Highlights
-
•
Methohexital-induced LPDs occur rarely during Wada testing in epilepsy.
-
•
Ipsilateral LPDs may impair memory testing during the Wada.
-
•
EEG monitoring during the Wada test is recommended.
Keywords: Epilepsy surgery, Brain tumors, Language lateralization, Neuropsychology, Interictal discharges
Abstract
Objective
The Wada test is used to evaluate language lateralization and memory performance after inactivation of an isolated cerebral hemisphere. Methohexital a short-acting barbiturate has a history of use to induce interictal discharges during intraoperative corticography. We report a new finding of activation of lateralized periodic discharges (LPDs) after Methohexital injection.
Methods
We retrospectively reviewed 174 consecutive adult patients who underwent Wada testing in preparation for epilepsy surgery (N = 129, 74%) or brain tumor resection (N = 45, 26%) at the University of Michigan to determine the frequency of induced periodic discharges by methohexital.
Results
Four epilepsy patients (2.29%) had methohexital-induced LPDs within a median of 2 s (1–99 s) of the injection and lasting a median of 4 min (3–10 min) after a total of 7 injections. All LPDs occurred ipsilateral to the injection hemisphere in the known region of interictal epileptiform discharges. LPDs were not induced in brain tumor patients. In one patient, LPDs occurred during memory testing, and this patient's memory performance was below expectation based on pre-test neuropsychological testing.
Conclusions
Methohexital can induce LPDs in ipsilateral hemisphere and that can potentially affect memory performance.
Significance
This observation indicates that concurrent EEG monitoring during the Wada test is important and that induced discharges should be considered when interpreting Wada test results.
1. Introduction
The Wada test, or intracarotid amobarbital procedure (IAP), is an established procedure to evaluate language lateralization and memory performance before surgery in epilepsy and brain tumor patients (Milner et al., 1962). The test was developed using amobarbital (Amytal), but methohexital (Brevital) is a short-acting barbiturate favored by many institutions because of its faster recovery time compared to amobarbital and the resultant decrease in procedure duration (Buchtel et al., 2002). Multiple shortages in amobarbital in mid-1998 and 2004 resulted in a more widespread use of methohexital. In one survey of 82 international epilepsy centers, 76% of the centers reported ability to perform the Wada test, and 43% reported they are likely to use Wada test for language lateralization (Benjamin et al., 2018).
Historically, methohexital was used during epilepsy surgery to induce interictal discharges identified by intraoperative electrocorticography with intravenous doses of 40–60 mg (Chui et al., 2013). Despite enhancement of existing spikes in 50–85% of patients, the practice fell out of favor due to inappropriate activation of distal spike foci in as many as 43% of the cases, raising concerns about its utility (Chui et al., 2013, Fiol et al., 1990). Methohexital was also used in the past to induce epileptiform activity during scalp EEG recording with intravenous administration of incremental doses of 5–50 mg, up to 1.9 mg/kg (Wilder et al., 1971), but this practice also fell out of favor because sleep alone was found to be similarly effective (Celesia and Paulsen, 1972).
A known rare complication of methohexital use is induction of seizures. During Wada testing, unintended induction of seizures complicated 0.5% of Wada tests as previously reported by our center (Beimer et al., 2015). Methohexital is a commonly used anesthetic in electroconvulsive therapy (ECT) (Stripp et al., 2018), and occasionally induces tonic-clonic seizures immediately after administration of methohexital and before the ECT treatment (Vande Voort et al., 2013).
The effect of methohexital on interictal epileptiform activity during the Wada test is not well reported. There is also an accumulating body of evidence suggesting a disturbing effect of interictal epileptiform discharges on neuropsychological testing performance, with multiple aspects affected – processing speed, psychomotor response, and multiple cognitive domains (Karakis et al., 2020), raising the possibility that this interictal epileptiform activity may confound performance on the Wada test.
2. Material and methods
We performed a retrospective review of consecutive Wada tests in adult patients, ages 18 and older, at the University of Michigan between 2010 and 2018. The recordings were reviewed for evidence of baseline epileptiform discharges and the effect of methohexital on the presence of epileptiform discharges. All the recordings were reviewed by experienced neurophysiologists board certified in clinical neurophysiology and/or epilepsy. When the recordings showed activation of periodic epileptiform discharges, the cognitive results of the Wada test were compared to the pretest comprehensive neuropsychological evaluation.
The Wada procedure at University of Michigan is adaptive, starting with an initial injection of 3 mg of methohexital over a 3-second period. A second injection of 2 mg over a 2-second period is given as soon as the patient begins to show signs of recovery from the first injection (usually resumption of contralateral grip strength from 0/5 to 2/5 or the beginnings of expressive language after injection into the speech hemisphere). Memory items are presented during the period in which the medication is considered in effect based on clinical response and concurrent EEG. A detailed description of our Wada procedure has been previously published (Buchtel et al., 2002).
The study was approved by the University of Michigan Institutional Review Board (no. HUM00004086).
3. Results
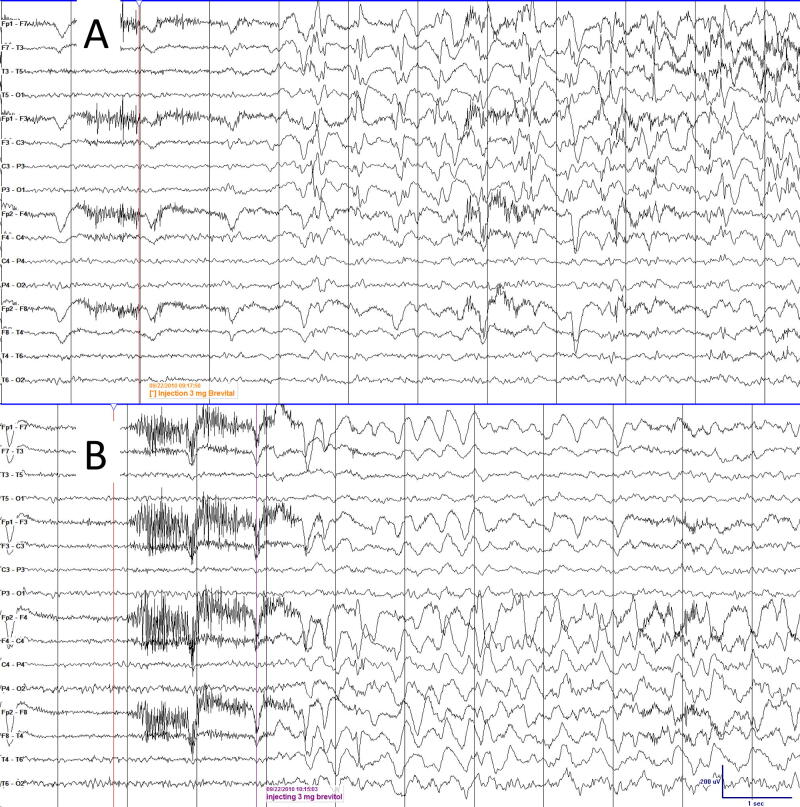
A total of 174 patients were identified who completed Wada testing in anticipation of surgery for epilepsy (n 129, 74%) or brain tumor (n 45, 26%). Four epilepsy cases (2.29 %) were identified in whom intra-arterial methohexital injection induced periodic epileptiform discharges within a median of 2 s (range 1–99 s) of the injection after a total of 7 injections, (Table 1, Table 2). The epileptiform discharges were ipsilateral to the injection site and abundant, in agreement with the American Clinical Neurophysiology Society (ACNS) criteria for lateralized periodic discharges (LPDs) (Hirsch et al., 2013), (Fig. 1A). The LPDs resolved within a median of 4 min (range 3–10 min) and none progressed to seizures. These LPDs were an induction of the patients’ typical interictal epileptiform discharges and did not induce any new distribution of epileptiform activity, (Fig. 1B). All LPDs were ipsilateral to the side of methohexital injection. In one patient with bilateral and broad field interictal discharges at baseline, discharges contralateral to the side of injection were induced but were not periodic.
Table 1.
Patient characteristics.
| ID | Age | Gender | Handedness | MRI Findings | Diagnosis | Baseline EEG IEDs |
|---|---|---|---|---|---|---|
| 1 | 41 | M | Right | Left MTS | Refractory focal epilepsy | No discharges |
| 2 | 25 | M | Mixed | Left superior temporal gyrus and insula | Refractory focal epilepsy/LGS | Bifrontal sharp waves |
| 3 | 24 | M | Right | Bilateral MTS | Refractory focal epilepsy | Right temporal sharp waves |
| 4 | 20 | F | Right | Nonlesional | Refractory focal epilepsy | Left temporal spikes |
IEDs, interictal discharges; MTS, mesial temporal sclerosis; LGS, Lennox-Gastaut Syndrome; M, male; F, female.
Table 2.
Results.
| ID | Side | Time (sec) | Duration (min) | Dose (mg) |
|---|---|---|---|---|
| 1 | Left | 7 | 10.3 | 3 + 2 |
| 2 | Left | 2 | 4 | 3 + 2 |
| Right | 1 | 0.3 | 3 + 2 | |
| Right | 1 | 0.8 | 3 + 2 | |
| 3 | Right | 99 | 4.7 | 3 |
| 4 | Left | 2 | 3.2 | 3 + 2 |
| Left | 1 | 2.7 | 3 + 2 |
Each injection is represented with side of injection, time after first injection to start of lateralized periodic discharges (LPDs), duration of LPDs, and dose of methohexital (typically given in two divided doses of 3 mg and 2 mg).
Fig. 1.
A. Lateralized periodic discharges (LPDs) after left internal carotid injection. B. Expected right hemispheric slowing and no LPDs after right carotid injection.
Three of four patients had their memory tested after the resolution of the LPDs, and the results were consistent with the previous neuropsychological evaluations. In one of these patients (# 2), the left internal carotid methohexital injection induced ipsilateral LPDs during the presentation of memory items. The Wada test confirmed left lateralization of language function in this patient. The resulting memory score for the right hemisphere was lower than expected (43.8%) and inconsistent with the pre-Wada neuropsychological evaluation which suggested that the patient’s non-dominant hemisphere (right in this case) would be capable of normal memory function. Specifically, the patient's memory for verbal material was in the impaired range while his memory for pictorial material was in the low-average range (Table 3, Table 4). After resolution of the left hemisphere LPDs, the right hemisphere was injected, and the patient’s memory performance was in the normal range.
Table 3.
Pre-Wada neuropsychological testing results.
| ID |
VCI (100 ± 15) |
PCI (100 ± 15) |
FSIQ (100 ± 15) |
WMS-IV (10 ± 3) |
WRAT-4 (100 ± 15) | MMSE (>23) | BNT(60) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Verbal Recall | Pictorial Recall | |||||||||
| 1 | 72 | 84 | 70 | Immediate: Delayed: |
7 4 |
Immediate: Delayed: |
11 11 |
70 | 27 | 45 |
| 2 | 83 | 79 | 68 | Immediate: Delayed: |
4 1 |
Immediate: Delayed: |
1 7 |
93 | 27 | 46 |
| 3 | 66 | 71 | 63 | Immediate: Delayed: |
** 1 |
Immediate: Delayed: |
** 2 |
65 | 16 | 30 |
| 4 | 107 | 117 | 108 | Immediate: Delayed: |
13 14 |
Immediate: Delayed: |
12 13 |
100 | 30 | 52 |
VCI, Verbal composite IQ score; PCI, Perceptual composite IQ score; FSIQ, full scale IQ; WRAT-4, Wide Range Achievement Test-4; MMSE, Mini-Mental State Exam; BN, Boston Naming Test; WMS-IV, Wechsler Memory Scale, 4th Edition; ** data not available.
Table 4.
Wada results.
| ID | Left side memory | Right side memory | Language lateralization |
|---|---|---|---|
| 1 | 28.5% | 87.5% | Left≫Right |
| 2 | 100% | 43.8% | Left |
| 3 | ** | ** | Left |
| 4 | 100% | 100% | Left |
** modality not tested; left-sided memory testing during right.
Internal carotid artery injection and vice-versa.
4. Discussion
We found that intra-arterial methohexital can rarely induce lateralized periodic discharges (LPDs) ipsilateral to the injection hemisphere in epilepsy patients undergoing the Wada test.
Lateralized periodic discharges after methohexital injection were identified only during Wada testing for epilepsy surgical evaluation and not in the one fourth of the cohort undergoing Wada strictly for brain tumor resection. This can be due to lack of epilepsy in vast majority of tumor patients and suggests that methohexital can induce LPDs in a more susceptible and established epileptic network in refractory epilepsy rather than newly diagnosed brain tumor. Three of the four cases found were in people of low intelligence quotient (FSIQ ≤ 70), supporting the idea that broader pathologic dysfunction predisposes to methohexital-induced LPDs.
In three of our patients, the administration of the objects for memory encoding happened after the resolution of the LPDs, and the results of the Wada test for memory were consistent with the previous neuropsychological evaluation. However, in one patient, LPDs occurred contralateral to the tested hemisphere during presentation of memory items, and the patient's recall for items was worse than expected. This is an unanticipated phenomenon with an unclear mechanism, suggesting that the effect of periodic interictal discharges can be beyond the local brain region from which it apparently originates. Although the relationship between LPDs and memory encoding during Wada testing is not definite, this finding suggests that interpretation of Wada results should take into consideration the presence of LPDs and is consistent with the emerging consensus that interictal epileptiform discharges are associated with cognitive dysfunction in epilepsy patients (Karakis et al., 2020, Glennon et al., 2016).
5. Conclusion
In conclusion, methohexital rarely induces ipsilateral periodic discharges in epilepsy patients undergoing Wada test, but when this does occur, it may complicate the interpretation of memory test results. Real-time EEG interpretation should be performed during the Wada test in patients with epilepsy to detect activation of interictal epileptiform discharges.
Although none of the tumor patients had LPDs induced by methohexital injection, this could not completely exclude this possibility and the EEG real-time review should be considered for this group as well.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7. Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Author Contributions
Omar Danoun: Conception and the design of the project, Data collection, Data analysis, Writing and reviewing.
Nicholas Beimer: Conception and the design of the project, Manuscript review, writing and editing.
Henry Buchtel: Manuscript review and editing, Data collection and analysis.
Simon Glynn: conception and the design of the project, manuscript review, writing and editing.
David Harris: Conception and the design of the project, Data collection and analysis, Manuscript review, Writing and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Beimer N.J., Buchtel H.A., Glynn S.M. One center's experience with complications during the Wada test. Epilepsia. 2015;56:e110–e113. doi: 10.1111/epi.13046. [DOI] [PubMed] [Google Scholar]
- Benjamin C.F.A., Dhingra I., Li A.X., Blumenfeld H., Alkawadri R., Bickel S., Helmstaedter C., Meletti S., Bronen R.A., Warfield S.K., Peters J.M., Reutens D., Połczyńska M.M., Hirsch L.J., Spencer D.D. Presurgical language fMRI: technical practices in epilepsy surgical planning. Hum. Brain Mapp. 2018;39(10):4032–4042. doi: 10.1002/hbm.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtel H.A., Passaro E., Selwa L.M., Deveikis J., Gomez-Hassan D. Methohexital (Brevital) as anesthetic in the Wada Test. Epilepsia. 2002;43:1056–1061. doi: 10.1046/j.1528-1157.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- Celesia G.G., Paulsen R.E. Electroencephalographic activation with sleep and methohexital. Comparative usefulness in the diagnosis of epilepsy. Arch. Neurol. 1972;27:361–363. doi: 10.1001/archneur.1972.00490160089013. [DOI] [PubMed] [Google Scholar]
- Chui J., Manninen P., Valiante T., Venkatraghavan L. The anesthetic considerations of intraoperative electrocorticography during epilepsy surgery. Anesth. Analg. 2013;117:479–486. doi: 10.1213/ANE.0b013e318297390c. [DOI] [PubMed] [Google Scholar]
- Fiol M.E., Torres F., Gates J.R., Maxwell R. Methohexital (Brevital) effect on electrocorticogram may be misleading. Epilepsia. 1990;31(5):524–528. doi: 10.1111/j.1528-1157.1990.tb06101.x. [DOI] [PubMed] [Google Scholar]
- Glennon J.M., Weiss-Croft L., Harrison S., Cross J.H., Boyd S.G., Baldeweg T. Interictal epileptiform discharges have an independent association with cognitive impairment in children with lesional epilepsy. Epilepsia. 2016;57:1436–1442. doi: 10.1111/epi.13479. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J. Clin. Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Karakis I., Lynam C., Taraschenko O., Staikova E., Drane D.L. Concurrent EEG monitoring helps interpret neuropsychological testing results in patients with epilepsy. Epilepsy Behav. 2020;111 doi: 10.1016/j.yebeh.2020.107275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B., Branch C., Rasmussen T. Study of short-term memory after intracarotid injection of Sodium Amytal. Trans. Am. Neurol. Assoc. 1962;87:224–226. [Google Scholar]
- Stripp T.K., Jorgensen M.B., Olsen N.V. Anaesthesia for electroconvulsive therapy - new tricks for old drugs: a systematic review. Acta Neuropsychiatr. 2018;30:61–69. doi: 10.1017/neu.2017.12. [DOI] [PubMed] [Google Scholar]
- Vande Voort J.L., Swintak C.C., Wall C.A., Rasmussen K.G., Jr. Methohexital-Induced Seizures During Electroconvulsive Therapy. J ECT. 2013;29:e4–e5. doi: 10.1097/YCT.0b013e3182610596. [DOI] [PubMed] [Google Scholar]
- Wilder B.J., Musella L., Van Horn G., Schmidt R.P. Activation of spike and wave discharge in patients with generalized seizures. Neurology. 1971;21:517–527. doi: 10.1212/wnl.21.5.517. [DOI] [PubMed] [Google Scholar]