Abstract
Introduction
Penile traction therapy (PTT) aims to non-surgically reduce curvature, enhance girth, and recover lost length. Available clinical practice guidelines however lack clear recommendations regarding their use.
Aim
To present a comprehensive review and recommendation regarding the available evidence to the use of PTT in Peyronie's disease (PD).
Methods
A systematic literature search was performed on Pubmed and Medline for relevant studies from all times until 2019. Studies of PTT (monotherapy and in combination) in patients with PD with any documented degree of curvature and in either the acute or chronic phase of the disease were included. Full texts not published in English language were excluded.
Main outcomes measures
Several scenarios, including preclinical data have been investigated. For each topic covered evidence was analyzed and expert opinion was stated.
Results
The paucity of high-level studies precluded any strong recommendations, however, specific statements on this topic, summarizing the ESSM position, were provided. The available data about the use of PTT in PD are still poor, and the impact of this therapy for the treatment of PD has not been clearly stablished. Available data in the clinical setting are still poor, and the impact of these devices on PD evolution has not been clearly established.
Conclusion
PTT seems to be a valid treatment option for PD, although there is not enough evidence to give any definitive recommendation in any clinical scenario. García-Gómez B, Aversa A, Alonso-Isa M et al. The Use of Penile Traction Devices for Peyronie's Disease: Position Statements from the European Society for Sexual Medicine. Sex Med 2021;9:100387.
Key Words: Peyronie's disease, Traction therapy, Penile traction, Conservative treatment
Introduction
Penile traction therapy (PTT) represents an emergent therapeutic option for men with Peyronie's disease (PD).1 The accessibility for clinicians and patients in terms of acquisition and ease of use has increased their popularity in recent years.
There are several penile traction devices easily available in the market with similar design, although most of them are not supported by any scientific background. The effect of very few has been specifically studied in the literature, as for instance FastSize Medical Extender (FastSize, Aliso Viejo, CA, USA)2, the PeniMaster Pro (MSC Concept, Berlin, Germany)3, or the RestoreX (PathRight Medical Inc, Plymouth, USA).4 The common stated clinical goals of all PTT are to non-surgically reduce curvature, enhance girth, and recover lost length, all of which are recognized concerns in patients suffering from PD.5 The American Urological Association Guideline on PD does not provide any recommendation related to PTT, acknowledging that the study samples were too small.6 The Evidence-Based Management Guidelines on PD, supported by the International Consultation on Sexual Medicine, however, stated that PTT may have some benefits in PD.7 Accordingly, the Guidelines on Sexual and Reproductive Health by the European Association of Urology state that PTT seems to be effective and safe for patients with PD, but there is still lack of evidence to give any definitive recommendation in terms of monotherapy for PD.8
A recent review by Avant et al9concluded that PTT has a potential role as a primary lengthening therapy (modest improvements); in curvature correction prior to penile prosthesis insertion; and after surgical correction of PD as part of post-operative rehabilitation. Whereas pre-operative and postoperative PTT can result in length preservation after surgery for PD10,11, the role of PTT in combination therapy with Clostridium Histolyticum (CCH) injections is still unclear.12
The aim of the present paper is to provide a detailed position statement of the European Society for Sexual Medicine (ESSM) on this topic, summarizing and emphasizing the current available evidence, any possible conflicting issues and the need of further clarifications.
Methods
A literature search of full text English language articles on the use of PTT in PD was performed using PubMed and Medline on the 09/01/2019 with the following MeSH terms ((((Peyronie's disease or penile induration) and (traction)) “English” [Language]) AND (“All times” [date-publication]: “August 2019” [date-publication]). Inclusion criteria included original papers investigating the outcomes of traction therapy on PD.
Exclusion criteria included posters or oral presentations, review articles, meta-analyses, expert opinions, and case reports.
The scientific committee of the ESSM selected the authors, based on their long-standing clinical experience and scientific involvement in the specific area. Data acquisition was performed by two independent reviewers, and their results collated and reviewed by a third. Possible conflicts were resolved after a discussion among the authors.
Results
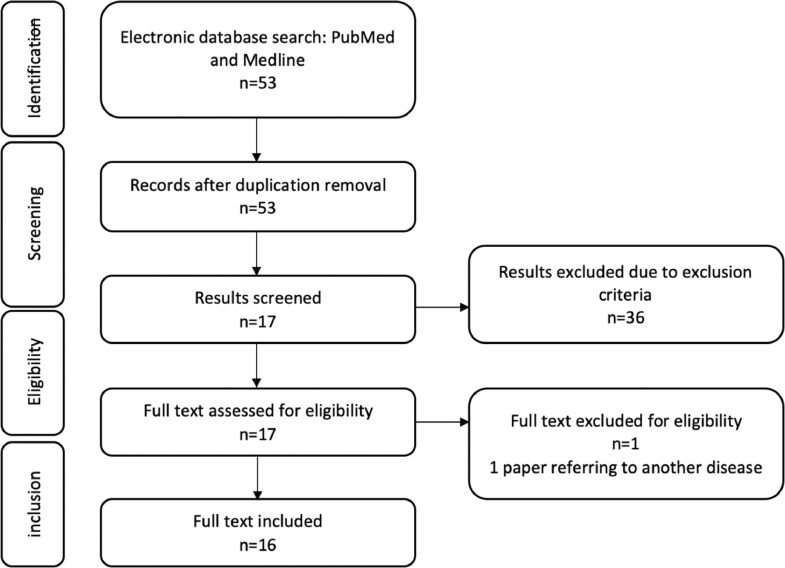
We identified 53 abstracts that met our search criteria, of which 1 was a clinical report, 1 referred to an ocular syndrome, 1 was a guideline (European Association of Urology), 4 were letters in response to an original article, and 30 were reviews or expert opinions. In total 16 papers were included in the review Figure 1 summarizes the data acquisition.
Figure 1.
Flow chart diagram of the data acquisition.
Due to limited evidence on clinical outcomes and the poor scientific quality of the identified studies, no recommendations based on the Oxford 2011 Levels of Evidence criteria were possible. However, specific statements on this topic are provided, which summarize the ESSM position, following a balanced assessment of the evidence by a panel of experts from the ESSM scientific committee and the ESSM executive board.
Preclinical Data
Statement 1. Although mechanical traction can modify connective tissue, more studies are needed to improve our understanding of the pathways involved in the PTT effect on PD.
Evidence
Limited evidence has suggested that the use of mechanical traction, also known as mechanotransduction, results in the alteration of connective tissue by cellular proliferation and expansion of the extracellular matrix.5,13 Chung et al5 investigated the cellular changes of PD plaques following traction forces. In vitro analysis of PD cells cultured in a mechanical strained environment showed an alteration in collagen and tissue metalloproteinase (MMP) expression, highlighting MMP as one possible underlying mechanisms of action of PTT in PD remodeling.2
Lin et al14 investigated the influence of PTT or a vacuum erectile device (VED) in a rat model of PD. PD plaques were induced by intra-tunica albuginea injection of a plaque-inducing agent (human recombinant trans-forming growth factor-b1 [TGF-b1]).The authors noted that the PTT group resulted in less penile curvature than the VED and control groups. They postulated that the observed improvement might be related to anti-apoptosis, anti-fibrosis, and smooth muscle preservation.11
Expert Opinion
It is important to recognize that the experimental conditions of the aforementioned studies do not resemble those present in a clinical scenario. In the study by Chung et al5, the strains applied to the model were not commensurate with those used in clinical practice (4-6 hours daily during a 6 months period).15 Moreover, the findings from Lin et al14 must be interpreted with caution since a rat model might not represent the actual human condition.
Despite aforementioned limitations, the available results provide a preliminary understanding of the underlying mechanisms involved in PTT in PD.
Clinical Evidence
The available evidence concerning the use of PPT in the treatment of PD with or without surgery will be separately discussed in the follow sections.
PTT as Primary Treatment for PD
Statement 2. PTT shows promising results for patients with PD. Further stratification in terms of patient and disease characteristics are still required in order to identify those subjects most likely to benefit from PTT. The limited evidence prevents any definitive recommendation.
Evidence
Five studies assessed the outcomes of PTT as primary treatment for PD (Table 1). Data interpretation was limited by the use of different devices (four studies used a conventional PTT device15, 16, 17, 18, whereas one used a novel ‘bending’ mechanism10). The recommended daily use ranged from 0.5 to 9 hours, and the follow-up period from 3 to 6 months. The latter trials identified described the use of PTT in different phases of PD: one included PD patients in the acute phase15, one in the chronic phase16, and the remaining three papers included mixed populations in both phases of the disease.18,17,10
Table 1.
Results with PTT as a primary treatment
| Author/year | No of patients | Study design | Disease phase | Device | Daily use (hours) | Treatment duration (months) | Mean SPL before (cm) | Gain in SPL (cm) | Mean curvature | Change in curvature |
|---|---|---|---|---|---|---|---|---|---|---|
| Levine/200818 | 10 | Cohort | Mixed | FastSize Penile Extender | 2-8 | 6 | 10.65 | 0.95 | 51° (30-85) | 22°/33% |
| Gontero/200917 | 19 | Cohort | Mixed | Andropenis | 2-8 | 6 | 10.66 | 0.83 | 31° (±1.55) | 4°/13% |
| Martínez-Salamanca/201415 | 55 | NRCT | Acute | Andropeyronie | 6-9 | 6 | 12.4 | 1.5 | 33° (10-90) | 13°/20% |
| Moncada/201916 | 93 | RCT | Chronic | Penimaster PRO | 3-8 | 3 | 11.9 | 1.8 | 72.3° (61-105) | 31°/41% |
| Ziegelmann/201910 | 90 | RCT | Mixed | RestoreX | 0.5-1.5 | 3 | 11.4 | 1.3 | 45.4° (±13.4) | 9°/18% |
PTT = penile traction therapy; NRCT = non-randomized controlled trial; RCT = randomized controlled trial; SPL = stretched penile length.
The studies also differed in design, number of patients involved, and baseline mean penile curvature. The overall reduction in penile curvature ranged from 4° to 31.2°, corresponding to a relative improvement of 12.9-41.1%, most of which reached statistical significance (Table 1).
All studies claimed a gain in the stretched penile length (SPL), although defined measurement protocols were absent uniformly.
Finally, a recent paper by Wymer et al19, compared the cost-effectiveness of surgery, CCH and PTT for the treatment of PD, concluding that traction with the RestoreX (PathRight Medical Inc, Plymouth, USA) PTT device represented the most cost-effective method for achieving ≥20% curvature improvement. As patients in this study were extracted from the Ziegelmann10 series, their data were not described separately.
Expert Opinion
Available data seem to support the use of PTT in either the acute or chronic phases of PD, resulting in improvement in penile curvature and SPLs. However, definitive conclusions were not possible due to the limited number of patients considered (267 in total), the heterogeneity in study design, and non-standardised inclusion and exclusion criteria. In addition, it is important to note that although the differences appreciated in the reduction of curvature may be statistically significant, they may not be clinically relevant. Patients as a consequence might need further treatment to ameliorate their curvature to achieve a satisfactory resolution of their functional deficit.
Unfortunately, most studies did not include patients with calcified plaques, hourglass or hinge deformities, which are theoretically less likely to respond to PTT. Most of the studies failed to identify how the curvature characteristics were assessed (ideally, with a goniometer after induction of artificial erection with intra cavernous vasoactive agent, as describe in the paper by Ziegelmann [10]). None of the studies used any tool to objectively measure the PTT time used by the patients.
PTT Prior to PD Surgery
Statement 3. Available data do not support the use of PPT for PD before surgery
Evidence
Only one study investigated the potential benefits of PTT prior to penile prosthesis implantation surgery in order to avoid postoperative length loss.1 Amongst the patients included, only 2 reported penile length loss due to PD (those who used the PTT 2 hours a day, for 2 months). Overall subjects experienced a penile length gain of 1.5 cm and 0.5 cm just before prosthesis implantation, with PTT and vacuum devices respectively. However, in only one of the patients the length gained persisted after prosthesis implantation, whereas the other reverted to pre-treatment length.
Expert Opinion
Using PTT prior to any PD surgery may be theoretically beneficial to optimize penile length and reduce curvature. However, available data are too limited to support the use of PTT before PD surgery.
PTT after PD Surgery
Statement 4. Although promising results from preliminary studies, available data do not support the use of PTT after PD surgery.
Evidence
PTT has been used as an adjuvant treatment following surgical correction (including penile shortening and lengthening procedures) for PD. Rybak et al11 retrospectively compared the results of 134 patients who underwent PD surgery with or without PTT. PTT was initiated 3-4 weeks after surgery, and was applied for 2 or more hours a day, for 3 months. They found statistically significant improvement in SPL after both shortening and lengthening procedures (a mean gain of 0.85 cm in the shortening procedures + PTT vs a mean loss of 0.53 cm in shortening procedure only; and a mean gain of 1.48 cm in the lengthening procedures + PTT vs a mean gain of 0.24 cm in lengthening procedure only).11 Other reports have recommended the use of PPT (along with the use of a VED) in the immediate postoperative period in order to avoid retraction of the graft after a lengthening procedure.20, 21, 22 The results of these studies suggest that PTT can be beneficial in improving the outcomes of the PD surgery, in terms of ameliorating penile length loss and preventing curvature recurrence.
Expert Opinion
PTT after a lengthening procedure may avoid retraction of the grafting material. However, it is important to recognize that the available evidence is limited and of poor quality. The results of Rybak et al11 may be promising, but due to its retrospective nature it is not possible to give any definitive recommendation. Further studies are warranted to elucidate the role of PTT after shortening PD procedures and its timing.
PTT in Combination With Oral or Intralesional Treatments
Statement 5. There is not enough data to recommend the use of PTT as a concomitant treatment with oral or intralesional therapy.
Evidence
The use of a PTT along with oral or intralesional therapies for PD became a popular approach especially after the approval of CCH for PD. Six studies evaluating the efficacy of PTT in combination with oral or intralesional treatments were identified (Table 2)).23One paper addressed the use of multimodal therapy in the acute phase of PD and five in the chronic phase.
Table 2.
Results of the PTT as a concomitant treatment
| Author/year | n | Type of therapy | PTD average daily use (hours) | PTD average duration (months) | Gain in SPL (cm, p) | Mean curvature ° (SD) | Change in curvature (°, %) |
|---|---|---|---|---|---|---|---|
| Gallo/201923 | 1. 76 2. 45 3. 56 |
1. L-arginine and pentoxifylline 2. L-arginine, pentoxifylline and verapamil 3. L-arginine, pentoxifylline, verapamil and PTD |
1. 0 2. 0 3. 2-8 |
6 | 1. -0.1 p=0.33 2. 0.1 p=0.37 3. 0.7 p=0.1 |
1. 24.2 (9) 2. 25.4 (16.8) 3. 34.3 (17.9) |
1. 0.5 (2.1) p=0.36 2. 1.3 (5.1) p=0.34 3. 8.2 (23.9) p=0.006 |
| Fernández-Pascual/201927 | 1. 50 2. 94 |
1. CCH + PNT +PTD modelling, tadalafil, pentoxifylline 2. CCH +PTD modelling, tadalafil, pentoxifylline |
1. 6-8 2. 6-8 |
2 | 1. +0.3 (0.5); p<0.001 2. +0.1 (0.3); p<0.01 |
1. 55.3 (14.5) 2. 50.6 (15.7) |
1. 19.2 (6.1)°, 36.2 (12.5)% 2. 12.7 (5.0)°, 28.1(14.5)% |
| Alom/201926 | 1. 52 2. 45 3. 16 |
1. CCH+ modelling 2. CCH modelling + PTD 3. CCH + modelling + RestoreX |
1. 0 2. 1,9 3. 0,9 |
6 | 1. -0.7 2. -0.4 3. +1.9 p=0.56 (group 2 vs group 1) p <0.0001 (group 3 vs group 1) p <0.001 (group 3 vs group 2) |
median (IQR): 1. 60 (45-75) 2. 61.5 (45-75) 3. 65 (50-85) |
1. 20,3 °, 31.2% 2. 19,2 °, 30.2% 3. 33,8 ° 49.4% |
| Ziegelmann/201712 | 1. 35 2. 16 |
1. CCH + modelling +PTD 2. CCH +modelling |
1. 1.7 2. 0 |
6 | 1. +0.4; p=0.25 2. -0.35; p=0.48 |
1. 67.4 (25.1) 2. 62.1 (24.9) |
1. 19.6 (16.1)°, 32.6 (26.8) % 2. 23.6 (19.9)°, 27.5 (30.1) % |
| Yafi/201525 | 1. 78 2. 34 |
1. IFN a-2b 2. IFN a-2b + PTD |
1. N/A 2. N/A |
N/A | 1. +1.3 (0.8) 2. +2.4 (0.9) p=0.5567 (group 1 vs group 2) |
1. 42.3 (20.8) 2. 42.2 (14.6) |
1. 9.9 (11.8) 2. 8.1 (16.0) p= 0.4894 (group 1 vs group 2) |
| Abern/201224 | 1. 39 2. 35 |
1. verapamil, PTD, oral pentoxifylline and L-arginine 2. verapamil, oral pentoxifylline and L-arginine |
1. 3.3 2. 0 |
6 | 1. +0,3; p = 0.06 2. -0,7; p = 0.46 |
1. 44.4 (40.0–48.7) 2. 36.6 (33.5–39.7) (95% CI) |
1. 11 ° 2. 15.1 |
SD = standard deviation; PTT = penile traction therapy; PTD = penile traction device; SPL = stretched penile length; CCH = collagenase clostridium histolyticum; PNT = percutaneous needle tunneling; IQR = interquartile range; IFN = interferon.
Gallo et al23 evaluated the results of multimodal treatment for acute PD. They compared the outcomes of 177 patients who underwent oral treatment alone (L-arginine and pentoxyfilline) vs oral treatment and 12 injections of intralesional verapamil vs oral and intralesional treatments in combination with PTT. Despite the limitations in the study design, the authors reported that oral therapy alone was successful in arresting disease progression, and that only oral and intralesional treatments in combination with PTT reduced penile curvature.
The data shown by the remaining studies are conflicting. Abern et al24 evaluated the results of intralesional verapamil combined with oral pentoxyfilline and L-arginine with or without PTT. They found that adding PTT resulted in improved penile curvature reduction (-26.9° vs -20.9°, P = 0.22), with higher (not statistically significant) SPL. Yafi et al25 found no differences in curvature or SPL in a group of PD patients treated with intralesional interferon with or without PTT. Similarly, Ziegelmann et al12 could not demonstrate any statistically significant differences in the reduction of curvature and SPL, in patients treated with a standard protocol of CCH with or without PTT. Alom et al26 found that only the use of a certain PTT device, the RestoreX (PathRight Medical Inc, Plymouth, USA), resulted in improved reduction of curvature and in SPL concomitantly with a protocol of CCH. This was not replicated with a conventional traction device, in spite of increased mean time of daily use.
Finally, more recently, the study by Fernandez-Pascual et al27 was designed to evaluate the differences in a new percutaneous technique along with CCH and PTT, so it is not possible to draw any conclusions about the interaction of PTT itself in the results observed.
Expert Opinion
PTT may enhance the efficacy of other PD treatments in reducing the penile curvature. However, it is not possible to give an evidence-based recommendation about its use, due to the inconsistence and heterogeneity of the available data. Ziegelmann et al12 found that adding PTT to CCH treatment protocol worsened the curvature in PD patients. The lack of improvement could be attributed to the low number of patients or short duration of PTT, but worsening of the curvature requires further evaluations before recommending PTT for PD patients. Alom et al26 found that only the use of the RestoreX device resulted in an improvement of the curvature, and not the use other PTT devices. This finding is consistent with the idea that this PTT device provokes a forced modeling that can be beneficial for CCH therapy. On the other hand, other studies found a general trend of SPL improvement with the use of the PTT.
PTT Safety
Statement 6. PTT adverse effects are mild and well tolerated.
Evidence
Not all the available studies describe adverse events related to the use of PTT. When reported, complications about the use of PTT are mild and well tolerated by the patients. Martínez-Salamanca et al15 reported that 25.4% of PD patients experienced discomfort with the PTT (Andropeyronie -Andromecial SL, Madrid, Spain-) and there were 2 (3.6%) cases of erythema. When using the Penimaster PRO (MSP Concept GmBH&Co, Berlin, Germany)16, 43% of the patients complained of episodic glans numbness, local discomfort or glans edema; whereas PD patients who used the RestoreX (PathRight Medical Inc, Plymouth, USA)10, 45.2% complained of erythema and discoloration, 53.2% mild penile pain, 32% swelling and 1.6% a new lump. Although several studies instructed patients not to use PTT for more than 2 hours, and advised patients to include a resting period of 30 minutes between applications in order to avoid glans ischemia15,10, there was no published data about this adverse event. In the paper by Ziegelmann et al10, they even excluded patients with severe diabetes mellitus to avoid glans necrosis.
Expert Opinion
Although the complications of the PTT are not systematically collected in every paper, they seem to be of mild importance, well tolerated by the patient and with no permanent consequences. Although there is no published report about glans ischemia, it seems reasonable to warn the patients about this risk.
Conclusions
Data about the use of PTT in patients with PD is still limited. There are many aspects to that require clarification through well designed trials before evidence-based recommendations can be made. The ideal PD patient for PTT is still undefined and requires investigation. Moreover, the role of adjuvant PTT also requires further well-designed trials before strong recommendations can be made for its use.
In summary, preliminary data suggests that PTT may be a promising treatment option for PD, although there is not enough evidence to give any definitive recommendation in any clinical scenario. Large well-designed and adequately powered RCTs are required to better clarify all these aspects.
Statement of Authorship
Category 1(a) Conception and Design: Borja García, Gómez, Javier Romero Otero, Antonio Aversa, Giovanni Corona, and Carlo Bettocchi.(b) Acquisition of Data and Literature Research: Javier Romero Otero, Borja García Gómez, and Manuel Alonso Isa.(c) Analysis and Interpretation of Data: Borja García Gómez, Manuel Alonso Isa, and Ege Serefoglu.Category 2(a) Drafting the Article: Borja García Gómez, Antonio Aversa, Ege Can Serefoglu, and Arie Stewart Parham.(b) Revising It for Intellectual Content: Arie Stewart Parham, Yacov Reisman, Carlo Bettocchi, and Giovanni Corona.Category 3(a) Final Approval of the Completed Article: Javier Romero Otero, Giovanni Corona, and Antonio Aversa.
Footnotes
Conflict of Interest: All the authors declare no conflict of interest for this paper.
References
- 1.Levine LA, Rybak J. Traction therapy for men with shortened penis prior to penile prosthesis implantation: a pilot study. J Sex Med. 2011;8:2112–2117. doi: 10.1111/j.1743-6109.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 2.Levine LA, Newell MM. FastSize Medical Extender for the treatment of Peyronie's disease. Expert Rev Med Devices. 2008;5:305–310. doi: 10.1586/17434440.5.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Moncada I, Krishnappa P, Romero J. Penile traction therapy with the new device “Penimaster PRO” is effective and safe in the stable phase of Peyronie's disease: a controlled multicentre study. BJU Int. 2019;123:694–702. doi: 10.1111/bju.14602. [DOI] [PubMed] [Google Scholar]
- 4.Ziegelmann M, Savage J, Toussi A. Outcomes of a Novel Penile Traction Device in Men with Peyronie's Disease: A Randomized, Single-Blind, Controlled Trial. J Urol. 2019;202:599–610. doi: 10.1097/JU.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 5.Chung E, De Young L, Solomon M. Peyronie's disease and mechanotransduction: an in vitro analysis of the cellular changes to Peyronie's disease in a cell-culture strain system. J Sex Med. 2013;10:1259–1267. doi: 10.1111/jsm.12082. [DOI] [PubMed] [Google Scholar]
- 6.Peyronie's Disease Guideline - American Urological Association [Internet]. [cited 2019 Oct 13]. Available at: https://www.auanet.org/guidelines/peyronies-disease-guideline. Accessed June 29, 2021.
- 7.Chung E, Ralph D, Kagioglu A. Evidence-Based Management Guidelines on Peyronie's Disease. J Sex Med. 2016;13:905–923. doi: 10.1016/j.jsxm.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 8.Professionals S-O. Sexual and Reproductive Health [Internet]. Uroweb. [cited 2020 May 6]. Available at: https://uroweb.org/guideline/sexual-and-reproductive-health/. Accessed June 29, 2021.
- 9.Avant RA, Ziegelmann M, Nehra A. Penile Traction Therapy and Vacuum Erection Devices in Peyronie's Disease. Sex Med Rev. 2019;7:338–348. doi: 10.1016/j.sxmr.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Ziegelmann M, Savage J, Toussi A. Outcomes of a Novel Penile Traction Device in Men with Peyronie's Disease: A Randomized, Single-Blind, Controlled Trial. J Urol. 2019;202:599–610. doi: 10.1097/JU.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 11.Rybak J, Papagiannopoulos D, Levine L. A retrospective comparative study of traction therapy vs. no traction following tunica albuginea plication or partial excision and grafting for Peyronie's disease: measured lengths and patient perceptions. J Sex Med. 2012;9:2396–2403. doi: 10.1111/j.1743-6109.2012.02849.x. [DOI] [PubMed] [Google Scholar]
- 12.Ziegelmann MJ, Viers BR, Montgomery BD. Clinical Experience With Penile Traction Therapy Among Men Undergoing Collagenase Clostridium histolyticum for Peyronie Disease. Urology. 2017;104:102–109. doi: 10.1016/j.urology.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE Signal Transduct Knowl Environ. 2002;2002:pe6. doi: 10.1126/stke.2002.119.pe6. 12. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Liu C, Wang R. Effect of Penile Traction and Vacuum Erectile Device for Peyronie's Disease in an Animal Model. J Sex Med. 2017;14:1270–1276. doi: 10.1016/j.jsxm.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Salamanca JI, Egui A, Moncada I. Acute phase Peyronie's disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med. 2014;11:506–515. doi: 10.1111/jsm.12400. [DOI] [PubMed] [Google Scholar]
- 16.Moncada I, Krishnappa P, Romero J. Penile traction therapy with the new device “Penimaster PRO” is effective and safe in the stable phase of Peyronie's disease: a controlled multicentre study. BJU Int. 2019;123:694–702. doi: 10.1111/bju.14602. [DOI] [PubMed] [Google Scholar]
- 17.Gontero P, Di Marco M, Giubilei G. Use of penile extender device in the treatment of penile curvature as a result of Peyronie's disease. Results of a phase II prospective study. J Sex Med. 2009;6:558–566. doi: 10.1111/j.1743-6109.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 18.Levine LA, Newell MM. FastSize Medical Extender for the treatment of Peyronie's disease. Expert Rev Med Devices. 2008;5:305–310. doi: 10.1586/17434440.5.3.305. [DOI] [PubMed] [Google Scholar]
- 19.Wymer K, Kohler T, Trost L. Comparative Cost-effectiveness of Surgery, Collagenase Clostridium Histolyticum, and Penile Traction Therapy in Men with Peyronie's Disease in an Era of Effective Clinical Treatment. J Sex Med. 2019;16:1421–1432. doi: 10.1016/j.jsxm.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Porena M, Mearini L, Mearini E. Peyronie's disease: corporoplasty using saphenous vein patch graft. Urol Int. 2002;68:91–94. doi: 10.1159/000048425. [DOI] [PubMed] [Google Scholar]
- 21.Hatzichristodoulou G, Tsambarlis P, Kübler H. Peyronie's graft surgery-tips and tricks from the masters in andrologic surgery. Transl Androl Urol. 2017;6:645–656. doi: 10.21037/tau.2017.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Gomez B, Ralph D, Levine L. Grafts for Peyronie's disease: a comprehensive review. Andrology. 2018;6:117–126. doi: 10.1111/andr.12421. [DOI] [PubMed] [Google Scholar]
- 23.Gallo L, Sarnacchiaro P. Ten-year experience with multimodal treatment for acute phase Peyronie's disease: A real life clinical report. Actas Urol Esp. 2019;43:182–189. doi: 10.1016/j.acuro.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie's disease. J Sex Med. 2012;9:288–295. doi: 10.1111/j.1743-6109.2011.02519.x. [DOI] [PubMed] [Google Scholar]
- 25.Yafi FA, Pinsky MR, Stewart C. The Effect of Duration of Penile Traction Therapy in Patients Undergoing Intralesional Injection Therapy for Peyronie's Disease. J Urol. 2015;194:754–758. doi: 10.1016/j.juro.2015.03.092. [DOI] [PubMed] [Google Scholar]
- 26.Alom M, Sharma KL, Toussi A. Efficacy of Combined Collagenase Clostridium histolyticum and RestoreX Penile Traction Therapy in Men with Peyronie's Disease. J Sex Med. 2019;16:891–900. doi: 10.1016/j.jsxm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Pascual E, González-García FJ, Angulo J. Optimizing collagenase Clostridium histolyticum therapy for Peyronie's disease using a novel approach with percutaneous needle tunnelling. BJU Int. 2019 doi: 10.1111/bju.14784. 29. [DOI] [PubMed] [Google Scholar]