Abstract
Introduction
Despite its role in social cognition and affiliative behavior, less is known about the role played by oxytocin in human sexual behavior.
Aim
In the present systematic review, we aimed to find the levels of oxytocin related to human sexual arousal and orgasm.
Methods
We conducted the study according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We performed a systematic search in the principal databases for studies that reported collection of salivary or plasmatic samples, with dosage of oxytocin in relation to sexual activity during induction of sexual arousal and orgasm.
Results
414 articles were obtained. After duplicates removal and the application of pre exclusion criteria, 16 articles were considered eligible and 13 articles were included with a Cohen's k of 0.827. Most of the studies used sexual self-stimulation and collected plasmatic or salivary samples to measure oxytocin. The sexual arousal and orgasm were assessed based on subjective reports.
Main Outcome Measure
The primary outcomes were the oxytocin levels collected during the induction of sexual arousal and orgasm.
Conclusions
Several studies collected only subjective reports about the sexual arousal and the orgasm. Most of the studies found higher levels of oxytocin during the orgasm or ejaculation. Given the sexual arousal evoked by self-stimulation in which sexual fantasies play an important role, it should be possible to postulate for a role of the oxytocin in sexual desire. In particular, we hypothesize a complex role of the oxytocin in the modulation of sexual fantasies and thoughts that are relevant in the sexual desire and help to trigger genital and sexual arousal.
Cera N, Vargas-Cáceres S, Oliveira C, et al. How Relevant is the Systemic Oxytocin Concentration for Human Sexual Behavior? A Systematic Review. Sex Med 2021;9:100370.
Key Words: Sexual Response, Orgasm, Oxytocin, Sexual Arousal
INTRODUCTION
During the last decades, the oxytocin attracted considerable attention from the scientific community due to its role in several cognitive and emotional functions.1 Paraventricular and supra optic nuclei of the hypothalamus synthesize the nonapeptide oxytocin, which is transported in axons to the neurohypophysis, where it is released into the blood circulation to reach remote and peripheral locations.2, 3, 4 Besides to its well-known roles in the stimulation of the uterine tone and contractions during labor and in milk ejection during lactation, it also plays an important role in the modulation of several cognitive and emotional functions most importantly in creating maternal bonding.5, 6, 7 Moreover, oxytocin has been found to be important in the modulation of several aspects of social behavior and in related pathological conditions. It is well documented that oxytocin promotes social cohesion and social contact between the individuals.8, 9, 10 Indeed, alterations in the level of oxytocin have been observed in borderline disorder, autism spectrum disorders and anxiety.11, 12, 13
As stated by Stoléru et al,14 sexual behavior is a complex set of components involving, at specific stages, several brain regions. Sexual behavior and, more specifically, sexual arousal involves autonomic, cognitive and emotional components. Among these, the endocrine component is strongly affected by the cognitive appraisal of the sexual stimulation. In this way, according to the Stoléru's hypothesis14 the cognitive component would be important to stimulate endocrine functions and the release of the sexual and non-sexual hormones.
Evidences from animal studies showed that the administration of oxytocin in the ventral tegmental area of the male rats induced erection and the stimulation of receptors in the meso-limbic dopaminergic pathway was relevant for the reward and drive.15 Interestingly, IsHak et al16 tested with good results intranasal oxytocin administration in a case of male treatment-resistant anorgasmia. In the same way, Muin et al,17 in a Randomized, double-blind, placebo-controlled, crossover trial, involving pre and post –menopausal women, the administration of intranasal oxytocin improved over time sexual function. According to Georgiadis and Kringelback,18 it is possible to frame the sexual behavior in a continuous process consisting of three stages. The “cycle of pleasure” model conceived sexual behavior as a process starting with the drive, followed by excitation or arousal and orgasm, and inhibited by the refractory periods or dysfunctions. The model relates the stages to the activity in specific brain areas, representing an evolution of the well-known model from Masters and Johnson.19 The first stage of the cycle implies a motivated, pro-sexual behavior, conceived as a readiness status for sexual behavior. It has a different connotation from the sexual desire, which represents a more complex process relying on the same brain pathways important for sexual arousal and sexual inhibition.20 Moreover, sexual desire is sexual context –independent.21, 22, 23
Despite the knowledge that sexual development, reproductive life and senescence are under the guidance of hormones, like oxytocin, less is known about their relationship with specific stages of the sexual arousal, desire and orgasm.
Consequently, we performed what we believe to be the first systematic review about the role played by oxytocin levels (plasmatic or salivary) in the male and female sexual behavior. In particular, we were interested in understanding (i) at which stages of sexual behavior previous studies found higher levels of oxytocin. (ii) Which is the best design and the best populations to study the role played by oxytocin in human sexual behavior and (iii) if the levels of oxytocin are related to subjective sexual arousal or to genital sexual arousal.
METHODS
We used the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24
The computer-based literature searches follow the PICO approach with combined terms related to oxytocin, sexual behavior, sexual activity, and sexual function. In particular, our research question was “What is the role played by the oxytocin in sexual behavior as described in the evidence (O) in the healthy population (P)?” The present review was limited to studies that measured the level of oxytocin in body fluids, like saliva or blood (I) during a task involving sexual activities (C). Table 1 shows the search terms have been defined based on the PICO question combined with the Boolean operators like “AND”, “OR” and “NOT”, according to the method that has been previously used in another systematic review.25
Table 1.
Search strategy used in the current systematic review
| Sexual Behavior/VSS 1. Sexual Behavior [Mesh] 2. Sexual activity 3. Sexual function [Title/Abstract] 4. Sexual arousal [Title/Abstract] 5. Visual sexual stimuli 6. Orgasm [Title/Abstract] 7. Ejaculation [Title/Abstract] 8. OR / 1-7 |
Study Design 12. Review [Publication Type] 13. Systematic review [Publication Type]) 14. OR / 12-13 |
| Oxytocin 9. Oxytocin [Title/Abstract] 10. Oxytocin [MeSH] 11. OR / 9-10 |
Combined search 15. #8 AND #11 NOT #14 |
We defined the inclusion and exclusion criteria based on the topic, study design and population (Table 2). Most importantly, concerning the population, we decided to exclude the studies in which neuropsychiatric patients have been included (Table 2). Studies about sexual dysfunctions has been included, given the presence of different oxytocin levels in the patients group could allow to disentangle its role in normal sexual behavior.
Table 2.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Experimental studies
|
Randomized clinical trial Intranasal oxytocin Animals Neuropsychiatric disease |
| Human Adults (> 18 yr.) Visual/audio sexual stimulation Self-stimulation Couple sexual activity |
We performed the computer-based literature search in order to retrieve all the published articles in English regarding the above-mentioned topic. The three principal databases: MEDLINE (PubMed), Scopus and Google Scholar were searched.
After the retrieval, all the studies included (see the inclusion criteria) in the present systematic review were screened in order to identify additional relevant bibliographic items. Moreover, the narrative and systematic publications that were retrieved, but considered off the topic for the outcome of the PICO, have also been screened to find relevant studies cited in the reference lists.
After the duplicate removal, the title and abstracts were manually screened to understand if they fulfilled the inclusion and/or exclusion criteria. After the retrieval of potentially relevant studies to be included, we read the full texts in order to confirm the eligibility.
The first and second author performed independently the literature search, screening and methodological evaluation. The consensus about the different stages was reached between the two authors discussing the results and the articles retrieved. If the consensus was not reached, a third opinion was obtained.
To assess the quality of the studies included in the present systematic review, we applied the “NIH/quality assessment tool for before-after (pre-post) studies with no control group” and the “Quality Assessment of Case-Control Studies”, following the instructions (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools;National Institutes of Health, 2014).26
Information has been extracted from each included study, following the above-mentioned guidelines. In particular, we extracted the characteristics of the participants, including the exclusion and inclusion criteria.
RESULTS
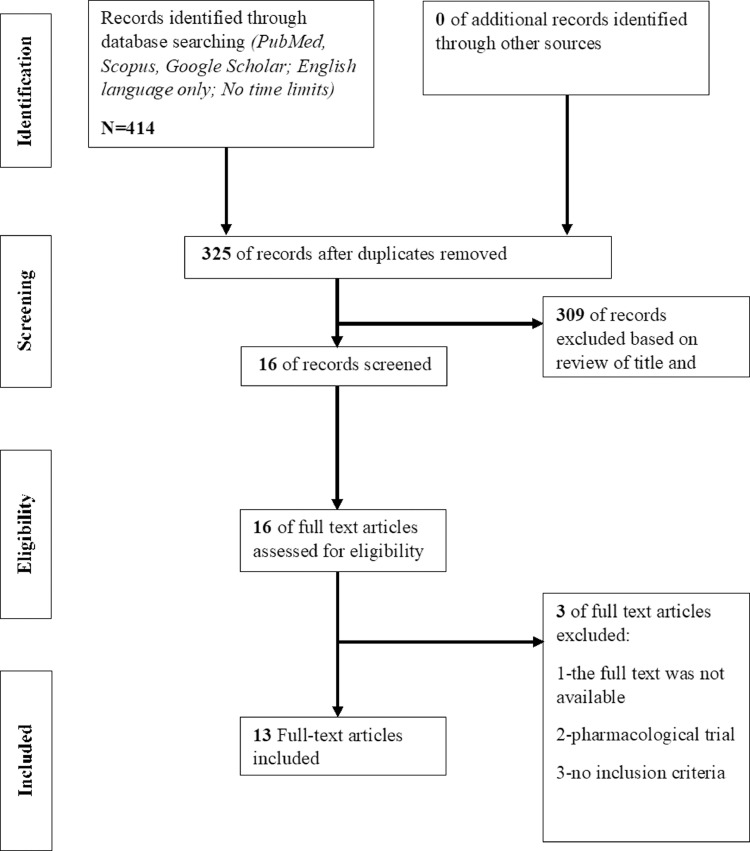
Flowchart (Figure 1) showed the selection process of the studies. After the consensus was reached, 13 published studies have been included in the systematic review. Following the guidelines from Landis & Koch27 we calculated Cohen's k with the 95 % (k = 0.827) indicating almost perfect agreement (https://idostatistics.com/cohen-kappa-free-calculator/).
Figure 1.
Flow diagram for identifying studies in the systematic review.
Tables 3 and 4 show the principal characteristics of the studies. We selected studies published from 1969 until 2019. Most of the included studies were conducted in different countries of Europe (61.5%; n = 8) and 325 subjects have been enrolled in the included studies. Most of the studies were crossover repeated measure studies, only two were cross sectional, longitudinal case control studies and only one a single case study. For this reason, the quality assessment was based on two different checklists. Tables 1S and 2S (supplementary information) showed the quality assessment of the included studies, by means of “NIH/quality assessment tool for before-after (pre-post) studies with no control group” and the “Quality Assessment of Case-Control Studies”, performed by the two above-mentioned reviewers.26 Radioimmunoassay (RIA) was used to determine the levels of oxytocin in 61.5 % of the studies. In most of the cases, the source of the oxytocin determinations has been plasmatic. Six studies also measured other hormones in the collected samples, namely cortisol, vasopressin and FSH, and LH (Table 4). Six studies (46.5%) used clinical assessment, by means of tests, or clinical interviews to evaluate several dimensions, like personality, anxiety and sexual functions of the participants enrolled. Three studies used a multi-task approach in the same study, in order to control confounding variables.
Table 3.
Demographic and psychometric tests applied in the included studies
| Source | Country | N° subjects | Men (%) | Age (yr) | Design | Psychological assessment |
|---|---|---|---|---|---|---|
| Fox et al., 196928 | UK | 1 | 0(0) | N/A | Single case/crossover study | N/A |
| Ogawa et al., 198029 | Japan | 17 | 17(100) | N/A Patients with abnormal spermatogenesis |
Crossover study. | N/A |
| Carmichael et al. 198730 | USA | 22 | 9(40.9) | 21-40 yr. M: Mean = 28 ± 4 S.D. W: Mean = 27.5 ± 4 SD |
Crossover study. correlational study | Psychological Interview MMPI BDI Spielberger Manifest Trait Anxiety Test. |
| Murphy et al.,198732 | UK | 13 | 13(100) | 22-32 yr. | Crossover study. | N/A |
| Carmichael et al. 199431 | USA | 23 | 10 (43.5) | 21-40 yr. M: Mean = 28 W: Mean = 27.5 |
Correlational study | Psychological interview MMPI BDI Spielberger Manifest Trait Anxiety Test. |
| Blaicher et al, 199933 | Austria | 12 | 0 (0) | 23-37yr. |
Crossover study. | Information from clinical history |
| Kruger et al., 200335 | Germany | 10 | 10 (100) | 18-30 yr. (Mean = 25.2 ± 1.21 S.D) |
Crossover study. | Semi-structured interview; Physical examination SIS/SES |
| Uckert et al., 200340 | Germany | 25 | 25(100) | Mean = 26 yr. | Crossover study | N/A |
| Salonia et al., 200541 | Italy | 30 (G1 = 20; G2 = 10) |
0(0) | G1 : 21-43 yr. (Mean = 33.8) G2 : 23-41 yr.(Mean = 32.4) |
Cross-sectional study. correlational study |
Semi structured interview (sexual function and medical history). G1= normally cycling fertile women G2= monophasic contraceptive pill |
| Caruso et al., 201836 | Italy | 31 (G1 = 15; G2 = 16) |
0(0) | G1: 25–34yr.; Mean = 28.8 ± 5.7 S.D G2: 24–35; Mean= 27.1 ± 4.4 S.D . |
Crossover study. | SHI FSFI; FSDS |
| de Jong et al., 201734 | Germany | 17 | 10(58.8) | M:Median = 28yr., 26-65 yr.; W: Median = 29 yr.; 23-52 yr. | Crossover study | N/A |
| Alley et al., 201937 | USA | 63 | 0(0) | 20-35 yr. | Crossover study. | N/A |
| Dickenson et al., 201938 | USA | 61 | 0(0) | 20-35 yr.; Mean = 27.2 yr. | Crossover study. | FFMQ |
BDI = Beck Depression Inventory; FFMQ = Five Facet Mindfulness Questionnaire; FSDS = Female Sexual Distress Scale; FSFI = Female Sexual Function Index; G = group; M = men; MMPI = Minnesota Multiphasic Personality Inventory; N/A = not available; SHI = sexual history interview; SIS/SES = System Inhibition/System Excitation Scale; W = women; yr=years
Table 4.
Tasks, hormonal assessment and Results described in the studies included in the systematic review
| Source | Task | Hormonal assessment | Determinationof hormonallevels | Results |
|---|---|---|---|---|
| Fox et al., 196928 | Couple sexual activity | Plasmatic oxytocin and vasopressin | N/A | Oxytocin was found in both cases in the blood sample taken after female orgasm but no activity was found in any of the samples from the male or any of the controls. |
| Ogawa et al., 198029 | Sexual self-stimulation | Plasmatic oxytocin | RIA | Oxytocin levels increased significantly from 3.1±1.9 pg/ml to 7.0 ± 4.5 pg/ml after ejaculation in 17 males (P < .01). No correlation with sperm count. |
| Carmichael et al. 198730 | Sexual self-stimulation continuing through orgasm /ejaculation | Plasmatic oxytocin collected 5 min. before starting; 5min post |
RIA | Significant levels increase between baseline and orgasm in men and monorgasmic women (P < .05). Significant increase in Multiorgasmic W: baseline =2 pg/ml; 1st orgasm= 2.7 pg/ml (P < .03); 2nd orgasm=3.4 pg/ml; post orgasm= 3.4pg/ml. Oxytocin self-stimulation = W: (3.4 pg/ml)>M: (2.2 pg/ml; P < .05). Oxytocin orgasm = W: (4.4 pg/ml)>M: (2.5 pg/ml; P < .05) |
| Murphy et al., 198732 | arousal induction task:using fantasies | Plasmatic oxytocin and vasopressin | RIA | Oxytocin levels increased at the time of arousal (ns) but increased with ejaculation; it increased from a baseline of 1.4 ± 0.3 to 7.3 ± 2.6 pmol/L (P < .01). The mean plasma level was still significantly elevated 10 min after ejaculation, reaching baseline in 30 min. |
| Carmichael et al., 199431 | Sexual self-stimulation | Plasmatic oxytocin collected at baseline, early SS, mid SS, late SS, orgasm and post orgasm. |
RIA | Oxytocin levels increased from baseline through the orgasm. |
| Blaicher et al, 199933 | Sexual self-stimulation | Plasmatic oxytocin | RIA | Oxytocin level increased in each subject after 1 minute following the orgasm (baseline = 11.53 pg/ml, 1 minutes after orgasm=14.00 pg/ml; P = .0033); Level after 5 Minutes =12.56 pg/ml. |
| Kruger et al., 200335 | Sexual self-stimulation; visual sexual stimulation | Plasmatic oxytocin, epinephrine, norepinephrine, vasopressin, prolactin, FSH, LH, testosterone and cortisol. | IRMA- oxytocin | Orgasm produced an increase in oxytocin plasma levels, which returned to basal values 10 min after orgasm. However, due to a large interindividual variance these alterations did not reach statistical significance (F(10,80)= 1•83, P = .068). |
| Uckert et al., 200340 | Sexual self-stimulation only in the glans; visual sexual stimulation | Plasmatic oxytocin | RIA | Two subjects feel pain during data collection; Two subjects did not terminate the experiment. Oxytocin plasma levels increased in the systemic and cavernous blood at the beginning of sexual arousal during penile tumescence (Corpus Cavernosum: from 66.7 ± 34 to 75 ± 44 pg/ml; Cubital Vein: from 71 ± 41 to 79 ± 49.5 pg/ml). Level of oxytocin increased during rigidity in the cavernous blood (to 81 ± 58.2 pg/ml), but unaltered in the systemic circulation (to 76.4 ± 44 pg/ml). Increase in the systemic blood (to 94±49 pg/ml). |
| Salonia et al., 200541 | N/A | Plasmatic oxytocin and other hormones. | RIA | Oxytocin levels correlated with the FSFI-lubrication subscale during the luteal (P = .007; r = 0.69) phase. Oxytocin correlated with both the arousal (P = .04; r = 0.72) and the lubrication (P = .009; r = 0.84) scales as measured with FSFI during the last week of assumption of the estroprogenistic pill. No significant difference among the mid luteal and mid follicular in FSFI. |
| Caruso et al., 201836 | Couple sexual activity | Plasmatic oxytocin | ELISA | At baseline, anorgasmic women showed lower levels of oxytocin than orgasmic women, 1.8 ± 0.2 pg/mL versus 2.1 ± 0.5 pg/mL, respectively (P < .04). At T1, anorgasmic women showed similar baseline levels of oxytocin. Orgasmic women had higher level of oxytocin than anorgasmic women, (P < .001). |
| de Jong et al., 201734 | Run, breastfeeding and sex tasks (ROC test): sexual self-stimulation | Salivary oxytocin | RIA | 10-min of sexual self-stimulation caused an increase inoxytocin levels (P < .001). Post-hoc comparisons revealed a significant increase of oxytocin concentrations 10 min after the start of sexual self-stimulation (P ≤ .001). No difference after 40 minutes (P = .438). |
| Alley et al., 201937 | audio sexual stimulation: arousal induction task | Salivary oxytocin and cortisol | EIA | No differences in oxytocin levels in response to the arousal task (mean difference = 0.18, t = 0.09, P = .94). Participants showed a significant increase in oxytocin from baseline to task for the arousal assessment (b = 0.11, P= .02), but no significant change from task to either the first recovery (b = 0.02, P= .87) or the second recovery (b = 0.10, P = .46). |
| Dickenson et al., 201938 | audio sexual stimulation: arousal induction task | Salivary oxytocin and cortisol | EIA | Significant random effect (P < .001), indicating that women varied in the extent to which oxytocin changed in response to the sexual arousal induction. No significant change in response to the sexual arousal induction (P > .1). None of the facets of trait mindfulness (FFMQ) moderated the change in oxytocin in response to the sexual arousal induction (P > .2). |
EIA= Enzyme immunoassay method; ELISA= Enzyme-linked immunosorbent assay; FSH=Follicle Stimulating Hormone; G= group; IRMA= Immuno radiometric assay; LH= Luteinizing Hormone; M= Men; RIA= Radioimmunoassay; SS= sexual stimulation; W= Women
The studies did not show complete homogeneity and consistency between results. This is congruent with the use of different samples, tasks and experimental designs.
Among the retrieved studies, most of them examined the oxytocin levels during orgasm and ejaculation. In the first published study.28 the authors showed, for the first time, the presence of oxytocin in the plasma before and after an intercourse between man and woman. Since the pioneer studies.29-33 until the most recent published one,34 all of them showed an increase of the oxytocin levels during, or immediately after, the orgasm or ejaculation. The task used has been the sexual self-stimulation that could be associated to visual sexual stimulation.30, 31, 32,35 Interestingly, most of the studies, assessing orgasm and ejaculation, did not use psychophysiological measures, only based on subjective reports. Carmichael et al31,32 assessed pelvic contractions during the arousal and orgasm by means of an anal device containing a photoplethysmograph. Noteworthy, the authors reported31,32 in first study no significant differences between men and women and, in the second one, significant positive correlations between the systolic pressure, electromyography levels and oxytocin during the baseline, arousal and orgasm or ejaculation. In the same way, Kruger et al35 assessed cardiovascular parameters (heart rate, systolic and diastolic blood pressure) continuously during the task. In particular, oxytocin showed a significant increase in the orgasm in monorgasmic and multiorgasmic women. In multiorgasmic women, the oxytocin level showed an increase between the first and second orgasm.31 Using a different methodological approach, deJong et al34 found an increase in oxytocin salivary levels after 10 minutes of sexual self-stimulation in men and women, without observing a specific gender effect. The studies that used self-stimulation tasks (ie masturbation) showed the highest levels of oxytocin in correspondence or after the orgasm or ejaculation. Interestingly, comparing anorgasmic and orgasmic women, Caruso et al 36 found that before and after the sexual intercourse anorgasmic women showed lower oxytocin levels than orgasmic ones. After coital activity, oxytocin levels did not change in anorgasmic women, which experience unpleasant and stressful sexual activity.
Despite the interest for the orgasm, three studies investigated oxytocin levels during sexual arousal. Two of the three studies involved women and used similar tasks. Alley et al,37 studying the oxytocin and cortisol levels during stress and sexual arousal elicited by audio sexual stimulation, found a significant increase in the oxytocin levels from baseline to arousal. Conversely, a second study38 found interesting results about oxytocinergic involvement in the attention related processes of sexual arousal. They found that women who were able to detect attentional shifts and women who reported greater levels of sexual arousal reported decrease in oxytocin in response to mindful breathing and were the only women to report an increase in oxytocin in response to sexual arousal induction. The study from Dickenson et al38 applied the Five-Facet Mindfulness Questionnaire (FFMQ)39
The third study investigated the relationship between genital arousal and oxytocin in men. Moreover, the interest was about the difference in oxytocin concentration in two different sites. Uckert et al40 collected the blood samples from cubital vein and corpora cavernosa during the different stages of erection form flaccid to rigidity during visual sexual stimulation and manual stimulation of the gland. From flaccidity, considered as the baseline, to tumescence oxytocin levels increased in both cubital vein and corpora cavernosa, but from tumescent to rigidity, it increased only in the corpora cavernosa. Indeed, the oxytocin levels recorded from the corpora cavernosa returned to the baseline, but the levels of oxytocin recorded in the cubital vein did not return to the baseline. Unfortunately, the authors did not report the time in minutes, but they did only reported that the blood collection was in correspondence of the detumescence.
Moreover, according to Salonia et al,41 in normal cycling women, oxytocin varied significantly during the menstrual cycle with significant lower levels during luteal phase and correlated with FSFI lubrification42. They found a significant correlation between the oxytocin levels and both the FSFI arousal 42 (P = .04; r = 0.72) and the lubrification 42 (P = .009; r = 0.84) in women under treatment with estroprogestinic oral contraceptives, during the last week of treatment. Carmichael et al,31 also reported an increase of oxytocin levels during luteal phase, but they did not observe a relationship between menstrual phases and orgasm for oxytocin.
To check the quality of the selected studies, we assessed how many of them reported the level of oxytocin after the task. This is important from a methodological point of view to know if the baseline is recovered after the task. Moreover, this information can help to establish future practices in studies that aim to collect hormonal data during an experimental session. Most of the studies (ten, 76,9%) reported the collection of samples to assess the return to the baseline of oxytocin after the tasks. Five (38,5%) studies indicated that blood or salivary samples having been collected after a period of time of 10-20 minutes following the task or the orgasm having been reached by the participants (Table 5).
Table 5.
Assessment of oxytocin baseline-recovery after tasks
| Source | Time in minutes |
|---|---|
| Fox et al., 196928 | N/A |
| Ogawa et al., 198029 | N/A |
| Carmichael et al. 198730 | 5 |
| Murphy et al.,198732 | 30 |
| Carmichael et al. 199431 | 5 |
| Blaicher et al, 199933 | 5 |
| Kruger et al., 200335 | 10 |
| Uckert et al., 200340 | N/A* |
| Salonia et al., 200541 | N/A⁎⁎ |
| Caruso et al., 201836 | 5 |
| de Jong et al., 201734 | 40⁎⁎ |
| Alley et al., 201937 | 20 |
| Dickenson et al., 201938 | 15-20 |
Uckert et al. collected blood samples after detumescence;
correlational study; ***40 minutes from the onset of the stimulus and 10 after orgasm.
DISCUSSION
Despite the relevance of the oxytocin in the social cognition and emotions, a limited number of studies investigated the role played by the oxytocin levels in human sexual behavior. The present systematic review took into account only studies that assessed the oxytocin levels without considering the randomized clinical trials in which the administration of synthetic oxytocin took place. Given the level of invasiveness of the procedure of collection of the oxytocin, several studies collected only subjective reports about the sexual arousal and the orgasm.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38,40,41 In this way, given the subjective reports, it was not possible to quantify the intensity or the exact moment in which sexual arousal and orgasm occurred. Previous studies used psychophysiological techniques to record the variation of the penile circumference and vasocongestion of the vaginal wall during tasks like visual sexual stimulation.43 Carmichael et al,10 in their pioneer study, recorded the muscle activity in the pubic area by mean of the recording of the anal blood-pulse amplitude and electromyographic activity that could be conceived as indirect measures of genital arousal but could also be useful to record pelvic contraction during orgasm.
Our first aim concerned the stage of sexual behavior in which the reviewed studies found higher levels of releasing oxytocin. Most of the studies found higher levels, or at least peaks of oxytocin levels during the orgasm or ejaculation.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Overall, this seems to be confirmed by indirect evidence about women affected by anorgasmia that showed lower levels than orgasmic women.36 Oxytocin is the hormone responsible for the uterine contractions during the labor 44,45 and it should be conceivable that a similar contractile mechanism during the orgasm is able to release, at the level of the neurohypophysis, higher amount of oxytocin. The role of oxytocin in the ejaculation has been investigated in pharmacological animal studies,46 showing contradictory results in shortening or prolonging the latency of the ejaculation.47 Also in a randomized, double-blind, placebo controlled study in men with premature ejaculation, high doses of Cligosiban, an oxytocin receptor antagonist, did not prolong the time of vaginal coitus and the latency of intravaginal intercourse.48 In this way, oxytocin might not play a direct role in the latency of the erection needed to a satisfactory intravaginal intercourse.
Importantly, reviewing these studies, we are able to hypothesize that besides to the effect in the orgasm, oxytocin also plays a role in the sexual arousal as confirmed by Alley et al38 who observed a significant change between the baseline and arousal during an audio sexual stimulation task in 63 participants. Similarly, Uckert40 found an increase of oxytocin levels in the corpora cavernosa during the tumescence that could reasonably be used as an index of genital arousal. Despite this, the self-stimulation task appears to include a series of sexual fantasies that represent uncontrollable confounding variables, given their level of subjectivity. In this way, responding to our second aim, one of the best options should be the use of a standardized audio and/or visual stimulation task in which it is possible to minimize the effect of uncontrollable confounding variables. At the same time, sexual fantasies, which are considered relevant for sexual desire, can be the trigger for the sexual arousal and orgasm. According to our review and the results obtained in the selected studies, it should be plausible that a mechanism involving empathy and reward could play an important role in these two stages of sexual behavior. Indeed, the studies that used self-stimulation tasks could confirm the hypothesis that sexual desire is a more complex process that goes beyond sexual arousal and orgasm, and it is different from the sexual drive needed to start sexual behavior.23 Oxytocin is primarily produced in the neurohypophysis and, so, a more central mechanism related to sexual or romantic interaction could be hypothesized. In this way, several studies highlighted its role in prosocial and positive emotions.49,50 In particular, oxytocin is important for the neuroendocrine mediation of the romantic love51 playing a role in the processes related to the initial stages of the romantic passion.52 This involvement could be explained in the light of the role played by the oxytocin in empathy, in the reward for positive social interaction53, 54, 55 and the bonding creation.
Not all the studies, with the exception of Carmichael et al,30,31 collected psychophysiological data, but only subjective ratings of sexual arousal (our third aim). Overall, according to the obtained information, the sexual arousal was not assessed with a Likert-like scale, but the participants indicated the exact time in which it occurred. Several studies showed that women’ subjective experiences of arousal are not automatically related to genital changes overall when the vaginal pulse amplitude is assessed56. Despite the self-detection of the sexual arousal could be easier in the male participants, women showed higher concentration levels of oxytocin than men as described by Marazziti et al.57
In summary, the present systematic review was not able to give a definitive response, in particular to determine the contribution of oxytocin in female sexual arousal. Indirectly, Uckert,37 reporting the values of the oxytocin during the penile tumescence allowed a better understanding of the complex role of the oxytocin in male sexual arousal.
CONCLUSIONS
Taking into account the limited number of studies that we were able to collect about the oxytocin levels during the different stages of human sexual arousal, and the heterogeneity in the tasks and population, our hypotheses have to be considered with caution.
However, the present systematic review highlighted the state of the art of the studies that assessed the role played by the oxytocin levels during the different stages of sexual behavior and, in particular, during sexual arousal and orgasm in men and women. In this way, thanks to the results here reported and described, several hypotheses about the role of oxytocin in the attraction, sexual fantasies and positive thoughts, plausibly regarding sexual interaction and intercourse could play an important role in the sexual arousal and in the genital response. To disentangle the role played by the oxytocin in the sexual desire, arousal and orgasm, further studies will be needed in which it should be important to better control the possible confounding variables, define the genital arousal and obtain a precise self-assessment of the sexual arousal as well as the orgasm timing.
According to our review, the future investigation in the area of the relations between the endocrine and the cognitive components underlying human sexual behavior can be promising.
STATEMENT OF AUTHORSHIP
Conceptualization, N.C. and S.V.C.; Methodology, N.C., S.V.C and C.O.; Investigation, N.C., S.V.C, J.M. and D.B.; Writing – Original Draft, N.C. and S.V.C.; Writing – Review & Editing, D. P. and S.R.; Funding Acquisition, N.C, C.O., J.M. and D.P.; Supervision, D. P. and S.R.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This study was funded by the Portuguese Science Foundation (Grant number: FCT-PTDC/PSI-GER/30520/2017; NORTE-01-0145-FEDER-030520).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.esxm.2021.100370.
Appendix. Supplementary materials
References
- 1.Heinrichs M., von Dawans B., Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Carter C.S. Oxytocin and sexual behavior. Neurosci & Biobehav Reviews. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 3.Carter C.S. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Carter C.S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 5.Meinlschmidt G., Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol. Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kendrick K.M., Keverne E.B., Baldwin B.A. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- 7.Porter R.H., Duchamp G., Nowak R. Induction of maternal behavior in non-parturient adoptive mares. Physiol. Behav. 2002;77:151–154. doi: 10.1016/s0031-9384(02)00819-3. [DOI] [PubMed] [Google Scholar]
- 8.Spengler F.B., Scheele D., Marsh N. Oxytocin facilitates reciprocity in social communication. Social Cognitive and Affective Neuroscience. 2017;12:1325–1333. doi: 10.1093/scan/nsx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L., Becker B., Kendrick K.M. Oxytocin facilitates social learning by promoting conformity to trusted individuals. Frontiers in Neuroscience. 2019;13:56. doi: 10.3389/fnins.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young L.J., Flanagan-Cato L.M. Editorial comment: oxytocin, vasopressin and social behavior. Horm. Behav. 2012;61:227. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki T., Hashimoto K., Oda Y. Decreased levels of serum oxytocin in pediatric patients with attention deficit/hyperactivity disorder. Psychiatry Res. 2015;228:746–751. doi: 10.1016/j.psychres.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Brüne M. On the role of oxytocin in borderline personality disorder. Br. J. Clin. Psychol. 2016;55:287–304. doi: 10.1111/bjc.12100. [DOI] [PubMed] [Google Scholar]
- 13.Scheele D., Kendrick K.M., Khouri C. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology. 2014;39:2078–2085. doi: 10.1038/npp.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoléru S., Fonteille V., Cornélis C. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci & Biobehav Reviews. 2012;36:1481–1509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Melis M.R., Melis T., Cocco C. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 16.IsHak W.W., Berman D.S., Peters A. Male anorgasmia treated with oxytocin. J. Sex. Med. 2008;5:1022–1024. doi: 10.1111/j.1743-6109.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 17.Muin D.A., Wolzt M., Marculescu R. Effect of long-term intranasal oxytocin on sexual dysfunction in premenopausal and postmenopausal women: a randomized trial. Fertil. Steril. 2015;104:715–723. doi: 10.1016/j.fertnstert.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Georgiadis J.R., Kringelbach M.L. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog. Neurobiol. 2012;98:49–81. doi: 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Masters W.H., Johnson V.E. Little, Brown.; 1966. Human Sexual Response. [Google Scholar]
- 20.Pfaus J.G. Reviews: pathways of sexual desire. The j. of sexual med. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 21.Laan E., Both S. What makes women experience desire? Feminism & Psychol. 2008;18:505–514. [Google Scholar]
- 22.Brotto L.A. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch. Sex. Behav. 2010;39:221–239. doi: 10.1007/s10508-009-9543-1. [DOI] [PubMed] [Google Scholar]
- 23.Goldey K.L., van Anders S.M. Sexual arousal and desire: interrelations and responses to three modalities of sexual stimuli. The j. of sexual med. 2012;9:2315–2329. doi: 10.1111/j.1743-6109.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Prisma Group Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys. Ther. 2009;89:873–880. [PubMed] [Google Scholar]
- 25.Vargas-Cáceres S., Cera N., Nobre P. The impact of psychosis on sexual functioning: a systematic review. The j. of sexual med. 2021;18:457–466. doi: 10.1016/j.jsxm.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health. (2014). Quality assessment tool for before-after (pre-post) studies with no control group. Systematic evidence reviews and clinical practice guidelines. Washington, DC: National Institutes of Health.
- 27.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Fox C.A., Knaggs G.S. Milk-ejection activity (oxytocin) in peripheral venous blood in man during lactation and in association with coitus. J. Endocrinol. 1969;45:145–146. doi: 10.1677/joe.0.0450145. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa S., Kudo S., Kitsunai Y. Increase in oxytocin secretion at ejaculation in male. Clin. Endocrinol. (Oxf) 1980;13:95–97. doi: 10.1111/j.1365-2265.1980.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael M.S., Humbert R., Dixen J. Plasma oxytocin increases in the human sexual response. The J. of Clin. Endocrinology & Metabolism. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael M.S., Warburton V.L., Dixen J. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch. Sex. Behav. 1994;23:59–79. doi: 10.1007/BF01541618. [DOI] [PubMed] [Google Scholar]
- 32.Murphy M.R., Seckl J.R., Burton S. Changes in oxytocin and vasopressin secretion during sexual activity in men. The J. of Clin. Endocrinology & Metabolism. 1987;65:738–741. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- 33.Blaicher W., Gruber D., Bieglmayer C. The role of oxytocin in relation to female sexual arousal. Gynecol. Obstet. Invest. 1999;47:125–126. doi: 10.1159/000010075. [DOI] [PubMed] [Google Scholar]
- 34.de Jong T.R., Menon R., Bludau A. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. doi: 10.1016/j.psyneuen.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Kruger T.H.C., Haake P., Chereath D. Specificity of the neuroendocrine response to orgasm during sexual arousal in men. J. Endocrinol. 2003;177:57. doi: 10.1677/joe.0.1770057. [DOI] [PubMed] [Google Scholar]
- 36.Caruso S., Mauro D., Scalia G. Oxytocin plasma levels in orgasmic and anorgasmic women. Gynecol. Endocrinol. 2018;34:69–72. doi: 10.1080/09513590.2017.1336219. [DOI] [PubMed] [Google Scholar]
- 37.Alley J., Diamond L.M., Lipschitz D.L. Associations between oxytocin and cortisol reactivity and recovery in response to psychological stress and sexual arousal. Psychoneuroendocrinology. 2019;106:47–56. doi: 10.1016/j.psyneuen.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Dickenson J.A., Alley J., Diamond L.M. Subjective and oxytocinergic responses to mindfulness are associated with subjective and oxytocinergic responses to sexual arousal. Frontiers in psychology. 2019;10:1101. doi: 10.3389/fpsyg.2019.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baer R.A., Smith G.T., Lykins E. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15:329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- 40.Ückert S., Becker A.J., Ness B.O. Oxytocin plasma levels in the levels of and cavernous blood of healthy males during different penile conditions. World J. Urol. 2003;20:323–326. doi: 10.1007/s00345-002-0300-5. [DOI] [PubMed] [Google Scholar]
- 41.Salonia A., Nappi R.E., Pontillo M. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm. Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Rosen C., Brown J., Heiman S. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 43.Tavares I.M., Vardasca R., Cera N. A review of infrared thermography as applied to human sexual psychophysiology. Int. J. Psychophysiol. 2018;133:28–40. doi: 10.1016/j.ijpsycho.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Dubin N.H., Ghodgaonkar R.B., King T.M. Role of prostaglandin production in spontaneous and oxytocin-induced uterine contractile activity in in vitro pregnant rat uteri. Endocrinology. 1979;105:47–51. doi: 10.1210/endo-105-1-47. [DOI] [PubMed] [Google Scholar]
- 45.Uvnäs-Moberg K., Ekström-Bergström A., Berg M. Maternal plasma levels of oxytocin during physiological childbirth–a systematic review with implications for uterine contractions and central actions of oxytocin. BMC pregnancy and childbirth. 2019;19:1–17. doi: 10.1186/s12884-019-2365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arletti R., Bazzani C., Castelli M. Oxytocin improves male copulatory performance in rats. Horm. Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- 47.Coolen L.M., Allard J., Truitt W.A. Central regulation of ejaculation. Physiol. Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Althof S., Osterloh I.H., Muirhead G.J. The oxytocin antagonist cligosiban fails to prolong intravaginal ejaculatory latency in men with lifelong premature ejaculation: Results of a randomized, double-blind, placebo-controlled phase IIb trial (PEDRIX) The j. of sexual med. 2019;16:1188–1198. doi: 10.1016/j.jsxm.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Striepens N., Kendrick K.M., Maier W. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front. Neuroendocrinol. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Turner R.A., Altemus M., Enos T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- 51.Carter SC. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology; 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 52.Hiller J. Gender differences in sexual motivation. J. of Men's Health and Gender. 2005;2:339–345. [Google Scholar]
- 53.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 54.Depue R.A., Morrone-Strupinsky J.V. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behav. Brain Sci. 2005;28:313–349. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- 55.Guastella A.J., Mitchell P.B., Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Everaerd W., Laan E., Both S. Psychological perspectives on human sexuality. John Wiley & Sons, Inc.; 2000. Female sexuality; pp. 101–146. [Google Scholar]
- 57.Marazziti D., Baroni S., Mucci F. Sex-related differences in plasma oxytocin levels in humans. Clin practice and epidemiol. in mental health: CP & EMH. 2019;15:58–63. doi: 10.2174/1745017901915010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.