Abstract
Background
Little is known about diffuse glioma patients infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2).
Methods
We performed a descriptive and retrospective analysis of 41 diffuse glioma patients with symptomatic SARS-CoV2 infection during the first wave of the COVID-19 pandemic.
Results
Confusion with or without fever was the most common neurological symptom (32%) supporting SARS-CoV2 testing in glioma patients with acute and unexplained confusion. Sixteen patients (39%) died after a median delay of 13 days. While multiple clinical, biological, and pathological features, COVID-19- or diffuse glioma-related, at hospital admission appeared to have a pejorative prognostic impact, none was significantly associated with death. Oncological treatments were interrupted at COVID-19 diagnosis and re-initiated with a median delay of 30 days after the end of COVID-19 symptoms.
Conclusions
Interestingly, our retrospective study describes for the first time the characteristics of a cohort of diffuse glioma patients with symptomatic COVID-19. Diffuse glioma patients with poorly symptomatic COVID-19 did not come to the attention of physicians and were not enrolled in the study skewing the denominator for prognostic analysis. Further studies are warranted to specify prognosis of overall population of diffuse glioma patients with COVID-19, including asymptomatic patients, and interactions of prognostic factors of both COVID-19 and diffuse gliomas.
Keywords: COVID-19, diffuse glioma, outcome, prognosis, SARS-CoV2
Key Points.
Unexplained confusion in diffuse glioma patient might reveal COVID-19 supporting SARS-CoV2 testing.
Mortality of diffuse glioma patients with COVID-19 seems high, though more evidence is needed.
Further studies are warranted to determine prognostic factors of COVID-19 in glioma patients.
Importance of the Study.
This descriptive and retrospective study aims to shed light on characteristics of diffuse glioma patients with symptomatic COVID-19 during the first wave of the pandemic. There is little information on clinical, biological, and pathological features and outcome of this subpopulation of cancer patients when infected by SARS-CoV2. Our study is the first cohort of diffuse glioma patients with COVID-19 and supports further studies.
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) emerged at the end of 2019 and spread rapidly throughout the world. SARS-CoV2 primarily targets the respiratory systemCITATION Asa20 \l 2058.1 The most common symptoms are fever (60%–98%), cough, fatigue, headaches, and dyspnea CITATION Hui20 \l 2058.2 In addition to respiratory symptoms, SARS-CoV2 is also able to affect multiple organs including kidneys, skin, eyes, and central nervous system (CNS)CITATION Mic20 \l 20583 CITATION Koy20 \l 20584 CITATION Lin20 \l 2058.5 Death has been reported in approximately 1% of cases and is mainly due to respiratory failure CITATION Hua20 \l 2058.6 The main prognostic factors of COVID-19 are: (i) old age, (ii) male gender, and (iii) co-morbidities CITATION Zho20 \l 2058.7
In cancer patients, the risk of severe infections could be increased due to steroids and anti-cancer treatment-induced immunosuppression CITATION JYo16 \l 20588; therefore, these patients may have a poorer prognosis in the setting of infectious diseases. In a study, including 1590 COVID-19 patients, 18 had a medical history of cancer. These patients had a higher risk of lethal COVID-19, especially if they received anti-cancer treatments within 1 month before the infectionCITATION Wen20 \l 2058.9 Therefore, multiple recommendations have been set up in hospitals to reduce exposure of cancer patients to: (i) immunosuppression including anti-cancer treatments and, (ii) hospital environment enriched for SARS-CoV2 CITATION Lin201 \l 2058.10 However, a more recent prospective cohort study suggested that mortality in cancer patients with COVID-19, estimated at 28–29%, is mainly predicted by age, gender, and co-morbidity. In contrast, cancer treatments do not appear to influence the outcome CITATION Len201 \l 205811 CITATION Ast201 \l 2058.12
Little is known about the impact of COVID-19 in diffuse glioma patients in terms of symptoms and prognosis. Therefore, in the Coco Neurosciences study group setting, we have conducted this retrospective study to describe clinical manifestations and outcome of diffuse glioma patients infected by SARS-CoV2 during the first wave of the pandemic in France.
Material and Methods
Patients
CoCo Neurosciences is an observational study based on data from medical records. Patients received written information about their participation and agreed to use their medical data following the French legislation. The study was sponsored by Assistance Publique-Hôpitaux de Paris and received approval from the Sorbonne Université Ethics Committee (CER-202028 on 24/04/2020). The study was registered on the clinicaltrial.gov website (NCT04362930).
We considered diffuse glioma patients enrolled in the Coco Neurosciences study (medRxiv 2020.10.21.20216747; doi: https://doi.org/10.1101/2020.10.21.20216747). This cohort enrolls patients with neurological or psychiatric disorders, diagnosed with COVID-19 and who came to the attention of physicians participating the study. During the first wave of the pandemic mainly patients with symptomatic COVID-19 were tested and diagnosed. In addition, we assume that only a subpopulation of these patients came to the attention of the physicians participating to the study for enrollment in the study. In parallel, most of them were also enrolled in the GCO-002 CACOVID-19 study CITATION Ast201 \l 2058,12 which is a cohort of patients with active cancer and diagnosed with SARS-CoV2 infection.
COVID-19 infection was defined by a positive SARS-CoV2 RT-PCR analysis after nasopharyngeal swab or by a chest CT scan highly suggestive of COVID-19 (i.e., multiple ground-glass abnormalities with crazy paving, absence of either lymphadenopathy or nodules). For each patient, the following parameters were collected at COVID-19 diagnosis using a standardized de-identified form: (i) clinical parameters: age, sex, body mass index (BMI), medical history, Karnofsky performance score (KPS), general symptoms, change in neurological symptoms, (ii) tumor and oncological characteristics: histologic type according to the WHO 2016 classification, date of the last oncological treatment, steroid treatment over the last month before COVID-19 diagnosis, oncological response according to Response Assessment in Neuro Oncology Criteria (RANO), (iii) biological parameters: complete blood cell count, blood chemical analysis, C-reactive protein (CRP), and (iv) both outcomes for COVID-19 and diffuse glioma CITATION Wen10 \l 2058.13
The severity of COVID-19 was determined using the following classification CITATION COV \l 205814: (i) asymptomatic: molecular diagnosis of SARS-CoV2 but no symptoms, (ii) mild illness: symptoms of COVID-19 without respiratory impact or abnormal chest imaging, (iii) moderate illness: clinical of radiological lower respiratory disease and a saturation of oxygen (SpO2) ≥94% on room air at sea level, (iv) severe illness: respiratory frequency >30 breaths per minute, SpO2 <94% on room air at sea level, the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg or, lung infiltrates >50%, and (v) critical illness: respiratory failure and/or multiple organ dysfunction.
Statistical Analysis
Statistical analysis was performed using SPSS version 20.0. Categorical variables were presented as frequencies and percentages, and continuous variables as medians and ranges. We performed the Wilcoxon or Student’s test according to the distribution of a variable, the P-value was considered significant if below .05.
Results
Characteristics of Patients and Covid-19
From December of 2019 to May 2020, we identified 41 diffuse glioma patients diagnosed with COVID-19, being 35 symptomatic and 6 asymptomatic at diagnosis. All were enrolled in the Coco Neurosciences cohort.
The RT-PCR test with nasopharyngeal swab was positive in 38 cases (93%). Chest CT-scan was consistent with the diagnosis of COVID-19 in 18 patients (44%); only 1 patient had a suggestive CT scan of SARS-CoV2 but a negative RT-PCR, a fibroscopy for pneumocystis carinii was performed to rule out this diagnosis. The serological test was positive in 6 patients (15%), although it was not performed in all cases (Table 1).
Table 1.
Patients and Tumors Characteristics
| Variables | N = 41* | ||
|---|---|---|---|
| Gender | Male | 25 | 61% |
| Age | 64* | (25–89)* | |
| Body mass index | 25* | (16–38)* | |
| Karnofsky Performance Status | pre-COVID19 (%) | 60 | (40–100) |
| Undetermined | 2 | 5% | |
| <60 | 15 | 37% | |
| 60–80 | 16 | 39% | |
| >80 | 8 | 20% | |
| Type of glioma | Glioblastoma (IDHmt) | 32 | 78% |
| Grade III Astrocytoma | 6 | 15% | |
| Grade III Oligodendroglioma | 2 | 5% | |
| Grade II Oligodendroglioma | 1 | 2% | |
| Comorbidities | Diabetes mellitus | 5 | 12% |
| Hypertension | 9 | 22% | |
| Cancer (excluding glioma) | 9 | 22% | |
| Rheumatologic | 5 | 12% | |
| Cardiac disease | 4 | 10% | |
| Metabolic | 5 | 12% | |
| Vascular | 5 | 12% | |
| Endocrinologic disease (excluding diabetes) | 4 | 10% | |
| Neurologic disease (excluding glioma) | 3 | 7% | |
| Hepatic | 2 | 5% | |
| Lung | 3 | 7% | |
| Digestive | 2 | 5% | |
| Chronic kidney failure | 2 | 5% | |
| Positive RT-PCR | 38 | 93% | |
| Positive Chest CT scan | 18 | 44% | |
| Serology positive | 6 | 15% | |
| Steroid Treatment | 31 | 76% | |
| Oncological treatment within 1 month prior to COVID-19 | Surgery | 1 | 2% |
| Chemotherapy | 24 | 59% | |
| Radiotherapy | 3 | 7% | |
| Status of tumor | Stable disease | 14 | 34% |
| Complete response | 0 | 0% | |
| Partial response | 3 | 7% | |
| Progressive disease | 18 | 44% | |
| Not specified | 6 | 15% | |
| Line of treatment | Newly diagnosed/First | 22 | 54% |
| Second | 12 | 29% | |
| Third | 4 | 10% | |
| Remission | 2 | 5% | |
| Palliative care | 1 | 2% | |
| In-patient care | 33 | 81% | |
| Impact of COVID-19 on oncological treatment | Oncological treatment delayed | 21 | 51% |
| Oncological treatment not delayed | 7 | 17% | |
| Patients monitored without oncological treatment | 13 | 32% |
*Data are presented as n (%), median, IQR as appropriate.
Most patients were males (25 cases, 61%). The median age at COVID-19 diagnosis was 63 years (25–89). The KPS was 60% or below in 15 patients (37%). The median BMI was 24 kg/m2 (16–38). Analysis of medical history showed diabetes mellitus, arterial hypertension, cardiac disease, and other chronic diseases in 5 (12%), 9 (22%), 4 (10%), and 11 (27%) patients, respectively. Overall, 24 patients (59%) had at least one co-morbidity.
Thirty-five patients (85%) experienced clinical symptoms as the initial manifestation of the disease, including 13 with systemic symptoms and 22 with both systemic and neurological signs. The most frequent systemic symptoms were fever, fatigue, cough, and dyspnea in 26 (63%), 15 (37%), 14 (34%), and 9 (22%) patients, respectively. Regarding neurological symptoms, confusion was reported in 15 cases (37%). Confusion was never an isolated presenting symptom and was most often associated with fever and fatigue. Headaches and vertigo/dizziness were reported in 3 (7%) and 2 (5%) patients, respectively. Thirteen patients had no modification of their clinical neurological status due to COVID-19 compared to the pre-COVID-19 period (Table 2). Six patients (15%) were asymptomatic.
Table 2.
New Clinical Symptoms Related to COVID-19
| N | % | ||
|---|---|---|---|
| Systemic | Fever | 26 | 63 |
| Unusual Fatigue | 15 | 37 | |
| Myalgia/arthralgia | 1 | 2 | |
| Respiratory | Cough | 14 | 34 |
| Sputum | 1 | 2 | |
| Rhinorrhea | 2 | 5 | |
| Dyspnea | 9 | 22 | |
| Tachypnea | 2 | 5 | |
| Desaturation | 5 | 12 | |
| Chest pain | 1 | 2 | |
| Digestive | Abdominal pain | 3 | 7 |
| Nausea / vomit | 3 | 7 | |
| Diarrhea | 1 | 2 | |
| Neurologic | Headache | 3 | 7 |
| Confusion | 15 | 37 | |
| Dysgeusia | 1 | 2 | |
| Dizziness / vertigo | 2 | 5 | |
| Anosmia | 1 | 2 | |
| Neurological clinical worsening compared to pre-COVID 19 period | 28 | 68 | |
| Asymptomatic | 6 | 15 |
Thirty-three (81%) patients were hospitalized because of extra-neurological and/or neurological symptoms (Table 1). Mild, moderate, severe, and critical forms of COVID-19 at admission were observed in 11, 20, 3 and, 1 patient, respectively. Three patients required oxygen and 1 patient was hospitalized in the intensive care unit (ICU).
At the end of data collection, 16 patients died (39%) due to respiratory complications of COVID-19 with a median delay of 12.7 days (range, 1–43 days) after COVID-19 diagnosis. During the COVID-19 course, 8 patients, including those referred at admission, were admitted to the ICU. In these 8 patients, the brain tumor was glioblastoma, grade III oligodendroglioma and grade III astrocytoma in 6, 1, and 1 patient(s), respectively. About oncological treatments, 5 patients were in first-line, 2 in second-line, and 1 in third-line treatment. Twenty-five patients survived COVID-19, 19 patients were alive at 2 months after diagnosis, and 6 patients died due to glioma progression.
Diffuse Glioma Course
The most frequent neuro-oncological diagnosis was glioblastoma in 32 cases (78%): (i) 29 IDH wild-type (IDHwt) and (ii) 3 IDHR132H positive. For the remaining patients, 6 suffered from grade III IDHwt astrocytoma (15%), one from grade II 1p/19q-codeleted IDH-mutant oligodendroglioma (2%), and 2 from grade III 1p/19q-codeleted IDH-mutant oligodendroglioma (5%) (Table 1). At COVID-19 diagnosis and according to RANO criteria, 3 (7%), 14 (34%), and 18 (44%) patients showed a partial response, stable, and progressive tumor disease, respectively. Over the month before COVID-19 diagnosis, 1 patient underwent glioma surgery, 24 patients received chemotherapy, 3 patients received brain radiotherapy, and 13 patients did not receive any anti-tumor treatment (Table 1).
Thirty-one patients (76%) were treated with steroids due to glioma-related symptoms, with a median equivalent-dose of prednisone of 45 mg per day, for at least 16 days during the month before the COVID-19 diagnosis.
Concerning tumor-related treatments, 22 patients (54%), 12 (29%), and 4 (10%) were in the first-line treatment, second-line treatment, and third-line treatment, respectively. Two patients (5%) were under surveillance and 1 patient (2%) was in palliative care.
Only 1 patient was considered to die due to glioma progression during the period of COVID-19 infection.
Clinical Outcome and Correlations
Although not statistically significant, COVID-19 surviving patients were younger. Also, they had fewer co-morbidities, higher KPS, lower BMI, a lower dose of steroids, lower CRP value, higher lymphocyte count, lower neutrophil count, and higher platelet count. They also exhibited less advanced and less progressive disease, according to RANO criteria. Finally, they were out of oncological treatment the month before the COVID-19 diagnosis compared to patients who have succumbed (Table 3). There was no difference between surviving and dead patients for the glioma histological type and severity of COVID-19 at admission (Table 3).
Table 3.
Characteristics of Surviving Versus Dead Patients
| Survivors (N = 25) | Non survivors (N = 16) | P value | ||||
|---|---|---|---|---|---|---|
| Clinical features | Age | 61.0 | (25–87) | 67.4 | (47–89) | NS |
| BMI | 23.8 | (16.1–37.6) | 25.6 | (20.2–35.2) | NS | |
| KPS pre COVID-19 | 70 | (40–100) | 60 | (40–90) | NS | |
| Diabetes | 2 | (8%) | 3 | (18%) | NS | |
| Arterial hypertension | 5 | (21%) | 4 | (24%) | NS | |
| Cardiac disease | 1 | (4%) | 3 | (18%) | NS | |
| Other comorbidity | 6 | (24%) | 5 | (31%) | NS | |
| At least one comorbidity | 13 | (52%) | 11 | (69%) | NS | |
| Treatment within1 month pre-COVID | Steroid treatment | 17 | (68%) | 14 | (88%) | NS |
| Dose equivalent prednisone(mg) | 38.0 | (7.5–80) | 52.5 | (10–120) | NS | |
| Chemotherapy | 13 | (52%) | 11 | (69%) | NS | |
| Radiotherapy | 2 | (8%) | 1 | (6%) | ||
| Surgery | 1 | (4 %) | 0 | (0%) | ||
| No treatment | 7 | (29%) | 4 | (24%) | ||
| Oncological treatment | Not specified | 10 | (42%) | 2 | (12%) | NS |
| Newly diagnosed/First line | 6 | (24%) | 8 | (50%) | ||
| Second line | 6 | (25%) | 2 | (12%) | ||
| Third line | 2 | (8%) | 2 | (12%) | ||
| Surveillance | 0 | (0%) | 2 | (12%) | ||
| Palliative care | 1 | (4%) | 0 | (0%) | ||
| Tumor status pre-COVID (RANO) | Partial response | 1 | (4%) | 2 | (12%) | NS |
| Stable disease | 9 | (36%) | 5 | (31%) | ||
| Progressive disease | 9 | (36%) | 9 | (56%) | ||
| Not specified | 7 | (30%) | - | - | ||
| Biopathological features | Grade II Oligodendroglioma | 0 | 1 | (6%) | NS | |
| Grade III Astrocytoma | 4 | (17%) | 2 | (12%) | ||
| Grade III Oligodendroglioma | 1 | (4%) | 1 | (6%) | ||
| Glioblastoma | 20 | (80%) | 12 | (75%) | ||
| C-Reactive Protein mg/L | 36.4 | (0–166) | 71.7 | (0–182) | NS | |
| Lymphocytes (Count, x10 9/L) | 1.66 | (0.3–14.4) | 0.89 | (0.40–2.31) | NS | |
| Neutrophils (Count, x10 9/L) | 3.43 | (0.2–8.01) | 5.80 | (2.43–9.97) | NS | |
| Platelets (Count, x10 9/L) | 143.4 | (4–247) | 126.1 | (44–221) | NS | |
| COVID-19 infection severity at admission | Not specified | 2 | (8%) | 2 | (12%) | NS |
| Mild | 8 | (33%) | 3 | (18%) | ||
| Moderate | 10 | (42%) | 10 | (59%) | ||
| Severe | 2 | (8%) | 1 | (6%) | ||
| Critical | 1 | (4%) | 0 | (0%) | ||
| ICU admission | 3 | (12%) | 5 | (31%) | NS | |
| Duration of COVID-19 | 20.8 | (1–63) | 12.6 | (1–43) | NS |
NS, not statistically significant.
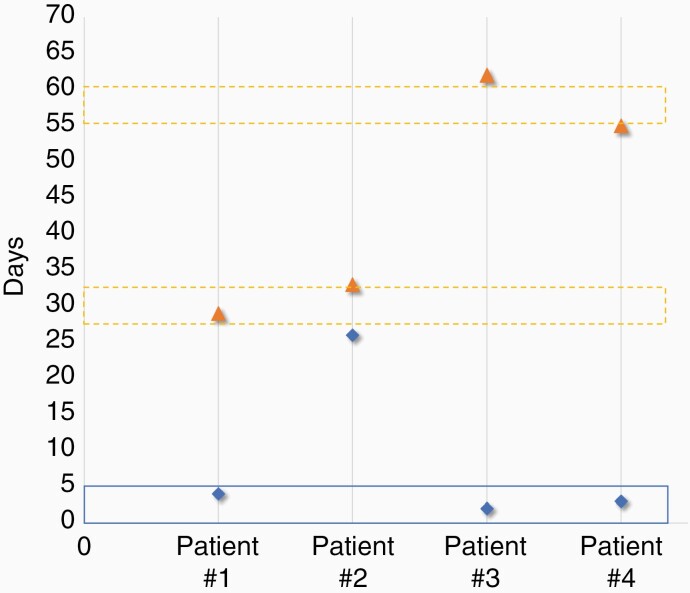
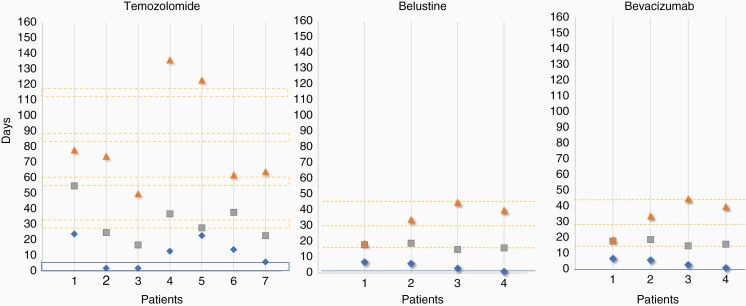
Among the 25 patients surviving COVID-19, tumor status was available in 22 patients. Ten patients (40%) showed stable disease and 11 (44%) showed progressive disease, only 1 patient showed a partial response (4%). About the time elapsed between COVID-19 and treatment continuation, there was a median interruption of treatment of (i) 25 days after COVID-19 diagnosis in asymptomatic (0.1–6 weeks) and (ii) 30 days after the end of COVID-19 symptoms in symptomatic patients (3–9 weeks; Figures 1 and 2).
Figure 1.
Oncological treatments delay in COVID-19 asymptomatic patients. The x-axis indicates the patients. All were treated with Temozolomide. The y-axis indicates the time in days. The blue diamond indicates the date of COVID-19 diagnosis. The blue line indicates the last chemotherapy cycle. The broken orange line indicates the theoretical date of the next chemotherapy cycle. The orange triangle indicates the first day of chemotherapy after COVID-19.
Figure 2.
Oncological treatments delay in COVID-19 symptomatic patients. The x-axis indicates the patients. Chemotherapy agents are indicated on the top of the panels. The y-axis indicates the time in days. The blue diamond indicates the date of COVID-19 diagnosis. The grey square indicates the clinical recovery of COVID-19. The blue line indicates the last chemotherapy cycle. The broken orange line indicates the theoretical date of the next chemotherapy cycle. The orange triangle indicates the first day of chemotherapy after COVID-19.
Discussion
The COVID-19 pandemic rapidly became a worldwide public health emergency due to its spread and respiratory morbidity and mortality overwhelming health care systems CITATION Wor20 \l 2058.15
The prognosis of COVID-19 patients is variable across countries. The WHO estimates the case fatality ratio (CFR) from less than 0.1% to over 25% CITATION Wor202 \l 2058.16 Multiple unfavorable prognostic factors have been identified in the general populationCITATION Moh20 \l 205817CITATION Ebo \l 2058.18 Cancer and oncological treatments are debated as risk factors of increased death in COVID-19 patients CITATION Vas20 \l 205819 CITATION Osa20 \l 2058.20 Consistent with this, little is known about the impacts of COVID-19 in diffuse glioma patients, in terms of clinical manifestations and outcome, supporting our retrospective descriptive study focused on this population. It is worth noting that the vast majority of our diffuse glioma patients with COVID-19 enrolled in our study developed symptoms at the beginning of SARS-CoV2 infection.
Our study suggests that acute/subacute confusion is a frequent neurological presenting symptom of COVID-19 (n = 15 patients, 37%) in diffuse glioma patients. When it is associated with fever (n = 12, 29%), this might reveal COVID-19 supporting urgent SARS-CoV2 testing. In non-diffuse glioma patients, confusion seems less frequent ~ 27% CITATION Ray21 \l 2058.21 Confusion is the second symptom revealing COVID-19 in diffuse glioma patients while it is the sixth in the general population CITATION Mau20 \l 2058.22 Other CNS complications related to SARS-CoV2 infection, including vasculopathy and encephalitis, were not detected in our cohort based on the MRI performed for tumor monitoring CITATION Cat20 \l 2058.23
The mortality rate in our cohort of diffuse glioma patients infected by SARS-CoV2 seems high (39%) compared to general cancer (28%–29%) and the non-cancer population. Our study with multiple limitations and several methodological biases does not allow robust final conclusions. Our findings, based on a retrospective study associated with missing data, cannot be extended elsewhere and should be taken with caution. Indeed, the limited number of patients enrolled in our study reduces its statistical power. The vast majority of diffuse glioma patients enrolled in the current study were symptomatic and/or hospitalized for COVID-19. The poorly symptomatic or asymptomatic diffuse glioma patients infected by SARS-CoV2 did not come to the attention of physicians and were not enrolled in the current study. Therefore, the denominator, that does not include poorly or asymptomatic patients, is skewed and mortality rate is overestimated and should be taken with caution.
In addition, most diffuse glioma patients aged over 50 years old suffered from well-known unfavorable prognostic factors of COVID-19. Compared to the 1289 solid cancer patients with COVID-19, our diffuse glioma patients with COVID-19 have more co-morbidities (59% vs 22% have at least one co-morbidity) and have higher BMI (25 vs 24). Therefore, although overestimated, the potential severity of COVID-19 in diffuse glioma patients could be related to the combination of multiple factors, although none of them, taken individually, reached statistical significance in our study. Unfortunately, some of them were not available for investigations in our cohort (eg, ferritin, IL-6, lactate dehydrogenase, experimental medications for COVID-19) reducing our prognostic analysis.
Due to limited ICU resources, the triage of patients as a factor of the high death rate of diffuse glioma patients with COVID-19 is difficult to interrogate. However, the observation that 20% of patients were admitted to ICU at some point of the course of their COVID-19 does not argue strongly for a systematic brake to the admission of diffuse glioma patients with COVID-19 in ICU whenever needed. This point needs to be addressed specifically in further studies dedicated to diffuse glioma patients.
In surviving patients, the median interruption of oncological treatments was approximately 4 weeks after diagnosis in asymptomatic patients and 4 weeks after COVID-19 clinical recovery in symptomatic patients. At this point, we do not have enough evidence for robust recommendations. The duration of oncological treatment interruption needs to personalize for each patient.
In the era of the COVID-19 pandemic, diffuse glioma patients with COVID-19 should be managed with: (i) strict respect of prevention measures, (ii) adapted oncological treatments, and (iii) appropriate extra-neurological management. Strict respect of WHO prevention measures include face mask, hand washing, physical distancing, and keeping rooms well ventilated. Adapted oncological treatments include standard of care paying attention to: (i) limit hospital visits, (ii) deliver optimal and personalized chemotherapy and steroid dosages considering the balance benefit/risk in the setting of the pandemic, and (iii) interrupting oncological treatments for only a limited period. Finally, appropriate systemic management including admission in ICU should be personalized to each patient regarding prognostic factors of both diffuse glioma and COVID-19.
In conclusion, our study describes for the first-time the characteristics of diffuse glioma patients with symptomatic COVID-19 during the first wave of the pandemic. Additional studies, including a larger number of patients, are warranted to specify our findings and to interrogate outcome of the overall population diffuse glioma patients with symptomatic and asymptomatic COVID-19 over the different waves of the pandemic.
Acknowledgments
The Cohort COVID-19 Neurosciences (CoCo Neurosciences) study supported by the APHP and funded by the generous support of the FIA (Fédération Internationale pour l’Automobile) Foundation and donors of the Paris Brain Institute – ICM. The authors thank the CoCo-Neurosciences study group for their participation in the data collection. The research leading to these results received funding from the program “Investissements d’avenir” ANR-10- IAIHU-06.
Steering Committee (Pitié-Salpêtrière Hospital, Paris): Cecile Delorme, Jean-Christophe Corvol, Jean-Yves Delattre, Stephanie Carvalho, Sandrine Sagnes. Scientific Committee (Pitié-Salpêtrière Hospital, Paris): Bruno Dubois, Vincent Navarro, Celine Louapre, Tanya Stojkovic, Ahmed Idbaih, Charlotte Rosso, David Grabli, Ana Zenovia Gales, Bruno Millet, Benjamin Rohaut, Eleonore Bayen, Sophie Dupont, Gaelle Bruneteau, Stephane Lehericy, Danielle Seilhean, Alexandra Durr, Aurelie Kas, Foudil Lamari, Marion Houot, Vanessa Batista Brochard. Principal investigators: Pitié-Salpêtrière Hospital (Paris): Sophie Dupont, Catherine Lubetzki, Danielle Seilhean, Pascale Pradat-Diehl, Charlotte Rosso, Khe Hoang-Xuan, Bertrand Fontaine, Lionel Naccache, Philippe Fossati, Isabelle Arnulf, Alexandra Durr, Alexandre Carpentier, Stephane Lehericy, Yves Edel; Foch Hospital (Suresnes): Anna Luisa Di Stefano; Rothschild Hospital (Paris): Gilberte Robain, Philippe Thoumie; Avicenne Hospital (Bobigny): Bertrand Degos; Sainte-Anne Hospital (Paris): Tarek Sharshar; Saint-Antoine Hospital (Paris): Sonia Alamowitch, Emmanuelle Apartis-Bourdieu, Charles-Siegried Peretti; Saint-Louis Hospital (Paris): Renata Ursu; Tenon Hospital (Paris): Nathalie Dzierzynski; Charles Foix Hospital (Ivry): Kiyoka Kinugawa Bourron, Joel Belmin, Bruno Oquendo, Eric Pautas, Marc Verny. Co-investigators: Pitié-Salpêtrière Hospital (Paris): Cecile Delorme, Jean-Christophe Corvol, Jean-Yves Delattre, Yves Samson, Sara Leder, Anne Leger, Sandrine Deltour, Flore Baronnet, Ana Zenovia Gales,Stephanie Bombois, Mehdi Touat, Ahmed Idbaih, Marc Sanson, Caroline Dehais, Caroline Houillier, Florence Laigle-Donadey, Dimitri Psimaras, Agusti Alenton, Nadia Younan, Nicolas Villain, David Grabli, Maria del Mar Amador, Gaelle Bruneteau, Celine Louapre, Louise-Laure Mariani, Nicolas Mezouar, Graziella Mangone, Aurelie Meneret, Andreas Hartmann, Clement Tarrano, David Bendetowicz, Pierre-François Pradat, Michel Baulac, Sara Sambin, François Salachas, Nadine Le Forestier, Phintip Pichit, Florence Chochon, Adele Hesters, Bastien HerlinAn Hung Nguyen, Valerie Procher, Alexandre Demoule, Elise Morawiec, Julien Mayaux, Morgan Faure, Claire Ewenczyk, Giulia Coarelli, Anna Heinzmann, Perrine Charles, Tanya Stojkovic, Marion Masingue, Guillaume Bassez, Vincent Navarro, Isabelle An, Yulia Worbe, Virginie Lambrecq, Rabab Debs, Esteban Munoz Musat, Timothee Lenglet, Virginie Lambrecq, Aurelie Hanin, Lydia Chougar, Nathalia Shor, Nadya Pyatigorskaya, Damien Galanaud, Delphine Leclercq, Sophie Demeret, Benjamin Rohaut, Albert Cao, Clemence Marois, Nicolas Weiss, Salimata Gassama, Loic Le Guennec, Vincent Degos, Alice Jacquens, Thomas Similowski, Capucine Morelot-Panzini, Jean-Yves Rotge, Bertrand Saudreau, Bruno Millet, Victor Pitron, Nassim Sarni, Nathalie Girault, Redwan Maatoug, Ana Zenovia Gales, Smaranda Leu, Eleonore Bayen, Lionel Thivard, Karima Mokhtari, Isabelle Plu; Sainte-Anne Hospital (Paris): Bruno Gonçalves; Saint-Antoine Hospital (Paris): Laure Bottin, Marion Yger; Rothschild Hospital (Paris): Gaelle Ouvrard, Rebecca Haddad; Charles Foix Hospital (Ivry): Flora Ketz, Carmelo Lafuente, Christel Oasi. Other Contributors: Associated centers (Lariboisière Hospital, Paris): Bruno Megabarne, Dominique Herve; Clinical Research Associates (ICM, Pitié-Salpêtrière Hospital, Paris): Haysam Salman, Armelle Rametti-Lacroux, Alize Chalançon, Anais Herve, Hugo Royer, Florence Beauzor, Valentine Maheo, Christelle Laganot, Camille Minelli, Aurelie Fekete, Abel Grine, Marie Biet, Rania Hilab, Aurore Besnard, Meriem Bouguerra, Gwen Goudard, Saida Houairi, Saba Al-Youssef, Christine Pires, Anissa Oukhedouma, Katarzyna Siuda-Krzywicka, Tal Seidel Malkinson; (Saint-Louis Hospital, Paris): Hanane Agguini; (Foch Hospital, Suresnes): Hassen Douzane; Data Manager (ICM, Paris): Safia Said; Statistician (ICM, Paris): Marion Houot.
Funding
The study was founded by the Assistance Publique-Hôpitaux de Paris.
Conflict of interest statement. There is no conflict of interest between the authors.
Authorship Statement. Analysis and interpretation: F.L.S., R.U., A.I., F.D., A.L.D.S., J.Y.D., N.Y., and M.T. Data analysis: F.L.S., R.U., and A.I. Data recompilation: F.L.S., R.U., A.I., F.D., A.L.D.S., M.G., H.A., C.B., L.G., and A.F.C.
References
- 1.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge H, Wang X, Yuan X, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng Kee Kwong KC, Mehta PR, Shukla G, Mehta AR. COVID-19, SARS and MERS: a neurological perspective. J Clin Neurosci. 2020;77:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157–76, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng L, Zagorac S, Stebbing J. Managing patients with cancer in the COVID-19 era. Eur J Cancer. 2020;132:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lièvre A, Turpin A, Ray-Coquard I, et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer. 2020;141:62–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed August 2020. [PubMed] [Google Scholar]
- 15.Worl Health Organization. 2020. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed July 2020.
- 16.World Health Organization. 2020. Estimating mortality from COVID-19. Available at: https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19. Accessed August 4, 2020.
- 17.Parohan M, Yaghoubi S, Seraji A, et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;23(5):1416–1424. [DOI] [PubMed] [Google Scholar]
- 18.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelis V, Tippu Z, Joshi K, et al. Defining the true impact of coronavirus disease 2019 in the at-risk population of patients with cancer. Eur J Cancer. 2020;136:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43(6):452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pranata R, Huang I, Lim MA, Yonas E, Vania R, Kuswardhani RAT. Delirium and mortality in coronavirus disease 2019 (COVID-19) - a systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;95:104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy M, Helfand BKI, Gou RY, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3(11):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassett CE, Gedansky A, Migdady I, Bhimraj A, Uchino K, Cho SM. Neurologic complications of COVID-19. Cleve Clin J Med. 2020;87:729–734. [DOI] [PubMed] [Google Scholar]