Abstract
Objectives
The development of a rapid diagnostic test for viable SARS-CoV-2 is important for infection control. Real-time RT-PCR assays detect non-viable virus, and cell culture differentiates viable virus but it takes several weeks and is labour-intensive. Subgenomic RNAs may reflect replication-competent virus. We therefore evaluated the usefulness of subgenomic RNAs for diagnosing viable SARS-CoV-2 in patients with COVID-19.
Methods
Patients with various severities of confirmed COVID-19 were enrolled at a tertiary hospital between February and December 2020. RT-PCR assay results for genomic and subgenomic RNA of SARS-CoV-2 from nasopharyngeal swab, sputum and saliva specimens were compared with cell culture results.
Results
A total 189 specimens from 20 COVID-19 patients were tested in genomic and subgenomic PCR assays and cultured on Vero cells. Of these 189 samples, 62 (33%) gave positive culture results, 93 (49%) negative results and the remaining 34 (18%) indeterminate results. Compared with cell culture results, the sensitivities of genomic RNA and subgenomic RNA of the N and S genes were comparable at 100%, but the specificity of subgenomic RNA (N, 65% and S, 68%) was higher than that of genomic RNA (N, 23% and S, 17%, p < 0.001). The mean durations of positive culture and subgenomic RNA were 11.39 ± 10.34 and 13.75 ± 11.22 days after symptom onset (p 0.437), respectively, while that of genomic RNA was 22.85 ± 11.83 days after symptom onset (p < 0.001).
Discussion
Our comparison of subgenomic RNA detection with symptom duration and SARS-CoV-2 culture positivity provides a significant advancement on the transmissibility-based approach beyond the detection of SARS-CoV-2 genomic RNA, and warrants further studies on the development of better diagnostic strategy.
Keywords: COVID-19, Infective viral shedding, SARS CoV-2, Subgenomic RNA, Virus culture
Introduction
Diagnosis of COVID-19 has relied on detection of SARS-CoV-2 viral RNA by real-time reverse transcription PCR (RT-PCR). This method is rapid and highly sensitive, but the viral RNA may be detectable for many weeks after clinical recovery [1,2], which could unnecessarily prolong patients' isolation. In addition, we do not know, on a real-time basis, the viability of SARS-CoV-2 in patients confirmed by conventional RT-PCR of SARS-CoV-2, so some unnecessary contact tracing with quarantine is inevitable. Therefore, the development of a rapid diagnostic test for viable SARS-CoV-2 is important for conserving resources, and compliance with quarantine and isolation polices.
Culture-based isolation of SARS-CoV-2 virus is the best indicator of the presence of replicating virus, but it is difficult, labour-intensive and time-consuming. In addition, it is further complicated by the need for Biosafety Level 3 facilities. Meanwhile, coronaviruses have a unique mechanism of discontinuous transcription involving the synthesis of subgenomic RNA [3]. Coronavirus subgenomic RNAs contain a nested set of negative-sense RNAs from the 3′ end of the virus genome joined to a common leader sequence of about 70 nucleotides derived from the 5′ end of the genomic RNA [4,5]. Therefore, the detection of subgenomic RNAs may better reflect replication-competent virus than detection of conventional genomic RNA [6]. However, there have been limited studies with regard to the correlation between replicative SARS-CoV-2 and subgenomic RNAs in respiratory samples [[6], [7], [8]]. We had a good opportunity to study COVID-19 patients of various severities including immunocompromised patients who were expected to shed viable virus for a prolonged period. We therefore evaluated the utility of subgenomic RNAs for diagnosing viable SARS-CoV-2 in patients with COVID-19.
Materials and methods
Patients and specimens
Respiratory specimens were collected from patients with confirmed COVID-19 who agreed to multiple serial sampling and were admitted to Asan Medical Center, a 2700-bed tertiary hospital in Seoul, Republic of Korea, between February 2020 and December 2020. Diagnosis of COVID-19 was confirmed with nasopharyngeal (NP) swab samples by RT-PCR for the RdRp, N and E genes of SARS-CoV-2 using the Allplex™ 2019-nCoV assay (Seegene, Seoul, Republic of Korea) or a PowerChek 2019-nCoV real-time PCR kit (Kogene Biotech, Seoul, Republic of Korea). NP swab samples, sputum and saliva samples were collected from the patients between the initial day of admission and the day of discharge, up to 50 days after symptom onset. NP swab samples in viral transport medium, and sputum and saliva samples collected in an airtight container, were aliquoted and stored in a –80°C deep freeze until use. This study was reviewed and approved by the ethics committee of the institutional review board of Asan Medical Center (IRB No. 2020-0297), and all participants gave written informed consent.
Measurement of viral load by real-time RT-PCR assay
The collected respiratory samples were inactivated at 65°C for 30 min in a special negative pressure laboratory. Viral RNA was extracted from the respiratory specimens using a QIAamp viral RNA Mini kit (Qiagen Inc., Hilden, Germany). To determine the SARS-CoV-2 viral RNA copy number, multiplex real-time RT-PCR assays targeting the S and N genes were developed; primer and probe sequences and detailed procedures are provided Table S1.
Detection of N and S gene subgenomic RNAs
SARS-CoV-2 N and S gene subgenomic RNAs were detected with RocketScript RT-PCR Premix (Bioneer Co. Daejeon, Republic of Korea). The shared forward primer was designed in the 5′ leader sequence, and reverse primers were located in the gene sequences coding for protein N and S (Table S2). RT-PCR reactions were performed as described in the supplementary materials.
Isolation of SARS-CoV-2 from specimen
Culture-based isolation of SARS-CoV-2 from respiratory specimens was performed by a plaque assay in a Biosafety Level 3 laboratory at Korea University College of Medicine, Seoul, Republic of Korea. Detailed procedures are described in the supplementary material.
Statistical analyses
Categorical variables were compared using Fisher's exact test, and continuous variables with the Mann–Whitney U-test, as appropriate. The diagnostic performance for each test was calculated and compared by using logistic regression with generalized estimating equation to account for the clustering effects. All tests of significance were 2-tailed; p ≤ 0.05 were considered statistically significant. IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) was used for these statistical analyses.
Results
Clinical characteristics of patients
Twenty confirmed COVID-19 patients were included in this study, and the clinical characteristics of these patients are presented in Table 1 . The symptoms of the patients were classified according to the NIH classification into four mild, for moderate and 12 severe and critical. Two patients with acute leukaemia and one patient with lymphoma were included among the 20 patients. Detailed clinical characteristics of the individuals are shown in Fig. S2.
Table 1.
Clinical characteristics of patients in this study
| Total (n = 20) | |
|---|---|
| Age, year | 60 (50–66) |
| Male | 9 (45) |
| Underlying disease | |
| Diabetes | 4 (20) |
| Hypertension | 6 (30) |
| Cardiovascular disease | 3 (15) |
| Chronic kidney disease | 0 (0) |
| Chronic lung disease | 1 (5) |
| Chronic liver disease | 1 (5) |
| Solid tumour | 1 (5) |
| Haematological malignancy | 3 (15) |
| Rheumatic disease | 0 (0) |
| Obesity (body mass index >25) | 1 (5) |
| Initial symptom | |
| Fever | 18 (90) |
| Chills or rigors | 3 (15) |
| Myalgia | 5 (25) |
| Headache | 2 (10) |
| Sore throat | 2 (10) |
| Nausea or vomiting | 3 (15) |
| Diarrhoea | 0 (0) |
| Fatigue | 3 (15) |
| Congestion or runny nose | 1 (5) |
| Cough | 9 (45) |
| Shortness of breathing | 3 (15) |
| Difficulty of breathing | 4 (20) |
| New olfactory disorder | 2 (10) |
| New taste disorder | 2 (10) |
| Hospital course | |
| Pneumonia | 16 (80) |
| Supplemental oxygen therapy | 11 (55) |
| Mechanical ventilation | 4 (20) |
| Extracorporeal membrane oxygenation | 1 (5) |
| In-hospital mortality | 2 (10) |
| Length of hospital stay | 29 (18-38) |
| Prolonged hospitalization (>14 days) | 15 (75) |
| Reason for prolonged hospitalization | |
| Severe COVID-19 | 10/15 (67) |
| Underlying medical condition | 3/15 (20) |
| Isolation until negative conversion of viral RNA | 2/15 (13) |
Data are expressed as the number (%) of patients or median (interquartile range).
Viral loads, subgenomic RNA, and isolation of SARS-CoV-2 by culture
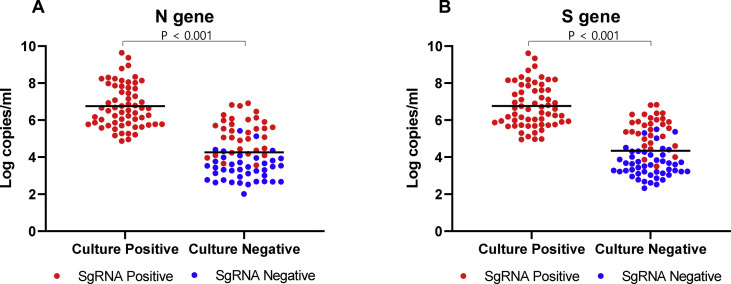
A total 189 respiratory specimens from the twenty COVID-19-confirmed patients were analysed. There were 74 nasopharyngeal swab specimens, 62 sputum specimens and 53 saliva specimens, and the collection times ranged from 1 to 49 days after symptom onset. The N and S genes were detected by genomic RNA RT-PCR assays (limits of detection: 2.6 log copies/mL of specimen) in 160 (N gene) and 161 (S gene) of the 189 specimens. Subgenomic RNA was detected in 108 (N gene) and 107 (S gene) specimens, and viral loads in the positive subgenomic RNA-specimens were 6.21 ± 1.32 log copies/mL and the viral loads in the negative subgenomic RNA specimens 3.35 ± 0.67 log copies/mL (p < 0.001, Mann–Whitney U-test). Sixty-two specimens were successfully cultured and viral loads were 6.76 ± 1.16 log copies/mL; 93 specimens were not cultured and the viral loads were 4.26 ± 1.24 log copies/mL (Fig. 1 , p < 0.001, Mann–Whitney U-test). The other 34 specimens gave invalid results due to bacterial contamination or cell detachment.
Fig. 1.
Plot of subgenomic RNA results stratified by SARS CoV-2 viral gene copy number (log copies/mL), and culture-isolation results for patients with COVID-19. (A) N gene; (B) S gene; red dot, positive subgenomic RNA; green dot, negative subgenomic RNA.
The prediction performances for viable SARS-CoV-2 were analysed with the culture-valid 155 samples (Table 2 ). All of the 62 culture-positive specimens were positive for genomic and subgenomic RNA, so the sensitivity of detection of genomic and subgenomic RNA in culture-positive cases was 100% (95% CI 93–100%) (Fig. 1 and Table 2). For the N gene, 21/93 and 60/93 culture negative specimens were negative for genomic and subgenomic RNA; thus the specificities of genomic and subgenomic RNA for culture negative specimens were 23% (95% CI 14–34%) and 65% (95% CI 53–75%), respectively (p < 0.001). For the S gene, the specificities for genomic and subgenomic RNAs among the culture negatives were 17% (16/93, 95% CI 11–26%) and 68% (95% CI 56–78%), respectively (p < 0.001). Depending on the sample site, NP swab samples showed the highest specificity of subgenomic RNA, 69% (N gene, 22/32, 95% CI 54–80%) and 72% (S gene, 23/32, 95% CI 57–83%), and saliva samples showed the lowest specificity of subgenomic RNA, 54% (N gene, 13/24, 95% CI 41–67%) and 58% (S gene, 14/24, 95% CI 44–71%) (Table 2).
Table 2.
Predictive performances of genomic and subgenomic RNA detection for culturable virus
| Sample | Genomic RNA detection |
Subgenomic RNA detection |
|||
|---|---|---|---|---|---|
| N gene | S Gene | N gene | S Gene | ||
| Sensitivity % (n/Na, 95% CI) | All NP swab Sputum Saliva |
100 (62/62, 93–100) 100 (31/31, 86–100) 100 (18/18, 78–100) 100 (13/13, 72–100) |
100 (62/62, 93–100) 100 (31/31, 86–100) 100 (18/18, 78–100) 100 (13/13, 72–100) |
100 (62/62, 93–100) 100 (31/31, 86–100) 100 (18/18, 78–100) 100 (13/13, 72–100) |
100 (62/62, 93–100) 100 (31/31, 86–100) 100 (18/18, 78–100) 100 (13/13, 72–100) |
| Specificity % (n/Nb, 95% CI) | All NP swab Sputum Saliva |
23 (21/93, 14–34) 25 (8/32, 14–40) 24 (9/37, 16–36) 17 (4/24, 8–31) |
17 (16/93, 11–26) 6 (2/32, 2–18) 22 (8/37, 12–35) 25 (6/24, 11–47) |
65 (60/93, 53–75) 69 (22/32, 54–80) 68 (25/37, 50–81) 54 (13/24, 41–67) |
68 (63/93, 56–78) 72 (23/32, 57–83) 70 (26/37, 53–83) 58 (14/24, 44–71) |
| Positive predictive value % (95% CI) | All NP swab Sputum Saliva |
46 (33–60) 56 (42–60) 39 (25–56) 39 (20–62) |
45 (31–59) 51 (36–66) 38 (24–55) 42 (21–66) |
65 (51–77) 76 (60–87) 60 (42–76) 54 (31–76) |
67 (53–79) 78 (61–88) 62 (43–78) 57 (33–78) |
| Negative predictive value % (95% CI) | All NP swab Sputum Saliva |
100 (81–100) 100 (60–100) 100 (63–100) 100 (40–100) |
100 (76–100) 100 (20–100) 100 (60–100) 100 (52–100) |
100 (93–100) 100 (82–100) 100 (83–100) 100 (72–100) |
100 (93–100) 100 (82–100) 100 (84–100) 100 (73–100) |
an/N, test positive n/culture positive N.
bn/N, test negative n/culture negative N.
This study included three patients with immunocompromised conditions. The sensitivity and specificity of genomic and subgenomic RNA in patients with or without immunocompromised condition are presented in Table S3. In the non-immunocompromised patients, the sensitivity and specificity of subgenomic RNA detection were comparable with that in total patients (sensitivity of N and S gene, 100% (42/42), 90–100%; specificity of N gene, 66 (55/83), 54–77%; specificity of S gene, 69% (57/83), 56–79%).
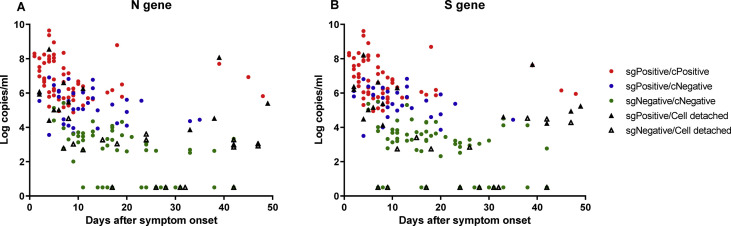
The distributions of positive subgenomic RNA and culture test results as a function of number of days after symptom onset are shown in Fig. 2 and Table 3 . The mean durations of positive culture and subgenomic RNA were 11.39 ± 10.34 and 13.75 ± 11.22 days after symptom onset, respectively (p 0.437, Mann–Whitney U-test), whereas that of genomic RNA was 22.60 ± 11.83 days after symptom onset (p < 0.001 between viral culture and genomic RNA detection, Mann–Whitney U-test). Excluding the three of immunocompromised patients, the mean durations of positive culture, subgenomic RNA and genomic RNA were 8.47 ± 3.93, 10.65 ± 5.70 and 20.35 ± 8.44. (between culture and subgenomic RNA, p 0.342; between culture and genomic RNA, p < 0.001). The results for all 189 specimens including contaminated or cell detached samples by cell culture as a function of days after symptom onset are shown in Table S4.
Fig. 2.
SARS CoV-2 viral gene copy number (log copies/mL), subgenomic RNA, and virus culture as a function of days after symptom onset for patients with COVID-19. (A) N gene; (B) S gene; red dot, positive subgenomic RNA and positive virus culture; blue dot, positive subgenomic RNA and negative virus culture; green dot, negative subgenomic RNA and negative virus culture; purple triangle, positive subgenomic RNA and invalid virus culture due to contamination or cell detachment; blank triangle, negative subgenomic RNA and invalid virus culture due to contamination or cell detachment.
Table 3.
Comparison of tests for Genomic RNA and Subgenomic RNA, and culture results as a function of days after symptom onset
| Assay | Days after symptom onset |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Positive/no. Tested (%) | |||||||||
| 1–5 days | 6–10 days | 11–15 days | 16–20 days | 21–30 days | 31–40 days | 41–50 days | Total | ||
| Genomic RNA | N gene | 35/35 | 44/44 | 20/23 (87) | 20/24 (83) | 6/17 (35) | 6/8 (75) | 3/4 (75) | 134/155 (86) |
| S gene | 35/35 | 41/44 (93) | 23/23 | 21/24 (88) | 11/17 (65) | 5/8 (63) | 3/4 (75) | 139/155 (90) | |
| Subgenomic RNA | N gene | 33/35 (94) | 35/44 (80) | 11/23 (48) | 10/24 (42) | 1/17 (6) | 3/8 (38) | 2/4 (50) | 95/155 (61) |
| S gene | 33/35 (94) | 33/44 (80) | 11/23 (48) | 10/24 (42) | 1/17 (6) | 2/8 (25) | 2/4 (50) | 92/155 (59) | |
| Culture | 28/35 (80) | 22/44 (50) | 4/23 (17) | 5/24 (21) | 0/17 | 1/8 (13) | 2/4 (50) | 62/155 (40) | |
SARS-CoV-2 virus was cultured from 23 of 25 specimens with viral loads ≥7.0 log copies/mL, 19 of 32 specimens with viral loads of 6.0–6.99 log copies/mL, 17 of 38 specimens with viral loads of 5.0–5.99 log copies/mL, three of 26 specimens with viral loads of 4.0–4.99 log copies/mL and none of 50 specimens with viral loads of <4.0 log copies/mL based on copy number of S gene (Table S5). The corresponding data for subgenomic RNAs were detected from 23 of 25 specimens with viral loads ≥7.0 log copies/mL, 28 of 32 specimens with viral loads 6.0–6.99 log copies/mL, 30 of 38 specimens with viral loads 5.0–5.99 log copies/mL, nine of 26 specimens with viral loads 4.0–4.99 log and two of 29 specimens with viral loads 3.0–3.99 log copies/mL, none of 39 specimens with viral loads <3.0 log copies/mL based on copy number of the S gene are also presented in Table S5.
Discussion
The idea of subgenomic RNA detection is not novel. Two studies have reported that replicative virus determined by viral culture and subgenomic RNA detection was not found beyond 8 or 9 days after symptom onset in patients with mild illness [6,7]. However, viral shedding has been observed for longer in patients with severe disease [[9], [10], [11]]. Van Kampen et al. detected culturable virus up to 20 days after symptom onset in patients with moderate-to-severe disease [12]. We extensively investigated whether the subgenomic RNA detect can reflect the viable virus by using various respiratory samples from patients with various severity of illness. The strength of our study is that we compared the positive rate of subgenomic RNA detection with other tests according to the duration of symptoms in patients with severe SARS-CoV-2 infection or immunocompromised patients with SARS-CoV-2 infection as well as in patients with non-severe SARS-CoV-2 infection. The sensitivity and specificity of virus culture as a surrogate for the transmissibility of SARS-CoV-2 is not perfect. So, the several samples including positive subgenomic RNA but the failed cell culture due to cell detachment or contamination may be likely to have viable SARS-CoV-2 especially from respiratory samples of patients at the earliest period from the symptom onset. Taken together, our study results indicate that the assay for subgenomic RNA predicts the duration of infective viral shedding accurately, as its duration was similar to that of culturable virus and its highest positive rate at the earliest period from the symptom onset.
Most of specimens with viral loads ≥6.0 log copies/mL were positive for both culture and subgenomic RNA, but numerous specimens with viral loads of 4.0–5.99 were classified as culture-negative and subgenomic RNA-positive. Two possible explanations for this are that (a) viral culture may be not sensitive enough to detect small amounts of viable virus or (b) subgenomic RNAs can still be detect in the absence of viable SARS-CoV-2. Indeed, 34 of 189 specimens (18%) resulted in culture failure due to bacterial contamination or cell detachment. In addition, 36 of 95 the specimens (38%) with high viral loads (>5 log copies/mL) resulted in culture-negative (Table S5). Although, in the cell culture system we used, viable virus could still be detected in dilutions containing only 1 PFU/mL [13], some invalid culture result could not rule out the possibility that some viable virus might only be detected by subgenomic RNA PCR. However, subgenomic SARS-CoV-2 RNA tightly associated with membrane structures might be protected from cellular RNases, so the detection of subgenomic RNAs from infected patients may not always be indicative of viable virus [8]. In addition, the previous study on the comparison of levels of viral subgenomic RNAs to viral genomic RNAs in the same sample indicated the decreasing ratio from specimens in patients with the late course of COVID-19 [7]. So, it is interesting whether a certain cut-off value of the ratio of viral subgenomic RNAs to viral genomic RNAs may more exactly reflect the viable viral shedding. Further studies are needed in this area.
The detection of genomic RNA has greatly contributed for the early diagnosis of patients with SARS-CoV-2 infection. However, the genomic RNA detection that cannot differentiate viable virus from non-viable virus inevitably results in the prolonged isolation of patients with SARS-CoV-2 infection and the unnecessary quarantine of contacts with those with SARS-CoV-2 infection who have less chance to transmit the virus. The recent studies that the rapid antigen test (RAT) but not genomic RNA detection was correlated with SARS-CoV-2 viral culture [14,15]. However, many other studies raised the issues on the suboptimal sensitivity of RAT [16,17]. In this context, our findings showing that the subgenomic RNA detection had comparable sensitivity to the genomic RNA detection and higher specificity for the viable virus than the genomic RNA detection further facilitate our venture toward the transmissibility-based approach instead of the diagnosis-based approach. For example, the surveillance genomic PCR test may be positive in asymptomatic individuals. If these persons do not have any known exposure to the confirmed SARS-CoV-2 infected patients, the subgenomic RNA detection may be useful for the epidemiologic investigation and isolation policy. In addition, the subgenomic RNA detection may objectively guide the de-isolation policy for immunocompromised patients with COVID-19 or those with severe COVID-19.
Our study has some limitations. First, the subgenomic RNA PCR assay we developed was not quantitative. A quantitative assay such as real-time PCR might provide more reliable information about the presence of viable virus. We are currently developing real-time PCR assays against subgenomic RNA. Second, some may argue that the cell culture procedure that is considered the gold standard for detecting viable SARS-CoV-2 is not so sensitive that underestimation of viable virus is possible. Therefore, a clinical parameter such as symptom duration might be more valuable; hence recent guidelines have symptom cessation as the indicator for discontinuing transmission-based precautions. We therefore analysed positivity for subgenomic RNA, genomic RNA and cell culture as a function of time of symptom onset (Table 3 and Table S4). However, it is difficult to estimate the time of symptom onset in some patients due to pauci- or null symptoms. Thus, our evidence that detection of subgenomic RNA is closely correlated with symptom duration may facilitate objective test-based discontinuation of precautions. Finally, we did not evaluate the diagnostic performance of various genetic variants including alpha-, beta-, gamma- and delta-variants because there were limited reported cases in South Korea during the study period.
In conclusion, this study shows that subgenomic RNA was detected for a few days after viral culture turns negative, but the mean duration of viral shedding assessed by subgenomic RNA detection was much more similar to that of virus culture in moderate-to-critical patients than that assessed by detecting genomic RNA. Therefore, subgenomic RNA could be a useful surrogate for predicting the duration of infectious viral shedding.
Transparency declaration
None. This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which is funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HW20C2062) and from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Republic of Korea (NRF-2018M3A9H4056537).
Author contributions
Writing – Original Draft: J.Y.K., S.B. and S.H.K.; Writing – Review and Editing: J.Y.K., S.B., S.H.K. and M.S.P.; Conceptualization: S.H.K., J.J., M.J.K. and M.S.P.; Investigation: J.Y.K., J.Y.B., H.H.C., J.S.K., C.C., H.P. and J.L.; Resources: S.B., M.H.S. and H.J.L.; Methodology: J.Y.K.; Data curation: J.J., M.J.K., M.H.S. and H.J.L.; Formal Analysis: J.Y.K., H.H.C. and J.S.K. All authors critically revised the manuscript for important intellectual content.
Acknowledgement
We thank Sung-Cheol Yun, PhD, who is a professor of Clinical Epidemiology and Biostatistics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, South Korea, for his assistance with the statistical analysis.
Editor: R. Chemaly
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.08.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Multimedia component 2
References
- 1.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao A.T., Tong Y.X., Gao C., Zhu L., Zhang Y.J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J Virol. 2007;81:20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sola I., Almazan F., Zuniga S., Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snijder E.J., Limpens R.W.A.L., de Wilde A.H., de Jong A.W.M., Zevenhoven-Dobbe J.C., Maier H.J., et al. A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera R., Tso E., Tsang O., Tsang D.N.C, Fung K., Leung Y.W.Y, et al. SARS-CoV-2 Virus culture and subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg Infect Dis. 2020;26:2701–2704. doi: 10.3201/eid2611.203219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 8.Alexandersen S., Chamings A., Bhatta T.R. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun. 2020;11:6059. doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J.T., Ran R.X., Lv Z.H., Feng L.N., Ran C.Y., Tong Y.Q., et al. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:2158–2166. doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J., Shi L.X., Xie Y., Zhang Y.J., Huang S.P., Li J.G., et al. Analysis of factors affecting the prognosis of COVID-19 patients and viral shedding duration. Epidemiol Infect. 2020;148:e125. doi: 10.1017/S0950268820001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J., Xiao J., Sun R., Tang X., Liang C., Lin H., et al. Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis. 2020;26:1834–1838. doi: 10.3201/eid2608.201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Kampen Jja, van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M.C., Cui C., Shin K.R., Bae J.Y., Kweon O.J., Lee M.K., et al. Duration of culturable SARS-CoV-2 in hospitalized patients with covid-19. N Engl J Med. 2021;384:671–673. doi: 10.1056/NEJMc2027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pekosz A., Parvu V., Li M., Andrews J.C., Manabe Y.C., Kodsi S., et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis. 2021;73(9):e2861–e2866. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince-Guerra J.L., Almendares O., Nolen L.D., Gunn J.K.L., Dale A.P., Buono S.A., et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 Infection at two community-based testing sites – pima County, Arizona, November 3-17, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:100–105. doi: 10.15585/mmwr.mm7003e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh S.M., Jeong H., Chang E., Choe P.G., Kang C.K., Park W.B., et al. Clinical application of the standard Q COVID-19 Ag test for the detection of SARS-CoV-2 infection. J Korean Med Sci. 2021;36 doi: 10.3346/jkms.2021.36.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack C.D., Osterholm M., Wasserman E.B., Petruski-Ivleva N., Anderson D.J., Myers E., et al. Optimizing SARS-CoV-2 surveillance in the United States: insights from the national football league occupational health program. Ann Intern Med. 2021;174:1081–1089. doi: 10.7326/M21-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Multimedia component 2