Abstract
Background
Rapid assessment of COVID-19 vaccine safety during pregnancy is urgently needed.
Methods
We conducted a rapid systematic review, to evaluate the safety of COVID-19 vaccines selected by the COVID-19 Vaccines Global Access-Maternal Immunization Working Group in August 2020, including their components and their technological platforms used in other vaccines for pregnant persons. We searched literature databases, COVID-19 vaccine pregnancy registries, and explored reference lists from the inception date to February 2021 without language restriction. Pairs of reviewers independently selected studies through COVIDENCE, and performed the data extraction and the risk of bias assessment. Discrepancies were resolved by consensus. Registered on PROSPERO (CRD42021234185).
Results
We retrieved 6757 records and 12 COVID-19 pregnancy registries from the search strategy; 38 clinical and non-clinical studies (involving 2,398,855 pregnant persons and 56 pregnant animals) were included. Most studies (89%) were conducted in high-income countries and were cohort studies (57%). Most studies (76%) compared vaccine exposures with no exposure during the three trimesters of pregnancy. The most frequent exposure was to AS03 adjuvant, in the context of A/H1N1 pandemic influenza vaccines, (n = 24) and aluminum-based adjuvants (n = 11). Only one study reported exposure to messenger RNA in lipid nanoparticles COVID-19 vaccines. Except for one preliminary report about A/H1N1 influenza vaccination (adjuvant AS03), corrected by the authors in a more thorough analysis, all studies concluded that there were no safety concerns.
Conclusion
This rapid review found no evidence of pregnancy-associated safety concerns of COVID-19 vaccines or of their components or platforms when used in other vaccines. However, the need for further data on several vaccine platforms and components is warranted, given their novelty. Our findings support current WHO guidelines recommending that pregnant persons may consider receiving COVID-19 vaccines, particularly if they are at high risk of exposure or have comorbidities that enhance the risk of severe disease.
Keywords: Pregnancy, COVID-19, Vaccine safety, Adjuvant, Systematic review
1. Background
The COVID-19 Vaccines Global Access Facility (COVAX) is a multilateral initiative to ensure that all countries have fair and equitable access to Coronavirus Disease 2019 (COVID-19) vaccines. Co-led by the GAVI Alliance (formerly the Global Alliance for Vaccines and Immunisation), the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), COVAX is a voluntary arrangement that enables countries to pool their resources and risk by collectively investing in vaccine candidates while developing the political and logistical infrastructure needed for vaccine distribution in a transparent and coordinated manner [1], [2], [3]. Preauthorization clinical trials of COVID-19 vaccines excluded pregnant persons, and only limited human data on their safety during pregnancy was available at the time of emergency use authorization [4]. However, pregnant persons with COVID-19 are at increased risk of adverse pregnancy and birth outcomes and severe illness compared to non-pregnant persons [5], [6], [7], [8], [9]. Many countries are vaccinating or considering vaccinating pregnant persons, especially if they are at risk of being exposed, even with limited available data about the safety of this strategy. Consequently, it is imperative to identify early safety concerns of COVID-19 vaccines, their components, or their platforms, defined as any underlying technology -a mechanism, delivery method, or cell line- that can be used to develop multiple vaccines: whole virus, protein, viral vector, or nucleic acid. To assist pregnant persons to make more fully informed decisions, we aimed to identify safety concerns during pregnancy associated with these exposures over a subset of COVID-19 vaccines selected for review by COVID-19 Vaccines Global Access - Maternal Immunization Working Group (COVAX-MIWG) in August 2020, through a rapid review of the literature databases as the first phase of an ongoing full systematic review. Given the urgency of the issue for current public health practice across the globe, we performed a rapid review as an interim analysis of the vaccines that the COVAX-MIWG selected in August 2020.
2. Objectives
To evaluate the effects of COVID-19 vaccines that the COVAX-MIWG selected in August 2020, or their components used in other vaccines, on pregnancy safety outcomes.
3. Methods
For this rapid review, we followed the Cochrane methods [10], [11] and the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12] for reporting results. This review was registered in PROSPERO (CRD42021234185).
3.1. Inclusion criteria
We included studies that used comparative or non-comparative study designs. Case series were only included if they reported more than 50 exposed pregnant persons. We also included experimental studies of any sample size with exposed pregnant animals. We excluded systematic reviews (SRs) but explored their reference lists as an additional primary study source.
The exposures or interventions of interest are the COVID-19 candidate vaccines that the COVAX-MIWG selected for review in August 2020; or the vaccine platforms (protein/subunit, vectored, nucleic acid/mRNA-LNP); or the components (antigen, vehicle, construct, adjuvants, lipid nanoparticles or other components) used by the selected COVID-19 vaccines (Table 1 ). At least one of these exposures was explicitly described in the report.
Table 1.
Main characteristics of the vaccines that were selected for review by the COVAX-MIWG# in August 2020.
| Platform | Developer/manufacturer | Vaccine candidates | Construct | Adjuvant | Dose/Schedule |
|---|---|---|---|---|---|
| Protein/subunit | Novavax | SARS-CoV-2 rS | Recombinant Spike Protein Nanoparticle vaccine Baculovirus Expressed trimeric Stabilized Spike, △F | Matrix-M™ | Two doses at 5 µg with/wo Matrix M (0,21 days) |
| Sanofi/GSK | Recombinant protein vaccine | Baculovirus Expressed trimeric Stabilized Spike | AS03 | 5 µg + AS03 (0, 21 days) | |
| Biological E (Bio E) Coalition for Epidemic Preparedness Innovations (CEPI) |
Protein antigen | SARS-CoV-2 Spike receptor binding domain (RBD) |
Alhydrogel (Alum)/CpG 1018 | Two doses (0,28 days) | |
| Clover Xiamen Innovax Biotech & GSK |
Recombinant protein vaccine | S-protein trimer | ASO3/CpG1018 (in CHO cells) | Two doses (0,21 days) | |
| Vectored | Merck Sharp & Dohme Corp.* | Recombinant replicating virus | Recombinant Vesicular stomatitis virus (rVSV)-ΔG-spike, (in MRC or Vero cells) | No | One dose (TBD) |
| Johnson & Johnson/Janssen | Non-replicating viral vector | Replication Incompetent Ad26; Stab. Spike; △F; TM | No | One dose at 5 × 1010 vp; 2 doses at 5 × 1010 (0,56 days) | |
| U Oxford/AstraZeneca | Non-replicating viral vector | ChAdOx1 wild type Spike; TM | No | Two doses at 5 × 1010 vp, (0,28 days) | |
| Nucleic acid/mRNA-LNP | Moderna | Encapsulated mRNA-1273 | mRNA: encodes 2P-stabilized Spike, TM, FI | No | Two doses at 100 µg (0,28 days) |
| BioNTech/Pfizer | BNT162a b2 | mRNA: encodes stabilized SARS-CoV-2 Spike | No | Two doses × 30 µg (0, 21 days) | |
| CureVac | mRNA nCoV-19 |
mRNA/LNP full-length S-protein stabilized | No | Two doses at 12 ug (0,28 days) |
# COVAX-MIWG: COVID-19 Vaccines Global Access - Maternal Immunization Working Group.
* Merck discontinued the development of this vaccine on January 25, 2021.
LNP: lipid nanoparticle; AS: Adjuvant System; CpG: Cytosine phosphoGuanosine; MRC: Human Fetal Lung Fibroblast Cells; CHO: Chinese hamster ovary; TM: transmembrane domain; S: Spike; FI: formalin-inactivated; rS: recombinant Spike.
We considered outcomes concerning exposure to the vaccines based on the reported gestational age at vaccination (based on validated methods including ultrasound or last menstrual period [LMP] for human studies). We used the 21 standardized case definitions developed by the Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA) of prioritized obstetric and neonatal outcomes based on the Brighton Collaboration process [13]. The ten GAIA obstetric outcomes include hypertensive disorders of pregnancy, maternal death, non-reassuring fetal status, pathways to preterm birth, postpartum hemorrhage, abortion/miscarriage, antenatal bleeding, gestational diabetes, dysfunctional labor, and fetal growth retardation. The 11 neonatal outcomes include congenital anomalies, neonatal death, neonatal infections, preterm birth, stillbirth, low birth weight, small for gestational age, neonatal encephalopathy, respiratory distress, failure to thrive, and microcephaly.
For this rapid review, we considered the integrative outcome “safety concerns” as any statistically significant adverse outcome reported in the comparative studies, or unexpected frequencies with respect to the published incidences in the peer-reviewed literature reported in uncontrolled studies. We described all the adverse events as they were reported by the authors of the original studies. For the full review, safety outcomes will be analyzed according to the US Food and Drug Administration (FDA) Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [14]. An adverse event (AE) is defined as any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product regardless of its causal relationship to the study treatment [15]. An AE can therefore be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product. These include local reactions at the injection site (pain, tenderness, erythema, edema, pruritus, other) and systemic reactions (fever ≥ 38 °C or 100.4°F, headache, malaise, myalgia, fatigue, etc.). We will also consider other post-vaccination medical events (unsolicited in the studies, reported by organ system as per Medical Dictionary for Regulatory Activities - MedDRA) [16].
We will use the classification in a four grade for the severity of AEs.
We also will consider other classifications of AEs commonly reported in safety studies, including:
-
–
Medically attended adverse events (MAEs): AEs leading to an otherwise unscheduled visit to or from medical personnel for any reason, including visits to an accident and emergency department.
-
–
Serious adverse events (SAEs): AEs that resulted in death, were life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in disability/incapacity or resulted in a congenital anomaly/birth defect in the child of a study participant.
-
–
Adverse events of special interest (AESIs): AEs worthy of closer follow-up over six months post-vaccination. These include vaccine-associated enhanced diseases such as multisystem inflammatory syndrome in children or adults (MIS-C/A).
The operative definition of each specific AE was reported elsewhere (PROSPERO- CRD42021234185).
3.2. Search strategy
We searched published and unpublished studies, without restrictions on language or publication status, from inception date to February 2021 (See the full search strategies and search terms in Appendix 1) in the Cochrane Library databases, MEDLINE, EMBASE, Latin American and Caribbean Health Sciences Literature (LILACS), Science Citation Index Expanded (SCI-EXPANDED), China Network Knowledge Information (CNKI), WHO Database of publications on SARS CoV2, TOXLine, preprint servers (ArXiv, BiorXiv, medRxiv, search.bioPreprint), and COVID-19 research websites (PregCOV-19LSR, Maternal and Child Health, Nutrition: John Hopkins Centre for Humanitarian health, the LOVE database).
We also searched reference lists of relevant primary studies and systematic reviews retrieved by the search strategy and the adverse events/safety reported in active COVID-19 pregnancy registries. The Food and Drug Administration (FDA), the European Medicines Agency (EMA), and clinical trials websites will be searched for the full review. We will then contact original authors and experts in the field for clarification or to obtain extra information. For the full review, we will re-run the search strategy, between March 2021 and the current date and time, to capture any new evidence in databases.
3.3. Selection of studies, data extraction, and assessment of the risk of bias in included studies
Pairs of authors independently screened each identified record by title and abstract and retrieved all the full texts of the potentially eligible studies. Pairs of review authors independently examined the full‐text articles for compliance with the inclusion criteria and selected the eligible studies. We resolved any disagreements by discussion. We documented the selection process with a PRISMA flow chart [12], conducted through COVIDENCE [17], a software for systematic reviews.
Pairs of review authors independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Any disagreements were resolved by discussion. Extracted data included study characteristics and outcome data. Where studies have multiple publications, we collated multiple reports of the same study under a single study ID with multiple references.
In Appendix 2, we describe the risk of bias assessment tools used for each study design. Briefly, we independently assessed the risk of bias of the included clinical trials using the Cochrane risk of bias assessment tool [18]. We used the Cochrane EPOC group tools [19] to assess controlled before‐after studies (CBAs), nationwide uncontrolled before‐after studies (UBAs), interrupted time series (ITSs), and controlled-ITSs (CITSs). We rated the risk of bias in each domain as “low”, “high”, or “unclear”. For observational cohort, case-control, cross-sectional, and case-series studies we used the NIH Quality Assessment Tool [20]. After answering the different signaling questions “Yes”, “No”, “Cannot determine”, “Not applicable”, or “Not reported”, the raters classified the study quality as “good”, “fair”, or “poor”. For consistency with the other designs, we use the classifications low, high, or unclear risk of bias, respectively.
3.4. Data synthesis
The primary analysis was the comparison of participants exposed and unexposed to the vaccines or their components. For this rapid review, we tabulated the study exposure characteristics and compared them against the unexposed. We analyzed the results of each study to determine any safety concerns as “Yes”, “No”, or “Unclear”.
Data from non-comparative studies, including registries, were collected and analyzed in the context of background rates of neonatal and obstetric outcomes. For specific indicators, we take into consideration group-specific definitions such as low-to-middle-income countries (LMICs).
We described the effect estimates as reported by the authors of the included studies. For dichotomous data, we used the numbers of events in the control and intervention groups of each study to calculate Risk Ratios (RRs), Hazard Ratios (HRs), or Mantel‐Haenszel Odds Ratios (ORs).
We planned to conduct meta-analysis and subgroup analyses by the trimester of exposure and sensitivity analysis restricted to studies with a low risk of bias. However, these were not pursued for this rapid review, given the lack of safety concerns identified. We plan to perform a meta-analysis and present GRADE 'Summary of findings' tables [10], [21] for the full review as was previously stated (PROSPERO- CRD42021234185).
4. Results
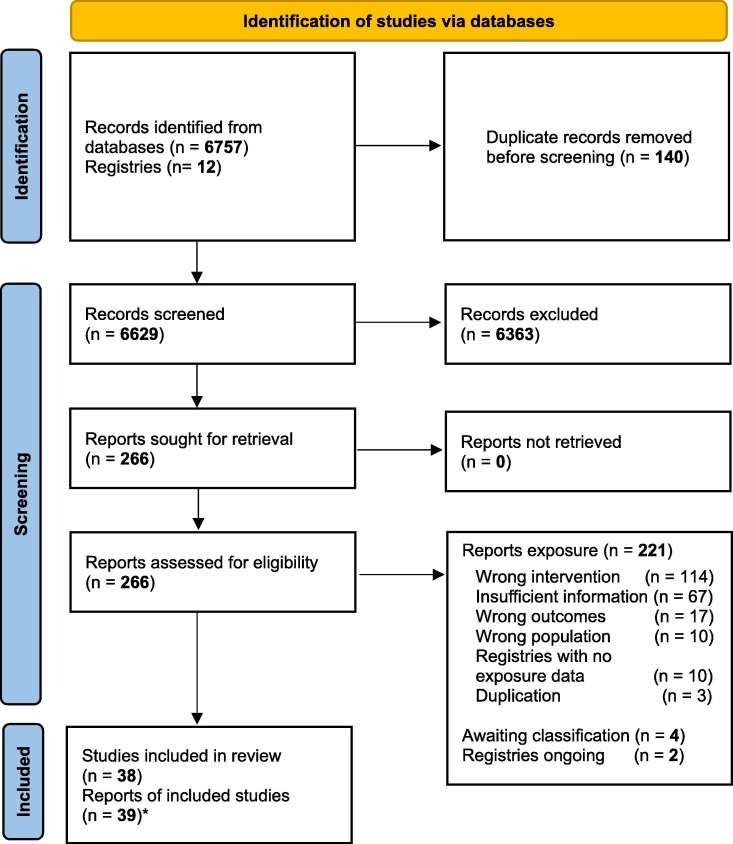
We retrieved 6756 records and 12 COVID-19 pregnancy registries from the search strategy,- 266 potentially eligible studies were assessed by full-text, and 227 were excluded, mainly because of wrong exposure or intervention (114) or insufficient information (67). We included 38 clinical and non-clinical studies, involving 2,398,855 pregnant persons and 56 pregnant animals from 39 reports.[4], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59] (Fig. 1 ). The list of excluded studies and the reasons for exclusion is presented in Appendix 3.
Fig. 1.
Study flow diagram.
4.1. Description of studies
The characteristics of included studies are described in Table 2 . The most frequent study design was cohort studies (n = 22) followed by surveillance studies (n = 8), controlled trials (n = 5), and registry analyses (n = 3). Twenty-nine of the included studies (76%) allowed comparisons between vaccinated and unvaccinated pregnant persons (n = 26) or were conducted in animals (n = 3). Nine out of the 38 studies (24%) were abstracts.
Table 2.
Main characteristics and results of included studies.
| Study ID | Study ID | N | Study design | Country | Population | Trimester exposure | Vaccine names | Exposure/intervention* | Control | Results (vaccinated vs. non-vaccinated pregnant women for comparative studies) | Original study authors s' conclusion |
Safety concerns |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baum 2015 [22] | Baum 2015[22] | 34,241 | Cohort studies | Finland | Pregnant women | 2 + 3 | Pandemrix | AS03 | No exposure | Stillbirth: aHR 1.05 (95% Confidence Interval [CI] 0.66–1.65) Early neonatal death: aHR 1.02 (0.43–2.40) Moderately preterm (28–36 weeks): aHR 1.00 (0.89–1.12) Very preterm (<28 weeks): aHR 0.90 (0.55–1.45) Moderately low birth weight (1500 g–2499 g): aHR 1.05 (0.90–1.21) Very low birth weight (<1500 g): aHR 0.84 (0.61–1.16) Fetal growth restriction: aHR 1.17 (0.98–1.40) |
The risk of adverse pregnancy outcomes was not associated with the exposure to the AS03 adjuvanted pandemic influenza vaccine. |
No |
| Celzo 2020[[23] | Celzo 2020[23] | 1,676 | Survei-llance | Belgium | Pregnant women | 1 + 2 + 3 | Havrix, Engerix-B or Twinrix | Alhydrogel (Alum)/CpG 1018 | No control | Pregnancy-related adverse event (Havrix 64/378; Engerix-B 23/339; Twinrix 103/199) Congenital anomalies/birth defects (Havrix 19/378; Engerix-B 29/339; Twinrix 10/199) Major birth defects (Havrix 17/19; Engerix-B 20/29; Twinrix 7/10) Spontaneous abortions (Havrix 43; Engerix-B 57; Twinrix 26) |
No indication of any concerning pattern of adverse pregnancy outcomes following exposure to any of the 3 vaccines during pregnancy | No |
| Chavant 2013[24] | Chavant 2013[24] |
2,415 | Survei-llance |
France | Pregnant women | NR | Pandemrix | AS03 | No control | Fever and Flu-like symptoms: 37/56 (65.9%) Headaches: 9/56 (17.6%) Local reactions: 37/56 (65.9%) Congenital anomalies: 1/56 (1.4%) |
Exposure to the A(H1N1)v2009 pandemic influenza vaccine during pregnancy does not increase the risk of adverse pregnancy outcomes. | No |
| Fell 2012[25] | Fell 2012[25] | 23,340 | Safety registry | Canada | Pregnant women | 2 + 3 | Pandemrix | AS03 | No exposure | Preterm birth (<37 weeks): aRR (95% CI) 0.95 (0.88, 1.02) Very preterm birth (<32 weeks): aRR 0.73 (0.58, 0.91) Small for gestational age: below 10th percentile: aRR 0.90 (0.85, 0.96) Small for gestational age: below 3rd percentile: aRR 0.81 (0.72, 0.92) 5-minute Apgar score below 7: aRR 0.97 (0.82, 1.14) Fetal death: aRR 0.66 (0.47, 0.91) |
Second- or third-trimester H1N1 vaccination was associated with improved fetal and neonatal outcomes during the recent pandemic. | No |
| Folkenberg 2011[26] | Folkenberg 2011[26] | 5,772 | Survei-llance | Denmark | Pregnant women | NR | Pandemrix | AS03 | No control | Uterine contractions: 2/12 Spontaneous abortions: 4/12 Stillbirth:1/12 |
No strong signals of any unknown or serious adverse events associated with influenza A/H1N1v vaccination in Denmark. | No |
| Galindo Santana 2011[27] | Galindo Santana 2011[27] | 80,317 | Cohort studies | Cuba | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No control | Adverse effects 615/80,317 (0.8%) of the vaccinated pregnant women (fever 32,4%; headache 30,3%; vomiting 12%; local reactions 9%; arthralgia 6,9%; dizziness 5%; allergic manifestations 3%; spontaneous abortions 0.3%; increase in uterine contractions 0.3%) | No safety problem is associated to the Pandemrix vaccine. | No |
| Glenn 2015[28] | Glenn 2015[28] | 71 | RCT | USA | Animals | 3 | RSV F vaccine | Protein/subunit; Nanoparticles; aluminum phosphate |
Another intervention & placebo | Delivery rate Placebo: 80%; RSV F: 80% and RSV F + AlP04: 90% 3 stillbirths placebo vs 3 stillbirths in the adjuvanted RSV F group |
The RSV F vaccine was safe. The rates of pregnancy and stillbirth were similar between controls and vaccinees. | No |
| Gray 2021[4] | Gray 2021[4] | 84 | Cohort studies | USA | Pregnant women | 1 + 2 + 3 | COVID-19 (Pfizer & Moderna) | Nucleic acid/mRNA | Not pregnant | Vaccine-related fevers/chills: 25/77 (32%) (8/16 [50%] in non-pregnant women; p = 0.25). Fetal growth restriction: 0/13; Preeclampsia/gestational hypertension: 0/13 Preterm delivery: 1/13; Death: 0/13 The cumulative symptom score after the 1st dose in all groups was low and after the 2nd dose, the cumulative symptom score (median (IQR) 2 (1–3), 3 (2–4), and 2.5 (1–4.5) in pregnant, lactating, and non– pregnant groups respectively, p = 0.40). |
There was no significant difference between pregnant, lactating, and non– pregnant groups respectively with respect to cumulative symptom score. | No |
| Groom 2018[29] | Groom 2018[29] | 1,399 | Cohort studies | USA | Pregnant women | 1 + 2 + 3 | Recombivax, Engerix or Twinrix | Aluminum hydrophosphate sulphate, Alhydrogel (Alum) Aluminum phosphate |
Not Hep B vaccinated (other vaccines or unvaccinated) | Gestational hypertension aOR (95%CI) 1.02 (0.80–1.30). Gestational diabetes: aOR 1.06 (0.91–1.23) Pre-eclampsia/eclampsia: aOR 1.07 (0.84–1.36) Cesarean delivery: aOR 1.01 (0.91–1.13) Pre-term birth (<37 weeks): aOR 1.14 (0.94–1.39) Low birth weight (<2500 g): aOR 1.21 (0.96–1.52) Small for gestational age at birth: aOR 1.13 (0.94–1.37) |
There were no significant associations between HepB exposure during pregnancy and maternal and neonatal outcomes. No increased risk for the adverse events that were observed among women or their offspring. |
No |
| Groom 2019[30] | Groom 2019 | 1140 | Cohort studies | USA | Pregnant women | 1 + 2 + 3 | Hepatitis A | Aluminium hydroxide | Not Hep A vaccinated (other vaccines or unvaccinated) |
Gestational hypertension: aOR 0.85 (0.64–1.15) Gestational diabetes: aOR 0.93 (0.78–1.11) Pre-eclampsia/eclampsia: aOR 0.92 (0.69–1.24) Cesarean delivery: aOR 1.01 (0.91–1.13) Pre-term birth (<37 wks): aOR 0.83 (0.65–1.07). Low birth weight (<2500 g): aOR 1.05 (0.81–1.37) Small for gestational: aOR 1.32 (1.09–1.60, likely due to unmeasured confounding) |
HepA vaccination during pregnancy was not associated with an increased risk for a range of adverse events examined among pregnancies resulting in live births, but an identified association between maternal HepA and SGA infant outcomes, while likely due to unmeasured confounding, warrants further exploration. | No |
| Guo 2010[31] | Guo 2010[30] | 875 | Cohort studiesA | Canada | Pregnant women | NR | Arepanrix | AS03 | No exposure | Fetal loss: 7/550 (1.3%) vaccinees vs 11/325 (3.3%) unvaccinated, P = 0.06 Premature birth: 31/359 (8.6%) vaccinated vs 23/185 (12%) unvaccinated P = .21 Of 261 vaccinees reporting weekly data, 11 (4.2%) reported an adverse event requiring missed work or an MD visit within 7 days of vaccination, most commonly acute respiratory illness (N = 7). Only one event (arm numbness) was thought to be vaccine related. No serious adverse events were reported. |
Results to date suggest that pandemic vaccines were safe. | No |
| Haberg 2013[32] | Haberg 2013[31] | 63,367 | Safety registry | Norway | Pregnant women | 2 + 3 | Pandemrix | AS03 | No exposure | Fetal death HR IC95% 0.88 (0.66–1.17) Preterm delivery HR 1.00 (0.93–1.09) Low birth weight at term HR 0.90 (0.76–1.08) Low Apgar score at term HR 1.08 (0.91–1.28) |
There is no evidence of association between vaccination and fetal death, preterm delivery, low birth weight at term, and low Apgar score at term | No |
| Heldens 2009[33] | Heldens 2009[32] | 10 | CT | Netherlands | Animals | 3 | Equilis Prequenza T | ISCOM-Matrix | No exposure | Local reaction (swelling): 3/10 (in each dose) Pyrexia: 0/10 Systemic reactions: 0/10 The effects in the non-intervention was not reported |
The vaccine was shown to be safe in pregnant mares, foals and is used safely since 2 years as a commercial vaccine in Europe. | No |
| Jonas 2015[34] | Jonas 2015[33] | 41,183 | Cohort studies | Sweden | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | Stillbirth: aHR IC95% 0.88 (0.59–1.30) Early neonatal death: aHR 0.82 (0.46–1.49) Later death: aHR 0.78 (0.52–1.19) |
AS03 adjuvanted H1N1 vaccination during pregnancy does not affect the risk of stillbirth, early neonatal death, or later mortality in the offspring. | No |
| Källén 2012[35] | Källén 2012[34] | 18,612 | Cohort studies | Sweden | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure & pre-vaccination group | Gestational diabetes aOR (IC95%) 0.94 (0.81–1.09) Pre-eclampsia: aOR 0.99 (0.92–1.07) Stillbirth: aOR 0.77 (0.57–1.03) Preterm birth: aOR 0.86 (0.77–0.96) Low birthweight: aOR 0.86 (0.77–0.96) Congenital malformations: aOR 1.01 (0.83–1.23) Small-for-gestational-age: aOR 1.04 (0.92–1.17) |
Vaccination during pregnancy with Pandemrix appeared to have no ill effects on the pregnancy. |
No |
| Katz 2016[36] | Katz 2016[35] | 1,845,379 | Safety registryA | Argentina | Pregnant women | 1 + 2 + 3 | Tdap | Protein/subunit & aluminum phosphate | No control | Adverse events following immunization (pregnant women): 1.46/100.000 Adverse events following immunization vaccine 0.43/100.000 |
Both vaccines presented a suitable safety profile. Since 2012 a downward trend in pertussis mortality was evident and no deaths from influenza in vaccinated were notified in pregnant women | No |
| Kushner 2020[37] | Kushner 2020[36] | 59 | Cohort studiesA | Australia | Pregnant women | 1 | Heplisav B | Aluminum phosphate/CpG1018 | Engerix-B | Healthy term deliveries: 24 (60%) Heplisav-B vs 11 (55%) Engerix-B Spontaneous abortions: 3 (7.5%) Heplisav-B vs 2 (10%) Engerix-B Congenital anomaly: 1 (2.5%) Heplisav-B vs 1 (5%) Engerix-B Stillbirths: 1 (2.5%) Heplisav-B vs 0 (0%) Engerix-B |
Heplisav-b shows similar fetal outcomes compared with Engerix-B. | No |
| Lacroix 2010[38] | Lacroix 2010[37] | 100,000 | Survei-llanceA | France | Pregnant women | NR | Pandemrix | AS03 | No control | The French National Pharmacovigilance of A(H1N1) vaccination in pregnant women between October 2009 and March 2010, reported 13 intra-uterine deaths and12 spontaneous abortions. | No causal relationship between immunization and in utero fetal death or spontaneous abortion was established. | No |
| Läkemedelsverket 2010[39] | Läkemedelsverket 2010[38] | 30,000 | Report | Sweden | Pregnant women | 1 + 2 | Pandemrix | AS03 | No control | Suspected adverse events: 50/30.000 (0.17%) Miscarriages: 31/30.000 (0.10%) Intrauterine fetal deaths: 7/30.000 (0.02%) |
The low number of reports with no defined risk profile would indicate that the vaccination with Pandemrix does not increase the risk for miscarriage or intrauterine fetal death. | No |
| Layton 2011[40] | Layton 2011[39] | 92 | Cohort studiesA | United Kingdom | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No control | Miscarriages: 4/92 (4.3%) Congenital problems: 6/92 (6.5%) |
No safety conclusion |
No |
| Levi 2012[41] | Levi 2012[40] | 6,989 | Cohort studies | Denmark | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | Serious congenital malformation (1st trimester): 5.5% vs 4.5% unvaccinated Premature birth or low birth weight was also equally common in both groups, regardless of it time of vaccination. |
It appears to be safe even during the pregnancy to be vaccinated against the H1N1- virus. | No |
| Ludvigsson 2013[43] | Ludvigsson 2013[42] | 13,297 | Cohort studies | Sweden | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | Low birth weight < 2,500 g: aOR (IC95%) 0.91 (0.79–1.04) Preterm birth < 37 weeks: aOR 0.99 (0.89–1.10) Small for gestational age: aOR 0.97 (0.90–1.05) Low Apgar score at 5 min < 7: aOR 1.05 (0.84–1.31) Caesarean section: aOR 0.94 (0.89–0.99) |
H1N1 AS03-adjuvanted vaccine during pregnancy, does not appear to adversely influence maternal or neonatal outcomes when used in different stages of pregnancy. |
No |
| Ludvigsson 2016[42] | Ludvigsson 2016[41] | 40,983 | Cohort studies | Sweden | Pregnant women | 1 | Pandemrix | AS03 | Siblings | Congenital malformation: aOR (IC95%) 0.98 (0.89–1.07) Congenital heart disease: aOR 0.98 (0.84–1.15) Oral cleft: aOR 1.14 (0.67–1.94) Limb deficiency: aOR 0.90 (0.36–2.28) |
When intrafamilial factors were taken into consideration, H1N1 vaccination during pregnancy did not seem to be linked to overall congenital malformation in offspring. | No |
| Mackenzie 2012[44] | Mackenzie 2012[43] | 128 | Cohort studies |
United Kingdom |
Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | Miscarriages: 4/97 (4.1%) Potentially congenital abnormalities: 6/97 (6.2%) Stillbirths: 0/97 (0%) |
Overall, no significant safety issues were identified. |
No |
| Madhi 2020[45] | Madhi 2020[44] |
4,636 | RCT |
Multi‐country# | Pregnant women |
2 + 3 |
RSV F vaccine |
Nanoparticle vaccine Baculovirus/Aluminum phosphate |
Placebo | Local injection-site reactions: 40.7% vs. 9.9% placebo; P < 0.001 Fever within 7 days: 1.2% vs 1.6% Systemic reaction: 41.2% vs 38.6% Serious adverse event: 29.8% vs 28.8% Any infant adverse event 82.3% vs 83% Serious adverse event: 44.3% vs 46.4% Serious adverse event with outcome of death: 0.6% vs 0.8% |
RSV F protein nanoparticle vaccination in pregnant women was safe |
No |
| McHugh 2019[46] | McHugh 2019[45] | 2,706 | Cohort studies | Australia | Pregnant women | 1 + 2 + 3 | Tdap | Protein/subunit & aluminum phosphate | No exposure | Preterm birth (<37 weeks): aRR (95% CI) 0.99 (0.75–1.32) Low birth weight at term (<2500 g): aRR 1.19 (0.61–1.11) Small for gestational age (<10th percentile): aRR 1.09 (0.86–1.37) |
No significant associations were found between pertussis vaccination in pregnancy and adverse birth outcomes, regardless of the trimester of pregnancy. | No |
| Moro 2014[47] | Moro 2014[46] | 139 | Survei-llance | USA | Pregnant women | 1 | Havrix, Vaqta, Twinrix | Aluminum hydrophosphate sulphate, Alhydrogel (Alum) Aluminum phosphate |
No control | Pregnancy AEs: 41/139 (29.4%) Non-pregnancy specific outcomes: 21/139 (15.1%) Infant/neonatal outcomes: 12/139 (8.6%) No AE reported: 65/139 (46.8%) |
This review of VAERS reports did not identify any concerning pattern of AEs in pregnant women or their infants following maternal Hep A or Hep AB immunizations during pregnancy | No |
| Moro 2018[48] | Moro 2018[47] | 192 | Survei-llance | USA | Pregnant women | 1 + 2 + 3 | Recombivax Engerix-b, Twinrix, Comvax, Pediarix |
Aluminum hydrophosphate sulphate, Alhydrogel (Alum) Aluminum phosphate |
No control | Pregnancy-specific AEs: 61 (55.4%) Non-pregnancy specific AEs: 35 (31.8%) Infant outcomes: 22 (20.0%) |
Our analysis of VAERS reports involving hepatitis B vaccination during pregnancy did not identify any new or unexpected safety concerns. | No |
| Muñoz 2019[49] | Muñoz 2019[48] |
50 | RCT |
USA |
Pregnant women | 3 | RSV F vaccine |
Nanoparticle vaccine Baculovirus/Aluminum phosphate |
Placebo |
Solicited AEs: 15/22 (68.2%) vs 10/28 (35.7%) Severe solicited AEs 0/22 vs 0/28 Local solicited AEs 13/22 (59.1%) vs1/28 (3.6%) Systemic solicited AEs 6/22 (27.3%) vs10/28 (35.7%) Any unsolicited AE 22/22 (100.0%) vs 28/28 (100.0%) Severe unsolicited AE 3/22 (13.6%) vs 4/28 (14.3%) Severe & related unsolicited AE 0/22 vs 0/28 Any medically attended AE 18/22 (81.8%) vs 25/28 (89.3%) Any serious AE (SAE) 3/22 (13.6%) vs1/28 (3.6%) |
The vaccine was well tolerated; no meaningful differences in pregnancy or infant outcomes were observed between study groups. Suggesting good tolerability of the RSV F vaccine among pregnant women and safety in their infants sufficient to justify larger trials. | No |
| Núñez Rojas 2010[50] | Núñez Rojas 2010[49] | 451 | Cohort studies | Cuba | Pregnant women | 1 | Pandemrix | AS03 | No exposure | 34/451 Vs control group 21/205 (OR:0.71) for some condition, minor or major. 64,7% of findings in the vaccinated group were located in the kidneys (62,2% were renal ecstasy, either unilateral or bilateral) |
Vaccination against influenza virus A H1N1 did not increase the risk of birth defects when applied during the first trimester of gestation in the sample studied |
No |
| Oppermann 2012[51] | Oppermann 2012[50] | 90 | Cohort studies | Germany | Pregnant women | 1 | Pandemrix | AS03 | No exposure | Systemic adverse reactions: 23/90 (25.6%) Local reactions: 64/90 (71.1%) Spontaneous abortions: 3/90 (3.3%) |
The results of our study do not indicate a risk for the pregnant woman and the developing embryo/fetus after H1N1 vaccination. | No |
| Pasternak 2012[52], [53] | Pasternak 2012[51], [52] | 54,585 | Cohort studies | Denmark | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | Propensity score | Major birth defects in gestational weeks 4–10: prevalence OR (POR) 1.24 (0.57–2.71) Preterm birth: POR: 0.99 (0.84–1.17) Small size for gestational age: POR: 0.97 (0.86–1.09) Fetal death: HR (95%CI) 0.79 (0.53–1.16) Spontaneous abortion HR 1.11 (0.71–1.73) Stillbirth: HR 0.44 (0.20–0.94) |
Exposure to an adjuvanted influenza A(H1N1) pdm09 vaccine during pregnancy was not associated with a significantly increased risk of major birth defects, preterm birth, or fetal growth restriction. | No |
| Ray 2014[54] | Ray 2014[53] | 509 | Cohort studies | Canada | Pregnant women | NR | Pandemrix | AS03 | Inactivated non-adjuvanted H1N1 vaccine | Peripartum complications: 83/199 (41.7%) nonadjuvanted vs 127/509 (25.1%) adjuvanted (aOR 1.55; IC95% 1.01–2.39) | The composite outcome of peripartum complications was more common in women who received the nonadjuvanted vaccine | No |
| Rega 2016[55] | Rega 2016[54] | 5,155 | Unclear | Australia | Pregnant women | NR | Aluminum phosphate | TIV & unvaccinated | Local reaction. 7.1% Tdap and 3.2% TIV | Active vaccine safety monitoring has not identified clinically significant issues. Pregnant woman vaccinated against influenza are less likely to experience stillbirth. | No | |
| Sammon 2011[56] | Sammon 2011[55] | 9,282 | Cohort studies | United Kingdom | Pregnant women | 1 + 2 + 3 | Influenza v. pandemic & seasonal | AS03 | No exposure | Spontaneous loss adjusted for age and chronic comorbidity: aRR 1.54; CI95% 1.36–1.74) | We identified an increased miscarriage risk associated with influenza vaccination during pregnancy possibility due to residual confounding | Unclear |
| Sammon 2012[57] | Sammon 2012[56] | 9,445 | Cohort studies | United Kingdom | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | Fetal death 9–12 weeks unadjusted HR 0.56; CI95 0.43–0.73) Fetal death 13–24 weeks unadjusted HR 0.45; CI95 0.28–0.73) Fetal loss 9–12 weeks unadjusted HR 0.74; CI95 0.62–0.88) Fetal loss 13–24 weeks unadjusted HR 0.59; CI95 0.45–0.77) |
Influenza vaccination during pregnancy does not appear to increase the risk of fetal death. | No |
| Stedman 2019[58] | Stedman 2019[57] | 16 | RCT | Netherlands | Animals | 2 | ChAdOx1 RVF | Vectored | Placebo | All ewes and does in the ChAdOx1 RVF (n = 8) and mock-vaccinated groups (n = 8) were in good health, with no clinical signs or other adverse events following vaccination | When administered to pregnant sheep and goats, ChAdOx1 RVF is safe | No |
| Tavares 2011[59] | Tavares 2011[58] | 267 | Cohort studies | United Kingdom | Pregnant women | 1 + 2 + 3 | Pandemrix | AS03 | No exposure | At least 1 MAE within the 31-daypost-vaccination: 59 (22.1 %) SAEs during the 181-day post-vaccination: 34 (12.7%) Observed/expected number of pregnancy outcomes by subgroup at vaccination: Spontaneous abortion: 4 (3.3%) (expected in the general population: 10–16%) Stillbirth :0 (expected in the general population: 0.51%) Congenital anomaly: 5 (1.9%) (expected in the general population: 2.09%) Preterm delivery (<37 weeks’ gestation): 14 (5.4%) (expected in the general population: 5.6%) Very pre-term delivery (<32 weeks’ gestation): 3 (1.1%) (expected in the general population: 1.7%) Low birth weight (<2.5 kg): 21 (8.1%) (expected in the general population: 7.1%) Very low birth weight (<1.5 kg): 4 (1.5%) (expected in the general population: 1.2%) |
The results of this analysis suggest that exposure to the AS03 adjuvanted H1N1 (2009) vaccine during pregnancy does not increase the risk of adverse pregnancy outcomes including spontaneous abortion, congenital anomalies, preterm delivery, low birth weight neonates, or maternal complications. |
No |
* See adjuvants, platforms and constructs in table 1; NR not reported; # Argentina, Australia, Chile, Bangladesh, Mexico, New Zealand, the Philippines, South Africa, Spain, the United Kingdom, and the United States.
A: Only available as abstract; RCT: Randomized Controlled Trial; aHR: adjusted Hazard Ratio; aRR: adjusted Relative Risk; aOR adjusted Odds Ratio; USA: United States of America; AE: Adverse Event; SAE: Serious AE; MAE: medically attended adverse event; RSV F: Respiratory Syncytial Virus Fusion; Alhydrogel is an aluminum hydroxide (referred to as alum).
The most frequent study location was the USA (n = 7), followed by Sweden and the United Kingdom (n = 5 each), Australia, Canada, and Denmark (n = 3 each), Cuba, France, and Netherlands (n = 2 each), and Argentina, Belgium, Finland, Germany, Norway, and multi‐country (n = 1 each). Only 4 out of 37 studies (11%) involved LMICs [27], [36], [45], [50].
Only 3 out of 37 studies were conducted on animals (8%)[28], [33], [58]. Most of the studies reported exposures during the three trimesters (n = 17), only the first trimester (n = 5), and the second and third trimester (n = 4). The time of exposure was not reported in six studies.
We only identified one COVID-19 vaccine study reporting exposure to mRNA-LNP from Pfizer & Moderna COVID-19 vaccines [4]. The most frequent exposures were to the AS03 adjuvant (536,240 pregnant participants from 23 studies) and aluminum-based adjuvants (1,861,462 pregnant participants from 11 studies) (Table 3 ). AS03 was the adjuvant of several A/H1N1 pandemic influenza vaccines (Pandemrix® and Arepanrix), while the influenza vaccine Equilis® used ISCOM-Matrix [32]. Aluminum phosphate was used in the testing of candidate Respiratory Syncytial Virus Fusion (RSV F) vaccines in pregnant persons [28], [44], [48] (n = 3). Aluminum phosphate was also used in Tdap vaccines [36], [46], [55] (n = 3). Different aluminum salts were used in Hepatitis vaccines [23], [29], [30], [37], [47], [48]. One study reported the use of the ChAdOx1 vector for a Rift Valley fever vaccine [58].
Table 3.
Adjusted relative effects comparing exposed vs. not exposed pregnant participants by vaccine components/platforms.
| Exposure References | Pregnant participants | Studies (%) | Adjusted relative effects# $ (exposed vs no exposed) |
|---|---|---|---|
| AS03 [22], [24], [25], [26], [27], [31], [32], [34], [35], [38], [39], [40], [41], [42], [43], [44], [50], [51], [52], [53], [54], [56], [57], [59] | 536,240 | 23 (60%) | Congenital malformation: 0.98–1.01 Fetal death 0.66–0.88 Early neonatal death: 0.82–1.02 Later death: 0.78 Preterm delivery 0.86–1.00 (Very preterm 0.73$–0.90) Low birth weight/small at term 0.86–1.04 (<10th & 3rd percentile: 0.90 & 0.81; very low birth weight 0.84) Low Apgar score at term 0.97–1.08 Gestational diabetes 0.94 Pre-eclampsia: 0.99 Stillbirth: 0.77 to1.05 Caesarean section: 0.94 Peripartum complications: 0.65$ |
| *Aluminum phosphate [28], [36], [45], [46], [49] | 1,852,842 | 5 (13%) | Preterm birth: 0.99 Low birth weight at term: 1.19 Small for gestational age (<10th percentile): 1.09 |
| *Aluminum salts only [29], [30], [47], [48], [55] | 8,025 | 5 (13%) | Stillbirth: 0.49$ Gestational hypertension 0.85–1.02 Gestational diabetes: 0.9–1.06 Pre-eclampsia/eclampsia: 0.92–1.07 Cesarean delivery: 1.01 Pre-term birth (<37 weeks): 0.83–1.14 Low birth weight (<2500 g): 1.05–1.21 Small for gestational age at birth: 1.13–1.32 |
| *CpG 1018 & Aluminum salts [23], [37] | 1,735 | 2 (5%) | Not available |
| ISCOM-Matrix [33] | 10 | 1 (3%) | Not available |
| mRNA-LNP [4] | 84 | 1 (3%) | Not available |
| ChAdOx1 RVF [58] | 16 | 1 (3%) | Not available |
* Any aluminum exposure 1,861,462 pregnant participants from 11 studies; LNP: lipid nanoparticle.
# Adjusted Hazard Ratio; Relative Risk or Odds Ratio; $ statistically significant.
The 12 COVID-19 and pregnancy registries identified (UKOS, PAN-COVID, BPSU, NPC-19, EPICENTRE, periCOVID, INTERCOVID, PregCOV-19LSR, PRIORITY, COVI-PREG), OTIS/MotherToBaby, CHOPAN, and V-safe registries) are presented in Appendix 4.
4.2. Risk of bias in included studies
The risk of bias for the included controlled trials is presented in Table 4 and for the included observational studies in Table 5 .
Table 4.
Risk of bias of clinical trials.
| Study ID | Adequate sequence generation | Allocation concealment | Blinding of participant & personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| Glenn 2015 | Unclear | Unclear | Low | Low | Low | Unclear | Unclear |
| Heldens 2009 | High | High | Unclear | Unclear | Low | Unclear | High |
| Madhi 2020 | High | High | Unclear | Unclear | Low | Unclear | Unclear |
| Muñoz 2019 | Unclear | Unclear | High | Low | Low | Unclear | Unclear |
| Stedman 2019 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
Table 5.
Risk of bias of observational studies.
| Study ID | Study design | Signaling questions* |
Global Quality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |||
| Baum 2015 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | No | Yes | No | Yes | Yes | Good |
| Galindo Santana 2011 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | No | Yes | No | NR | No | Fair |
| Gray 2021 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | No | Fair |
| Groom 2018 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | Yes | Good |
| Groom 2019 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | Yes | Good |
| Guo 2010 | Cohort studies | Yes | CD | CD | CD | CD | Yes | Yes | NA | CD | NA | CD | NR | Yes | No | Fair |
| Jonas 2015 | Cohort studies | Yes | Yes | Yes | Yes | NR | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | Yes | Good |
| Källén 2012 | Cohort studies | Yes | Yes | Yes | Yes | NR | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | NR | Fair |
| Kushner 2020 | Cohort studies | Yes | Yes | Yes | NR | No | Yes | Yes | No | Yes | Yes | Yes | NR | NR | No | Fair |
| Layton 2011 | Cohort studies | Yes | CD | CD | CD | CD | Yes | CD | NA | CD | NA | CD | CD | NR | No | Fair |
| Levi 2012 | Cohort studies | Yes | Yes | CD | CD | CD | Yes | Yes | NA | CD | NA | CD | CD | NR | CD | Fair |
| Ludvigsson 2013 | Cohort studies | Yes | Yes | Yes | Yes | NR | Yes | Yes | NA | Yes | NA | Yes | NR | No | Yes | Good |
| Ludvigsson 2016 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | Yes | Good |
| Mackenzie 2012 | Cohort studies | Yes | Yes | Yes | Yes | NR | Yes | Yes | NA | Yes | No | Yes | NR | Yes | No | Fair |
| McHugh 2019 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NR | Yes | Yes | Good |
| Núñez Rojas 2010 | Cohort studies | Yes | Yes | NR | No | No | Yes | Yes | NA | Yes | NA | No | NR | NR | No | Poor |
| Oppermann 2012 | Cohort studies | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | NA | Yes | No | Yes | Yes | Good |
| Pasternak 2012 | Cohort studies | Yes | Yes | Yes | Yes | NR | Yes | Yes | NA | Yes | No | Yes | NR | Yes | Yes | Good |
| Pasternak 2012 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NA | NA | Yes | Good |
| Ray 2014 | Cohort studies | No | No | CD | Yes | NR | Yes | CD | NA | No | No | No | NR | CD | NR | Poor |
| Sammon 2011 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | CD | NA | Yes | NA | No | NA | NA | No | Poor |
| Sammon 2012 | Cohort studies | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | No | Yes | No | NR | Yes | Good |
| Tavares 2011 | Cohort studies | Yes | Yes | CD | Yes | No | Yes | Yes | NA | Yes | No | Yes | No | No | No | Fair |
| Fell 2012 | Registry analysis | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NA | Yes | Yes | Good |
| Haberg 2013 | Registry analysis | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NA | No | Yes | Good |
| Katz 2016 | Registry analysis | Yes | Yes | CD | Yes | No | Yes | CD | NA | Yes | NA | Yes | NA | NA | NA | Fair |
| Celzo 2020 | Surveillance | Yes | Yes | CD | No | No | Yes | NR | NR | Yes | NA | Yes | NA | NA | No | Fair |
| Chavant 2013 | Surveillance | Yes | Yes | NA | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | CD | No | Poor |
| Folkenberg 2011 | Surveillance | Yes | Yes | CD | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NA | NA | NA | Good |
| Lacroix 2010 | Surveillance | Yes | Yes | CD | Yes | No | No | Yes | NA | Yes | No | Yes | NA | NA | No | Fair |
| Läkemedelsverket 2010 | Surveillance | Yes | Yes | CD | CD | No | Yes | Yes | NA | Yes | NA | No | No | NA | No | Poor |
| Moro 2014 | Surveillance | Yes | Yes | CD | Yes | No | Yes | Yes | NA | Yes | NA | Yes | NA | NA | NA | Good |
| Moro 2018 | Surveillance | Yes | Yes | NA | Yes | No | Yes | Yes | NA | No | No | Yes | NA | NA | NA | Poor |
| Rega 2016 | Surveillance | No | No | Yes | NR | No | Yes | NR | NA | Yes | NA | NR | NA | NA | No | Poor |
NA: not applicable, NR: not reported, CD: cannot be determined.
*Signaling questions.
1. Was the research question or objective clearly stated in this study?
2. Was the study population clearly specified and defined?
3. Was the participation of eligible persons at least 50%?
4. Were all subjects selected or recruited from the same or similar populations (including the same time frame)? Were the inclusion and exclusion criteria pre-specified and applied to participate in the study of uniformly to all participants?
5. Was a justification of the sample size, a description of the power, or estimates of variance provided and effect?
6. For the analysis in this study, were the exposure (s) of interest measured before the outcome (s) were measured?
7. Was the follow-up period long enough for one to reasonably expect to see an association between exposure and result if it exists?
8. For exposures that can vary in quantity or level, did the study examine different levels of exposure in relation to with the outcome (for example, exposure categories or exposure measured as a continuous variable)?
9. Were the exposure measures (independent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
10. Were the exposure (s) evaluated more than once over time?
11. Were the outcome measures (dependent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
12. Were the outcome assessors blinded to the exposure status of the participants?
13. Was the loss to follow-up after the start of the study 20% or less?
14. Were potential key confounding variables statistically measured and adjusted for their impact on the relationship between exposure (s) and outcome (s)?
We assessed the 38 included reports. Among the five RCTs, two (40%) presented a high risk of bias in the randomization process, and one (20%) in the blinding of participants and personnel. Among the 33 observational study reports, 14 were classified as “good” (43%), 12 as “fair” (36%), and seven as “poor” (21%).
4.3. Outcomes of exposures
The results of included studies are described in Table 2. There were 13 pregnancy-related outcomes (26 reports), eight neonatal outcomes (19 reports), and nine maternal outcomes (13 reports). The most-reported pregnancy outcomes were preterm delivery (n = 12), stillbirth (n = 9), spontaneous abortion (n = 9), fetal growth restriction/small gestational age (n = 8), and fetal death (n = 6). The most reported neonatal outcomes were congenital anomalies (n = 9) and low birth weight (n = 8), and the most reported maternal outcomes were local reactions (n = 7), systemic reactions (n = 5), and serious adverse events (n = 6).
The adjusted relative effects comparing exposed vs. not exposed pregnant participants by vaccine components/platforms were summarized in Table 3. None of the available exposures, including AS03, aluminum phosphate, or aluminum salts only, was statistically associated with adverse outcomes. AS03 showed a statistically lower frequency of very preterm aRR 0.73 (95%CI 0.58–0.91)[25] and peripartum complications aOR 0.65 (95%CI 0.42–0.99)[54], and aluminum salts showed lower stillbirth aHR 0.49 (95%CI 0.29–0.84)[55]. The lack of more comparative information regarding “safety concerns” precludes further subgroup analysis by exposure.
Of the 37 included studies, 36 (97%) concluded that there was no evidence of safety concerns. Only one study [56], reported as abstract, mentioned unclear safety concerns regarding the 9,026 pregnancies ending in a delivery that had a record of the swine flu vaccine during or just before their pregnancy. The authors reported that they may not have captured early pregnancy losses, that some misclassification of outcome may have occurred, or residual confounding may have been present after adjusting for age and chronic comorbidity. However, the full-text manuscript reported one year later by these authors [57], including 9,445 persons vaccinated with the swine flu vaccine before or during pregnancy, found no difference in the hazard of fetal loss during weeks 25–43 and a lower hazard of fetal loss than unvaccinated pregnancies in gestational weeks 9–12 and 13–24.
The planned subgroup analyses by the trimester of exposure and sensitivity analysis, restricted to studies with low risk of bias, were not conducted, given the lack of reported safety concerns in every study.
Table 4 shows the characteristics of the 12 identified COVID-19 and pregnancy registries, with potential data on safety/adverse events. The USA and the UK were the most represented countries. Some large registries are multinational, such as EPICENTRE, COVI-PREG, or PAN-COVID, which gathers data from 42 countries. Most registries include information on obstetric/pregnancy outcomes like early pregnancy loss, fetal growth, stillbirths, and delivery outcomes. All of them include neonatal and infant outcomes. Additionally, UKOSS and V-safe include specific vaccination information on the pregnant population. PeriCOVID was the only registry that collected blood samples. More detailed information on the relevant information from these registries will be described in the full systematic review, which is currently ongoing.
We also identified three ongoing studies in the COVID-19 vaccine tracker, developed by the Vaccine Centre at the London School of Hygiene and Tropical Medicine, which contains information from the WHO, the Milken Institute, and clinicaltrials.gov databases [60]. A phase-2 trial, assessing the Ad26.COV2.S vaccine (a monovalent vaccine composed of a recombinant, replication-incompetent adenovirus type 26 vector) [61], and a phase-2/3 trial, assessing the BNT162b2 vaccine (an RNA vaccine) [62], are being conducted in the United States, Australia, Brazil, Canada, Finland, South Africa, Spain, and in the United Kingdom. In addition, a phase-4 nonrandomized controlled study is being conducted in Belgium to verify if SARS-Cov-2 specific antibodies can be found in blood serum and milk of lactating mothers vaccinated with the CX-024414 vaccine (mRNA vaccine) [63].
5. Discussion
Through this rapid review of studies of vaccine components and platforms also used by COVID-19 vaccines, we found no evidence of safety concerns regarding the COVID-19 vaccines that the COVAX MIWG selected for review in August 2020, their components, or platforms used in other vaccines during pregnancy.
None of the adjusted relative effects comparing exposed vs. not exposed pregnant participants of the available exposure results were statistically associated with adverse outcomes. Only AS03 showed a statistically lower frequency of very preterm [25] and peripartum complications [54], and aluminum salts showed lower stillbirth aHR 0.49 (95%CI 0.29–0.84) [55]. Uncontrolled studies, in general, reported low frequencies of adverse outcomes. One study [56], reported as an abstract, suggested safety concerns regarding the swine flu vaccine (AS03 adjuvant) during or just before pregnancy, but the authors recognized potential bias for this finding. The authors published the full-text manuscript [56] one year later, and after a complete analysis, they concluded that there is no evidence of safety concerns.
Nine systematic reviews consistently supported the safety of influenza vaccines during pregnancy [64], [65], [66], [67], [68], [69], [70], [71], [72]. In general, cohort studies showed the benefits of vaccination during pregnancy, such as significantly decreased risks for preterm birth, small for gestational age, and fetal death. However, after adjusting for the season at the time of vaccination and countries' income level, only the reduction of fetal death remained significant [68]. There is no evidence of an association between influenza vaccination and serious adverse events in the comparative studies [69]. When assessing only major malformations, no increased risk was detected after immunization at any trimester. Neither adjuvanted nor unadjuvanted vaccines were associated with an increased risk for congenital anomalies [71].
Other systematic reviews also assessed the safety of different vaccines. One SR evaluated the safety of the hepatitis B vaccine, the pneumococcal polysaccharide vaccine, and the meningococcal polysaccharide vaccine during pregnancy and found no clear association with a teratogenic effect on the fetus, preterm labor, or spontaneous abortion [73]. Another SR evaluated the safety of vaccines frequently given to travelers on pregnant persons, such as yellow fever, MMR (mumps, measles, and rubella), influenza, Tdap (tetanus, diphtheria, and pertussis), meningococcus, or hepatitis A and B [74]. The authors concluded the safety of the influenza vaccine is supported by high-quality evidence. For the Tdap vaccine, no evidence of any unexpected harm was found in the meta-analysis of RCTs. Meningococcal vaccines are probably safe during pregnancy, as supported by RCTs comparing meningococcal vaccines to other vaccines. Data supported the safety of hepatitis A and hepatitis B vaccines during pregnancy. In summary, primary and secondary evidence of studies of vaccine components and platforms also used by COVID-19 vaccines supports the safety of COVID-19 vaccines, their components, or their platforms used in other vaccines during pregnancy.
Three recent studies about mRNA-LNP vaccines in pregnant persons, published after this rapid review was finalized, reinforced these findings [75], [76], [77]. Shimabukuro et al. published preliminary results from the U.S. surveillance review of the safety of mRNA COVID-19 vaccines during pregnancy [77]. The local and systemic reactions reported were similar among persons who identified as pregnant and non-pregnant persons. Prabhu et al. studied the antibody response of 122 pregnant persons and their neonates at the time of birth who had received one or both doses of an mRNA-based COVID-19 vaccine [75]. COVID-19 vaccination during pregnancy induced a robust maternal immune response, with transplacental antibody transfer detectable as early as 16 days after the first dose. Rottenstreich et al. reported on 20 pregnant persons who received two doses of the SARS-CoV-2 BNT162b2 (Pfizer/BioNTech) mRNA vaccine and found a similar antibody response [76]. No safety concern was reported in any of these studies. Also, the proportions of adverse pregnancy and neonatal outcomes among completed pregnancies in the registry were similar to the published incidences in pregnant populations studies before the COVID-19 pandemic [78], [79], [80], [81], [82], [83], [84].
This rapid review has several strengths. First, we included reports without time, language, or publication type restriction in humans and animals, to provide a timely answer to a hot topic. Second, we adhered to rigorous recommended quality standards to conduct rapid reviews [11] including independent, data extraction and risk of bias assessment, and a sensitive and comprehensive search strategy on literature databases to reduce the risk of missing relevant studies. Third, we categorized the exposure to the vaccine components and platforms, which was a challenging issue that frequently demanded exploring additional sources. Finally, we summarized and critically appraised a considerable amount of evidence to conclude if there are safety concerns of the components or platforms used by the vaccines that the COVAX MIWG selected for review in August 2020. The vaccine availability has changed over time [85], but we plan to update the search strategy covering the new vaccines for the ongoing full systematic review.
Our study is not exempt from limitations. Twenty-four percent of the included studies were reported as abstracts.
Only 11% of the total body of evidence comes from LMICs, limiting the generalizability to these settings. Additionally, only 76% of included studies allowed comparisons between vaccinated and unvaccinated pregnant persons, and only five of them were RCTs. Therefore, most of this evidence is observational. Nevertheless, the absence of safety concerns regardless of the study design and publication type suggest that this could not be a major limitation. Adverse events were reported by the classification used by authors of the original studies; however, we plan to analyze them in the ongoing full review accordingly to our protocol.
Moreover, the set of non-controlled studies do not show unexpected figures with respect to the incidences published in the peer-reviewed literature of neonatal or obstetric outcomes [77]. Regardless of the exposure, all reported rates of spontaneous abortion in exposed pregnant persons, described in Table 2, are below the reported highest global incidence of 31%, or 10%, when considering only losses occurring in clinically recognized pregnancies [78]. Tavares 2011, reported a rate of congenital anomalies of 1.9%, in line with the reported rate in the general population of approximately 2–4% of live births [79], [80], [81], [82], [83]. Regarding fetal death, rates reported by Läkemedelsverket 2010, (0.2%) in Sweden, are consistent with the reported rates of stillbirth for high-income countries: approximately 3 deaths per 1000 live births [84]. None of the included studies conducted in LMICs reported stillbirth rates, which have been reported to be higher than in HIC: approximately 21 deaths per 1000 live births in low-income countries [84].
We are aware that the list of Tdap vaccines included in our review is incomplete due to the focus of our research question. This vaccine contains aluminum phosphate as an adjuvant, which is not used for the COVID-19 vaccines under study, like the alhydrogel adjuvant. Therefore, our search strategy did not include the term “Tdap”. Nevertheless, any aluminum adjuvant retrieved by our search strategy was included and reported.
The nature of this rapid review did not allow us to search in FDA, the EMA websites, and clinical trials registers, or to contact authors and experts in the field to obtain additional data. For the same reason, we could not conduct the meta-analysis that is planned for the full review phase. Regarding COVID-19 and pregnancy registries, we identified 12 national or international databases with potentially helpful information on safety outcomes. These will be further inspected in the next phase of this work.
Based on existing data, it seems that there are no evident safety risks of COVID-19 vaccines, their components, or the technological platforms used for pregnant persons. It is reasonable to consider COVID-19 vaccination in pregnant persons because of their higher risk of adverse outcomes. The next full review phase will add more robust evidence over this critical public health issue.
Future experimental data will be needed to assess the pregnancy-related maternal and neonatal COVID-19 vaccine safety. Good quality safety registries, ideally with active surveillance, would also provide extremely useful evidence from real-world data.
Financial support
This work was supported, in whole, by the Bill & Melinda Gates Foundation [INV008443]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The sponsors had no role in conducting the present study.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Buekens Pierre M. reports financial support was provided by Bill & Melinda Gates Foundation.
Acknowledgement
We want to thank Ajoke Sobanjo-ter Meulen for her supervision and general support and Oduyebo Titilope for her feedback and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.08.034.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Thex L. Access to COVID-19 vaccines: looking beyond COVAX. Lancet. 2021;397:941. doi: 10.1016/S0140-6736(21)00617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eccleston-Turner M., Upton H. International Collaboration to Ensure Equitable Access to Vaccines for COVID-19: The ACT-Accelerator and the COVAX Facility. Milbank Q. 2021 doi: 10.1111/1468-0009.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog L.M., Norheim O.F., Emanuel E.J., McCoy M.S. Covax must go beyond proportional allocation of covid vaccines to ensure fair and equitable access. BMJ. 2021;372 doi: 10.1136/bmj.m4853. [DOI] [PubMed] [Google Scholar]
- 4.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. medRxiv : the preprint server for health sciences. 2021. COVID-19 vaccine response in pregnant and lactating women: a cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueiro-Filho E.A., Yudin M., Farine D. COVID-19 during pregnancy: an overview of maternal characteristics, clinical symptoms, maternal and neonatal outcomes of 10,996 cases described in 15 countries. J Perinat Med. 2020;48:900–911. doi: 10.1515/jpm-2020-0364. [DOI] [PubMed] [Google Scholar]
- 7.Vergara‐Merino L., Meza N., Couve‐Pérez C., Carrasco C., Ortiz‐Muñoz L., Madrid E., et al. Maternal and perinatal outcomes related to COVID-19 and pregnancy: An overview of systematic reviews. Acta Obstet Gynecol Scand. 2021;100(7):1200–1218. doi: 10.1111/aogs.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciapponi A., Bardach A., Comandé D., Berrueta M., Argento F.J., Rodriguez Cairoli F., et al. COVID-19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0253974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J, Thomas J, Chandler J, MS. C, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). In: Cochrane, editor. Cochrane, 2019.
- 11.Garritty C., Gartlehner G., Nussbaumer-Streit B., King V.J., Hamel C., Kamel C., et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. doi: 10.1016/j.jclinepi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohl K.S., Bonhoeffer J., Chen R., Duclos P., Heijbel H., Heininger U., et al. The Brighton Collaboration: enhancing comparability of vaccine safety data. Pharmacoepidemiol Drug Saf. 2003;12(4):335–340. doi: 10.1002/pds.851. [DOI] [PubMed] [Google Scholar]
- 14.CBER-FDA. In: Services USDoHaH, Administration F-FaD, Research C-CfBEa, editors. Guidance for Industry -Toxicity Grading Scale or Healthy Adult and Adolescent-Volunteers Enrolled in Preventive Vaccine Clinical Trials; 2007.
- 15.EMA. ICH Topic E 2 A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting EMA - European Medical Agency; 1995.
- 16.Fescharek Reinhard, K??bler J??rgen, Elsasser Ulrich, Frank Monika, G??thlein Petra. Medical Dictionary for Regulatory Activities (MedDRA) Int J Pharmaceut Med. 2004;18(5):259–269. [Google Scholar]
- 17.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation.
- 18.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane; 2011.
- 19.EPOC EPOoCCG. What study designs should be included in an EPOC review? EPOC Resources for review authors; 2017.
- 20.NIH. Study Quality Assessment Tools. NIH National Heart L, and Blood Institute (NHLBI); 2020.
- 21.Jan Brozek AO, Holger Schünemann. The GRADE Working Group GRADEpro. 3.2.2 for Windows. Updated March 2009; 2009.
- 22.Baum Ulrike, Leino Tuija, Gissler Mika, Kilpi Terhi, Jokinen Jukka. Perinatal survival and health after maternal influenza A(H1N1)pdm09 vaccination: A cohort study of pregnancies stratified by trimester of vaccination. Vaccine. 2015;33(38):4850–4857. doi: 10.1016/j.vaccine.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Celzo Froilan, Buyse Hubert, Welby Sarah, Ibrahimi Abdelilah. Safety evaluation of adverse events following vaccination with Havrix, Engerix-B or Twinrix during pregnancy. Vaccine. 2020;38(40):6215–6223. doi: 10.1016/j.vaccine.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Chavant F., Ingrand I., Jonville-Bera A.P., Plazanet C., Gras-Champel V., Lagarce L., et al. The PREGVAXGRIP study: a cohort study to assess foetal and neonatal consequences of in utero exposure to vaccination against A(H1N1)v2009 influenza. Drug Saf. 2013;36:455–465. doi: 10.1007/s40264-013-0030-1. [DOI] [PubMed] [Google Scholar]
- 25.Fell D.B., Sprague A.E., Liu N., Yasseen A.S., 3rd, Wen S.-W., Smith G., et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–e40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkenberg Maja, Callréus Torbjörn, Svanström Henrik, Valentiner-Branth Palle, Hviid Anders. Spontaneous reporting of adverse events following immunisation against pandemic influenza in Denmark November 2009-March 2010. Vaccine. 2011;29(6):1180–1184. doi: 10.1016/j.vaccine.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Galindo Santana B.M., Pelaez Sanchez O.R., Galindo Sardina M.A., Leon Villafuerte M., Concepcion Diaz D., Estruch Rancano L., et al. Active surveillance of adverse effects of Pandemrix vaccine to prevent influenza A(H1N1) in Cuba Vigilancia activa de eventos adversos a la vacuna Pandemrixpara prevenir la influenza AH1N1 en Cuba. 2011;63:231–238. [PubMed] [Google Scholar]
- 28.Glenn Gregory M., Fries Louis F., Smith Gale, Kpamegan Eloi, Lu Hanxin, Guebre-Xabier Mimi, et al. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Special Issue: Advancing maternal immunization programs through research in low and medium income countries. 2015;33(47):6488–6492. doi: 10.1016/j.vaccine.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Groom Holly C., Irving Stephanie A., Koppolu Padma, Smith Ning, Vazquez-Benitez Gabriela, Kharbanda Elyse O., et al. Uptake and safety of Hepatitis B vaccination during pregnancy: a Vaccine Safety Datalink study. Vaccine. 2018;36(41):6111–6116. doi: 10.1016/j.vaccine.2018.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groom Holly C., Smith Ning, Irving Stephanie A., Koppolu Padma, Vazquez- Benitez Gabriela, Kharbanda Elyse O., et al. Uptake and safety of hepatitis A vaccination during pregnancy: A Vaccine Safety Datalink study. Vaccine. 2019;37(44):6648–6655. doi: 10.1016/j.vaccine.2019.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y., Allen V., Bujold E., Coleman B., Drews S., Gouin K., et al. Efficacy and safety of pandemic influenza vaccine in pregnancy. Can J Infect Dis Med Microbiol. 2010;21:209. [Google Scholar]
- 32.Håberg Siri E., Trogstad Lill, Gunnes Nina, Wilcox Allen J., Gjessing Håkon K., Samuelsen Sven Ove, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. New Engl J Med. 2013;368(4):333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldens J.G.M., Pouwels H.G.W., Derks C.G.G., Van de Zande S.M.A., Hoeijmakers M.J.H. The first safe inactivated equine influenza vaccine formulation adjuvanted with ISCOM-Matrix that closes the immunity gap. Vaccine. 2009;27(40):5530–5537. doi: 10.1016/j.vaccine.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 34.Jonas F., Peter S., Cecilia L., Sven C., Anders E., Örtqvist Å., et al. Maternal vaccination against H1N1 influenza and offspring mortality: Population based cohort study and sibling design. BMJ (Online) 2015;351 doi: 10.1136/bmj.h5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Källén B., Olausson P.O. Vaccination against H1N1 influenza with Pandemrix® during pregnancy and delivery outcome: A Swedish register study. BJOG: Int J Obstetr Gynaecol. 2012;119:1583–1590. doi: 10.1111/j.1471-0528.2012.03470.x. [DOI] [PubMed] [Google Scholar]
- 36.Katz N., Neyro S., Carrega M.E.P., Del Valle Juarez M, Rancaño C., Pasinovich M., et al. Maternal immunization in argentina: The importance of a safety profile analysis. Open Forum. Infect Dis. 2016;3 [Google Scholar]
- 37.Kushner T., Youhanna J., Walker R., Erby K., Janssen R.S. Safety and immunogenicity of Heplisav-B in pregnancy. Hepatology. 2020;72:469A–470A. [Google Scholar]
- 38.Lacroix I., Damase-Michel C., Kreft-Jais C., Castot A.C., Montastruc J.L. H1N1 influenza vaccine in pregnant women: French pharmacovigilance survey. Drug Saf. 2010;33:908–909. doi: 10.1016/j.vaccine.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Läkemedelsverket. Läkemedelsverket. Final summary of adverse drug reaction reports in Sweden with Pandemrix through October 2009-mid April 2010. June 2, 2010; 2010. Accessed 23 May 2011 from www.lakemedelsverket.se.
- 40.Layton D., Dryburgh M., MacDonald T.M., Shakir S.A., MacKenzie I.S. Pilot swine flu vaccination active surveillance study: Final results. Drug Saf. 2011;34:889–890. [Google Scholar]
- 41.Levi M. Vaccination against influenza A(H1N1) virus is also safe during pregnancy. Ned Tijdschr Geneeskd. 2012;156 [Google Scholar]
- 42.Ludvigsson J.F., Strom P., Lundholm C., Cnattingius S., Ekbom A., Ortqvist A., et al. Risk for congenital malformation with H1N1 influenza vaccine: a cohort study with sibling analysis. Ann Inter Med. 2016;165:848–855. doi: 10.7326/M16-0139. [DOI] [PubMed] [Google Scholar]
- 43.Ludvigsson Jonas F., Zugna Daniela, Cnattingius Sven, Richiardi Lorenzo, Ekbom Anders, Örtqvist Åke, et al. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol. 2013;28(7):579–588. doi: 10.1007/s10654-013-9813-z. [DOI] [PubMed] [Google Scholar]
- 44.Mackenzie Isla S., MacDonald Thomas M., Shakir Saad, Dryburgh Moira, Mantay Brian J., McDonnell Patrick, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol. 2012;73(5):801–811. doi: 10.1111/j.1365-2125.2011.04142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madhi S.A., Polack F.P., Piedra P.A., Munoz F.M., Trenholme A.A., Simoes E.A., et al. Vaccination of pregnant women with respiratory syncytial virus vaccine and protection of their infants. N Engl J Med. 2020;383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHugh L., Marshall H.S., Perrett K.P., Nolan T., Wood N., Lambert S.B., et al. The safety of influenza and pertussis vaccination in pregnancy in a cohort of Australian mother-infant pairs, 2012–2015: the FluMum study. Clin Infect Dis. 2019;68:402–408. doi: 10.1093/cid/ciy517. [DOI] [PubMed] [Google Scholar]
- 47.Moro P.L., Museru O.I., Niu M., Lewis P., Broder K. Reports to the vaccine adverse event reporting system after hepatitis a and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.12.036. 561.e1–561.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro Pedro L., Zheteyeva Yenlik, Barash Faith, Lewis Paige, Cano Maria. Assessing the safety of hepatitis B vaccination during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990–2016. Vaccine. 2018;36(1):50–54. doi: 10.1016/j.vaccine.2017.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munoz F.M., Swamy G.K., Hickman S.P., Agrawal S., Piedra P.A., Glenn G.M., et al. Safety and Immunogenicity of a Respiratory Syncytial Virus Fusion (F) Protein Nanoparticle Vaccine in Healthy Third-Trimester Pregnant Women and Their Infants. J Infect Dis. 2019;220:1802–1815. doi: 10.1093/infdis/jiz390. [DOI] [PubMed] [Google Scholar]
- 50.Núñez Rojas Y., Orive Rodríguez N., Varona De La Peña F., Bermúdez Velásquez Y., Raad López A.F., Muñoz Martínez Y., et al. Vaccination against influenza a H1N1 and the risk of birth defects. VacciMonitor. 2010;19:209. [Google Scholar]
- 51.Oppermann Marc, Fritzsche Juliane, Weber-Schoendorfer Corinna, Keller-Stanislawski Brigitte, Allignol Arthur, Meister Reinhard, et al. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;30(30):4445–4452. doi: 10.1016/j.vaccine.2012.04.081. [DOI] [PubMed] [Google Scholar]
- 52.Pasternak B., Svanstrom H., Molgaard-Nielsen D., Krause T.G., Emborg H.D., Melbye M., et al. influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2009;2012:344. doi: 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasternak B., Svanstrom H., Molgaard-Nielsen D., Krause T.G., Emborg H.D., Melbye M., et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA J Am Med Assoc. 2012;308:165–174. doi: 10.1001/jama.2012.6131. [DOI] [PubMed] [Google Scholar]
- 54.Ray Joel G., McGeer Allison J., Blake Jennifer M., Lebovic Gerald, Smith Graeme N., Yudin Mark H. Peripartum outcomes: non-adjuvanted v. adjuvanted H1N1 vaccination. CMAJ : Can Med Assoc J = journal de l'Association medicale canadienne. 2014;186(2):137.1–137. doi: 10.1503/cmaj.114-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rega A., Moore H., De Klerk N., Effler P. Maternal vaccinations in Australia-uptake, safety and impact. Aust N Z J Obstet Gynaecol. 2016;56:13–14. [Google Scholar]
- 56.Sammon C.J., McGrogan A., Snowball J., De Vries C.S. Swine flu vaccination in pregnancy and associated miscarriage risk. Pharmacoepidemiol Drug Saf. 2011;20:S58–S59. [Google Scholar]
- 57.Sammon C.J., Snowball J., McGrogan A., de Vries C.S. Evaluating the Hazard of Foetal Death following H1N1 Influenza Vaccination; A Population Based Cohort Study in the UK GPRD. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0051734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stedman A., Wright D., Schreur P.J.W., Clark M.H.A., Hill A.V.S., Gilbert S.C., et al. Safety and efficacy of ChAdOx1 RVF vaccine against Rift Valley fever in pregnant sheep and goats. npj Vaccines. 2019;4 doi: 10.1038/s41541-019-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tavares Fernanda, Nazareth Irwin, Monegal Javier Sawchik, Kolte Ida, Verstraeten Thomas, Bauchau Vincent. Pregnancy and safety outcomes in women vaccinated with an AS03-adjuvanted split virion H1N1 (2009) pandemic influenza vaccine during pregnancy: a prospective cohort study. Vaccine. 2011;29(37):6358–6365. doi: 10.1016/j.vaccine.2011.04.114. [DOI] [PubMed] [Google Scholar]
- 60.COVID-19 vaccine tracker. Vaccine Centre at the London School of Hygiene & Tropical Medicine; 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ [Accessed May 20th 2021].
- 61.A Study of Ad26.COV2.S in Healthy Pregnant Participants (COVID-19); 2021. https://ClinicalTrials.gov/show/NCT04765384.
- 62.Study to Evaluate the Safety, Tolerability, and Immunogenicity of SARS CoV-2 RNA Vaccine Candidate (BNT162b2) Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older; 2021. https://ClinicalTrials.gov/show/NCT04754594.
- 63.COVID-19: study to detect transfer of SARS-Cov-2 antibodies in breastmilk; 2021. https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-000893-27/BE.
- 64.Bratton K.N., Wardle M.T., Orenstein W.A., Omer S.B. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis. 2015;60:e11–e19. doi: 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- 65.Demicheli V., Jefferson T., Ferroni E., Rivetti A., Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;2:CD001269. doi: 10.1002/14651858.CD001269.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG. 2015;122(1):17–26. doi: 10.1111/1471-0528.12977. [DOI] [PubMed] [Google Scholar]
- 67.Giles Michelle L., Krishnaswamy Sushena, Macartney Kristine, Cheng Allen. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccin Immunother. 2019;15(3):687–699. doi: 10.1080/21645515.2018.1540807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeong S., Jang E.J., Jo J., Jang S. Effects of maternal influenza vaccination on adverse birth outcomes: A systematic review and Bayesian meta-analysis. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0220910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michiels Barbara, Govaerts Frans, Remmen Roy, Vermeire Etienne, Coenen Samuel. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29(49):9159–9170. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Nunes Marta, Aqil Anushka, Omer Saad, Madhi Shabir. The Effects of Influenza Vaccination during Pregnancy on Birth Outcomes: A Systematic Review and Meta-Analysis. Am J Perinatol. 2016;33(11):1104–1114. doi: 10.1055/s-0036-1586101. [DOI] [PubMed] [Google Scholar]
- 71.Polyzos K.A., Konstantelias A.A., Pitsa C.E., Falagas M.E. Maternal Influenza Vaccination and Risk for Congenital Malformations: A Systematic Review and Meta-analysis. Obstet Gynecol. 2015;126:1075–1084. doi: 10.1097/AOG.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Chuan, Wang Xiaodong, Liu Dan, Zhang Lingli, Sun Xin. A systematic review and meta-analysis of fetal outcomes following the administration of influenza A/H1N1 vaccination during pregnancy. Int J Gynaecol Obstet. 2018;141(2):141–150. doi: 10.1002/ijgo.12394. [DOI] [PubMed] [Google Scholar]
- 73.Makris Marinos C., Polyzos Konstantinos A., Mavros Michael N., Athanasiou Stavros, Rafailidis Petros I., Falagas Matthew E. Safety of hepatitis B, pneumococcal polysaccharide and meningococcal polysaccharide vaccines in pregnancy: a systematic review. Drug Saf. 2012;35(1):1–14. doi: 10.2165/11595670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.Nasser R., Rakedzon S., Dickstein Y., Mousa A., Solt I., Peterisel N., et al. Are all vaccines safe for the pregnant traveller? A systematic review and meta-analysis. J Travel Med. 2020;27 doi: 10.1093/jtm/taz074. [DOI] [PubMed] [Google Scholar]
- 75.Prabhu M., Murphy E.A., Sukhu A.C., Yee J., Singh S., Eng D., et al. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet Gynecol. 2021 doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magnus M.C., Wilcox A.J., Morken N.H., Weinberg C.R., Haberg S.E. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364 doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marden Philip M., Smith David W., McDonald Michael J. Congenital anomalies in the newborn infant, including minor variations. a study of 4,412 babies by surface examination for anomalies and buccal smear for sex chromatin. J Pediatr. 1964;64(3):357–371. doi: 10.1016/s0022-3476(64)80188-8. [DOI] [PubMed] [Google Scholar]
- 80.Leppig Kathleen A., Werler Martha M., Cann Cristina I., Cook Catherine A., Holmes Lewis B. Predictive value of minor anomalies. I. Association with major malformations. J Pediatr. 1987;110(4):531–537. doi: 10.1016/s0022-3476(87)80543-7. [DOI] [PubMed] [Google Scholar]
- 81.Holmes Lewis B. Current concepts in genetics. Congenital malformations. N Engl J Med. 1976;295(4):204–207. doi: 10.1056/NEJM197607222950406. [DOI] [PubMed] [Google Scholar]
- 82.Mai Cara T., Isenburg Jennifer L., Canfield Mark A., Meyer Robert E., Correa Adolfo, Alverson Clinton J., et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111(18):1420–1435. doi: 10.1002/bdr2.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldkamp M.L., Carey J.C., Byrne J.L.B., Krikov S., Botto L.D. Etiology and clinical presentation of birth defects: population based study. BMJ. 2017;357 doi: 10.1136/bmj.j2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H., Bhutta Z.A., Coates M.M., Coggeshall M., Dandona L.D.K., et al. Global, regional, national, and selectedsubnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: asystematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1725. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.WHO. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process; 2021. Accessed 12 May 2011 from: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_04May2021.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.