphenethyl)-3-methoxy-4-hydroxycinnamamide | C19H21NO4 | 327.38 | (Hu et al., 2015) |
| 66 | 8S-10-O-demethylbocconoline | C21H19NO5 | 365.38 | (Sukieum et al., 2018) |
| 67 | 8-Acetonyldihydronitidine | C24H23NO5 | 405.45 | (Lin and Chen, 2020) |
| 68 | 8-Acetonyldihydroavicine | C23H19NO5 | 389.41 | (Lin and Chen, 2020) |
| 69 | Decarine | C19H13NO4 | 319.32 | (Lin and Chen, 2020) |
Fig. 3.
Structure and corresponding chemical name of alkaloids from T. asiatica.
2.3. Terpenoids
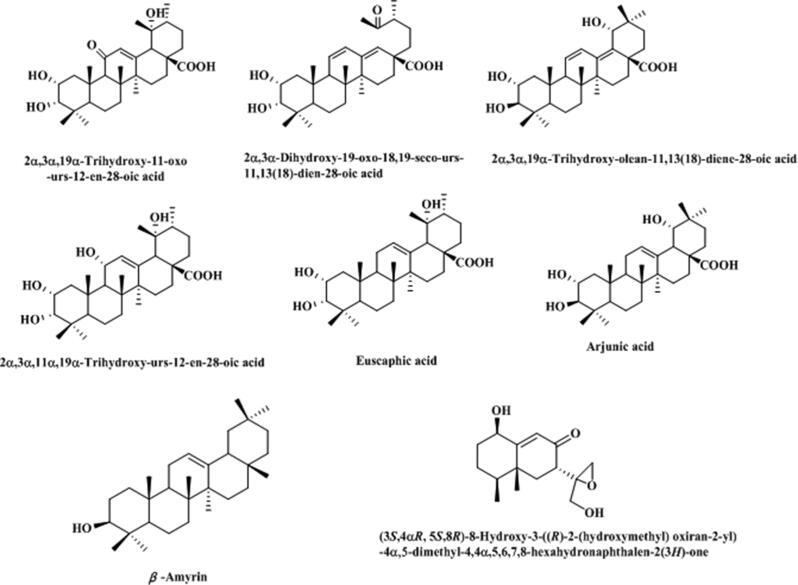
Except alkaloids and coumarins in T. asiatica, terpenoids, an important source of bioactive ingredients of natural medicines, are another important natural monomeric component in this medicinal material, summarized in Table 3 and Fig. 4. Huang et al (Huang et al., 2005) collected six triterpenoids that were 2α,3α,19α-trihydroxy-11-oxo-urs-12-en-28-oic acid, 2α,3α-dihydroxy-19-oxo-18,19-seco-urs-11,13(18)-diene-28-oic acid, 2α,3β,19α-trihydroxy-olean-11, 13(18)-dien-28-oic acid, 2α,3α,1lα,19α-tetrahydroxy-urs-12-en-28-oic acid, euscaphic acid, and arjunic acid from the ethanol extract of T. asiatica stem from Baiji Township, Nanning City, Guangxi Zhuang Autonomous Region, China. Moreover, another triterpene compound, β-amyrin (Ishii et al., 1983), was isolated from T. asiatica. Recently, a new sesquiterpene, (3S,4aR, 5S,8R)-8-Hydroxy-3-((R)-2-(hydroxymethyl) oxiran-2-yl) −4a,5-dimethyl-4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one, was firstly separated from T. asiatica (Lin and Chen, 2020).
Table 3.
Terpenoids reported from T. asiatica.
| Num. | Compound name | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | β-Amyrin | C30H50O | 426.72 | (Ishii et al., 1983) |
| 2 | 2α,3α,19α-Trihydroxy-11-oxo-urs-12-en-28-oic acid | C30H46O6 | 502.68 | (Huang et al., 2005) |
| 3 | 2α,3α-Dihydroxy-19-oxo-18,19-seco-urs-11, 13(18)-diene-28-oicacid | C30H46O5 | 486.68 | (Huang et al., 2005) |
| 4 | 2α, 3β,19α-Trihydroxy-olean-11, 13(18)-dien-28-oic acid | C30H46O5 | 486.68 | (Huang et al., 2005) |
| 5 | 2α,3α,1lα,19α-Tetrahydroxy-urs-12-en-28-oic acid | C30H48O6 | 504.70 | (Huang et al., 2005) |
| 6 | Euscaphic acid | C30H48O5 | 488.70 | (Huang et al., 2005) |
| 7 | Arjunic acid | C30H48O5 | 488.70 | (Huang et al., 2005) |
| 8 | (3S,4aR, 5S,8R)-8-Hydroxy-3-((R)-2-(hydroxymethyl) oxiran-2-yl) −4a,5-dimethyl-4,4a,5,6,7,8- hexahydronaphthalen-2(3H)-one | C15H22O4 | 266.34 | (Lin and Chen, 2020) |
Fig. 4.
Structure and corresponding chemical name of terpenoids from T. asiatica.
2.4. Flavonoids
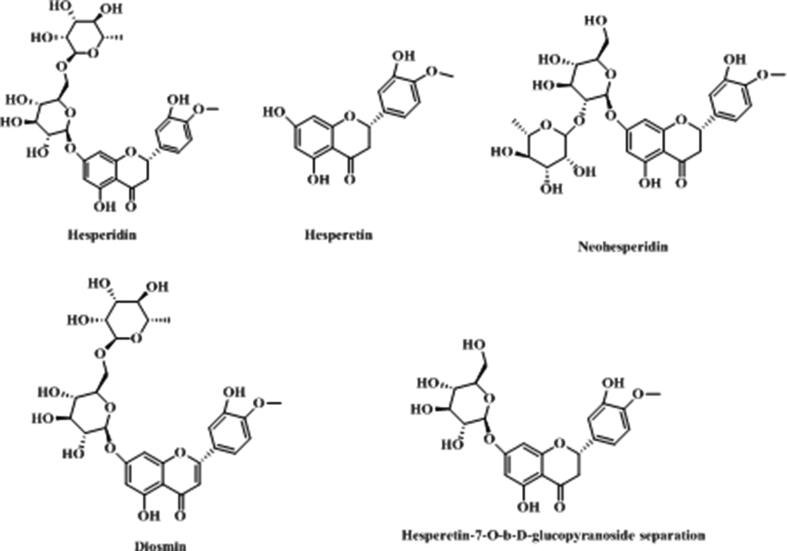
Flavonoids are an important member in natural products. It also existed in T. asiatica, including hesperidin, hesperetin, neohesperidin, diosmin and hesperetin-7-O-β-D-glucopyranoside, seen in Table 4 and Fig. 5. (Chen et al., 2013, Shi et al., 2014).
Table 4.
Flavonoids reported from T. asiatica.
| Num | Compound name | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | Hesperetin | C16H14O6 | 302.28 | (Chen et al., 2013) |
| 2 | Hesperidin | C28H34O15 | 610.57 | (Shi et al., 2014) |
| 3 | Neohesperidin | C28H34O15 | 610.57 | (Shi et al., 2014) |
| 4 | Diosmin | C28H32O15 | 608.54 | (Shi et al., 2014) |
| 5 | Hesperetin-7-O-β-D-glucopyranoside | C22H24O11 | 464.42 | (Shi et al., 2014) |
Fig. 5.
Structure and corresponding chemical name of flavonoids from T. asiatica.
2.5. Other compounds
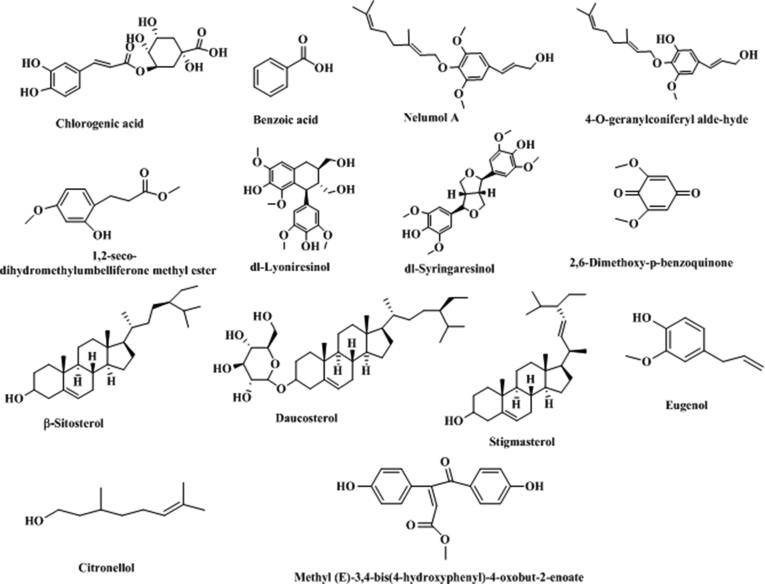
Except the above-mentioned major components, T. asiatica also contains phenolic compounds such as chlorogenic acid, benzoic acid, and nelumol A; Lignin compounds: dl-lyoniresinol, and dl-syringaresinol; Quinones: 2,6-dimethoxy-p-benzoquinone; Steroids: β-sitosterol, daucosterol and stigmasterol; Fatty acids: octadecanoic acid, oleic acid, linoleic acid, and hexacosanoic acid. These compounds were summarized in Table 5 and Fig. 6.
Table 5.
Other compounds reported in T. asiatica.
| Num. | Compound name | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | dl-Lyoniresinol | C22H28O8 | 420.45 | (Tsai et al., 1998) |
| 2 | dl-Syringaresinol | C22H26O8 | 418.44 | (Tsai et al., 1998) |
| 3 | 2,6-Dimethoxy-p-benzoquinone | C8H8O4 | 168.15 | (Tsai et al., 1998) |
| 4 | Benzoic acid | C7H6O2 | 122.12 | (Jain et al., 2006) |
| 5 | Stigmasterol | C29H48O | 412.70 | (Jain et al., 2006) |
| 6 | β-Sitosterol | C29H50O | 414.71 | (Shi et al., 2012) |
| 7 | Citronellol | C10H20O | 156.26 | (Chen et al., 2013) |
| 8 | Chlorogenic acid | C16H18O9 | 354.31 | (Liu et al., 2014) |
| 9 | Daucosterol | C35H60O6 | 576.90 | (Shi et al., 2014) |
| 10 | Eugenol | C10H12O2 | 164.20 | (Shi et al., 2014) |
| 11 | Nelumol A | C21H30O4 | 346.46 | (Phatchana and Yenjai, 2014) |
| 12 | 4-O-geranylconiferyl alde-hyde | C19H26O3 | 302.41 | (Phatchana and Yenjai, 2014) |
| 13 | 1,2-seco-Dihydromethylumbelliferonem methyl ester | C11H14O4 | 210.23 | (Phatchana and Yenjai, 2014) |
| 14 | Methyl(E)-3,4-bis(4-hydroxyphenyl)-4-oxobut-2-enoate | C17H14O5 | 298.08 | (Li et al., 2017) |
Fig. 6.
Structure and corresponding chemical name of other compounds from T. asiatica.
3. Pharmacological effects of T. asiatica
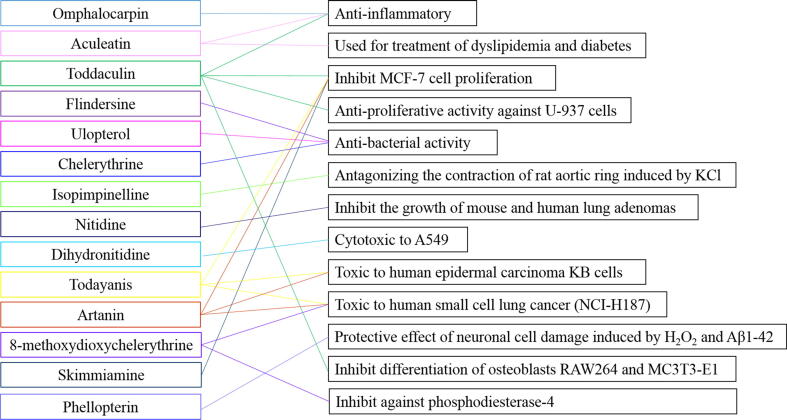
At present, researches on the pharmacological activities of T. asiatica show that extracts and chemical components of T. asiatica have some biological activities including anti-inflammatory and analgesic, hemostatic coagulation, antibacterial, anti-oxidant and anti-tumor effects and so forth. To the best of our knowledge, pharmacological effects were mainly focused on the extracts of alcohol, water and methanol and so on. Just few monomeric compounds on their pharmacological effects were reported summarized in Fig. 7. The following will elaborate on these activities in turn.
Fig. 7.
Relationship between chemical compounds from T. asiatica and their pharmacological effects.
3.1. Anti-inflammatory analgesic effect
Researches (Lu et al., 2015, Zhang et al., 2019) found that the alcohol, water and n-butanol extracts of T. asiatica root had analgesic effects and the former had better analgesic effects than the two latter. Its mechanism may be related to increasing the content of serum β-endor endorphin (β-EP), decreasing the content of prostaglandin E2 (PGE2) and nitric oxide (NO), up-regulating the expression of β-EP receptor and down-regulating the expression of PGE2 receptor. Yang et al., (Yang et al., 2013) found that feeding arthritis mice with type II bovine collagen injected with ethanol and ethyl acetate extracts of T. asiatica could relieve swelling of paws and joints. Histopathological examination showed that the extracts could protect the knee joint from the erosion and deformation of bone and cartilage, significantly decrease the concentration of tumor necrosis factor-α (TNF- α), interleukin-1-1 β (IL-1 β) and interleukin-6 (IL-6), and increase the concentration of interleukin-10 (IL-10), compared with the control group, which showed similar results according with the consequence obtained by Tian et al., (Tian et al., 2018). The anti-inflammation mechanism of T. asiatica extract maybe involve the balance between Th17 and Treg in the rat model with wind-chill and dampness adjuvant arthritis (Wang et al., 2016, Liu et al., 2018a, Liu et al., 2018b).
Tong et al., (Tong et al., 2014) used RAW264.7 as the test cell to study the anti-inflammatory activity of coumarin omphalocarpin. The consequences suggested that omphalocarpin could reduce the release of NO induced by lipopolysaccharide and the secretion of TNF- α and IL-6 inflammatory factors, strongly inhibit the expression and activity of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and inhibit the transfer of NF- κ B to the nucleus. Kumagai et al., (Kumagai et al., 2018) also found that aculeatin and toddaculin isolated from T. asiatica could also play important roles in anti-inflammation in LPS-stimulated RAW264 macrophages via different mechanisms, which further demonstrated that extracts from T. asiatica own significant anti-inflammatory effect.
Hao et al., (Hao et al., 2004) found that the total alkaloids from the root bark of T. asiatica can significantly inhibit xylene-induced ear swelling and agar-induced foot swelling in mice, and significantly inhibit the migration of hemameba in abdominal cavity induced by sodium carboxymethyl cellulose and the writhing reaction induced by acetic acid in mice, which showed no damage to the liver when given for a long time. Wang et al., (Wang et al., 2007) found that the water extract from the rhizome of T. asiatica had anti-inflammatory and analgesic effects, and could also reduce the number of writhing induced by glacial acetic acid in mice and prolong the latent period of licking hind feet in mice through hot plate test. It could significantly inhibit the foot swelling induced by carrageenan and the ear swelling induced by xylene in mice. Liu et al., (Liu et al., 2007) investigated the analgesic and anti-inflammatory effects of alcohol extract and water extract of T. asiatica. The results showed that both alcohol extract and water extract had certain analgesic and anti-inflammatory effects, but the analgesic and anti-inflammatory effect of alcohol extract was stronger than that of water extract. Balasubramaniam et al., (Balasubramaniam et al., 2012) found that 50% ethanol extract from T. asiatica had good analgesic and anti-inflammatory effects through carrageenan-induced foot swelling and cotton ball-induced granuloma in rats. Kariuki et al., (Kariuki et al., 2013) using formalin-induced pain test and carrageenan-induced foot swelling test investigated the analgesic and anti-inflammatory effects of methane/methanol (1:1) extract from T. asiatica. The results showed that the methane/methanol (1:1) extract from T. asiatica had good analgesic and anti-inflammatory effects under 100 mg/kg. Hu et al., (Hu et al., 2000) found that the ethanol extract from T. asiatica could reduce the writhing times of mice induced by acetic acid and alleviate the foot swelling induced by agar, both of which were dose-dependent. Besides, investigation performed by Qin et al., (Qin et al., 2020) found that 60% ethanol extract from T. asiatica could inhibit macrophage migration through high throughput screening. Those consequences showed that compounds from T. asiatica had played an important role in anti-inflammatory effect.
3.2. Bacteriostatic effect
Ding et al., (Ding et al., 2007) selected four different polar solvents (distilled water, anhydrous ethanol, ethyl acetate, petroleum ether) and two different extraction methods (cold soaking method and heating reflux method) to extract the root of T. asiatica. It was found that anhydrous ethanol and ethyl acetate extracts could significantly inhibit the growth of Bacillus subtilis, Shigella dysentery and Saccharomyces cerevisiae. At the same time, the alkaloids isolated from the ethanol extract of T. asiatica root by Hu et al., (Hu et al., 2014) can inhibit the growth of bacteria and fungi. Narod et al., (Narod et al., 2004) found that aqueous extracts of T. asiatica stems and leaves could inhibit the proliferation of Pseudomonas aeruginosa and Staphylococcus aureus; the extract of methanol/chloroform (1:1) from stem can inhibit the proliferation of P. aeruginosa, S. aureus and Aspergillus; the extract of n-hexane from leaves can inhibit the growth of P. aeruginosa, S. aureus, Aspergillus and Candida albicans. Continuous extraction was used by Duraipandiyan et al (Duraipandiyan and Ignacimuthu, 2009) to extract the leaves and root of T. asiatica, and found that ethyl acetate extract had the best antibacterial activity. Moreover, flindersine was further isolated from the ethyl acetate layer extract, and demonstrated to inhibit the growth of S. aureus, Bacillus subtilis, S. epidermidis, Enterococcus faecalis, P. aeruginosa, Acinetobacter baumannii, Trichophyton rubrum, T. dermatophytes and C. albicans. Karunau Raj et al., (Karunai Raj et al., 2012) also found a coumarin compound (Ulopterol), from the ethyl acetate extract of the leaves of T. asiatica, which could inhibit the growth of S. epidermidis, Enterobacter aerogenes, Klebsiella pneumoniae, Escherichia coli, A. flavus, C. Cruise, Botrytis cinerea etc.. Indirect immunofluorescence was used to observe the effects of different herbs on the adhesion of C. albicans to oral mucosal epithelial cells in vitro. It was found that T. asiatica had the dual effects of anti- C. albicans and anti- C. albicans adhesion (Hou and Wang, 1990). Microbroth dilution method (Xu et al., 2012) was used to investigate the inhibitory effect of ethanol extract of T. asiatica in vitro, the consequences of which showed that the minimum inhibitory concentration of ethanol extract against C. albicans was 7.5 mg/mL, while the minimum bactericidal concentration was 15.0 mg/mL. With the increase of drug concentration, the expression of virulence factors SNF2 and PDE2 decreased obviously, which inferred that the antibacterial effect is mainly due to inhibiting the formation of pathogenic mycelium and destroying the integrity of bacterial cell wall. It has also been confirmed that the metabolites of T. asiatica had broad-spectrum bacteriostatic activity (Guo et al., 2014). Furthermore, He et al., (He et al., 2018) investigated the antibacterial mechanism of chelerythrine isolated from T. asiatica root and discovered that chelerythrine could play a role in antibacterial activity via destroying the bacterial cell wall and membrance, and inhibiting protein biosynthesis.
3.3. Antioxidant activity
Tian et al., (Tian et al., 2011) using Fenton and 1,1-Diphenyl-2-picrylhydrazine (DPPH) method, found that polysaccharides from the root of T. asiatica could scavenge hydroxyl radical and DPPH free radical, and the scavenging ability of the two free radicals was positively correlated in the concentration range of 0.2–0.4 g/L and 5 × (10−3–10−1) g/L, respectively. The scavenging rate of hydroxyl radical was 73.7% when the concentration of 0.4 g/L was 0.5 g/L, and the scavenging rate of DPPH free radical was 79.5%. With vitamin C (VC) and tea polyphenols as positive controls, the ability of scavenging hydroxyl free radicals of polysaccharides from T. asiatica was studied by flow injection chemiluminescence method. The scavenging ability of 0.1–500 mg/L to hydroxyl radical was related to the concentration, and the IC50 values were 5.69, 2.19 and 0.745 mg/L, respectively. The free radical scavenging rate was as high as 94% when the concentration of polysaccharides was up to 500 mg/L, and the photostability was stronger than that of VC and tea polyphenols (Tian et al., 2011). Chen et al., (Chen and Long, 2013) investigated the antioxidant activity of the extract from T. asiatica stem by Fenton method, DPPH method and Fe2+- cysteine reaction and found that the n-butanol extraction exhibits strong hydroxyl radical scavenging ability; Ethyl acetate extract has the strongest ability of scavenging DPPH; 70% ethanol extract and n-butanol extract showed strong anti-lipid peroxidation activity, which indicated that the extracts of different polar solvents had certain antioxidant activity. Balasubramaniam et al., (Balasubramaniam et al., 2012) found that 50% ethanol extract of T. asiatica stem can scavenge hydroxyl radical, diphenylpicryl hydrazide radical and nitric oxide radical, and also has the ability of chelating divalent iron ion, which indicates that 50% ethanol extract of T. asiatica stem has antioxidant activity in vitro. Stephen Irudayaraj et al., (Stephen Irudayaraj et al., 2012) found that the activities of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) were low in diabetic rats, while the activities of the three enzymes returned to the normal range in intragastric administration of ethyl acetate extract from T. asiatica leaves of diabetic rats, indicating that the ethyl acetate extract has antioxidant activity.
3.4. Cardiovascular protective effect
Ren et al., (Ren et al., 1993) performed a systematic study on the cardiovascular protective effect of T. asiatica and found that Long Jing 1 (L01, extract from T. asiatica) could reduce the blood pressure of anesthetized rats and relax the contractile smooth muscle induced by KCl in vitro, and its mechanism might be related to inhibition of calcium influx. Iso-anise coumarin (isopimpinelline) found in ethanol extract could antagonize the contraction of rat aortic ring induced by KCl (Guo et al., 1998). In addition, Fei Long 1 (F01), aqueous extract from T. asiatica, has protective effects on coronary artery contraction induced by pituitrin, myocardial overexcitation caused by isoproterenol, and acute myocardial ischemia caused by coronary artery occlusion and ligation (He and Ren, 1998, Ren and He, 1998, He and Ren, 1999). F01 can significantly reduce the work and oxygen consumption of acute ischemic myocardium in New Zealand rabbits without ligation of the left anterior descending branch of pleural coronary artery, regulate the balance of oxygen supply and demand, and improve the function of pumping blood, so as to protect the ischemic myocardium (He et al., 2000). Through the study of cardiac function and hemodynamics in normal domestic cats, it is proved that F01 can inhibit the heart and dilate peripheral blood vessels, and reduce cardiac afterload as well as myocardial work and oxygen consumption, which may be another anti-myocardial ischemia mechanism (Ren et al., 2000). Rats were fed with high fat diet and injected isoprenaline hydrochloride (Iso) once a day for consecutive three days at the end of the 4-weeks treatment and the results suggested that T. asiatica extract could significantly improve the Iso-induced electrocardiogram changes in rats, reduce the heart, liver and fat indexes, the level of total cholesterol (TC), triglyceride (TG), low density (LDL), TNF-α, interferon-γ (INF-γ) and IL-6, increase the contents of high density lipoprotein (HDL) and IL-10, and improve the pathological damages of myocardium, which may regulate the balance of anti-inflammatory and proinflammatory cytokines in protecting rat model from cardiovascular diseases (Liu et al., 2018a, Liu et al., 2018b).
3.5. Antimalarial activity
Orwa et al., (Orwa et al., 2013) carried out antimalarial experiments on extracts from different parts of the T. asiatica, using chloroquine-sensitive D6 and chloroquine-resistant Plasmodium falciparum strain W2 incorporated 50% radioisotope to determine the inhibitory concentration of antimalarial parasite IC50 in vitro. Through the experiment of mice infected with P. berghei, quinine hydrochloride used as positive control, it was found that the ethyl acetate extract from T. asiatica fruit showed high activity against chloroquine-resistant P. falciparum strain with IC50 1.87 ug/mL, followed by the water extract of root bark with IC50 2.43 µg/mL. The inhibitory activity of ethyl acetate extract (500 mg/kg) and root bark water extract (250 mg/kg) of fruit against P. berghei in vivo were 81.34% and 56.8%, respectively. The root bark extracts of T. asiatica and the isopimpinellin, geraniol and D-limonene separated from T. asiatica (Liu et al., 2013) were found to have antimalarial and insecticidal effect.
3.6. Inhibitory effect on the proliferation of human cancer cells
Iwasaki et al., (Iwasaki et al., 2006, Iwasaki et al., 2010) found that two alkaloids, nitidine and dihydronitidine, which were isolated from T. asiatica could inhibit the proliferation of mouse and human lung adenocarcinoma cells in vitro. Results have shown that nitidine can effectively inhibit the growth of mouse and human lung adenomas in subcutaneous xenograft models without any significant side effects; dihydronitidine is highly specific cytotoxic to human lung adenocarcinoma (A549) cells. It can up-regulate apoptosis-related genes and regulate cell cycle gene expression, that is, inhibiting cell proliferation. Furthermore, researchers isolated 18 compounds from T. asiatica, named todayanin, toddayanis, artanin, coumurrayin, toddaculin, toddanol, toddalolactone, isopimpinellin, phellopterin, 5-methoxy-8-geranyloxypsoralen, 8-methoxydihydrochelerythrine, methoxynorchelerythrine, skimmiamine, norchelerythrine, chelerythrine, oplopanone, nelumol and p-isopentenoxybenzenepropanoic acid. Among them, todayanis is toxic to human epidermal carcinoma KB cells, breast cancer cell line MCF-7 cells, and human small cell lung cancer (NCI-H187) cell lines, with IC50 of 32.2, 5.8, and 17.6 μg/mL, respectively. Artanin is toxic to all three cancer cells, with IC50 ranging from 7.4 to 31.0 μg/mL. Moreover, 8-methoxydioxychelerythrine is the most toxic to NCI-H187 cells with IC50 of 0.8 μg/mL. In addition, toddaculin and skimmiamine can inhibit MCF-7 cell proliferation with IC50 of 23.4 and 8.7 μg/mL, separately, which have been suggested that the two compounds are the most likely anticancer lead compounds (Murakami et al., 2000, Hirunwong et al., 2016). Vázquez et al., (Vazquez et al., 2012) isolated six isoprene-substituted coumarins from T. asiatica, among which toddaculin had the strongest cytotoxicity and anti-proliferative activity against U-937 cells. Further research found that toddaculin had dual effects, which could induce apoptosis of U-937 cells at a drug concentration of 250 μmol/L, and promote cell differentiation at a concentration of 50 μmol/L.
3.7. Inhibiting the pathogenesis of Alzheimer’s disease (AD)
Alzheimer’s disease (AD) is the most common cause of dementia and a chronic and progressive neurodegenerative disorder following with memory impairment, gradual loss of attention, emotions, mental capacity and the learning ability and what not. Takomthong et al., (Takomthong et al., 2020) investigated that seven out of nine coumarins isolated from T. asiatica were identified as the multifunctional agents which could inhibit the pathogenesis of AD, especially the phellopterin that showed significant protective effect of neuronal cell damage induced by H2O2 and Aβ1-42 toxicity. With the intensification of aging in the whole world, the number of AD patients is increasing, which aggravates the burden of families and social medical treatment, specially there are no specific medicine used in clinic. Therefore, traditional Chinese herb is another effective breach that could provide a new treatment for AD patients, which has also attracted extensive attention of scholars engaged in medical researches.
3.8. Other pharmacological effects
T. asiatica has also played an important role in other pharmacological effects other than the above six pharmacological effects. Using mouse tail amputation and capillary glass tube method, the bleeding time, bleeding volume and clotting time were used to investigate the hemostatic activity of different polar parts of blood root bark (Zhao et al., 2016). The average bleeding time, bleeding volume and clotting time of ethyl acetate in cold extract were (59.67 ± 12.31) s, (4.42 ± 1.67) mg and (79.67 ± 5.57) s, respectively. It was also found that the hemostatic activity of ethyl acetate in cold immersion was better than the other polar extracts. Ethanol extract can significantly shorten the mice’s bleeding and clotting time, and the hemostatic time is similar to that of Panax notoginseng powder which has better hemostatic effect at present. Further studies found that the hemostatic effect of T. asiatica may be related to the increase of fibrinogen content (p < 0.05), the promotion of endogenous coagulation pathway and the change of platelet morphology (Liu et al., 2016). To observe the bleeding and coagulation activity of different polar parts of T. asiatica in mice, methanol part has the best coagulation effect, which can improve the coagulation function of the body and enhance the hemostatic effect (Shi et al., 2010). Research carried out by Tsai et al., (Tsai et al., 1998) showed that seven compounds had anti-platelet aggregation effect in vitro after biological activity guided the separation of chemical constituents from T. asiatica. Watanabe et al., (Watanabe et al., 2014) found that extracted from T. asiatica could increase the differentiation and lipolysis of adipocytes at the same time, and be used in the treatment of dyslipidemia and diabetes. They then found that the toddaculin in T. asiatica acted on osteoclasts RAW264 and osteoblasts MC3T3-E1 in vitro via inhibition of osteoclast differentiation by activating NF- κ B, ERK1/2 and p38MAPK signal transduction pathways (Watanabe et al., 2015). Otherwise, T. asiatica. also has the effects of antiviral (Tan et al., 1991, Mao et al., 2002, Li et al., 2005), antispasmodic (Lakshmi et al., 2002), diuretic (Liu et al., 2007), anti-HIV (Rashid et al., 2014) and larvicidal (Borah et al., 2010).
4. Conclusion and future perspectives
T. asiatica is a traditional Chinese herbal medicine in China, which is a characteristic ethnic medicine in southwest China, Wuling Mountainous areas, such as Guizhou, Yunnan, Guangxi and Enshi, Hubei province and others. Its unique geographical advantages and abundant medicinal resources have laid a solid foundation for its sustainable development and utilization. However, there has been a lack of theoretical guidance for the study of modern medicine. It is of great significance for the development of new drugs with reliable efficacy to carry out the systematic pharmaceutical research on T. asiatica. So far, large-scale clinical application of T. asiatica is still relatively rare, mainly in anti-inflammation and analgesia, hemostasis and coagulation. Moreover, pharmacological research is still in the basic research stage. With the rapid development of modern medical science and technology, the active material basis, pharmacological effects and mechanisms of T. asiatica would be explored more deeply. A small number of unknown active natural compounds, novel pharmacology and pharmacological mechanisms will also be found. Otherwise, there are few reports on the safety of T. asiatica, which needs to be further strengthened and improved in the future, so as to provide a scientific basis for the development and rational application of the medicinal resources.
Based on the bioactivities and pharmacokinetics, coumarins and alkaloids are the main compounds in T. asiatica as there are 138 compositions isolated from the plant. On one hand, these compounds provide framework for biosynthesis; on the other hand, they play an important role in pharmacological activities in vitro, which lay a foundation for clinic application, although there is still a long way to go. As shown in Fig. 7, pharmacological mechanisms of monoclonal compounds are still defective and more work could be done in future. In addition, the resource of T. asiatica is abundant, but the officinal part of this plant is root and stem, which would lead to decrease the number of the wild T. asiatica. Therefore, more works need to be done to fill gaps in protection of the medicine resource.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are thankful to the Hubei Natural Science Foundation (2019CFB630) and National Natural Science Foundation of China (NSFC 81860757).
We thank academic staff members in Lin Yuan’s group at Hubei Provincial Key Laboratory of Occurrence and Intervention of Rhumatic Disease, Hubei Minzu University. Thanks for Qiulong Zhang who is studying for his PhD at Sun Yat-sen University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Balasubramaniam A., Manivannan R., Paramaguru R., Mazumder P.M., Vijayakumar M. Evaluation of anti-inflammatory and antioxidant activities of stem bark of Todalia asiatica (L) Lam. using different experimental models. Pharmacologia. 2012;3:144–149. doi: 10.5567/pharmacologia.2012.144-149. [DOI] [Google Scholar]
- Borah R., Kalita M.C., Kar A., Talukdar A.K. Larvicidal efficacy of Toddalia asiatica (Linn.) Lam against two mosquito vectors Aedes aegypti and Culex quinquefasciatus. Afr. J. Biotechnol. 2010;9:2527–2530. [Google Scholar]
- Cao C., Du P., Zhu X., Yan H., Song X., Zhu H., Geng Y., Wang D. Rapid screening and purification of potential alkaloid neuraminidase inhibitors from Toddalia asiatica (Linn.) Lam. roots via ultrafiltration liquid chromatography combined with stepwise flow rate counter-current chromatography. J. Sep. Sci. 2019;42:2621–2627. doi: 10.1002/jssc.201900379. [DOI] [PubMed] [Google Scholar]
- Chen I.S., Tsai I.L., Wu S.J., Sheen W.S., Ishikawa T., Ishii H. Toddaquinoline from formosan Toddalia asiatica. Phytochemistry. 1993;34:1449–1451. doi: 10.1016/0031-9422(91)80052-3. [DOI] [Google Scholar]
- Chen X.X., Long S.J. Study on antioxidant activities of Toddalia asiatica in vitro. Northwest Pharmac. J. 2013;1:27–29. doi: 10.3969/j.issn.1004-2407.2013.01.011. [DOI] [Google Scholar]
- Chen X.X., Simayi R., Long S.J. Studies on chemical constituents of Toddalia asiatica stems. Northwest Pharmac. J. 2013;28:337–339. doi: 10.3969/j.issn.1004-2407.2013.04.004. [DOI] [Google Scholar]
- Deshmukh M.N., Deshpande V.H., Rama Rao A.V. Two new coumarins from Toddalia aculeata. Phytochemistry. 1976;15:1419–1420. doi: 10.1016/S0031-9422(00)97133-4. [DOI] [Google Scholar]
- Ding W., Wen C.F., Chen J.H., Huang K.X., Dong A.W. Primary study on antibacterial activity of extracts from Toddalia asiatica (Linn) Lam. Biomass Chem. Eng. 2007;41:33–35. [Google Scholar]
- Duraipandiyan V., Ignacimuthu S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol. 2009;123:494–498. doi: 10.1016/j.jep.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Guo J.Y., Sun Y., Wang Y.K., Liu X. Study on isolation of endophytic fungi from Toddalia asiatica and their antimicrobial activity. J. Guangdong Pharmac. Univ. 2014;30:422–426. doi: 10.3969/j.issn.1006-8783.2014.04.007. [DOI] [Google Scholar]
- Guo S.H., Li S.M., Peng Z.Y., Ren X.D. Isolation and identification of active constituent of Toddalia asiatica in cardiovascular system. J. Chin. Med. 1998 Materials515-516. [PubMed] [Google Scholar]
- Hao X.Y., Peng L., Ye L., Huang N.H., Shen Y.M. A study on anti-inflammatory and analgesic effects of alkaloids of Toddalia asiatica. J. Chin. Integr. Med. 2004;2:450–452. doi: 10.3736/jcim20040615. [DOI] [PubMed] [Google Scholar]
- He N., Wang P., Wang P., Ma C., Kang W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complem. Altern. M. 2018;18 doi: 10.1186/s12906-018-2317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.P., Ren X.D. Protective effects of Toddalia asiatic aqueous extract on ischemic myocardium induced by pituitrin in rats. Chin. J. Pathophysiol. 1998;14:283–286. [Google Scholar]
- He X.P., Ren X.D. Protective effects of aqueous extract of Toddalia asiatica on experimental myocardial infarction in rats. J. Jinan Univ. (Med. Ed.) 1999;20:15–18. [Google Scholar]
- He Y.K., Da R.X., Hua X.A., Xiu Y.Y. Effects of aqueous extract from Toddalia asiatica on cardiac function and hemodynamics in myocardial ischemic rabbits. Chin. J. Pathophysiol. 2000;16:606–609. [Google Scholar]
- Hirunwong C., Sukieum S., Phatchana R., Yenjai C. Cytotoxic and antimalarial constituents from the roots of Toddalia asiatica. Phytochem. Lett. 2016;17:242–246. doi: 10.1016/j.phytol.2016.08.008. [DOI] [Google Scholar]
- Hou Y.H., Wang Z.W. Effect of seven kinds of Chinese herbal medicines on the adherence of C. albicans and their ultrastructure observation. Chin. J. Dermatovenereol. 1990;4:136–140. [Google Scholar]
- Hu J., Shi X., Chen J., Mao X., Zhu L., Yu L., Shi J. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014;148:437–444. doi: 10.1016/j.foodchem.2012.12.058. [DOI] [PubMed] [Google Scholar]
- Hu J., Shi X., Mao X., Chen J., Li H. Amides from the Roots of Toddalia asiatica. Chem. Nat. Compd. 2015;51:726–729. doi: 10.1007/s10600-015-1393-6. [DOI] [Google Scholar]
- Hu X.Y., Zeng F.B., Cui X.R., Li Y.R. Studies on analgesic and anti-inflammatory effects and toxicity of ethanol extract from Toddalia asiatica. Chin. J. Tradit. Med. Sci. Technol. 2000;7:231–232. [Google Scholar]
- Huang P., Karagianis G., Wei S.X., Walerman P.G. Triterpene acids from Toddalia asiatica. Natl. Prod. Res. Develop. 2005;04:404–408. [Google Scholar]
- Ishii H., Kobayashi J.-I., Ishikawa T. Toddacoumalone, a novel mixed dimer of coumarin and quinolone from Toddalia asiatica (L.) Lam. (T. aculeata Pers) Tetrahedron Lett. 1991;132:6907–6910. doi: 10.1016/0040-4039(91)80441-8. [DOI] [Google Scholar]
- Ishii H., Kobayashi J., Ishikawa M., Haginiwa J., Ishikawa T. Studies on the chemical constituents of Rutaceae++ plants. LXVI. The chemical constituents of Toddalia asiatica (L.) Lam. (T. aculeata Pers.). (1). Chemical constituents of the root bark. Yakugaku Zasshi. 1991;111:365–375. doi: 10.1248/yakushi1947.111.7_365. [DOI] [PubMed] [Google Scholar]
- Ishii H., Kobayashi J.I., Ishikawa T. Toddalenone: A new coumarin from Toddalia asiatica (T. aculeata) structural establishment based on the chemical conversion of limettin into toddalenone. Chem. Pharm. Bull. 1983 doi: 10.1248/cpb.31.3330. [DOI] [Google Scholar]
- Iwasaki H., Okabe T., Takara K., Toda T., Shimatani M., Oku H. Tumor-selective cytotoxicity of benzo[c]phenanthridine derivatives from Toddalia asiatica Lam. Cancer Chemother. Pharmacol. 2010;65:719–726. doi: 10.1007/s00280-009-1077-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Oku H., Takara R., Miyahira H., Hanashiro K., Yoshida Y., Kamada Y., Toyokawa T., Takara K., Inafuku M. The tumor specific cytotoxicity of dihydronitidine from Toddalia asiatica Lam. Cancer Chemother. Pharmacol. 2006;58:451–459. doi: 10.1007/s00280-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Jain S.C., Pandey M.K., Upadhyay R.K., Kumar R., Hundal G., Hundal M.S. Alkaloids from Toddalia aculeata. Phytochemistry. 2006;67:1005–1010. doi: 10.1016/j.phytochem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kariuki H.N., Kanui T.I., Yenesew A., Patel N., Mbugua P.M. Antinocieptive and anti-inflammatory effects of Toddalia asiatica (L) Lam. (Rutaceae) root extract in Swiss albino mice. Pan. Afr. Med. J. 2013;14:133. doi: 10.11604/pamj.2013.14.133.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunai Raj M., Balachandran C., Duraipandiyan V., Agastian P., Ignacimuthu S. Antimicrobial activity of Ulopterol isolated from Toddalia asiatica (L.) Lam.: a traditional medicinal plant. J. Ethnopharmacol. 2012;140:161–165. doi: 10.1016/j.jep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Kumagai M., Watanabe A., Yoshida I., Mishima T., Nakamura M., Nishikawa K., Morimoto Y. Evaluation of aculeatin and toddaculin isolated from Toddalia asiatica as anti-inflammatory agents in LPS-stimulated RAW264 macropgages. Biol. Pharm. Bull. 2018;41:132–137. doi: 10.1248/bpb.b17-00607. [DOI] [PubMed] [Google Scholar]
- Lakshmi V., Kapoor S., Pandey K., Patnaik G.K. Spasmolytic activity of Toddalia asiatica Var. floribunda. Phytother. Res. 2002;16:281–282. doi: 10.1002/ptr.844. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Qiao Y.J., Xiao P.G., Tan X.H. Identification of antiviral activity of Toddalia asiatica against influenza type A virus. China J. Chin. Materia Medica. 2005;30:998–1001. [PubMed] [Google Scholar]
- Li W., Zhang J.-S., Huang J.-L., Jiang M.-H., Xu Y.-K., Ahmed A., Yin S., Tang G.-H. New prenylated coumarins from the stems of Toddalia asiatica. RSC Adv. 2017;7:31061–31068. doi: 10.1039/c7ra04794k. [DOI] [Google Scholar]
- Li X., Qiu Z., Jin Q., Chen G., Guo M. Cell cycle arrest and apoptosis in HT-29 cells induced by dichloromethane fraction from Toddalia asiatica (L.) Lam. Front. Pharmacol. 2018;9:629. doi: 10.3389/fphar.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun S.W., Zhang X.Y., Liu Y., Liu X.H., Zhang S., Wang W., Wang J., Wang W. Separation of new coumarin glycosides from Toddalia asiatica using offline two-dimensional high-performance liquid chromatography. Plants (Basel) 2020;9 doi: 10.3390/plants9040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-T., Chen G. A new sesquiterpene and known alkaloids from Toddalia asiatica and their inhibitions against phosphodiesterase-4. Rec. Nat. Prod. 2020;14:207–212. doi: 10.25135/rnp.157.1907.1356. [DOI] [Google Scholar]
- Lin T.T., Huang Y.Y., Tang G.H., Cheng Z.B., Liu X., Luo H.B., Yin S. Prenylated coumarins: natural phosphodiesterase-4 inhibitors from Toddalia asiatica. J. Nat. Prod. 2014;77:955–962. doi: 10.1021/np401040d. [DOI] [PubMed] [Google Scholar]
- Liu M., Liu Y., Deng Y., Ding Y., Wu F. Effects of extract from Toddalia asiatica on Th17/Treg balance in rats with wind-chill and dampness adjuvant arthritis. Pharmacol. Clin. Chin. Mater. Med. 2018;34:108–111. doi: 10.13412/j.cnki.zyyl.2018.03.026. [DOI] [Google Scholar]
- Liu M., Liu Y., Deng Y., Hu Z.L. Effects of Toddalia asiatica extract on inflammatory cytokines in rats with myocardial ischemia and hyperlipidemia. Chin. J. Comp. Med. 2018;28:64–68. 10.3969.j.issn.1671-7856.2018.02.011. [Google Scholar]
- Liu M., Luo C.L., Zhang Y.P., Qiu D.W. Studies on analgesic, anti-inflammatory and diuretic effects of Polygonum capitatum and Toddalia asiatica (in Chinese) Guizhou Med. J. 2007;31:370–371. [Google Scholar]
- Liu X.C., Dong H.W., Zhou L., Du S.S., Liu Z.L. Essential oil composition and larvicidal activity of Toddalia asiatica roots against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2013;112:1197–1203. doi: 10.1007/s00436-012-3251-9. [DOI] [PubMed] [Google Scholar]
- Liu Z.G., Wang X.Y., Mao B.P., Xie X.L. Study on chemical constituents of Toddalia asiatica. J. Chin. Med. Mater. 2014;37:1600–1603. [PubMed] [Google Scholar]
- Liu Z.G., Wang X.Y., Mao B.P., Xie X.L. Study on the hemostatic mechanism of Toddalia asiatica extracts. Chin. J. Pharm. 2016;31:157–159. doi: 10.13375/j.cnki.wejps.2016.02.015. [DOI] [Google Scholar]
- Lu, Y., Zhu, Y.Z., Guo, C.X., Han, C., Zhu, G.F., Pan, Y.Y., 2015. Analgesic effect of Toddalia asiatica's extract and its peripheral analgesic mechnism.
- Mao P.C., Mouscadet J.F., Leh H., Auclair C., Hsu L.Y. Chemical modification of coumarin dimer and HIV-1 integrase inhibitory activity. Chem. Pharm. Bull. (Tokyo) 2002;50:1634–1637. doi: 10.1248/cpb.50.1634. [DOI] [PubMed] [Google Scholar]
- Murakami A., Nakamura Y., Tanaka T., Kawabata K., Takahashi D., Koshimizu K., Ohigashi H. Suppression by citrus auraptene of phorbol ester-and endotoxin-induced inflammatory responses: role of attenuation of leukocyte activation. Carcinogenesis. 2000;21:1843–1850. doi: 10.1093/carcin/21.10.1843. [DOI] [PubMed] [Google Scholar]
- Narod F.B., Fakim A.G., Subratty A.H. Biological investigations into Antidesma madagascariense Lam. (Euphorbiaceae), Faujasiopsis flexuosa (Lam.) C. Jeffrey (Asteraceae), Toddalia asiatica (L.) Lam. and Vepris lanceolata (Lam.) G. Don. J. Mol. Cell. Biol. 2004;3:15–21. [Google Scholar]
- Nyahanga T., Jondiko J.I., Manguro L.O.A., Orwa J.A. Antiplasmodial and larvicidal compounds of Toddalia asiatica root bark. J. Chem. Sci. 2013;125:1115–1121. doi: 10.1007/s12039-013-0483-x. [DOI] [Google Scholar]
- Oketch-Rabah H.A., Mwangi J.W., Lisgarten J., Mberu E.K. A new antiplasmodial coumarin from Toddalia asiatica roots. Fitoterapia. 2000;71:636–640. doi: 10.1016/s0367-326x(00)00222-7. [DOI] [PubMed] [Google Scholar]
- Orwa J.A., Ngeny L., Mwikwabe N.M., Ondicho J., Jondiko I.J. Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae) J. Ethnopharmacol. 2013;145:587–590. doi: 10.1016/j.jep.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Phatchana R., Yenjai C. Cytotoxic coumarins from Toddalia asiatica. Planta Med. 2014;80:719–722. doi: 10.1055/s-0034-1368568. [DOI] [PubMed] [Google Scholar]
- Qin S., Zhang Y.P., Chen X., Xia J.Y., Chen Y.D., Kong D.M. Researches on high throughput screening of drugs to inhibit macrophage migration (in Chinese) J. Guizhou Univ. Tradit. Chin. Med. 2020;42:87–90. doi: 10.16588/j.cnki.issn1002-1108.2020.03.021. [DOI] [Google Scholar]
- Qiu H., Xiao X., Li G. Separation and purification of furanocoumarins from Toddalia asiatica (L.) Lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 2012;35:901–906. doi: 10.1002/jssc.201100995. [DOI] [PubMed] [Google Scholar]
- Rashid M.A., Gustafson K.R., Kashman Y., Ll J.H.C., Mcmahon J.B., Boyd M.R. Anti-HIV Alkaloids from Toddalia asiatica. Nat. Prod. Lett. 2014;6:153–156. doi: 10.1080/10575639508044104. [DOI] [Google Scholar]
- Ren X.D., He X.P. Protective effects of aqueous extract from Toddalia asiatica on myocardial ischemia induced by isoprenaline in rats. J. Jinan Univ. (Med. Ed.) 1998;19:22–25. [Google Scholar]
- Ren X.D., Wang D.W., Zhong L., Li S.M., Guo S.Y. Effects of Long Jing 1 on isolated rat thoracic aortic rings. Chin. J. Pathophysiol. 1993;9:129–132. [Google Scholar]
- Ren X.D., Ye K.H., Xiong A.H., Yang Y.X. The effects of aqueous extract from Toddalia asiatica on cardiac function and hemodynamics in normal cats. J. Jinan Univ. (Med. Ed.) 2000;21:1–4. [Google Scholar]
- Sharma P.N., Shoeb A., Kapii R.S., Popli S.P. 8-hydroxydihydrochelerythrine and arnottianamide from roots of Toddalia asiatica. Phytochemistry. 1982;21:252–253. doi: 10.1016/0031-9422(82)80068-X. [DOI] [Google Scholar]
- Sharma P.N., Shoeb A., Kapil R.S., Popli S.P. Toddasin, a new dimeric coumarin from Toddalia asiatica. Phytochemistry. 1980;19:1258–1260. doi: 10.1016/0031-9422(80)83106-2. [DOI] [Google Scholar]
- Sharma P.N., Shoeb A., Kapil R.S., Popli S.P. Toddalidimerine, a dimeric benzophenanthridine alkaloid from Toddalia asiatica. Phytochemistry. 1981;20:2781–2783. doi: 10.1016/0031-9422(81)85291-0. [DOI] [Google Scholar]
- Sharma P.N., Shoeb A., Kapil R.S., Popli S.P. Toddanol and toddanone, two coumarins from Toddalia asiatica. Phytochemistry. 1981;20:335–336. doi: 10.1016/0031-9422(81)85120-5. [DOI] [Google Scholar]
- Shi L., Ji Z.Q., Yu Q.Y., Li Y.M. Chemical constituents in methanol parts of Toddalia asiatica (Linn) Lam. China Pharmac. 2014;17:534–537. [Google Scholar]
- Shi L., Ji Z.Q., Zhang Z.W., Li Y.Y. Chemical constituents in ethyl acetate parts of Toddalia asiatica (Linn) Lam. China Pharmac. 2013;16:1293–1295. [Google Scholar]
- Shi L., Li C.Q., Lian T.T., Xu Q.T., Kang W.Y. Effect of Toddalia asiatica on bleeding time and clotting time in mice. China Pharmacy. 2010;21:4424–4425. [Google Scholar]
- Shi L., Li D., Kang W.Y. Research progress of chemical constituents and pharmacological effects of Toddalia asiatica. China Pharmacy. 2011;22:666–668. [Google Scholar]
- Shi L., Wang W., Ji Z.Q., Kang W.Y. Study on chemical constituents in petroleum parts of Toddalia asiatica. China Pharmacy. 2012;23:2531–2532. doi: 10.6039/j.issn.1001-0408.2012.27.13. [DOI] [Google Scholar]
- Stephen Irudayaraj S., Sunil C., Duraipandiyan V., Ignacimuthu S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. leaves in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2012;143:515–523. doi: 10.1016/j.jep.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Sukieum S., Sang-Aroon W., Yenjai C. Coumarins and alkaloids from the roots of Toddalia asiatica. Nat. Prod. Res. 2018;32:944–952. doi: 10.1080/14786419.2017.1374264. [DOI] [PubMed] [Google Scholar]
- Takomthong P., Waiwut P., Yenjai C., Sripanidkulchai B., Reubroycharoen P., Lai R., Kamau P., Boonyarat C. Structure-activity analysis and molecular docking studies of coumarins from Toddalia asiatica as multifunctional agents for Alzheimer's disease. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.T., Pezzuto J.M., Kinghorn A.D. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991;54:143–154. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- Tang Z.H., Liu Y.B., Ma S.G., Li L., Li Y., Jiang J.D., Qu J., Yu S.S. Antiviral Spirotriscoumarins A and B: two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from Toddalia asiatica. Org. Lett. 2016;18:5146–5149. doi: 10.1021/acs.orglett.6b02572. [DOI] [PubMed] [Google Scholar]
- Tian C.L., Jiang F.K., Wen C.F. Study on free radical scavenging efficency of Toddalia asiatica polysaccharides. Sci. Technol. Food Ind. 2011;11:106–108. doi: 10.13386/j.issn1002-0306.2011.11.009. [DOI] [Google Scholar]
- Tian C.L., Kang L.C., Wang P., Gong Z.Q. Determination of hydroxyl radical scavenging capacity of polysaccharides from roots of Toddalia asiatica by flow injection chemiluminescence method. Food Sci. 2013;34:79–82. doi: 10.7506/spkx1002-6630-201313017. [DOI] [Google Scholar]
- Tian P.Y., Yu Y.S., Deng Z.G., Chen Y.K., Zhu X. Effects of Miao medicine Toddalia asiatica on acute gouty arthris in rats. Chin. J. Gerontol. 2018;3019-3022 doi: 10.3969/j.issn.1005-9202.2018.12.078. [DOI] [Google Scholar]
- Tong L., Chen T., Chen Z., Zhang P., Pi H., Ruan H., Wu J. Anti-inflammatory activity of omphalocarpin isolated from Radix Toddaliae Asiaticae. J. Ethnopharmacol. 2014;155:1553–1560. doi: 10.1016/j.jep.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Tsai I.L., Fang S.C., Ishikawa T., Chang C.T., Chen I.S. N-cyclohexyl amides and a dimeric coumarin from formosan Toddalia asiatica. Phytochemistry. 1997;44:1383–1386. doi: 10.1016/S0031-9422(96)00724-8. [DOI] [Google Scholar]
- Tsai I.L., Wun M.F., Teng C.M., Ishikawa T., Chen I.S. Anti-platelet aggregation constituents from Formosan Toddalia asiatica. Phytochemistry. 1998;48:1377–1382. doi: 10.1016/s0031-9422(97)00678-x. [DOI] [PubMed] [Google Scholar]
- Vazquez R., Riveiro M.E., Vermeulen M., Mondillo C., Coombes P.H., Crouch N.R., Ismail F., Mulholland D.A., Baldi A., Shayo C., Davio C. Toddaculin, a natural coumarin from Toddalia asiatica, induces differentiation and apoptosis in U-937 leukemic cells. Phytomedicine. 2012;19:737–746. doi: 10.1016/j.phymed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Wang F., Xu Y., Liu J.K. New geranyloxycoumarins from Toddalia asiatica. J. Asian Nat. Prod. Res. 2009;11:752–756. doi: 10.1080/10286020903048975. [DOI] [PubMed] [Google Scholar]
- Wang Q.J., Lu H., Lv W.W., Liu F., Liu J. The experimental research on analgesia and anti-inflammation of aqueous extract from Toddalia asiatica Lam. Chin. J. Exp. Tradit. Med. Formulae. 2007;13:35–37. [Google Scholar]
- Wang X.K., Li P., Ren Y., Liang Z.C., Yang Z.B. Effects of alcohol extract of Toddalia asiatica on the inflammation-associated cytokines of model rats with adjuvant arthritis. China Pharmacy. 2016;27:3524–3527. doi: 10.6039/j.issn.1001-0408.2016.25.21. [DOI] [Google Scholar]
- Watanabe A., Kato T., Ito Y., Yoshida I., Harada T., Mishima T., Fujita K., Watai M., Nakagawa K., Miyazawa T. Aculeatin, a coumarin derived from Toddalia asiatica (L.) Lam., enhances differentiation and lipolysis of 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2014;453:787–792. doi: 10.1016/j.bbrc.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Kumagai M., Mishima T., Ito J., Otoki Y., Harada T., Kato T., Yoshida M., Suzuki M., Yoshida I., Fujita K., Watai M., Nakagawa K., Miyazawa T. Toddaculin, Isolated from of Toddalia asiatica (L.) Lam., Inhibited Osteoclastogenesis in RAW 264 Cells and Enhanced Osteoblastogenesis in MC3T3-E1 Cells. PLoS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0127158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q., Liu Y. Pharmacognosy identification of ethno-methcine Toddalia asiatica (L.) Lam. J. Southwest Univ. National.: Natl. Sci. Ed. 2007;33:1101–1103. [Google Scholar]
- Xu Y., Guo J.Y., Liu X., Xi Y.L., Fan S.X. The antibacterial activity of the ethanol extract Toddalia asiatica against Candida albicans. Chin. J. Exp. Tradit. Med. Formul. 2012;18:270–274. doi: 10.13422/j.cnki.syfjx.2012.10.079. [DOI] [Google Scholar]
- Yang K., Tong L., Chen C., Zhang P., Pi H., Ruan H., Wu J. Therapeutic effects of extracts from Radix Toddaliae Asiaticae on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol. 2013;146:355–362. doi: 10.1016/j.jep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang X., Sun W., Yang Z., Liang Y., Zhou W., Tang L. Hemostatic chemical constituents from natural medicine Toddalia asiatica root bark by LC-ESI Q-TOF MS(E) Chem. Cent. J. 2017;11:55. doi: 10.1186/s13065-017-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.W., Hu Z.L., Luo Y.B., Chen L.J., Chen A.L., Liu Y., Liu M., Hu W.F. Study on the activity screening and mecheanism of the effective analgesic part of ptrropterus chinensis. Asia-Pacific Tradit. Med. 2019;15:13–15. doi: 10.11954/ytctyy.201906005. [DOI] [Google Scholar]
- Zhao M.X., Zhang X.Y., Liu S.H., He M.Q., Liang Y., Hao X.Y., Zhou W. Pharmacognostic identification and hemostatic activity of Toddalia asiatica root bark. Chin. J. Exp. Tradit. Med. Formulae. 2016;221:32–36. doi: 10.13422/j.cnki.syfjx.2016240032. [DOI] [Google Scholar]