Abstract
Introduction
Certain hematologic parameters related to blood cells, known as the biomarkers that predict cardiovascular disease, might be potential predictors of erectile dysfunction (ED) due to the shared pathophysiology between ED and cardiovascular disease .
Aim
To investigate the relationship between ED and these hematologic parameters and the clinical significance of hematologic parameters for the diagnosis of ED.
Methods
A total of 113 male patients diagnosed with ED were included in this study. Blood samples were collected before 10:00 AM for blood cells examination, biochemical tests, and sex hormone analysis. Another 212 healthy controls without ED from the health management center was included as the control group. The relationship between hematologic parameters and ED was assessed by comparing differences in body mass index (BMI), biochemical indexes and hematologic parameters between the 2 groups, and the diagnostic value of hematologic parameters for ED was also examined and compared.
Main outcome measures
International Index of Erectile Function, hematologic parameters
Results
The neutrophil count (NC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in ED patients were significantly higher than those in healthy controls, whereas the lymphocyte count (LC) was significantly lower than that in healthy controls. After adjusting for age, BMI, uric acid (UA), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), increases in the NC, NLR, and PLR and a decrease in the LC were shown to be independent risk factors for ED. Receiver operating characteristic (ROC) curve analysis showed that the NLR exhibited better diagnostic performance for ED than the other parameters.
Conclusion
Increases in the NC, NLR, and PLR and a decrease in the LC significantly increased the risk of ED. The NC, LC, NLR and PLR could contribute to the diagnosis and assessment of ED. Zhangcheng L, Yuxin T, Xiucheng L and Dongjie L, et al. The Relationship Between Hematologic Parameters and Erectile Dysfunction. Sex Med 2021;9:100401.
Key Words: Erectile Dysfunction (ED), Neutrophil Count (NC), Lymphocyte Count (LC), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR)
INTRODUCTION
Erectile dysfunction (ED), a common condition, is defined as a persistent inability to reach and maintain an erection hard enough for successful vaginal intercourse.1 The particularly close association between ED and cardiovascular disease (CVD) has been widely discussed.2 ED and cardiovascular disease share common risk factors, including age, hypertension, insulin resistance and diabetes, hypercholesterolemia, obesity, metabolic syndrome, smoking, sedentary lifestyle, and depression.3
The underlying common pathophysiology (endothelial dysfunction) of ED and CVD or other vascular diseases has been illustrated in previous studies.4 In endothelial dysfunction, which is a systematic disorder, major vascular beds are uniformly invaded,5 and the size and diameter of arteries differ in various areas (eg, arteries in the penis are smaller than those in the heart, brain, and lower limbs). Therefore, because arteries in the penis are less tolerant to the same amount of vessel plaque due to their smaller size, sexual symptoms develop first in patients with endothelial dysfunction.6 Consequently, coronary artery disease (CAD), stroke, and peripheral artery disease usually occur after the onset of ED, following a significant period ranging from 2–5 years in patients (3 years on average).3
As one of the most basic clinical examinations, hematologic tests related to blood cells provide a comprehensive analysis of the red blood cell (RBC), white blood cell (WBC) and platelet systems. Studies have shown that hematologic parameters, such as erythrocytes, hemoglobin (HGB), neutrophils, and platelets, are closely related to the development of endothelial dysfunction and atherosclerosis.7,8 These parameters can be used not only to predict the occurrence of cardiovascular, cerebrovascular, and peripheral vascular diseases but also to assess the severity and even the prognosis of such diseases.9, 10, 11
As CVD or other vascular diseases and ED share the same pathophysiology, is there any potential relationship between these hematologic parameters and ED? It has not been clearly demonstrated in previous studies, and the association between the lymphocyte count (LC) and ED was not previously reported. In our study, we comprehensively analysed and investigated the relationship between hematologic parameters and ED. Independent risk factors for ED were evaluated after adjusting for confounding factors. Furthermore, the sensitivity and specificity of parameters related to ED prediction were assessed and compared. We also explored trends of these parameters in patients with mild, moderate and severe ED.
METHODS
Participants
This cross-sectional study, conducted from October 2018–September 2019, was approved by the Ethics Committee of Xiangya Hospital and Third Xiangya Hospital, Central South University. All patients were diagnosed with ED by the same andrologist at Xiangya Hospital using the simplified International Index of Erectile Function (IIEF-5), and all patients had IIEF-5 scores ≤21. All patients had engaged in sexual activities within the past 3 months, and in more than 50% of the incidences of sexual activity, the patients were unable to obtain and/or maintain an erection sufficient to complete satisfactory sexual intercourse. The severity of ED was assessed according to the IIEF score as follows: Mild, 12–21; moderate, 8–11; and severe, 5–7. For all patients, a detailed inquiry including ED as well as previous and current medical conditions was performed, and a physical examination and laboratory tests were carried out. An ED-related medical history was recorded in detail to help determine the presence of any vasculogenic, hormonal, neurogenic, anatomic, structural or other potential factors. Additionally, conditions including pelvic or perineal trauma, spinal cord injury, rectal surgery, genitourinary or pelvic radiotherapy, alcohol consumption, cigarette smoking, and consumption of any drugs, such as antihypertensives, antiandrogens, antipsychotics, antidepressants, and recreational or addictive drugs, that might interfere with erectile function were documented. Physical examinations consisting of a visual examination and palpation of the external genitalia, perineum and inguinal region were conducted. Other measurements, including blood pressure, height and weight, were obtained. Blood samples were drawn before 10:00 a.m. for blood cells examination and other laboratory parameters, such as fasting blood glucose (FBG), blood lipids, sex hormones, and thyroid hormones. Doppler ultrasound of the genital system, including the bilateral testicles, epididymides, prostate, and spermatic veins, was carried out for all patients, and if necessary, measurement of nocturnal penile tumescence and rigidity and/or a penile vascular ultrasound examination were performed. Healthy males from the health management center of Third Xiangya Hospital with IIEF-5 scores >21 was included as the control group. Detailed history taking, a hematologic examination and other relevant laboratory measurements were also carried out for the participants in the control group.
To avoid possible age-related factors affecting the accuracy of the results, only participants aged 18–60 years were included. The exclusion criteria were as follows:
(i) Neurologic dysfunction, including stroke, intracranial tumor, traumatic brain injury, spinal cord injury, paralysis, Alzheimer's disease, or Parkinson's disease; (ii) genital anatomic or structural defects, including micropenis, penile tumor, balanoposthitis, or penile cavernous sclerosis; (iii) severe cardiovascular and cerebrovascular diseases, including heart failure and cerebral infarction; (iv) systemic immune diseases, including ankylosing spondylitis, systemic lupus erythematosus, and rheumatoid arthritis; (v) endocrine diseases, including hyperprolactinemia and hypogonadism; (vi) acute and chronic infectious or inflammatory diseases, including hepatitis and tuberculosis; (vii) a history of psychiatric/psychogenic diseases or antidepressants, antipsychotics administration; (viii) hematologic diseases, including anemia (HGB <115 g/L), leukemia, and lymphoma; and (ix) major urogenital surgery including prostatectomy or radical prostatectomy.
Statistical Analysis
The Kolmogorov-Smirnov (K-S) test was used to calculate the normality of the data distribution. Numerical variables are shown as the mean ± SD when normally distributed and as the median (first quartile–third quartile) when non-normally distributed. Categorical variables are expressed as numbers and percentages. Student's t test and the Mann-Whitney U test were utilized to analyze normally and non-normally distributed data, respectively. Univariate and multivariate logistic regression analyses were conducted to determine potential predictors of ED. The sensitivity and specificity of related parameters for predicting ED were assessed by receiver operating characteristic (ROC) curve analysis.
The statistical analysis was performed with SPSS Statistics version 23.0 (IBM, SPSS, Chicago, IL, USA) and a value of P < .05 was considered statistically significant.
RESULTS
According to the inclusion and exclusion criteria, a total of 113 male patients and 212 healthy males in the control group were included in this study. No statistically significant difference was found in age, body mass index (BMI), FBG, total cholesterol (TC), or triglyceride (TG) level between the ED group and the control group. However, the high-density lipoprotein (HDL) level in the ED group was significantly lower than that in the control group (1.15 vs 1.25 mmol/L, P < .001). The uric acid (UA) (373.89 vs 355.87 µmol/L, P =.036) and low-density lipoprotein (LDL) levels (2.97 vs 2.86 mmol/L, P =.038) were significantly higher in the ED group than in the control group.
Regarding blood cells parameters, the LC in the ED group was significantly lower than that in the control group (1.7 vs 2.1, P < .001), while the neutrophil count (NC) (3.8 vs 3.3, P < .001), neutrophil-to-lymphocyte ratio (NLR) (2.06 vs 1.57, P < .001), and platelet-to-lymphocyte ratio (PLR) (111.30 vs 99.76, P =.001) were significantly higher than those in the control group. There were no significant differences in the WBC, RBC, and platelet counts, HGB concentration, or red blood cell distribution width (RDW) between the groups, as shown in Table 1.
Table 1.
Comparison of clinical and demographic feature between ED and control group
| Characteristics | ED (n = 113) | Control (n = 212) | P |
|---|---|---|---|
| Age (year)† | 32 (29-37) | 33 (29-38) | .443 |
| BMI (kg/m2)* | 23.57±3.13 | 24.12±2.96 | .119 |
| UA (µmol/L)* | 373.89±76.36 | 355.87±71.36 | .036 |
| FBG (mmol/L)† | 5.27 (4.85-5.87) | 5.33 (5.09-5.62) | .164 |
| TC (mmol/L)† | 4.84 (4.27-5.60) | 4.94 (4.25-5.57) | .938 |
| TG (mmol/L)† | 1.60 (1.08-2.10) | 1.35 (0.95-2.00) | .101 |
| HDL (mmol/L)† | 1.15 (1.00-1.31) | 1.25 (1.11-1.40) | <.001 |
| LDL (mmol/L)† | 2.97 (2.57-3.49) | 2.86 (2.34-3.48) | .038 |
| WBC (× 109/L)† | 6.2 (5.3-7.3) | 5.9 (5.1-7.2) | .282 |
| NC (× 109/L)† | 3.8 (3.2-4.6) | 3.3 (2.7-3.9) | <.001 |
| LC (× 109/L)† | 1.7 (1.5-2.2) | 2.1 (1.8-2.5) | <.001 |
| RBC (× 1012/L)* | 5.2±0.4 | 5.1±0.3 | .079 |
| HGB (g/L)† | 156 (149-161) | 158 (151-164) | .059 |
| RDW (%)† | 12.3 (11.9-12.9) | 12.5 (12.2-12.8) | .099 |
| PLT (× 109/L)† | 198 (161-239) | 215 (185-238) | .060 |
| NLR† | 2.06 (1.64-2.72) | 1.57 (1.25-1.91) | <.001 |
| PLR† | 111.30 (93.42-139.67) | 99.76 (85.21-121.85) | .001 |
BMI = body mass index; FBG = fasting blood-glucose; HDL = high‐density lipoprotein; LC = lymphocyte count; LDL = low‐density lipoprotein; NC = neutrophilic cell count; NLR = Neutrophil-to-lymphocyte ratio; PLT = platelet count; PLR = platelet-to-lymphocyte ratio; RDW = red cell distribution; TC = total cholesterol; TG = triglyceride; UA = uric acid; WBC = white blood cell.
Student's t test.
Mann–Whitney U test.
Univariate and multivariate logistic regression analyses were used to predict risk factors for ED, as depicted in Figure 1. After a djusting for age, BMI, UA, FBG, TC, TG, HDL and LDL values, the NC (OR = 1.34, P =.014), NLR (OR = 2.43, P < .001), and PLR (OR = 1.01, P =.001) were identified as independent risk factors for ED, whereas the LC was identified as a protective factor (OR = 0.26, P < .001).
Figure 1.
Univariate (a) and multivariate (b) logistic regression analyses of predictors for erectile dysfunction.
ROC curve analyses demonstrated the sensitivity and specificity of the NC, LC, NLR, and PLR for predicting ED. The area under the curve (AUC) was highest for NLR (0.718, confidence interval (CI): 0.659-0.778, P < .001) among the 4 parameters, while the AUC was lowest for the PLR (AUC: 0.609, CI: 0.545-0.673, P =.001) (Figure 2 and Table 2). The optimal cut-off value of NLR to predict ED was 1.94, with a sensitivity of 60.2% and specificity of 76.9% (Table 3).
Figure 2.
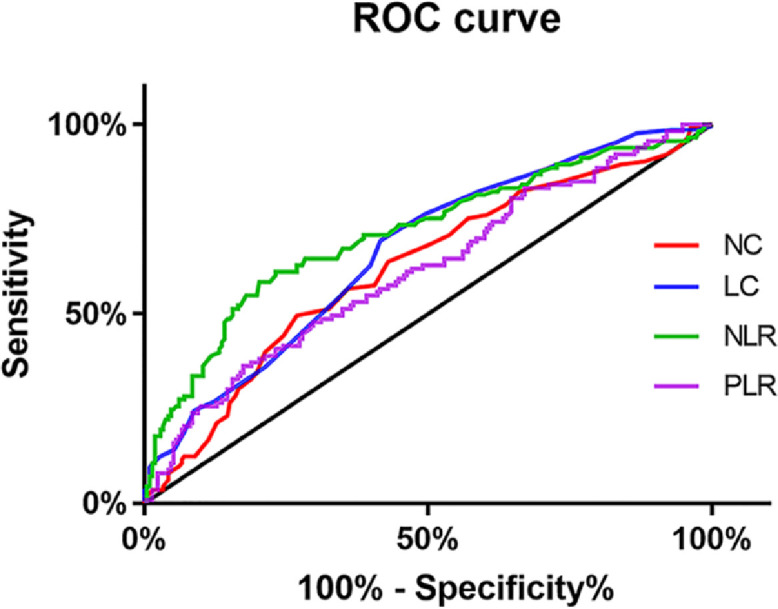
Receiver operator characteristic (ROC) curve analysis of Neutrophil count (NC), neutrophil-tolymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte count (LC) in the prediction of ED.
Table 2.
Area under the ROC curve
| Test result variables | Area | SD | P value | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| NC | 0.622 | 0.033 | P < .001 | 0.558 | 0.686 |
| LC | 0.662 | 0.032 | P < .001 | 0.600 | 0.72 |
| NLR | 0.718 | 0.030 | P < .001 | 0.659 | 0.778 |
| PLR | 0.609 | 0.033 | P =.001 | 0.545 | 0.673 |
CI = confidence interval; LC = lymphocyte count; NC = neutrophilic cell count; NLR = Neutrophil-to-lymphocyte ratio; PLT = platelet count; PLR = platelet-to-lymphocyte ratio; SD = standard deviation.
Table 3.
The cutoff value, sensitivity and specificity to predict ED
| Parameters | Cutoff value | Sensibility (n%) | Specificity (n%) |
|---|---|---|---|
| NC | 3.89 | 49.6 | 73.6 |
| LC | 1.71 | 80.2 | 49.6 |
| NLR | 1.94 | 60.2 | 76.9 |
| PLR | 95.66 | 74.3 | 43.4 |
LC = lymphocyte count; NC = neutrophilic cell count; NLR = Neutrophil-to-lymphocyte ratio; PLT = platelet count; PLR = platelet-to-lymphocyte ratio.
Figure 3 clearly illustrates a relatively flat trend of the NC, LC, NLR and PLR in patients with mild and moderate ED but the NC, NLR, and PLR were significantly increased and the LC was significantly decreased in patients with severe ED.
Figure 3.
The changing trend of Neutrophil count (NC), neutrophil-to-lymphocyte ratio (NLR), platelet-tolymphocyte ratio (PLR) and lymphocyte count (LC) in mild, moderate and severe ED.
DISCUSSION
Penile erection is a complex neurovascular phenomenon with multivariable factors that can be classified as psychogenic, organic, or mixed psychogenic and organic.12 Previously, ED was considered a purely or primarily psychological symptom in most cases.13 However, with the development of diagnostic approaches, current evidence indicates the existence of potential organic risk factors or aetiologies in more than 80% of cases.14 Indeed, it has been widely recognized that vasculogenic factors are extremely common in ED.15 Due to the close association between ED and CVD or other vascular diseases, it is hypothesized that relevant hematologic parameters that can be used to predict or evaluate CVD may also have great clinical significance for ED. A hematologic examination related to blood cells, as one of the most common and basic examinations in clinical work, may become a time-effective, cost-effective, and convenient method contributing to the prediction, diagnosis and even prognosis of ED. Data from a previous study provide evidence that the WBC count is an independent risk factor for CAD,16 and it was further demonstrated in another study that the WBC count is associated with vascular atherosclerosis and related to early and advanced measurements.17 According to a community-based study,18 the NC is strongly and independently related to death and heart failure after myocardial infarction and can predict death and heart failure events. A review19 illustrated that poor outcomes in patients with various heart diseases, such as coronary heart disease, acute coronary syndromes and heart failure, are associated with a low LC, although the relevant underlying pathophysiological mechanisms remain unclear.
In our study, the UA level was significantly increased in ED patients, as clearly indicated in previous research.20 Increased LDL and decreased HDL levels in ED patients have also been reported21. Although we observed no significant association between the WBC count and ED, the NC was significantly increased (P < .001) and the LC was significantly decreased (P < .001) in the ED group. An increased NC (OR = 1.34, P =.014) and a decreased LC (OR = 0.26, P < .001) were also independent risk factors for ED after adjusting for age, BMI, UA, FBG, TC, TG, HDL and LDL. Few studies have reported a significant association between the NC or LC and ED, but other studies may illustrate the potential mechanisms of the relationship of the NC and LC with ED. An increased NC, triggered by hyperlipidaemia, was proven to initiate and promote early atherosclerosis,22 and proteases and oxygen radicals derived from neutrophils induce vascular endothelial cell injury and dysfunction.23 A reduction in or deficiency of B lymphocytes may promote atherosclerosis progression.24 However, more research and evidence are needed to elucidate the mechanisms involved.
Previous studies have shown that the NLR and PLR are predictors and markers for poor outcomes of cardiovascular and other vascular diseases.25,26 In an age-matched study, the NLR and PLR were increased in patients with heart failure, and mortality due to heart failure during follow-up was predicted by the NLR.27 The potential pathophysiological mechanism was demonstrated in further studies. The NLR and PLR are known to be potential markers of inflammation in vascular diseases,28 and inflammation plays a critical role in the initiation and development of vascular endothelial dysfunction and atherosclerosis.29
In the present study, we demonstrated that patients with ED had a significant higher NLR (2.06 vs 1.57, P<0.001) and PLR (111.30 vs 99.76, P =.001) than control subjects without ED. Furthermore, according to the ROC curve analysis, the NLR better predicted ED than the PLR, NC, and LC, with a sensitivity of 60.2% and a specificity of 76.9% under the optimal cut-off of 1.94. There have been few studies exploring the association between the NLR or PLR and ED, and the association has not yet been determined. A study from Turkey showed that the PLR was significantly higher in ED patients but that the NLR was not significantly associated with ED.30 Sambel M, et al.31 found that both the NLR and PLR were significantly correlated with ED, although the PLR rather than the NLR was identified as an independent predictor for ED in the multivariate analysis. Nevertheless, in another study, the NLR was noted as an independent risk factor for ED based on multivariate logistic regression analysis, but this study did not investigate the role of the PLR.32 Our control group included healthy participants from the health management centre, while the control participants in Sambel M's study31 were patients who visited the outpatient centre for complaints other than ED. The control group of another 2 studies30,32 included non-ED participants, but their sources were not described in detail. In our study, both the NLR and PLR were identified as independent risk factors for ED and could be used to predict the occurrence of ED. In addition, the NLR was more accurate in predicting the occurrence of ED than the PLR. Similarly, the NLR has been reported to be more significant for cardiovascular and other vascular diseases, such as heart failure, than the PLR.27,33 However, the exact association between the NLR and PLR and ED and even the correlation of the NLR and PLR with the severity of ED need to be further examined in future studies.
An NLR over 1.94 was predictive of ED, with 60.2% sensitivity and 76.9% specificity in our study. In a cross-sectional study to evaluate the predictive value of the NLR for coronary chronic total occlusions, a cut-off of 2.09 was used, which yielded a sensitivity of 61% and a specificity of 69.3%.34 It seems that the sensitivity for NLR to predict ED is similar to that for NLR to predict coronary chronic total occlusions but the specificity for NLR to predict ED is better. While in another study, the sensitivity of the NLR to predict cerebral ischaemic stroke with a cut-off of 2.55 was 55.3%, but the specificity was much higher (93.6%).35 Compared to predicting cerebral ischaemic stroke, the result of our study showed that NLR might predict ED with a better sensitivity but a lower specificity. Therefore, additional evidence is required to systematically compare and demonstrate the predictive value of the NLR for ED and vascular diseases.
One of the limitations of our study is that the results may have been more accurate and convincing if the sample size had been larger. In addition, hematologic parameters can be affected by many other factors, such as active infection or inflammation. These factors might have influenced the accuracy of our results, although we adopted exclusion criteria to avoid potential influences as much as possible. And the potential differences in hematologic parameters between vascular ED and non-vascular ED were not illustrated in our study and should be further investigated in future research.
CONCLUSION
This study indicates that the NC, NLR, and PLR are significantly increased and that the LC is significantly decreased in ED patients. An increased NC, NLR, and LC and a decreased LC are independent risk factors for ED and may contribute to the diagnosis and evaluation of ED.
STATEMENT OF AUTHORSHIP
Z.L.: Conceptualization, Methodology, Investigation, Acquisition of Data, Formal Analysis, Writing – Original Draft, Writing – Review & Editing; Y.T.: Methodology, Investigation, Writing – Original Draft, Funding Acquisition; X.L.: Investigation, Acquisition of Data; D.L.: Writing – Review & Editing, Funding Acquisition, Supervision.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest of regarding the publication of this paper.
Funding: This work was supported by the National Natural Science Foundation of China (Number: 82071636), the National Natural Science Foundation of China (Number: 82071636), the Natural Science Foundation of Hunan Province, China (2020JJ5906) and the China Postdoctoral Science Foundation (2020M670107ZX).
References
- 1.Burnett AL, Nehra A, Breau RH. Erectile Dysfunction: AUA Guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Gandaglia G, Briganti A, Jackson G. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli MF, Hoes AW, Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the european association for cardiovascular prevention & rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks E, Joshy G, Abhayaratna WP. Erectile dysfunction severity as a risk marker for cardiovascular disease hospitalisation and all-cause mortality: a prospective cohort study. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montorsi P, Ravagnani PM, Galli S. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol. 2005;96:19m–23m. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Montorsi P, Montorsi F, Schulman CC. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur Urol. 2003;44:352–354. doi: 10.1016/s0302-2838(03)00307-5. [DOI] [PubMed] [Google Scholar]
- 7.Elkind MS, Sciacca RR, Boden-Albala B. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Yarnell JW, Baker IA, Sweetnam PM. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation. 1991;83:836–844. doi: 10.1161/01.cir.83.3.836. [DOI] [PubMed] [Google Scholar]
- 10.Elkind MS, Cheng J, Boden-Albala B. Elevated white blood cell count and carotid plaque thickness: the northern manhattan stroke study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 11.Puddu PE, Lanti M, Menotti A. Red blood cell count in short-term prediction of cardiovascular disease incidence in the Gubbio population study. Acta Cardiol. 2002;57:177–185. doi: 10.2143/AC.57.3.2005387. [DOI] [PubMed] [Google Scholar]
- 12.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 13.Aghighi A, Grigoryan V, Delavar A. Psychological determinants of erectile dysfunction among middle-aged men. Int J Impot Res. 2015;27:63–68. doi: 10.1038/ijir.2014.34. [DOI] [PubMed] [Google Scholar]
- 14.Yafi FA, Jenkins L, Albersen M. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:1–20. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004;171:2341–2345. doi: 10.1097/01.ju.0000125198.32936.38. [DOI] [PubMed] [Google Scholar]
- 16.Twig G, Afek A, Shamiss A. White blood cell count and the risk for coronary artery disease in young adults. PloS One. 2012;7:e47183. doi: 10.1371/journal.pone.0047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega E, Gilabert R, Nuñez I. White blood cell count is associated with carotid and femoral atherosclerosis. Atherosclerosis. 2012;221:275–281. doi: 10.1016/j.atherosclerosis.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Arruda-Olson AM, Reeder GS, Bell MR. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Núñez J, Miñana G, Bodí V. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18:3226–3233. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 20.Salem S, Mehrsai A, Heydari R. Serum uric acid as a risk predictor for erectile dysfunction. J Sex Med. 2014;11:1118–1124. doi: 10.1111/jsm.12495. [DOI] [PubMed] [Google Scholar]
- 21.Vrentzos GE, Paraskevas KI, Mikhailidis DP. Dyslipidemia as a risk factor for erectile dysfunction. Curr Med Chem. 2007;14:1765–1770. doi: 10.2174/092986707781058931. [DOI] [PubMed] [Google Scholar]
- 22.Drechsler M, Megens RT, van Zandvoort M. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 23.Boehme MW, Galle P, Stremmel W. Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology. 2002;107:340–349. doi: 10.1046/j.1365-2567.2002.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 25.Tamhane UU, Aneja S, Montgomery D. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Liu N, Sheng J, Pan T. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with lower extremity vascular lesions in chinese patients with type 2 diabetes. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180804. [DOI] [PubMed] [Google Scholar]
- 27.Durmus E, Kivrak T, Gerin F. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105:606–613. doi: 10.5935/abc.20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin B, Ma N, Tang Q. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26:372–376. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 29.Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132:1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 30.Akbas A, Gulpinar MT, Sancak EB. The relationship between platelet-lymphocyte ratio and severity of erectile dysfunction. Kaohsiung J Med Sci. 2016;32:91–95. doi: 10.1016/j.kjms.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambel M, Kilic M, Demirbas M. Relationship between erectile dysfunction and the neutrophil to lymphocyte and platelet to lymphocyte ratios. Int J Impot Res. 2018;30:27–35. doi: 10.1038/s41443-017-0007-1. [DOI] [PubMed] [Google Scholar]
- 32.Aslan A, Kaya Y, Cirakoglu A. Neutrophil-lymphocyte ratio could be a marker for erectile dysfunction. Urol J. 2019;16:216–220. doi: 10.22037/uj.v16i2.5011. [DOI] [PubMed] [Google Scholar]
- 33.Alan S, Tuna S, Türkoğlu EB. The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behçet’s syndrome. Kaohsiung J Med Sci. 2015;31:626–631. doi: 10.1016/j.kjms.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demir K, Avci A, Altunkeser BB. The relation between neutrophil-to-lymphocyte ratio and coronary chronic total occlusions. BMC Cardiovasc Disord. 2014;14:130. doi: 10.1186/1471-2261-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akıl E, Akıl MA, Varol S. Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio are novel inflammatory predictors of cerebral ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:2328–2334. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.028. [DOI] [PubMed] [Google Scholar]




