Abstract
Objectives
This study was aimed to identify the number, shape and distribution of megakaryocytes and to recognize blast cells and its location.Further, to identify presence of fibrosis or increased microvascularity.Also,study correlation between histological findings.
Methods
A retrospective was conducted between January 2016 to December 2018 at al-mouwasat university hospital. A total of 44 cases of myeloproliferative disorders using H&E and IHC stains were studied.Chi-square test was performed with descriptive statistics.
Results
Most of our patients were men younger than 75 years of age. 40 was the most prevalent as a median number of megakaryocytes in bone marrow biopsy with normal shape and diffuse pattern, most of biopsies were fibrotic, paratrabecular pattern and absent of hemosiderin deposits that correlate significantly to women patients.Minimal blast cells were more common with diffuse pattern.
Conclusion
Bone marrow biopsy is a useful investigation in myeloproliferative disorders. Evaluation of megakaryopoiesis, fibrosis, and localization of blasts are possible on a bone marrow biopsy.
Keywords: Myeloproliferative disorders, Chronic myeloid leukemia, Essential thrombocytosis, Polycythemia vera, Marrow fibrosis
Highlights
-
•
Myeloproliferative disorders originate in multipotent myeloid progenitors
•There is increased production of one or more types of blood cells
•The term “myeloproliferative disorders” include chronic myeloid leukemia, polycytethemia vera, essential thrombocythemia and marrow fibrosis.
•The main treatment for myeloproliferative disorders is chemotherapy and bone marrow transplantation.
1. Introduction
Increased proliferative drive in the bone marrow is a common feature of myeloproliferative disorders.Also, variable transformation either to marrow fibrosis or acute leukemia.myeloproliferative disorders include chronic myeloid leukemia, polycythemia vera, essential thrombocytosis, and marrow fibrosis. Most originate from multipotent myeloid progenitors. there is considerable degree of clinical and morphological overlap among myeloproliferative disorders [1]. Under the category of myeloproliferative neoplasms (MPNs), the new edition of the 2016 World Health Organization (WHO) classification system for tumors of the hematopoietic and lymphoid tissues includes seven subcategories: chronic myeloid leukemia, chronic neutrophilic leukemia, polycythemia vera (PV), primary myelofibrosis (PMF), essential thrombocythemia (ET), chronic eosinophilic leukemia-not otherwise specified and MPN, unclassifiable (MPN-U) [3]. The current problem remains to develop our understanding of histological findings of bone marrow biopsy and use it as a diagnostic and evaluating tool for patients with myeloproliferative disorders. (see Chart (1), Chart (2))
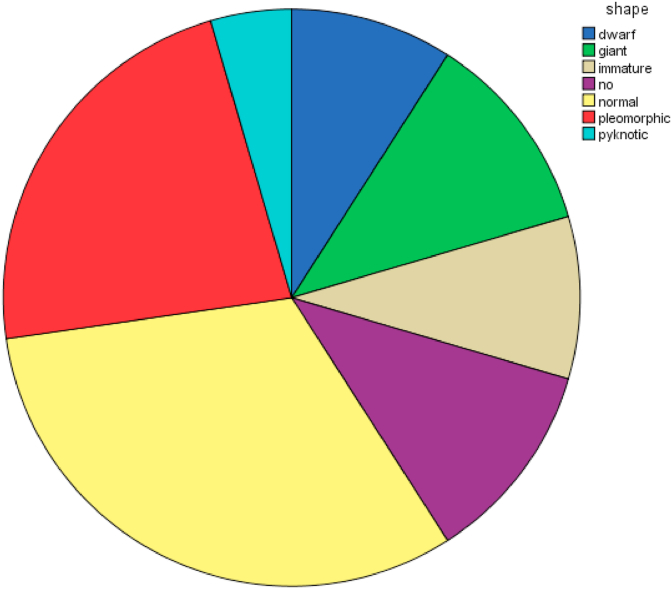
Chart (1).
This chart shows percentage of each morphology of megakaryocytes.
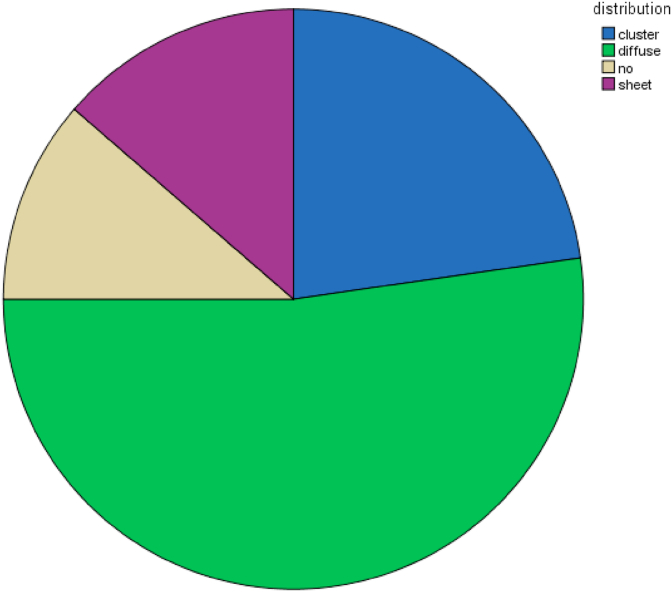
Chart (2).
This chart shows percentage of distribution of megakaryocytes.
2. Materials and methods
This is a retrospective cohort study. Bone marrow biopsy was done in forty four patients of myeloproliferative disorders. None of the patients were received any therapy at time of biopsy. The biopsy was stained with haematoxylin and eosin. Immunohistochemistry and special stains could not be done and genetic data were not available due to financial difficulties. This study was reported in line with STROCSS criteria [4].
A detailed study of the morphology and distribution of megakaryocytes was done as described by Burkhardt et al. [2] Megakaryocytes were classified as normal, dwarf, immature, giant, pleomorphic, blastic and pyknotic.The distribution was noted as diffuse, sheets or clustered.the fibrosis evaluated by increased angiogenesis.Blast cells identified by IHC and H&E stains. Statistical analysis was done by chi-square test. Also, descriptive statistics were performed. Chi-square test was done among different variables which are: Number,shape and distribution of megakaryocytes, fibrosis and its location, blasts and their location,hemosiderin and its location,age and sex.
3. Results
The main median number of megakaryocytes was 40 [Table 1] with normal shape being most common (n = 14, 31.8%) [Table 2] followed by pleomorphic shape (n = 10, 22.7%) [Table 2]. The most common distribution of megakaryocytes is diffuse type (n = 23, 52%) [Table 3] followed by cluster type (n = 10, 22.7%) [Table 3]. The highest cellularity ranged between 95% and 0% with the most common cellularity was 90% (n = 15, 34.1%) [Table 4] followed by 80% (n = 7, 15.9%) [Table 4].The most common finding in bone marrow biopsies was presence of fibrosis (n = 28, 63,3%) [Table 5] with location of trabecular pattern [Table 6] followed by diffuse fibrosis [Table 6]. Minimal blasts was more prevalent than high frequency of blasts (n = 37,84.1%) [Table 7] with diffuse type (n = 6,13.65%) [Table 8]. Hemosiderin deposition was less common than hemosiderin depletion (n = 27,61.4%) [Table 9 and Table 10]. Age of patients ranged between 75 and 1 year old [Table 11]. Most of patients were male (n = 23, 52.3%) [Table 12].
Table 1.
This table reveals median number of megakaryocytes.
| Statistics | ||
|---|---|---|
| median | ||
| N | Valid | 44 |
| Missing | 0 | |
| Mode | 1.00 | |
| Range | 40.00 | |
| Minimum | .00 | |
| Maximum | 40.00 | |
Table 2.
This table reveals different shapes of megakaryocytes.
| shape | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | dwarf | 4 | 9.1 | 9.1 | 9.1 |
| giant | 5 | 11.4 | 11.4 | 20.5 | |
| immature | 4 | 9.1 | 9.1 | 29.5 | |
| no | 5 | 11.4 | 11.4 | 40.9 | |
| normal | 14 | 31.8 | 31.8 | 72.7 | |
| pleomorphic | 10 | 22.7 | 22.7 | 95.5 | |
| pyknotic | 2 | 4.5 | 4.5 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 3.
This table reveals distribution of megakaryocytes.
| distribution | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | cluster | 10 | 22.7 | 22.7 | 22.7 |
| diffuse | 23 | 52.3 | 52.3 | 75.0 | |
| no | 5 | 11.4 | 11.4 | 86.4 | |
| sheet | 6 | 13.6 | 13.6 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 4.
This table reveals bone marrow cellularity.
| cellularity | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | .00 | 5 | 11.4 | 11.4 | 11.4 |
| .30 | 1 | 2.3 | 2.3 | 13.6 | |
| .40 | 1 | 2.3 | 2.3 | 15.9 | |
| .50 | 2 | 4.5 | 4.5 | 20.5 | |
| .70 | 5 | 11.4 | 11.4 | 31.8 | |
| .75 | 2 | 4.5 | 4.5 | 36.4 | |
| .80 | 7 | 15.9 | 15.9 | 52.3 | |
| .85 | 2 | 4.5 | 4.5 | 56.8 | |
| .90 | 15 | 34.1 | 34.1 | 90.9 | |
| .95 | 4 | 9.1 | 9.1 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 5.
This table reveals presence and abscence of fibrosis.
| fibrosis | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | no | 16 | 36.4 | 36.4 | 36.4 |
| yes | 28 | 63.6 | 63.6 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 6.
This table reveals location of fibrosis.
| location of fibrosis | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | cells | 5 | 11.4 | 11.4 | 11.4 |
| diffuse | 7 | 15.9 | 15.9 | 27.3 | |
| multifocal | 1 | 2.3 | 2.3 | 29.5 | |
| no | 16 | 36.4 | 36.4 | 65.9 | |
| paratrabecular | 9 | 20.5 | 20.5 | 86.4 | |
| perivascular | 3 | 6.8 | 6.8 | 93.2 | |
| unifocal | 3 | 6.8 | 6.8 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 7.
This table reveals maximal and minimal number of blasts.
| blasts | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | no | 37 | 84.1 | 84.1 | 84.1 |
| yes | 7 | 15.9 | 15.9 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 8.
This table reveals location of blasts.
| location of blasts | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | diffuse | 6 | 13.6 | 13.6 | 13.6 |
| no | 37 | 84.1 | 84.1 | 97.7 | |
| perivascular | 1 | 2.3 | 2.3 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 9.
This table reveals hemosiderin deposits.
| hemosiderin | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | no | 27 | 61.4 | 61.4 | 61.4 |
| yes | 17 | 38.6 | 38.6 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 10.
This table reveals location of hemosiderin.
| hemosiderin location | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | central | 8 | 18.2 | 18.2 | 18.2 |
| no | 27 | 61.4 | 61.4 | 79.5 | |
| peripheral | 9 | 20.5 | 20.5 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Table 11.
This table reveals age of study patients.
| Statistics | ||
|---|---|---|
| Age | ||
| N | Valid | 31 |
| Missing | 13 | |
| Mode | 50.00 | |
| Range | 74.00 | |
| Minimum | 1.00 | |
| Maximum | 75.00 | |
Table 12.
This table reveals sex category of study patients.
| sex | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | F | 21 | 47.7 | 47.7 | 47.7 |
| M | 23 | 52.3 | 52.3 | 100.0 | |
| Total | 44 | 100.0 | 100.0 | ||
Significant association was found in the relationship between:
Median number and shape of megakaryocytes [Table 13].
Table 13.
This table reveals chi square tests between different variables.
| value | df | A sym.sig.(2-sided) | |
|---|---|---|---|
| median ∧ shape | 131.117 | 102 | 0.028 |
| median ∧ distribution | 92.549 | 51 | 0 |
| median ∧ cellularity | 191.348 | 153 | 0.019 |
| median ∧ fibrosis | 18.38 | 17 | 0.365 |
| median ∧ blasts | 17.66 | 17 | 0.411 |
| median ∧ location of blasts | 57.698 | 34 | 0.006 |
| median ∧ hemosiderin | 19.195 | 17 | 0.317 |
| median ∧ Age | 344.875 | 330 | 0.275 |
| median ∧ sex | 20.237 | 17 | 0.262 |
| shape ∧ distribution | 60.771 | 18 | 0 |
| shape ∧ cellularity | 76.334 | 54 | 0.024 |
| shape ∧ fibrosis | 3.101 | 6 | 0.796 |
| shape ∧blasts | 6.198 | 6 | 0.401 |
| shape ∧hemosiderin | 2.665 | 6 | 0.85 |
| shape ∧age | 115.918 | 132 | 0.839 |
| shape ∧sex | 1.999 | 6 | 0.92 |
| distribution ∧ cellularity | 42.101 | 27 | 0.032 |
| distribution ∧ fibrosis | 4.193 | 3 | 0.241 |
| distribution ∧ blasts | 2.043 | 3 | 0.564 |
| distribution ∧ hemosiderin | 2.359 | 3 | 0.501 |
| distribution ∧ age | 70.846 | 66 | 0.319 |
| distribution ∧ sex | 0.821 | 3 | 0.844 |
| cellularity ∧ fibrosis | 7.71 | 9 | 0.564 |
| cellularity ∧ blasts | 6.198 | 9 | 0.72 |
| cellularity ∧ hemosiderin | 8.369 | 9 | 0.49 |
| cellularity ∧ age | 177.968 | 176 | 0.444 |
| cellularity ∧ sex | 9.071 | 9 | 0.431 |
| fibrosis ∧ location of fibrosis | 44 | 6 | 0 |
| fibrosis ∧ blasts | 4.423 | 1 | 0.035 |
| fibrosis ∧ hemosiderin | 0.277 | 1 | 0.598 |
| fibrosis ∧ age | 22.264 | 22 | 0.444 |
| fibrosis ∧ sex | 0.052 | 1 | 0.82 |
| blasts ∧ location of blasts | 44 | 2 | 0 |
| blasts ∧ hemosiderin | 0.036 | 1 | 0.803 |
| blasts ∧ age | 19.912 | 22 | 0.589 |
| blasts ∧ sex | 1.224 | 1 | 0.269 |
Median number and distribution of megakaryocytes [Table 13].
Median number of megakaryocytes and total cellularity [Table 13].
Median number of megakaryocytes and location of blasts [Table 13].
Shape of megakaryocytes and its distribution [Table 13].
Shape of megakaryocytes and total cellularity [Table 13].
Distribution of megakaryocytes and total cellularity [Table 13].
Fibrosis and location of fibrosis [Table 13].
Fibrosis and blasts [Table 13].
Blasts and location of blasts [Table 13].
Hemosiderin and its location [Table 14].
Table 14.
This table reveals chi square tests between available variables.
| value | df | A sym.sig.(2-sided) | |
|---|---|---|---|
| hemosiderin ∧ location of hemosiderin | 44 | 2 | 0 |
| hemosiderin ∧ age | 21.516 | 22 | 0.489 |
| hemosiderin ∧ sex | 3.201 | 1 | 0.074 |
| age ∧ sex | 18.988 | 22 | 0.646 |
Hemosiderin and sex [Table 14].
4. Discussion
In this study, we investigated the number,shape,and distribution of megakaryocytes in bone marrow biopsy from patients with myeloproliferative disorders and also evaluated the presence of fibrosis and blasts.Our study found that the average age of patients was 51.The most common distribution of megakaryocytes was in diffuse pattern followed by cluster pattern in contrast to the german study which the megakaryocytes were clusters rather than diffusely distributed [2]. Most of our cases were men who were younger than 75 years of age in contrast to german study [2] which were women comprising the majority.It is important to note that in the present study 63.3% of cases were fibrotic paratrabecular pattern which consists with the german study [2].Majority of cases that we encountered showed absence of blasts with high frequency and most being presented in diffuse pattern. Also, consists with the german study [2]. Our study has some limitations are lack of clinical information and insufficient immunohistochemical stains.
5. Conclusion
Evaluation of megakaryopoiesis,fibrosis and blasts with their location are novel findings should be studied in every bone marrow biopsy so we recommend to use bone marrow biopsy in patients with myeloproliferative disorders as it provides many information that maybe of clinical significance.
Sources of funding
No funds
Ethical approval
This is retrospective cohort study. Ethical approval not needed.
Trial registry number.
-
1.
Name of the registry: Research Registry.
-
2.
Unique Identifying number or registration ID: Research registry 6952
Consent
No consent obtained as consent form was not applicable to our study.
This is retrospective study, collecting data from file of patients.
Declaration of competing interest
Author has no conflict of interest.
Provenance and peer review.
Not commissioned, externally peer reviewed.
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Registration of research studies
In accordance with the Declaration of Helsinki 2013, all research involving human participants has to be registered in a publicly accessible database. Please enter the name of the registry and the unique identifying number (UIN) of your study.
Annals of medicine and surgery
The following information is required for submission. Please note that failure to respond to these questions/statements will mean your submission will be returned. If you have nothing to declare in any of these categories then this should be stated.
Please state any conflicts of interest
All authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
All the authors declare that they have no conflicts of interest.
Ethical approval
Research studies involving patients require ethical approval. Please state whether approval has been given, name the relevant ethics committee and the state the reference number for their judgement.
No ethical approval was needed.
Please state any sources of funding for your research
All sources of funding should be declared as an acknowledgement at the end of the text. Authors should declare the role of study sponsors, if any, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. If the study sponsors had no such involvement, the authors should so state.
There was no funding.
Author contribution
Please specify the contribution of each author to the paper, e.g. study concept or design, data collection, data analysis or interpretation, writing the paper, others, who have contributed in other ways should be listed as contributors.
Consent
Studies on patients or volunteers require ethics committee approval and fully informed written consent which should be documented in the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102628.
Contributor Information
Rihan Mhmed Ali, Email: ryhanmhmdly490@gmail.com, ryhanmhmdly95@gmail.com.
Verra Masoud, Email: vera_yusuf@yahoo.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Robbins S.L., Kumar V., Cotran R.S. eighth ed. Saunders/Elsevier; Philadelphia, PA: 2010. Robbins and Cotran Pathologic Basis of Disease. [Google Scholar]
- 2.Burkhardt R., Bartl R., Jager K., Frisch B., Kettner G., Mahl G., Sund M. Working classification of chronic myeloproliferative disorders based on histological, haematological, and clinical findings. J. Clin. Pathol. 1986;39(3):237–252. doi: 10.1136/jcp.39.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbui T., Thiele J., Gisslinger H., Kvasnicka H.M., Vannucchi A.M., Guglielmelli P., Orazi A., Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Canc. J. 2018 Feb 9;8(2):15. doi: 10.1038/s41408-018-0054-y. PMID: 29426921; PMCID: PMC5807384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., for the Strocss Group The STROCSS 2019 guideline: strengthening the reporting of cohort studies in Surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.