Abstract
Layered transition metal oxides are the most important cathode materials for Li/Na/K ion batteries. Suppressing undesirable phase transformations during charge-discharge processes is a critical and fundamental challenge towards the rational design of high-performance layered oxide cathodes. Here we report a shale-like NaxMnO2 (S-NMO) electrode that is derived from a simple but effective water-mediated strategy. This strategy expands the Na+ layer spacings of P2-type Na0.67MnO2 and transforms the particles into accordion-like morphology. Therefore, the S-NMO electrode exhibits improved Na+ mobility and near-zero-strain property during charge-discharge processes, which leads to outstanding rate capability (100 mAh g−1 at the operation time of 6 min) and cycling stability (>3000 cycles). In addition, the water-mediated strategy is feasible to other layered sodium oxides and the obtained S-NMO electrode has an excellent tolerance to humidity. This work demonstrates that engineering the spacings of alkali-metal layer is an effective strategy to stabilize the structure of layered transition metal oxides.
Subject terms: Batteries, Batteries
Suppressing phase transitions is crucial for the layered lithium/sodium transition metal oxide cathodes in batteries. Here, the authors report a water-mediated strategy to mitigate the phase transitions and boost electrochemical performances of manganese-based layered cathodes for cost-effective Na-ion batteries.
Introduction
Lithium-ion batteries (LIBs) dominate the markets for portable electronics and electric vehicles1,2. As complementary candidates to LIBs, sodium-ion batteries (NIBs) hold the promise for large-scale energy storage, such as national and smart grids3–6. Composed of the ordered stacking of alkali-metal (Li+/Na+) layers and transition metal (TmO2) layers, the layered transition metal oxides (LiTmO2 and NaxTmO2) are prevailing cathodes for both LIBs and NIBs owing to their scalable synthesis, great variety in compositions, superior theoretical specific capacity, and reliable electrochemical performances7–10. However, the charge–discharge processes of LiTmO2 and NaxTmO2 cathodes are typically accompanied by undesirable phase transformations and the subsequent unsatisfied specific capacity and cycling stability. The major causes for such phase transformations include the migration of Tm ions that results in layer-to-spinel transformation, the anisotropic change of Tm–O bonds during the redox reactions of Tm/O ions, and the evolutions of electrostatic interactions between O–O, Na(Li)–O, and Tm–O.
Since utilized as the positive electrodes in alkaline Zn/MnO2 batteries in the early 1900s11, the Mn-based oxides have attracted enormous attention as battery materials due to the advantages of high abundance in the earth’s crust (11.3-folds of Ni and 38.1-folds of Co), large annual production, and low cost of Mn sources. The present LiTmO2 cathodes, however, rely heavily on scarce, expensive, and thermally unstable Co and Ni elements, because the Mn-redox active LiTmO2 suffers from the interlayer migration of Mn ions during repeated Li+ (de-)intercalation and rapid capacity decay12,13. However, the Mn-based NaxTmO2, such as P2-Na0.67MnO213 and P2-Na-Fe-Mn-O14 series, exhibited high specific capacity and better redox reversibility, which highly meet the requirements for sustainable and cost-effective NIBs.
To address the challenge of phase transformations in Mn-based NaxTmO2, various design and modification strategies have been developed. Substituting Jahn–Teller center (Mn3+) with inert elements, such as Li+ 15,16, Mg2+ 17–19, Zn2+ 20,21, and Al3+ 22,23, is reported to improve the structural and cycling stability, but usually compromises the specific capacity and energy density. Another effective strategy is to regulate the pristine structure of layered oxides. For example, Tm vacancies, whose concentration can be regulated by adopting different cooling processes, have been reported to influence the reversible capacity and structural changes of NaxTmO2 greatly13,24,25. However, the application of the Mn-based NaxTmO2 is still thwarted by unsatisfied electrochemical performances. Therefore, developing new designing strategies to tackle these issues are necessary and urgent.
Electrostatic interaction is a decisive factor for understanding the phase transformations of layered oxides. For pristine structure, the localized electron distributions in layered transition metal oxides determine the stacking modes (P- or O-type stacking) of layered sodium oxides26,27. During charge and discharge processes, the adjacent TmO2 layers glide at the highly charged states to balance the O–O repulsion and Li(Na)–O attraction, such as the O3-H1-3 transition in LiCoO228–30, collapse of c parameter in LiNixCoyMnz-x-yO231–33, P2-O2 transition in P2-Na0.67Ni0.33Mn0.67O210, and P2-OP4 transformation in P2-Na0.67Zn0.2Mn0.8O220. Besides Tm types and the Li+/Na+ contents, the electrostatic interactions of layered oxides (Fig. 1), including O–O repulsion, O-Na(Li) attraction, and van der Waals (vdW) force, are greatly influenced by the Li+/Na+ layer spacings (dO–O). Taking P2-Na0.67MnO2 as an example (Supplementary Fig. 1), as Na+ layer spacings increase from 3.8 to 6.0 Å, the electrostatic repulsion of O1-O2 atoms (297 eV) and electrostatic attraction of Na1-O2 atoms (−157 eV) decay by 18% (54 eV) and 12% (−18 eV), respectively. Simultaneously, the vdW interaction also decreases gradually with the increment of Na+ layer spacings (Supplementary Fig. 2), although the strength of vdW interaction (0–3.5 eV) is much weaker than that of the O–O and Na-O bonds. Therefore, by increasing the alkali-metal (Li+/Na+) layer spacings, the coulombic interactions in layered oxides decrease considerably, which provides a possibility to facilitate their Li+/Na+ mobility, suppress their phase transformations, and improve their electrochemical performances.
Fig. 1. Electrostatic interactions.
Schematic illustration of the electrostatic interactions of a NaxTmO2 and b LiTmO2.
This work uses P2-Na0.67MnO2 as a model material to study the influence of the changes of Na+ layers spacings on the electrochemical properties of layered oxides. We propose a nonhazardous water-mediated strategy to increase the Na+ layer spacings in P2-Na0.67MnO2. The obtained sample exhibits a shale-like structure (S-NMO), accordion-like morphology, and exceptional electrochemical performances. Without any further electrode/electrolyte modifications, the S-NMO delivers a high specific capacity of 181 mAh g−1 with no obvious capacity fade after 100 cycles at 0.1 C, outstanding rate performance, and long cycling stability of 3000 cycles with 83% capacity retention. In situ synchrotron X-ray diffraction (XRD) and ex situ magic-angle-spinning nuclear magnetic resonance spectroscopy (MAS NMR) results reveal that the S-NMO electrode exhibits a zero-strain property along the c axis with only 1.96% volume change during charge–discharge processes. This water-mediated engineering strategy is applicable for other Mn- and Fe-based layered oxides, such as Na0.67Zn0.1Mn0.9O2 and Na0.67Fe0.1Mn0.9O2. In addition, the obtained S-NMO cathodes can be stored in moist atmospheres and thus lowering the manufacture and storage cost of Mn-based layered oxides.
Results
Materials synthesis and characterizations
A schematic illustration of the facile water-mediated process is shown in Fig. 2, along with the structural diagrams and morphologies of NaxMnO2 materials at different stages. Synthesized Na0.67MnO2 particles have an irregular shape and smooth surface. XRD patterns in Fig. 3a reveal that the pristine Na0.67MnO2 has a P2-type structure with P63/mmc space group (JCPDF no. 27-251). After aging in H2O + CO2 atmosphere for 6 days, some Na+ ions are extracted from the bulk Na0.67MnO2 and the NaHCO3 compound is formed, as demonstrated by the XRD and Fourier transformed infrared spectroscopy (FTIR) results in Fig. 3a, b. Simultaneously, H2O is inserted into the Na+ layer, thus broadening the c parameter from 11.0 to 14.2 Å and transforming Na0.67MnO2 into the hydration phase (Fig. 3a, b)34. SEM images show that the particless’ surfaces of hydration phase are coated by NaHCO3 (Fig. 2 and Supplementary Fig. 3). Moreover, due to the large volume expansion during hydration (~30%)34, the Na0.67MnO2 particle breaks into multiple two-dimensional nanoflakes that stack along the c axis (Fig. 2 and Supplementary Fig. 3). To obtain S-NMO, the hydration phase was stirred in distilled water for 10 min and then dehydrated at 160 °C for 1 h to remove the NaHCO3 impurities and inserted H2O molecules, respectively.
Fig. 2. Water-mediated strategy.
Schematic illustration of the preparation process of S-NMO.
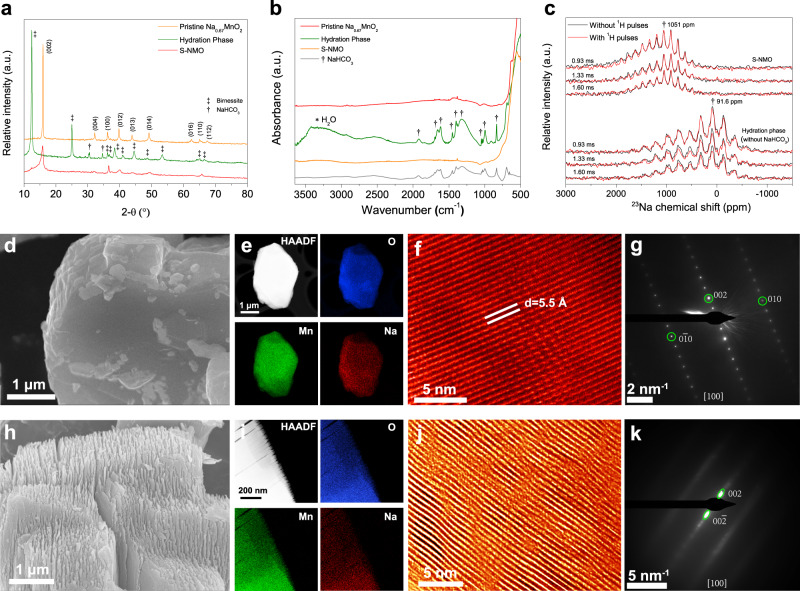
Fig. 3. Structural characteristics of S-NMO.
a XRD patterns and b FTIR spectra of pristine Na0.67MnO2, S-NMO, and hydration phase. c 23Na{1H} REDOR-dephased MAS NMR spectra of S-NMO and hydration phase. d SEM, e EDS mapping, f TEM, and g SAED results of pristine P2-type Na0.67MnO2 material. h SEM, i EDS mapping, j TEM, and k SAED results of S-NMO material.
To verify whether NaHCO3 and H2O impurities have been successfully removed, multiple characterizations were carried out. As shown in Fig. 3a–c, the NaHCO3 signals in XRD pattern (30.4° and 34.4°), FTIR spectrum (600–2000 cm−1), and 23Na MAS NMR spectra (0 ppm) of S-NMO disappear, suggesting that the NaHCO3 impurity has been removed. The absence of O–H stretching signal at 2500–3500 cm−1 of FTIR spectrum of S-NMO (Fig. 3b) demonstrates that the water molecules have been also eliminated. According to our previous results34, during the annealing processes, the hydrated Na0.67MnO2 undergoes NaHCO3 decomposition and dehydration processes in the temperature range of ~30–130 °C. As shown in the thermogravimetric analysis (TGA) results (Supplementary Fig. 4), no mass loss is observed during 30–300 °C of S-NMO, indicating that NaHCO3 and H2O have been removed (Supplementary Note 1), which coincides well with the XRD and FTIR results. In addition, 23Na{1H} rotational-echo double-resonance (REDOR) spectroscopy was used to inspect the residual protons (H+) in S-NMO34. The shift of 23Na signal from 91.6 ppm of hydration phase to 1051 ppm of S-NMO corresponds to the contraction of the Na+ layer spacings due to the dehydration process. For hydration phase (Fig. 3c), the 23Na{1H} REDOR-dephased signal intensities with 1H pulses decrease as the spin-echo time increases from 0.93 to 1.60 ms as opposed to that without 1H pulses, indicating the existence of protons in Na+ layers. For S-NMO material, both 23Na{1H} REDOR-dephased signals with and without 1H pulses at different spin-echo times remain the same, implying that no hydrogen atoms either in proton or H2O states reside closely to the Na+. The above results demonstrate that NaHCO3, H2O, and inserted protons have been successfully removed from S-NMO electrode.
SEM (Fig. 3h) and TEM (Supplementary Fig. 5) images confirm that the S-NMO is composed of two-dimensional nanoflakes and the thickness of each nanoflake is about 10–30 nm. Inductively coupled plasma-atomic emission spectrometry (ICP-AES, Supplementary Table 1) reveals that the chemical composition of S-NMO is Na0.21MnO2. As shown in Fig. 3a and Supplementary Fig. 7, compared to the XRD pattern of pristine Na0.67MnO2, the (002) reflection of S-NMO asymmetrically broadens, ranging from 15.8° to 11.8° (2θ, Cu Kα radiation), which corresponds to the lattice parameter c of 11.2–15.0 Å (3.8–5.8 Å for Na+ layer spacing), much larger than that of Na0.67MnO2 (11.0 Å for c and 3.6 Å for Na+ layer spacing). Simultaneously, (100) diffraction peak shifts from 36.18° (pristine Na0.67MnO2) to 36.54° (S-NMO), demonstrating that the lattice parameter a shrinks from 2.864 to 2.837 due to the oxidation of Mn ions. Moreover, because of the expansion of Na+ layers, all of the (hkl) (l ≠ 0) peaks broaden and lose their intensity, such as (004), (01l), and (112), while the (hk0) peaks, such as (100) and (110), shift slightly to higher angles and maintain their intensity.
The migration of Mn ions from Tm layers to Na+ layers might also lead to the changes of diffraction peaks in the XRD pattern. Therefore, XRD simulations considering the interlayer migration of Mn ions and expansion of interlayer spacing were carried out in a separately way. In P2-type NaxMnO2 material, Na ions are located in two different prismatic sites, i.e., Nae (1/3, 2/3, 3/4) and Naf (0, 0, 1/4). When Mn ions migrate from Mn site (0, 0, 0) to Nae sites, the relative intensities of (012) and (013) peaks become relatively stronger than (002) peak with the increment in the contents of migrated Mn ions (Supplementary Fig. 7a). When Mn ions migrate from Mn site to Naf sites, the relative intensity of (004), (100), and (015) peaks become stronger with the increase of migrated Mn ions (Supplementary Fig. 7b). These trends do not fit the difference between the XRD patterns of pristine Na0.67MnO2 and S-NMO (Fig. 3a). In contrast, with the increase in the fractions of expanded Na layers, the relative intensities of (hkl) (l ≠ 0) peaks decrease, while that of (hk0) peaks remain unchanged (Supplementary Fig. 8), and this fact coincides well with the experimental results.
The atomistic structure of NaxMnO2 has been illustrated (Supplementary Fig. 9) to clarify the change of XRD patterns. As it is observed, the (hk0) planes are perpendicular to the (002) plane and the intensities of (hk0) diffraction peaks are not influenced by the expansion of Na+ layers. However, the inhomogeneous broadening of the (002) plane reduces the atomic ordering degree of (hkl) (l ≠ 0) planes and results in the weakening of (hkl) (l ≠ 0) diffraction peaks.
To further probe the structures of pristine Na0.67MnO2 (Fig. 3d) and S-NMO (Fig. 3h) materials, TEM characterization was carried out. Elemental mapping results (Fig. 3e, i) indicate that Na, Mn, and O elements are homogeneously distributed in both pristine Na0.67MnO2 and S-NMO materials. High-resolution TEM images (Supplementary Fig. 10a, c) and selected area electron diffraction (SAED) patterns (Supplementary Fig. 10b, d) show that the atomic ordering and hexagonal crystal symmetry of S-NMO along [001] direction are identical to that of Pristine Na0.67MnO2, suggesting that the atomic ordering along c axis is well preserved during the water-mediated treatment. As shown in Fig. 3f, the pristine Na0.67MnO2 presents the straight (002) lattice fringes with a uniform spacing of 5.5 Å. By contrast, the (002) planes of S-NMO are slightly curved with varied interplanar spacings (Fig. 3j), as also confirmed by the SAED patterns. Compared to the typical SAED pattern of Na0.67MnO2 along [100] direction (Fig. 3g), only (002) and (00) reflections of S-NMO can be clearly identified (Fig. 3k), indicating a reduced atomic ordering in the (100) plane. Moreover, the (002) and (00) spots in Fig. 3k are elongated toward the central (000) spot, corresponding to the varied (002) d-spacings with different expansion rates. Thus, both high-resolution TEM and SAED analyses confirm that the Na+ layer spacings expand after the water-mediated treatment, well consistent with the XRD results. The above results reveal that the S-NMO exhibits typical shale-like structural features35.
Besides the structural changes at the lattice level, the change of particle size during water-mediated process is also observed. As shown in Supplementary Fig. 11, the median particle sizes (D50) of pristine Na0.67MnO2, hydration phase, and S-NMO are about 5.5, 7.3, and 3.9 μm, respectively. The particle size is expanded by ~32% from pristine Na0.67MnO2 to hydration phase, which coincides well with the XRD patterns (Fig. 3a) and the previous results34. Due to the repeated volume expansion and contraction during the water-mediated process, the particles split along the grain boundaries of Na0.67MnO2 polycrystal (Supplementary Fig. 12), thus resulting in the smaller D50 value of S-NMO than pristine Na0.67MnO2. The elimination of grain boundaries is beneficial to maintain the particle integrity during the electrochemical cycling processes.
Electrochemical performances of S-NMO
The Na-storage electrochemical characteristics of S-NMO electrode were carried out in sodium half-cells with 1 M NaPF6-98% + PC-2% fluoroethylene carbonate (FEC) solution as electrolytes.
To explore the redox behavior of pristine Na0.67MnO2 and S-NMO, the dQ/dV plots and cyclic voltammetry (CV) curves have been obtained and shown in Fig. 4. According to the redox and phase transition mechanisms9,13,20,22, the pristine Na0.67MnO2 electrode can be briefly separated into three regions: anionic redox region, P2-phase region, and Jahn–Teller distortion region. The anionic redox reactions occur at ~4.0–4.4 V, which are usually accompanied by P–O phase transitions9,20. At ~2.5–4.0 V, the Na0.67MnO2 electrode shows P2 structure and exhibits fast kinetics and high redox reversibility20,22. The redox peaks at this stage could be attributed to the local structural transitions of Na vacancy and Mn3+/Mn4+ orderings36. Due to the Mn3+/Mn4+ redox reaction, the Jahn–Teller distortion occurs at the voltages below 2.5 V, which has been also termed as P2-P2′ phase transformation13.
Fig. 4. Redox behavior of pristine Na0.67MnO2 and S-NMO.
a dQ/dV profiles within 2.0–4.4 V obtained from galvanostatic curves at 24 mA g−1. b CV curves at 0.1 mV s−1 from 1.5 to 4.4 V. c CV curves at the sweep rates of 0.1, 0.5, and 1 mV s−1 within 1.5–4.4 V.
As compared to pristine Na0.67MnO2, the S-NMO electrode shows much smoother dQ/dV plots and CV curves, indicating the local structural transitions of Na0.67MnO2 are mitigated. Moreover, as shown in Fig. 4a, b, the contribution of specific capacity by the anionic redox reactions and Jahn–Teller distortion regions decreases after the water-mediated treatment. As shown in Fig. 4c, the overpotential of Mn3+/Mn4+ and anionic redox reactions of pristine Na0.67MnO2 electrode increases drastically on increasing the scan rates. At the scan rates of 0.1, 0.5, and 1 mV s−1, the anodic peaks of Mn3+/Mn4+ redox couple of pristine Na0.67MnO2 increase from 2.46 to 2.64 and 2.74 V, respectively, and the potential polarization of anionic redox reactions increases from 0.05 V (4.20/4.15 V) to 0.42 (4.28/3.86 V) and 0.61 V (4.35/3.74 V), respectively, demonstrating the slow kinetics of both Mn3+/Mn4+ and anionic redox reactions. By contrast, the anodic peaks of Mn3+/Mn4+ redox couple of the S-NMO electrode are located at the lower voltages of 2.47, 2.56, and 2.61 V at the scan rates of 0.1, 0.5, and 1 mV s−1, respectively.
Furthermore, to check the capacitive contributions in both NMO and S-NMO electrodes, we analyzed the anodic peaks located at ~2.5 V (Mn3+/Mn4+ redox peak) and 3.5 V as37
| 1 |
where i corresponds to the current densities at various scan rates (v, mV s−1) and a and b are adjustable constants. When the b value approaches 0.5 and 1, the redox reactions tend to be diffusion-controlled or surface-controlled, respectively38,39. As shown in Supplementary Fig. 16, the b value of NMO and S-NMO electrodes at 2.5 V are 0.60 and 0.69, respectively, which suggests a diffusion-controlled characteristic for Mn3+/Mn4+ redox reactions. When the potential increases to 3.5 V (Supplementary Fig. 17), the b value increases to 0.67 and 0.89 for NMO and S-NMO electrodes, respectively. Compared to the NMO electrode, the S-NMO electrode exhibits greater surface faradaic contribution due to the open morphology (Fig. 3h).
According to the CV results, the ameliorated electrochemical polarization of S-NMO could be attributed to the improved reaction kinetics, the increased surface faradaic capacity contribution, and more favorable pathways for Na+ diffusion because of the expanded Na+ layers spacings.
To further understand the differences between pristine Na0.67MnO2 and S-NMO, electrochemical impedance spectroscopy (EIS) measurements were carried out. Supplementary Fig. 13 shows the Nyquist plots of pristine Na0.67MnO2 and S-NMO electrodes at the discharged 2.8 V states20,40. The ohmic resistances (Rohm) of pristine Na0.67MnO2 and S-NMO are 25.4 and 27.8 Ω, respectively, implying the water-mediated strategy is beneficial for enhancing the electronic conductivity. At the medium- and high-frequency region (1 Hz–100 kHz), the electrode impedances consist of charge transfer resistances and surface resistances of both cathode and anode (RCT+SR). As shown in Supplementary Fig. 13, the RCT+SR of S-NMO is lower than pristine Na0.67MnO2, demonstrating the faster kinetic of redox reactions of S-NMO than pristine Na0.67MnO2.
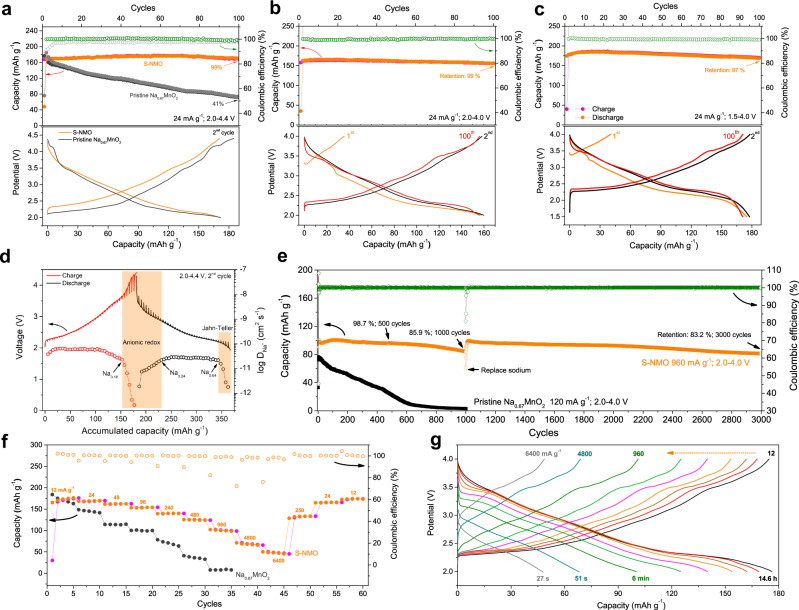
As shown in Fig. 5a, the S-NMO electrode shows improved coulombic efficiency and smoother charge–discharge profiles than pristine Na0.67MnO220,41. At the current density of 24 mA g−1 (0.2 C) within the voltage range of 2.0–4.4 V, the pristine Na0.67MnO2 electrode exhibits rapid capacity decay with only 41% capacity retention after 100 cycles. At the same testing conditions, S-NMO electrode shows no obvious capacity decay (Fig. 5a). At the potential ranges of 2.0–4.0 V (Fig. 5b), 2.0–4.4 V (Fig. 5a), and 1.5–4.0 V (Fig. 5c), the delivered specific capacities of S-NMO electrode at 0.2 C (24 mA g−1) are 162, 170, and 181 mAh g−1, and the corresponding capacity retentions after 100 cycles are 99%, 99%, and 97%, respectively. No obvious capacity and voltage decay are observed in the charge–discharge profiles of 2nd, and 1000th cycles (Fig. 5b, c), demonstrating the excellent cycling stability of S-NMO electrode.
Fig. 5. Electrochemical performances of S-NMO.
The cycling performances and charge–discharge curves of S-NMO electrode at 24 mA g−1 within the voltage ranges of a 2.0–4.4 V, b 2.0–4.0 V, and c 1.5–4.0 V. d GITT results of S-NMO electrode. e The comparison of the cycling performance of pristine Na0.67MnO2 and S-NMO electrodes at 120 and 960 mA g−1, respectively. f The rate performance of pristine Na0.67MnO2 and S-NMO at the voltage range of 2.0–4.0 V. g The charge–discharge profiles of S-NMO at different current densities.
To investigate the kinetic characteristics of the S-NMO electrode, we carried out the galvanostatic intermittent titration technique (GITT) analysis and calculated the corresponding diffusion coefficients of Na+ (DNa+)40. As shown in Fig. 5d, the DNa+ of S-NMO at the voltage range of 2.0–3.5 V remains stable with the geomean DNa+ value of 5.1 × 10−11 cm2 s−1. When the sodium contents of S-NMO decrease from 0.18 to 0.08 (the end of charge), the DNa+ value decreases rapidly from 2.28 × 10−11 to 3.33 × 10−13 cm2 s−1, probably resulted from the increased electrostatic interactions of Na+–O bonds and the slower reaction kinetics of possible anionic redox reactions. At the initial stage of the discharge process, the DNa+ value increases gradually and reaches 2.2 × 10−11 cm2 s−1 at the sodium content of 0.24. Then, the DNa+ value remains constant with the geomean value of 2.64 × 10−11 cm2 s−1 until the sodium content reaches 0.64. Due to the Jahn–Teller effects and increased Na+–Na+ electrochemical interactions, the DNa+ value decreases from 2.18 × 10−11 to 1.8 × 10−12 cm2 s−1 at the end of the discharge process. The more sluggish diffusion of Na+ during anionic redox reactions and Jahn–Teller transitions demonstrate that the capacity delivered at the high current densities mainly originates from the sodium contents of 0.2–0.65 (~130 mAh g−1). Compared to P2-type Na0.67MnO2, the calculated geomean DNa+ values of S-NMO are 11 times higher during the charge process and 2 times higher during the discharge process20.
As shown in Fig. 5e, at 120 mA g−1, the specific capacity of pristine Na0.67MnO2 electrode reduces to only 4% (3 mAh g−1) of the initial capacity after 1000 cycles. Moreover, the CV curves in Supplementary Fig. 14 show that both the cathodic and anodic peaks disappear, revealing the complete collapse of the pristine Na0.67MnO2 electrode. In contrary, the S-NMO electrode exhibits an initial discharge capacity of 98 mAh g−1 at 960 mA g−1 (8 C, 2.0–4.0 V). After 500 and 1000 cycles, 99 and 86% of the initial capacity is maintained, respectively (Fig. 5e). As shown in Supplementary Fig. 15, the redox characteristics of S-NMO during the charge process remain almost unchanged except for the slight shift of the redox peaks and the minor capacity degradation, demonstrating that the redox behavior of S-NMO is well maintained after 1000 cycles.
To investigate the degradation mechanisms of S-NMO electrodes during long cycling, the top-view SEM images of S-NMO electrodes before and after 1000 cycles have been obtained. As shown in Supplementary Fig. 18a, b, the pristine S-NMO electrode is composed of conductive carbon and S-NMO particles. After 1000 cycles (Supplementary Fig. 18c, d), the surface of the S-NMO electrode is covered by a cathode electrolyte interphase (CEI) layer, suggesting the electrolyte decomposition might be the main cause that leads to the degradation of S-NMO electrode. Therefore, we assembled a new half-cell based on the cycled S-NMO electrode. As shown in Fig. 5e, after replacing with a fresh electrolyte and sodium counter electrode, the S-NMO electrode recovers to 99 mAh g−1 at 960 mA g−1 and retains 94 and 83% of the original capacity after 2000 and 3000 cycles, respectively. In addition, Supplementary Fig. 19 shows that the charge–discharge profiles of S-NMO over 3000 cycles at 960 mA g−1 remain practically unchanged, revealing the excellent structural stability during long cycling.
Figure 5f compares the rate performances of Na0.67MnO2 and S-NMO electrodes within 2.0–4.0 V. At the current density of 12 mA g−1 (0.1 C), S-NMO delivers a high specific capacity of 175 mAh g−1, which is comparable to that of Na0.67MnO2. When the current density increases by 4-, 8-, 20-, 40-, and 80-folds, S-NMO maintains 92%, 87%, 80%, 71%, and 57% (100 mAh g−1) of the capacity delivered at 12 mA g−1, respectively. In contrast, the specific capacity of Na0.67MnO2 degrades rapidly at the first ten cycles at 12 and 24 mA g−1. At the current densities of 960 mA g−1 (8 C), S-NMO electrode delivers 12.5-folds of specific capacity of Na0.67MnO2, demonstrating a greatly improved rate performance after the water-mediated strategy. The charge–discharge profiles of S-NMO electrode at various current densities ranging from 12–6400 mA g−1 are shown in Fig. 5g, showing that high specific capacities of 100, 68, and 47 mAh g−1 can be achieved even at the very short operation time of 6 min, 51 s, and 27 s, respectively.
The above electrochemical performances reveal excellent electrochemical reversibility and structural stability of S-NMO electrode, demonstrating lattice engineering is a promising strategy for layered oxide cathodes. With further electrode/electrolyte modifications, such as doping, coating, oxide-carbon composites, or electrolyte modulations, the S-NMO will become a promising cost-effective NIBs electrode for practical applications.
The structural evolutions of S-NMO during charge and discharge
Layered oxide cathodes usually undergo various phase transformations during charge–discharge processes. For example, Na0.67MnO2 experiences C2/c-P2, P2-P2′, and P2-OP4 phase transformations thus resulting in electrode degradations and capacity decay13,20,22. To unravel the origin of the improved electrochemical performances of S-NMO electrode, in situ lab-source/synchrotron XRD and 23Na MAS NMR were performed to investigate the structural transformations during cycling.
Firstly, in situ lab-source XRD patterns with Cu Kα radiation (λ = 1.5405 Å) are recorded. As shown in Supplementary Fig. 20, the shift and intensity changes of (002) peak are negligible during the charge–discharge processes. Other diffraction peaks, such as (004), (100), (103), and (104) are also found in the in situ XRD patterns, but their intensities are too low to be analyzed due to the structural changes during the water-mediated process (Fig. 3a). Synchrotron X-ray is much brighter than the lab-source X-ray. Moreover, the transmission mode of the in situ synchrotron XRD setup strengthens the intensity of (hkl) (h + k ≠ 0) diffraction peaks9. Therefore, in situ synchrotron XRD (λ = 0.6887 Å) was further utilized to track the structural changes of S-NMO.
The in situ synchrotron XRD patterns and the corresponding contour map are shown in Fig. 6a, b, respectively. No new peak appears, indicating that the P2 structure is well maintained during the whole charge–discharge processes. Both position and intensity of the (002) diffraction peak remain static during the initial two cycles, which coincides well with the in situ lab-source XRD patterns. The other diffraction peaks, including (100), (110), (102), and (112), contract during charge and expand during discharge because of the oxidation and reduction of Mn ions, respectively. The variations in lattice parameters and volume during the first two cycles were calculated (Fig. 6c and Supplementary Note 2). During the charge process, the parameter c of Mn-based layered sodium oxides usually increases firstly and then contracts, due to the increased repulsion of adjacent oxygen layers and the following glides of TmO2 slabs13,22. As shown in Fig. 6c, parameter c remains unchanged and the lattice parameter a and volume (V) experience maximum changes of 0.98% and 1.96%, respectively, demonstrating a near-zero-strain insertion/extraction property during charge–discharge process. The volume change of S-NMO electrode is larger than that of the zero-strain Li4Ti5O1242 due to the redox reaction of Mn ions. However, it is much smaller than that of the typical commercial cathodes for LIBs, such as LiMn2O4 (V: ~5.6%)41, 4.3 V LiCoO2 (c: ~2.6%)43 and LiFePO4 (V: ~6.8%)44, which explains the outstanding electrochemical performances of S-NMO electrode.
Fig. 6. Structural evolution mechanisms of S-NMO.
a In situ XRD patterns, b in situ XRD contour map, c calculated lattice parameters, and d ex situ 23Na MAS NMR spectra of S-NMO during the charge–discharge processes. e The comparison of XRD patterns of S-NMO at different cycles.
The structural transformations during the first and second cycles were further studied by ex situ 23Na MAS NMR. As shown in Fig. 6d, the NMR signals that are located at 0, 200–500, 800–900, and 900–1450 ppm correspond to the Na+ in diamagnetic sodium salts (such as CEI), hydration, P2′ (Mn3+-rich environment), and P2 phases, respectively, and the signals located at 1700–1900 ppm most probably arise from the Mn4+-rich local environments19,20,22. Trace amounts of hydration phase at the discharged 3.75 V state of the first cycle and the charged 4.0 V state of the second cycle are introduced during the cell disassembling processes.
The NMR spectrum of pristine S-NMO electrode shows two signals located at 1813 and 1143 ppm, corresponding to the local Mn4+-rich and P2 environments, respectively20,22,45. During the charge process, the intensities of two signals decrease progressively due to the extraction of Na+ from both P2-type and Mn4+-rich environments. During the discharge process within 4.4–2.25 V, these two signals become stronger gradually. With the further intercalation of Na+, Mn4+-rich local environment disappears and a new Mn3+-rich signal (i.e., local P2′ phase) that is located at ~830 ppm emerges, corresponding to the reduction of Mn ions. At the start of the charge process of the second cycle, the local P2′ phase disappears and the Mn4+-rich environment reappears. During the second charge process, the P2-phase signal keeps decreasing. Meanwhile, the signal of the Mn4+-rich environment gradually recovers before the voltage reaches 3.5 V and then becomes weaker with the further extraction of Na+. The P2′-type phase (Mn3+-rich environment) appears in MAS NMR spectra at 2.0 V (Fig. 6d) but is absent in the observed in situ synchrotron XRD patterns (Fig. 6a), indicating the disruption of the long-range Jahn–Teller distortion by the water-mediated treatment.
To further probe the stability of S-NMO electrode, ex situ synchrotron XRD patterns at different cycles were studied, as shown in Fig. 6e. These electrodes are tested at the current density of 24 mA g−1 (0.2 C) within 2.0–4.4 V (Fig. 5a). Previous results indicate that the (002) diffraction peak of Mn-based layered sodium oxides shifts to a lower angle due to the irreversible redox reactions20. As shown in Fig. 6e, at the charged 4.4 V state of both the first and the fifth cycles, all of the diffraction peaks fit well with the P2-type structure, indicating the P-type to O-type structural transformations are inhibited. At the discharged 2.0 V state of the 1st, 2nd, 5th, 20th, 50th, and 100th cycles, all of the diffraction peaks fit well with neither shift nor extra peaks can be observed, demonstrating the excellent structural reversibility under repeated Na+ intercalation and extraction processes.
Altogether, the above XRD and NMR results demonstrate that the S-NMO electrode shows a near-zero-strain property with high structural and electrochemical reversibility during cycling.
Discussions
Due to the high abundancy, cost-effectivity, and environmental friendliness, Mn-based layered sodium oxides possess the qualifications to meet the low-cost requirement of NIBs. However, the electrochemical performances of these promising electrodes are still far from satisfactory due to the Jahn–Teller effect and the migration of adjacent TmO2 layers. The modifications of P2-type NaxMnO2 electrodes are mainly focused on doping (substitution), coating, and electrolyte modulation. In this work, by using a water-mediated method, we successfully expanded the Na+ layer spacings of P2-type Na0.67MnO2, improved the structural stability and Na+ mobility, and obtained the electrochemically stable S-NMO electrode.
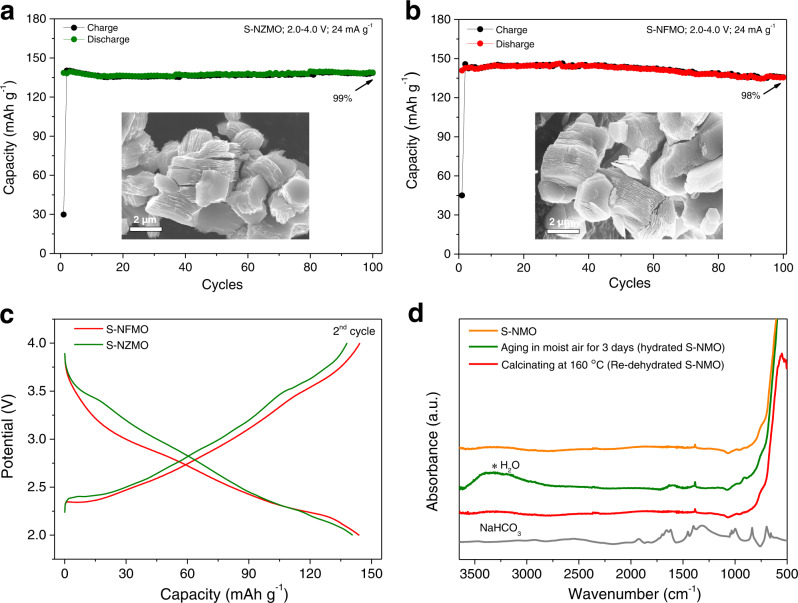
The water-mediated strategy has been also applied in Na0.67Zn0.1Mn0.9O2 and Na0.67Fe0.1Mn0.9O2 materials to verify its feasibility on other layered sodium oxides. As shown in Fig. 7a, b, similar shale-like structure with accordion-like morphologies of S-NMO are observed for treated Na0.67Zn0.1Mn0.9O2 (S-NZMO) and Na0.67Fe0.1Mn0.9O2 (S-NFMO) materials. At the current density of 24 mA g−1 within 2.0–4.0 V, the delivered discharge capacities of S-NZMO and S-NFMO electrodes are 140 and 144 mAh g−1, respectively, with 99% and 98% of the original discharge capacities retained after 100 cycles, much better than the pristine electrodes. As shown in Fig. 7c, the S-NZMO and S-NFMO electrodes show similar charge–discharge curves as S-NMO with smooth slope within 2.0–4.0 V.
Fig. 7. Merits of the water-mediated method.
The morphologies and cycling stability of a S-NZMO and b S-NFMO. c Charge–discharge curves of S-NFMO and S-NZMO within the voltage range of 2.0–4.0 V. d FTIR spectra of S-NMO, hydrated S-NMO, and re-dehydrated S-NMO.
Air stability is an important criterion to evaluate a qualified electrode material. Therefore, a survey of moisture tolerance of S-NMO electrode was conducted by FTIR and the results are shown in Fig. 7d. After aging S-NMO in RH 93% + CO2 for 3 days, the O–H stretching signal located at 3000–3500 cm−1 emerges, suggesting that the S-NMO is hydrated at the moist atmosphere. The absence of Na2CO3 and NaHCO3 characteristic peak at 600–2000 cm−1 suggests that Na+ in S-NMO is highly stable. After dehydrating the hydrated S-NMO material at 160 °C in air for 2 h, the FTIR spectrum of re-dehydrated S-NMO material fits well with that of pristine S-NMO with neither O–H stretching signal nor NaHCO3 appears (Fig. 7d), indicating the hydrated S-NMO can be easily recovered by mild annealing process. The above results suggest that the S-NMO material has excellent moisture tolerance and can be stored at ambient atmospheres.
In summary, a S-NMO electrode has been developed by a water-mediated method. This electrode exhibits outstanding electrochemical performances and excellent structural stability with near-zero-stain property. Notably, the S-NMO electrode shows superior moisture tolerance. It can be stored in highly humid environments and lowers the cost of the preparation and storage of layered sodium oxides. Furthermore, the feasibility of this water-mediated strategy to other layered transition metal oxides is validated. This work presents a simple but effective strategy to eliminate the phase transformations of NaxTmO2 and provides new guidelines for future design in reining the structure of layered transition metal cathodes.
Methods
Preparation of pristine layered sodium oxides
The pristine Na0.67MnO2, Na0.67Fe0.1Mn0.9O2, and Na0.67Zn0.1Mn0.9O2 samples were synthesized via high-temperature solid-state reaction40. Stoichiometric amounts of MnO2 (99.95%, Aladdin), Na2CO3 (99.99%, Aladdin), Fe2O3 (99.95%, Aladdin), and ZnO (99.99%, Aladdin) were mixed homogeneously by ball milling with acetone at 350 rpm min−1 for 12 h. The mixture was dried at 120 °C for 12 h, pressed into pellets, and then sintered at 900 °C for 15 h in air. The pellets were removed to Ar-filled glove box at 150 °C, ground into powder, and stored in the Ar-filled glove box.
Preparation of Shale-like layered sodium oxides
To prepared Shale-like layered sodium oxides, the above synthesized powder was aged on the atmosphere of relative humidity of 93% with the presence of CO2 (RH 93% + CO2) at 40 °C for 6 days34. One gram of exposed sample was stirred in 30 mL distilled water for 10 min at room temperature, centrifuged and washed with ethanol, dried at 120 °C for 6 h, and dehydrated at 160 °C for 12 h. The final samples were stored at Ar-filled glove box.
Theoretical calculations
The interaction potential energies between one atom of x and multiple (N) atoms of y were calculated via Eq. (2). During the calculation, we regard Na and O as point charges with charge amounts of +q and −2q, respectively
| 2 |
where x = Na1, O1 and y = O2 (Supplementary Fig. 1a). Ux-y represents the interaction energy between atoms x and y; qx, qyi, and ri correspond to the charge of the x atom, the charge of the y atom, and the distance between the x and yi atoms, respectively; N is the amounts of y atoms whose distance to the x atom is lower than 10 Å.
The plane-wave PAW DFT calculations (Supplementary Fig. 2) were carried out by the Vienna ab initio Simulation Package46,47 with the Perdew−Burke−Ernzerhof functional (GGA-PBE) formula48. The cutoff energy of 500 eV was employed and the SCF convergence energy was set to 10−7 eV. DFT+U method was adopted and the Ueff of Mn 3d states were set to 4.9 eV. The Brillouin zone was sampled with 7 × 7 × 2 Monkhorst-Pack grids. In addition, vdW-DF2 was used to calculate vdW energy49. The layer spacing dO–O is adjusted in the range of 1.5–7 Å. The vdW energy at the d-spacings of Na+ layers (dO–O) of 50 Å was set to 0 eV.
Characterizations
The powder XRD patterns were collected on a BrukerD8 Discover A25 diffractometer with Cu Kα radiation (λ = 1.5406 Å) at a scanning rate of 8° min−1. XRD simulation was performed via CrystalMaker (version 2.3.2) with the structure of P2-type Na0.2MnO2 (space group: P63/mmc)13,20,22. The SEM images of prepared samples were characterized by Hitachi S-4800 (HITACHI, Japan) microscope and Zeiss Sigma 300. The high-resolution TEM, energy dispersive X-ray spectroscopy (EDS) elemental mapping, and SAED experiments were conducted via FEI Talos-F200s TEM instrument. The composition of samples was tested via ICP-AES (Iris Intrepid II XSP, Thermo Electron). The 23Na ss-NMR spectra were acquired on a Bruker AVANCE III 400 MHz spectrometer using a double-resonance 1.3 mm MAS probe at a spinning rate of 55 kHz with a Hahn-echo pulse sequence (90° pulse – τ – 180° pulse – τ). The 90° pulse length for 23Na was 1.2 μs and the recycle delay was 2 ms. The 23Na shifts were referenced to 1 M NaCl aqueous solution (0 ppm). The REDOR NMR sequence50 is applied to confirm the existence of H2O and H+ in the prepared materials. Geometry-independent information about the dipole couplings between the observation nuclear species S (23Na) and the heteronuclear species I (1H) can be conveniently obtained from the experimental curve of the signal attenuation (1-scale) versus dipolar evolution time (T). FTIR spectra were recorded on a Nicolet is50 FTIR (Thermo Fisher Scientific Inc., Madison, USA) spectrometer. TGA experiments were performed in a STA 409 PC analyzer (Netzsch).
In situ XRD
All of the in situ XRD experiments were performed at room temperature with 2025-type coin cell within 2.0-4.45 V. Lab-source in situ XRD experiments were carried out on a BrukerD8 Discover diffractometer that equipped with a Cu Kα radiation. In situ synchrotron XRD patterns were acquired at BL14B1 of the Shanghai Synchrotron Radiation Facility (SSRF)51. A monochromatic X-ray beam with an energy of 18 keV (λ = 0.6887 Å) and a beam size of 180 μm (width) × 200 μm (height) was adopted. The Mythen 1 K linear detector was adapted for high-resolution powder diffraction data acquisition in Debye–Scherrer mode. The wavelength of the X-ray was calibrated using LaB6 standard from NIST (660b). The diffraction peaks’ FWHM of standard LaB6 is less than 0.02° 52. The lattice parameters were calculated according to Bragg equation
| 3 |
Specifically, a and c were obtained based on the position (2θ) of (100) and (002) diffraction peaks, respectively. The position at the highest intensity of (002) diffraction peak has been selected to calculate the lattice parameter c (Supplementary Note 2).
Electrochemical tests
The sodium half-cells were assembled in Ar-filled glove box with 1 M NaPF6 in propylene carbonate (PC, 98 vol%) and fluoroethylene carbonate (2 vol%) as the electrolyte and Whatman glass fiber filter as the separator. The cathodes were composed of 10 wt% acetylene carbon black, 80 wt% of active materials (layered oxides), and 10 wt% of polyvinylidene fluoride. The mass loading of active materials was ~3 mg cm−1. The electrochemical testing (galvanostatic charge–discharge and GITT) of sodium-ion half-cells were performed at 30 °C using LAND battery testing system (CT2001A, Wuhan, China) in 2025-type coin cells. The GITT of the S-NMO at the second cycle was applied by charging and discharging the testing cell at 9 mA g−1 for 20 min, and then relax it for 1 h. The Na+ diffusion coefficient was calculated as
| 4 |
where mb, Mb, Vm, and A correspond to the electrode mass, molecular weight, molar volume of the compound, and the contact area between the electrolyte and the electrode, respectively. In this article, the geometric mean (geomean) was adopted to measure the average value of sodium-ion diffusion coefficients during charge or discharge process, which were calculated as
| 5 |
Both CV and EIS were applied at a four-channel multifunctional electrochemical workstation (Versa STAT MC. America). The EIS measurements were tested in the frequency range of 0.01 Hz–100 kHz and at the cell voltage of 2.8 V.
Supplementary information
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (grant nos 2018YFB0905400, 2016YFB0901502), National Natural Science Foundation of China (grant nos 21761132030, 21935009, U1932201), the Fundamental Research Funds for the Central Universities (grant no. 20720200075), and the “Double-First Class” Foundation of Materials and Intelligent Manufacturing Discipline of Xiamen University. The authors thank Dr. Dong Su for the kind help and insightful discussions. W.Z. acknowledges the research fellowship from the Alexander von Humboldt Foundation. G.F.O. acknowledges the financial support from Spanish ministry of science and innovation (project nº MAT2017-84002-C2-1-R). R.F. acknowledges the support from the National High Magnetic Field Laboratory which is supported by NSF Cooperative Agreement DMR-1644779 and the State of Florida. The authors gratefully acknowledge the valuable beamtime from beamline BL14B1 of the SSRF.
Source data
Author contributions
W.Z., M.-S.W., and Y.Y. conceived the project. Y.Y. supervised the project. W.Z., J.Q., X.L., Y.L., M.-S.W., and Y.Y. designed the experiments. W.Z. and J.Q. performed the materials synthesis and electrochemical testing. W.Z., J.Q., X.L., Z.X., J.X., J.W., G.F.O., W.W., and Y.L. performed the characterizations. D.Z. and S.W. completed the computational calculations. W.Z., J.Q., X.L., F.R., D.Z., Z.X., Y.L., S.W., R.F., M.-S.W., and Y.Y. analyzed the data and proposed the mechanisms. W.Z. and Y.Y. wrote the initial paper. All authors discussed the results and commented on the manuscript.
Data availability
The data are available within the paper and its Supplementary information file or from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks Surendra Martha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming-Sheng Wang, Email: mswang@xmu.edu.cn.
Yong Yang, Email: yyang@xmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25074-9.
References
- 1.Whittingham MS. Lithium batteries and cathode materials. Chem. Rev. 2004;104:4271–4302. doi: 10.1021/cr020731c. [DOI] [PubMed] [Google Scholar]
- 2.Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010;22:587–603. doi: 10.1021/cm901452z. [DOI] [Google Scholar]
- 3.Vaalma C, Buchholz D, Weil M, Passerini S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018;3:18013. doi: 10.1038/natrevmats.2018.13. [DOI] [Google Scholar]
- 4.Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research development on sodium-ion batteries. Chem. Rev. 2014;114:11636–11682. doi: 10.1021/cr500192f. [DOI] [PubMed] [Google Scholar]
- 5.Nayak PK, Yang L, Brehm W, Adelhelm P. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew. Chem., Int. Ed. 2018;57:102–120. doi: 10.1002/anie.201703772. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Zhao L, Li P, Zhang Q, Wang M-S. In-situ electron microscopy observation of electrochemical sodium plating and stripping dynamics on carbon nanofiber current collectors. Nano Energy. 2017;42:122–128. doi: 10.1016/j.nanoen.2017.10.050. [DOI] [Google Scholar]
- 7.Mizushima K, Jones PC, Wiseman PJ, Goodenough JB. LixCoO2 (0 < x < 1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 1980;15:783–789. doi: 10.1016/0025-5408(80)90012-4. [DOI] [Google Scholar]
- 8.Myung S-T, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett. 2016;2:196–223. doi: 10.1021/acsenergylett.6b00594. [DOI] [Google Scholar]
- 9.Zuo W, et al. Li-rich cathodes for rechargeable Li-based batteries: reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020;13:4450–4497. doi: 10.1039/D0EE01694B. [DOI] [Google Scholar]
- 10.Lu Z, Dahn JR. In situ X-ray diffraction study of P2-Na2/3Ni1/3Mn2/3O2. J. Electrochem. Soc. 2001;148:A1225. doi: 10.1149/1.1407247. [DOI] [Google Scholar]
- 11.Zu C-X, Li H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011;4:2614. doi: 10.1039/c0ee00777c. [DOI] [Google Scholar]
- 12.Ammundsen B, Paulsen J. Novel lithium‐ion cathode materials based on layered manganese oxides. Adv. Mater. 2001;13:943–956. doi: 10.1002/1521-4095(200107)13:12/13<943::AID-ADMA943>3.0.CO;2-J. [DOI] [Google Scholar]
- 13.Kumakura S, Tahara Y, Kubota K, Chihara K, Komaba S. Sodium and manganese stoichiometry of P2-type Na2/3MnO2. Angew. Chem., Int. Ed. 2016;55:12760–12763. doi: 10.1002/anie.201606415. [DOI] [PubMed] [Google Scholar]
- 14.Yabuuchi N, et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012;11:512–517. doi: 10.1038/nmat3309. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, et al. Lithium-doping stabilized high-performance P2-Na0.66Li0.18Fe0.12Mn0.7O2 cathode for sodium ion batteries. J. Am. Chem. Soc. 2019;141:6680–6689. doi: 10.1021/jacs.9b01855. [DOI] [PubMed] [Google Scholar]
- 16.Kwon MS, et al. P2 orthorhombic Na0.7[Mn1-xLix]O2+y as cathode materials for Na-ion batteries. ACS Appl. Mater. Interfaces. 2017;9:14758–14768. doi: 10.1021/acsami.7b00058. [DOI] [PubMed] [Google Scholar]
- 17.Billaud J, et al. Na0.67Mn1−xMgxO2 (0 ≤ x ≤ 0.2): a high capacity cathode for sodium-ion batteries. Energy Environ. Sci. 2014;7:1387–1391. doi: 10.1039/C4EE00465E. [DOI] [Google Scholar]
- 18.Buchholz D, Vaalma C, Chagas LG, Passerini S. Mg-doping for improved long-term cyclability of layered Na-ion cathode materials—the example of P2-type NaxMg0.11Mn0.89O2. J. Power Sources. 2015;282:581–585. doi: 10.1016/j.jpowsour.2015.02.069. [DOI] [Google Scholar]
- 19.Clément RJ, et al. Structurally stable Mg-doped P2-Na2/3Mn1−yMgyO2 sodium-ion battery cathodes with high rate performance: insights from electrochemical, NMR and diffraction studies. Energy Environ. Sci. 2016;9:3240–3251. doi: 10.1039/C6EE01750A. [DOI] [Google Scholar]
- 20.Zuo W, et al. Highly-stable P2–Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering. Energy Storage Mater. 2020;26:503–512. doi: 10.1016/j.ensm.2019.11.024. [DOI] [Google Scholar]
- 21.Wu X, et al. P2-type Na0.66Ni0.33–xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. J. Power Sources. 2015;281:18–26. doi: 10.1016/j.jpowsour.2014.12.083. [DOI] [Google Scholar]
- 22.Liu X, et al. P2-Na0.67AlxMn1-xO2: cost-effective, stable and high-rate sodium electrodes by suppressing phase transitions and enhancing Na+ mobility. Angew. Chem., Int. Ed. 2019;58:18086–18095. doi: 10.1002/anie.201911698. [DOI] [PubMed] [Google Scholar]
- 23.Han DW, et al. Aluminum manganese oxides with mixed crystal structure: high-energy-density cathodes for rechargeable sodium batteries. ChemSusChem. 2014;7:1870–1875. doi: 10.1002/cssc.201301254. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, et al. Al and Fe-containing Mn-based layered cathode with controlled vacancies for high-rate sodium ion batteries. Nano Energy. 2020;76:104997. doi: 10.1016/j.nanoen.2020.104997. [DOI] [Google Scholar]
- 25.Bordet-Le Guenne L, Deniard P, Biensan P, Siret C, Brec R. Structural study of two layered phases in the NaxMnyO2 system. Electrochemical behavior of their lithium substituted derivatives. J. Mater. Chem. 2000;10:2201–2206. doi: 10.1039/b004598p. [DOI] [Google Scholar]
- 26.Zhao C, et al. Rational design of layered oxide materials for sodium-ion batteries. Science. 2020;370:708–711. doi: 10.1126/science.aay9972. [DOI] [PubMed] [Google Scholar]
- 27.Delmas C, Fouassier C, Hagenmuller P. Structural classification and properties of the layered oxides. Phys. B+C. 1980;99:81–85. doi: 10.1016/0378-4363(80)90214-4. [DOI] [Google Scholar]
- 28.Wang K, et al. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-ion batteries. J. Power Sources. 2020;460:228062. doi: 10.1016/j.jpowsour.2020.228062. [DOI] [Google Scholar]
- 29.Zhang J-N, et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy. 2019;4:594–603. doi: 10.1038/s41560-019-0409-z. [DOI] [Google Scholar]
- 30.Ohzuku T, Ueda A. Solid‐state redox reactions of LiCoO2 (R3m) for 4 Volt secondary lithium cells. J. Electrochem. Soc. 2019;141:2972–2977. doi: 10.1149/1.2059267. [DOI] [Google Scholar]
- 31.Li W, Asl HY, Xie Q, Manthiram A. Collapse of LiNi1-x-yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 2019;141:5097–5101. doi: 10.1021/jacs.8b13798. [DOI] [PubMed] [Google Scholar]
- 32.Lebens-Higgins ZW, et al. Revisiting the charge compensation mechanisms in LiNi0.8Co0.2−yAlyO2 systems. Mater. Horiz. 2019;6:2112–2123. doi: 10.1039/C9MH00765B. [DOI] [Google Scholar]
- 33.Bi Y, et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science. 2020;370:1313–1317. doi: 10.1126/science.abc3167. [DOI] [PubMed] [Google Scholar]
- 34.Zuo W, et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres. Nat. Commun. 2020;11:3544. doi: 10.1038/s41467-020-17290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gale JFW, Laubach SE, Olson JE, Eichhuble P, Fall A. Natural fractures in shale: a review and new observations. AAPG Bull. 2014;98:2165–2216. doi: 10.1306/08121413151. [DOI] [Google Scholar]
- 36.Clément RJ, Bruce PG, Grey CP. Review—manganese-based P2-type transition metal oxides as sodium-ion battery cathode materials. J. Electrochem. Soc. 2015;162:A2589–A2604. doi: 10.1149/2.0201514jes. [DOI] [Google Scholar]
- 37.Conway BE, Birss V, Wojtowicz J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources. 1997;66:1–14. doi: 10.1016/S0378-7753(96)02474-3. [DOI] [Google Scholar]
- 38.Augustyn V, et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013;12:518–522. doi: 10.1038/nmat3601. [DOI] [PubMed] [Google Scholar]
- 39.Li R, et al. Direct growth of Fe3O4-MoO2 hybrid nanofilm anode with enhanced electrochemical performance in neutral aqueous electrolyte. Prog. Nat. Sci.: Mater. Int. 2016;26:258–263. doi: 10.1016/j.pnsc.2016.05.003. [DOI] [Google Scholar]
- 40.Zuo W, et al. Structure-performance relationship of Zn2+ substitution in P2–Na0.66Ni0.33Mn0.67O2 with different Ni/Mn ratios for high-energy sodium-ion batteries. ACS Appl. Energy Mater. 2019;2:4914–4924. doi: 10.1021/acsaem.9b00614. [DOI] [Google Scholar]
- 41.Ohzuku T, Kitagawa M, Hirai T. Electrochemistry of manganese dioxide in lithium nonaqueous cell: III. X-ray diffractional study on the reduction of spinel-related manganese dioxide. J. Electrochem. Soc. 1990;137:769–775. doi: 10.1149/1.2086552. [DOI] [Google Scholar]
- 42.Ohzuku T, Ueda A, Yamamoto N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995;142:1431–1435. doi: 10.1149/1.2048592. [DOI] [Google Scholar]
- 43.Cho J, Kim YJ, Kim T-J, Park B. Zero-strain intercalation cathode for rechargeable Li-Ion cell. Angew. Chem. 2001;113:3471–3473. doi: 10.1002/1521-3757(20010917)113:18<3471::AID-ANGE3471>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 44.Delmas C, Maccario M, Croguennec L, Le Cras F, Weill F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nat. Mater. 2008;7:665–671. doi: 10.1038/nmat2230. [DOI] [PubMed] [Google Scholar]
- 45.Clément RJ, et al. Direct evidence for high Na+ mobility and high voltage structural processes in P2-Nax[LiyNizMn1−y−z]O2 (x, y, z ≤ 1) cathodes from solid-state NMR and DFT calculations. J. Mater. Chem. A. 2017;5:4129–4143. doi: 10.1039/C6TA09601H. [DOI] [Google Scholar]
- 46.Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens Matter. 1993;47:558–561. doi: 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- 47.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 48.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 49.Lee, K., Murray, É. D., Kong, L., Lundqvist, B. I. & Langreth, D. C. Higher-accuracy van der Waals density functional. Phys. Rev. B82, 081101 (2010).
- 50.Laurencin D, et al. Probing the calcium and sodium local environment in bones and teeth using multinuclear solid state NMR and X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2010;12:1081–1091. doi: 10.1039/B915708E. [DOI] [PubMed] [Google Scholar]
- 51.Yang T, et al. Introduction of the X-ray diffraction beamline of SSRF. Nucl. Sci. Technol. 2015;26:020101. [Google Scholar]
- 52.Gao M, et al. Facile usage of a MYTHEN 1K with a Huber 5021 diffractometer and angular calibration in operando experiments. J. Appl. Crystallogr. 2016;49:1182–1189. doi: 10.1107/S1600576716008566. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available within the paper and its Supplementary information file or from the corresponding author upon reasonable request. Source data are provided with this paper.