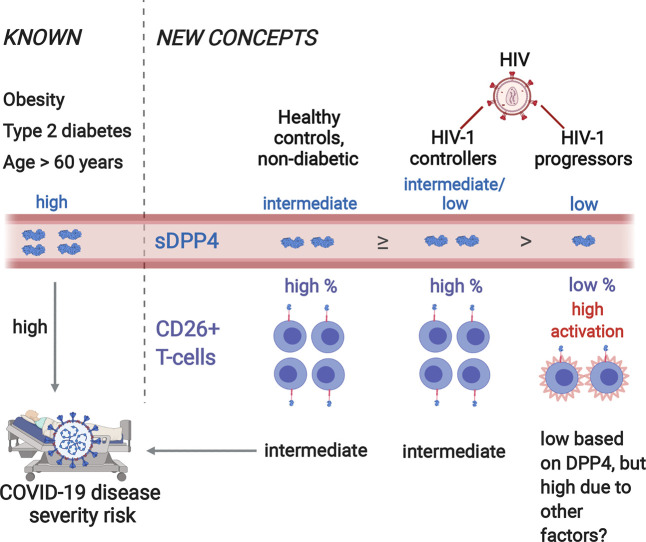
Abstract
The current intersection of the COVID-19 and HIV-1 pandemics, has raised concerns about the risk for poor COVID-19 outcomes particularly in regions like sub-Saharan Africa, disproportionally affected by HIV. DPP4/CD26 has been suggested to be a potential therapeutic target and a biomarker for risk in COVID-19 patients with high risk co-morbidities. We therefore evaluated soluble DPP4 (sDPP4) levels and activity in plasma of 131 HIV-infected and 20 HIV-uninfected South African individuals. Flow cytometry was performed to compare cell surface expression of DPP4/CD26 and activation markers on peripheral blood mononuclear cells of extreme clinical phenotypes. Progressors had lower specific DPP4 activity and lower frequency of CD3+ T-cells expressing CD26 than HIV-1 controllers, but more activated CD3+CD26+ T-cells. The frequency of CD26-expressing T-cells negatively correlated with HLA-DR+ and CD38+ T-cells. Divergent DPP4/CD26 expression between HIV-1 controllers and progressors may have implications for risk and treatment of COVID-19 in people living with HIV.
Keywords: DPP4, CD26, HIV-1, Elite controllers, Progressors, COVID-19
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic of coronavirus disease (COVID-19) [1,2]. Patients with co-morbidities (e.g. diabetes, hypertension, cardiovascular disease, obesity and immunosuppression) present with more severe disease and have a greater risk of death [3]. In the United Kingdom, people living with HIV had higher risk of COVID-19 death with adjusted hazard ratio (HR) 2.59 after controlling for confounders [4]. The growing spread of COVID-19 in sub-Saharan Africa, a population with the highest burden of HIV-1 infection, raises concerns as to whether COVID-19 will have a higher morbidity and mortality rate in people living with HIV [5,6]. Consequently, there is an urgent need to understand factors including the prevailing immune environment in HIV-1 infected individuals - that may predispose coinfected patients to a greater risk of poor clinical outcomes and inform interventions for prevention and treatment. Viral entry into the host cell is an essential component of transmission of coronaviruses. Coronaviruses have a surface spike glycoprotein which binds host-cell receptor and mediates viral entry [7]. Functional studies show that SARS-CoV and SARS-CoV-2 gain entry by binding angiotensin-converting enzyme (ACE2) host receptor, while MERS-CoV binds dipeptidyl peptidase 4 (DPP4) [8]. Structural studies predicted a potential interaction between SARS-CoV-2 spike glycoproteins and DPP4 [9] but binding has not been confirmed in an experimental model [8,10].
DPP4, also known as CD26, is a multifunctional protein [11] with immune modulatory properties and has been associated with type 2 diabetes, obesity, aging and HIV-1 [[12], [13], [14], [15]] – high risk groups for COVID-19 disease severity [3,16]. DPP4 is expressed by multiple cell types [17] and is present in two forms; (i) bound to the cell-membrane and (ii) soluble circulating in blood [18]. Soluble DPP4 (sDPP4) is produced as a result of a post-translational modification of membrane-bound CD26 which is then released into peripheral blood [18]. DPP4 enzymatic activity regulates receptor specificity and the function of various chemokines [19]. Recent discussions on the potential role of DPP4 in COVID-19 immunopathogenesis [20,21] have suggested that DPP4 may be a determinant of COVID-19 disease severity [15]. It has been proposed that DPP4 could be a potential marker for risk stratification and disease progression in COVID-19 patients with diabetes and other highly susceptible populations [21]. However, when considering a biomarker for disease progression or therapeutic interventions, it is crucial to characterize the candidate marker/target in groups that are at high risk for COVID-19 disease severity. Evidence suggests that sDPP4 levels and activity may be associated with HIV-1 disease progression and has the potential to be used as a biomarker in HIV cure research [22,23]. Previous studies have shown that sDPP4 activity is reduced in HIV-1 infection [[22], [23], [24]]. It has also been shown that low sDPP4 activity is a predictor of rapid disease progression [23]. Others have suggested that DPP4/CD26 may play a protective role against HIV-1 acquisition [25]. Investigation into a Kenyan sex worker cohort showed that HIV-1 exposed seronegative female sex workers had higher membrane expression of DPP4/CD26 on CD4+ T-cells and higher sDPP4 levels in plasma [25].
With the current intersection of the COVID-19 pandemic and HIV-1 epidemic in South Africa, it is important to characterize DPP4 in this immunocompromised population. However, to date, no studies have assessed the role of DPP4 in HIV-1 disease progression in sub-Saharan Africa. We also need to consider that people living with HIV with or without combination antiretrovirals (cART) have different phenotypes of HIV disease progression. There are individuals, termed progressors, who are symptomatic with low CD4 counts and HIV-1 high viral loads requiring cART. There are also groups of individuals who are able to inherently limit the impact of HIV infection on their immune system, or even naturally control replication of HIV without ART [[26], [27], [28], [29]]. Long-term nonprogressors (LTNPs) are individuals who remain asymptomatic for a prolonged period without treatment and maintain CD4 counts >500 cells/μl in the absence of therapy for >7 years [29]. Unlike long-term surviving children [30], adult viremic nonprogressors (AVPs) [[31], [32], [33]] – also termed high viral load LTNPs (HVL LTNPs) in our studies [34,35] are very rare. They appear to possess mechanisms that protect CD4 T-cells but do not control virus replication [33], a feature reminiscent of the natural hosts of non-pathogenic simian immunodeficiency virus (SIV) infection, such as sooty mangabeys and African green monkeys [32,36,37]. Groups controlling viral loads to levels <2000 RNA molecules/ml are viraemic controllers (VCs), while those who maintain HIV viral loads of <50 RNA copies/ml for at least 1 year are termed elite controllers (ECs) [27]. The study of these different HIV-1 phenotypes of disease progression may provide insights into whether DPP4 may be a useful biomarker or candidate therapeutic in COVID-19 patients with HIV-1. This study, therefore aimed to characterize sDPP4 levels and activity and cell-membrane bound DPP4/CD26 expression in a cohort of South African HIV-1 controllers (LTNPs and ECs) and progressors.
2. Materials and methods
2.1. Study participants
This cross-sectional study comprised a cohort of 20 HIV-1 uninfected healthy controls (HC) and 131 HIV-1 infected black South Africans. The HIV-1 group was classified according to different clinical phenotypes of HIV-1 controllers and progressors. The controllers were further categorized as elite controllers (ECs), high viral load long-term nonprogessors (HVL LTNPs) and viraemic controllers (VCs). ECs were individuals with viral load <50 RNA copies/ml in the absence of cART, and CD4 counts >500 cells/μl. HVL LTNPs were defined as having viral loads >10,000 RNA copies/ml and CD4 counts >500 cells/μl in the absence of cART for >7 years. VCs had low viral loads >50 RNA copies/ml but <2000 RNA copies/ml. The progressor group was identified in a prospective cohort [38]. The participants that were included were recruited from two prior studies; a randomized trial of preventative treatment against tuberculosis (TB) [39] and the Tshisimane Wellness cohort [40,41]. Progressors were HIV-infected with CD4 < 200 cells/μl and viral load >10,000 RNA copies/ml thus requiring cART. We used samples from the time-point prior to therapy initiation. Data for body mass index (BMI) was available for these patients. Peripheral blood, collected from the individuals in EDTA, was processed immediately by standard procedures and the plasma fraction stored at −80 °C until assays were carried out. Plasma samples from all 151 individuals were used for CD26/DPP4 analysis and peripheral blood mononuclear cells (PBMCs) from 5 healthy controls, 5 ECs and 6 progressors were used for flow cytometry analysis. Ethics approval for this study was granted by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand and informed written consent was obtained from all individuals prior to enrolment.
2.2. HIV-1 viral load and CD4 counts
The viral load determinations were performed using the Roche Amplicor RNA Monitor assay version 1.5 (Roche Diagnostic systems) and the CD4 T-cell counts were determined using the commercially available FACS Count System from Becton Dickinson (San Jose, CA, USA).
2.3. Quantitative determination of soluble DPP4/CD26 levels
The RayBio® Human CD26 ELISA kit (catalog # ELH-CD26; RayBiotech, Georgia, USA) was used for quantifying sDPP4 levels in plasma by following the manufacturer's recommendation. Samples were diluted 1:2000 and run in duplicate. This colorimetric assay was measured at 450 nm using a VersaMax™ microplate reader (Molecular Devices, California, USA), and optical densities calculated from the mean values of the standard curve were used to determine sample concentrations. The minimum detectable dose of human sDPP4/CD26 using this ELISA is 25 pg/ml.
2.4. Determination of plasma DPP4 activity
Plasma sDPP4 activity was measured by a fluorometric assay using the fluorogenic substrate H-Gly-Pro-AMC (catalog # MAK088, Sigma-Aldrich, Missouri, USA). This assay is based on the cleavage of 7-amino-4-methylcoumarin (AMC) moiety from the C-terminus of the peptide substrate, which increases its fluorescence intensity at 460 nm. In addition, a specific DPP4 inhibitor (Sitagliptin) was used to account for any activity not contributed by DPP4. Fluorescence intensity (λ excitation, 360 nm; λ emission, 460 nm) was measured by the Fluoroskan Ascent microplate fluorometer (Labsystems, Helsinki, Finland) in kinetic mode at 0, 4, 8, 12, 16 and 20-min time points. A standard curve was determined by using AMC fluorescence measurement and DPP4 activity was expressed as nmol of substrate converted per minute. We normalized total DPP4 activity to sDPP4 protein levels to account for individual variation, defined here as specific DPP4 activity expressed as nmol/min/ng of sDPP4. Results for both total DPP4 activity and specific DPP4 activity are presented.
2.5. Peripheral blood mononuclear cell staining and Flow Cytometry
PBMCs were isolated from EDTA anticoagulated whole blood by Ficoll density gradient separation using a standard protocol and stored in liquid nitrogen. Cryopreserved PBMCs from HCs (n = 5), ECs (n = 5) and progressors (n = 6) were rapidly thawed and washed twice with RPMI-1640 culture medium supplemented with 10 mM HEPES, 1% penicillin/streptomycin and 10% heat inactivated fetal bovine serum. After thawing, cell viability was determined with trypan blue staining. For each donor, DPP4/CD26 cell surface expression was assessed on different cell subsets in the following antibody panel: CD3-APC-H7, CD4-BV786, CD8-PerCP, CD26-PE, CD16-Pacific Blue, CD56-PeCy7, CD38-BV510, HLA-DR-PeCy5.5, CD14-FITC and the cell viability stain propidium iodide. The HLA-DR-PeCy5.5 (Invitrogen™, California, USA) antibody and propidium iodide (Molecular Probes™, Oregon, USA) were obtained from Thermo Fisher Scientific, RSA. All other antibodies were obtained from BD Pharmingen (BD Biosciences, California, USA). The average cell viability of the lymphocyte and monocyte populations were 98% and 87%, respectively. Multicolour flow cytometry was performed and at least 100,000 events were acquired on a four laser BD LSRFortessa™ X-20 cell analyzer (BD Biosciences) using BD FACSDiva™ software (BD Biosciences). Eight-peak Rainbow Calibration Particles (BD Biosciences) were run to standardize mean fluorescence intensity between experiments. Compensation was performed for each experiment using BD™ CompBeads (BD Biosciences) stained individually with each fluorochrome included in the panel. Fluorescence minus one (FMO) staining was used to set gates to differentiate negative and positive populations for the following antibodies: CD26-PE, CD16-Pacific Blue, CD56-PeCy7, CD38-BV510 and HLA-DR-PeCy5.5. Data were analyzed using FlowJo 7.6.1 (Tree Star, California, USA). Cell doublets were excluded using forward scatter-area versus forward scatter-height parameters. The lymphocyte population was identified based on forward and side scatter parameters. T-cells were defined as CD3-expressing lymphocytes and were further classified as CD4+ or CD8+ T-cells. Natural killer (NK) cells were defined as lymphocytes negative for CD3 and positive for CD56 and positive/negative for CD16 expression. The monocyte population was also first identified based on forward and side scatter parameters and then defined as CD14+ CD3− cells.
2.6. Statistical analyses
Mann–Whitney U tests were performed for comparisons between the HIV-1 infected and uninfected groups (Fig. 1 ). For Fig. 2, Fig. 4 , Kruskal-Wallis test with Dunn's multiple comparison post-test were used for comparisons between three or more groups. Adjusted P values are shown and P < 0.05 was considered significant. Spearman's rank coefficients (rs) were calculated to determine correlations between variables. GraphPad Prism version 7.03 was used for the Mann–Whitney U tests, Kruskal-Wallis tests and Spearman's rank correlation coefficient analyses.
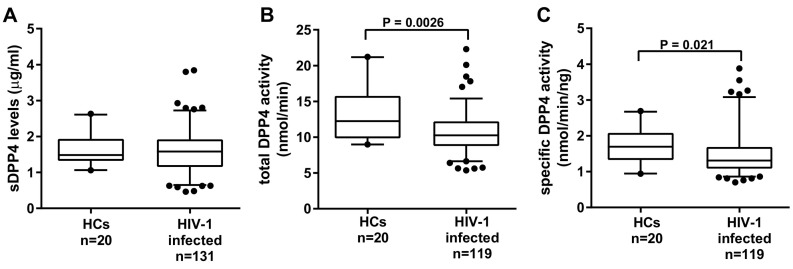
Fig. 1.
Characterization of sDPP4 levels activity in HIV-1 infection. (A) sDPP4 levels (B) total DPP4 activity and (C) specific DPP4 activity were measured in HIV-1 uninfected healthy controls (HC) and HIV infected group. Box-whisker plots depict the median (horizontal black line), 25th and 75th percentile (margins of the box) and the 5th and 95th percentiles (whiskers). Dots represent outliers.
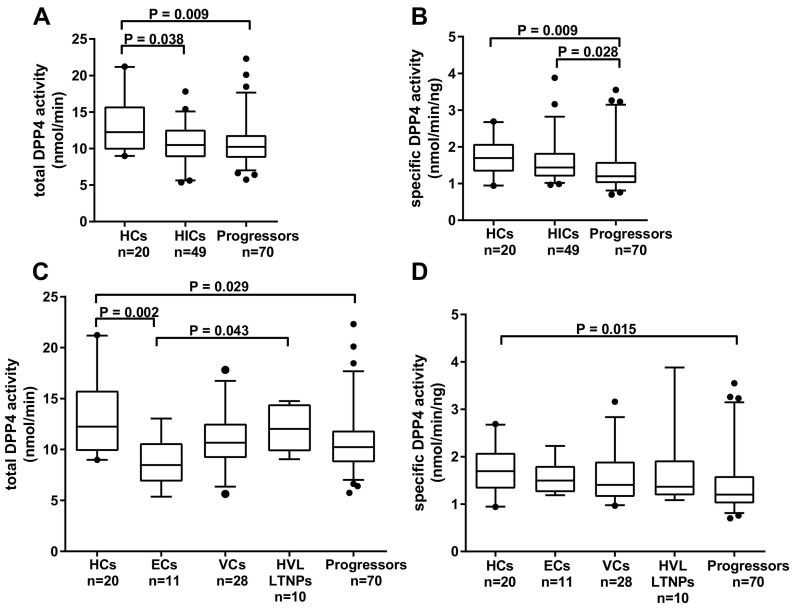
Fig. 2.
Analysis of DPP4 activity in HIV controllers and progressors. Comparisons between HIV-1 uninfected healthy controls (HCs) and the HIV-1 infected subgroups; HIV-1 controllers (HICs) and progressors were performed for (A) total DPP4 activity and (B) specific DPP4 activity. (C) Total DPP4 activity and (D) specific DPP4 activity were compared amongst the HIC subgroups; elite controllers (ECs), viraemic controllers (VCs) and high viral load long term non-progressors (HVL LTNPs) and between the progressors. Box-whisker plots depict the median (horizontal black line), 25th and 75th percentile (margins of the box) and the 5th and 95th percentiles (whiskers). Dots represent outliers.
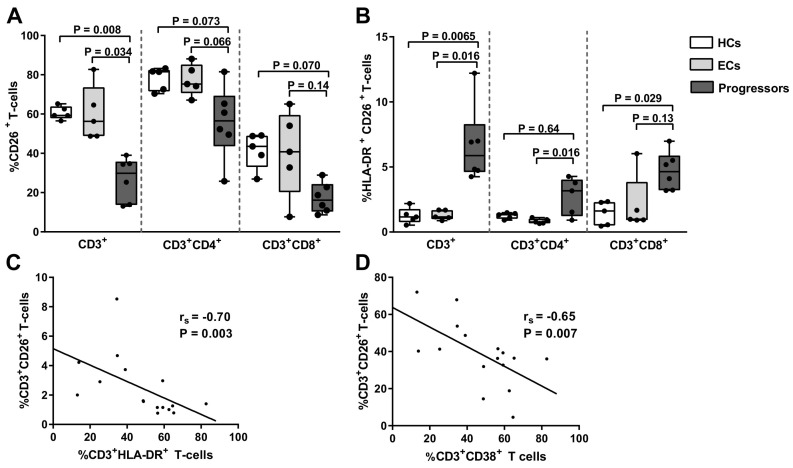
Fig. 4.
Relationship between T-cell activation and CD26 expression in elite controllers and progressors. (A) Frequency of CD3+CD26+ T-cells, CD4+CD26+ T-cells and CD8+CD26+ T-cells were compared amongst HIV-1 uninfected healthy controls (HCs) (n = 5), elite controllers (ECs) (n = 5) and progressors (n = 6). Percentage of (B) CD3+CD26+HLA-DR+ T-cells, CD4+CD26+HLA-DR+ T-cells and CD8+CD26+HLA-DR+ T-cells were compared amongst HCs (n = 5), ECs (n = 5) and progressors (n = 6). Box-whisker plots depict the median (horizontal black line), 25th and 75th percentile (margins of the box) and the min and max (whiskers). Dots represent all individuals. Correlation analysis was performed with all samples (n = 16) between frequency of CD3+CD26+ T-cells and (C) CD3+HLA-DR+ T-cells and (D) CD3+CD38+ T-cells.
3. Results
3.1. Characterization of study population
The healthy controls (HCs) group were HIV uninfected, non-diabetic and had a median age of 32 (range: 23–44) and was comprised entirely of females. Although no BMI data was available, none of the HCs were obese. The HIV-1 infected cohort had a median age of 38 (range: 23–65) and was comprised of 101 (85%) females and 18 (15%) males. Detailed characteristics of the study groups are described in Table 1. The distribution of females for each of the phenotypic groups was at least 80%. The median age of the HCs was lower than the ECs (P < 0.01) and HVL LTNPs (P < 0.01). There were no significant differences in age between the HIV-1 controller groups and the progressors. Correlation analysis showed no significant relationship between age and sDPP4 plasma levels (rs = 0.09, P = 0.23) and total activity (rs = −0.02, P = 0.79) in our cohort (Supplementary Fig. 1A and B). There was no significant correlation between BMI and sDPP4 levels but a significant weak positive relationship between BMI and total DPP4 activity (rs = 0.24, P = 0.048) in our progressor group (Supplementary Fig. 1C and D). Amongst progressors, 45.6%, 27.9%, and 26.5% had normal, overweight, and obese (> 30 kg/m2) BMIs, respectively (Supplementary Table 1). For flow cytometric analyses of membrane-bound DPP4/CD26, patients with extreme clinical phenotypes were selected: 5 elite controllers (median age 41 [range: 30–52], median CD4 515 [IQR: 505–822], viral load all <20 RNA copies/ml) and 6 progressors (median age 37 [range:27–43], median CD4 162 [IQR 159–179]), median viral load 67,832 [IQR 39,738–138, 836]. There were 4 females in each group.
Table 1.
Characteristics of the study population.
| Study group | n | Age (years) median (min - max) | Biological sex (% female) | CD4 count (cells/μl) median and IQR | Viral load (RNA copies/ml) median and IQR |
|---|---|---|---|---|---|
| HCs | 20 | 32 (23–44) | 100 | ||
| ECs | 16 | 43 (23–58) | 82.4 | 772 (594–924) | 20 (<20–95) |
| VCs | 28 | 39 (23–45) | 92.9 | 612 (558–826) | 473 (350–1076) |
| HVL LTNPs | 10 | 44 (35–55) | 80 | 680 (622–722) | 14,861 (11519–34,967) |
| Progressors | 76 | 37 (23–65) | 81.6 | 179 (152–220) | 42,975 (20145–110,710) |
HCs = healthy controls, ECs = elite controllers, VCs = viraemic controllers, HVL LTNPs = high viral load long term nonprogressors, IQR = interquartile range.
3.2. Specific DPP4 activity is associated with HIV-1 control
Previous evidence suggests high sDPP4 levels are protective against HIV-1 acquisition [25]. To determine whether sDPP4 is associated with HIV-1 control we characterized sDPP4 levels, total DPP4 activity and specific DPP4 activity in healthy controls (HCs) and HIV-1 infected individuals. sDPP4 levels did not differ between HIV-1 infected and HC groups (P = 0.69) (Fig. 1A). However, both total DPP4 activity and specific DPP4 activity were significantly lower in the presence of HIV-1 infection (Fig. 1B, P = 0.0026 and Fig. 1C, P = 0.021). Correlation analysis revealed a positive relationship between sDPP4 levels and total DPP4 activity in the HC individuals (rs = 0.53, P = 0.016) and HIV-1 infection did not alter this relationship (rs = 0.48, P < 0.0001) (Supplementary Fig. 2A and B). When the HIV-1 group was stratified into two subgroups; HIV-1 controllers (HICs) and progressors - sDPP4 levels did not differ between these subgroups (data not shown). They also shared similar total DPP4 activity, each in turn lower than HCs (P = 0.038 and P = 0.009, respectively) (Fig. 2A). Progressors had significantly lower specific DPP4 activity than HICs (P = 0.028) and HCs (P = 0.009) (Fig. 2B). However, there was no significant difference in specific DPP4 activity between HICs and HCs. To further characterize sDPP4 in HIV-1 control, the HIC group was stratified by viral load into EC, VC and HVL LTNP subgroups. As for the comparison of HICs and progressors sDPP4 protein levels did not differ between each of the HIV-1 controller subgroups and the progressors (data not shown). The ECs had lower total DPP4 activity compared to HVL LTNPs and to HCs (P = 0.043 and P = 0.002, respectively) (Fig. 2C). However, once DPP4 activity was normalized to sDPP4 levels this difference did not remain (Fig. 2D). This would suggest that the differences in total activity may be due to variation in sDPP4 levels and not enzyme function. Progressors had lower specific activity than HCs (P = 0.015), while there were no significant differences in specific activity between the controller subgroups and HCs (Fig. 2D). Given that the HC group was comprised only of females, we questioned whether biological sex had an influence on our group comparisons. Therefore, we analyzed the specific activity using only the female dataset. However, exclusion of the male dataset did not change the trend of the data (data not shown).
3.3. Specific DPP4 activity inversely correlated with viral load and positively correlated with CD4 count
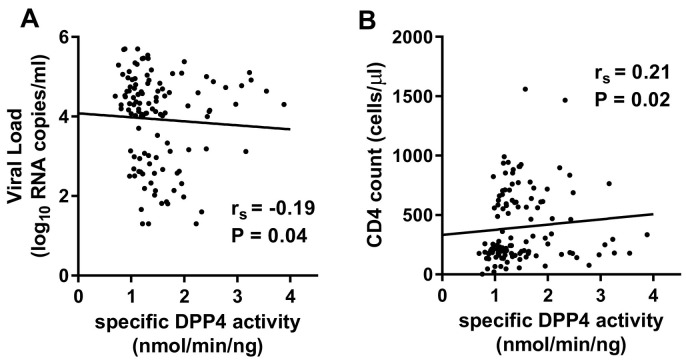
It has been previously suggested that low total and specific DPP4 activity is associated with HIV-1 disease progression [22,23]. Hence, we investigated the relationship between sDPP4, HIV-1 viral load and CD4 count. There were no significant correlations between sDPP4 levels and viral load and total DPP4 activity and viral load. However, there was a weak negative correlation between specific DPP4 activity and viral load (rs = −0.19, P = 0.04) (Fig. 3A). Similarly, there were no significant relationships between sDPP4 levels and CD4 counts and total DPP4 activity and CD4 counts. However, there was a weak positive correlation between CD4 counts and specific DPP4 activity (rs = 0.21, P = 0.02) (Fig. 3B).
Fig. 3.
Correlation between specific DPP4 activity, HIV-1 viral load and CD4 counts. (A) Specific DPP4 activity inversely correlated with HIV-1 viral load and (B) positively correlated with CD4 counts.
3.4. High DPP4/CD26 expression correlated with low T-cell activation
Since high cell surface DPP4/CD26 expression has been suggested to be protective against HIV-1 acquisition [25], we hypothesized that HIV-1 controllers have higher CD26 cell surface expression than progressors. To test this hypothesis, we selected a small subset of patients with the extreme clinical phenotypes; ECs and progressors and compared cell surface CD26 expression. Selecting extreme phenotypes allows for a greater likelihood of detecting differences on small sample numbers. Previous studies suggest that in PBMCs, CD26 is predominantly expressed on T-cells [42,43]. In agreement, in the HCs, we found that CD26 was expressed by a median of 52.6% CD3+ T-cells while only 2.9% CD3− lymphocytes and 0.07% CD14+ monocytes expressed CD26. CD26 was also expressed by a median of 8.6% CD56+CD16− and 0.5% CD56+CD16+ NK cells, and by an estimated maximum of 3.9% B cells (CD3−CD56−CD16−). Significant differences in CD26 expression were noted between ECs and progressors, in terms of percentage T-cells that express CD26. ECs and HIV-uninfected controls had similar median frequencies of CD3+ (56.3% vs. 59.3%), CD4+ (75.3% vs. 81.5%) and CD8+ (40.8% vs. 43.5%) T-cells expressing CD26 (Fig. 4A). Progressors had substantially reduced median frequencies of CD3+ T-cells expressing CD26 than both ECs (29.9% vs. 56.3%, P = 0.034) and HIV-uninfected controls (29.9% vs. 59.3%, P = 0.008) (Fig. 4A). Similar trends were seen for CD4+ and CD8+ T-cells. There were no differences in CD26 median fluorescence intensity (MFI) between the groups (data not shown).
A lowered immune activation state has also been suggested to be protective against HIV-1 acquisition [44], and it is well-established that higher immune activation associates with more progressive HIV-1 disease [45,46]. Using HLA-DR and CD38 as markers of cellular activation we found no significant differences in NK cell and monocyte activation between the HCs, ECs and progressors (data not shown). However, there were significant differences evident in T-cell activation between the three groups. As expected, progressors had higher proportions of CD3+ T-cells expressing HLA-DR and CD38 than HCs and ECs (data not shown). Furthermore, progressors had significantly more CD3+CD26+ and CD8+CD26+ T-cells expressing HLA-DR than HCs (P = 0.0065 and P = 0.029) (Fig. 4B). A similar trend was seen for CD4+CD26+ T-cells (Fig. 4B). Progressors also had significantly higher frequencies of CD3+CD26+ T-cells and CD4+CD26+ T-cells expressing HLA-DR than ECs (P = 0.016 and P = 0.016). There were no significant differences in frequency of CD26+CD38+ T-cells between the groups (data not shown). We next investigated the relationship between CD26 expression and T-cell activation. There were no significant correlations between sDPP4 levels and %HLA-DR+ T-cells (rs = 0.004, P = 0.86) or %CD38+ T-cells (rs = 0.21, P = 0.54). However, there was a strong inverse correlation between frequency of CD26-expressing T-cells and that of HLA-DR-expressing T-cells (rs = −0.7, P = 0.003) (Fig. 4C). Moreover, the frequency of CD26-expressing T-cells strongly negatively correlated with CD38-expressing T-cells (rs = −0.65, P = 0.007) (Fig. 4D).
4. Discussion
With the current COVID-19 pandemic, there is a need to identify biomarkers that could predict more severe disease outcomes of SARS-CoV-2 infection in high risk populations. It has been suggested that DPP4 could be a potential marker for COVID-19 risk stratification and disease progression in aging patients, those with diabetes and possibly other highly susceptible populations [21]. HIV-infected individuals are thought to be at greater risk of more severe COVID-19 outcomes; however, this remains to be conclusively established. In order to gain insights into this risk based on DPP4, we characterized different DPP4 measures (plasma levels, total and specific enzyme activity and DPP4/CD26 surface expression), in a cohort of untreated South African HIV-1 infected patients compared to healthy HIV-uninfected controls. In agreement with findings from a Japanese cohort [22] and two French cohorts [23,24], we showed that total DPP4 activity and specific DPP4 activity is reduced in HIV-1 infection. Consistent with previous findings [22], we also showed that specific DPP4 activity inversely correlated with HIV-1 viral load. These results suggest HIV-1 may inhibit DPP4 activity and are supported by multiple functional studies that show HIV-1 viral protein Tat binds to DPP4/CD26 thereby inhibiting DPP4 activity [[47], [48], [49], [50], [51], [52]]. It has also been proposed that hypersialylation of DPP4/CD26 in HIV-1 infection promotes binding of HIV peptides, Tat and gp120, through their cationic domains to the sialic acid residues of DPP4/CD26 [52]. Although gp120 also binds DPP4/CD26 [[52], [53], [54]], evidence on whether this interaction results in inhibition of DPP4 activity is less clear [52].
Previous studies suggested DPP4/CD26 could be used as a potential biomarker in the field of HIV cure/remission research [23,55]. sDPP4 levels has been suggested to be a surrogate marker for ART efficacy in children whereby a study in a Kenyan cohort showed sDPP4 levels increased after cART initiation which correlated with a decrease in viral load and increase in CD4 counts [55]. However, in adults, sDPP4 levels did not appear to be a suitable marker for ART efficacy. In a French adult cohort (ANRS COPANA), sDPP4 levels did not increase after cART initiation [23]. For disease progression, sDPP4 levels did not appear to be an insightful marker in our adult cohort since sDPP4 levels were not different between healthy controls, HIV-1 controllers and progressors. However, DPP4 enzyme activity showed to be a promising marker of disease progression in both our cohort and the French cohort [23]. In our study, progressors had lower specific DPP4 activity than HIV-1 controllers and uninfected individuals, while there was no difference between HIV-1 controllers and uninfected controls. Our results therefore suggest progressive infection is associated with lowered DPP4 activity, while HIV-1 control is associated with preserved DPP4 activity. The French study also suggested that higher DPP4 activity was associated with protection against HIV-1 whereby HIV-1 patients with low levels of total or specific DPP4 activity had an increased risk of rapid disease progression, and rapid progressors had lower DPP4 activity than slow progressors [23].
A potential mechanism of DPP4 protection against HIV-1 may be linked to the role of DPP4 in Th17-mediated immune response. DPP4 is expressed by Th17 cells in peripheral blood and inflamed mucosal tissue – with a larger proportion of Th17 cells and DPP4+ Th17 cells in mucosal tissue [56]. Mucosal Th17 cells play a crucial role in maintaining mucosal barrier integrity and preventing inflammation and have been suggested to be protective against HIV-1 [57,58]. In addition, peripheral Th17 cells have been linked to HIV-1 control [59] and HIV-induced loss of peripheral Th17 cells has been suggested to impair mucosal immunity reconstitution [60,61]. It has been suggested that DPP4 enzyme activity contributes to the immune response mobilized by Th17 cells [56]. It has also been suggested that peripheral DPP4 activity could be a surrogate marker for HIV-induced loss of mucosal Th17 cells [23]. Ploquin and colleagues showed that in the gut of SIV-infected non-human primates, peripheral DPP4 activity was increased in animals treated with IL-21 and that this increase was associated with restoration of the Th17 compartment and reduced inflammation [23].
Our findings show an association between high DPP4 activity and protection against HIV-1 and is supported by previous biological evidence on DPP4 mechanism of action. Previous biological studies show DPP4 activity increases the potency of CCR1 and CCR5 agonists which leads to protection against HIV-1 [62,63]. Lower DPP4 activity would result in less efficient cleavage of these receptor agonists which may lead to increased viral replication. Preserved DPP4 activity may also be crucial for the protective properties of interferon gamma-induced protein 10 (CXCL-10/IP-10) against HIV-1. IP-10 is a CXC chemokine which recruits activated memory T-cells to sites of inflammation and is shown to be a predictor of HIV-1 disease progression [64]. However, IP-10 can be cleaved by DPP4 to produce short IP-10 which acts an antagonist of native IP-10 and therefore inhibits recruitment of activated T-cells and may reduce infection. Consistent with lower DPP4 activity, previous evidence shows rapid progressors have lower levels of short IP-10 than slow progressors [65].
Based on prior studies on DPP4/CD26 and HIV-1 resistance [25,66], we hypothesized that HIV-1 controllers may have higher CD26 cell-membrane bound expression than progressors. We showed progressors have lower CD26 expression on T-cells, but higher proportions of activated T-cells compared to elite controllers and uninfected individuals, while elite controllers had similar CD26 expression to uninfected individuals. Although flow cytometric analyses were performed on a small number of individuals, and broad variation in percentages of some cell subsets was observed, there was no overlap in CD26 expression data when comparing total CD3+ T-cells of elite controllers or uninfected individuals with progressors. Our results suggest HIV-1 control is associated with higher cell surface CD26 expression, but low T-cell activation, as evidenced by strong inverse relationships between T-cells expressing CD26 and T-cells expressing HLA-DR or CD38. Our findings are supported by biological evidence that show CD26+ (DPP4+) Jurkat cells are more resistant to HIV-1 infection than CD26+ (DPP4−) cells [66]. Consistent with our findings, it has been suggested that higher CD26 expression and a quiescent immune activation state may be protective against HIV-1 [25,44]. Songok and colleagues showed in a Kenyan sex worker cohort that HIV-1 resistant women had higher cell surface expression and soluble CD26 levels compared to HIV-positive and negative women [25]. Further analysis showed that these HIV-1 resistant women also had a gene expression signature suggestive of a lowered immune activation state [44]. Activated CD4+ T-cells are more prone to HIV-1 infection and promote higher levels of viral replication than resting cells [67,68] therefore low levels of these target cells may be advantageous in both control of HIV-1 disease progression and protection against HIV-1 infection.
Previous data from our larger cohort showed that HIV-1 infected individuals with BMIs in the obese and overweight range had reduced risk of mortality and infection with M. tuberculosis (TB) [41]. Findings from our progressors, a subset of this larger cohort [41], showed that BMI was positively correlated with total DPP4 activity - consistent with DPP4 being a biological driver for the protective effects against mortality and TB. A limitation of our study was that we did not have BMI data for all individuals and therefore could not compare BMI between our groups. Nevertheless, a biological influence of BMI on HIV-1 disease progression may potentially be mediated by DPP4. Biological studies show that DPP4 is an adipokine (a signalling protein via which adipose tissue cells communicate with other organs/cells), and increased release of DPP4 correlates with size of fat cells [69]. Considering DPP4 is associated with type 2 diabetes, another potential limitation of our study is that we did not have data for glucose levels for our participants. However, it is well-known that DPP4 regulates glucose homeostasis [70,71]. Therefore, similarly to BMI, a potential influence of glucose levels on mechanisms of HIV-1 control may be biologically mediated via DPP4. Taken together, findings from our study support the hypothesis that high circulating sDPP4 activity and high membrane-bound CD26 expression with low T-cell activation may be important for controlling HIV-1 disease progression.
Risk factors for developing severe COVID-19 include obesity, type 2 diabetes, aging and potentially HIV-1 – all linked to DPP4. In HIV-1, we show that high DPP4/CD26 has potentially protective effects against disease progression. However, this is in contrast to type 2 diabetes, obesity and aging. High circulating sDPP4 levels and activity are characteristic of type 2 diabetes [72,73]. In obesity, DPP4 promotes adipose inflammation and insulin resistance [74]. Evidence also suggests, high DPP4 activity is associated with aging [75,76] and impaired cognitive function in the elderly >60 years old [[77], [78], [79]]. Severe COVID-19 is a hyperinflammatory disease [80]. Therefore it is likely that high DPP4 activity may be linked to regulation of immune activation and hyperinflammatory disease in severe and critically ill COVID-19 patients [15]. In this regard, there has been discussion about the potential use of DPP4 inhibitors in COVID-19 patients with diabetes and markers of hyperinflammation [15,20,[81], [82], [83], [84]]. DPP4 inhibitors have been clinically tested in HIV-1 infected non-diabetic individuals for potential treatment of cardiometabolic disorders [85,86]. These studies showed that DPP4 inhibitors had no effect on immune activation [85,86] but improved glycemia [85] in HIV-1 infected virally suppressed individuals on ART. However, if based on sDPP4 activity alone, it could be argued that HIV-1 infected individuals, particularly progressors, might be protected from developing severe outcomes of COVID-19. However, it is unlikely that any one factor alone would drive such a response. For example, progressors are more likely to have poor HIV-specific CD4 and CD8 T-cell responses and are more immunosuppressed, factors likely to impact their susceptibility to any secondary infections. Future studies of HIV-1 infected individuals coinfected with SARS-CoV-2 are needed to understand the apparent divergent effects of levels of sDPP4 activity, and to establish the extent to which HIV-infected individuals of various clinical phenotypes (controllers, progressors, virologically suppressed on ART) are vulnerable to developing severe COVID-19.
The following are the supplementary data related to this article.
Body mass Index (BMI) characteristics of HIV-1 Progressors.
Relationship between sDPP4, age and body mass index (BMI). Correlation analysis was performed between age of all participants and (A) sDPP4 levels and (B) total DPP4 activity. Correlation between BMI and (C) sDPP4 levels and (D) total DPP4 activity was performed using data from the HIV-1 progressor group.
Relationship between sDPP4 levels and total DPP4 activity. sDPP4 levels positively correlated with total DPP4 activity in both (A) HIV-1 uninfected and (B) HIV-1 infected groups.
Funding
This work is based on the research supported by the Strategic Health Innovation Partnerships (SHIP) Unit of the South African Medical Research Council (SA MRC), a grantee of the Bill & Melinda Gates Foundation, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, the Poliomyelitis Research Foundation and the SA MRC through the Soweto Matlosana Centre for HIV/AIDS and TB Research (SoMCHAT). The study of HIV-Associated lung infections in Soweto (progressor group) was funded by the National Institutes of Health, USA (R01HL090312 and P30AI094189: R. E. Chaisson).
Conflicts of interest
All authors report no conflicts of interest.
Acknowledgements
We thank all the individuals who participated in the study.
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. Nature Microbiology. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y., Ho W., Huang Y., et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet (London, England) 2020;395(10228):949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Fang X., Cai Z., et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Washington, DC) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskaran K., Rentsch C.T., MacKenna B., et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The Lancet HIV. 2021;8(1):e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drain P.K., Garrett N. SARS-CoV-2 pandemic expanding in sub-Saharan Africa: considerations for COVID-19 in people living with HIV. EClinicalMedicine. 2020:100342. doi: 10.1016/j.eclinm.2020.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies M.A. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. medRxiv. 2020:1–21. doi: 10.1101/2020.07.02.20145185. the preprint server for health sciences. [DOI] [Google Scholar]
- 7.Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020;9(1):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aliyari Serej Z., Ebrahimi Kalan A., Mehdipour A., Nozad Charoudeh H. Regulation and roles of CD26/DPPIV in hematopoiesis and diseases. Biomed. Pharmacother. = Biomed. Pharmacother. 2017;91:88–94. doi: 10.1016/j.biopha.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuki T., Tsuda H., Morimoto C. Good or evil: CD26 and HIV infection. J. Dermatol. Sci. 2000;22(3):152–160. doi: 10.1016/s0923-1811(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 13.Shao S., Xu Q., Yu X., Pan R., Chen Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020;209:107503. doi: 10.1016/j.pharmthera.2020.107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nistala R., Savin V. Diabetes, hypertension, and chronic kidney disease progression: role of DPP4. Am. J. Physiol. Ren. Physiol. 2017;312(4):F661–F670. doi: 10.1152/ajprenal.00316.2016. [DOI] [PubMed] [Google Scholar]
- 15.Bassendine M.F., Bridge S.H., McCaughan G.W., Gorrell M.D. Covid-19 and co-morbidities: a role for Dipeptidyl Peptidase 4 (DPP4) in disease severity? J. Diabetes. 2020;12(9):649–658. doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- 16.Malavazos A.E., Corsi Romanelli M.M., Bandera F., Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity. 2020;28(7):1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorrell M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005;108(4):277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 18.Gorrell M.D., Gysbers V., McCaughan G.W. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 2001;54(3):249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto C., Schlossman S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 20.Strollo R., Pozzilli P. DPP4 inhibition: preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab. Res. Rev. 2020;163 doi: 10.1016/j.diabres.2020.108165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barchetta I., Cavallo M.G., Baroni M.G. COVID-19 and diabetes: is this association driven by the DPP4 receptor? Potential clinical and therapeutic implications. Diabetes Res. Clin. Pract. 2020;163:108165. doi: 10.1016/j.diabres.2020.108165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosono O., Homma T., Kobayashi H., et al. Decreased dipeptidyl peptidase IV enzyme activity of plasma soluble CD26 and its inverse correlation with HIV-1 RNA in HIV-1 infected individuals. Clin. Immunol. 1999;91(3):283–295. doi: 10.1006/clim.1999.4711. [DOI] [PubMed] [Google Scholar]
- 23.Ploquin M.J., Casrouge A., Madec Y., et al. Systemic DPP4 activity is reduced during primary HIV-1 infection and is associated with intestinal RORC(+) CD4(+) cell levels: a surrogate marker candidate of HIV-induced intestinal damage. J. Int. AIDS Soc. 2018;21(7) doi: 10.1002/jia2.25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gougeon M.L., Lecoeur H., Callebaut C., et al. Selective loss of the CD4+/CD26+ T-cell subset during HIV infection. Res. Immunol. 1996;147(1):5–8. doi: 10.1016/0923-2494(96)81544-6. [DOI] [PubMed] [Google Scholar]
- 25.Songok E.M., Osero B., McKinnon L., et al. CD26/dipeptidyl peptidase IV (CD26/DPPIV) is highly expressed in peripheral blood of HIV-1 exposed uninfected female sex workers. Virol. J. 2010;7:343. doi: 10.1186/1743-422X-7-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell K.A., Bailey J.R., Blankson J.N. Elucidating the elite: mechanisms of control in HIV-1 infection. Trends Pharmacol. Sci. 2009;30(12):631–637. doi: 10.1016/j.tips.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Theze J., Chakrabarti L.A., Vingert B., Porichis F., Kaufmann D.E. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin. Immunol. 2011;141(1):15–30. doi: 10.1016/j.clim.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalo-Gil E., Ikediobi U., Sutton R.E. Mechanisms of virologic control and clinical characteristics of HIV+ elite/Viremic controllers. Yale J. Biol. Med. 2017;90(2):245–259. [PMC free article] [PubMed] [Google Scholar]
- 29.Sabin C.A., Lundgren J.D. The natural history of HIV infection. Curr. Opin. HIV AIDS. 2013;8(4):311–317. doi: 10.1097/COH.0b013e328361fa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muenchhoff M., Adland E., Karimanzira O., et al. Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci. Transl. Med. 2016;8(358):358ra125. doi: 10.1126/scitranslmed.aag1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhary S.K., Vrisekoop N., Jansen C.A., et al. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J. Virol. 2007;81(16):8838–8842. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotger M., Dalmau J., Rauch A., et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J. Clin. Invest. 2011;121(6):2391–2400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klatt N.R., Bosinger S.E., Peck M., et al. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS Pathog. 2014;10(8) doi: 10.1371/journal.ppat.1004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picton A.C.P., Paximadis M., Chaisson R.E., Martinson N.A., Tiemessen C.T. CXCR6 gene characterization in two ethnically distinct South African populations and association with viraemic disease control in HIV-1-infected black South African individuals. Clin. Immunol. 2017;180:69–79. doi: 10.1016/j.clim.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koor G.W., Paximadis M., Picton A.C.P., et al. Cis-regulatory genetic variants in the CCR5 gene and natural HIV-1 control in black South Africans. Clin. Immunol. 2019;205:16–24. doi: 10.1016/j.clim.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestri G. AIDS pathogenesis: a tale of two monkeys. J. Med. Primatol. 2008;37(Suppl. 2):6–12. doi: 10.1111/j.1600-0684.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 37.He T., Brocca-Cofano E., Gillespie D.G., et al. Critical role for the adenosine pathway in controlling simian immunodeficiency virus-related immune activation and inflammation in gut mucosal tissues. J. Virol. 2015;89(18):9616–9630. doi: 10.1128/JVI.01196-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupte A.N., Wong M.L., Msandiwa R., et al. Factors associated with pulmonary impairment in HIV-infected South African adults. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinson N.A., Barnes G.L., Moulton L.H., et al. New regimens to prevent tuberculosis in adults with HIV infection. N. Engl. J. Med. 2011;365(1):11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golub J.E., Pronyk P., Mohapi L., et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. Aids. 2009;23(5):631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanrahan C.F., Golub J.E., Mohapi L., et al. Body mass index and risk of tuberculosis and death. Aids. 2010;24(10):1501–1508. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierson D.M., Jones D., Muzzafar T., et al. Utility of CD26 in flow cytometric immunophenotyping of T-cell lymphomas in tissue and body fluid specimens. Cytometry B Clin. Cytom. 2008;74(6):341–348. doi: 10.1002/cyto.b.20431. [DOI] [PubMed] [Google Scholar]
- 43.Ohnuma K., Dang N.H., Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29(6):295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Songok E.M., Luo M., Liang B., et al. Microarray analysis of HIV resistant female sex workers reveal a gene expression signature pattern reminiscent of a lowered immune activation state. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn S.D., Butera S.T., Folks T.M. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 2001;14(4):753–777. doi: 10.1128/CMR.14.4.753-777.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utay N.S., Hunt P.W. Role of immune activation in progression to AIDS. Curr. Opin. HIV AIDS. 2016;11(2):131–137. doi: 10.1097/COH.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutheil W.G., Subramanyam M., Flentke G.R., et al. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat’s immunosuppressive activity. Proc. Natl. Acad. Sci. U. S. A. 1994;91(14):6594–6598. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wrenger S., Reinhold D., Hoffmann T., et al. The N-terminal X-X-Pro sequence of the HIV-1 Tat protein is important for the inhibition of dipeptidyl peptidase IV (DP IV/CD26) and the suppression of mitogen-induced proliferation of human T cells. FEBS Lett. 1996;383(3):145–149. doi: 10.1016/0014-5793(96)00221-9. [DOI] [PubMed] [Google Scholar]
- 49.Wrenger S., Hoffmann T., Faust J., et al. The N-terminal structure of HIV-1 Tat is required for suppression of CD26-dependent T cell growth. J. Biol. Chem. 1997;272(48):30283–30288. doi: 10.1074/jbc.272.48.30283. [DOI] [PubMed] [Google Scholar]
- 50.Weihofen W.A., Liu J., Reutter W., Saenger W., Fan H. Crystal structures of HIV-1 Tat-derived nonapeptides Tat-(1-9) and Trp2-Tat-(1-9) bound to the active site of dipeptidyl-peptidase IV (CD26) J. Biol. Chem. 2005;280(15):14911–14917. doi: 10.1074/jbc.M413400200. [DOI] [PubMed] [Google Scholar]
- 51.Tansi F.L., Blanchard V., Berger M., Tauber R., Reutter W., Fan H. Interaction of human dipeptidyl peptidase IV and human immunodeficiency virus type-1 transcription transactivator in Sf9 cells. Virol. J. 2010;7:267. doi: 10.1186/1743-422X-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith R.E., Talhouk J.W., Brown E.E., Edgar S.E. The significance of hypersialylation of dipeptidyl peptidase IV (CD26) in the inhibition of its activity by Tat and other cationic peptides. CD26: a subverted adhesion molecule for HIV peptide binding. AIDS Res. Hum. Retrovir. 1998;14(10):851–868. doi: 10.1089/aid.1998.14.851. [DOI] [PubMed] [Google Scholar]
- 53.Valenzuela A., Blanco J., Callebaut C., et al. Adenosine deaminase binding to human CD26 is inhibited by HIV-1 envelope glycoprotein gp120 and viral particles. J. Immunol. 1997;158(8):3721–3729. [PubMed] [Google Scholar]
- 54.Blanco J., Valenzuela A., Herrera C., Lluis C., Hovanessian A.G., Franco R. The HIV-1 gp120 inhibits the binding of adenosine deaminase to CD26 by a mechanism modulated by CD4 and CXCR4 expression. FEBS Lett. 2000;477(1–2):123–128. doi: 10.1016/s0014-5793(00)01751-8. [DOI] [PubMed] [Google Scholar]
- 55.Maina A., Mbugua J., Trajtman A., et al. The potential for DPPIV/CD26 usage as a surrogate marker for antiretroviral therapy efficacy in HIV infected populations. Afr. J. Pharmacol. Ther. 2016;5(4):263–271. [Google Scholar]
- 56.Bengsch B., Seigel B., Flecken T., Wolanski J., Blum H.E., Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26) J. Immunol. 2012;188(11):5438–5447. doi: 10.4049/jimmunol.1103801. [DOI] [PubMed] [Google Scholar]
- 57.Schuetz A., Deleage C., Sereti I., et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12) doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandiyan P., Younes S.A., Ribeiro S.P., et al. Mucosal regulatory T cells and T helper 17 cells in HIV-associated immune activation. Front. Immunol. 2016;7:228. doi: 10.3389/fimmu.2016.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salgado M., Rallon N.I., Rodes B., Lopez M., Soriano V., Benito J.M. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin. Immunol. 2011;139(2):110–114. doi: 10.1016/j.clim.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Marquez-Coello M., Montes-de-Oca Arjona M., Fernandez-Gutierrez Del Alamo C., Ruiz-Sanchez C., Giron-Gonzalez J.A. Peripheral Th17 cells expressing beta7 intestinal homing receptor in recent and chronic HIV infections. Clin. Exp. Immunol. 2018;194(3):350–360. doi: 10.1111/cei.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prendergast A., Prado J.G., Kang Y.H., et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24(4):491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 62.Proost P., De Meester I., Schols D., et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J. Biol. Chem. 1998;273(13):7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 63.Proost P., Menten P., Struyf S., Schutyser E., De Meester I., Van Damme J. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96(5):1674–1680. [PubMed] [Google Scholar]
- 64.Liovat A.S., Rey-Cuille M.A., Lecuroux C., et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Müller-trutwin M., Ploquin M., Albert M., Madec Y., Goujard C., Meyer L., Inventors; Institut Pasteur (Paris, FR), assignee . 2015. Levels of CXCL-10/IP-10 Forms and Soluble CD26/DPPIV Activity as Early Predictive Biomarkers for HIV/SIV Associated Mucosal Inflammation and Progression Towards AIDS. [Google Scholar]
- 66.Morimoto C., Lord C.I., Zhang C., Duke-Cohan J.S., Letvin N.L., Schlossman S.F. Role of CD26/dipeptidyl peptidase IV in human immunodeficiency virus type 1 infection and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1994;91(21):9960–9964. doi: 10.1073/pnas.91.21.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevenson M., Stanwick T.L., Dempsey M.P., Lamonica C.A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meditz A.L., Haas M.K., Folkvord J.M., et al. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J. Virol. 2011;85(19):10189–10200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamers D., Famulla S., Wronkowitz N., et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60(7):1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trzaskalski N.A., Fadzeyeva E., Mulvihill E.E. Dipeptidyl peptidase-4 at the interface between inflammation and metabolism. Clin. Med. Insights Endocrinol. Diabetes. 2020;13 doi: 10.1177/1179551420912972. 1179551420912972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drucker D.J. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.A., Kim Y.R., Yang E.J., et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2013;98(6):2553–2561. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 73.Barchetta I., Ciccarelli G., Barone E., et al. Greater circulating DPP4 activity is associated with impaired flow-mediated dilatation in adults with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2019;29(10):1087–1094. doi: 10.1016/j.numecd.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Ghorpade D.S., Ozcan L., Zheng Z., et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555(7698):673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa Y., Hayashi K., Takemoto Y., et al. DPP-4 inhibition with linagliptin ameliorates the progression of premature aging in klotho−/− mice. Cardiovasc. Diabetol. 2017;16(1):154. doi: 10.1186/s12933-017-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim K.M., Noh J.H., Bodogai M., et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 2017;31(15):1529–1534. doi: 10.1101/gad.302570.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B., Zheng T., Qin L., et al. Strong association between plasma dipeptidyl Peptidase-4 activity and impaired cognitive function in elderly population with normal glucose tolerance. Front. Aging Neurosci. 2017;9:247. doi: 10.3389/fnagi.2017.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao L., Ge B., Chen X., et al. The relationship between plasma DPP4 activity to BDNF ratio and mild cognitive impairment in elderly population with normal glucose tolerance. Front. Aging Neurosci. 2019;11:33. doi: 10.3389/fnagi.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng T., Qin L., Chen B., et al. Association of Plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care. 2016;39(9):1594–1601. doi: 10.2337/dc16-0316. [DOI] [PubMed] [Google Scholar]
- 80.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceriello A., Stoian A.P., Rizzo M. COVID-19 and diabetes management: what should be considered? Diabetes Res. Clin. Pract. 2020;163:108151. doi: 10.1016/j.diabres.2020.108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C.F., Chien C.H., Yang Y.P., et al. Role of dipeptidyl peptidase 4 inhibitors in diabetic patients with Coronavirus-19 infection. J. Chin. Med. Assoc. 2020;83(8):710–711. doi: 10.1097/JCMA.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drucker D.J. Coronavirus infections and Type 2 Diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020;41(3) doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020;162:108125. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodwin S.R., Reeds D.N., Royal M., Struthers H., Laciny E., Yarasheski K.E. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J. Clin. Endocrinol. Metab. 2013;98(2):743–751. doi: 10.1210/jc.2012-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dube M.P., Chan E.S., Lake J.E., et al. A randomized, double-blinded, placebo-controlled trial of Sitagliptin for reducing inflammation and immune activation in treated and suppressed human immunodeficiency virus infection. Clin. Infect. Dis. 2019;69(7):1165–1172. doi: 10.1093/cid/ciy1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body mass Index (BMI) characteristics of HIV-1 Progressors.
Relationship between sDPP4, age and body mass index (BMI). Correlation analysis was performed between age of all participants and (A) sDPP4 levels and (B) total DPP4 activity. Correlation between BMI and (C) sDPP4 levels and (D) total DPP4 activity was performed using data from the HIV-1 progressor group.
Relationship between sDPP4 levels and total DPP4 activity. sDPP4 levels positively correlated with total DPP4 activity in both (A) HIV-1 uninfected and (B) HIV-1 infected groups.